Abstract
Platinum salts are active against metastatic triple negative breast cancer (mTNBC), and biomarkers to predict their effectiveness are urgently needed. In recent years, the neutrophil-to-lymphocyte ratio (NLR) and the platelet-to-lymphocyte ratio (PLR) have emerged as prognostic biomarkers in many malignancies, but their predictive role in platinum-treated mTNBC patients remains unexplored. We performed a retrospective, single centre study to evaluate the association between baseline NLR or PLR and progression free survival (PFS) of mTNBC patients treated with platinum-based chemotherapy. As a control population, we analysed data from patients with hormone receptor-positive HER2-negative (HR+ HER2−) metastatic breast cancer. Among 57 mTNBC patients treated with the carboplatin-paclitaxel or carboplatin-gemcitabine combination, high NLR and PLR were associated with significantly lower PFS at both univariate and multivariable analysis. Conversely, we did not find a significant association between NLR or PLR and the PFS of 148 patients in the control population. Our findings suggest that the NLR and PLR are predictive of benefit from platinum-containing chemotherapy specifically in mTNBC patients. If validated in larger prospective studies, these easy-to-measure parameters could be combined with emerging predictive biomarkers, such as BRCA 1/2 mutations, to improve the selection of mTNBC patients more likely to benefit from platinum-based chemotherapy.
Introduction
Breast cancer (BC) is the most common malignancy among women and also one of the leading causes of cancer-related death1. Metastatic triple negative breast cancer (mTNBC) accounts for 10–20% of metastatic BC (mBC) cases, and is characterized by an aggressive course, the lack of therapeutic targets and high lethality2,3. Even in recently published prospective trials, median progression free survival (PFS) and overall survival (mOS) did not exceed 6 months and 12–18 months, respectively4,5.
Cytotoxic chemotherapy (ChT) remains the mainstay of treatment for mTNBC, with taxane and platinum salts being among the most effective compounds when used alone, or in combination with bevacizumab or other chemotherapeutical agents4–9. In a recent randomized study, carboplatin demonstrated superior activity compared to docetaxel in mTNBC patients bearing germline mutations in BRCA1 or BRCA2 genes, thus suggesting that platinum compounds should be preferred treatment options in this patient population6. While such results await confirmation in larger studies, new predictive biomarkers are needed that are low-cost, routinely assessable with standardized techniques, well reproducible across different laboratories, and capable of predicting benefit from platinum-based ChT.
Many evidences highlight the importance of immune system activation in TNBC control: first, tumor-infiltrating lymphocytes (TILs) and other immune markers correlate with higher rates of pathological complete response (pCR), as well as with better patient disease-free survival (DFS) and OS, after neoadjuvant ChT10–13; second, the programmed death 1 (PD-1) inhibitor pembrolizumab and the PD-1 ligand (PD-L1) inhibitor atezolizumab are active in subgroups of heavily pre-treated TNBC patients14–16; lastly, combining nab-paclitaxel with atezolizumab has shown promising clinical activity in TNBC17,18, and taxanes-atezolizumab combinations are being evaluated in larger prospective trials [NCT02425891; NCT03125902].
Recent studies have revealed the prognostic role of parameters that reflect systemic inflammation or the status of antitumor immunity, such as the neutrophil-to-lymphocyte ratio (NLR) and the platelet-to-lymphocyte ratio (PLR), in different solid malignancies, including colorectal, renal and lung carcinomas19,20. High NLR has also been associated with an increased risk of death in heterogeneous BC patient populations21, including patients with limited-stage TNBC22,23. However, none of these studies specifically assessed the role of NLR in mTNBC and, even in those studies including different proportions of patients with mBC (4.2–13.9%), subgroup analyses in specific tumor biology subgroups, or based on the type of ChT, were not reported24–28.
In this work, we assessed for the first time the potential role of NLR and PLR as biomarkers predictive of PFS in mTNBC patients treated with platinum-based ChT. As a control population, we analysed data from patients with hormone receptor-positive HER2-negative (HR+ HER2−) mBC treated with the same platinum-containing regimens. To discriminate between a predictive and a purely prognostic role of these parameters, we also evaluated patient OS.
Results
Patient characteristics
Between July 2007 and July 2017, 62 mTNBC patients were treated at our Institution with platinum-based ChT combinations. Among these patients, 57 fulfilled the inclusion criteria and were evaluable for the biomarkers of interest. At the moment of data lock and analysis, 53 (93%) patients had progressed and 48 (84%) had died.
Characteristics of evaluated patients are described in Table 1. Median age among TNBC patients was 56 years (range 33.7–78.9). Most patients (84%) received carboplatin-paclitaxel ChT, while the remaining ones (16%) were treated with the carboplatin-gemcitabine combination. All mTNBC patients received platinum-containing ChT as their first- (88%) or second-line (12%) treatment.
Table 1.
Characteristics of patients with mTNBC and the HR+ mBC control population.
| TNBC | ER/PgR-positive HER2-negative | |
|---|---|---|
| N. pts | 57 | 148 |
| Median age (years, range) | 56 (33.7–78.9) | 58 (29.8–79.3) |
| Previous taxane exposure | ||
| Yes | 43 (75.4%) | 102 (68.9%) |
| No | 14 (24.6%) | 46 (31.1%) |
| N. chemotherapy line | ||
| 1st–2nd | 57 (100%) | 104 (70.3%) |
| >2nd | 0 (0%) | 44 (29.7%) |
| N. disease sites: | ||
| 1–2 sites | 36 (63.2%) | 88 (59.5%) |
| >2 sites | 21 (36.8%) | 60 (40.5%) |
| Presence of visceral disease | 37 (65%) | 99 (66.9%) |
| Type of treatment | ||
| paclitaxel | 48 (84.2%) | 112 (75.7%) |
| gemcitabine | 9 (15.8%) | 36 (24.3%) |
| Maintenance therapy | 14 (24.6%) | 65 (43.9%) |
In the control population of HR+ HER2− patients, median age was 58 years (range 29.8–79.3); most patients (75.7%) were treated with carboplatin-paclitaxel and the remaining ones (24.3%) received carboplatin-gemcitabine. Of these patients, 70.3% received platinum-containing ChT as their first- or second-line therapy.
Impact of NLR and PLR on PFS
Median PFS in the TNBC population was 204 days. Higher neutrophils (4000/µl) were associated with non-significantly lower PFS (p = 0.081), while platelets above 300000/µl or lymphocytes below 1500/µl correlated with significantly shorter PFS (p = 0.041 and p = 0.005, respectively) (Fig. S1).
We then investigated the potential predictive role of NLR and PLR. Based on previous studies, we chose a NLR threshold of 2.5 and a PLR threshold of 20021,29. Both NLR and PLR were inversely associated with patient age (p < 0.001 and p = 0.048, respectively), while they did not correlate with previous taxane exposure (p = 0.076 and p = 0.68, respectively), presence of visceral disease (p = 0.9 and p = 0.6, respectively), type of ChT received (p = 0.48 and p = 0.7, respectively) and number of metastatic sites (p = 0.22 and p = 0.39, respectively) (Table 2). Finally, high NLR correlated with lower probability of receiving maintenance ChT (p = 0.017), while PLR did not (p = 0.097).
Table 2.
Characteristics of mTNBC patients by NLR and PLR.
| Total (n = 57) | NLR <2.5 (n = 25) | NLR ≥2.5 (n = 32) | p value | PLR <200 (n = 34) | PLR ≥200 (n = 23) | p value | |
|---|---|---|---|---|---|---|---|
| N (%) | N (%) | N (%) | N (%) | N (%) | |||
| Pts age | |||||||
| <50 yrs | 21 (36.8%) | 3 (5.2%) | 18 (31.6%) | <0.001 | 9 (15.8%) | 12 (21%) | 0.048 |
| >50 yrs | 36 (63.2%) | 22 (38.6%) | 14 (24.6%) | 25 (43.9%) | 11 (19.3%) | ||
| Previous taxane | |||||||
| Yes | 43 (75.4%) | 16 (28%) | 27 (47.4%) | 0.076 | 25 (43.8%) | 18 (31.6%) | 0.68 |
| No | 14 (24.6%) | 9 (15.8%) | 5 (8.8%) | 9 (15.8%) | 5 (8.8%) | ||
| Visceral disease | |||||||
| Yes | 37 (64.9%) | 16 (28%) | 21 (36.8%) | 0.9 | 23 (40.3%) | 14 (24.6%) | 0.6 |
| No | 20 (35.1%) | 9 (15.8%) | 11 (19.4%) | 11 (19.3%) | 9 (15.8%) | ||
| N. metastatic sites | |||||||
| 1–2 | 36 (63.2%) | 18 (31.6%) | 18 (31.6%) | 0.22 | 23 (40.3%) | 13 (22.8%) | 0.39 |
| >2 | 21 (36.8%) | 7 (12.3%) | 14 (24.5%) | 11 (19.3%) | 10 (17.6%) | ||
| ChT type | |||||||
| Taxane | 48 (84.2%) | 20 (35.1%) | 28 (49.1%) | 0.48* | 28 (49.1%) | 20 (35.1%) | 0.7* |
| Gemcitabine | 9 (15.8%) | 5 (8.8%) | 4 (7%) | 6 (10.5%) | 3 (5.3%) | ||
| Maintenance ChT | |||||||
| Yes | 14 (24.6%) | 10 (17.6%) | 4 (7%) | 0.017 | 11 (19.3%) | 3 (5.3%) | 0.097 |
| No | 43 (75.4%) | 15 (26.3%) | 28 (49.1%) | 23 (40.3%) | 20 (35.1%) | ||
Pts = patients; ChT = chemotherapy.
*Fisher’s exact test.
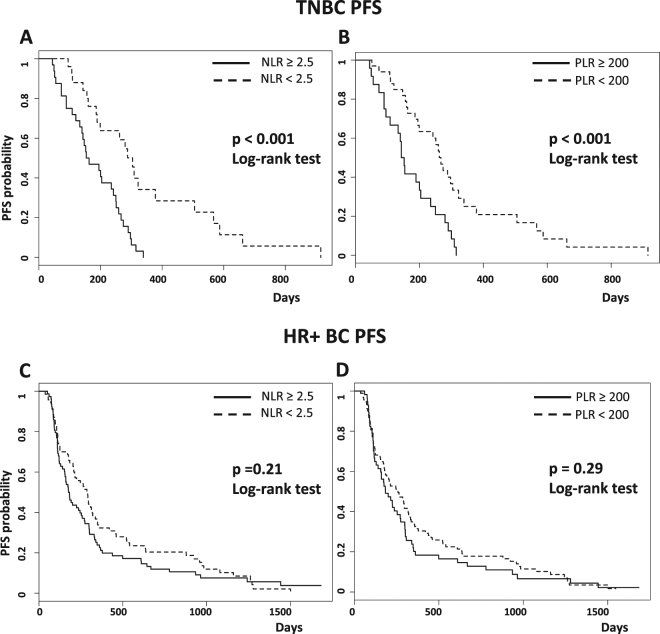
Median PFS was 304 days in patients with NLR < 2.5 and 158 days in those with NLR ≥ 2.5 (HR 3.25, 95% CI 1.72–6.25; p < 0.001) (Fig. 1A). Similar results were obtained by choosing a threshold of 3.3 (274 vs 148 days, p < 0.0001) or 2 (309 vs 186 days, p = 0.0015) (data not shown). Regarding the PLR, PFS was longer in patients with baseline PLR < 200 as compared to PLR ≥ 200 (HR 2.75, 95% CI 1.52–4.99; p < 0.001) (Fig. 1B). Baseline LMR ≥ 4.5 was also associated with significantly better PFS in TNBC patients, although the association was less strong than in the case of NLR and PLR (HR 2.22, 95% CI 1.22–4.05, p = 0.009; data not shown).
Figure 1.
Kaplan-Meier curves of progression free survival (PFS) of TNBC and HR+ mBC patients according to baseline NLR (A and C) and PLR (B and D).
When the same parameters were evaluated before the administration of the third treatment cycle, NLR < 2.5 was still associated with reduced risk of disease progression (140 vs 262 days, p < 0.001), while PLR < 200 was not (259 vs 204 days, p = 0.48) (data not shown).
In the control population of HR+ HER2− mBC patients, mPFS was 220 days. Baseline higher NLR and PLR were non-significantly associated with the risk of disease progression (p = 0.21 and p = 0.29) (Fig. 1C and D).
Independent predictive role of NLR and PLR on PFS
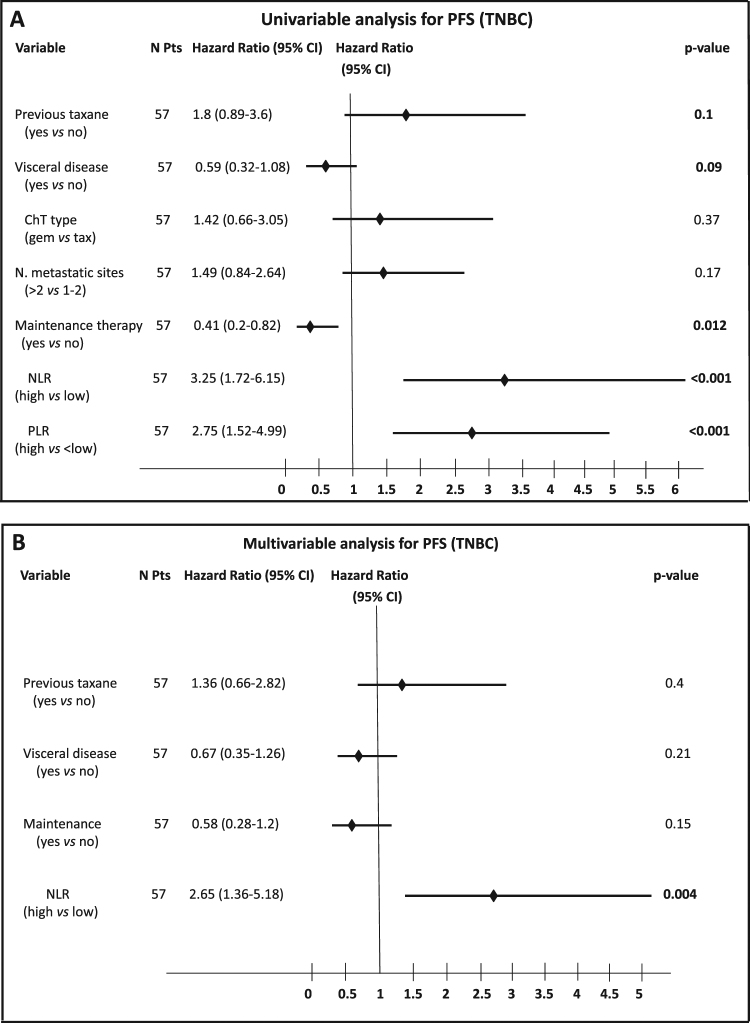
Factors associated with the risk of disease progression in mTNBC were: previous exposure to taxanes, the presence of visceral metastases, having received maintenance ChT, NLR ≥ 2.5 and PLR ≥ 200 (Fig. 2A). As previously described27, NLR and PLR positively correlated with each other (Pearson coefficient regression = 0.49; p < 0.001); therefore, only one of these parameters was evaluated at multivariable analysis.
Figure 2.
Forest plot illustrating the results of univariable (A) and multivariable (B) analysis of covariates associated with the risk of disease progression in mTNBC.
In the multivariable model including NLR as a covariate, NLR ≥ 2.5 was associated with significantly lower PFS (HR 2.65; p = 0.004), while other covariates were not statistically significantly associated with PFS (Fig. 2B). When we tested PLR at multivariable analysis, PLR ≥ 200 was independently associated with poorer patient prognosis (HR 2.33; p = 0.007), while the other covariates were not, with the exception of maintenance ChT, which correlated with reduced risk of progression (HR 0.45; p = 0.027) (data not shown).
Impact of peripheral blood parameters on OS
Median OS was 483 days in mTNBC patients and 653 days in the control population of HR+ HER2− patients.
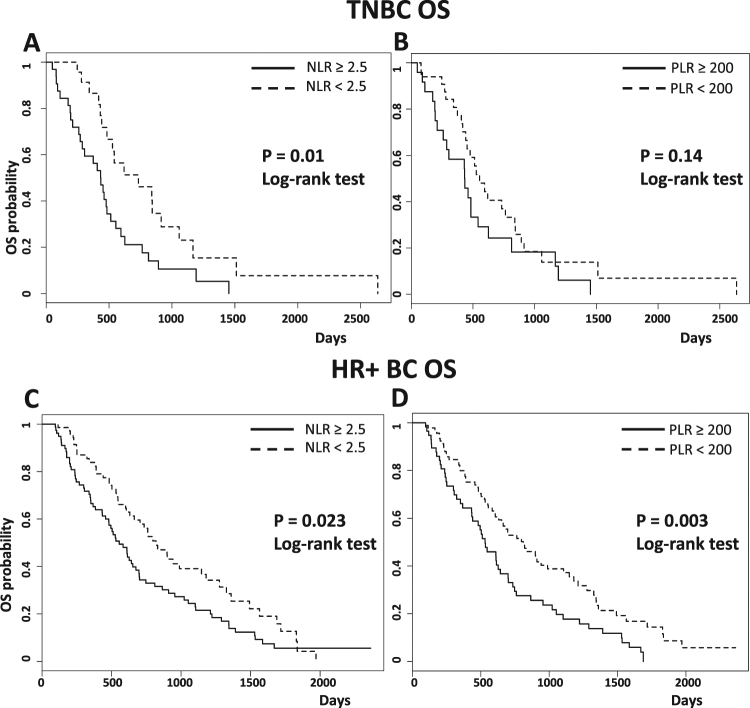
In mTNBC patients, mOS was significantly longer in patients with NLR < 2.5 compared to those with NLR ≥ 2.5 (p = 0.01), while PLR values were not associated with mOS (p = 0.14) (Fig. 3, upper panels). In HR+ HER2− BC patients, both NLR < 2.5 and PLR < 200 were associated with significantly better mOS (p = 0.023 and p = 0.003, respectively) (Fig. 3, lower panels).
Figure 3.
Kaplan-Meier curves of overall survival (PFS) of TNBC and HR+ mBC patients according to baseline NLR (A and C) and PLR (B and D).
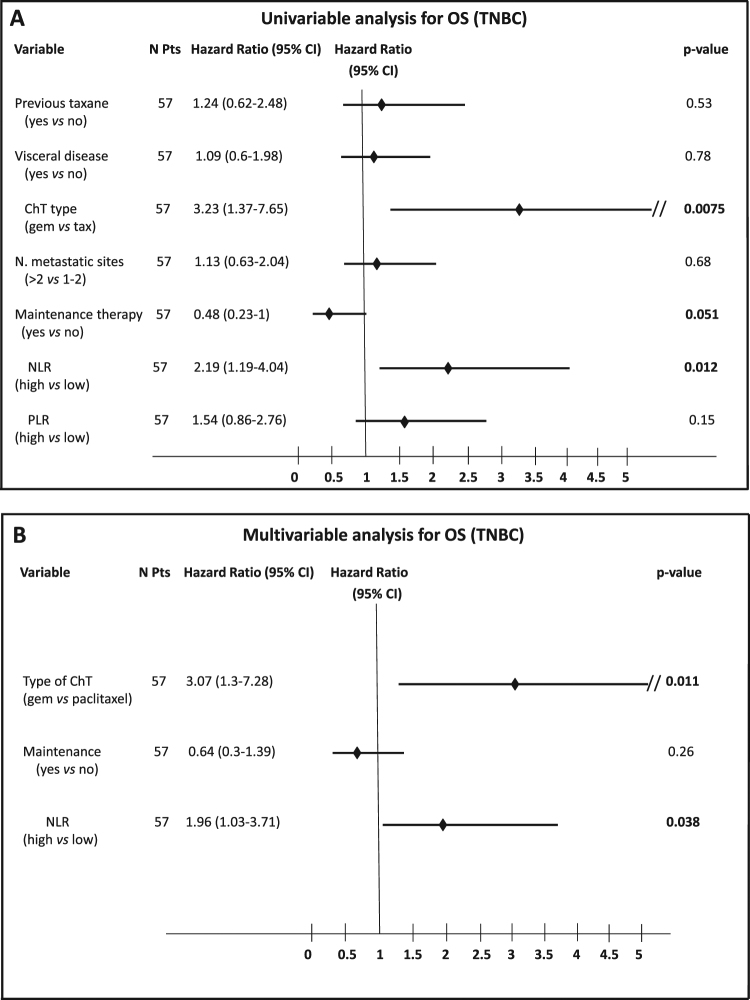
At univariate analysis, factors associated with worse OS in mTNBC patients were NLR ≥ 2.5 and having received gemcitabine in combination with carboplatin, while maintenance ChT correlated with better PFS (Fig. 4A). At multivariable analysis, NLR ≥ 2.5 and gemcitabine treatment were independently associated with worse outcomes (Fig. 4B).
Figure 4.
Forest plot illustrating the results of univariable (A) and multivariable (B) analysis of covariates associated with the overall survival in mTNBC.
In the control population, factors associated with lower OS were: NLR ≥ 2.5, presence of visceral disease, previous taxane exposure and more advanced (>2nd) treatment lines, while having received maintenance therapy correlated with lower risk of death (Fig. S2, upper panel). At multivariable analysis, high NLR and visceral involvement were independently associated with lower survival, while maintenance treatment correlated with better outcome (Fig. S2, lower panel).
Impact of germline BRCA 1/2 mutations on TNBC patient PFS
Among mTNBC patients included in our study, only 19 had undergone analysis of germline mutations of the BRCA1/2 genes. Of them, 6 were carriers of a pathogenic germline BRCA1/2 mutation, while the remaining 13 patients had wild-type BRCA1/2 gene. Median PFS was 126 days in carriers of BRCA1/2 mutations and 163 days in non-mutated ones (HR = 1.09, 50% CI 0.38–3.12, p = 0.87).
Discussion
Although platinum-based ChT is active against mTNBC, many patients fail to respond, and no biomarkers are currently available to predict treatment effectiveness. In this study, we found for the first time an association between higher NLR or PLR and worse PFS in mTNBC patients receiving carboplatin-paclitaxel or carboplatin-gemcitabine, but not in a control population of HR+ HER2− patients treated with the same regimens.
The fact that specific blood cell populations reflect the inflammatory/immune contexture is quite a well-established concept. Indeed, high neutrophils can be associated with systemic inflammation or immune suppression30; high platelets reflect systemic inflammation as well, but can be also associated with increased metastatization of neoplastic cells via platelet clots30–32; finally, low lymphocyte counts can be associated with impaired activation of adaptive immunity or poor nutritional status33,34. In this study, the association between clinical outcomes and NLR/PLR was stronger than in the case of individual cell counts. This is not surprising, since parameter combinations are more stable to changes in single parameters (e.g. neutrophils or platelets can increase during acute infections or glucocorticoid administration) and may capture more aspects of the tumor-immune system interplay. Notably, the HRs associated to NLR and PLR at multivariable analysis for PFS were similar, and these parameters also correlated with each other. This suggests that both NLR and PLR well reflect the inflammatory/immune contexture in mTNBC, and may be redundant as predictive biomarkers.
In TNBC patients, higher NLR correlated with lower OS, but the association was weaker than in the case of PFS; moreover, no statistically significant association was found between PLR and OS. These results can be due to the fact that, different from PFS, OS is affected by the whole treatment course, including therapies administered after platinum-based ChT; therefore, the association between NLR/PLR and PFS during platinum-based ChT could be diluted by subsequent treatments for which the same parameters are not predictive.
Conversely, both NLR and PLR correlated with lower OS in the control population of HR+ HER2− mBC patients, despite the fact that they were not predictive of PFS during platinum-based ChT. This could be due to the fact that NLR and PLR are associated to benefit from treatments administered after platinum ChT; therefore, their effect may only emerge when evaluating OS as an endpoint. Alternatively, these parameters may be generally prognostic in HR+ BC, but not predictive of benefit from specific treatments. Based on our data, we are unable to discriminate between these two hypotheses.
Studies conducted in recent years have revealed a previously unrecognized complexity of the number and functional status of different immune system subpopulations35,36. For instance, circulating blood lymphocytes include phenotypically and functionally different cells, such as CD8+ cytotoxic lymphocytes, regulatory T cells and exhausted lymphocytes, whose balance can determine the predominantly antitumor or protumor activity of adaptive immunity35. In parallel, blood monocytes include both cells that differentiate into antitumor M1 macrophages at the tissue level, and myeloid-derived suppressive cells (MDSCs) that exert pro-tumor effects by inhibiting the activity of antitumor T lymphocytes37. Coherently with a previous research in localized TNBC23, in this study we also found an association between the LMR and patient PFS, but this was weaker than in the case of NLR and PLR, and similar to the association between absolute lymphocyte counts and PFS. This finding suggests that monocytes may be poorly predictive of survival in mTNBC.
Investigating the potential association between specific immune cell populations and patient prognosis could reveal more reliable and predictive parameters to improve treatment selection in mTNBC. In this perspective, we recently started a prospective observational study to assess the role of baseline immunological parameters, as well as their on-treatment modifications, on the PFS of mTNBC patients receiving platinum-based ChT.
In this study, we did not find any significant difference in PFS duration between patients with or without BRCA1/2 germline mutations, which have recently emerged as associated with higher tumor response rates during carboplatin ChT6. However, the number of subjects amenable to this analysis was too low, and larger studies are required to assess the independent predictive role of NLR/PLR and BRCA1/2 mutations on the PFS of mTNBC receiving platinum-based ChT.
Strengths of this study consist in the monocentric patient cohort, which guarantees more reproducible assessment of tumor response and coherent collection of patient laboratory data by different investigators, as well as the homogeneity of ChT regimens used and the TNBC patient cohort. In particular, the fact that all TNBC patients received carboplatin-based ChT as first- or second-line treatment makes our results more robust compared to previously published data in patients treated with different regimens in different treatment lines. Weaknesses of our study consist in the retrospective design, the limited number of TNBC patients included in the analysis and the lack of data on tumor-infiltrating immune cells, which did not allow us to establish a direct, mechanistic link between peripheral blood parameters and immune cell populations in tumor microenvironment or directly infiltrating the tumor. However, the NLR and PLR calculated at the initiation of platinum-based ChT may reflect the systemic inflammatory and immunological status much more reliably than immune cells detected in tumor specimens biopsied/removed months/years before treatment administration. Moreover, the NLR and PLR could offer a more global picture of the immune contexture, thus circumventing the spatial heterogeneity in tumor-infiltrating immune cell populations.
In conclusion, the NLR and PLR are predictive of benefit from platinum-containing ChT specifically in mTNBC patients. They also confirm to have a generally prognostic role independently from tumor biology. If validated in larger prospective studies, these easy-to-measure parameters could be combined with emerging predictive biomarkers, such as germline or somatic BRCA 1/2 gene mutations, to improve the selection of mTNBC patients more likely to benefit from platinum-based ChT.
Methods
Study setting
This was a monocentric, retrospective study on patients with mTNBC treated between July 2007 and July 2017 at Fondazione IRCCS Istituto Nazionale dei Tumori (Milan, Italy) with platinum-based ChT. The study was approved by the Institutional Review Board of Fondazione IRCCS Istituto Nazionale dei Tumori (Milano, Italy). The study was performed in accordance to relevant guidelines and regulations. Patients alive at the time of data collection and/or analysis signed an informed consent for the use of their personal data for research purposes.
Eligibility criteria were: (1) age ≥18 years; (2) pathologically or cytologically confirmed diagnosis of unresectable, locally recurrent or metastatic TNBC, as defined by ER <1% and PgR <1% expression at immunohistochemistry (IHC) analysis and an IHC score for HER2 of 0, 1+, or 2+ with negative in situ hybridization (ISH); (3) ECOG performance status (PS) of 0–1; (4) treatment with one the following ChT schedules: carboplatin area under the concentration-time curve (AUC) of 2 plus paclitaxel 80 mg/m2, or carboplatin AUC 2 plus gemcitabine 800 mg/m2, both given on days 1 and 8 of every-three weeks cycles; (5) availability of baseline (pre-treatment) absolute peripheral blood neutrophil, lymphocyte and platelet counts; (6) available information about previous treatment(s) for limited-stage or advanced disease; (7) available information on the date of disease progression and patient death; (8) absence of acute infections or documented bone marrow infiltration at the time of peripheral blood cell count assessment.
As a control population, we selected patients with HR+ HER2− mBC treated with the same platinum-containing regimens in the July 2007-July 2017 decade at our Institution.
All subjects fulfilling these criteria were evaluated, regardless of line of treatment for mBC.
Objectives
The main objective of the study was to investigate the potential association between baseline NLR/PLR and clinical outcome. The primary clinical endpoint was PFS, as defined as the time between treatment initiation and disease progression or death from any cause. OS was a secondary endpoint, and was defined as the time between treatment initiation and death from any cause.
Assessment of response
Response was assessed every 3 ChT cycles, but tumor re-evaluation was anticipated in patients with worsening symptoms or other signs suggestive of progressive disease (PD). Tumor response was evaluated according to the Response Evaluation Criteria in Solid Tumors (RECIST 1.1). Patients with only superficial, measurable disease were evaluated also clinically by measuring lesion diameters every three weeks.
Evaluation of biomarkers
We collected data on absolute counts of peripheral blood neutrophils, lymphocytes, platelets and monocytes. We calculated the following parameters: (a) NLR by dividing neutrophil by lymphocyte counts; (b) PLR by dividing platelet by lymphocyte counts. As an exploratory analysis, we also assessed the lymphocyte-to-monocyte ratio (LMR). Blood parameters were evaluated before initiation of platinum-based ChT. Parameters measured within one month before the initiation of platinum-based ChT were considered acceptable, provided that the patient was not receiving any concomitant anticancer treatment. Patients whose blood parameters were measured more than one month before ChT initiation, or after having received the first dose of platinum-based ChT, were excluded from this study. As an exploratory analysis, we also evaluated the NLR and PLR before the administration of the third treatment cycle.
Genetic analysis
Analysis of BRCA1/2 germline mutations was carried out on the DNA extracted from peripheral blood leukocytes. All coding exons and flanking regions of BRCA1 and BRCA2 genes were sequenced through direct sequencing, followed in most cases by multiple ligation-dependent probe amplification (MLPA) to detect large genomic rearrangements. Identified genetic variants were classified according to the IARC 5-tier scheme, following the guidelines of the Evidence-based Network for the Interpretation of Germline Mutant Alleles (ENIGMA; http://enigmaconsortium.org/)38.
Statistical analysis
Patients’ characteristics were analysed by descriptive statistics. The χ2 or Fisher’s exact tests were used to assess the association between categorical variables, while linear correlation was used for continuous variables. PFS and OS were calculated according to the Kaplan-Meier method, and the log-rank test was used to compare survival between different patient populations. The impact of known prognostic factors on PFS was first assessed at univariate analysis. Covariates significantly associated with the risk of progression (p < 0.1) were then included in a Cox proportional hazard model to assess their independent association with survival. Based on previously published data, the following categorical covariates were tested: previous exposure to taxanes (yes vs no); visceral disease (yes vs no); the number of metastatic sites (1–2 vs >2); having received maintenance treatment (yes vs no)9. The type of chemotherapeutical agent combined with carboplatin (i.e. gemcitabine vs paclitaxel) was also tested as a covariate. All statistical analyses were performed using the software R (version 3.3.2 (2016-10-31)), while the package “survival” was used for survival analyses. A p value of 0.05 was chosen as a threshold level for statistical significance.
Data availability
The datasets generated and/or analysed during the current study are available from the corresponding author on reasonable request.
Electronic supplementary material
Author Contributions
C.V., A.M., M.P., M.M., C.M., L.R., M.S.C., B.F., G.M., G.B. and G.C. collected and reviewed patient data and clinical history. S.M. collected data of genetic analyses. V.H. and L.R. reviewed biological data. C.V. performed statistical analyses. C.V. wrote the main manuscript text and prepared Figures 1, 3 and Supplementary Figure 1. A.M. prepared Figure Nos 2, 4, Supplementary Figure 2. F.d.B. supervised the whole project. All authors reviewed the manuscript.
Competing Interests
The authors declare no competing interests.
Footnotes
Electronic supplementary material
Supplementary information accompanies this paper at 10.1038/s41598-018-27075-z.
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Society, A. C. Cancer Facts & Figures. American Cancer Society, Atlanta (2017).
- 2.Malorni L, et al. Clinical and biologic features of triple-negative breast cancers in a large cohort of patients with long-term follow-up. Breast Cancer Res Treat. 2012;136(3):795–804. doi: 10.1007/s10549-012-2315-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bianchini G, Balko JM, Mayer IA, Sanders ME, Gianni L. Triple-negative breast cancer: challenges and opportunities of a heterogeneous disease. Nat Rev Clin Oncol. 2016;13(11):674–690. doi: 10.1038/nrclinonc.2016.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.O’Shaughnessy J, et al. Phase III study of iniparib plus gemcitabine and carboplatin versus gemcitabine and carboplatin in patients with metastatic triple-negative breast cancer. J Clin Oncol. 2014;32(34):3840–3847. doi: 10.1200/JCO.2014.55.2984. [DOI] [PubMed] [Google Scholar]
- 5.Yardley, D. C. R. et al. nab -paclitaxel + carboplatin or gemcitabine vs gemcitabine/carboplatin as first-line treatment for patients with triple-negative metastatic breast cancer: Results from the randomized phase 2 portion of the tnAcity trial. Abstract P5-15-03 Presented at: 2016 San Antonio Breast Cancer Symposium, San Antonio (2016).
- 6.Tutt, A. E. P. et al. Carboplatin in BRCA1/2-mutated and triple-negative breast cancer BRCAness subgroups: the TNT Trial. Nat Med.24(5), 628–637 (2018). [DOI] [PMC free article] [PubMed]
- 7.Miles DW, et al. First-line bevacizumab in combination with chemotherapy for HER2-negative metastatic breast cancer: pooled and subgroup analyses of data from 2447 patients. Ann Oncol. 2013;24(11):2773–2780. doi: 10.1093/annonc/mdt276. [DOI] [PubMed] [Google Scholar]
- 8.Hu XC, et al. Cisplatin plus gemcitabine versus paclitaxel plus gemcitabine as first-line therapy for metastatic triple-negative breast cancer (CBCSG006): a randomised, open-label, multicentre, phase 3 trial. Lancet Oncol. 2015;16(4):436–446. doi: 10.1016/S1470-2045(15)70064-1. [DOI] [PubMed] [Google Scholar]
- 9.Vernieri C, et al. Antitumor activity and safety profile of weekly carboplatin plus paclitaxel in metastatic breast cancer: a ten-year, monocentric, retrospective study. Breast Cancer Res Treat. 2017;165(2):365–373. doi: 10.1007/s10549-017-4336-z. [DOI] [PubMed] [Google Scholar]
- 10.Loi S, et al. Prognostic and predictive value of tumor-infiltrating lymphocytes in a phase III randomized adjuvant breast cancer trial in node-positive breast cancer comparing the addition of docetaxel to doxorubicin with doxorubicin-based chemotherapy: BIG 02-98. J Clin Oncol. 2013;31(7):860–867. doi: 10.1200/JCO.2011.41.0902. [DOI] [PubMed] [Google Scholar]
- 11.West NR, et al. Tumor-infiltrating lymphocytes predict response to anthracycline-based chemotherapy in estrogen receptor-negative breast cancer. Breast Cancer Res. 2011;13(6):R126. doi: 10.1186/bcr3072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Denkert C, et al. Tumor-infiltrating lymphocytes and response to neoadjuvant chemotherapy with or without carboplatin in human epidermal growth factor receptor 2-positive and triple-negative primary breast cancers. J Clin Oncol. 2015;33(9):983–991. doi: 10.1200/JCO.2014.58.1967. [DOI] [PubMed] [Google Scholar]
- 13.Dieci MV, et al. Prognostic and predictive value of tumor-infiltrating lymphocytes in two phase III randomized adjuvant breast cancer trials. Ann Oncol. 2015;26(8):1698–1704. doi: 10.1093/annonc/mdv239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nanda R, et al. Pembrolizumab in Patients With Advanced Triple-Negative Breast Cancer: Phase Ib KEYNOTE-012 Study. J Clin Oncol. 2016;34(21):2460–2467. doi: 10.1200/JCO.2015.64.8931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Emens, L. A. B. et al. Inhibition of PD-L1 by MPDL3280A leads to clinical activity in patients with metastatic triple-negative breast cancer [abstract]. San Antonio Breast Cancer Symposium: 2014 Dec 9–13; San Antonio, TX (2014).
- 16.Schmid, P. C. et al. Atezolizumab in metastatic TNBC (mTNBC): Long-term clinical outcomes and biomarker analyses AACR Annual Meeting Abstract 2986 Presented April 3, 2017 (2017).
- 17.Adams, S. D. et al. Phase Ib trial of atezolizumab in combination with nab-paclitaxel in patients with metastatic triple-negative breast cancer (mTNBC). J Clin Oncol34, (suppl, abstr1009) (2016).
- 18.Adams, S. D. et al. Safety and clinical activity of atezolizumab (anti-PDL1) in combination with nab-paclitaxel in patients with metastatic triple-negative breast cancer. [abstract] In: Proceedings of the Thirty-Eighth Annual CTRC-AACR San Antonio Breast Cancer Symposium: 2015 Dec 8–12; San Antonio, TX Philadelphia (PA): AACR; Cancer Res 76 (4 Suppl): Abstract nrP2-11-06 (2016).
- 19.Templeton AJ, et al. Prognostic role of neutrophil-to-lymphocyte ratio in solid tumors: a systematic review and meta-analysis. J Natl Cancer Inst. 2014;106(6):dju124. doi: 10.1093/jnci/dju124. [DOI] [PubMed] [Google Scholar]
- 20.Templeton AJ, et al. Prognostic role of platelet to lymphocyte ratio in solid tumors: a systematic review and meta-analysis. Cancer Epidemiol Biomarkers Prev. 2014;23(7):1204–1212. doi: 10.1158/1055-9965.EPI-14-0146. [DOI] [PubMed] [Google Scholar]
- 21.Ethier JL, Desautels D, Templeton A, Shah PS, Amir E. Prognostic role of neutrophil-to-lymphocyte ratio in breast cancer: a systematic review and meta-analysis. Breast Cancer Res. 2017;19(1):2. doi: 10.1186/s13058-016-0794-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pistelli M, et al. Pre-treatment neutrophil to lymphocyte ratio may be a useful tool in predicting survival in early triple negative breast cancer patients. BMC Cancer. 2015;15:195. doi: 10.1186/s12885-015-1204-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jia W, et al. The Peripheral Blood Neutrophil-To-Lymphocyte Ratio Is Superior to the Lymphocyte-To-Monocyte Ratio for Predicting the Long-Term Survival of Triple-Negative Breast Cancer Patients. PLoS One. 2015;10(11):e0143061. doi: 10.1371/journal.pone.0143061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Azab B, et al. Usefulness of the neutrophil-to-lymphocyte ratio in predicting short- and long-term mortality in breast cancer patients. Ann Surg Oncol. 2012;19(1):217–224. doi: 10.1245/s10434-011-1814-0. [DOI] [PubMed] [Google Scholar]
- 25.Azab B, et al. Pretreatment neutrophil/lymphocyte ratio is superior to platelet/lymphocyte ratio as a predictor of long-term mortality in breast cancer patients. Med Oncol. 2013;30(1):432. doi: 10.1007/s12032-012-0432-4. [DOI] [PubMed] [Google Scholar]
- 26.Dirican A, et al. Do the derived neutrophil to lymphocyte ratio and the neutrophil to lymphocyte ratio predict prognosis in breast cancer? Int J Clin Oncol. 2015;20(1):70–81. doi: 10.1007/s10147-014-0672-8. [DOI] [PubMed] [Google Scholar]
- 27.Koh CH, et al. Utility of pre-treatment neutrophil-lymphocyte ratio and platelet-lymphocyte ratio as prognostic factors in breast cancer. Br J Cancer. 2015;113(1):150–158. doi: 10.1038/bjc.2015.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rimando J, Campbell J, Kim JH, Tang SC, Kim S. The Pretreatment Neutrophil/Lymphocyte Ratio Is Associated with All-Cause Mortality in Black and White Patients with Non-metastatic Breast Cancer. Front Oncol. 2016;6:81. doi: 10.3389/fonc.2016.00081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Plon SE, et al. Sequence variant classification and reporting: recommendations for improving the interpretation of cancer susceptibility genetic test results. Hum Mutat. 2008;29(11):1282–1291. doi: 10.1002/humu.20880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Raungkaewmanee S, Tangjitgamol S, Manusirivithaya S, Srijaipracharoen S, Thavaramara T. Platelet to lymphocyte ratio as a prognostic factor for epithelial ovarian cancer. J Gynecol Oncol. 2012;23(4):265–273. doi: 10.3802/jgo.2012.23.4.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Roxburgh CS, McMillan DC. Cancer and systemic inflammation: treat the tumour and treat the host. Br J Cancer. 2014;110(6):1409–1412. doi: 10.1038/bjc.2014.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gay LJ, Felding-Habermann B. Contribution of platelets to tumour metastasis. Nat Rev Cancer. 2011;11(2):123–134. doi: 10.1038/nrc3004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wagner DD. New links between inflammation and thrombosis. Arterioscler Thromb Vasc Biol. 2005;25(7):1321–1324. doi: 10.1161/01.ATV.0000166521.90532.44. [DOI] [PubMed] [Google Scholar]
- 34.Gross RL, Newberne PM. Role of nutrition in immunologic function. Physiol Rev. 1980;60(1):188–302. doi: 10.1152/physrev.1980.60.1.188. [DOI] [PubMed] [Google Scholar]
- 35.Dunki Jacobs PB, Ruevekamp M, Hart GA, de Graaf PW. Dietary influences on cell proliferation in bone marrow. Eur J Cancer Clin Oncol. 1989;25(6):953–957. doi: 10.1016/0277-5379(89)90153-3. [DOI] [PubMed] [Google Scholar]
- 36.Mariucci S, et al. Lymphocyte subpopulation and dendritic cell phenotyping during antineoplastic therapy in human solid tumors. Clin Exp Med. 2011;11(4):199–210. doi: 10.1007/s10238-010-0120-7. [DOI] [PubMed] [Google Scholar]
- 37.Camisaschi, C. et al. Targeting Immune Regulatory Networks to Counteract Immune Suppression in Cancer. Vaccines (Basel) 4(4) (2016). [DOI] [PMC free article] [PubMed]
- 38.Filipazzi P, et al. Identification of a new subset of myeloid suppressor cells in peripheral blood of melanoma patients with modulation by a granulocyte-macrophage colony-stimulation factor-based antitumor vaccine. J Clin Oncol. 2007;25(18):2546–2553. doi: 10.1200/JCO.2006.08.5829. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated and/or analysed during the current study are available from the corresponding author on reasonable request.