Abstract
The transforming growth factor-β (TGF-β) family plays major pleiotropic roles by regulating many physiological processes in development and tissue homeostasis. The TGF-β signaling pathway outcome relies on the control of the spatial and temporal expression of >500 genes, which depend on the functions of the Smad protein along with those of diverse modulators of this signaling pathway, such as transcriptional factors and cofactors. Ski (Sloan-Kettering Institute) and SnoN (Ski novel) are Smad-interacting proteins that negatively regulate the TGF-β signaling pathway by disrupting the formation of R-Smad/Smad4 complexes, as well as by inhibiting Smad association with the p300/CBP coactivators. The Ski and SnoN transcriptional cofactors recruit diverse corepressors and histone deacetylases to repress gene transcription. The TGF-β/Smad pathway and coregulators Ski and SnoN clearly regulate each other through several positive and negative feedback mechanisms. Thus, these cross-regulatory processes finely modify the TGF-β signaling outcome as they control the magnitude and duration of the TGF-β signals. As a result, any alteration in these regulatory mechanisms may lead to disease development. Therefore, the design of targeted therapies to exert tight control of the levels of negative modulators of the TGF-β pathway, such as Ski and SnoN, is critical to restore cell homeostasis under the specific pathological conditions in which these cofactors are deregulated, such as fibrosis and cancer.
Growth suppressors: Antagonistic proteins offer drug targets for fibrosis and cancer
Proteins that repress molecular signaling through the transforming growth factor-beta (TGF-β) pathway offer promising targets for treating cancer and fibrosis. Marina Macías-Silva and colleagues from the National Autonomous University of Mexico in Mexico City review the ways in which a pair of proteins, called Ski and SnoN, interact with downstream mediators of TGF-β to inhibit the effects of this master growth factor. Aberrant levels of Ski and SnoN have been linked to diverse range of diseases involving cell proliferation run amok, and therapies that regulate the expression of these proteins could help normalize TGF-β signaling to healthier physiological levels. For decades, drug companies have tried to target the TGF-β pathway, with limited success. Altering the activity of these repressors instead could provide a roundabout way of remedying pathogenic TGF-β activity in fibrosis and oncology.
Introduction
The transforming growth factor-beta (TGF-β) superfamily comprises a large group of related growth factors, such as TGF-βs, activins, inhibins, bone morphogenetic proteins (BMPs), myostatin, nodal, lefty, anti-Müllerian hormone/Müllerian inhibiting substance (AMH/MIS), and growth and differentiation factors (GDFs).1 This family of cytokines and differentiation factors has major pleiotropic activities in development and tissue homeostasis and exerts altered functions in diverse pathologies.2–5 Upon ligand binding to type II and type I receptors, the active ligand-heterotetrameric receptor complex signals through downstream transcriptional factors named Smads (Fig. 1).6–10 The Smad protein family is divided into three groups: R-Smad (Smad1, Smad2, Smad3, Smad5, and Smad8), co-Smad (Smad4), and I-Smad (Smad6 and Smad7). Receptor-regulated Smads (R-Smad) have an SSXS motif in their C-terminal region that is phosphorylated by type I receptors; R-Smad phosphorylation (p-R-Smad) allows their association with Smad4.11–18 After translocation of the p-R-Smad/Smad4 heterotrimeric complex into the nucleus, Smads associate with other transcriptional factors and coregulators to regulate the expression of specific target genes. TGF-β signaling regulates the transcription of >500 genes, which may contain one or more Smad binding elements (SBEs) in their promoter region (Fig. 1). Multiple Smad-interacting transcription factors may cooperate with Smads to modulate-specific target gene expression, depending on the cellular type, in both physiological and pathological conditions.19,20
Fig. 1.

Regulation of the TGF-β/Smad signaling pathway by Ski and SnoN. In the absence of TGF-β, the Ski and SnoN proteins interact with Smad4 to inhibit the expression of TGF-β target genes, such as Smad7 and Skil, by recruiting other corepressors and HDACs to their promoters. Then, TGF-β induces the phosphorylation of Smad2/3 proteins to form the R-Smad/Smad4 complex, which associates with specific transcription factors and cofactors to modulate the expression of its target genes
The luminescence-based mammalian interactome mapping (LUMIER) dataset comprises >100 proteins associated with the TGF-β receptor complex or with Smad proteins; although, the biological relevance of each interaction remains to be elucidated. The LUMIER dataset is available to support the investigation of TGF-β signaling networks in different cell types.21 Furthermore, the activity and stability of Smad proteins are regulated by diverse post-translational modifications (PTMs), such as phosphorylation, acetylation, ubiquitination, sumoylation, and parylation; thus, the variety of Smad isoforms generated by PTMs increases the complexity of TGF-β signal transduction networks.20,22–24 The TGF-β signaling pathway is also modulated by multiple mechanisms, including the actions of diverse positive and negative regulators, which orchestrate temporal and spatial actions of TGF-β signaling.25,26 TGF-β signals may also regulate the expression of their own regulators, generating negative feedback loops to control the magnitude and duration of TGF-β signaling. Among the negative regulators of TGF-β signals are TGIF, Ski, and SnoN transcriptional cofactors, which can inhibit Smad transcriptional activity to exert differential regulation of TGF-β-targeted gene expression (Fig. 1).27–30
Ski and snon are major negative regulators of the Tgf-β signaling pathway
The homologous Ski and SnoN proteins belong to the Ski protein family; they are mainly localized in the nucleus and may induce cell transformation when overexpressed.29–33 The Ski protein family includes several members, such as Ski, SnoN, SnoN2, SnoI, SnoA, CORL-1, DACH1/Dach1, DACH2/Dach2, Fussel-15 (SKOR1; LBXCOR1), Fussel-18 (SKOR2; CORL-2), DAF-5, Dachshund, and Fusel.34–48 Presently, Ski and SnoN have been identified as key transcriptional corepressors of Smad proteins and exhibit vital biological functions by controlling the TGF-β/Smad signaling pathway during embryogenesis and tissue homeostasis.29,30,33 Ski and SnoN act as Smad corepressors by indirect binding to the consensus sequence 5′-GTCTAGAC-3′ (known as SBE) through their association with Smad proteins.49,50 According to the current model, Ski and SnoN block TGF-β signaling by forming an inhibitory complex with Smad proteins on SBEs of the TGF-β-target gene promoters, and such complexes recruit histone deacetylases (HDACs) and additional corepressors to inhibit gene expression (Fig. 1).29,30,33
Ski functions rather as a transcriptional coregulator since it binds only to DNA in a complex with other transcriptional factors, which is essential for target-gene activation or repression.51–55 Notably, Ski acts as a potent inhibitor of transcriptional factors, such as Smads and Gli3, in mouse embryos through its association with HDACs, NCoR, and mSin3A.51,56–58 The Ski protein disturbs TGF-β signaling by competing with the CREB-binding protein (CBP) for the binding of activated Smads.59 Ski may also repress the expression of some BMP target genes by its interaction with the homeodomain-interacting protein kinase 2 (HIPK2) in mouse myoblasts.60 Additional examples include the inhibition of Smad2 transcriptional activity via the interaction with Ski and c-Jun;61 inactivation of the vitamin-D-receptor (VDR) signaling pathway by the Ski/NCoR complex;62 inhibition of the retinoic acid (RA) signaling and the transcription factor PU.1 expression by Ski/HDAC3;63,64 decreased Ctip2 gene expression by the Ski/HDAC1 and Satb2/MTA2 complexes;65,66 Ski inhibition of GATA1 binding to DNA and its transcriptional activity;67 Ski and SIRT1 inhibition of p53 actions;68 and Hippo pathway inhibition through the recruitment of NCoR by Ski to the TEAD/TAZ protein complex.69 Transcriptional gene silencing is also mediated by the interactions of Ski with other factors, such as PRMT5, HDAC3, Rb, MeCP2, and Mad, as well as with the thyroid hormone receptor β (TRβ).54,70–72
Likewise, the SnoN protein also functions as a transcriptional coregulator; it associates with Smads to inhibit gene expression, including the silencing of its own gene Skil (Fig. 1).73 To date, a few genes have been identified as targets of the SnoN/Smad complex, including those encoding Smad7, SnoN, FGF8, GSC, MIXL1, and AFP.54,73–76 Other genes that are regulated by SnoN encode miR720, miR274A, miR1274B, ADAM12, PLSCR1, Ccd1, and pS2.77–83
Ski function as a coactivator became evident after the demonstration of its association with the nuclear factor 1 (NF1) family of transcription factors.84,85 Ski favors β-catenin signaling to promote the expression of Mitf and Nr-CAM genes in melanoma.86 Ski also favors myogenin gene expression and myogenesis by forming a complex with specific transcription factors, such as MyoD,87 as well as with Six1 and Eya3.88 SnoN can also act as a coactivator for Smads and for other transcriptional factors, but this ability depends on specific target genes;77,78 for instance, SnoN is a coactivator for estrogen receptors (ER) and enhances the estrogen-signaling pathway in breast cancer cells.80 Nevertheless, more studies are required to reveal the versatility of Ski and SnoN mechanisms to control gene transcription.
Structure and regulation of Ski and Skil genes
The viral form v-Ski was identified as a gene inserted into the avian Sloan-Kettering retroviruses (SKVs) genome.89,90 Later, a homologous proto-oncogene was identified in chicken and denoted c-Ski (cellular gene).90 Ski homologs have been detected in several vertebrate species from fish to humans.90–94 The human Ski gene is localized at chromosome 1 and generates at least two transcripts that encode a 728-amino acid (aa) protein.91,95 Thus, there is no evidence of Ski mRNA splicing, and it appears that Ski transcript differences rely on the length of the 3′-UTR (untranslated region).91,96 Regulation of Ski gene expression has been poorly studied; however, some factors or signals have been identified as potential regulators of Ski expression, such as serum-response factor (SRF), peroxisome proliferator-activated receptor δ (PPARδ), and RA signaling (Fig. 2).97–100 Recently, it has been reported that the Ski gene is hypermethylated and silenced in human primary lung cancer tissues, supporting Ski function as a tumor suppressor.101 Ski mRNA levels are also modulated by different miRNAs, such as miR-21, miR-29a, miR-155, and miR-127-3p (Fig. 2).102–107
Fig. 2.

Regulation of Ski and SnoN expression by different mechanisms. Several signaling pathways and factors induce Ski and Skil gene expression; whereas, the TGF-β signaling pathway enhances or inhibits Skil gene expression via Smad2/4 or Smad3/4, respectively. Moreover, some miRNAs control the mRNA levels of these genes. The Ski and SnoN protein levels are also regulated by different signals
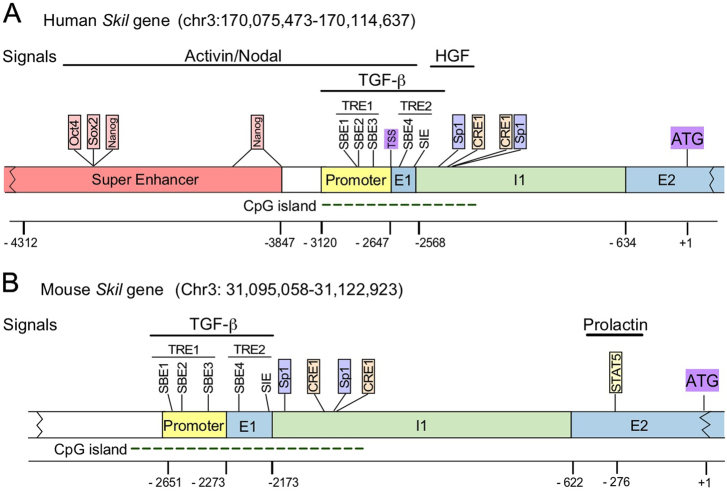
Human and mouse Skil genes are localized on chromosome 3 and encode for all SnoN isoforms. There is a limited characterization of the 5′ end regulatory sequences of both human and mouse Skil genes, including promoters and enhancers, due to the complex organization and modulation of these genes (Fig. 3). Both human and mouse Skil genes harbor two TGF-β-responsive elements (TRE) localized ~2 kb upstream of the translation starting site (ATG). TRE1 is localized in the core promoter and formed by three SBE sequences: SBE1, SBE2, and SBE3,73,108 whereas TRE2 is localized at exon 1 and contains the SBE4 and a Smad inhibitory element (SIE); all the SBEs are recognized by the Smad2/Smad4 complex to induce Skil gene expression, whereas SIE is recognized by the Smad3/Smad4 complex, which inhibits Skil gene expression (Fig. 3).73,108 Regarding the 5′-UTR of Skil mRNA, E1 (exon 1 with 170 bp) and a fragment of E2 (exon 2 with 633 bp) encode for the 5′-UTR of Skil mRNA and are separated by a large intron (1.9 kb).73
Fig. 3.
Regulatory regions of human and mouse Skil genes. The Skil gene promoter includes two TGF-β-responsive elements (TRE) containing several Smad binding elements (SBEs and SIE). The human Skil gene superenhancer region contains binding sites for Oct4, Sox2, and Nanog proteins. The mouse Skil gene promoter includes the SBEs and SIE, as well as a binding site for STAT5 that is controlled by prolactin. The positions of response elements and transcription start site (TSS) are indicated with respect to the ATG (+1)
The TGF-β signaling pathway controls the expression of the Skil gene through diverse mechanisms: under basal conditions, the SnoN/Ski/Smad4 complex binds and represses the Skil gene promoter; then, after a short period of TGF-β stimulation, SnoN and Ski protein degradation occurs via the proteasome; whereas, the activated Smad2/Smad4 complex replaces the SnoN/Ski complex on the Skil gene promoter and promotes its induction. Both SnoN mRNA and protein levels are increased after TGF-β treatment for ~1.5 h. Subsequently, a negative feedback loop is generated, in which the SnoN protein binds the Skil gene promoter to inhibit its own expression.73 Interestingly, human Skil gene also contains a super-enhancer localized ~3.6 kb upstream from the ATG (+1) and ~1 kb upstream from the TRE1; the coordinated regulation of Skil super-enhancer by Oct4/Sox2/Nanog (OSN) transcriptional factors and Skil promoter by activated Smads seems to be essential for the maintenance of stem cell pluripotency (Figs. 2 and 3).74
TGF-β/Smad, activin, nodal, BMP7, HGF/CREB, and prolactin/STAT5 are the best-known signals that regulate Skil gene expression at the transcriptional level (Fig. 2).73,109–113 Other less studied pathways also appear to regulate Skil gene expression, such stimulation of the PI3K/Akt pathway with arsenic trioxide (As2O3), and inhibition of Smad3 phosphorylation with endocrine disrupting chemicals (EDC), such as 4-nonylphenol (NP) and bisphenol A (BPA).114,115 Furthermore, Skil expression is also regulated by miRNAs: miR-17 and miR-23a are potential regulators of the 3′-UTR of human Skil mRNA (Fig. 2);116,117 interestingly, miR-17 is a member of the miR-17-19-130 superfamily targeting tumor suppressors, such as the TGF-β, PI3K, and p53 pathways.
Molecular structure and PTMs of the Ski and SnoN proteins
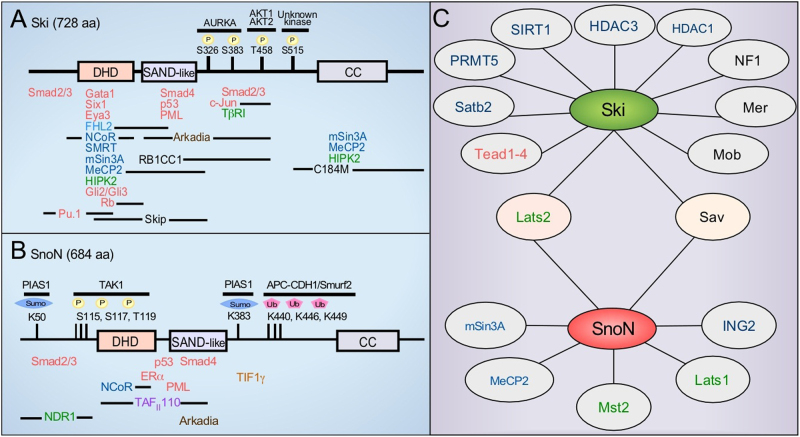
The human Ski protein has 728 aa and three main domains (Fig. 4). The N-terminal Ski-dachshund homology domain (Ski-DHD) comprises a region of residues 91–192 with a folding pattern of mixed α/β structures; this domain has lost the ability to bind directly to DNA, but instead behaves as an interacting domain that binds R-Smads and other transcriptional regulators, such as NCoR and Skip.51,56,58,71,118–121 The Ski protein also has a SAND-like domain (residues 219–312) that includes an extended I-loop motif that is responsible for Smad4 binding (Fig. 4).122 Two other N-terminal regions in the Ski protein include a proline-enriched stretch (between residues 61 and 89) and the transformation domain that is localized in the first 304 residues; this last domain is responsible for some Ski activities, such as cell transformation and transcriptional gene repression.49,50,123,124 The Ski- and SnoN-DHD crystal structure reveals that the transformation domain is important for recruiting multiple partners (Fig. 4).121,125,126 The Ski protein differs at its C-terminus sequence, where it has a helical domain that forms a coiled-coil (CC) region constituting two motifs: a region of five tandem repeat (TR) motifs with 25 residues in each, and a leucine zipper (LZ) motif of six heptad repeats; both structural motifs are implicated in the formation of Ski homodimers and Ski/SnoN heterodimers.124,127,128
Fig. 4.
Molecular structure, posttranslational modifications, and protein–protein interactions of the Ski and SnoN proteins. a, b The domains that define the Ski and SnoN proteins include: Dachshund homology domain (DHD), SAND-like domain, and coiled-coil domain (CC). Both the Ski and SnoN proteins are regulated by PTMs, as catalyzed by several enzymes at specific residues that are indicated. Known regions of interaction with some partners for Ski and SnoN are indicated. c Several proteins are also partners for Ski and/or SnoN but the interacting domains are not identified. Kinases are shown in green, transcriptional coregulators in blue, transcription factors in red, and other proteins in black
SnoN is a protein of 684 aa with four conserved domains in its N-terminus: minimal transformation domain, SAND-like domain, DHD domain, and Smad-binding domain. The C-terminus of the SnoN protein is variable and contains a domain for SnoN homodimerization and Ski/SnoN heterodimerization (Fig. 4).122,127,129–131 Four SnoN isoforms have been identified: SnoN (non-alu-containing), SnoN2, SnoA (alu-containing), and SnoI (insertion). All SnoN isoforms result from alternative splicing and almost all are ubiquitously expressed, except SnoI, which is mainly expressed in skeletal muscle. SnoN2 differs from SnoN by a deletion of 138 nucleotides; whereas, SnoI and SnoA encode truncated proteins of 399 and 415 aa, respectively. Although both SnoI and SnoA proteins lack the dimerization domain, only SnoA has a unique domain in its C-terminal region.91,132,133 The four SnoN isoforms have been identified in Drosophila and humans; whereas, SnoN and SnoN2 are the only isoforms expressed in mice.133–135 Nevertheless, the localization, regulation, and function of all SnoN isoforms have not yet been completely revealed.
Ski was initially described as a phosphorylated protein in serine residues.32 Growth factors and hormones, such as hepatocyte growth factor (HGF), insulin-like growth factor-1 (IGF-1), and insulin promote Ski protein degradation by inducing its phosphorylation at the Thr458 residue by AKT kinase.136 The Ski protein residue Ser515 is also phosphorylated, but the kinase involved has not been identified; however, mutation of Ser515 does not affect Ski activity as a transcriptional corepressor.137 Ski also interacts with Aurora A kinase (Aurka) through its C-terminal region; Aurka phosphorylates Ski at Ser326 and Ser383 to decrease Ski stability (Fig. 4).138,139 By contrast, TAK1 kinase promotes SnoN protein phosphorylation at residues Ser115, Ser117, and Thr119 after TGF-β treatment, which causes polyubiquitination and posterior degradation of the SnoN protein (Fig. 4).140
Ski and SnoN protein polyubiquitination causes their degradation via the ubiquitin-proteasome system (UPS). To date, three main E3 ubiquitin-ligases interacting with SnoN have been identified: the anaphase-promoting complex (APC), Smad-ubiquitination-related-factor 2 (Smurf2), and Arkadia all catalyze SnoN polyubiquitination (Figs. 4 and 5).141–148 The Smurf2 WW domains interact with the Smad2 PY motif, and this Smurf2/Smad2 complex recruits the SnoN protein.141,149 SnoN polyubiquitination by Smurf2 requires two segments of the SnoN protein: one region (residues 1–97) binds the Smad2 MH2 domain, and the second region (residues 366–684) harbors the target lysines for ubiquitination.141 APC binds Smad2 and Smad3 proteins to promote SnoN polyubiquitination; in this case, SnoN has a D-box motif between residues 164 and 172 (RLCLPQVLN), which is equivalent to the destruction box of most APC substrates. SnoN protein residues K440, K446, and K449 are the targets for polyubiquitination by APC (Figs. 4 and 5),141–143,150 whereas in the case of Ski, the polyubiquitinated lysine residues have not yet been identified. Arkadia was identified as a major positive regulator of TGF-β signaling because it causes the downregulation of Smad7, Ski, and SnoN negative regulators. Arkadia interacts with Ski (at residues 211–490) and SnoN (at residues 263–355) and catalyzes their polyubiquitination in a manner dependent on the recruitment of activated Smad2/Smad3.144–146,148 Recently, it was shown that Smurf2 induces Smad4 monoubiquitination to inhibit its function as mediator of TGF-β signaling, whereas formation of the Ski/Smad4 complex blocks Smad4 monoubiquitination.151
Fig. 5.
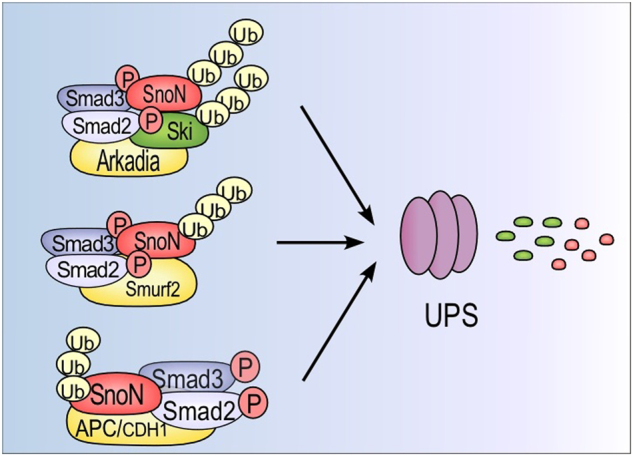
Regulation of Ski and SnoN protein stability by the TGF-β/Smad signaling pathway. TGF-β induces Ski and SnoN protein degradation by the ubiquitin-proteasome system (UPS) after 15–30 min of treatment because activated R-Smads are adapters for ubiquitin E3-ligases APC, Smurf2, and Arkadia
SnoN protein sumoylation requires the participation of Ubc9 (SUMO E2-conjugating enzyme) and PIAS1 or TIF1γ (SUMO E3 ligases); the addition of SUMO occurs at the K50 and K383 residues of the SnoN protein (Fig. 4).152–155 This SnoN sumoylation is important for SnoN-mediated repression of key genes, such as myogenin, in the C2C12 myoblast cell line and for the negative regulation of myogenesis.152,153 Sumoylation of SnoN does not appear to affect its function as a transcriptional corepressor of Smad since the sumoylated SnoN protein can still inhibit the TGF-β-dependent repression of E-cadherin during the epithelial-mesenchymal transition (EMT) process. TGF-β signaling inhibits SnoN sumoylation by decreasing PIAS1 levels during EMT of the mammary epithelial NMuMG cell line.154 By contrast, sumoylation of SnoN1 by TIF1γ is necessary during acinar morphogenesis of NMuMG cells to inhibit EMT induced by TGF-β.155 TIF1γ catalyzes only the sumoylation of SnoN1, but not of SnoN2. Regarding Ski, there is lack of evidence about its sumoylation, although Ski interacts with the Ubc9 enzyme to enhance its activity. Thus, Ubc9 promotes MDM2 mono-sumoylation, which in turn promotes p53 protein polyubiquitination and degradation.156
Regulation of subcellular localization and stability of the Ski and Snon proteins
The Ski and SnoN proteins can be localized in both the cytoplasm and nucleus; intriguingly, Ski and SnoN appear to have an exclusive nuclear localization in most cancer cell lines. Ski and SnoN have non-homologous nuclear localization signals (NLS); the SnoN NLS is localized at its N-terminus and depends on residues K30 and K31,157 whereas residues 452–458 (sequence PRKRKLT) comprise the Ski NLS.158 Ski and SnoN mutants in their respective NLSs exhibit high stability and may sequester Smad proteins in the cytoplasm.157,158
Some proteins retain Ski and SnoN in the cytoplasm; for instance, the interaction of Ski with the C184M protein in the cytoplasm may block Smad2 translocation to the nucleus despite the activation of TGF-β signaling, in both the liver and lenses.159,160 The Ski and SnoN proteins can also interact with the TβRI receptor (or ALK5) in the cytoplasm, inducing failure of the R-Smad/Smad4 complex to translocate to the nucleus.161 The Ski protein was found first in both nuclear and cytoplasmic fractions of melanoma cells.162,163 SnoN levels are modified during mouse mammary gland development; however, the localization of SnoN remains cytoplasmic in normal human breast cells, but not in breast cancer tissues.157,164,165 Vitamin C treatment decreases cytosolic Ski protein levels in rat kidney mesangial cells.166
In the peripheral nervous system, nuclear Ski regulates Schwann cell proliferation and differentiation by promoting normal myelination of axons.167 The TGF-β signaling may promote Ski relocalization into the cytoplasm, but without any protein degradation in Schwann cells; thus, the Ski protein is localized at early endosomes to sequester the phosphorylated retinoblastoma protein (pRb).168 TGF-β signaling also promotes Ski protein shuttling from the cytoplasm to the nucleus in cardiac myofibroblasts,169 whereas MG132 treatment causes accumulation of the Ski protein in the cytoplasm of human cervical cancer HeLa cells.158 Furthermore, the Ski and SnoN proteins associate with members of the Hippo pathway in the human mammary epithelial cells MDA-MB-231 and MCF10A; if these cells are grown at a high density, then Lats2 kinase promotes a decrease in the SnoN protein levels.69,170
In the liver, the Ski protein has been localized in multivesicular endosomes (MVE); recently, we reported that cytoplasmic Ski is present in lipid raft-rich endosomes of normal hepatocytes and co-localizes with markers of multivesicular bodies, such as CD63 and Alix. Intriguingly, TGF-β signaling and proteasome inhibition by MG132 promote the localization of the Ski protein at these lipid raft-rich vesicles.171 The Ski and SnoN proteins are localized in both the cytoplasm and nucleus of C9 cells (rat hepatocyte clone 9) and primary rat hepatocytes.172 In this case, the stability of the Ski and SnoN proteins is regulated by actin-cytoskeleton dynamics (Fig. 2).171,172 The Ski protein levels are differentially modulated by GPCR (G-protein-coupled receptors) signals, i.e., GPCR signals associated with the G12/13/Rho/ROCK axis, which promote actin polymerization, downregulating the Ski protein, whereas GPCR signals associated with the Gs/adenylate cyclase/cAMP axis, leading to the depolymerization of actin filaments, stabilizing the Ski protein. By contrast, the SnoN protein is stabilized by signals that promote actin polymerization in hepatocytes.171–173 Interestingly, Ski and SnoN regulation mediated by actin polymerization dynamics is lost in hepatoma cells, affecting notably the TGF-β signaling outcome.172 Other studies have shown that cAMP also increases the Ski protein levels, but in rat Schwann cells.168 Therefore, it is clear that cell density, polarity, and actin-cytoskeleton dynamics regulate Ski and SnoN protein stability, as well as their subcellular localization (Fig. 2).
The Ski and SnoN proteins stability can change during the cell cycle since protein levels decay rapidly during the transition from the G1 to S phases of the cell cycle, and both proteins are accumulated in the G2 phase and mitosis. The Ski protein is polyubiquitinated and degraded during the G1 phase, whereas it is temporally stabilized by phosphorylation during mitosis.143,174,175 The Ski protein may bind α- and γ-tubulin, and this interaction mediates Ski localization at the mitotic spindle and centrosomes during the cell cycle.175 Ski localization in the mitotic spindle is important in chromosome segregation; loss of both Ski alleles (Ski −/−) causes aneuploidy in mouse embryonic fibroblasts (MEFs).176 The Ski C-terminal domain is important for its co-localization with Aurka in centrosomes; this association regulates centrosomal amplification and multipolar mitosis in MEFs.138,139 Intriguingly, the number of complexes between Ski and SnoN with R-Smads appears to increase during mitosis.177 The role of Ski and SnoN during cell proliferation is unclear, thus meriting further studies.
Role of Ski and Snon in development and tissue homeostasis
TGF-β family members play crucial roles in regulating embryo development and tissue homeostasis. During these physiological processes, Ski and SnoN are strongly involved in controlling the spatio-temporal effects of the TGF-β pathway, as well as the magnitude and duration of the TGF-β signal. Ski and SnoN may also simultaneously regulate other signaling pathways during embryonic development or tissue homeostasis (Fig. 6). The role of SnoN during development has been partially determined based on the varied phenotypes exhibited by Skil knockout (KO) mice.178,179 Two Skil KO mice were generated by the deletion of exon 1: one of them resulted in embryo lethality;178 whereas, the other was viable but showed alterations in T-cell functions.179 A third Skil KO mouse was generated by deleting the Skil gene promoter, resulting in alterations in T-cell activation.179 The knock-in mouse, expressing a mutant SnoN protein that lacks the ability to interact with Smads, showed a phenotype with partial embryo lethality due to defects in vascular system development. Studies using this transgenic mouse revealed a relevant function of SnoN to promote angiogenesis since SnoN interacts with the ALK1 receptor to increase the TGF-β/BMP9-dependent activation of Smad1 and Smad5.180 The expression of the SnoN protein is induced by TGF-β, activin, and nodal in human pluripotent embryonic stem cells, particularly to repress mesendodermal and primitive streak genes. Thus, the maintenance of embryonic stem-cell pluripotency is impaired by SnoN downregulation.74
Fig. 6.

Ski and SnoN functions in health and disease. The specific or common biological functions of Ski and SnoN are linked to some physiological (black text) or pathological processes (white text)
Ski also exerts an important activity throughout embryonic development, specifically in formation of the central nervous system (CNS), skeletal muscle, and limbs.30 Endogenous Ski expression is increased during mouse embryonic development in several regions of the CNS; thus, Ski-KO mice show embryo lethality because closure of the cranial neural tube is impaired; there are also eye malformations, abnormalities in craniofacial structures, and a decrease in skeletal muscle mass. Likewise, Ski-knockdown (KD) mice show defects in eye and neural tube formation.181 Ski-KO mice mimic the same signs/symptoms of an anomaly named persistent hyperplastic primary vitreous (PHPV) observed in human and mouse models, which involves some ocular abnormalities, such as retinal malformations and microphtalmia.182 By contrast, overexpression of the Ski isoforms SkiA and SkiB gives rise to gastrulation alterations in zebrafish.94
Neurogenesis
Ski and SnoN are important regulators during neurogenesis and for maintaining the homeostasis of the nervous system.183–185 Ski overexpression increases neural axis formation.186 Ski is needed for normal myelination of axons in the peripheral nervous system because it regulates Schwann cell proliferation and differentiation.167 In rats with a spinal cord injury, Ski is upregulated in reactive astrocytes, but not in neurons.187 The inhibition of Smad1 and Smad2 by Ski induces the expression of some neural markers;188 whereas, Ski-KO mice exhibit reduced expression of nestin, an intermediate filament protein of neuroepithelial cells and myocytes precursors;189,190 embryos of Ski-KO mice also exhibit extra vestigial-digits.190 Ski appears to maintain the identity of callosal neurons in the developing neurocortex by blocking Ctip2 gene expression through a Ski/Stab2/HDAC1 complex.65,66 By contrast, SnoN modulates neuronal branching and positioning during axonal growth and regeneration (Fig. 6).79,191,192
Hematopoiesis
Ski and SnoN are important regulators of cell differentiation; thus, they become key factors in the regulation of hematopoiesis.193 Ski induces the expression of genes involved in myeloid differentiation.194 Ski expression has been observed in megakaryocyte/erythrocyte dual-lineage progenitors, as well as in mature macrophages, mast cells, and B- and T-lineage cells.193 Ski mainly inhibits erythroid differentiation by inhibiting GATA1 activity or the RA signaling pathway in hematopoietic cells.67,195 Phorbol 12-myristate 3-acetate (PMA) can enhance Ski expression in human megakaryoblastic CHRF-288-11 cells that specifically undergo megakaryocytic differentiation;196 whereas, the process of megakaryopoiesis is stimulated by PMA, which favors activin A/Smad signaling and SnoN protein degradation (Fig. 6).197
Myogenesis
Ski promotes muscular development by enhancing the expression of muscle regulatory factors; for instance, Ski overexpression in myoblasts induces expression of genes encoding muscle creatine kinase (Mck) and myosin light chain (Mlc).198 High Ski mRNA levels are observed during the late stages of muscle differentiation of mouse embryos and in cell lines.199,200 Overexpression of v-Ski induces MyoD and myogenin expression and promotes myogenesis.87,201–203 Ski-overexpressing transgenic mice exhibit an increased skeletal muscular mass;204,205 these mice exhibit high Ski mRNA levels in hind leg muscles.206 By contrast, transgenic cattle overexpressing chicken Ski also develop muscular hypertrophy, but later show muscle degeneration.207 Ski transgene overexpression in mice produces skeletal muscle hypertrophy; whereas, Ski overexpression in osteocytes causes skeletal abnormalities.205,206 Furthermore, Ski plays an important role in muscle regeneration; thus, higher Ski levels promote the proliferation of muscle cells.208 Other studies have shown an upregulation of Ski in some stages of axolotl embryogenesis, such as limb formation, as well as during axolotl limb regeneration.92 SnoN is involved in muscle cell differentiation by regulating the formation of muscle fibers in SnoN transgenic mice.209 TGF-β decreases Ihh mRNA levels in the neonatal growth plate via Smad2/SnoN and Ski/Smad3 complexes; this study shows that TGF-β signals antagonize Ihh expression, a key regulator of the proliferation and differentiation of chondrocytes (Fig. 6).210
Metabolism
Ski is a key factor in the induction of myogenesis, but Ski may also decrease body fat mass by inducing oxidative metabolism of fatty acids. Transgenic mice overexpressing Ski show increased growth at early postnatal stages, as well as an altered body composition with decreased body fat and an enhanced lean body mass. The skeletal muscle of these transgenic mice has an enhanced fatty oxidative capacity and enhanced activity of both cytochrome C oxidase and citrate synthase; whereas, Ski represses some key genes in metabolism, such as Srebp1 and Pparγ.211 Ski overexpression alters glycolysis and lactate production and enhances mitochondrial biogenesis and fatty acid oxidation; these effects may result after the upregulation of PPARγ. Ski overexpression also induces the expression of some PPARγ target genes that are implicated in lipid uptake, transport, and oxidation.212 Ski inhibits AKT phosphorylation induced by insulin in Ski-transgenic mice with resistance to diet-induced obesity.213
Regulation of Tgf-β/smad signaling by Ski and Snon
The role of Ski and SnoN in the TGF-β signaling network
The canonical TGF-β/Smad signaling pathway establishes a crosstalk with other pathways to efficiently achieve most of its biological functions, including the Wnt, Notch, Hippo, PI3K-AKT, PKC, MAPKs, and JAK-STAT signaling pathways. The regulation of Ski and SnoN protein expression is part of this crosstalk; thus, the TGF-β/R-Smad axis, activin, nodal, HGF/CREB/Sp1 axis, and prolactin/Stat5 axis are some of the known pathways that enhance SnoN expression in specific cell types (Fig. 2). Many other signals also control the SnoN protein levels, but the molecular mechanisms involved are unknown; for instance, certain EDCs increase SnoN in ovarian cancer cells, whereas oxymatrine and MG132 treatments induce SnoN in the damaged kidney of diabetic rats (Fig. 2).115,214,215 NFAT stabilizes SnoN to control TGF-β-induced EMT in MDA-MB-231 breast cancer cells.216 By contrast, other treatments are linked to SnoN downregulation; for example, HGF treatment decreases the SnoN protein levels in proliferating renal tubular epithelial HK2 cells.217 PMA induces activin A production, which activates Smad2 and Smad3 to promote SnoN degradation in myelogenous leukemia cells.197 Smurf2 and MAD2B proteins cause SnoN downregulation in the fibrotic kidney.218,219 The antibiotics anisomycin and puromycin induce Ski and SnoN degradation via UPS only in specific human cell types, such as A549 lung cancer cells, AD293 embryonic kidney cells, and A7 melanoma cells.220–222
TGF-β induces SnoN expression to inhibit the BMP2 and BMP7 signaling pathways in osteoblasts from patients undergoing total hip replacement.223 SnoN may also stabilize proteins, such as p53, TAZ, and STAT5. High levels of the SnoN/PML (promyelocytic leukemia) complex stabilize p53 and induce senescence in MEFs; whereas, the SnoN/p53 complex enhances p53-mediated transcription in MEFs.45,224 SnoN stabilizes TAZ protein by inhibiting its phosphorylation, which enhances TAZ/TEAD complex target gene expression during cell proliferation. SnoN cooperates with the Hippo pathway to induce transformation and promote EMT of breast cancer cells.170 SnoN is also induced by TGF-β and prolactin via Stat5 in late pregnancy; thus, SnoN blocks TGF-β signaling and stabilizes STAT5 protein to activate the prolactin pathway in lactogenesis.112
Ski also negatively controls the BMP, Hippo, Hedgehog, vitamin D, and RA signaling pathways. The Ski protein interacts with Lats2 and enhances its activity to destabilize the TAZ protein in mammary epithelial cells,69 whereas, in lung cancer, the Ski gene is silenced by methylation and, consequently, Ski is unable to inhibit TAZ.101 Ski and SnoN negatively regulate the expression of the Indian hedgehog (Ihh) gene in primary chondrocytes via different transcriptional complexes: SnoN/Smad2 and HDAC4 form a complex with high affinity; whereas, the Ski/Smad3 complex has a lower affinity.210 Furthermore, gefitinib is a drug that is used to treat lung cancer; thus, the IL-6 cytokine induces Stat3 phosphorylation to repress Smad3 expression, promoting gefitinib resistance, and Stat3 interacts with Ski and SnoN to inhibit the R-Smad/co-Smad complex.225
In summary, TGF-β participates in a crosstalk with many other pathways that may regulate the expression and/or abundance of the Ski and SnoN proteins, and concomitantly, Ski and SnoN exert diverse effects on the outcome of those signaling pathways through regulatory feedback loops that control the different cellular responses.
The role of Ski and SnoN in the regulation of TGF-β signaling in disease
The physiological and pathological relevance of Ski and SnoN transcriptional cofactors has been clearly documented, but there is scarce information about their target genes and about the biological relevance of such gene regulation. Ski and SnoN may regulate gene expression in both Smad-dependent and -independent manners, and they may also depend on many other transcription factors to regulate gene transcription. This biological function of Ski and SnoN is important in tissue homeostasis, whereas alterations in Ski and SnoN expression and function have been linked to some diseases.
Wound healing and fibrosis
TGF-β superfamily members exert key roles during wound healing and fibrosis, regulating processes, such as myofibroblast (MFB) differentiation and EMT promotion.226–228 Ski and SnoN also exert differential effects during wound healing and tissue remodeling, depending on the cell context (Fig. 6). A few examples include the following: Ski and SnoN expression is enhanced during liver regeneration, probably to neutralize TGF-β/Smad antiproliferative actions;171,229 the regeneration of tendon-to-bone insertion is promoted by both SnoN overexpression and Tgif gene induction;230 Ski inhibits Smad3-induced apoptosis to promote cell proliferation in skin fibroblasts during wound healing;231 and Ski reduces scarring when it is used for gene therapy in rats.232 Ski also inhibits Smad3 activation but stimulates the p38 pathway to block TGF-β-induced vascular smooth muscle cell proliferation after vascular injury.233
Ski and SnoN cofactors may also mediate antifibrotic responses by blocking TGF-β signals. The renal model of fibrosis induced by unilateral urethral obstruction (UUO) exhibits an activated TGF-β pathway as a consequence of increased expression of Smurf2 and proteosomal degradation of the Ski and SnoN proteins.150 TGF-β profibrotic activity is inhibited after Ski and SnoN overexpression in renal cells, whereas the absence of SnoN sensitizes tubular epithelial HKC cells to TGF-β signaling.234 Smurf2 overexpression promotes SnoN downregulation in renal proximal tubule epithelial cells when they are cultured under high-glucose conditions;218 whereas, SnoN overexpression protects these tubular epithelial cells from undergoing EMT.235 Furthermore, the alkaloid oxymatrine extracted from the herb Sephora japonica prevents SnoN downregulation in renal tubules during the EMT process induced by high glucose.215 By contrast, enhancement of SnoN protein stability due to treatment with MG132 decreases kidney damage in diabetic rats.214 Likewise, Smurf2 knockdown increases SnoN levels and blocks TGF-β signaling in rats with obstructive nephropathy.236 It is clear that the polyubiquitination and degradation of SnoN via Smurf2 induced by the TGF-β/Smad pathway is critical in diabetic nephropathy.237 BMP7 enhances the levels of SnoN mRNA and protein, which is important for controlling diabetic nephropathy and renal fibrosis.113 Moreover, the upregulation of SnoN inhibits EMT and renal fibrogenesis induced by high glucose. The miR-23a causes SnoN downregulation, whereas depletion of miR-23a increases the SnoN protein levels and decreases EMT and renal fibrogenesis.117 Interestingly, the expression of SnoN, TGF-β, and Arkadia can be increased in renal tubular cells treated with high glucose, whereas the SnoN protein levels are decreased; however, the SnoN protein levels can be increased if Arkadia is downregulated, leading to the inhibition of high glucose-induced EMT.238 Similarly, fibrogenesis in different tissues correlates with changes in SnoN levels, as occurs in the fibrotic lung239 and during renal fibrosis.219 Thus, regulation of SnoN protein abundance seems to be key in the implementation of therapeutic strategies for diabetic nephropathy.
TGF-β induces cellular matrix production by activating MFB during cardiac fibrogenesis; however, transient Ski overexpression in MFB decreases Smad2 phosphorylation, which results in a decrease in type I collagen synthesis and α-SMA levels.169,240 In this case, stable overexpression of Ski promotes cardiac MFB apoptosis.241 Bone marrow progenitor cell therapy modulates cardiac fibrosis in diabetic hearts by decreasing miR-155 levels, whereas overexpression of miR-155 in cardiac fibroblasts inhibits Ski and SnoN expression.242 Ski overexpression also induces Meox2 gene expression and blocks Zeb2 gene expression, affecting the cardiac MFB phenotype.243 Furthermore, TGF-β can induce endothelial-mesenchymal transition (EndMT) of human coronary artery endothelial cells (HCAECs) to generate matrix-producing fibroblasts and promote cardiac fibrosis. Importantly, Ski overexpression inhibits the TGF-β-induced EndMT of HCAECs via a mechanism that involves the regulation of miR-155.107 During liver fibrogenesis induced by TGF-β and acetaldehyde, an ethanol metabolite, increased collagen synthesis occurs in hepatic stellate cells (HSC); in these cells, the Ski/Smad4 complex is translocated to the cytoplasm, and the Ski protein is degraded via UPS.244 In systemic sclerosis, the levels of the Ski and SnoN proteins are increased in scleroderma fibroblasts, although they fail to inhibit the TGF-β pathway because they are unable to compete with the p300 coactivator.245 Regarding dermal tissues, Ski transgenic overexpression promotes wound healing in rat dermal wounds and inhibits scar formation in rabbit ears.246
Carcinogenesis
The TGF-β cytokine exerts tumor-suppressive actions that include inhibition of cellular proliferation and immortalization, and it also promotes apoptosis in normal cells and early carcinomas. By contrast, the tumor-promoting effects of TGF-β include the promotion of EMT, cell migration, invasion, and metastasis.247–249 Thus, TGF-β cytostatic and protective effects are frequently lost as tumors develop. Loss-of-TGF-β signaling is involved in hyperproliferative disorders, inflammation, autoimmune diseases, and tumor formation; whereas, gain of TGF-β signaling promotes immunosuppression and tumor metastasis.3 In addition, most tumors may arise after mutations or deletions in genes encoding components of the TGF-β signaling pathway.3,250
Ski and SnoN were initially described as proto-oncoproteins because of their ability to induce cellular transformation in vitro.50,89,201,202,251,252 Interestingly, the Ski and SnoN heterodimers are better able to induce cellular transformation than their respective homodimers or monomers.50,130 Thus, SnoN and Ski are up- or downregulated in many types of cancer cells, including leukemia, lymphoma, melanoma, breast cancer, cervical cancer, esophageal squamous-cell carcinoma, colorectal carcinoma, pancreatic cancer, and gastrointestinal tumors.85,104,178,253–272 However, it is worth mentioning that no mutations of Ski and SnoN have been found in any of the different cancer types studied to date.
Currently, it has been observed that Ski and SnoN are differentially expressed in normal and cancerous cells, and some evidence also supports the alteration of their localization, abundance, and function in cancer. Skil heterozygous mice develop spontaneous lymphoma and are quite sensitive to carcinogens, revealing the anti-oncogenic activity of SnoN. The T-cells, B-cells, and fibroblasts of these mice exhibit resistance to apoptosis and cell cycle arrest, supporting the role of SnoN as a tumor suppressor.178 Likewise, Ski tumor suppression activity has been observed in Ski-deficient mice, which exhibit higher sensitivity to tumor formation induced with carcinogens.273 Ski loss increases MEF proliferation, whereas Ski overexpression inhibits MEF proliferation via transcriptional gene repression in association with Rb and MAD.273 Both SnoN and Ski seem to be important in some cancer types. A correlation between β-catenin activation and upregulation of the SnoN and Ski proteins has been observed in early stages of colorectal cancer.262 Another study has shown that the p53 protein is stabilized after the SnoN protein is recruited to PML nuclear bodies through PML, which induces cellular senescence in a Smad-independent manner and inhibits tumorigenesis;45,224 by contrast, Ski inhibits p53 activity and promotes p53 protein degradation.68,156 Defective control of TGF-β signaling is responsible for cancer induction in Barret´s esophagus; patients with low-grade dysplasia exhibit low Ski and SnoN protein levels in dysplasic areas, whereas these proteins are absent in patients with high-grade dysplasia/adenocarcinoma.274
The mechanisms linked to SnoN deregulation in cancer have been better studied. Altered Skil gene transcriptional regulation, Skil gene amplification, or higher SnoN protein stability are among the main causes of SnoN upregulation, whereas SnoN can be decreased by allelic loss in some cancer types. Hence, SnoN expression in human colorectal cancer is downregulated in <40% of tumors with high-level microsatellite instability, whereas 50% of microsatellite-stable tumors present an upregulation of SnoN.275 Furthermore, in 179 human colorectal tumor biopsies, 55.2% of tumors have either partial or complete allelic loss of the Skil gene, whereas 15.1% of tumors present amplification of the Skil gene.256 Skil gene amplification was initially observed in esophagus squamous cell carcinoma,254 but it has also been observed in immortalized human mammary epithelial cells, where Skil and Tloc1 may cooperate to promote cell proliferation, transformation, invasion, and tumor growth.276 By contrast, TGF-β is unable to induce SnoN protein degradation in human esophageal cancer cell lines and in rat AS-30D hepatoma cells; indeed, high levels of the SnoN protein have been detected in both cancer types.172,260 Skil and Ski genes are often co-amplified with other genes with oncogenic activity. For instance, Skil and Tloc1 genes are contained in the 3q26 chromosome, specifically in a region that is frequently amplified in several cancer types.276 The progression of nasopharyngeal carcinoma is associated with co-amplification of Gpr160 and Skil genes, both of which are localized at 3q26.2-q26.32, along with deletion of the AdamtS9 and Lrig1 genes, which are localized at 3p12.3-p14.2.277 The 3q26 region is often amplified in ovarian cancer, increasing the mRNA levels of some genes, such as Skil, Evi1, and Plscr1.81,278,279 The Ski gene is co-amplified with the Mel1 gene in MKN28 gastric cancer cells; both genes are localized at 1p36 and cooperate to inhibit TGF-β anti-proliferative actions.267
Metastasis
The role of Ski in cancer metastasis depends on the context and cellular type.273 The Ski protein levels are decreased in metastatic non-small lung cancer cells (NSCLCs) and lung cancer tissues, whereas Ski overexpression blocks the TGF-β-mediated EMT process.280 Ski downregulation by RNAi inhibits human melanoma growth in vivo,264 whereas it has a dual effect on pancreatic-cancer tumorigenesis because Ski-KD inhibits tumor growth and enhances metastasis to the lung; additionally, Ski downregulation alters the expression of TGF-β target genes linked to pancreatic cancer cell metastasis.268 Ski also participates in maintaining the stemness of pancreatic cancer stem cells by promoting the expression of components of the sonic hedgehog (Shh) pathway, such as Shh, Ptch-1, Smo, Gli-1, and Gli-2.281 Ski overexpression has been observed in acute myeloid leukemia (AML), where Ski inhibits retinoic acid receptor (RAR) signaling.282 Ski upregulation activates cancer-associated fibroblasts (CAFs) in breast tumors to promote breast cancer cell invasion.283 In addition, the expression of HPV16 genes is mediated by NF1 in association with Ski in cervical carcinoma.85 Ski is a negative prognostic marker in primary esophageal squamous carcinoma,257 pancreatic ductal adenocarcinoma (PDAC),268 colorectal carcinoma,256 and gastric cancer.269 Untreated patients with chronic lymphocytic leukemia (CLL) have a two-gene signature consisting of Ski and Slamf1 that predicts time to treatment and survival.284 High levels of cytoplasmic Ski negatively correlate with tumor size, stage, and lymph node status in invasive breast carcinomas and positively with survival.285 Interestingly, the Ski gene is methylated in lung cancer, which correlates with the progression of this type of cancer. Under this scenario, Ski fails to block TAZ activity and the proliferation of lung cancer cells.101 Moreover, one of the treatments to regulate Ski protein levels includes the use of the TGF-β inhibitory P144 peptide, which is derived from the extracellular sequence of the human TGF-β type III receptor, and interestingly, P144 promotes Ski downregulation in human glioblastoma cell lines, such as A172 and U-87 MG.286
SnoN duality as an oncoprotein and tumor suppressor was confirmed through xenotransplantation in athymic mice of lung A549 and breast MDA-MB-231 cancer cells expressing either high or low SnoN protein levels. Surprisingly, xenotransplant of breast cancer cells expressing low SnoN levels exhibited high cell metastasis to the bones and lungs, but poor tumor development.287 SnoN is an important regulator of EMT induced by TGF-β in MDA-MB231 breast cancer cells. During EMT, upregulated NFAT modifies SnoN subcellular localization from the cytoplasm to the nucleus and prevents SnoN degradation by sequestering Smad3. NFAT cooperates with SnoN to promote TGF-β-induced EMT by increasing the expression of genes encoding MMP-2, MMP-9, and N-cadherin.216 Moreover, sumoylation of SnoN by PIAS1 has an inhibitory effect on the invasive capacity of MDA-MB231 cells.288 Particularly for SnoN, it has been reported that this protein regulates cell differentiation in normal skin and in benign skin tumors, but it promotes squamous cell carcinoma.289 SnoN induces proliferation of ovarian cancer cells, whereas it promotes cell cycle arrest and senescence in non-transformed ovarian epithelial cells.279 SnoN oncogenic activity can induce cellular proliferation and inhibit apoptosis of other cancer cells, such as pancreatic cancer cells272 (Table 1).
Table 1.
Ski and SnoN levels in cancer
| Cancer types | Cofactor | Expression | Mechanism | References |
|---|---|---|---|---|
| Acute myeloid leukemia | Ski | Upregulated | Loss-of-Ski regulation by miR-29a | 104,282 |
| Breast cancer | SnoN | Upregulated | N.D. | 255 |
| Cervical | Ski | Upregulated | N.D. | 85,271 |
| Chronic Myelogenus Leukemia | Ski | Upregulated | N.D. | 258 |
| Colorectal | Ski | Downregulated | Partial or complete allelic loss | 256,262 |
| Upregulated | Ski gene amplification | |||
| SnoN | Downregulated | Partial or complete allelic loss | 256,275 | |
| Upregulated | Skil gene amplification | |||
| Esophageal | Ski | Upregulated | N.D. | 257,274 |
| SnoN | Upregulated | Stability of SnoN protein | 254,260,274 | |
| Skil gene amplification | ||||
| Gastrointestinal | Ski/SnoN | Upregulated | N.D. | 265,267,269,270 |
| Hemangioma | Ski | Upregulated | N.D. | 266 |
| Hepatocarcinoma | SnoN/Ski | Upregulated | N.D. | 172 |
| Lymphoma | SnoN | Downregulated | sno-heterozygous mice | 178 |
| Lung cancer | Ski | Downregulated | Ski gene methylation | 101 |
| Melanoma | Ski/SnoN | Upregulated | N.D. | 162,163,253,259,263,264 |
| Nasopharyngeal | SnoN | Upregulated | Skil gene amplification | 277 |
| Ovarian | SnoN | Upregulated | Skil gene amplification | 81,279 |
| Pancreatic | Ski/SnoN | Upregulated | N.D. | 261,268,272,281 |
N.D. Not Demonstrated
Human genetic diseases
Shprintzen-Goldberg syndrome (SGS) is a human disorder characterized by craniofacial, cardiovascular, neuromuscular, and skeletal anomalies.290,291 The specific mutation consists of an in-frame deletion in exon 1 of the human Ski gene; this mutation falls within the Ski protein region of interaction with R-Smad.290–292 Thus, SGS alterations correlate with uncontrolled TGF-β signaling activation since mutant Ski cannot bind Smad proteins. Analysis of the mutation frequency in patients with SGS demonstrates that the Smad-binding domain of Ski is a hot spot for multiple de novo mutations.291,293 Intriguingly, Ski-KO mouse and Ski-KD zebrafish have similar phenotypes to those observed in SGS, i.e., craniofacial abnormalities and aortic aneurysms, among others (Fig. 6).220,293
Ski and SnoN as targets in therapies to regulate TGF-β signaling
The TGF-β signaling pathway is deregulated in different diseases, such as fibrosis and cancer. Multiple pharmacological compounds have been used to target the components of the TGF-β pathway, such as anti-sense oligonucleotides, antibodies, and kinase inhibitors, among others. However, it has been a real challenge to target the components of this pathway due to its pleiotropic nature and because of the side effects generated by the pharmacological agents.294 Furthermore, the applicability of TGF-β pathway inhibitors for therapeutic treatments must be carefully considered since TGF-β signaling blockade has been demonstrated to cause an upregulation of Ski and SnoN as a result of the loss of stability regulation of these proteins exerted by Smads.
Like TGF-β signaling, Ski and SnoN also participate in the homeostasis of many physiological processes; consequently, the deregulation of these cofactors is associated with disease development, such as fibrosis and cancer. Fibrosis of some organs, such as the kidney, lung, and liver, seems to be linked to the reduction of Ski and SnoN levels and activation of the TGF-β pathway.218,219,234,236,239 The treatments for fibrosis and associated diseases, such as diabetic nephropathy, will benefit from strategies to upregulate Ski and SnoN expression to antagonize TGF-β pro-fibrotic actions.235 For instance, it has been suggested that Ski and SnoN accumulation after treatment with proteasome inhibitors (such as MG132) is associated with the prevention of fibrotic damage in the kidney of diabetic rats.214 In addition, the use of alkaloids, such as oxymatrine, blocks SnoN downregulation in a cellular model of kidney tubulo-interstitial fibrosis,215 whereas treatment with omega-3 fatty acid increases SnoN expression in fibrotic lungs.239 Regarding tissue regeneration, it has been hypothesized that the upregulation of Ski and SnoN may promote liver regeneration by antagonizing the antiproliferative actions of TGF-β;171,172,229 likewise, the upregulation of SnoN stimulates axonal growth and regeneration.79,191,192
In carcinogenesis, Ski and SnoN act as oncoproteins when overexpressed; SnoN can be upregulated by gene amplification, elevated protein stability, or by enhanced Skil gene transcription, whereas SnoN can be downregulated by partial or complete allelic loss of the Skil gene. Ski and SnoN levels are also upregulated in some cancer types, where some of their main regulators, such as Smad2/3/4 or E3 ubiquitin ligases, are mutated or non-functional.295 By contrast, SnoN expression is increased, whereas Ski expression is downregulated, after Ski gene methylation in lung cancer. Therefore, the design of strategies focused on the downregulation of these cofactors might be useful to control tumor growth. For instance, the downregulation of SnoN with specific interfering RNAs reduces proliferation and promotes apoptosis of hepatoma and pancreatic cancer cells.272,296 Of note, there is a pitfall associated with a significant reduction of Ski or SnoN levels since this approach can lead to the promotion of tumor metastasis, as has been shown in breast, lung, and pancreatic cancer cells, and thus the use of this strategy as an anti-cancer therapy can result in a serious disadvantage.268,287
Concluding remarks
The Ski and SnoN proteins play critical roles in health and disease by controlling the outcome of TGF-β and other signaling pathways.28–30,33,297 Therefore, the maintenance of balanced Ski and SnoN expression levels must be tightly controlled to normalize the TGF-β signaling outcome in diverse pathologies. Therefore, Ski and SnoN are potential therapeutic targets under the pathological conditions in which they are deregulated. The design of therapeutic strategies must be focused on restoring the expression levels of Ski and SnoN, with the purpose of recovering the function of TGF-β signaling and, perhaps, other signaling pathways, as well as re-establishing cellular homeostasis. Notably, the Ski and SnoN proteins are downregulated in fibrosis and cancer metastasis and upregulated in tumor growth; thus, it is important to develop diverse therapeutic strategies to target Ski and SnoN to regulate the TGF-β signaling outcome by attacking only the pro-fibrotic and tumor-promoting effects of this cytokine.
Acknowledgements
We thank B.Sc. Marcela Sosa-Garrocho for helpful discussions. Our work is supported by grants from CONACYT (No. 240224 to M.M.-S.) and PAPIIT/DGAPA/UNAM (IN208115 and IN208118 to M.M.-S., and IA200916 to A.C.T.-C.). We thank Ingrid Mascher for grammatical corrections.
Competing interests
The authors declare no competing interests.
References
- 1.Hinck AP, Mueller TD, Springer TA. Structural biology and evolution of the TGF-β family. Cold Spring Harb. Perspect. Biol. 2016;8:a022103. doi: 10.1101/cshperspect.a022103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Moses, H. L., Roberts, A. B. The discovery of TGF-β: A historical perspective. In: Derynck R., Miyazono K. (eds). The TGF-β Family, vol. 50. Cold Spring Harbor Laboratory Press: New York, 2008, pp 1–28.
- 3.Massagué J, Blain SW, Lo RS. TGFβ signaling in growth control, cancer, and heritable disorders. Cell. 2000;103:295–309. doi: 10.1016/S0092-8674(00)00121-5. [DOI] [PubMed] [Google Scholar]
- 4.Wrana, J. L., Ozdamar, B., Le Roy, C., Benchabane, H. Signaling Receptors of the TGF β family. In: Derynck R., Miyazono K. (eds). The TGF-β Family, vol. 50. Cold Spring Harbor Laboratory Press: New York, 2008, pp 151–178.
- 5.Heldin CH, Moustakas A. Signaling receptors for TGF-β family members. Cold Spring Harb. Perspect. Biol. 2016;8:A022053. doi: 10.1101/cshperspect.a022053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wrana JL, Attisano L, Wieser R, Ventura F, Massagué J. Mechanism of activation of the TGF-β receptor. Nature. 1994;370:341–347. doi: 10.1038/370341a0. [DOI] [PubMed] [Google Scholar]
- 7.Wieser R, Wrana JL, Massagué J. GS domain mutations that constitutively activate T beta R-I, the downstream signaling component in the TGF-beta receptor complex. EMBO J. 1995;14:2199–2208. doi: 10.1002/j.1460-2075.1995.tb07214.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Raftery LA, Twombly V, Wharton K, Gelbart WM. Genetic screens to identify elements of the decapentaplegic signaling pathway in Drosophila. Genetics. 1995;139:241–254. doi: 10.1093/genetics/139.1.241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hoodless PA, et al. MADR1, a MAD-related protein that functions in BMP2 signaling pathways. Cell. 1996;85:489–500. doi: 10.1016/S0092-8674(00)81250-7. [DOI] [PubMed] [Google Scholar]
- 10.Savage C, et al. Caenorhabditis elegans genes sma-2, sma-3, and sma-4 define a conserved family of transforming growth factor beta pathway components. Proc. Natl Acad. Sci. USA. 1996;93:790–794. doi: 10.1073/pnas.93.2.790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Macías-Silva M, et al. MADR2 is a substrate of the TGF-β receptor and its phosphorylation is required for nuclear accumulation and signaling. Cell. 1996;87:1215–1224. doi: 10.1016/S0092-8674(00)81817-6. [DOI] [PubMed] [Google Scholar]
- 12.Lagna G, Hata A, Hemmati-Brivanlou A, Massagué J. Partnership between DPC4 and SMAD proteins in TGF-beta signalling pathways. Nature. 1996;383:832–836. doi: 10.1038/383832a0. [DOI] [PubMed] [Google Scholar]
- 13.Abdollah S, et al. TbetaRI phosphorylation of SMAD2 on Ser465 and Ser467 is required for SMAD2-SMAD4 complex formation and signaling. J. Biol. Chem. 1997;272:27678–27685. doi: 10.1074/jbc.272.44.27678. [DOI] [PubMed] [Google Scholar]
- 14.Souchelnytskyi S, et al. Phosphorylation of Ser465 and Ser467 in the C terminus of SMAD2 mediates interaction with SMAD4 and is required for transforming growth factor-beta signaling. J. Biol. Chem. 1997;272:28107–28115. doi: 10.1074/jbc.272.44.28107. [DOI] [PubMed] [Google Scholar]
- 15.Liu X, et al. Transforming growth factor beta-induced phosphorylation of SMAD3 is required for growth inhibition and transcriptional induction in epithelial cells. Proc. Natl Acad. Sci. USA. 1997;94:10669–10674. doi: 10.1073/pnas.94.20.10669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kretzschmar M, Liu F, Hata A, Doody J, Massagué J. The TGF-beta family mediator SMAD1 is phosphorylated directly and activated functionally by the BMP receptor kinase. Genes Dev. 1997;11:984–995. doi: 10.1101/gad.11.8.984. [DOI] [PubMed] [Google Scholar]
- 17.Macías-Silva M, Hoodless PA, Tang SJ, Buchwald M, Wrana JL. Specific activation of SMAD1 signaling pathways by the BMP7 type I receptor, ALK2. J. Biol. Chem. 1998;273:25628–25636. doi: 10.1074/jbc.273.40.25628. [DOI] [PubMed] [Google Scholar]
- 18.Wrana JL. Phosphoserine-dependent regulation of protein-protein interactions in the SMAD pathway. Structure. 2002;10:5–7. doi: 10.1016/S0969-2126(01)00702-X. [DOI] [PubMed] [Google Scholar]
- 19.Lin X, Chen G, Feng H. Transcriptional control via SMADs. In: Derynck R, Miyazono K, editors. The TGF-β Family. New York: Cold Spring Harbor Laboratory Press; 2008. pp. 287–332. [Google Scholar]
- 20.Gaarenstroom T, Hill CS. TGF-β signaling to chromatin: How SMADs regulate transcription during self-renewal and differentiation. Semin. Cell Dev. Biol. 2014;32:107–118. doi: 10.1016/j.semcdb.2014.01.009. [DOI] [PubMed] [Google Scholar]
- 21.Barrios-Rodiles M, et al. High-throughput mapping of a dynamic signaling network in mammalian cells. Science. 2005;307:1621–1625. doi: 10.1126/science.1105776. [DOI] [PubMed] [Google Scholar]
- 22.Aragon E, et al. A SMAD action turnover switch operated by WW domain readers of a phosphoserine code. Genes Dev. 2011;25:1275–1288. doi: 10.1101/gad.2060811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Macias MJ, Martin-Malpartida P, Massagué J. Structural determinants of SMAD function in TGF-β signaling. Trends Biochem. Sci. 2015;40:296–308. doi: 10.1016/j.tibs.2015.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Xu P, Lin X, Feng XH. Posttranslational regulation of SMADs. Cold Spring Harb. Perspect. Biol. 2016;8:a022087. doi: 10.1101/cshperspect.a022087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Miyazono K. TGF-beta signaling by SMAD proteins. Cytokine Growth Factor Rev. 2000;11:15–22. doi: 10.1016/S1359-6101(99)00025-8. [DOI] [PubMed] [Google Scholar]
- 26.Vizan P, et al. Controlling long-term signaling: receptor dynamics determine attenuation and refractory behavior of the TGF-β pathway. Sci. Signal. 2013;6:ra106. doi: 10.1126/scisignal.2004416. [DOI] [PubMed] [Google Scholar]
- 27.Stroschein SL, Wang W, Zhou S, Zhou Q, Luo K. Negative feedback regulation of TGF-beta signaling by the SnoN oncoprotein. Science. 1999;286:771–774. doi: 10.1126/science.286.5440.771. [DOI] [PubMed] [Google Scholar]
- 28.Liu X, Sun Y, Weinberg RA, Lodish HF. Ski/Sno and TGF-β signaling. Cytokine Growth Factor Rev. 2001;12:1–8. doi: 10.1016/S1359-6101(00)00031-9. [DOI] [PubMed] [Google Scholar]
- 29.Luo K. Ski and SnoN: negative regulators of TGF-β signaling. Curr. Opin. Genet. Dev. 2004;14:65–70. doi: 10.1016/j.gde.2003.11.003. [DOI] [PubMed] [Google Scholar]
- 30.Bonnon C, Atanasoski S. c-Ski in health and disease. Cell Tissue Res. 2012;347:51–64. doi: 10.1007/s00441-011-1180-z. [DOI] [PubMed] [Google Scholar]
- 31.Stavnezer E, Barkas AE, Brennan LA, Brodeur D, Li Y. Transforming Sloan-Kettering viruses generated from the cloned v-ski oncogene by in vitro and in vivo recombinations. J. Virol. 1986;57:1073–1083. doi: 10.1128/jvi.57.3.1073-1083.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sutrave P, Copeland TD, Showalter SD, Hughes SH. Characterization of chicken c-ski oncogene products expressed by retrovirus vectors. Mol. Cell Biol. 1990;10:3137–3144. doi: 10.1128/MCB.10.6.3137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Deheuninck J, Luo K. Ski and SnoN, potent negative regulators of TGF-β signaling. Cell Res. 2009;19:47–57. doi: 10.1038/cr.2008.324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mardon G, Solomon NM, Rubin GM. Dachshund encodes a nuclear protein required for normal eye and leg development in Drosophila. Development. 1994;120:3473–3486. doi: 10.1242/dev.120.12.3473. [DOI] [PubMed] [Google Scholar]
- 35.Hammond KL, Hanson IM, Brown AG, Lettice LA, Hill RE. Mammalian and Drosophila dachshund genes are related to the Ski proto-oncogene and are expressed in eye and limb. Mech. Dev. 1998;74:121–131. doi: 10.1016/S0925-4773(98)00071-9. [DOI] [PubMed] [Google Scholar]
- 36.Kozmik Z, et al. Molecular cloning and expression of the human and mouse homologues of the Drosophila dachshund gene. Dev. Genes. Evol. 1999;209:537–445. doi: 10.1007/s004270050286. [DOI] [PubMed] [Google Scholar]
- 37.Caubit X, et al. Mouse Dac, a novel nuclear factor with homology to Drosophila dachshund shows a dynamic expression in the neural crest, the eye, the neocortex, and the limb bud. Dev. Dyn. 1999;214:66–80. doi: 10.1002/(SICI)1097-0177(199901)214:1<66::AID-DVDY7>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 38.Davis RJ, Shen W, Heanue TA, Mardon G. Mouse Dach, a homologue of Drosophila dachshund, is expressed in the developing retina, brain and limbs. Dev. Genes Evol. 1999;209:526–536. doi: 10.1007/s004270050285. [DOI] [PubMed] [Google Scholar]
- 39.Davis RJ, Shen W, Sandler YI, Heanue TA, Mardon G. Characterization of mouse Dach2, a homologue of Drosophila dachshund. Mech. Dev. 2001;102:169–179. doi: 10.1016/S0925-4773(01)00307-0. [DOI] [PubMed] [Google Scholar]
- 40.Da Graca LS, et al. DAF-5 is a Ski oncoprotein homolog that functions in a neuronal TGFβ pathway to regulate C. elegans dauer development. Development. 2004;131:435–446. doi: 10.1242/dev.00922. [DOI] [PubMed] [Google Scholar]
- 41.Tewari M, et al. Systematic interactome mapping and genetic perturbation analysis of a C. elegans TGF-β signaling network. Mol. Cell. 2004;13:469–482. doi: 10.1016/S1097-2765(04)00033-4. [DOI] [PubMed] [Google Scholar]
- 42.Tavsanli BC, et al. Structure-function analysis of the Drosophila retinal determination protein Dachshund. Dev. Biol. 2004;272:231–247. doi: 10.1016/j.ydbio.2004.05.005. [DOI] [PubMed] [Google Scholar]
- 43.Arndt S, Poser I, Schubert T, Moser M, Bosserhoff AK. Cloning and functional characterization of a new Ski homolog, Fussel-18, specifically expressed in neuronal tissues. Lab. Invest. 2005;85:1330–1341. doi: 10.1038/labinvest.3700344. [DOI] [PubMed] [Google Scholar]
- 44.Arndt S, Poser I, Moser M, Bosserhoff AK. Fussel-15, a novel Ski/Sno homolog protein, antagonizes BMP signaling. Mol. Cell Neurosci. 2007;34:603–611. doi: 10.1016/j.mcn.2007.01.002. [DOI] [PubMed] [Google Scholar]
- 45.Pan D, Zhu Q, Luo K. SnoN functions as a tumour suppressor by inducing premature senescence. EMBO J. 2009;28:3500–3513. doi: 10.1038/emboj.2009.250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Arndt S, Schmidt J, Wacker E, Karrer S, Bosserhoff AK. Fussel-15, a new player in wound healing, is deregulated in keloid and localized scleroderma. Am. J. Pathol. 2011;178:2622–2631. doi: 10.1016/j.ajpath.2011.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Fischer S, et al. Fussel (fuss)-A negative regulator of BMP signaling in Drosophila melanogaster. PLoS ONE. 2012;7:e42349. doi: 10.1371/journal.pone.0042349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Takaesu NT, et al. Drosophila CORL is required for SMAD2-mediated activation of Ecdysone Receptor expression in the mushroom body. Development. 2012;139:3392–3401. doi: 10.1242/dev.079442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Nicol R, Stavnezer E. Transcriptional repression by v-Ski and c-Ski mediated by a specific DNA binding site. J. Biol. Chem. 1998;273:3588–3597. doi: 10.1074/jbc.273.6.3588. [DOI] [PubMed] [Google Scholar]
- 50.Nicol R, Zheng G, Sutrave P, Foster DN, Stavnezer E. Association of specific DNA binding and transcriptional repression with the transforming and myogenic activities of c-Ski. Cell Growth Differ. 1999;10:243–254. [PubMed] [Google Scholar]
- 51.Luo K, et al. The Ski oncoprotein interacts with the SMAD proteins to repress TGFβ signaling. Genes Dev. 1999;13:2196–2206. doi: 10.1101/gad.13.17.2196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sun Y, et al. Interaction of the Ski oncoprotein with SMAD3 regulates TGF- β signaling. Mol. Cell. 1999;4:499–509. doi: 10.1016/S1097-2765(00)80201-4. [DOI] [PubMed] [Google Scholar]
- 53.Xu W, et al. Ski acts as a co-repressor with SMAD2 and SMAD3 to regulate the response to type β transforming growth factor. Proc. Natl Acad. Sci. USA. 2000;97:5924–5929. doi: 10.1073/pnas.090097797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Tabata T, Kokura K, ten Dijke P, Ishii S. Ski co-repressor complexes maintain the basal repressed state of the TGF-β target gene, SMAD7, via HDAC3 and PRMT5. Genes Cell. 2009;14:17–28. doi: 10.1111/j.1365-2443.2008.01246.x. [DOI] [PubMed] [Google Scholar]
- 55.Nagase T, et al. Requirement of protein co-factor for the DNA-binding function of the human ski proto-oncogene product. Nucleic Acids Res. 1990;18:337–343. doi: 10.1093/nar/18.2.337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Akiyoshi S, et al. c-Ski acts as a transcriptional co-repressor in transforming growth factor-β signaling through interaction with SMADs. J. Biol. Chem. 1999;274:35269–35277. doi: 10.1074/jbc.274.49.35269. [DOI] [PubMed] [Google Scholar]
- 57.Dai P, et al. Ski is involved in transcriptional regulation by the repressor and full-length forms of Gli3. Genes Dev. 2002;16:2843–2848. doi: 10.1101/gad.1017302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ueki N, Hayman MJ. Direct interaction of Ski with either SMAD3 or SMAD4 is necessary and sufficient for Ski-mediated repression of transforming growth factor-beta signaling. J. Biol. Chem. 2003;278:32489–32492. doi: 10.1074/jbc.C300276200. [DOI] [PubMed] [Google Scholar]
- 59.Chen W, et al. Competition between Ski and CREB-binding protein for binding to SMAD proteins in transforming growth factor- β signaling. J. Biol. Chem. 2007;282:11365–11376. doi: 10.1074/jbc.M700186200. [DOI] [PubMed] [Google Scholar]
- 60.Harada J, et al. Requirement of the co-repressor homeodomain-interacting protein kinase 2 for ski-mediated inhibition of bone morphogenetic protein-induced transcriptional activation. J. Biol. Chem. 2003;278:38998–39005. doi: 10.1074/jbc.M307112200. [DOI] [PubMed] [Google Scholar]
- 61.Pessah M, et al. C-Jun associate with the oncoprotein Ski and suppresses SMAD2 transcriptional activity. J. Biol. Chem. 2002;277:29094–291000. doi: 10.1074/jbc.M202831200. [DOI] [PubMed] [Google Scholar]
- 62.Ueki N, Hayman MJ. Signal-dependent N-CoR requirement for repression by the Ski oncoprotein. J. Biol. Chem. 2003;278:24858–24864. doi: 10.1074/jbc.M303447200. [DOI] [PubMed] [Google Scholar]
- 63.Ueki N, Zhang L, Hayman MJ. Ski can negatively regulates macrophage differentiation through its interaction with PU.1. Oncogene. 2008;27:300–307. doi: 10.1038/sj.onc.1210654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zhao HL, Ueki N, Marcelain K, Hayman MJ. The Ski protein can inhibit ligand induced RARα and HDAC3 degradation in the retinoic acid signaling pathway. Biochem. Biophys. Res. Commun. 2009;383:119–124. doi: 10.1016/j.bbrc.2009.03.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Baranek C, Atanasoski S. Modulating epigenetic mechanisms: the diverse functions of Ski during cortical development. Epigenetics. 2012;7:676–679. doi: 10.4161/epi.20590. [DOI] [PubMed] [Google Scholar]
- 66.Baranek C, et al. Protooncogene Ski cooperates with the chromatin-remodeling factor Satb2 in specifying callosal neurons. Proc. Natl Acad. Sci. USA. 2012;109:3546–3551. doi: 10.1073/pnas.1108718109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ueki N, Zhang L, Hayman MJ. Ski negatively regulates erythroid differentiation through its interaction with GATA1. Mol. Cell Biol. 2004;24:10118–10125. doi: 10.1128/MCB.24.23.10118-10125.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Inoue Y, Iemura S, Natsume T, Miyazawa K, Imamura T. Suppression of p53 activity through the cooperative action of Ski and histone deacetylase SIRT1. J. Biol. Chem. 2011;286:6311–6320. doi: 10.1074/jbc.M110.177683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Rashidian J, et al. Ski regulates Hippo and TAZ signaling to suppress breast cancer progression. Sci. Signal. 2015;8:ra14. doi: 10.1126/scisignal.2005735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Tokitou F, et al. Viral ski inhibits retinoblastoma protein (Rb)-mediated transcriptional repression in a dominant negative fashion. J. Biol. Chem. 1999;274:4485–4488. doi: 10.1074/jbc.274.8.4485. [DOI] [PubMed] [Google Scholar]
- 71.Nomura T, et al. Ski is a component of the histone deacetylase complex required for transcriptional repression by Mad and thyroid hormone receptor. Genes Dev. 1999;13:412–423. doi: 10.1101/gad.13.4.412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kokura K, et al. The Ski protein family is required for MeCP2-mediated transcriptional repression. J. Biol. Chem. 2001;276:34115–34121. doi: 10.1074/jbc.M105747200. [DOI] [PubMed] [Google Scholar]
- 73.Tecalco-Cruz AC, et al. Transforming growth factor-β/SMAD Target gene SKIL is negatively regulated by the transcriptional cofactor complex SNON-SMAD4. J. Biol. Chem. 2012;287:26764–26776. doi: 10.1074/jbc.M112.386599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Tsuneyoshi N, et al. The SMAD2/3 corepressor SNON maintains pluripotency through selective repression of mesendodermal genes in human ES cells. Genes Dev. 2012;26:2471–2476. doi: 10.1101/gad.201772.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Briones-Orta MA, Sosa-Garrocho M, Moreno-Alvarez P, Fonseca-Sánchez MA, Macías-Silva M. SnoN co-repressor binds and represses SMAD7 gene promoter. Biochem. Biophys. Res. Commun. 2006;341:889–894. doi: 10.1016/j.bbrc.2006.01.041. [DOI] [PubMed] [Google Scholar]
- 76.Wilkinson DS, Tsai WW, Schumacher MA, Barton MC. Chromatin-bound p53 anchors activated SMADs and the mSin3A corepressor to confer Transforming-Growth-Factor-β-mediated transcription repression. Mol. Cell Biol. 2008;28:1988–1998. doi: 10.1128/MCB.01442-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Sarker KP, Wilson SM, Bonni S. SnoN is a cell type-specific mediator of transforming growth factor-beta responses. J. Biol. Chem. 2005;280:13037–13046. doi: 10.1074/jbc.M409367200. [DOI] [PubMed] [Google Scholar]
- 78.Sarker KP, et al. ING2 as a novel mediator of transforming growth factor-β-dependent responses in epithelial cells. J. Biol. Chem. 2008;283:13269–13279. doi: 10.1074/jbc.M708834200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Ikeuchi Y, et al. A SnoN-Ccd1 pathway promotes axonal morphogenesis in the mammalian brain. J. Neurosci. 2009;29:4312–4321. doi: 10.1523/JNEUROSCI.0126-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Band AM, Laiho M. SnoN oncoprotein enhances estrogen receptor-α transcriptional activity. Cell Signal. 2012;24:922–930. doi: 10.1016/j.cellsig.2011.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Kodigepalli KM, Anur P, Spellman P, Sims PJ, Nanjundan M. Phospholipid Scramblase 1, an interferon-regulated gene located at 3q23, is regulated by SnoN/SkiL in ovarian cancer cells. Mol. Cancer. 2013;12:32. doi: 10.1186/1476-4598-12-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Solomon E, Li H, Duhachek Muggy S, Syta E, Zolkiewska A. The role of SnoN in transforming growth factor beta1-induced expression of metalloprotease-disintegrin ADAM12. J. Biol. Chem. 2010;285:21969–21977. doi: 10.1074/jbc.M110.133314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Shinozuka E, et al. SnoN/SKIL modulates proliferation through control of hsa-miR-720 transcription in esophageal cancer cells. Biochem. Biophys. Res. Commun. 2013;430:101–106. doi: 10.1016/j.bbrc.2012.11.005. [DOI] [PubMed] [Google Scholar]
- 84.Tarapore P, et al. DNA binding and transcriptional activation by the Ski oncoprotein mediated by interaction with NFI. Nucleic Acids Res. 1997;25:3895–3903. doi: 10.1093/nar/25.19.3895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Baldwin A, Pirisi L, Creek KE. NFI-Ski interactions mediate Transforming Growth Factor β modulation of human papillomavirus type 16 early gene expression. J. Virol. 2004;78:3953–3964. doi: 10.1128/JVI.78.8.3953-3964.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Chen D, et al. SKI activates Wnt/beta-catenin signaling in human melanoma. Cancer Res. 2003;63:6626–6634. [PubMed] [Google Scholar]
- 87.Kobayashi N, et al. c-Ski activates MyoD in the nucleus of myoblastic cells through suppression of histone deacetylases. Genes Cell. 2007;12:375–385. doi: 10.1111/j.1365-2443.2007.01052.x. [DOI] [PubMed] [Google Scholar]
- 88.Zhang H, Stavnezer E. Ski regulates muscle terminal differentiation by transcriptional activation of Myog in a complex with Six1 and Eya3. J. Biol. Chem. 2009;284:2867–2879. doi: 10.1074/jbc.M807526200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Stavnezer E, Gerhard DS, Binari RC, Balazs I. Generation of transforming viruses in cultures of chicken fibroblasts infected with an avian leukosis virus. J. Virol. 1981;39:920–934. doi: 10.1128/jvi.39.3.920-934.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Li Y, Turck CM, Teumer JK, Stavnezer E. Unique sequence, ski, in Sloan-Kettering avian retroviruses with properties of a new cell-derived oncogene. J. Virol. 1986;57:1065–1072. doi: 10.1128/jvi.57.3.1065-1072.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Nomura N, et al. Isolation of human cDNA clones of ski and the ski-related gene, sno. Nucleic Acids Res. 1989;17:5489–5500. doi: 10.1093/nar/17.14.5489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Ludolph DC, et al. Cloning and expression of the axolotl proto-oncogene ski. Biochim. Biophys. Acta. 1995;1260:102–104. doi: 10.1016/0167-4781(94)00194-8. [DOI] [PubMed] [Google Scholar]
- 93.Huang CJ, Lin JY, Tsai HJ. Two distinct c-ski cDNAs of fish, tilapia (Oreochromis aurea) Mol. Reprod. Dev. 1999;54:223–231. doi: 10.1002/(SICI)1098-2795(199911)54:3<223::AID-MRD3>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 94.Kaufman CD, Martínez-Rodríguez G, Hackett PB. Ectopic expression of c-ski disrupts gastrulation and neural patterning in zebrafish. Mech. Dev. 2000;95:147–162. doi: 10.1016/S0925-4773(00)00351-8. [DOI] [PubMed] [Google Scholar]
- 95.Grimes HL, Szente BE, Goodenow MM. C-ski cDNAs are encoded by eight exons, six of which are closely linked within the chicken genome. Nucleic Acids Res. 1992;20:1511–1516. doi: 10.1093/nar/20.7.1511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Grimes HL, Ambrose MR, Goodenow MM. C-ski transcripts with and without exon 2 are expressed in skeletal muscle and throughout chick embryogenesis. Oncogene. 1993;8:2863–2868. [PubMed] [Google Scholar]
- 97.Leferovich JM, Lana DP, Sutrave P, Hughes SH, Kelly AM. Regulation of c-ski transgene expression in developing and mature mice. J. Neurosci. 1995;15:596–603. doi: 10.1523/JNEUROSCI.15-01-00596.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Zhang SX, et al. Identification of direct serum-response factor gene targets during Me2SO-induced P19 cardiac cell differentiation. J. Biol. Chem. 2005;280:19115–19126. doi: 10.1074/jbc.M413793200. [DOI] [PubMed] [Google Scholar]
- 99.Li J, et al. Upregulation of ski in fibroblast is implicated in the peroxisome proliferator-activated receptor δ-mediated wound healing. Cell Physiol. Biochem. 2012;30:1059–1071. doi: 10.1159/000341482. [DOI] [PubMed] [Google Scholar]
- 100.Melling MA, Friendship CR, Shepherd TG, Drysdale TA. Expression of Ski can act as a negative feedback mechanism on retinoic acid signaling. Dev. Dyn. 2013;242:604–613. doi: 10.1002/dvdy.23954. [DOI] [PubMed] [Google Scholar]
- 101.Xie M, Wu X, Zhang J, Li X. Ski regulates Smads and TAZ signaling to suppress lung cancer progression. Mol. Carcinog. 2017;56:2178–2189. doi: 10.1002/mc.22661. [DOI] [PubMed] [Google Scholar]
- 102.Imig J, et al. microRNA profiling in Epstein-Barr virus-associated B-cell lymphoma. Nucleic Acids Res. 2011;39:1880–1893. doi: 10.1093/nar/gkq1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Levati L, et al. MicroRNA-155 targets the SKI gene in human melanoma cell lines. Pigment Cell Melanoma Res. 2011;24:538–550. doi: 10.1111/j.1755-148X.2011.00857.x. [DOI] [PubMed] [Google Scholar]
- 104.Teichler S, et al. MicroRNA29a regulates the expression of the nuclear oncogene Ski. Blood. 2011;118:1899–1902. doi: 10.1182/blood-2010-09-306258. [DOI] [PubMed] [Google Scholar]
- 105.Jiang H, et al. Next generation sequencing analysis of miRNAs: MiR-127-3p inhibits glioblastoma proliferation and activates TGF-β signaling by targeting SKI. OMICS. 2014;18:196–206. doi: 10.1089/omi.2013.0122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Li J, Zhao L, He X, Yang T, Yang K. MiR-21 inhibits c-Ski signaling to promote the proliferation of rat vascular smooth muscle cells. Cell Signal. 2014;26:724–729. doi: 10.1016/j.cellsig.2013.12.013. [DOI] [PubMed] [Google Scholar]
- 107.Wang J, et al. The mechanism of TGF-β/miR-155/c-Ski regulates endothelial-mesenchymal transition in human coronary artery endothelial cells. Biosci. Rep. 2017;37:BSR20160603. doi: 10.1042/BSR20160603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Zhu Q, Pearson-White S, Luo K. Requirement for the SnoN oncoprotein in transforming growth factor β-induced oncogenic transformation of fibroblast cells. Mol. Cell Biol. 2005;25:10731–10744. doi: 10.1128/MCB.25.24.10731-10744.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Denissova NG, Liu F. Repression of endogenous SMAD7 by Ski. J. Biol. Chem. 2004;279:28143–28148. doi: 10.1074/jbc.M404961200. [DOI] [PubMed] [Google Scholar]
- 110.Tan R, Zhang X, Yang J, Li Y, Liu Y. Molecular basis for the cell type specific induction of SnoN expression by hepatocyte growth factor. J. Am. Soc. Nephrol. 2007;18:2340–2349. doi: 10.1681/ASN.2007010128. [DOI] [PubMed] [Google Scholar]
- 111.Mayoral R, et al. Impairment of transforming growth factor β signaling in caveolin-1-deficient hepatocytes: role in liver regeneration. J. Biol. Chem. 2010;285:3633–3642. doi: 10.1074/jbc.M109.072900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Jahchan NS, Wang D, Bissell MJ, Luo K. SnoN regulates mammary gland alveologenesis and onset of lactation by promoting prolactin/Stat5 signaling. Development. 2012;139:3147–3156. doi: 10.1242/dev.079616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Wang Y, et al. BMP-7 enhances SnoN mRNA expression in renal tubular epithelial cells under high-glucose conditions. Mol. Med. Rep. 2017;16:3308–3314. doi: 10.3892/mmr.2017.7025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Kodigepalli KM, Dutta PS, Bauckman KA, Nanjundan M. SnoN/SkiL expression is modulated via arsenic trioxide-induced activation of the PI3K/AKT pathway in ovarian cancer cells. FEBS Lett. 2013;587:5–16. doi: 10.1016/j.febslet.2012.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Park MA, Choi KC. Effects of 4-nonylphenol and bisphenol A on stimulation of cell growth via disruption of the Transforming Growth Factor-β signaling pathway in ovarian cancer models. Chem. Res. Toxicol. 2014;27:119–128. doi: 10.1021/tx400365z. [DOI] [PubMed] [Google Scholar]
- 116.Hamilton MP, et al. Identification of a pan-cancer oncogenic microRNA superfamily anchored by a central core seed motif. Nat. Commun. 2013;4:2730. doi: 10.1038/ncomms3730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Xu H, Sun F, Li X, Sun L. Down-regulation of miR-23a inhibits high glucose-induced EMT and renal fibrogenesis by up-regulation of SnoN. Hum. Cell. 2017;30:1–11. doi: 10.1007/s13577-016-0149-3. [DOI] [PubMed] [Google Scholar]
- 118.Dahl R, Wani B, Hayman MJ. The Ski oncoprotein interacts with Skip, the human homolog of Drosophila Bx42. Oncogene. 1998;16:1579–1586. doi: 10.1038/sj.onc.1201687. [DOI] [PubMed] [Google Scholar]
- 119.Prathapam T, Kuhne C, Hayman M, Banks L. Ski interacts with the evolutionary conserved SNW domain of Skip. Nucleic Acids Res. 2001;29:3469–3476. doi: 10.1093/nar/29.17.3469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Kim SS, et al. Structure of the retinal determination protein Dachshund reveals a DNA binding motif. Structure. 2002;10:787–795. doi: 10.1016/S0969-2126(02)00769-4. [DOI] [PubMed] [Google Scholar]
- 121.Wilson JJ, Malakhova M, Zhang R, Joachimiak A, Hegde RS. Crystal structure of the dachshund homology domain of human SKI. Structure. 2004;12:785–792. doi: 10.1016/j.str.2004.02.035. [DOI] [PubMed] [Google Scholar]
- 122.Wu JW, et al. Structural mechanism of SMAD4 recognition by the nuclear oncoprotein Ski: insights on Ski-mediated repression of TGF-β signaling. Cell. 2002;111:357–367. doi: 10.1016/S0092-8674(02)01006-1. [DOI] [PubMed] [Google Scholar]
- 123.Stavnezer E, Brodeur D, Brennan LA. The v-ski oncogene encodes a truncated set of c-ski coding exons with limited sequence and structural relatedness to v-myc. Mol. Cell Biol. 1989;9:4038–4045. doi: 10.1128/MCB.9.9.4038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Nagase T, Nomura N, Ishii S. Complex formation between proteins encoded by the ski gene family. J. Biol. Chem. 1993;268:13710–13716. [PubMed] [Google Scholar]
- 125.Nyman T, et al. The crystal structure of the dachshund domain of human SnoN reveals flexibility in the putative protein interaction surface. PLoS ONE. 2010;5:e12907. doi: 10.1371/journal.pone.0012907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Walldén K, Nyman T, Hällberg BM. SnoN stabilizes the SMAD3/SMAD4 protein complex. Sci. Rep. 2017;7:46370. doi: 10.1038/srep46370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Heyman HC, Stavnezer E. A carboxyl-terminal region of the ski oncoprotein mediates homodimerization as well as heterodimerization with the related protein SnoN. J. Biol. Chem. 1994;269:26996–27003. [PubMed] [Google Scholar]
- 128.Zheng G, et al. High affinity dimerization by Ski involves parallel pairing of a novel bipartite α-helical domain. J. Biol. Chem. 1997;272:31855–31864. doi: 10.1074/jbc.272.50.31855. [DOI] [PubMed] [Google Scholar]
- 129.Cohen SB, Nicol R, Stavnezer E. A domain necessary for the transforming activity of SnoN is required for specific DNA binding, transcriptional repression and interaction with TAF(II)110. Oncogene. 1998;17:2505–2513. doi: 10.1038/sj.onc.1202177. [DOI] [PubMed] [Google Scholar]
- 130.Cohen SB, Zheng G, Heyman HC, Stavnezer E. Heterodimers of the SnoN and Ski oncoproteins form preferentially over homodimers and are more potent transforming agents. Nucleic Acids Res. 1999;27:1006–1014. doi: 10.1093/nar/27.4.1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.He J, Tegen SB, Krawitz AR, Martin GS, Luo K. The transforming activity of Ski and SnoN is dependent on their ability to repress the activity of SMAD proteins. J. Biol. Chem. 2003;278:30540–30547. doi: 10.1074/jbc.M304016200. [DOI] [PubMed] [Google Scholar]
- 132.Pearson-White S. SnoI, a novel alternatively spliced isoform of the ski protooncogene homolog, sno. Nucleic Acids Res. 1993;21:4632–4638. doi: 10.1093/nar/21.19.4632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Pearson-White S, Crittenden R. Proto-oncogene Sno expression, alternative isoforms and immediate early serum response. Nucleic Acids Res. 1997;25:2930–2937. doi: 10.1093/nar/25.14.2930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Takaesu NT, et al. dSno facilitates baboon signaling in the Drosophila brain by switching the affinity of Medea away from Mad and toward dSMAD2. Genetics. 2006;174:1299–1313. doi: 10.1534/genetics.106.064956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Ramel MC, et al. Drosophila SnoN modulates growth and patterning by antagonizing TGF-β signalling. Mech. Dev. 2007;124:304–317. doi: 10.1016/j.mod.2006.12.006. [DOI] [PubMed] [Google Scholar]
- 136.Band AM, Bjorklund M, Laiho M. The phosphatidylinositol 3-kinase/Akt pathway regulates transforming growth factor-β signaling by destabilizing Ski and inducing SMAD7. J. Biol. Chem. 2009;284:35441–35449. doi: 10.1074/jbc.M109.029488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Nagata M, et al. Identification of a phosphorylation site in c-Ski as serine 515. J. Biochem. 2010;148:423–427. doi: 10.1093/jb/mvq076. [DOI] [PubMed] [Google Scholar]
- 138.Mosquera J, et al. Identification of Ski as a target for Aurora A kinase. Biochem. Biophys. Res. Commun. 2011;409:539–543. doi: 10.1016/j.bbrc.2011.05.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Rivas S, et al. The Ski protein is involved in the transformation pathway of aurora kinase A. J. Cell Biochem. 2016;117:334–343. doi: 10.1002/jcb.25275. [DOI] [PubMed] [Google Scholar]
- 140.Kajino T, Omori E, Ishii S, Matsumoto K, Ninomiya-Tsuji J. TAK1 MAPK kinase kinase mediates Transforming Growth Factor-β signaling by targeting SnoN oncoprotein for degradation. J. Biol. Chem. 2007;282:9475–9481. doi: 10.1074/jbc.M700875200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Bonni S, et al. TGF-β induces assembly of a SMAD2-Smurf2 ubiquitin ligase complex that targets SnoN for degradation. Nat. Cell Biol. 2001;3:587–595. doi: 10.1038/35078562. [DOI] [PubMed] [Google Scholar]
- 142.Stroschein SL, Bonni S, Wrana JL, Luo K. SMAD3 recruits the anaphase-promoting complex for ubiquitination and degradation of SnoN. Genes Dev. 2001;15:2822–2836. doi: 10.1101/gad.912901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Wan Y, Liu X, Kirschner MW. The anaphase-promoting complex mediates TGF-beta signaling by targeting SnoN for destruction. Mol. Cell. 2001;8:1027–1039. doi: 10.1016/S1097-2765(01)00382-3. [DOI] [PubMed] [Google Scholar]
- 144.Koinuma D, et al. Arkadia amplifies TGF-β superfamily signalling through degradation of SMAD7. EMBO J. 2003;22:6458–6470. doi: 10.1093/emboj/cdg632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Levy L, et al. Arkadia activates SMAD3/SMAD4-dependent transcription by triggering signal-induced SnoN degradation. Mol. Cell Biol. 2007;27:6068–6083. doi: 10.1128/MCB.00664-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Nagano Y, et al. Arkadia induces degradation of SnoN and c-Ski to enhance transforming growth factor-β signaling. J. Biol. Chem. 2007;282:20492–20501. doi: 10.1074/jbc.M701294200. [DOI] [PubMed] [Google Scholar]
- 147.Stegmüller J, Huynh MA, Yuan Z, Konishi Y, Bonni A. TGF-β-SMAD2 signaling regulates the Cdh1-APC/SnoN pathway of axonal morphogenesis. J. Neurosci. 2008;28:1961–1969. doi: 10.1523/JNEUROSCI.3061-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Koinuma D, et al. RB1CC1 protein positively regulates Transforming Growth Factor-β signaling through the modulation of Arkadia E3 ubiquitin ligase activity. J. Biol. Chem. 2011;286:32502–32512. doi: 10.1074/jbc.M111.227561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Mizuide M, et al. Two short segments of SMAD3 are important for specific interaction of SMAD3 with c-Ski and SnoN. J. Biol. Chem. 2003;278:531–536. doi: 10.1074/jbc.C200596200. [DOI] [PubMed] [Google Scholar]
- 150.Fukasawa H, et al. Ubiquitin-dependent degradation of SnoN and Ski is increased in renal fibrosis induced by obstructive injury. Kidney Int. 2006;69:1733–1740. doi: 10.1038/sj.ki.5000261. [DOI] [PubMed] [Google Scholar]
- 151.Zhou F, et al. USP4 inhibits SMAD4 monoubiquitination and promotes activin and BMP signaling. EMBO J. 2017;36:1623–1639. doi: 10.15252/embj.201695372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Hsu YH, et al. Sumoylated SnoN represses transcription in a promoter-specific manner. J. Biol. Chem. 2006;281:33008–33018. doi: 10.1074/jbc.M604380200. [DOI] [PubMed] [Google Scholar]
- 153.Wrighton KH, et al. Transforming growth factor-β-independent regulation of myogenesis by SnoN sumoylation. J. Biol. Chem. 2007;282:6517–6524. doi: 10.1074/jbc.M610206200. [DOI] [PubMed] [Google Scholar]
- 154.Netherton SJ, Bonni S. Suppression of TGFβ-induced epithelial-mesenchymal transition like phenotype by a PIAS1 regulated sumoylation pathway in NMuMG epithelial cells. PLoS ONE. 2010;5:e13971. doi: 10.1371/journal.pone.0013971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Ikeuchi Y, et al. TIF1γ protein regulates epithelial-mesenchymal transition by operating as a small ubiquitin-like modifier (SUMO) E3 ligase for the transcriptional regulator SnoN1. J. Biol. Chem. 2014;289:25067–25078. doi: 10.1074/jbc.M114.575878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Ding B, Sun Y, Huang J. Overexpression of SKI oncoprotein leads to p53 degradation through regulation of MDM2 protein sumoylation. J. Biol. Chem. 2012;287:14621–14630. doi: 10.1074/jbc.M111.301523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Krakowski AR, Laboureau J, Mauviel A, Bissell MJ, Luo K. Cytoplasmic SnoN in normal tissues and nonmalignant cells antagonizes TGF-β signaling by sequestration of the SMAD proteins. Proc. Natl Acad. Sci. USA. 2005;102:12437–12442. doi: 10.1073/pnas.0504107102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Nagata M, et al. Nuclear and cytoplasmic c-Ski differently modulate cellular functions. Genes Cell. 2006;11:1267–1280. doi: 10.1111/j.1365-2443.2006.01018.x. [DOI] [PubMed] [Google Scholar]
- 159.Kokura K, et al. The Ski-binding protein C184M negatively regulates tumor growth factor-β signaling by sequestering the SMAD proteins in the cytoplasm. J. Biol. Chem. 2003;278:20133–20139. doi: 10.1074/jbc.M210855200. [DOI] [PubMed] [Google Scholar]
- 160.Rajagopal R, Ishii S, Beebe DC. Intracellular mediators of transforming growth factor beta superfamily signaling localize to endosomes in chicken embryo and mouse lenses in vivo. BMC Cell Biol. 2007;8:25. doi: 10.1186/1471-2121-8-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Ferrand N, Atfi A, Prunier C. The oncoprotein c-ski functions as a direct antagonist of the Transforming Growth Factor-β type I receptor. Cancer Res. 2010;70:8457–8466. doi: 10.1158/0008-5472.CAN-09-4088. [DOI] [PubMed] [Google Scholar]
- 162.Javelaud D, et al. Efficient TGF-β/Smad signaling in human melanoma cells associated with high c-Ski/SnoN expression. Mol. Cancer. 2011;10:2. doi: 10.1186/1476-4598-10-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Reed JA, et al. Cytoplasmic localization of the oncogenic protein Ski in human cutaneous melanomas in vivo: functional implications for transforming growth factor β signaling. Cancer Res. 2001;61:8074–8078. [PubMed] [Google Scholar]
- 164.Jahchan NS, You YH, Muller WJ, Luo K. Transforming Growth Factor-β regulator SnoN modulates mammary gland branching morphogenesis, postlactational involution, and mammary tumorigenesis. Cancer Res. 2010;70:4204–4213. doi: 10.1158/0008-5472.CAN-10-0135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Jahchan NS, Ouyang G, Luo K. Expression profiles of SnoN in normal and cancerous human tissues support its tumor suppressor role in human cancer. PLoS ONE. 2013;8:e55794. doi: 10.1371/journal.pone.0055794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Ji X, et al. Vitamin C deficiency exacerbates diabetic glomerualr injury through activation of transforming growth factor-β signaling. Biochim. Biophys. Acta. 2017;1861:2186–2195. doi: 10.1016/j.bbagen.2017.06.018. [DOI] [PubMed] [Google Scholar]
- 167.Atanasoski S, et al. The protooncogene Ski controls Schwann cell proliferation and myelination. Neuron. 2004;43:499–511. doi: 10.1016/j.neuron.2004.08.001. [DOI] [PubMed] [Google Scholar]
- 168.Jacob C, Grabner H, Atanasoski S, Suter U. Expression and localization of Ski determine cell type-specific TGFβ signaling effects on the cell cycle. J. Cell. Biol. 2008;182:519–530. doi: 10.1083/jcb.200710161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Cunnington RH, et al. Antifibrotic properties of c-Ski and its regulation of cardiac myofibroblast phenotype and contractility. Am. J. Physiol. Cell Physiol. 2011;300:176–186. doi: 10.1152/ajpcell.00050.2010. [DOI] [PubMed] [Google Scholar]
- 170.Zhu Q, et al. SnoN Antagonizes the hippo kinase complex to promote TAZ signaling during breast carcinogenesis. Dev. Cell. 2016;37:399–412. doi: 10.1016/j.devcel.2016.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Vázquez-Victorio G, et al. Novel regulation of Ski protein stability and endosomal sorting by actin cytoskeleton dynamics in hepatocytes. J. Biol. Chem. 2015;290:4487–4499. doi: 10.1074/jbc.M114.579532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Caligaris C, et al. Actin-cytoskeleton polymerization differentially controls the stability of Ski and SnoN co-repressors in normal but not in transformed hepatocytes. Biochim. Biophys. Acta. 2015;1850:1832–1841. doi: 10.1016/j.bbagen.2015.05.012. [DOI] [PubMed] [Google Scholar]
- 173.Vázquez-Victorio G, González-Espinosa C, Espinosa-Riquer ZP, Macías-Silva M. GPCRs and actin-cytoskeletal dynamics. Method Cell Biol. 2016;132:165–188. doi: 10.1016/bs.mcb.2015.10.003. [DOI] [PubMed] [Google Scholar]
- 174.Macdonald M, et al. Control of cell cycle-dependent degradation of c-Ski proto-oncoprotein by Cdc34. Oncogene. 2004;23:5643–5653. doi: 10.1038/sj.onc.1207733. [DOI] [PubMed] [Google Scholar]
- 175.Marcelain K, Hayman MJ. The Ski oncoprotein is upregulated and localized at the centrosomes and mitotic spindle during mitosis. Oncogene. 2005;24:4321–4329. doi: 10.1038/sj.onc.1208631. [DOI] [PubMed] [Google Scholar]
- 176.Marcelain K, et al. Chromosomal instability in mouse embryonic fibroblasts null for the transcriptional co-repressor Ski. J. Cell Physiol. 2012;227:278–287. doi: 10.1002/jcp.22733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Zieba A, et al. Intercellular variation in signaling through the TGF-β pathway and its relation to cell density and cell cycle phase. Mol. Cell Prot. 2012;11:M111013482. doi: 10.1074/mcp.M111.013482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Shinagawa T, Dong HD, Xu M, Maekawa T, Ishii S. The sno gene, which encodes a component of the histone deacetylase complex, acts as a tumor suppressor in mice. EMBO J. 2000;19:2280–2291. doi: 10.1093/emboj/19.10.2280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Pearson-White S, McDuffie M. Defective T-cell activation is associated with augmented transforming growth factor β sensitivity in mice with mutations in the Sno gene. Mol. Cell Biol. 2003;23:5446–5459. doi: 10.1128/MCB.23.15.5446-5459.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Zhu Q, Kim YH, Wang D, Oh SP, Luo K. SnoN facilitates ALK1-SMAD1/5 signaling during embryonic angiogenesis. J. Cell Biol. 2013;202:937–950. doi: 10.1083/jcb.201208113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Shinagawa T, Ishii S. Generation of Ski-knockdown mice by expressing a long double-strand RNA from an RNA polymerase II promoter. Genes Dev. 2003;17:1340–1345. doi: 10.1101/gad.1073003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.McGannon P, Miyazaki Y, Gupta PC, Traboulsi EI, Colmenares C. Ocular abnormalities in mice lacking the Ski proto-oncogene. Invest. Ophthalmol. Vis. Sci. 2006;47:4231–4237. doi: 10.1167/iovs.05-1543. [DOI] [PubMed] [Google Scholar]
- 183.Lyons GE, et al. Protooncogene c-ski is expressed in both proliferating and postmitotic neuronal populations. Dev. Dyn. 1994;201:354–365. doi: 10.1002/aja.1002010407. [DOI] [PubMed] [Google Scholar]
- 184.Pot I, Ikeuchi Y, Bonni A, Bonni S. SnoN: Bridging neurobiology and cancer biology. Curr. Mol. Med. 2010;10:667–673. doi: 10.2174/156652410792630616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Bonni S, Bonni A. SnoN signaling in proliferating cells and postmitotic neurons. FEBS Lett. 2012;586:1977–1983. doi: 10.1016/j.febslet.2012.02.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Amaravadi LS, Neff AW, Sleeman JP, Smith RC. Autonomous neural axis formation by ectopic expression of the protooncogene c-ski. Dev. Biol. 1997;192:392–404. doi: 10.1006/dbio.1997.8780. [DOI] [PubMed] [Google Scholar]
- 187.Zhou K, et al. Spatiotemporal expression of Ski after rat spinal cord injury. Neuroreport. 2017;28:149–157. doi: 10.1097/WNR.0000000000000729. [DOI] [PubMed] [Google Scholar]
- 188.Chang C, Harland RM. Neural induction requires continued suppression of both SMAD1 and SMAD2 signals during gastrulation. Development. 2007;134:3861–3872. doi: 10.1242/dev.007179. [DOI] [PubMed] [Google Scholar]
- 189.Berk M, Desai SY, Heyman HC, Colmenares C. Mice lacking the ski proto-oncogene have defects in neurulation, craniofacial, patterning, and skeletal muscle development. Genes Dev. 1997;11:2029–2039. doi: 10.1101/gad.11.16.2029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Colmenares C, et al. Loss of the SKI proto-oncogene in individuals affected with 1p36 deletion syndrome is predicted by strain-dependent defects in Ski-/- mice. Nat. Genet. 2002;30:106–109. doi: 10.1038/ng770. [DOI] [PubMed] [Google Scholar]
- 191.Huynh MA, et al. An isoform-specific SnoN1-FOXO1 repressor complex controls neuronal morphogenesis and positioning in the mammalian brain. Neuron. 2011;69:930–944. doi: 10.1016/j.neuron.2011.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Do JL, Bonni A, Tuszynski MH. SnoN facilitates axonal regeneration after spinal cord injury. PLoS ONE. 2013;8:e71906. doi: 10.1371/journal.pone.0071906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Pearson-White S, et al. The ski/sno protooncogene family in hematopoietic development. Blood. 1995;86:2146–2155. [PubMed] [Google Scholar]
- 194.Singbrant S, et al. The SKI proto-oncogene enhances the in vivo repopulation of hematopoietic stem cells and causes myeloproliferative disease. Haematologica. 2014;99:647–655. doi: 10.3324/haematol.2013.093971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Dahl R, Kieslinger M, Beug H, Hayman MJ. Transformation of hematopoietic cells by the Ski oncoprotein involves repression of retinoic acid receptor signaling. Proc. Natl Acad. Sci. USA. 1998;95:11187–11192. doi: 10.1073/pnas.95.19.11187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Namciu S, Lieberman MA, Stavnezer E. Induction of the c-ski proto-oncogene by phorbol ester correlates with induction of megakaryocyte differentiation. Oncogene. 1994;9:1407–1416. [PubMed] [Google Scholar]
- 197.Li C, et al. PMA induces SnoN proteolysis and CD61 expression through an autocrine mechanism. Cell Signal. 2014;26:1369–1378. doi: 10.1016/j.cellsig.2014.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Engert JC, Servaes S, Sutrave P, Hughes SH, Rosenthal N. Activation of a muscle-specific enhancer by the Ski proto-oncogene. Nucleic Acids Res. 1995;23:2988–2994. doi: 10.1093/nar/23.15.2988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Ambrose MR, Bottazzi ME, Goodenow MM. Expression of the c-ski proto-oncogene during cell cycle arrest and myogenic differentiation. DNA Cell Biol. 1995;14:701–707. doi: 10.1089/dna.1995.14.701. [DOI] [PubMed] [Google Scholar]
- 200.Namciu S, et al. Enhanced expression of mouse c-ski accompanies terminal skeletal muscle differentiation in vivo and in vitro. Dev. Dyn. 1995;204:291–300. doi: 10.1002/aja.1002040307. [DOI] [PubMed] [Google Scholar]
- 201.Colmenares C, Stavnezer E. The ski oncogene induces muscle differentiation in quail embryo cells. Cell. 1989;59:293–303. doi: 10.1016/0092-8674(89)90291-2. [DOI] [PubMed] [Google Scholar]
- 202.Colmenares C, Teumer JK, Stavnezer E. Transformation-defective v-ski induces MyoD and myogenin expression but not myotube formation. Mol. Cell Biol. 1991;11:1167–1170. doi: 10.1128/MCB.11.2.1167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Ichikawa K, Nagase T, Ishii S, Asano A, Mimura N. Trans-regulation of myogenin promoter/enhancer activity by c-ski during skeletal-muscle differentiation: the C-terminus of the c-Ski protein is essential for transcriptional regulatory activity in myotubes. Biochem. J. 1997;328:607–613. doi: 10.1042/bj3280607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Sutrave P, Kelly AM, Hughes SH. Ski can cause selective growth of skeletal muscle in transgenic mice. Genes Dev. 1990;4:1462–1472. doi: 10.1101/gad.4.9.1462. [DOI] [PubMed] [Google Scholar]
- 205.Costelli P, et al. Reduced protein degradation rates and low expression of proteolytic systems support skeletal muscle hypertrophy in transgenic mice overexpressing the c-ski oncogene. Cancer Lett. 2003;200:153–160. doi: 10.1016/S0304-3835(03)00415-4. [DOI] [PubMed] [Google Scholar]
- 206.Lana DP, Leferovich JM, Kelly AM, Hughes SH. Selective expression of a ski transgene affects IIb fast muscles and skeletal structure. Dev. Dyn. 1996;205:13–23. doi: 10.1002/(SICI)1097-0177(199601)205:1<13::AID-AJA2>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- 207.Bowen RA, et al. Transgenic cattle resulting from biopsied embryos: expression of c-ski in a transgenic calf. Biol. Reprod. 1994;50:664–668. doi: 10.1095/biolreprod50.3.664. [DOI] [PubMed] [Google Scholar]
- 208.Soeta C, et al. Possible role for the c-ski gene in the proliferation of myogenic cells in regenerating skeletal muscles of rats. Dev. Growth Differ. 2001;43:155–164. doi: 10.1046/j.1440-169X.2001.00565.x. [DOI] [PubMed] [Google Scholar]
- 209.Kano K, et al. C. Skeletal muscles of transgenic mice expressing human SnoN, a homologue of c-ski. J. Reprod. Dev. 1998;44:253–260. doi: 10.1262/jrd.44.253. [DOI] [Google Scholar]
- 210.Wang Y, et al. Smad2 and Smad3 regulate chondrocyte proliferation and differentiation in the growth plate. PLoS Genet. 2016;12:e1006352. doi: 10.1371/journal.pgen.1006352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Leong GM, et al. The Ski proto-oncogene regulates body composition and suppresses lipogenesis. Int. J. Obes. 2010;34:524–536. doi: 10.1038/ijo.2009.265. [DOI] [PubMed] [Google Scholar]
- 212.Ye F, et al. Peroxisome proliferator-activated receptor gamma (PPARγ) mediates a Ski oncogene-induced shift from glycolysis to oxidative energy metabolism. J. Biol. Chem. 2011;286:40013–40024. doi: 10.1074/jbc.M111.292029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Diaz M, et al. Ski overexpression in skeletal muscle modulates genetic programs that control susceptibility to diet-induced obesity and insulin signaling. Obesity. 2012;20:2157–2167. doi: 10.1038/oby.2012.101. [DOI] [PubMed] [Google Scholar]
- 214.Huang W, et al. The proteasome inhibitor, MG132, attenuates diabetic nephropathy by inhibiting SnoN degradation in vivo and in vitro. Biomed. Res. Int. 2014;2014:684765. doi: 10.1155/2014/684765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Liu L, et al. Oxymatrine inhibits renal tubular EMT induced by high glucose via upregulation of SnoN and inhibition of TGF-β1/SMAD signaling pathway. PLoS ONE. 2016;11:e0151986. doi: 10.1371/journal.pone.0151986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Sengupta S, Jana S, Biswas S, Mandal PK, Bhattacharyya A. Cooperative involvement of NFAT and SnoN mediates transforming growth factor-β (TGF-β) induced EMT in metastatic breast cancer (MDA-MB 231) cells. Clin. Exp. Metastas. 2013;30:1019–1031. doi: 10.1007/s10585-013-9600-y. [DOI] [PubMed] [Google Scholar]
- 217.Esposito C, et al. The antifibrogenic effect of hepatocyte growth factor (HGF) on renal tubular (HK-2) cells is dependent on cell growth. Growth Factors. 2009;27:173–180. doi: 10.1080/08977190902834077. [DOI] [PubMed] [Google Scholar]
- 218.Li X, et al. The downregulation of SnoN expression in human renal proximal tubule epithelial cells under high-glucose conditions is mediated by an increase in Smurf2 expression through TGF-β1 signaling. Int. J. Mol. Med. 2016;37:415–422. doi: 10.3892/ijmm.2015.2448. [DOI] [PubMed] [Google Scholar]
- 219.Tang H, et al. MAD2B-mediated SnoN downregulation is implicated in fibroblast activation and tubulointerstitial fibrosis. Am. J. Physiol. Ren. Physiol. 2016;311:207–216. doi: 10.1152/ajprenal.00600.2015. [DOI] [PubMed] [Google Scholar]
- 220.Vázquez-Macías A, Ruiz-Mendoza AB, Fonseca-Sánchez MA, Briones-Orta MA, Macías-Silva M. Downregulation of Ski and SnoN co-repressors by anisomycin. FEBS Lett. 2005;579:3701–3706. doi: 10.1016/j.febslet.2005.05.057. [DOI] [PubMed] [Google Scholar]
- 221.Macías-Silva M, Vázquez-Victorio G, Hernández-Damián J. Anisomycin is a multifunctional drug: more than just a tool to inhibit protein synthesis. Curr. Chem. Biol. 2010;42:124–132. [Google Scholar]
- 222.Hernández-Damián J, et al. Downregulation of SnoN oncoprotein induced by antibiotics anisomycin and puromycin positively regulates transforming growth factor-β signals. Biochim. Biophys. Acta. 2013;1830:5049–5058. doi: 10.1016/j.bbagen.2013.07.006. [DOI] [PubMed] [Google Scholar]
- 223.Ehnert S, et al. Transforming growth factor β1 inhibits bone morphogenic protein (BMP)-2 and BMP-7 signaling via upregulation of Ski-related novel protein N (SnoN): possible mechanism for the failure of BMP therapy? BMC Med. 2012;10:101. doi: 10.1186/1741-7015-10-101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Pan D, Zhu Q, Conboy MJ, Conboy IM, Luo K. SnoN activates p53 directly to regulate aging and tumorigenesis. Aging Cell. 2012;11:902–911. doi: 10.1111/j.1474-9726.2012.00857.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Makino Y, et al. Repression of Smad3 by Stat3 and c-Ski/SnoN induces gefitinib resistance in lung adenocarcinoma. Biochem. Biophys. Res. Commun. 2017;484:269–277. doi: 10.1016/j.bbrc.2017.01.093. [DOI] [PubMed] [Google Scholar]
- 226.López-Novoa JM, Nieto MA. Inflammation and EMT: an alliance towards organ fibrosis and cancer progression. EMBO Mol. Med. 2009;1:303–314. doi: 10.1002/emmm.200900043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Tan EJ, Olsson AK, Moustakas A. Reprogramming during epithelial to mesenchymal transition under the control of TGFβ. Cell Adh. Migr. 2015;9:233–246. doi: 10.4161/19336918.2014.983794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Moustakas A, Heldin CH. Mechanisms of TGFβ-induced epithelial-mesenchymal transition. J. Clin. Med. 2016;5:63. doi: 10.3390/jcm5070063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Macías-Silva M, Li W, Leu JI, Crissey MA, Taub R. Up-regulated transcriptional repressors SnoN and Ski bind SMAD proteins to antagonize transforming growth factor-β signals during liver regeneration. J. Biol. Chem. 2002;277:28483–28490. doi: 10.1074/jbc.M202403200. [DOI] [PubMed] [Google Scholar]
- 230.Zhang Q, Zhou J, Ge H, Cheng B. Tgif1 and SnoN modified chondrocytes or stem cells for tendon-bone insertion regeneration. Med. Hypotheses. 2013;81:163–166. doi: 10.1016/j.mehy.2013.05.017. [DOI] [PubMed] [Google Scholar]
- 231.Liu X, et al. Expression and possible mechanism of c-ski, a novel tissue repair-related gene during normal and radiation-impaired wound healing. Wound Repair Regen. 2006;14:162–171. doi: 10.1111/j.1743-6109.2006.00106.x. [DOI] [PubMed] [Google Scholar]
- 232.Peng Y, et al. Comparative evaluation of the wound-healing potency of recombinant bFGF and ski gene therapy in rats. Growth Factors. 2016;34:119–127. doi: 10.1080/08977194.2016.1200570. [DOI] [PubMed] [Google Scholar]
- 233.Li J, et al. c-Ski inhibits the proliferation of vascular smooth muscle cells via suppressing SMAD3 signaling but stimulating p38 pathway. Cell Signal. 2013;25:159–167. doi: 10.1016/j.cellsig.2012.09.001. [DOI] [PubMed] [Google Scholar]
- 234.Yang J, Zhang X, Li Y, Liu Y. Downregulation of SMAD transcriptional corepressors SnoN and Ski in the fibrotic kidney: an amplification mechanism for TGF-β1 signaling. J. Am. Soc. Nephrol. 2003;14:3167–3177. doi: 10.1097/01.ASN.0000099373.33259.B2. [DOI] [PubMed] [Google Scholar]
- 235.Liu R, et al. SnoN as a key regulator of the high glucose-induced epithelial-mesenchymal transition in cells of the proximal tubule. Kidney Blood Press Res. 2012;35:517–528. doi: 10.1159/000339172. [DOI] [PubMed] [Google Scholar]
- 236.Tan R, et al. Downregulation of SnoN expression in obstructive nephropathy is mediated by an enhanced ubiquitin-dependent degradation. J. Am. Soc. Nephrol. 2006;17:2781–2791. doi: 10.1681/ASN.2005101055. [DOI] [PubMed] [Google Scholar]
- 237.Xu Z, Diao Z, Liu R, Liu W. Molecular mechanism of smurf2 in regulating the expression of SnoN in diabetic nephropathy. Mol. Med. Rep. 2017;15:2560–2566. doi: 10.3892/mmr.2017.6307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Liu L, et al. SnoN upregulation ameliorates renal fibrosis in diabetic nephropathy. PLoS ONE. 2017;12:e0174471. doi: 10.1371/journal.pone.0174471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Chen J, et al. Docosahexaenoic acid (DHA) ameliorates paraquat-induced pulmonary fibrosis in rats possibly through up-regulation of SMAD 7 and SnoN. Food Chem. Toxicol. 2013;57:330–337. doi: 10.1016/j.fct.2013.03.045. [DOI] [PubMed] [Google Scholar]
- 240.Wang J, et al. The role of c-SKI in regulation of TGF-β-induced human cardiac fibroblast proliferation and ECM protein expression. J. Cell Biochem. 2017;118:1911–1920. doi: 10.1002/jcb.25935. [DOI] [PubMed] [Google Scholar]
- 241.Zeglinski MR, et al. Chronic expression of Ski induces apoptosis and represses autophagy in cardiac myofibroblasts. Biochim. Biophys. Acta. 2016;1863:1261–1268. doi: 10.1016/j.bbamcr.2016.03.027. [DOI] [PubMed] [Google Scholar]
- 242.Kishore R, et al. Bone marrow progenitor cell therapy-mediated paracrine regulation of cardiac miRNA-155 modulates fibrotic response in diabetic hearts. PLoS ONE. 2013;8:e60161. doi: 10.1371/journal.pone.0060161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Cunnington RH, et al. The Ski-Zeb2-Meox2 pathway provides a novel mechanism for regulation of the cardiac myofibroblast phenotype. J. Cell Sci. 2014;127:40–49. doi: 10.1242/jcs.126722. [DOI] [PubMed] [Google Scholar]
- 244.Reyes-Gordillo K, et al. Mechanisms of action of acetaldehyde in the up-regulation of the human alpha2(I) collagen gene in hepatic stellate cells: key roles of Ski, SMAD3, SMAD4, and SMAD7. Am. J. Pathol. 2014;184:1458–1467. doi: 10.1016/j.ajpath.2014.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Jinnin M, Ihn H, Mimura Y, Asano Y, Tamaki K. Involvement of the constitutive complex formation of c-Ski/SnoN with SMADs in the impaired negative feedback regulation of transforming growth factor β signaling in scleroderma fibroblasts. Arthr. Rheum. 2007;56:1694–1705. doi: 10.1002/art.22588. [DOI] [PubMed] [Google Scholar]
- 246.Li P, et al. Ski, a modulator of wound healing and scar formation in the rat skin and rabbit ear. J. Pathol. 2011;223:659–671. doi: 10.1002/path.2831. [DOI] [PubMed] [Google Scholar]
- 247.Obenauf AC, Massagué J. Surviving at a distance: organ specific metastasis. Trends Cancer. 2015;1:76–91. doi: 10.1016/j.trecan.2015.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.David CJ, et al. TGF-β Tumor Suppression through a Lethal EMT. Cell. 2016;164:1015–1030. doi: 10.1016/j.cell.2016.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Nieto MA, Huang RY, Jackson RA, Thiery JP. Emt: 2016. Cell. 2016;166:21–45. doi: 10.1016/j.cell.2016.06.028. [DOI] [PubMed] [Google Scholar]
- 250.Lebrun JJ. The dual role of TGFβ in human cancer: from tumor suppression to cancer metastasis. ISRN Mol. Biol. 2012;2012:381428. doi: 10.5402/2012/381428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Colmenares C, Sutrave P, Hughes SH, Stavnezer E. Activation of the c-ski oncogene by overexpression. J. Virol. 1991;65:4929–4935. doi: 10.1128/jvi.65.9.4929-4935.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Zheng G, Teumer J, Colmenares C, Richmond C, Stavnezer E. Identification of a core functional and structural domain of the v-Ski oncoprotein responsible for both transformation and myogenesis. Oncogene. 1997;15:459–471. doi: 10.1038/sj.onc.1201205. [DOI] [PubMed] [Google Scholar]
- 253.Fumagalli S, Doneda L, Nomura N, Larizza L. Expression of the c-ski proto-oncogene in human melanoma cell lines. Melanoma Res. 1993;3:23–27. doi: 10.1097/00008390-199304000-00004. [DOI] [PubMed] [Google Scholar]
- 254.Imoto I, et al. SNO is a probable target for gene amplification at 3q26 in squamous-cell carcinomas of the esophagus. Biochem. Biophys. Res. Commun. 2001;286:559–565. doi: 10.1006/bbrc.2001.5428. [DOI] [PubMed] [Google Scholar]
- 255.Zhang F, et al. Ski-related novel protein N (SnoN), a negative controller of transforming growth factor-β signaling, is a prognostic marker in estrogen receptor-positive breast carcinomas. Cancer Res. 2003;63:5005–5010. [PubMed] [Google Scholar]
- 256.Buess M, et al. Amplification of SKI is a prognostic marker in early colorectal cancer. Neoplasia. 2004;6:207–212. doi: 10.1593/neo.03442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Fukuchi M, et al. Increased expression of c-Ski as a co-repressor in transforming growth factor-β signaling correlates with progression of esophageal squamous cell carcinoma. Int. J. Cancer. 2004;108:818–824. doi: 10.1002/ijc.11651. [DOI] [PubMed] [Google Scholar]
- 258.Kronenwett R, et al. Distinct molecular phenotype of malignant CD34(+) hematopoietic stem and progenitor cells in chronic myelogenous leukemia. Oncogene. 2005;24:5313–5324. doi: 10.1038/sj.onc.1208596. [DOI] [PubMed] [Google Scholar]
- 259.Poser I, Rothhammer T, Dooley S, Weiskirchen R, Bosserhoff AK. Characterization of Sno expression in malignant melanoma. Int. J. Oncol. 2005;26:1411–1417. [PubMed] [Google Scholar]
- 260.Edmiston JS, Yeudall WA, Chung TD, Lebman DA. Inability of transforming growth factor-β to cause SnoN degradation leads to resistance to Transforming Growth Factor-β-induced growth arrest in esophageal cancer cells. Cancer Res. 2005;65:4782–4788. doi: 10.1158/0008-5472.CAN-04-4354. [DOI] [PubMed] [Google Scholar]
- 261.Heider TR, Lyman S, Schoonhoven R, Behrns KE. Ski promotes tumor growth through abrogation of Transforming Growth Factor-β signaling in pancreatic cancer. Ann. Surg. 2007;246:61–68. doi: 10.1097/SLA.0b013e318070cafa. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Bravou V, et al. TGF-β repressors SnoN and Ski are implicated in human colorectal carcinogenesis. Cell. Oncol. 2009;31:41–51. doi: 10.3233/CLO-2009-0460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Boone B, Haspeslagh M, Brochez L. Clinical significance of the expression of c-Ski and SnoN, possible mediators in TGF-β resistance in primary cutaneous melanoma. J. Dermatol. Sci. 2009;53:26–33. doi: 10.1016/j.jdermsci.2008.07.010. [DOI] [PubMed] [Google Scholar]
- 264.Chen D, et al. SKI knockdown inhibits human melanoma tumor growth in vivo. Pigment. Cell. Melanoma Res. 2009;22:761–772. doi: 10.1111/j.1755-148X.2009.00603.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Kiyono K, et al. c-Ski overexpression promotes tumor growth and angiogenesis through inhibition of transforming growth factor-β signaling in diffuse-type gastric carcinoma. Cancer Sci. 2009;100:1809–1816. doi: 10.1111/j.1349-7006.2009.01248.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.O TM, et al. Differential expression of SKI oncogene protein in hemangiomas. Otolaryngol. Head Neck Surg. 2009;141:213–218. doi: 10.1016/j.otohns.2009.05.005. [DOI] [PubMed] [Google Scholar]
- 267.Takahata M, et al. SKI and MEL1 cooperate to inhibit transforming growth factor-beta signal in gastric cancer cells. J. Biol. Chem. 2009;284:3334–3344. doi: 10.1074/jbc.M808989200. [DOI] [PubMed] [Google Scholar]
- 268.Wang P, et al. Dual role of Ski in pancreatic cancer cells: tumor-promoting versus metastasis-suppressive function. Carcinogenesis. 2009;30:1497–1506. doi: 10.1093/carcin/bgp154. [DOI] [PubMed] [Google Scholar]
- 269.Nakao T, et al. Expression of thrombospondin-1 and Ski are prognostic factors in advanced gastric cancer. Int. J. Clin. Oncol. 2011;16:145–152. doi: 10.1007/s10147-010-0147-5. [DOI] [PubMed] [Google Scholar]
- 270.Bravou V, et al. Transforming growth factor β repressor, SnoN, is overexpressed in human gastrointestinal stromal tumors. J. Buon. 2012;17:684–690. [PubMed] [Google Scholar]
- 271.Chen Y, Pirisi L, Creek KE. Ski protein levels increase during in vitro progression of HPV16-immortalized human keratinocytes and in cervical cancer. Virology. 2013;444:100–108. doi: 10.1016/j.virol.2013.05.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272.Liu C, et al. The influence of SnoN gene silencing by siRNA on the cell proliferation and apoptosis of human pancreatic cancer cells. Diagn. Pathol. 2015;10:30. doi: 10.1186/s13000-015-0267-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273.Shinagawa T, et al. Increased susceptibility to tumorigenesis of ski-deficient heterozygous mice. Oncogene. 2001;20:8100–8108. doi: 10.1038/sj.onc.1204987. [DOI] [PubMed] [Google Scholar]
- 274.Villanacci V, et al. Ski/SnoN expression in the sequence metaplasia-dysplasia-adenocarcinoma of Barrett’s esophagus. Hum. Pathol. 2008;39:403–409. doi: 10.1016/j.humpath.2007.07.009. [DOI] [PubMed] [Google Scholar]
- 275.Chia JA, et al. SnoN expression is differently regulated in microsatellite unstable compared with microsatellite stable colorectal cancers. BMC Cancer. 2006;6:252. doi: 10.1186/1471-2407-6-252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276.Hagerstrand D, et al. Systematic interrogation of 3q26 identifies TLOC1 and SKIL as cancer drivers. Cancer Discov. 2013;3:1044–1057. doi: 10.1158/2159-8290.CD-12-0592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277.Sheu JJC, et al. Chromosome 3p12.3-p14.2 and 3q26.2-q26.32 are genomic markers for prognosis of advanced nasopharyngeal carcinoma. Cancer Epidemiol. Biomark. Prev. 2009;18:2709–2716. doi: 10.1158/1055-9965.EPI-09-0349. [DOI] [PubMed] [Google Scholar]
- 278.Nanjundan M, et al. Amplification of MDS1/EVI1 and EVI1, located in the 3q26.2 amplicon, is associated with favorable patient prognosis in ovarian cancer. Cancer Res. 2007;67:3074–3084. doi: 10.1158/0008-5472.CAN-06-2366. [DOI] [PubMed] [Google Scholar]
- 279.Nanjundan M, et al. Overexpression of SnoN/SkiL, amplified at the 3q26.2 locus, in ovarian cancers: a role in ovarian pathogenesis. Mol. Oncol. 2008;2:164–181. doi: 10.1016/j.molonc.2008.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280.Yang H, et al. Ski prevents TGF-β-induced EMT and cell invasion by repressing SMAD-dependent signaling in non-small cell lung cancer. Oncol. Rep. 2015;34:87–94. doi: 10.3892/or.2015.3961. [DOI] [PubMed] [Google Scholar]
- 281.Song L, et al. Ski modulate the characteristics of pancreatic cancer stem cells via regulating sonic hedgehog signaling pathway. Tumor Biol. 2016;37:16115–16125. doi: 10.1007/s13277-016-5461-8. [DOI] [PubMed] [Google Scholar]
- 282.Ritter M, et al. A. Inhibition of retinoic acid receptor signaling by Ski in acute myeloid leukemia. Leukemia. 2006;20:437–443. doi: 10.1038/sj.leu.2404093. [DOI] [PubMed] [Google Scholar]
- 283.Wang L, et al. c-Ski activates cancer-associated fibroblasts to regulate breast cancer cell invasion. Mol. Oncol. 2013;7:1116–1128. doi: 10.1016/j.molonc.2013.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 284.Schweighofer CD, et al. A two-gene signature, SKI and SLAMF1, predicts time-to-treatment in previously untreated patients with chronic lymphocytic leukemia. PLoS ONE. 2011;6:e28277. doi: 10.1371/journal.pone.0028277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 285.Theohari I, et al. Differential effect of the expression of TGF-β pathway inhibitors, SMAD-7 and Ski, on invasive breast carcinomas: relation to biologic behavior. APMIS. 2012;120:92–100. doi: 10.1111/j.1600-0463.2011.02814.x. [DOI] [PubMed] [Google Scholar]
- 286.Gallo-Oller G, et al. P144, a transforming growth factor beta inhibitor peptide, generates antitumoral effects and modifies SMAD7 and SKI levels in human glioblastoma cell lines. Cancer Lett. 2016;381:67–75. doi: 10.1016/j.canlet.2016.07.029. [DOI] [PubMed] [Google Scholar]
- 287.Zhu Q, et al. Dual role of SnoN in mammalian tumorigenesis. Mol. Cell Biol. 2007;27:324–339. doi: 10.1128/MCB.01394-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 288.Chanda A, et al. Identification of the SUMO E3 ligase PIAS1 as a potential survival biomarker in breast cancer. PLoS ONE. 2017;12:e0177639. doi: 10.1371/journal.pone.0177639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 289.Zhang X, Egawa K, Xie Y, Ihn H. The expression of SnoN in normal human skin and cutaneous keratinous neoplasms. Int. J. Dermatol. 2009;48:579–583. doi: 10.1111/j.1365-4632.2009.03685.x. [DOI] [PubMed] [Google Scholar]
- 290.Zhu X, et al. 576 kb deletion in 1p36.33-p36.32 containing SKI is associated with limb malformation, congenital heart disease and epilepsy. Gene. 2013;528:352–355. doi: 10.1016/j.gene.2013.07.024. [DOI] [PubMed] [Google Scholar]
- 291.Schepers D, et al. The SMAD-binding domain of SKI: a hotspot for de novo mutations causing Shprintzen-Goldberg syndrome. Eur. J. Hum. Genet. 2015;23:224–228. doi: 10.1038/ejhg.2014.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 292.Carmignac V, et al. In-frame nutations in exon 1 of SKI cause dominant Shpritzen-Goldberg syndrome. Am. J. Hum. Genet. 2012;91:950–957. doi: 10.1016/j.ajhg.2012.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 293.Doyle AJ, et al. Mutations in the TGF-β repressor SKI cause Shprintzen-Goldberg syndrome with aortic aneurysm. Nat. Genet. 2012;44:1249–1254. doi: 10.1038/ng.2421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 294.Colak S, ten Dijke P. Targeting TGF-β signaling in cancer. Trends Cancer. 2016;3:56–71. doi: 10.1016/j.trecan.2016.11.008. [DOI] [PubMed] [Google Scholar]
- 295.Inoue Y, Imamura T. Regulation of TGF-β family signaling by E3 ubiquitin ligases. Cancer Sci. 2008;99:2107–2112. doi: 10.1111/j.1349-7006.2008.00925.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 296.Wang W, Liu C, Wang Y, Cao L. Effects of the downregulation of SnoN expression on HepG2 cell proliferation and apoptosis. Mol. Med. Rep. 2013;7:1324–1328. doi: 10.3892/mmr.2013.1340. [DOI] [PubMed] [Google Scholar]
- 297.Vázquez-Victorio, G., Rosales-Alvarez, R. E., Ríos-López, D. G., Tecalco-Cruz, A. C., Macías-Silva, M. In Advances in health and disease, Vol. 1 (ed Duncan, L. T.) (Nova Science Publishers: New York, USA, NY, 2017) pp 63–135.