Abstract
Sperm entering the epididymis are immotile and cannot respond to stimuli that will enable them to fertilize. The epididymis is a highly complex organ, with multiple histological zones and cell types that together change the composition and functional abilities of sperm through poorly understood mechanisms. Sperm take up taurine during epididymal transit, which may play antioxidant or osmoregulatory roles. Cysteine dioxygenase (CDO) is a critical enzyme for taurine synthesis. A previous study reported that male CDO−/− mice exhibit idiopathic infertility, prompting us to investigate the functions of CDO in male fertility. Immunoblotting and quantitative reverse transcription-polymerase chain reaction analysis of epididymal segments showed that androgen-dependent CDO expression was highest in the caput epididymidis. CDO−/− mouse sperm demonstrated a severe lack of in vitro fertilization ability. Acrosome exocytosis and tyrosine phosphorylation profiles in response to stimuli were normal, suggesting normal functioning of pathways associated with capacitation. CDO−/− sperm had a slight increase in head abnormalities. Taurine and hypotaurine concentrations in CDO−/− sperm decreased in the epididymal intraluminal fluid and sperm cytosol. We found no evidence of antioxidant protection against lipid peroxidation. However, CDO−/− sperm exhibited severe defects in volume regulation, swelling in response to the relatively hypo-osmotic conditions found in the female reproductive tract. Our findings suggest that epididymal CDO plays a key role in post-testicular sperm maturation, enabling sperm to osmoregulate as they transition from the male to the female reproductive tract, and provide new understanding of the compartmentalized functions of the epididymis.
Keywords: Epididymal maturation, fertilization, sperm, taurine
Summary
Mammalian sperm develop the osmoregulatory capability during epididymal transit. Cysteine dioxygenase (CDO) is a critical enzyme for taurine synthesis. Using genetically engineered mice, this study demonstrates that epididymal CDO plays a key role in post-testicular sperm maturation by producing taurine for sperm osmoregulation as they transition from the male to the female reproductive tract with different tonicities.
INTRODUCTION
Mammalian sperm leaving the testis are morphologically mature but must develop functionally through two distinct maturational processes, in the epididymis and the female reproductive tract. Epididymal maturation is poorly understood, despite being essential for enabling the signaling pathways that govern fertilization ability, as well as for acquisition of motility and the development of the osmoregulatory capability that allows the sperm to move among the different fluid environments in the male and female reproductive tracts [1, 2]. Epididymal maturation involves complex interactions between sperm and the intraluminal milieu, regulated by absorptive and secretory activities of the epididymal epithelium under androgenic control. Gene expression in the epididymal epithelium is region specific, resulting in different compositions of epididymal intraluminal fluid among epididymal regions [2].
Mammalian sperm experience considerable changes in their osmotic environment after ejaculation. For example, murine sperm normally encounter a decrease in osmotic pressure when they enter the uterus (osmotic pressure, 320 mOsm) [3] from the cauda epididymis (420 mOsm) [4]. This drastic change necessitates that sperm osmoregulate to avoid excessive changes in volume. Previous studies that characterized the osmotic tolerance of sperm, from the caput, corpus, and cauda regions, demonstrated the ability of sperm to regulate volume changes in response to osmotic challenges that develop during epididymal maturation [5, 6].
Epididymal intraluminal fluid contains high concentrations of various organic components that act as intracellular osmolytes in other cells. Of these, taurine is one of the most abundant [7, 8] and plays important roles in osmoregulation and antioxidant defense in several cell types [9, 10]. In fact, its precursor, hypotaurine, is a more potent oxygen free radical scavenger [11]. The roles of taurine and hypotaurine in sperm include antioxidation [12], enhanced motility [13–16], and increased fertilization potential [13]. Despite the important roles of taurine and hypotaurine in regulating sperm function, the mechanisms by which these organic components support the fertilizing potential of sperm are poorly understood.
Taurine is synthesized via a pathway that involves dioxygenation of cysteine into cysteine sulfinate by cysteine dioxygenase (CDO). Cysteine sulfinate is then metabolized by decarboxylation to produce hypotaurine, which is further oxidized into taurine. This is believed to be the major pathway for synthesizing hypotaurine and taurine in the body [17, 18]. The abundance and activity of CDO respond to the availability of cysteine in vitro and in vivo [19–21], which appears to result from the actions of cysteine on downregulation of CDO ubiquitination and degradation, as well as post-translational production of a CDO protein cofactor, which is dependent on substrate (cysteine) turnover by CDO [21]. CDO is expressed in the liver, adipose tissue, pancreas, kidneys, and lungs [22].
The physiological importance of CDO has been investigated using CDO null (CDO−/−) mice. CDO−/− mice have a higher incidence of postnatal mortality, exhibit a postnatal growth deficit, have very low levels of tissue and plasma taurine, and show signs of connective tissue pathology [23]. Interestingly, severe male infertility was observed when reproductive ability was tested by natural mating of CDO−/− males with wild-type (WT) females, despite the presence of mature sperm in the epididymis [23], suggesting that CDO may play an important role in male fertility, possibly through the physiological actions of taurine and hypotaurine. However, the exact mechanisms by which loss of CDO causes male infertility remain unclear and constitute the central objective of the current study.
In this study, we found that CDO is primarily expressed in the caput epididymidis and essential for epididymal taurine and hypotaurine production. Furthermore, HPLC analysis of epididymal luminal fluid and sperm cytosol showed that taurine and hypotaurine are taken up by sperm during epididymal transit. Although there was no difference in sperm lipid peroxidation between genotypes, osmotic challenge test showed that CDO−/− sperm resulted in tail angulation due to defects in osmoregulation. Our results provide new insight into epididymal maturation and sperm function.
RESULTS
CDO protein and mRNA expression in different epididymal segments
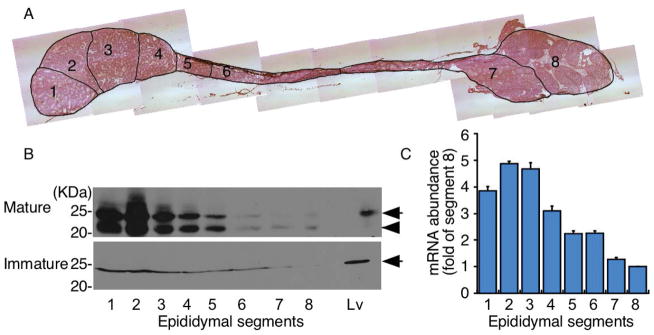
Epididymal gene expression is highly regulated by androgens [24]. To obtain the CDO expression profile and examine the association between changes involved in sexual maturation and CDO expression, epididymides were collected from sexually mature (retired breeder) and immature (4-week-old) mice. They were separated into eight segments based on connective tissue septae, observable with transmitted light under a dissecting microscope (Fig. 1A), and the presence of CDO was assessed by immunoblotting. CDO expression was very high in segments 1–3, but decreased significantly by segment 8 (Fig. 1B top). Two distinct bands were observed from 20–25 kDa in the epididymis collected from mature mice (Fig. 1B top). These two bands are consistent with a previous study demonstrating the formation of a cysteine-tyrosine cross-linked cofactor (lower band) that migrated below the immature, non-cofactor-containing form (upper band) [21]. Interestingly, we only detected a single CDO-immunoreactive band corresponding to the non-cofactor form in epididymis collected from immature mice (Fig. 1B bottom). In addition, CDO expression levels were much lower in the epididymis of immature versus mature mice. No CDO was detected in sperm by immunoblotting (data not shown).
Figure 1.
Characterization of epididymal cysteine deoxygenase (CDO) expression. (A) Histology of mouse epididymides collected from sexually mature mice showing segments 1–8. (B) Comparison of CDO protein expression in the epididymis of mature and immature (4-week-old) male mice. CDO in the liver (Lv) is shown for comparison [22]. Each lane was loaded with 100 μg epididymal proteins. Immunoblotting for CDO revealed that CDO expression (arrow) was abundant in segments 1–3 of the caput region, but was dramatically lower in subsequent segments towards the cauda region. Higher CDO expression was found in mature versus immature mice. CDO expression in mature mice was accompanied by the appearance of the non-cofactor (arrow) and cysteine-tyrosine cross-linked cofactor (arrow head) forms (B, top). In contrast, only the non-cofactor form of CDO was detected in immature mice (B, bottom). (C) CDO mRNA quantification in sections of epididymides from sexually mature mice was consistent with the high CDO expression level in the caput compared with that in the corpus and cauda regions. Data are means ± standard error (SE) of five independent replicates.
To confirm the CDO expression pattern in the epididymis of sexually mature mice, CDO mRNA levels were also assessed in all segments using quantitative RT-PCR. Consistent with the immunoblotting results, these data showed a high abundance of CDO in segments 1–3 in the epididymis of mature mice (Fig. 1C).
CDO localization in the epididymis of WT and CDO−/− mice
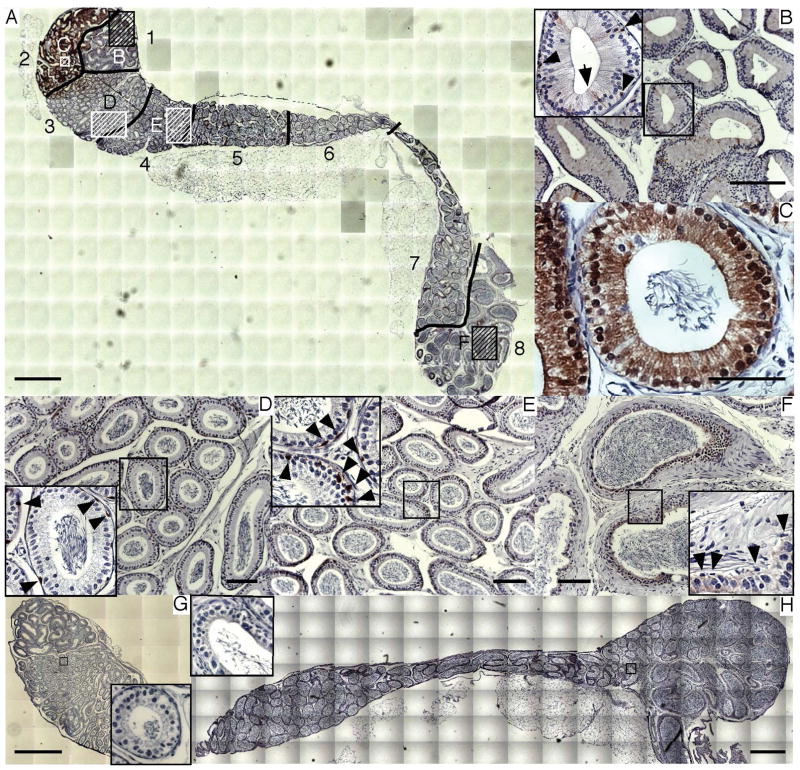
The epididymis is composed of several different cell types. To determine localization of CDO within the epididymis, immunohistochemistry was performed on paraffin-embedded sections of whole epididymis using anti-CDO antibody. The epididymis from CDO−/− mice was also stained as a negative control. As results, high CDO expression was detected in segments 1–3, consistent with the immunoblotting and quantitative RT-PCR results (Fig. 2A). When segment 1 was observed at high magnification, CDO was detected in multiple cell types, including narrow (arrow) and basal cells (arrowhead) adjacent to smooth muscle cells, which comprise the outer layer of tubules (Fig. 2B inset). Interestingly, CDO was localized in the cytoplasm and nuclei of epithelial cells in segment 2 (Fig. 2C). CDO was also localized in the basal cells (arrow heads) of segments 3 (Fig. 2D, inset) and 4 (Fig. 2E, inset). However, CDO expression was not detected in the basal cells of segment 8, although very faint expression was observed in the epithelial cytoplasm (Fig. 2F, inset). In contrast, CDO−/− epididymis was morphologically normal but no CDO expression was observed in the caput, corpus, or cauda regions (Fig. 2G and H). In addition, no immunoreactivity was detected when WT epididymis was labelled with secondary antibody alone, confirming specificity of the anti-CDO antibody. No CDO expression was detected in paraffin-embedded testes collected from WT mice (data not shown).
Figure 2.
CDO localization in the epididymis. Immunohistochemistry was performed in wild-type (WT) (A) and CDO−/− mice (G, H). The entire epididymis, consisting of segments 1–8 (see Fig. 1), was captured using sets of tiled images. CDO was expressed specifically in the narrow (arrow) and basal cells (arrow head) adjacent to smooth muscle cells in segment 1 (b, inset). CDO was most abundant in segment 2, consistent with Figure 1(B and C). Notably, CDO was localized in both the cytoplasm and nucleus. Segment 3 exhibited less CDO expression compared with that in segment 2 (D). CDO localization was observed in basal cells in segments 3 and 4 (arrowhead; D and E, inset). However, CDO expression in basal cells disappeared in segment 8 (F, inset). As a control for staining specificity, CDO was not detected in either the caput (G) or corpus and cauda regions (H) of CDO−/− epididymis. Bars = 1 mm (A, G, H), 100 μm(B, D, E, F), 50 μm (C).
Fertility assays with CDO null sperm in vitro
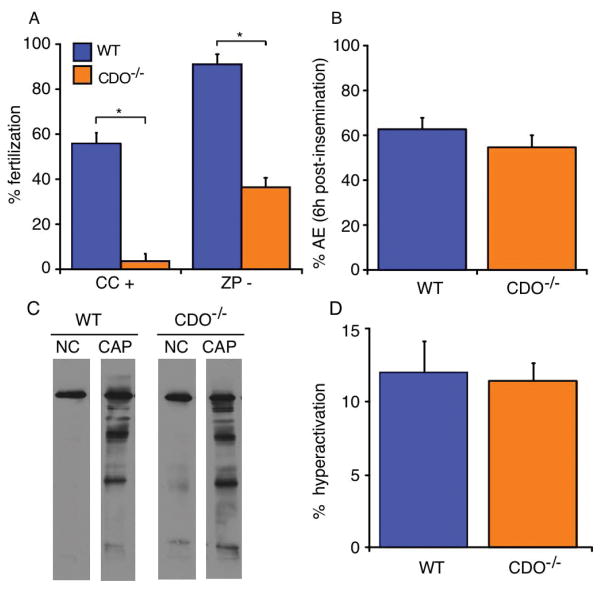
A previous study reported that mating CDO−/− males with WT females does not result in pregnancy [23]. However, a robust analysis of male CDO−/− fertility was not performed; thus, infertility could have resulted from factors other than sperm function, such as behavioral deficiencies, defects in ejaculation, production of seminal plasma, etc. Therefore, we performed IVF tests using WT and CDO−/− sperm. Surprisingly, CDO−/− sperm showed significantly lower fertility (3.5%) than that of WT (55.9%) using cumulus-intact oocytes for IVF (P < 0.05) (Fig. 3A). The fertilizing capability of CDO−/− sperm (36.3%) remained significantly lower than that of WT (91.1%; P < 0.05) when zona-free oocytes were inseminated, suggesting that the principal defect was not in zona binding or penetration. Taken together with the previous results of natural mating [23], these results clearly demonstrate impaired fertility with CDO−/− sperm.
Figure 3.
In vitro fertilization (IVF) with cumulus intact (CC+) and zona pellucida-free (ZP−) oocytes. (A) CDO−/− sperm had lower fertilizing ability compared with that of WT sperm using either CC+ or ZP− oocytes (*P < 0.05). Data are means ± SE of five independent replicates for each experiment. (B) Acrosome exocytosis (AE) of WT and CDO−/− sperm co-incubated with CC+ for 6 h were not significantly different. Data are means ± SE from four independent replicates. (C) Tyrosine phosphorylation of WT and CDO−/− sperm proteins after incubation under non-capacitating (NC) or capacitating conditions (CAP). No difference was observed in the capacitation-induced phosphorylation profile of WT and CDO−/− sperm. A representative immunoblot is shown from three replicate experiments. (D) Hyperactivation of WT and CDO−/− sperm incubated under CAP condition. No difference was observed between genotypes. Data are means ± SE from four independent replicates.
To examine the cause of the impaired fertilization, we performed an acrosome exocytosis (AE) assay using sperm co-incubated with cumulus-intact oocytes for 6 h. Cumulus cells surrounding oocytes and zona pellucida are a physiological stimulator of AE in murine sperm [25, 26]. No significant difference in the percentage of sperm that underwent AE before insemination was detected between WT and CDO−/− sperm (data not shown). Similarly, the percentage of sperm that underwent AE was not different between WT (62.6%) and CDO−/− sperm (54.5%; Fig. 3B) when sperm were incubated with cumulus-intact oocytes for 6 h, suggesting that the impaired fertility in CDO−/− mice was not due to a reduced ability of sperm to undergo AE.
In addition, we examined the ability of CDO−/− sperm to respond to capacitation stimuli by evaluating tyrosine phosphorylation of sperm proteins (Fig. 3C) and hyperactivated motility (Fig. 3D). No difference in the extent of tyrosine phosphorylation of proteins was observed between WT and CDO−/− sperm (Fig. 3C). Consistently, percentage of hyperactivation in CDO−/− sperm (11.4%) did not differ from that in WT sperm (12.0%; Fig. 3D) although sperm representing hyperactivation motion significantly increased in response to capacitation stimuli (data not shown). Previous studies showed in sperm of several species that flagellar beating pattern and head yaw can be influenced by fluid viscosity [27, 28]. Although the hyperactivation assay of CDO−/− sperm was conducted using a culture medium which has different viscosity from the mucus, we found the consistency of severe infertility between our IVF experiments and previous natural mating test [23]. This, combined with the results of tyrosine phosphorylation status and AE assay, suggests normal ability of CDO−/− sperm to undergo capacitation.
Morphological analysis
A strict morphological comparison of sperm revealed slightly higher percentage of abnormalities in CDO−/− sperm compared with that in WT sperm (68.7 vs. 53.8%, respectively, P < 0.05; Fig. 4A). CDO−/− mice had a significantly increased percentage of sperm with abnormal heads (71.9%) than that of WT mice (59.7%; P < 0.05). Sperm head abnormalities mainly included minor distortion involving the apical and distal parts of the sperm head. No difference in the percentage of sperm carrying abnormal mid- or principle pieces or cytoplasmic droplets was detected (Fig. 4B). These results showed a slight increase in the frequency of sperm head morphological abnormalities in CDO−/− mice.
Figure 4.
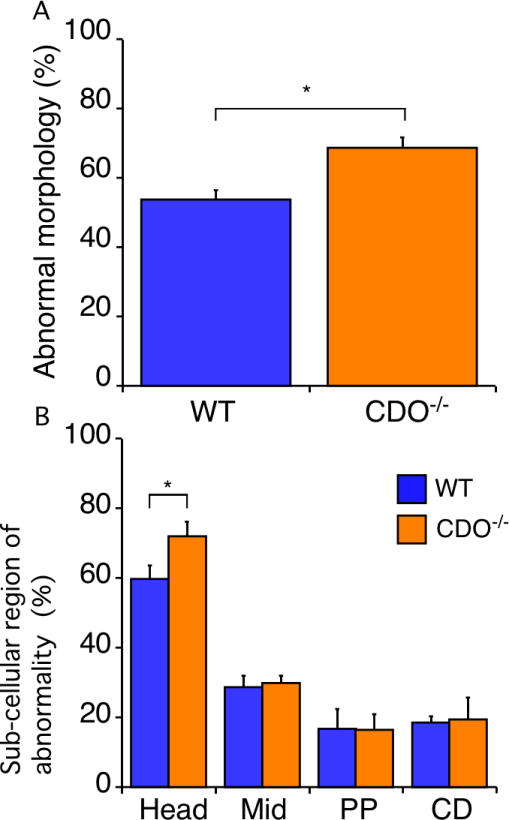
Morphological analysis. The morphologies of WT and CDO−/− sperm were classified as normal, deformed sperm head (head), midpiece deformed or bent at the distal midpiece or annulus ring (Mid), principal piece deformed or kinked (PP), or containing cytoplasmic droplets (CD). (A) Gross abnormalities were higher in CDO−/− versus WT sperm (*P < 0.05). Data are means ± SE from five independent replicates. (B) Morphological classification of abnormal sperm. CDO−/− sperm had a higher rate of head segment abnormalities compared with WT sperm (*P < 0.05). Data are means ± SE.
Quantification of epididymal taurine and hypotaurine in CDO−/− mice
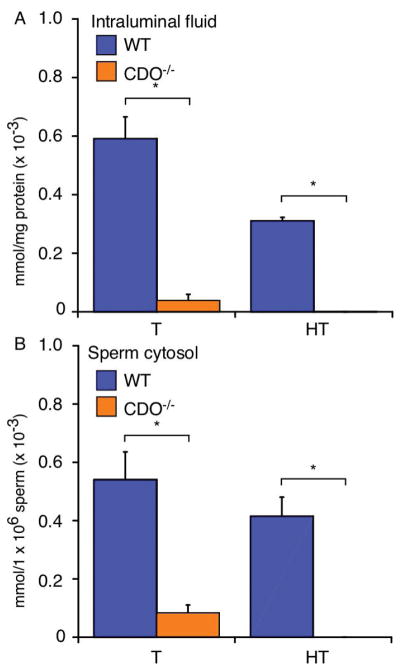
Taurine and hypotaurine play roles in osmoregulation and exert antioxidant effects in several cell types [9]. They are abundant in epididymal intraluminal fluid [7, 29] and sperm [29, 30]. Combined with our immunohistochemistry and IVF results, this suggests that taurine and hypotaurine may play roles in epididymal maturation. Therefore, we measured taurine and hypotaurine levels in epididymal intraluminal fluid, as well as in sperm collected from WT and CDO−/− mice. The levels of both were markedly higher in epididymal intraluminal fluid from WT mice (taurine 0.59 × 10−3 mmol/mg protein; hypotaurine, 0.31 × 10−3 mmol/mg protein) compared with those in CDO−/− mice (hypotaurine, 0.001 × 10−3 mmol/mg protein; taurine, 0.07 × 10−3 mmol/mg protein) (P < 0.05) (Fig. 5A). The sperm cytosol revealed markedly higher levels of both amino acids in WT (taurine, 0.54 mmol/1 × 106 cells; hypotaurine, 0.41 × 10−3 mmol/1 × 106 cells) versus those in CDO−/− mice (taurine, 0.09 mmol/1 × 106 cells; hypotaurine, not detected; P < 0.05; Fig. 5B). These results suggest that CDO−/− mice had a severe lack of taurine and hypotaurine in their epididymal intraluminal fluid and sperm.
Figure 5.
Quantification of epididymal taurine and hypotaurine contents. (A) Epididymal intraluminal fluid. CDO−/− mice had significantly less taurine and hypotaurine in the intraluminal fluid compared with WT mice (*P < 0.05). Data are means ± SE from four independent replicates. (B) Sperm cytosolic fraction. Cytosolic taurine and hypotaurine contents were greater in WT versus CDO−/− sperm. Data are means ± SE of four independent replicates.
Roles of taurine and hypotaurine in sperm during fertilization
Taurine and hypotaurine are antioxidants [9, 10, 31] and organic osmolytes [9, 31] in several cell types. For example, both taurine and hypotaurine prevent loss of motility in rabbit sperm by reducing the generation of superoxide leading to lipid peroxidation [12]. Therefore, we examined the degree of membrane lipid peroxidation in WT and CDO−/− sperm, but initial experiments detected no difference in the quantity of lipid hydroperoxides between genotypes (data not shown).
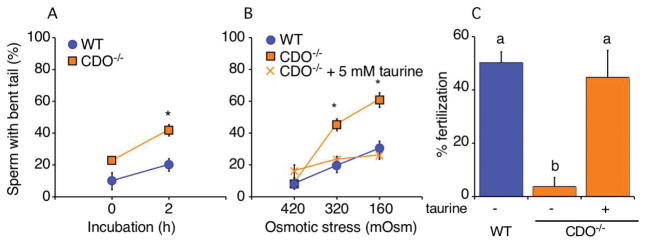
It has been hypothesized that organic osmolytes are important for sperm to regulate cell volume in response to the acute osmotic change that occurs when they move into the female reproductive tract after ejaculation. Previous studies using sperm from c-ros- and aquaporin3-null mice demonstrated that failure to regulate cell volume manifests as flagellar coiling or angulation in the sperm (particularly bending at the annulus), and that these abnormalities result in infertility [4, 32]. To assess this possibility in CDO−/− mice, we incubated sperm from knockout and WT mice under NC conditions for 2 h and assessed the number of sperm with bent tails. Use of NC medium allowed us to distinguish problems in volume regulation versus flagellar bending, which can occur in response to incubation with sterol efflux mediators. Significantly more CDO−/− sperm had bent tails (45.0%) compared with WT sperm (20.0%; P < 0.05) under NC conditions, although no significant difference between genotypes was detected at time 0 prior to incubation (Fig. 6A). These results led us to hypothesize that the lack of taurine and hypotaurine in CDO−/− sperm resulted in a reduced capacity to regulate volume.
Figure 6.
Tail angulation assay as a proxy for defects in volume regulation. (A) Tail angulation in WT and CDO−/− sperm incubated for 2 h under NC conditions (260 mOsm). Following the incubation, more CDO−/− sperm manifested bent tails compared with WT sperm (*P < 0.05). Data are means ± SE of three independent replicates. (B) Tail angulation in WT and CDO−/− sperm after a 15-min incubation in TYH of different osmotic pressures (420, 320, or 160 mOsm). To assess whether exogenous taurine could rescue/prevent sperm tail angulation at the lower tonicities, CDO−/− sperm were also incubated in the presence of 5 mM taurine. The number of sperm with bent tails increased dramatically in CDO−/− compared with WT sperm when they were incubated at 320 or 160 mOsm (*P < 0.05, WT versus CDO−/−). Supplementing with 5 mM taurine completely abolished the increased tail angulation in CDO−/− sperm at the lower osmotic pressures. Data are means ± SE from five independent replicates for the sperm tail angulation observations under different osmotic pressures, and four independent replicates for the taurine-rescue experiment. (C) IVF of WT and CDO−/− sperm. To examine the roles of taurine in sperm, CDO−/− sperm were pre-incubated and inseminated under the presence or absence of 5 mM taurine. CDO−/− sperm showed lower fertility than WT sperm. However, supplementing taurine to pre-incubation and insemination rescued the impaired fertility (abP < 0.05). Data are means ± SE from five independent replicates for IVF experiment.
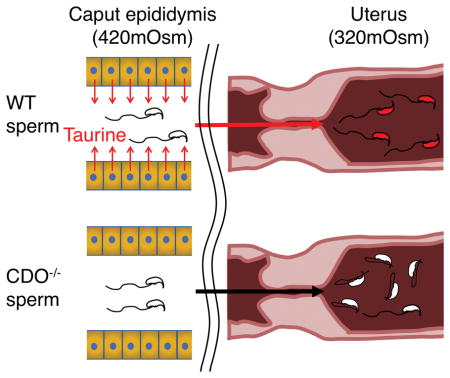
After ejaculation, sperm encounter a dramatic decrease in osmotic pressure as they move from the cauda epididymal fluid (420 mOsm) [4] into the uterine fluid (320 mOsm) [3]. To confirm our hypothesis, we examined the osmoadaptation capacity of WT and CDO−/− sperm by evaluating the number of sperm with bent tails after incubation in medium of different osmolarities (420, 320, and 160 mOsm) with or without 5 mM taurine. As results, no difference in tail bending was observed between WT and CDO−/− sperm incubated in 420 mOsm medium, which mimicked the osmolarity of the intraluminal fluid of the cauda epididymidis (7–16%; Fig. 6B). However, CDO−/− sperm incubated in 320 mOsm, which mimicked the uterine fluid osmolarity, or at 160 mOsm, showed significantly increased tail bending (42.6 and 60.8% at 320 and 160 mOsm, respectively) than that in WT sperm (22.5 and 33.5% at 320 and 160 mOsm, respectively). Interestingly, 5 mM taurine rescued these osmoregulatory defects at 320 mOsm (23.3% vs. 42.6%) and at 160 mOsm (25.9% vs. 60.8%). These results suggest that sperm take up taurine to regulate cell volume. To confirm the physiological importance of taurine uptake in sperm, we performed IVF with WT and CDO−/− sperm under presence or absence of 5 mM taurine. Same as above, CDO−/− sperm showed significantly lower fertility than WT sperm (3.6% vs 50.2%). However, the fertilizing ability in CDO−/− sperm was improved to the same level as WT sperm when 5 mM taurine was added during both pre-incubation and insemination (44.8%). These results demonstrate that infertility of CDO−/− sperm results from lack in taurine that causes flagellar angulation.
DISCUSSION
We found that CDO expressed primarily in the caput epididymidis played a key role in sperm function, by producing taurine critical for sperm osmoregulation as they moved from environments with different tonicities, such as from the male into the female reproductive tract. These findings add to our knowledge of the compartmentalized functions of the epididymis, as well as providing a mechanism that explains several other observations reported previously.
CDO is a major enzyme involved in synthesizing taurine, which is highly abundant in epididymal intraluminal fluid. Epididymal gene expression is characterized by strong regional specificity [33]. The absorptive and secretory activities of the epididymal epithelium result in a unique intraluminal environment that differs between segments. Our immunoblotting, quantitative RT-PCR, and immunohistochemical data from eight epididymal segments demonstrated that CDO was most abundantly expressed in the narrow and basal cells of segments 1 and 3 of the caput region, and all cells in segment 2 of the caput. Interestingly, immunohistochemical staining demonstrated that CDO was abundantly localized in the cytoplasm and nuclei of various cell types in segment 2. Consistent with these findings, CDO localizes in the cytoplasm and nucleus in rat mammary adipocytes [34]. In addition, taurine is localized in the cytoplasm and nucleus of Leydig and epididymal epithelial cells [35], showing that taurine is present in the nuclear compartment. These differences among segments within the caput region reflect the highly specialized functions that vary from segment to segment even within the same cell type, providing opportunities to improve our understanding of epididymal physiology.
Consistent with our expression and localization findings, a previous study reported that the next enzyme in the taurine metabolic pathway, cysteine sulfinate decarboxylase (CSD), is also expressed in various cell types in the caput [36]. CDO and CSD mRNA expression decrease after orchidectomy [24, 37], showing the androgen dependence of these enzymes. Surprisingly, immunoblotting revealed that only the non-cofactor form of epididymal CDO is present in immature mice, whereas the cofactor-containing and non-cofactor forms can be extracted from mature mice, consistent with the pattern seen in other mammalian tissues [20, 38, 39]. Production of the cofactor-containing form increases the catalytic efficiency and half-life of CDO at physiologically relevant cysteine concentrations [21, 38]. Therefore, the functional activity of epididymal CDO may also be regulated by sexual maturation.
Phospholemman, a taurine channel, and multiple taurine transporter isoforms are expressed at higher levels in the caput region than in the corpus and cauda regions [40]. This suggests that taurine is synthesized and secreted into the intraluminal compartment at higher levels in the caput region compared with other areas. However, the mechanisms involved in taurine and hypotaurine uptake into sperm during epididymal transit remain unknown. One possibility is that the hypertonic environment of epididymal fluid is associated with regulation of this process. Sperm of many mammals are exposed to increasing tonicity of the extracellular fluid as they move from the rete testis to the cauda epididymidis [1], which reduces sperm cell volume [41, 42]. Cells that shrink due to a difference in osmotic pressure undergo a regulatory volume increase (RVI) to recover their volume by taking up water and intracellular osmolytes, including taurine [43]. Incubating boar sperm under a hypertonic condition in vitro demonstrated that RVI occurs to some extent against osmotic shrinkage [44]. Together with previous studies showing that taurine and hypotaurine share transport systems in other cells [45, 46], these findings suggest that taurine and hypotaurine may be taken up by sperm during RVI as they move toward the cauda region.
One of our most interesting findings was that CDO−/− sperm were less capable of fertilizing oocytes with accompanying cumulus cells and oocytes stripped of cumulus cells and the zona pellucida. When coupled with the ability of CDO−/− and WT mice to undergo normal capacitation, AE and hyperactivation, these results suggest that the infertility of CDO−/− mice reported previously was unlikely to be due to a defect in binding or penetration of the zona. However, it cannot be ruled out that the ability to fuse with the oocyte plasma membrane is deteriorated to some extent in CDO−/− sperm. In support of this view, a rigorous analysis of sperm morphology demonstrated that CDO−/− mice had a slightly higher rate of sperm head abnormalities, which is where membrane fusion with the oocyte plasma membrane is initiated [47]. A recent study using genetically engineered mice suggested that epididymal maturation involves changes in sperm structural elements [48]. Therefore, we examined morphological abnormalities of testicular WT and CDO−/− sperm and found no difference (21% vs 24%, data not shown). Considering the lack of CDO in mature sperm and testis, and the severe infertility of CDO−/− males mated with WT females [23], our findings are consistent with the infertility phenotype being caused by defective sperm epididymal maturation.
A long-standing hypothesis is that organic osmolytes are taken up from the extracellular environment by sperm during epididymal passage [1, 49]. Our quantification of taurine and hypotaurine verified this hypothesis by demonstrating that much less taurine and hypotaurine contents are in epididymal intraluminal fluid and sperm cytosol of CDO−/− versus WT mice. Previous analyses of sperm from c-ros null mice and transgenic mice with Gpx5 promoter-driven expression of Simian virus 40 large and small T-antigens indicate their infertility [4, 50]. Despite differences in manipulated genes, both mice types consistently showed misshapen epididymal epithelial cells at the initial segment (segment 1) and bending of the flagellum, caused by excess volume expansion of cytoplasmic droplets [4, 5, 32, 51]. In contrast, the histological analysis of the CDO−/− epididymis in this study did not detect any morphological abnormality in the epithelial layer, suggesting multiple mechanisms for flagellar bending in response to failure to regulate the volume of cytoplasmic droplets.
Based on these observations, we assessed whether CDO−/− sperm were capable of adapting to physiologically relevant conditions with varying tonicities, as experienced when sperm move from the male to the female reproductive tract. Incubating sperm in 420 mOsm medium (equivalent to the osmotic conditions of the intraluminal fluid of the cauda epididymis; [1] did not affect tail morphology. However, decreasing osmotic pressure to 320 mOsm (equivalent to the osmotic conditions in the uterine cavity; [3] resulted in an increased rate of tail angulation in CDO−/− sperm, suggesting defective osmotic tolerance to hypo-osmotic conditions. Reducing osmolarity to 160 mOsm resulted in an even higher prevalence of bending, consistent with volume regulation being the nature of the defect.
Osmotically swollen cells undergo a regulatory volume decrease (RVD) by releasing KCl, organic osmolytes, and water to reduce cell volume back to the original size [52]. Although it has been hypothesized that accumulated organic osmolytes in maturing sperm could be expended for RVD after hypotonicity-induced cell swelling [1], only indirect evidence demonstrates the involvement of taurine and/or hypotaurine in sperm RVD. Our data suggest that taurine acts as a critical osmoregulator for RVD in sperm. Importantly, we demonstrated that supplementing CDO−/− sperm with taurine abolished defective osmotic tolerance and fertility. These results also demonstrate that CDO−/− sperm do not have any other unanticipated defects in volume regulation.
Our results provide mechanistic insights into epididymal maturation and sperm function, showing that luminal taurine has a critical function in sperm osmoregulation. The CDO expression and localization data show segmental variations, associated compartmentalized functions, and variations from segment to segment within the same cell types in the caput region. Although these data improve our knowledge of epididymal function and physiology, they highlight the sophisticated specialization of this organ, responsible for maturing and storing sperm, and preparing them for their journey into the female reproductive tract.
MATERIALS AND METHODS
Ethics
All animal experiments were performed with approval of the Institutional Animal Care and Use Committees of the University of Tsukuba and Cornell University, and in accordance with the NIH guidelines for the Care and Use of Laboratory Animals.
Reagents and animals
An affinity-purified polyclonal anti-CDO antibody was generated and characterized previously [34]. CD-1 retired breeder and 4-week-old CD-1 male mice were purchased from Charles River Laboratories (Kingston, NY, USA). CDO−/− and WT mice were prepared by crossing heterozygous C57BL/6 mice for CDO knockout, as described previously [23]. All mice in the breeding colony were raised as described previously [53].
Immunoblotting with anti-CDO antibody
Sperm were collected from the cauda epididymis by the swim-out procedure in modified Whitten’s medium (MW) [54]. Sperm (2 × 106) were boiled in sample buffer for 10 min and separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE).
Epididymides were excised from retired breeder and 4-week-old male mice. Each epididymis was divided into eight segments under a stereomicroscope, as shown in Fig. 1A. The tissue segments were homogenized in sample buffer containing a protease inhibitor cocktail (Roche Applied Science, Mannheim, Germany) that lacked DTT. Protein contents were quantified using a BCA protein assay (Thermo Scientific, Rockford, IL, USA), and 100 μg protein from each segment was boiled with 100 mM DTT and separated by SDS-PAGE [55]. CDO was detected by transfer and immunoblotting [54].
Immunohistochemistry
Mice were anesthetized using isoflurane. Epididymides were fixed using intracardial perfusion with 4% (v/v) paraformaldehyde in phosphate-buffered saline (PBS) for 15 min. The tissues were excised, embedded in paraffin, and sectioned. Immunostaining was performed as described previously [56], and images were captured using an inverted microscope (Axio Observer; Carl Zeiss, Gottingen, Germany). Serial images depicting the entire epididymis were captured and assembled using Axio Vision Microscopy software (Carl Zeiss).
RNA extraction and quantitative reverse transcription-polymerase chain reaction (RT-PCR)
Epididymides were excised immediately and placed on ice. Epididymal segments 1–8 (depicted in Fig. 1A) were dissected under a stereoscope and frozen immediately in liquid nitrogen. RNA was extracted as described previously [57], reverse transcribed using the High Capacity Reverse Transcription Kit (Life Technologies, Carlsbad, CA, USA), and quantified using Power SYBR (Life Technologies) and the LightCycler®480 with Advanced Relative Quantitation software (ver. 1.5; Roche Applied Science, Indianapolis, IN, USA). Values for mRNA abundance are reported relative to the amount of mRNA in segment 8.
In vitro fertilization (IVF)
IVF was performed as described previously [57]. The zona pellucida was removed by exposure to Tyrode’s solution (Irvine Scientific, Santa Ana, CA, USA), and the resulting zona-free oocytes were washed and used for IVF. For taurine rescue experiment, CDO−/− sperm were pre-incubated and inseminated under the presence or absence of 5 mM taurine.
Acrosome exocytosis (AE) assay
AE was stimulated by co-incubating sperm with cumulus-oocyte complexes for IVF. Sample preparation, Coomassie Blue staining, and microscopy were performed as described previously [57]. Acrosomal status was assessed from at least 200 sperm [58].
Tyrosine phosphorylation assay for sperm capacitation
Sperm (1 × 106) were incubated for 60 min under either non-capacitating (NC) (MW supplemented with 5.5 mM D-glucose) or capacitating (CAP) (MW supplemented with 5.5 mM D-glucose, 10 mM NaHCO3, and 3 mM 2-OHCD) conditions. CAP conditions have been demonstrated to stimulate capacitation-associated tyrosine phosphorylation and in vitro fertility [59]. After incubation, the samples were processed for SDS-PAGE, and phosphotyrosine residues were detected by immunoblotting.
Hyperactivated motility analysis
The proportion of sperm representing hyperactivated motion was determined using the Sperm Motility Analyzer System (SMAS, DITECT Co. Ltd., Tokyo, Japan). Sperm (1 x 106) were incubated under NC or CAP conditions for 60 min and loaded into a standard counting chamber (Leja, Nieuw-Vennet, Netherlands). At least 200 sperm were analyzed by the SMAS according to parameters previously identified as hyperactivated motion in mouse: curvilinear velocity > 90 μm/s, linearity < 20% and an amplitude of lateral head > 7 μm [60].
Measurement of taurine and hypotaurine contents
Epididymides were excised from the 0.8-in section along the vas deferens connected to the distal cauda region. Cauda epididymides were wiped carefully to remove blood and divided at the tissue septa separating segments 7 and 8 (Fig. 1A) under a stereoscope. The vas deferens was cannulated using a microcannula (0.4 mm OD × 0.2 mm ID; Kent Scientific Co., Torrington, CT, USA) connected to a 1-ml syringe filled with 500 μl PBS. The tissue was perfused manually at a flow rate of 200 μl/min to extrude the intraluminal contents. The intraluminal contents were centrifuged at 10,000 × g for 10 min at 4°C to separate the supernatant (diluted intraluminal fluid) from the sperm pellet. The sperm cytosol was extracted from 2.3 × 106 sperm by sonication on ice followed by centrifugation at 10,000 × g for 10 min at 4°C to obtain the supernatant, which contained the sperm cytosol. Taurine and hypotaurine contents of the intraluminal fluid and sperm cytosol fractions were analyzed by high performance liquid chromatography [53]. Data are expressed as the amount of taurine or hypotaurine per mg protein in epididymal intraluminal fluid, and values for sperm cytosol were expressed as quantity per 1 × 106 sperm.
Sperm morphology
Sperm were fixed in 4% paraformaldehyde and 0.1% glutaraldehyde in PBS containing 5 mM CaCl2 [61], and sperm morphology was assessed using a phase-contrast microscope (×1,000 magnification). Sperm morphology was scored as normal (no obvious deformation), head abnormality (deformed sperm head), midpiece abnormality (deformed midpiece or hairpin shape in distal midpiece or at the annulus ring), principal piece abnormality (deformation or kink in the principal piece), or the presence of cytoplasmic droplets (residual cytoplasm in the proximal or distal midpiece). At least 200 sperm were evaluated from each sample. Gross abnormality was calculated by dividing the number of abnormal sperm by the total number of sperm examined. The segmental abnormalitiy was calculated by dividing the number of sperm with each regional abnormality by the total number of abnormal sperm.
Lipid peroxidation analysis
Taurine and hypotaurine have a scavenging effect against oxygen free radicals, leading to membrane lipid peroxidation in sperm [12]. Therefore, lipid peroxidation status in sperm was determined using the Lipid Hydroperoxide Assay Kit (Cayman Chemical, Ann Arbor, MI, USA), according to the manufacturer’s instructions. Sperm were incubated under NC conditions for 1 h. Sperm (1 × 107) lipids at 0 and 1 h post-incubation were extracted using extraction R saturated methanol and chloroform, and fractionated by centrifugation at 1,500 × g for 5 min at 0˚C. The bottom layer, enriched in lipids, was collected and processed to measure hydroperoxide content by spectrophotometry at an absorbance of 500 nm.
Measuring sperm tail angulation
Angulation of the sperm flagellum was assessed as an indication of cell swelling [62]. Sperm (2 × 106) were incubated under NC or CAP conditions for 2 h, which is the length of time required for sperm to penetrate the oocyte [63]. Sperm (15 μl) were examined under a phase-contrast microscope (×400 magnification) equipped with a slide heater at 0 and 2 h post-incubation. At least 100 live sperm were counted, and the shapes of their tails were classified as straight (no angulation) or bent (including angulation and hairpin).
Assessment of the sperm tail response to different osmotic stresses
The change in tail angulation in response to different osmotic conditions was examined. TYH medium was used to prepare 420, 320, and 160 mOsm solutions by adding the appropriate NaCl concentration [63]. Sperm were recovered and washed in 420 mOsm solution. Sperm (2 × 106 in 15 μl) were added to 285 μl TYH media and incubated for 15 min at 37°C. To assess the effects of taurine compensation, 5 mM taurine was added to these media prior to adding sperm. Sperm tail angulation was assessed as described above.
Statistical analysis
Pairwise comparisons between groups were performed using the t-test. Multiple comparisons were performed using two-way analysis of variance and Tukey’s HSD test when Bartlett’s test and the Kolmogorov–Smirnov/Lilliefors test confirmed equal variation and normality.
Acknowledgments
The authors thank Dr. Roy Cohen and Chinatsu Mukai for technical assistance with the microscope. The Program to Disseminate the Tenure-Track System, MEXT, Japan (A. A.), National Institute of Health Grants DP-OD-006431 (A. J. T.) and DK056649 (M.H.S.) and the Baker Institute for Animal Health supported this work.
ABBREVIATIONS
- CDO
cysteine dioxygenase
- CDO−/−
CDO null
- IVF
in vitro fertilization
- WT
wild-type
- AE
acrosome exocytosis
- CSD
cysteine sulfinate decarboxylase
- RVI
regulatory volume increase
- RVD
regulatory volume decrease
- MW
modified Whitten’s medium
Footnotes
Conflicts of interest
The authors declare no conflicts of interest associated with this manuscript.
AUTHOR CONTRIBUTIONS
A.A. and A.J.T. planned experiments: A.A., H.B.R., L.L.H., A.U., J.L.N., and M.M.H. performed experiments: A.A., M.H.S., and A.J.T. analyzed data: A.A. and A.J.T. wrote the paper.
References
- 1.Cooper TG, Yeung CH. Acquisition of volume regulatory response of sperm upon maturation in the epididymis and the role of the cytoplasmic droplet. Microsc Res Tech. 2003;61:28–38. doi: 10.1002/jemt.10314. [DOI] [PubMed] [Google Scholar]
- 2.Cornwall GA. New insights into epididymal biology and function. Human Reprod update. 2009;15:213–27. doi: 10.1093/humupd/dmn055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yeung CH, Wagenfeld A, Nieschlag E, Cooper TG. The cause of infertility of male c-ros tyrosine kinase receptor knockout mice. Biol Reprod. 2000;63:612–8. doi: 10.1095/biolreprod63.2.612. [DOI] [PubMed] [Google Scholar]
- 4.Yeung CH, Sonnenberg-Riethmacher E, Cooper TG. Infertile spermatozoa of c-ros tyrosine kinase receptor knockout mice show flagellar angulation and maturational defects in cell volume regulatory mechanisms. Biol Reprod. 1999;61:1062–9. doi: 10.1095/biolreprod61.4.1062. [DOI] [PubMed] [Google Scholar]
- 5.Yeung CH, Anapolski M, Sipila P, Wagenfeld A, Poutanen M, Huhtaniemi I, Nieschlag E, Cooper TG. Sperm volume regulation: maturational changes in fertile and infertile transgenic mice and association with kinematics and tail angulation. Biol Reprod. 2002;67:269–75. doi: 10.1095/biolreprod67.1.269. [DOI] [PubMed] [Google Scholar]
- 6.Yeung CH, Anapolski M, Setiawan I, Lang F, Cooper TG. Effects of putative epididymal osmolytes on sperm volume regulation of fertile and infertile c-ros transgenic Mice. J Androl. 2004;25:216–23. doi: 10.1002/j.1939-4640.2004.tb02781.x. [DOI] [PubMed] [Google Scholar]
- 7.Hinton BT. The testicular and epididymal luminal amino acid microenvironment in the rat. J Androl. 1990;11:498–505. [PubMed] [Google Scholar]
- 8.Johnson LA, Pursel VG, Gerrits RJ, Thomas CH. Free amino acid composition of porcine seminal, epididymal and seminal vesicle fluids. J Anim Sci. 1972;34:430–4. doi: 10.2527/jas1972.343430x. [DOI] [PubMed] [Google Scholar]
- 9.Lambert IH. Regulation of the cellular content of the organic osmolyte taurine in mammalian cells. Neurochem Res. 2004;29:27–63. doi: 10.1023/b:nere.0000010433.08577.96. [DOI] [PubMed] [Google Scholar]
- 10.Redmond HP, Stapleton PP, Neary P, Bouchier-Hayes D. Immunonutrition: the role of taurine. Nutrition. 1998;14:599–604. doi: 10.1016/s0899-9007(98)00097-5. [DOI] [PubMed] [Google Scholar]
- 11.Aruoma OI, Halliwell B, Hoey BM, Butler J. The antioxidant action of taurine, hypotaurine and their metabolic precursors. Biochem J. 1988;256:251–5. doi: 10.1042/bj2560251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Alvarez JG, Storey BT. Taurine, hypotaurine, epinephrine and albumin inhibit lipid peroxidation in rabbit spermatozoa and protect against loss of motility. Biol Reprod. 1983;29:548–55. doi: 10.1095/biolreprod29.3.548. [DOI] [PubMed] [Google Scholar]
- 13.Fraser LR. Both taurine and albumin support mouse sperm motility and fertilizing ability in vitro but there is no obligatory requirement for taurine. J Reprod Fertil. 1986;77:271–80. doi: 10.1530/jrf.0.0770271. [DOI] [PubMed] [Google Scholar]
- 14.Mrsny RJ, Waxman L, Meizel S. Taurine maintains and stimulates motility of hamster sperm during capacitation in vitro. J Exp Zool. 1979;210:123–8. doi: 10.1002/jez.1402100113. [DOI] [PubMed] [Google Scholar]
- 15.Boatman DE, Bavister BD, Cruz E. Addition of hypotaurine can reactivate immotile golden hamster spermatozoa. J Androl. 1990;11:66–72. [PubMed] [Google Scholar]
- 16.Meizel S, Lui CW, Working PK, Mrsny RJ. Taurine and hypotaurine: Their effects on motility, capacitation and the acrosome reaction of hamster sperm in vitro and their presence in sperm and reproductive tract fluids of several mammals. Dev Growth Differ. 1980;22:483–494. doi: 10.1111/j.1440-169X.1980.00483.x. [DOI] [PubMed] [Google Scholar]
- 17.Stipanuk MH. Sulfur amino acid metabolism: pathways for production and removal of homocysteine and cysteine. Annu Rev Nutr. 2004;24:539–77. doi: 10.1146/annurev.nutr.24.012003.132418. [DOI] [PubMed] [Google Scholar]
- 18.Stipanuk MH. Metabolism of sulfur-containing amino acids. Annu Rev Nutr. 1986;6:179–209. doi: 10.1146/annurev.nu.06.070186.001143. [DOI] [PubMed] [Google Scholar]
- 19.Cresenzi CL, Lee JI, Stipanuk MH. Cysteine is the metabolic signal responsible for dietary regulation of hepatic cysteine dioxygenase and glutamate cysteine ligase in intact rats. J Nutr. 2003;133:2697–702. doi: 10.1093/jn/133.9.2697. [DOI] [PubMed] [Google Scholar]
- 20.Dominy JE, Jr, Hirschberger LL, Coloso RM, Stipanuk MH. Regulation of cysteine dioxygenase degradation is mediated by intracellular cysteine levels and the ubiquitin-26 S proteasome system in the living rat. Biochem J. 2006;394:267–73. doi: 10.1042/BJ20051510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dominy JE, Jr, Hwang J, Guo S, Hirschberger LL, Zhang S, Stipanuk MH. Synthesis of amino acid cofactor in cysteine dioxygenase is regulated by substrate and represents a novel post-translational regulation of activity. J Biol Chem. 2008;283:12188–201. doi: 10.1074/jbc.M800044200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Stipanuk MH, Ueki I. Dealing with methionine/homocysteine sulfur: cysteine metabolism to taurine and inorganic sulfur. J Inherit Metab Dis. 2011;34:17–32. doi: 10.1007/s10545-009-9006-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ueki I, Roman HB, Valli A, Fieselmann K, Lam J, Peters R, Hirschberger LL, Stipanuk MH. Knockout of the murine cysteine dioxygenase gene results in severe impairment in ability to synthesize taurine and an increased catabolism of cysteine to hydrogen sulfide. Am J Physiol Endocrinol Metab. 2011;301:E668–84. doi: 10.1152/ajpendo.00151.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sipila P, Pujianto DA, Shariatmadari R, Nikkila J, Lehtoranta M, Huhtaniemi IT, Poutanen M. Differential endocrine regulation of genes enriched in initial segment and distal caput of the mouse epididymis as revealed by genome-wide expression profiling. Biol Reprod. 2006;75:240–51. doi: 10.1095/biolreprod.105.047811. [DOI] [PubMed] [Google Scholar]
- 25.Tanigawa M, Miyamoto K, Kobayashi S, Sato M, Akutsu H, Okabe M, Mekada E, Sakakibara K, Miyado M, Umezawa A, Miyado K. Possible involvement of CD81 in acrosome reaction of sperm in mice. Mol Reprod Dev. 2008;75:150–5. doi: 10.1002/mrd.20709. [DOI] [PubMed] [Google Scholar]
- 26.Jungnickel MK, Marrero H, Birnbaumer L, Lemos JR, Florman HM. Trp2 regulates entry of Ca2+ into mouse sperm triggered by egg ZP3. Nat Cell Biol. 2001;3:499–502. doi: 10.1038/35074570. [DOI] [PubMed] [Google Scholar]
- 27.Suarez SS, Dai X. Hyperactivation enhances mouse sperm capacity for penetrating viscoelastic media. Biol Reprod. 1992;46:686–91. doi: 10.1095/biolreprod46.4.686. [DOI] [PubMed] [Google Scholar]
- 28.Smith DJ, Gaffney EA, Gadelha H, Kapur N, Kirkman-Brown JC. Bend propagation in the flagella of migrating human sperm, and its modulation by viscosity. Cell Motil Cytoskeleton. 2009;66:220–36. doi: 10.1002/cm.20345. [DOI] [PubMed] [Google Scholar]
- 29.Buff S, Donze A, Guerin P, Guillaud J, Fontbonne A, Menezo Y. Taurine and hypotaurine in spermatozoa and epididymal fluid of cats. J Reprod Fertil Suppl. 2001;57:93–5. [PubMed] [Google Scholar]
- 30.Velazquez A, Delgado NM, Rosado A. Taurine content and amino acid composition of human acrosome. Life Sci. 1986;38:991–5. doi: 10.1016/0024-3205(86)90232-8. [DOI] [PubMed] [Google Scholar]
- 31.Huxtable RJ. Physiological actions of taurine. Physiol Rev. 1992;72:101–63. doi: 10.1152/physrev.1992.72.1.101. [DOI] [PubMed] [Google Scholar]
- 32.Chen Q, Peng H, Lei L, Zhang Y, Kuang H, Cao Y, Shi QX, Ma T, Duan E. Aquaporin3 is a sperm water channel essential for postcopulatory sperm osmoadaptation and migration. Cell Res. 2011;21:922–33. doi: 10.1038/cr.2010.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Johnston DS, Jelinsky SA, Bang HJ, DiCandeloro P, Wilson E, Kopf GS, Turner TT. The mouse epididymal transcriptome: transcriptional profiling of segmental gene expression in the epididymis. Biol Reprod. 2005;73:404–13. doi: 10.1095/biolreprod.105.039719. [DOI] [PubMed] [Google Scholar]
- 34.Ueki I, Stipanuk MH. Enzymes of the taurine biosynthetic pathway are expressed in rat mammary gland. J Nutr. 2007;137:1887–94. doi: 10.1093/jn/137.8.1887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lobo MV, Alonso FJ, del Rio RM. Immunohistochemical localization of taurine in the male reproductive organs of the rat. J Histochem Cytochem. 2000;48:313–20. doi: 10.1177/002215540004800301. [DOI] [PubMed] [Google Scholar]
- 36.Li JH, Ling YQ, Fan JJ, Zhang XP, Cui S. Expression of cysteine sulfinate decarboxylase (CSD) in male reproductive organs of mice. Histochem Cell Biol. 2006;125:607–13. doi: 10.1007/s00418-005-0095-8. [DOI] [PubMed] [Google Scholar]
- 37.Hamzeh M, Robaire B. Identification of early response genes and pathway activated by androgens in the initial segment and caput regions of the regressed rat epididymis. Endocrinology. 2010;151:4504–14. doi: 10.1210/en.2010-0023. [DOI] [PubMed] [Google Scholar]
- 38.Stipanuk MH, Hirschberger LL, Londono MP, Cresenzi CL, Yu AF. The ubiquitin-proteasome system is responsible for cysteine-responsive regulation of cysteine dioxygenase concentration in liver. Am J Physiol Endocrinol Metab. 2004;286:E439–48. doi: 10.1152/ajpendo.00336.2003. [DOI] [PubMed] [Google Scholar]
- 39.Stipanuk MH, Londono M, Hirschberger LL, Hickey C, Thiel DJ, Wang L. Evidence for expression of a single distinct form of mammalian cysteine dioxygenase. Amino acids. 2004;26:99–106. doi: 10.1007/s00726-003-0001-4. [DOI] [PubMed] [Google Scholar]
- 40.Xu YX, Wagenfeld A, Yeung CH, Lehnert W, Cooper TG. Expression and location of taurine transporters and channels in the epididymis of infertile c-ros receptor tyrosine kinase-deficient and fertile heterozygous mice. Mol Reprod Dev. 2003;64:144–51. doi: 10.1002/mrd.10250. [DOI] [PubMed] [Google Scholar]
- 41.Damm OS, Cooper TG. Maturation of sperm volume regulation in the rat epididymis. Asian J Androl. 2010;12:578–90. doi: 10.1038/aja.2010.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Brotherton J. Difference in size between spermatozoa from the cauda epididymidis and the caput epididymidis of the rat. J Reprod Fertil. 1976;48:365–6. doi: 10.1530/jrf.0.0480365. [DOI] [PubMed] [Google Scholar]
- 43.Jentsch TJ. VRACs and other ion channels and transporters in the regulation of cell volume and beyond. Nat Rev Mol Cell Biol. 2016;17:293–307. doi: 10.1038/nrm.2016.29. [DOI] [PubMed] [Google Scholar]
- 44.Petrunkina AM, Jebe E, Topfer-Petersen E. Regulatory and necrotic volume increase in boar spermatozoa. J Cell Physiol. 2005;204:508–21. doi: 10.1002/jcp.20317. [DOI] [PubMed] [Google Scholar]
- 45.Holopainen I, Kontro P, Frey HJ, Oja SS. Taurine, hypotaurine, and GABA uptake by cultured neuroblastoma cells. J Neurosci Res. 1983;10:83–92. doi: 10.1002/jnr.490100110. [DOI] [PubMed] [Google Scholar]
- 46.Tiruppathi C, Brandsch M, Miyamoto Y, Ganapathy V, Leibach FH. Constitutive expression of the taurine transporter in a human colon carcinoma cell line. Am J Physiol. 1992;263:G625–31. doi: 10.1152/ajpgi.1992.263.5.G625. [DOI] [PubMed] [Google Scholar]
- 47.Ikawa M, Inoue N, Benham AM, Okabe M. Fertilization: a sperm’s journey to and interaction with the oocyte. J Clin Invest. 2010;120:984–94. doi: 10.1172/JCI41585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Simerly C, Castro C, Hartnett C, Lin CC, Sukhwani M, Orwig K, Schatten G. Post-Testicular Sperm Maturation: Centriole Pairs, Found in Upper Epididymis, are Destroyed Prior to Sperm’s Release at Ejaculation. Sci Rep. 2016;6:31816. doi: 10.1038/srep31816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cooper TG. Sperm maturation in the epididymis: a new look at an old problem. Asian J Androl. 2007;9:533–9. doi: 10.1111/j.1745-7262.2007.00285.x. [DOI] [PubMed] [Google Scholar]
- 50.Sipila P, Cooper TG, Yeung CH, Mustonen M, Penttinen J, Drevet J, Huhtaniemi I, Poutanen M. Epididymal dysfunction initiated by the expression of simian virus 40 T-antigen leads to angulated sperm flagella and infertility in transgenic mice. Mol Endocrinol. 2002;16:2603–17. doi: 10.1210/me.2002-0100. [DOI] [PubMed] [Google Scholar]
- 51.Cooper TG, Yeung CH, Wagenfeld A, Nieschlag E, Poutanen M, Huhtaniemi I, Sipila P. Mouse models of infertility due to swollen spermatozoa. Mol Cell Endocrinol. 2004;216:55–63. doi: 10.1016/j.mce.2003.10.076. [DOI] [PubMed] [Google Scholar]
- 52.Hoffmann EK, Lambert IH, Pedersen SF. Physiology of cell volume regulation in vertebrates. Physiol Rev. 2009;89:193–277. doi: 10.1152/physrev.00037.2007. [DOI] [PubMed] [Google Scholar]
- 53.Ueki I, Roman HB, Hirschberger LL, Junior CC, Stipanuk MH. Extrahepatic Tissues Compensate for Loss of Hepatic Taurine Synthesis in Mice with Liver-Specific Knockout of Cysteine Dioxygenase. Am J Physiol Endocrinol Metab. 2012 doi: 10.1152/ajpendo.00589.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Travis AJ, Merdiushev T, Vargas LA, Jones BH, Purdon MA, Nipper RW, Galatioto J, Moss SB, Hunnicutt GR, Kopf GS. Expression and localization of caveolin-1, and the presence of membrane rafts, in mouse and Guinea pig spermatozoa. Dev Biol. 2001;240:599–610. doi: 10.1006/dbio.2001.0475. [DOI] [PubMed] [Google Scholar]
- 55.Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–5. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 56.Roman HB, Hirschberger LL, Krijt J, Valli A, Kozich V, Stipanuk MH. The cysteine dioxgenase knockout mouse: altered cysteine metabolism in nonhepatic tissues leads to excess H2S/HS(-) production and evidence of pancreatic and lung toxicity. Antioxid Redox Signal. 2013;19:1321–36. doi: 10.1089/ars.2012.5010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Asano A, Nelson-Harrington JL, Travis AJ. Phospholipase B Is Activated in Response to Sterol Removal and Stimulates Acrosome Exocytosis in Murine Sperm. J Biol Chem. 2013;288:28104–28115. doi: 10.1074/jbc.M113.450981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Larson JL, Miller DJ. Simple histochemical stain for acrosomes on sperm from several species. Mol Reprod Dev. 1999;52:445–9. doi: 10.1002/(SICI)1098-2795(199904)52:4<445::AID-MRD14>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 59.Travis AJ, Tutuncu L, Jorgez CJ, Ord TS, Jones BH, Kopf GS, Williams CJ. Requirements for glucose beyond sperm capacitation during in vitro fertilization in the mouse. Biol Reprod. 2004;71:139–45. doi: 10.1095/biolreprod.103.025809. [DOI] [PubMed] [Google Scholar]
- 60.Itach SB, Finklestein M, Etkovitz N, Breitbart H. Hyper-activated motility in sperm capacitation is mediated by phospholipase D-dependent actin polymerization. Dev Biol. 2012;362:154–61. doi: 10.1016/j.ydbio.2011.12.002. [DOI] [PubMed] [Google Scholar]
- 61.Selvaraj V, Asano A, Buttke DE, McElwee JL, Nelson JL, Wolff CA, Merdiushev T, Fornes MW, Cohen AW, Lisanti MP, Rothblat GH, Kopf GS, Travis AJ. Segregation of micron-scale membrane sub-domains in live murine sperm. J Cell Physiol. 2006;206:636–46. doi: 10.1002/jcp.20504. [DOI] [PubMed] [Google Scholar]
- 62.Willoughby CE, Mazur P, Peter AT, Critser JK. Osmotic tolerance limits and properties of murine spermatozoa. Biol Reprod. 1996;55:715–27. doi: 10.1095/biolreprod55.3.715. [DOI] [PubMed] [Google Scholar]
- 63.Toyoda Y, Yokoyama M, Hoshi T. Studies on the fertilization of mouse eggs in vitro. I. In vitro fertilization of eggs by fresh epididymal sperms. Jpn J Anim Reprod. 1971;16:147–151. [Google Scholar]