Abstract
BACKGROUND AND OBJECTIVES
Primary liver sarcomas(PLS) are rare. Published series are limited by small numbers of patients.
METHODS
We reviewed the National Cancer Database (2004-2014) for patients who underwent surgical resection of PLS.
RESULTS
Of 237 patients identified, the majority were female(60.8%), with median age of 52 years. Histologies were:epithelioid hemangioendothelioma(n=67), angiosarcoma(n=64), leiomyosarcoma(n=33), embryonal rhabdomyosarcoma(n=31), carcinosarcoma(n=16), giant cell sarcoma(n=14), spindle cell sarcoma(n=12). Ninety-seven(40.9%) patients underwent lobectomies or extended lobectomies,41 patients(17.3%) underwent transplantation. Surgical margins were negative in 82.9%. Tumors were well differentiated in 11.3%. Histology type correlated with outcome with the best prognosis for epithelioid hemangioendothelioma (OS:not reached, similar for resection and transplantation) and the worst for angiosarcoma(OS:10.8mo with resection;6mo with transplantation;p=0.09). Resections with microscopically negative margins were associated with improved survival (58.7movs11.3mo for positive margins;p<0.001). Chemotherapy and radiation therapy were used in a minority of patients(32.9% and 4.3% respectively) with no improvement in outcomes.
CONCLUSIONS
Both hepatic resection and liver transplantation can be associated with long term survival for selected primary liver sarcomas such as epitheliod hemangioendotheliomas. Histology type and the ability to resect the tumor with negative margins correlate with outcomes and the decision to operate should be carefully weighed for subtypes with particularly dismal prognosis such as angiosarcomas.
Keywords: Primary liver sarcomas, resection, transplantation
Introduction
Primary liver sarcomas (PLS) are very rare, representing less than 0.1% of all primary liver tumors[1,2]. They are a heterogeneous group of malignancies with their biology ranging from the indolent behavior of epithelioid hemangioendothelioma with five-year survival of 55-75% after transplantation or resection, to the aggressive angiosarcoma associated with dismal prognosis and rarely survival beyond 2 years regardless of treatment modality[2–4].
The optimal treatment approach for PLS is not well defined. Surgery is the only treatment modality that can offer a potential cure. However, existing studies of outcomes after resection of liver sarcomas are limited by small patient numbers treated over extended periods of time[5,6]. Amongst patients who undergo surgery, the indications for resection versus transplantation are not defined. Moreover, the benefit of adjuvant treatment remains questionable with existing literature reporting minimal if any benefit[2].
In an effort to circumvent the above limitations, we examined the National Cancer Database, as it captures approximately 70% of new cancer cases treated nationwide with detailed clinicopathologic and treatment data[7]. The aims of the present study were to determine outcomes after resection of primary liver sarcomas, utilizing a large contemporary cohort, to examine whether transplantation is associated with an improvement in survival over resection, and to determine whether adjuvant treatment is associated with an improved outcome.
Materials and Methods
The National Cancer Database (NCDB) is a hospital-based cancer registry sponsored by a joint program between the American College of Surgeons Commission on Cancer (CoC) and the American Cancer Society. It captures data from more than 1,500 hospitals[7]. The Participant Use Data Files (PUF) are Health Insurance Portability and Accountability Act (HIPAA)-compliant data files containing de-identified data. Institutional review board (IRB) approval was not required for this study as no protected health information was examined. For the purpose of this study we reviewed the relevant PUF for liver.
For the purpose of this study, we reviewed the period of 2004-2014, for patients 18 years of age or older who underwent surgical resection for a liver sarcoma and included pathologies with more than ten resected cases (International Classification of Diseases for Oncology, 3rd edition (ICD-O-3) topographical code C22.0: liver and morphological codes 8801: spindle cell sarcoma, 8802: giant cell sarcoma, 8890: leiomyosarcoma, 8980: carcinosarcoma, 8991: embryonal rhabdomyosarcoma, 9120: angiosarcoma, 9133: epithelioid hemangioendothelioma). Carcinosarcomas were included because they are included in the histologic differential diagnosis of primary liver sarcomas as well as for survival comparison reasons. Patients with metastatic disease were excluded from this study.
The patient demographics examined included age, gender, race, insurance status and median income. The effect of patient comorbidity was examined with the Charlson Comorbidity Index (CCI)[8]. Facility types were categorized as Academic/Research National Cancer Institute (NCI)-designated, community, comprehensive community and integrated cancer network. Postoperative outcomes examined included length of stay, readmission within 30 days, 30-day and 90-day mortality. Long term outcome was examined with overall survival comparisons for patients with follow up of at least 1 month.
Statistical Analysis
Continuous variables are presented as median and range or mean and standard deviation as appropriate. Categorical variables are presented as proportions. We assessed group differences using Fisher exact or Pearson x2 test for categorical variables. Continuous variables were compared with the student’s t test when the distribution was normal, or the Wilcoxon rank-sum test and Kruskal-Wallis test when the distribution was not normal. Survival curves were constructed with the Kaplan Meier method and differences assessed with the log rank test. Statistical analysis was performed using SPSS Statistics v23 software (IBM Corp., Armonk, NY).
Results
Clinicopathologic characteristics
During the period 2004-2014, we identified 237 patients who underwent liver resection for a primary liver sarcoma or carcinosarcoma. The majority of patients were female (60.8%) and white (81%) with a Charlson comorbidity index of 0 (76.8%). Median age was 52 years. Table 1 illustrates the clinicopathologic data of this cohort.
Table 1.
Clinicopathologic Characteristics of 237 patients who underwent liver resection for a primary liver sarcoma.
| Variable | n(%) |
|---|---|
| Age, median, (range), y | 52(18-84) |
|
| |
| Female gender | 144(60.8) |
|
| |
| Race | |
| White | 192(81) |
| African American | 29(12.2) |
| Other | 16(6.8) |
|
| |
| Insurance | |
| Private | 132(55.7) |
| Medicaid | 19(8) |
| Medicare | 65(27.4) |
| Other | 21(8.9) |
|
| |
| Median Income | (N=231) |
| <38,000 | 40(16.9) |
| 38,000-47,999 | 55(23.2) |
| 48,000-62,999 | 59(24.9) |
| >63,000 | 80(33.8) |
|
| |
| Charlson-Deyo Comorbidity Score | |
| 0 | 182(76.8) |
| 1 | 38(16) |
| 2 | 17(7.2) |
|
| |
| Histology | |
| Epithelioid hemangioendothelioma | 67(28.3) |
| Angiosarcoma | 64(27) |
| Leiomyosarcoma | 33(13.9) |
| Embryonal Rhabdomyosarcoma | 31(13.1) |
| Carcinosarcoma | 16(6.8) |
| Giant Cell Sarcoma | 14(5.9) |
| Spindle Cell Sarcoma | 12(5.1) |
|
| |
| Tumor size, median (range), cm | 10.7(0.4-99) |
|
| |
| Grade | N=124 |
| Well | 14(11.3) |
| Moderate | 13(10.5) |
| Poor/undifferentiated | 97(78.2) |
|
| |
| Examined Lymph nodes | N=62 |
| Negative lymph nodes | 43(69.4) |
|
| |
| Surgical Margins | N=211 |
| R0 | 175(82.9) |
| R1/R2 | 36(17.1) |
|
| |
| Hospital Type | N=179 |
| Academic/Research NCI designated | 132(55.7) |
| Integrated Network Cancer | 11(4.6) |
| Comprehensive Community Cancer | 32(13.5) |
| Community Cancer | 4(1.7) |
|
| |
| In-hospital stay, median (range), d | 4(1-109) |
|
| |
| Readmission within 30d | N=231 16(6.8) |
|
| |
| 30d Mortality | N=216 8(3.4) |
|
| |
| 90d Mortality | N=216 17(7.2) |
y=years, cm=centimeters, d= days
The most common histologies were epithelioid hemangioendothelioma (28.3%), and angiosarcoma (27%). Lobectomies or extended lobectomies were performed in 40.9% of patients, whereas liver transplantation was performed in 17.3% of patients, almost exclusively consisting of angiosarcomas and epithelioid hemangioendotheliomas (97.6%).
Surgical margins were negative in 82.9%. The median tumor diameter was 10.7cm. Lymph nodes were evaluated in 26.2% and were negative in 69.4%. Tumor grade was available for 52.3%. Tumors were well differentiated in 11.3%, but almost all of these (93%) were angiosarcomas and epithelioid hemangioendotheliomas.
The median length of hospital stay was 4 days; the 30-day readmission and mortality rates were 6.8% and 3.4%, whereas 90-day mortality rate was 7.2%. Only a minority of patients received chemotherapy (30.4%) or radiation (4.5%).
Long term outcome
Overall, 209(88.2%) patients had follow up of at least 1 month. The median overall survival (OS) of these patients was 4 years. There were 52 five-year survivors (25%). Of these, most had either epithelioid hemangioendothelioma (n=25 or 48%) or embryonal rhabdomyosarcoma (n=10 or 19.2%). When examining survival as a function of histologic subtype, epithelioid hemangioendothelioma conferred the best prognosis (median OS not reached), whereas angiosarcoma conferred the worst prognosis (median OS 8.6mo, Figure 1).
Figure 1.
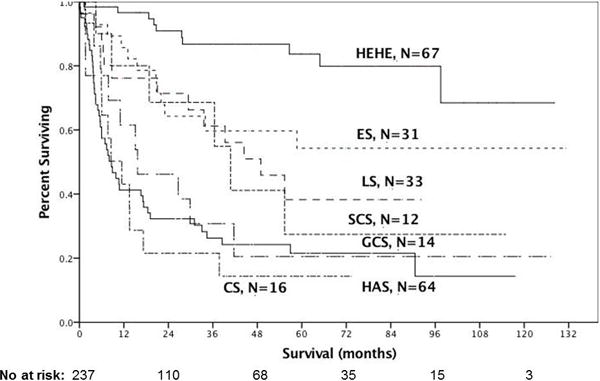
Survival of 237 primary liver sarcoma and carcinosarcoma patients according to histology
(HEHE: epithelioid hemangioendothelioma median OS: not reached, ES: embryonal sarcoma median OS not reached, LS: leiomyosarcoma median OS 48.9mo, SCS: spindle cell sarcoma median OS 40.8mo, GCS: giant cell sarcoma median OS 15.7mo, AS: angiosarcoma median OS 8.7mo, CS: carcinosarcoma median OS 11.4mo)
Resections with microscopically negative margins were associated with improved survival (58.7mo vs 11.3mo for positive margins; p<0.001). Use of chemotherapy was not associated with an improved outcome (median OS: 57mo without chemotherapy versus 33.6mo with chemotherapy; p=0.3).
Angiosarcomas and epithelioid hemangioendotheliomas: resection versus transplantation
We examined separately the 131 patients with angiosarcoma and epithelioid hemangioendothelioma who underwent resection or transplantation. Table 2 illustrates the clinicopathologic characteristics of these patients. Patients who underwent transplantation were younger, had larger tumors and more frequent nodal disease.
Table 2.
Resection versus Transplantation for 131 Epithelioid Hemangioendotheliomas and Angiosarcomas
| Variable | Resection N=91(%) |
Transplantation N=40(%) |
p |
|---|---|---|---|
| Age, mean, (SD), y | 53.9(17) | 40(11.6) | 0.01 |
| Female gender | 54(59.3) | 24(60) | NS |
| Race | NS | ||
| White | 83(91.2) | 33(82.5) | |
| African American | 4(4.4) | 2(5) | |
| Other | 4(4.4) | 5(12.5) | |
| Charlson Comorbidity Index | 0.03 | ||
| 0 | 68(74.7) | 24(60) | |
| 1 | 17(18.7) | 7(17.5) | |
| 2 | 6(6.6) | 9(22.5) | |
| Histology | NS | ||
| Epithelioid Hemangioendothelioma | 48(52.7) | 16(40) | |
| Angiosarcoma | 43(47.3) | 24(60) | |
| Tumor size, mean (SD), cm | 14.8 | 44.6 | <0.001 |
| Grade: Well-Differentiated | N=32 11(34.4) |
N=16 2(12.5) |
NS |
| Positive Lymph Nodes | N=13 2(15.4) |
N=17 13(76.5) |
0.003 |
| R0 Surgical Margins | N=84 68(81) |
N=32 29(90.6) |
NS |
| In-hospital stay, mean (SD), d | 9.3 | 23.2 | 0.01 |
| Readmission within 30d | N=87 6(6.9) |
N=40 5(12.5) |
NS |
| 90d Mortality | N=84 9(10.7) |
N=38 2(5.3) |
NS |
Overall median survival was similar for resection versus transplantation for angiosarcomas and epithelioid hemangioendotheliomas (Figure 2). When they were examined separately, angiosarcomas had a trend for improved survival with resection versus transplantation (median OS: 16.6 mo vs 6 mo, respectively; p=0.07) whereas epithelioid hemangioendotheliomas had similar great outcomes (median OS not reached for either group; p=0.3).
Figure 2.
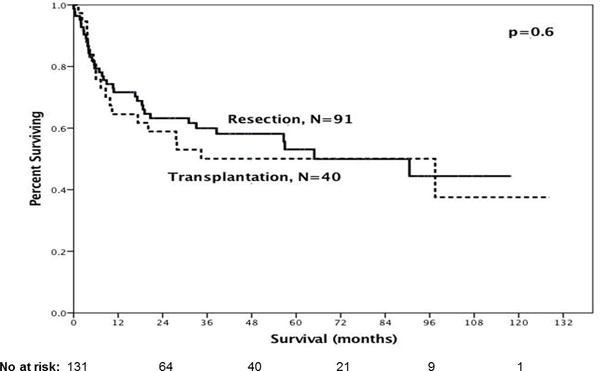
Overall Survival of primary liver sarcomas (epithelioid hemangioendotheliomas (n=67) or angiosarcomas (n=64) who underwent resection or transplantation was similar (median OS: 90.5mo vs 97.5mo respectively; p=0.6)
Well-differentiated tumors were associated with improved survival over moderately/poorly differentiated for angiosarcomas (median OS: not reached versus 8.6mo; p=0.02), with a trend for improved survival for epithelioid hemangioendotheliomas (97.5mo versus 56.6mo; p=0.7).
Discussion
Primary liver sarcomas (PLS) are extremely rare[1]. Therapeutic options with curative intent for patients with PLS are surgical resection and transplantation, whereas chemotherapy and radiation have questionable, if any benefit[2]. The present study is unique in its inclusion of a large number of patients treated fairly recently (2004-2014 period). Moreover, this study demonstrated that outcome can be stratified according to different tumor histologies. Twenty-five percent of the patients were 5-year survivors, the majority of whom had either hepatic epithelioid hemangioendotheliomas (HEHE) or embryonal rhabdomyosarcomas. The outcomes of resection and transplantation for hepatic epithelioid hemangioendotheliomas (HEHE) and hepatic angiosarcomas (HAS) appear to be similar however transplanted patients had more advanced disease. Angiosarcomas and carcinosarcomas were associated with overall survival of less than a year which should be taken into account in considering resection for these patients. Chemotherapy was not associated with improved outcome.
Previous reports on the outcomes of surgical resection for PLS are limited by a small number of patients treated over extended periods of time, during which major changes in liver surgery occurred[5,6]. Weitz et al.[6] reported on 30 patients (only 16 underwent surgical exploration) treated over a period of more than 2 decades, the majority of whom (66%) harbored HEHE, HAS or embryonal rhabdomyosarcomas. Long-term (>3 years) survival was only possible if R0-resection was achieved, except for patients with HEHE, which were treated conservatively due to their indolent disease. The importance of tumor grade was illustrated as no recurrences were observed for low grade tumors resected with clear margins. In a similar study, Poggio et al.[5] report 20 adult patients who underwent resection during a period spanning almost two decades and found that the grade of the sarcoma was the only factor associated with survival. It is worth noting that this cohort was significantly different, with the majority of these patients (12/20) harboring leiomyosarcomas, malignant solitary fibrous tumors or HEHE. The contemporary analysis herein showed improved survival with an R0 resection along with well-differentiated angiosarcomas versus more poorly differentiated histology.
Liver transplantation (LT) should be used with caution in PLS. Almost all LT in the current study occurred for hepatic epithelioid HEHE and HAS. However, survival benefit of transplantation over resection was not shown for either histology in this analysis. A major limitation of this comparison is that transplantation was utilized for larger tumors with frequent nodal spread, the volume of disease was therefore different. Orlando et al. in a study utilizing the European Liver Transplant Registry on 22 patients who underwent LT for HAS reported extremely poor outcomes, with 5/22 patients dying of early infections and the remaining succumbing to early recurrence of their disease, with overall survival of 7.2 months and no patient surviving more than 2 years[9]. It is important to emphasize that only 30% of patients had a correct pre-transplant diagnosis of HAS; more patients were incorrectly diagnosed as having HEHE. Our study similarly found very poor outcomes for LT, whereas resection was associated with an observable trend toward better survival. The outcomes of transplantation are much more encouraging for HEHE, which is regarded as a low-grade angiosarcoma with indolent behavior even in the presence of lymph node and distant extrahepatic disease[3]. In a study of 59 patients who underwent LT for HEHE from the same transplant registry, the 10 year recurrence free survival and overall survival were 64% and 72% respectively[10]. However, whether these excellent outcomes are a direct effect of the transplantation or result from the indolent nature of the tumor is unclear. This study did not identify any survival difference between resection and transplantation and therefore we do not favor transplantation especially as patients with indolent disease can have a prolonged survival even in the presence of disease.
Even though the National Cancer Database is a robust population-based database, there are inherent limitations to the use of retrospective analyses of large clinical databases with the possibility of reporting errors and bias[11]. However, the data collection, validation and reporting process for the NCDB is standardized, monitored, and reviewed[7]. The liver module includes only patients who underwent liver resection for a primary liver neoplasm; however, liver invasion from a retroperitoneal sarcoma necessitating hepatectomy cannot be excluded. Data on specific chemotherapy agents and treatment of recurrent disease are lacking. Survival is measured as overall survival rather than disease-specific survival. Comorbidity is assessed with the Charlson comorbidity index, however the exact nature of the comorbid conditions is not known, which may affect the decision for surgery. The study is further limited by small sample size in some histologic subgroups, which makes producing meaningful results very difficult. Despite these weaknesses, the NCDB provides a substantial amount of clinicopathologic, oncologic, and treatment data. This relatively large, contemporary cohort is reflective of practice patterns in the United States. Given the rarity of this disease, NCDB and other population-based data sets are an excellent adjunct to small scale institutional data. Moreover, the data in this study will be invaluable in counseling liver sarcoma patients with regards to expectations and outcomes.
Conclusions
In conclusion, the current study suggests that surgical resection represents the mainstay of treatment for primary liver sarcomas. Survival is closely related to the histologic subtype. Transplantation should be utilized with caution as it does not improve the dismal prognosis of angiosarcomas and it might not provide additional benefit to resection for epithelioid hemangioendotheliomas.
Synopsis.
Liver Sarcomas in the Modern Era. An NCDB experience
Acknowledgments
Funding: Research reported in this publication was supported in part by the National Cancer Institute of the National Institutes of Health under awards number NIH 5K12CA001727-20 and P30CA033572. The content is solely the responsibility of L.M. and does not necessarily represent the official views of the National Institutes of Health
Footnotes
Presented at: Oral Presentation, American College of Surgeons Clinical Congress, October 22-26, 2017, San Diego, CA
References
- 1.Primary liver cancer in Japan. Clinicopathologic features and results of surgical treatment. Ann Surg. 1990;211:277–287. [PMC free article] [PubMed] [Google Scholar]
- 2.Kim HR, Rha SY, Cheon SH, et al. Clinical features and treatment outcomes of advanced stage primary hepatic angiosarcoma. Ann Oncol. 2009;20:780–787. doi: 10.1093/annonc/mdn702. [DOI] [PubMed] [Google Scholar]
- 3.Mehrabi A, Kashfi A, Fonouni H, et al. Primary malignant hepatic epithelioid hemangioendothelioma: a comprehensive review of the literature with emphasis on the surgical therapy. Cancer. 2006;107:2108–2121. doi: 10.1002/cncr.22225. [DOI] [PubMed] [Google Scholar]
- 4.Weiss SW, Enzinger FM. Epithelioid hemangioendothelioma: a vascular tumor often mistaken for a carcinoma. Cancer. 1982;50:970–981. doi: 10.1002/1097-0142(19820901)50:5<970::aid-cncr2820500527>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 5.Poggio JL, Nagorney DM, Nascimento AG, et al. Surgical treatment of adult primary hepatic sarcoma. Br J Surg. 2000;87:1500–1505. doi: 10.1046/j.1365-2168.2000.01564.x. [DOI] [PubMed] [Google Scholar]
- 6.Weitz J, Klimstra DS, Cymes K, et al. Management of primary liver sarcomas. Cancer. 2007;109:1391–1396. doi: 10.1002/cncr.22530. [DOI] [PubMed] [Google Scholar]
- 7.Winchester DP, Stewart AK, Phillips JL, Ward EE. The national cancer data base: past, present, and future. Ann Surg Oncol. 2010;17:4–7. doi: 10.1245/s10434-009-0771-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Deyo RA, Cherkin DC, Ciol MA. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J Clin Epidemiol. 1992;45:613–619. doi: 10.1016/0895-4356(92)90133-8. [DOI] [PubMed] [Google Scholar]
- 9.Orlando G, Adam R, Mirza D, et al. Hepatic hemangiosarcoma: an absolute contraindication to liver transplantation–the European Liver Transplant Registry experience. Transplantation. 2013;95:872–877. doi: 10.1097/TP.0b013e318281b902. [DOI] [PubMed] [Google Scholar]
- 10.Lerut JP, Orlando G, Adam R, et al. The place of liver transplantation in the treatment of hepatic epitheloid hemangioendothelioma: report of the European liver transplant registry. Ann Surg. 2007;246:949–957. doi: 10.1097/SLA.0b013e31815c2a70. discussion 957. [DOI] [PubMed] [Google Scholar]
- 11.Bilimoria KY, Bentrem DJ, Stewart AK, et al. Comparison of commission on cancer-approved and -nonapproved hospitals in the United States: implications for studies that use the National Cancer Data Base. J Clin Oncol. 2009;27:4177–4181. doi: 10.1200/JCO.2008.21.7018. [DOI] [PubMed] [Google Scholar]


