Abstract
Background
Lactulose is the first-line drug for hepatic encephalopathy (HE), but its acceptance widely differs between Western and Eastern studies. Patient preference for lactulose between different parts of the world has not been examined systematically.
Aim
To define the preferences and reasons behind acceptance of lactulose in patients from USA and India.
Methods
A discrete-choice questionnaire with six hypothetical scenarios was constructed. Situations 1–3 studied preference for lactulose vs no-lactulose, while 4–6 studied preference for high-dose vs low-dose lactulose varying the overt HE prevention at 6 months and adverse event rates in each situation. This was administered to outpatient cirrhotics without prior/current experience with lactulose after dedicated education.
Results
100 patients (50 Indian, 50 USA) with similar MELD scores were included. A significantly higher proportion of Indian respondents agreed to lactulose in all situations compared to Americans. While their acceptance of lactulose decreased in the situation with the least difference in overt HE prevention, it was consistently higher than Americans. In the high-dose vs low-dose scenario, the relative proportion of American respondents accepting high-dose increased with the higher presented protection against overt HE. On the other hand, Indian respondents remained largely consistent with low-dose lactulose option.
Conclusions
There are significant variations in the acceptance of lactulose in Indian and American populations. The acceptance increases with a more favorable perceived benefit/risk profile, which is strongly influenced by socio-cultural factors. These results have important implications when designing, comparing and interpreting HE trials from different parts of the world.
Abbreviations: CHE, covert hepatic encephalopathy; DCE, discrete choice experiments; DCQ, discrete choice questionnaire; HE, hepatic encephalopathy; OHE, overt hepatic encephalopathy
Keywords: cirrhosis, discrete choice questionnaire, adverse events, patient-reported outcomes
Hepatic encephalopathy (HE) is a common and disabling neuropsychiatric manifestation of cirrhosis.1 Even its mildest forms i.e. covert HE (CHE) is associated with higher progression to overt HE (OHE), poor quality of life, driving difficulties, and poor workplace productivity.2, 3, 4 Moreover, each episode of OHE is associated with an additive/cumulative effect on cognitive decline and can increase mortality independent of other cirrhosis complications.5, 6
Non-absorbable disaccharides like lactulose are the cornerstone of the treatment and prophylaxis of HE across the spectrum for decades.7 Lactulose is indicated for the treatment of an acute OHE episode and the secondary prophylaxis of OHE.1 However, recommendations for treatment of CHE and for prevention of first episode are less clear.1 Lactulose is metabolized by colonic lactose fermenters, producing short chain fatty acids, colonic acidification and faster intestinal transit. This can lead to diarrhea, bloating, and abdominal pain. Though lactulose therapy has been shown to improve CHE, health-related quality of life and prevent the first episode of OHE, its adverse effect profile limits its utility in clinical practice.8, 9 Between 12 and 28% patients on lactulose therapy discontinue due to adverse effects, which varies between Western studies and those from India.10, 11, 12 This range of patient discontinuation of lactulose may stem from socio-cultural differences and perceptions related to bowel movement frequency. Given the importance of continued adherence and importance of cultural factors in medical effectiveness, it is relevant to define reasons behind potential non-adherence systematically, as called for in recent publications.1, 13 Discrete choice experiments (DCE) are a widely used quantitative technique for eliciting preferences that can be used in the absence of revealed preference data.14 This is based on Lancaster's economic theory of value and involves asking subjects to define their preferences designed around hypothetical alternative scenarios.
Our aim was to elucidate the preference and reasons behind acceptance of lactulose in cirrhotic patients from USA and India who have never experienced overt HE using a discrete choice questionnaire (DCQ).
Materials and Methods
We designed a cohort study with risk factor being two different cultural backgrounds and outcomes being the responses to the use of lactulose. We then presented educational materials and questionnaires that changed according to their risk/benefit of lactulose therapy to elucidate a potential dose response relationship of this preference between patients from India and USA.
We prospectively recruited patients with cirrhosis between the ages of 21 and 65 years from two institutions, Virginia Commonwealth University Medical Center and PGIMER after informed consent. Cirrhosis was diagnosed using liver biopsy, liver elastography or using varices/radiologic features of cirrhosis in patients with chronic liver disease. Subjects were excluded if: (1) they never had an episode of HE and/or were ever treated, (2) were currently on therapy for overt or covert HE, (3) had an unclear diagnosis of cirrhosis, (4) were actively abusing alcohol or illicit drugs, (5) were already on lactulose for constipation, (6) suffered from diseases in which lactulose would not ordinarily be prescribed due to pre-existing gastroenterological issues (Inflammatory bowel disease, irritable bowel syndrome-diarrhea predominant, other diarrheal illnesses) or (7) were unable to consent or follow the directions of the DCQ.
After informed consent, all patients were provided a standardized patient education presentation regarding HE and lactulose through printouts from the Uptodate website.15, 16 After their questions regarding HE and lactulose were answered, a gambler's choice DCQ was then administered with the scenarios outlined in Table 1. This questionnaire was constructed under neuropsychological guidance (JBW). The DCQ (Appendix) focused on six hypothetical situations with the ultimate goal of using lactulose to prevent the first episode of overt HE.
Table 1.
Discrete Choice Questionnaire Situations Presented to the Subjects.
| Presented Situation number |
Theoretical OHE development rates |
Theoretical rates of adverse events with lactulose |
|||
|---|---|---|---|---|---|
| With lactulose | Without lactulose | Specific issues | With lactulose | Without lactulose | |
| 1 | 8–12% | 20–30% | Daily bowel movements | 2–3 | 1 or <1 |
| 2 | 8–12% | 30–40% | Bloating and gas | 40–50% | None |
| 3 | 8–12% | 10–20% | Unpleasant taste | 10–20% | None |
| Presented Situation number |
Theoretical OHE development rates |
Theoretical rates of adverse events with lactulose |
|||
|---|---|---|---|---|---|
| With low-dose lactulose | With high-dose lactulose | Specific issues | With low-dose lactulose | With high-dose lactulose | |
| 4 | 8–12% | 8–20% | Daily bowel movements | 2–3 | 4–5 |
| 5 | 10–20% | 5–10% | Bloating and gas | 40–50% | 50–70% |
| 6 | 10–20% | 0–5% | Unpleasant taste | 10–20% | 20–30% |
OHE: overt hepatic encephalopathy. These were the decision points presented to the American and Indian cohorts at each situation for them to make their choices for accepting lactulose or not and if accepting lactulose, then high-dose or low-dose.
Situations 1–3 studied lactulose vs. no-treatment, while situations 4–6 studied the comparison between high vs. low-dose lactulose. Assumptions were made as to the development of overt HE in all of these situations using published literature and the rates of adverse events (diarrhea, gas, bloating) with lactulose were correspondingly changed between the high and low-dose states. Specific reasons for their decisions were presented and respondents were asked to rank the reason behind their decisions. Those who declined lactulose in situations 1–3 were not administered the DCQ for situations 4–6. Comparisons were made between Indian and American respondents at each stage of the questionnaire process using Chi-square tests, while demographic variables were compared using parametric and non-parametric tests as applicable. The sample size was based on prior studies of acceptance of lactulose in clinical trials from India that was >90%, and that from a real-life experience in the USA after HE development that was around 50–60%.8, 11 We would require 32 subjects in each group for 80% power and α = 0.05. To extend this into patients who had never had HE before, we increased the sample size to 50 per group.
Results
We recruited 100 consecutive cirrhotic patients, 50 each from India and USA, without prior OHE. The severity of liver disease and etiology pattern was similar between groups. Patients in the Indian cohort were younger, had lower years of formal education, and more likely to be men (Table 2).
Table 2.
Demographics.
| India (n = 50) | USA (n = 50) | P value | |
|---|---|---|---|
| Age (mean ± SD) | 50.3 ± 10.5 | 60.0 ± 6.2 | <0.0001 |
| Gender (male) | 45 | 33 | 0.004 |
| Education (years) | 9 ± 4 | 14 ± 3 | <0.0001 |
| MELD (mean ± SD) | 11.5 ± 5.1 | 10.1 ± 3.5 | 0.12 |
| Etiology of cirrhosis | |||
| Alcohol | 20 | 10 | 0.17 |
| Viral hepatitis | 17 | 23 | |
| NASH | 8 | 11 | |
| Other | 5 | 7 | |
Choice Between Lactulose and No Lactulose (Situations 1–3)
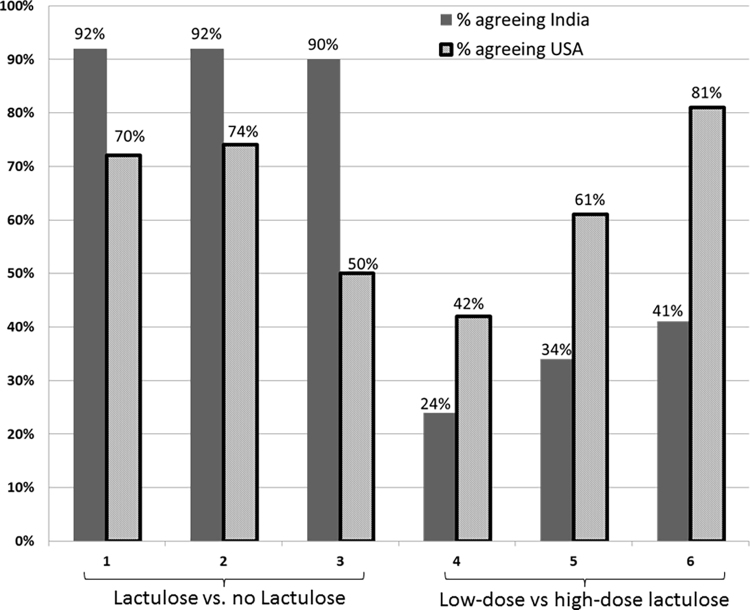
When given a choice between accepting lactulose or rejecting prophylaxis for prevention of the first HE episode, a significantly higher proportion of Indian respondents agreed to lactulose (Figure 1). This pattern was uniform across the three hypothetical risk-situations. Americans were more likely to decline lactulose prophylaxis. The difference between the populations was the most when presented with situation 3, where the difference in risk of developing OHE between the two choices was the lowest and the chances for adverse events were presented as the greatest between lactulose and no-lactulose.
Figure 1.
Acceptance of lactulose between the cohorts. Y-axis shows percentage of patients in both cohorts who accepted lactulose (situations 1–3) and high-dose lactulose (situations 4–6). X-axis demonstrates the specific situations. A significantly higher proportion of Indian respondents accepted lactulose, however the proportion of Americans accepting high-dose lactulose increased as the risk/benefit ratio became more favorable. Solid bars: American respondents, striped bars: Indian respondents.
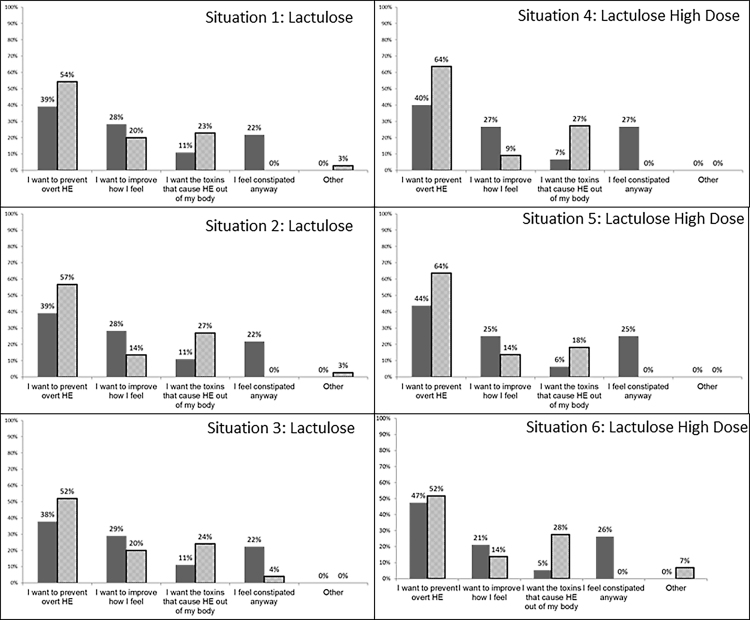
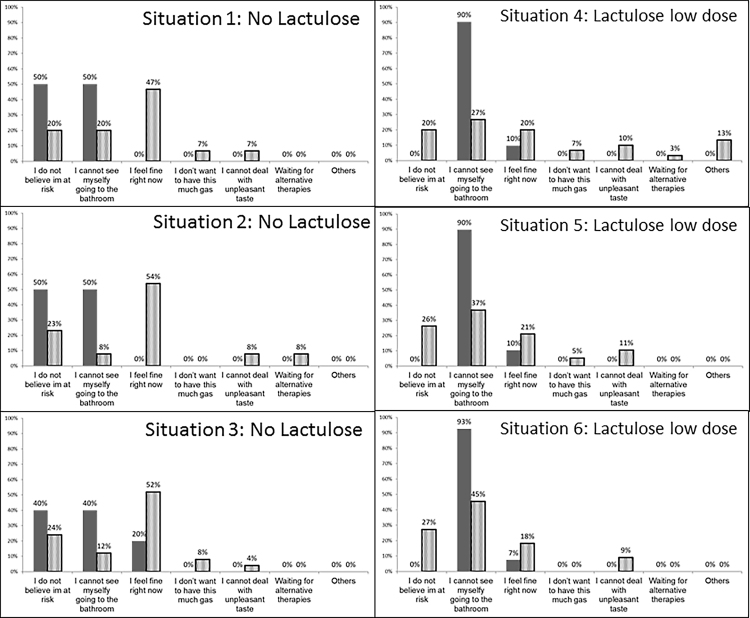
The main reasons for choice of lactulose vs no lactulose in Indian and American populations were similar across situations 1–3 (Figure 2, Figure 3).
Figure 2.
Reasons for acceptance of lactulose in each situation. The reasons for accepting lactulose in situations 1–3 and accepting high-dose lactulose in situations 4–6 is shown in this figure. Data is shown as the percentage of patients who accepted lactulose in situations 1–3 or high-dose lactulose in situations 4–6. Solid bars: American respondents, striped bars: Indian respondents.
Figure 3.
Reasons for not accepting of lactulose in each situation. The reasons for not accepting lactulose in situations 1–3 and accepting high-dose lactulose in situations 4–6 is shown in this figure. Data is shown as the percentage of patients who did not accept lactulose in situations 1–3 or high-dose lactulose in situations 4–6; less than 10% of Indian respondents rejected lactulose. Solid bars: American respondents, striped bars: Indian respondents.
Choice Between Low-dose and High-dose Lactulose (Situations 4–6)
When given a choice between high-dose vs low-dose lactulose for preventing the first HE episode, the proportion of American respondents accepting higher-dose lactulose was significantly higher than Indians. This number further increased with the higher presented protection against OHE (Figure 1). Indian respondents were largely consistent with low-dose lactulose option. The leading reasons to accept higher dose were “to prevent OHE” 50% and “improve how I feel” 20%, while to reject high-dose lactulose were “don’t want to have diarrhea” 60% and “don’t believe I am at risk” 20% and “I feel fine” 16%.
The main reasons for choice of high dose vs low dose lactulose in Indian and American populations were similar across situations 4–6 (Figure 2, Figure 3).
Discussion
Our results demonstrate that the two separate populations of cirrhotic patients, who were not yet exposed to lactulose, had very different responses to prescription of this medication. Specifically, the cohort in India was more accepting of lactulose compared to the US-based group. Moreover, the acceptance rates varied with changes in the scenarios presented between the groups, with the US-based cohort more likely to change their acceptance based on the assumptions provided.
These two cohorts were chosen because of the published differences in adherence to lactulose, which can affect adoption of trials published in India into clinical practice in the USA and vice-versa.10, 11, 17, 18, 19, 20 Lactulose use involves the patients and caregivers titrating dose based on alteration of mental status in conjunction with bowel movement frequency.1 This requires constant feedback and education between the clinical team and caregivers. If not properly carried out, this can result in HE episodes either due Lactulose overuse or underuse.11 Given its success in preventing and treating HE, and its relatively low cost compared to alternatives such as rifaximin, it is often considered first-line therapy.1 Therefore, factors that can affect its adherence, especially in covert HE, are important to determine.
The existing evidence on lactulose compliance is quite heterogeneous between Western and Eastern medical literature. Studies from the USA and Sweden emphasize lactulose discontinuation due to diarrhea severity, overt HE precipitation and/or deterioration in quality of life.11, 12, 20 Conversely, in various Indian trials, while diarrhea has been associated with lactulose in almost a quarter of the cases, it was reported to improve with dose-reduction alone, and none of the patients required cessation of drug9, 10, 18 and lactulose improved quality of life in Indian patients.8 Therefore, reconciliation of these extremes in patient acceptance rates requires study of the medical and socio-cultural issues that underlies these differences.
Most patients in either cohort, who wanted lactulose therapy, cited the prevention of overt HE and feeling better as the major reason behind their decision. This was also similar for those who accepted higher doses of lactulose. These are important considerations because the DCQ was administered after a dedicated educational session about HE and lactulose therapy, and shows that time spent on education helps patients make informed decisions regardless of social cultural differences.
However, it was also clear that a significantly higher proportion of Indian patients accepted lactulose compared to the US-cohort. Apart from knowledge imparted from the clinical interview, socio-cultural factors, including formal educational status, can profoundly affect acceptance of medications and also the patient-doctor relationship.21 This was evident in our comparison of the cohorts. When specific reasons for acceptance of lactulose were studied, the Indian cohort believed that it would help their baseline constipation, which was not the case in most US-based respondents. This could partly be due to the perception of normal stool frequency for each population. A study done in healthy, asymptomatic subjects from eastern India found the median frequency to be as high as 14 stools per week.22 Such a frequency may be perceived as diarrhea in many western countries and is indeed much higher than what is found in the US population.23 This may be a strong reason for relative ease in accepting lactulose treatment in the Indian population. Indians would therefore likely perceive constipation as a greater change in their quality of life compared to any resultant diarrhea from lactulose.
Socio-cultural differences may also affect the doctor–patient relationship.24 In Western countries such as the USA, most healthcare delivery puts the patient in the center of the decision-making process after counseling and education regarding the alternatives. This patient-centered model of healthcare was reflected by the ability of the US-based cohort to modify their acceptance of lactulose when the theoretical the risk-benefit ratio tilted toward benefit, even in the high-lactulose level. In addition, the reasons behind rejection of lactulose were largely based on their personal risk perception for overt HE development and them “feeling fine”. This indicates a population that is largely used to making their decisions regarding their clinical outcomes and medication strategies based on processing available data. On the other hand, it is likely that a doctor-centric model, where the patients follow the clinicians’ recommendations regarding therapy compounded by a relatively lower formal educational status, results in a potentially paternalistic healthcare delivery, was seen in the Indian cohort.24, 25 This is a socio-cultural phenomenon, which could explain the very few Indian respondents who refused lactulose and their constant acceptance of high-dose vs low-dose lactulose despite changes in the theoretical risk-benefit ratio.
Recent systematic reviews have determined lactulose to be an efficacious therapy for HE but most recent trials included have been from Indian centers.7 While this certainly reflects practice there, these positive results may not be as applicable to centers in Western countries. The factors demonstrated in our study could give insight into the difference between efficacy and effectiveness of lactulose in Indian versus Western studies. As the scope of HE goes beyond inpatient and secondary prophylaxis into the primary prophylaxis or CHE treatment realm, these results have a substantial bearing on the formulation of prophylaxis guidelines. Instead of a blanket approach based on a single population group, it sets the stage to allow a more liberal use of lactulose prophylaxis in populations more likely to accept it and a lower threshold for using alternatives or short-term trials in other populations that would have an a priori high likelihood of rejecting the therapy. However, while the results are striking, these remain theoretical opinions of a selected patient group that could change once they are actually faced with overt HE development. We avoided including patients already on lactulose and rifaximin because their experience of the drugs, the HE severity, the precipitating factors and its impact of their daily life could potentially cloud their opinion. However future studies are required in already experienced patients between Eastern and Western countries. Given the excellent adherence to rifaximin in most patients, we focused on lactulose alone, which because of the cost-differential, is used as the de facto first line therapy for HE.
We conclude that there are significant variations in the acceptance of lactulose between Indian and American cirrhotic patients for the prevention of the first episode of overt HE. This is likely due to socio-cultural differences centered on bowel movement frequency and the doctor–patient relationship. These results have important implications when designing, comparing and interpreting HE trials from different parts of the world.
Financial Support
This was partly supported by VA Merit Review Grant number I0CX001076 to JSB.
Authors’ Declaration of Personal Interests
JSB has served as a consultant and advisory board member for Abbott, Norgine, Alfa-Wasserman and Valent, and has received research funding from Valeant, which was not for this project.
Writing and data analysis was performed by the authors.
Authorship Statement
-
(i)
Guarantor of the article: JSB is the guarantor of the article.
-
(ii)
Specific author contributions: JSB and RKD conceptualized the study, JBW helped with the questionnaire creation, SR, AF, MBW, CA, DG administered the questionnaires, SR, JSB and AF were involved in the data analysis. All authors helped with drafting and editing the manuscript.
-
(iii)
All authors approved the final version of the manuscript.
Conflicts of Interest
The authors have none to declare.
Footnotes
Supplementary data associated with this article can be found, in the online version, at doi:10.1016/j.jceh.2017.11.010.
Appendix A. Supplementary data
The following are the supplementary data to this article:
References
- 1.Vilstrup H., Amodio P., Bajaj J. Hepatic encephalopathy in chronic liver disease: 2014 Practice Guideline by the American Association for the Study of Liver Diseases and the European Association for the Study of the Liver. Hepatology. 2014;60(2):715–735. doi: 10.1002/hep.27210. [DOI] [PubMed] [Google Scholar]
- 2.Ampuero J., Simon M., Montoliu C. Minimal hepatic encephalopathy and critical flicker frequency are associated with survival of patients with cirrhosis. Gastroenterology. 2015;149(6):1483–1489. doi: 10.1053/j.gastro.2015.07.067. [DOI] [PubMed] [Google Scholar]
- 3.Bajaj J.S., Cordoba J., Mullen K.D. Review article: the design of clinical trials in hepatic encephalopathy – an International Society for Hepatic Encephalopathy and Nitrogen Metabolism (ISHEN) consensus statement. Aliment Pharmacol Ther. 2011;33(7):739–747. doi: 10.1111/j.1365-2036.2011.04590.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Patidar K.R., Thacker L.R., Wade J.B. Covert hepatic encephalopathy is independently associated with poor survival and increased risk of hospitalization. Am J Gastroenterol. 2014;109(11):1757–1763. doi: 10.1038/ajg.2014.264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Riggio O., Ridola L., Pasquale C. Evidence of persistent cognitive impairment after resolution of overt hepatic encephalopathy. Clin Gastroenterol Hepatol. 2011;9(2):181–183. doi: 10.1016/j.cgh.2010.10.002. [DOI] [PubMed] [Google Scholar]
- 6.Bajaj J.S., Schubert C.M., Heuman D.M. Persistence of cognitive impairment after resolution of overt hepatic encephalopathy. Gastroenterology. 2010;138(7):2332–2340. doi: 10.1053/j.gastro.2010.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gluud L.L., Vilstrup H., Morgan M.Y. Nonabsorbable disaccharides for hepatic encephalopathy: a systematic review and meta-analysis. Hepatology. 2016;64(3):908–922. doi: 10.1002/hep.28598. [DOI] [PubMed] [Google Scholar]
- 8.Prasad S., Dhiman R.K., Duseja A., Chawla Y.K., Sharma A., Agarwal R. Lactulose improves cognitive functions and health-related quality of life in patients with cirrhosis who have minimal hepatic encephalopathy. Hepatology. 2007;45(3):549–559. doi: 10.1002/hep.21533. [DOI] [PubMed] [Google Scholar]
- 9.Sharma P., Sharma B.C., Agrawal A., Sarin S.K. Primary prophylaxis of overt hepatic encephalopathy in patients with cirrhosis: an open labeled randomized controlled trial of lactulose versus no lactulose. J Gastroenterol Hepatol. 2012;27(8):1329–1335. doi: 10.1111/j.1440-1746.2012.07186.x. [DOI] [PubMed] [Google Scholar]
- 10.Sharma B.C., Sharma P., Agrawal A., Sarin S.K. Secondary prophylaxis of hepatic encephalopathy: an open-label randomized controlled trial of lactulose versus placebo. Gastroenterology. 2009;137(3):885–891. doi: 10.1053/j.gastro.2009.05.056. 891 e1. [DOI] [PubMed] [Google Scholar]
- 11.Bajaj J.S., Sanyal A.J., Bell D., Gilles H., Heuman D.M. Predictors of the recurrence of hepatic encephalopathy in lactulose-treated patients. Aliment Pharmacol Ther. 2010;31(9):1012–1017. doi: 10.1111/j.1365-2036.2010.04257.x. [DOI] [PubMed] [Google Scholar]
- 12.Kalaitzakis E., Simren M., Olsson R. Gastrointestinal symptoms in patients with liver cirrhosis: associations with nutritional status and health-related quality of life. Scand J Gastroenterol. 2006;41(12):1464–1472. doi: 10.1080/00365520600825117. [DOI] [PubMed] [Google Scholar]
- 13.Patidar K.R., Bajaj J.S. Covert and overt hepatic encephalopathy: diagnosis and management. Clin Gastroenterol Hepatol. 2015;13(12):2048–2061. doi: 10.1016/j.cgh.2015.06.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ryan M., Gerard K. Using discrete choice experiments to value health care programmes: current practice and future research reflections. Appl Health Econ Health Policy. 2003;2(1):55–64. [PubMed] [Google Scholar]
- 15.https://www.uptodate.com/contents/lactulose-patient-drug-information?source=see_link.In.
- 16.https://www.uptodate.com/contents/hepatic-encephalopathy-the-basics?source=search_result&search=hepatic%20encephalopathy%20patient%20info&selectedTitle=1∼150.In.
- 17.Landis C.S., Ghabril M., Rustgi V. Prospective multicenter observational study of overt hepatic encephalopathy. Dig Dis Sci. 2016;61(6):1728–1734. doi: 10.1007/s10620-016-4031-7. [DOI] [PubMed] [Google Scholar]
- 18.Sharma B.C., Sharma P., Lunia M.K., Srivastava S., Goyal R., Sarin S.K. A randomized, double-blind, controlled trial comparing rifaximin plus lactulose with lactulose alone in treatment of overt hepatic encephalopathy. Am J Gastroenterol. 2013;108(9):1458–1463. doi: 10.1038/ajg.2013.219. [DOI] [PubMed] [Google Scholar]
- 19.Bass N.M., Mullen K.D., Sanyal A. Rifaximin treatment in hepatic encephalopathy. N Engl J Med. 2010;362(12):1071–1081. doi: 10.1056/NEJMoa0907893. [DOI] [PubMed] [Google Scholar]
- 20.Leevy C.B., Phillips J.A. Hospitalizations during the use of rifaximin versus lactulose for the treatment of hepatic encephalopathy. Dig Dis Sci. 2007;52(3):737–741. doi: 10.1007/s10620-006-9442-4. [DOI] [PubMed] [Google Scholar]
- 21.https://www.ahrq.gov/professionals/quality-patient-safety/quality-resources/tools/literacy-toolkit/healthlittoolkit2-tool10.html.In.
- 22.Panigrahi M.K., Kar S.K., Singh S.P., Ghoshal U.C. Defecation frequency and stool form in a coastal eastern Indian population. J Neurogastroenterol Motil. 2013;19(3):374–380. doi: 10.5056/jnm.2013.19.3.374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mitsuhashi S., Ballou S., Jiang Z.G. Characterizing normal bowel frequency and consistency in a representative sample of adults in the United States (NHANES) Am J Gastroenterol. 2017 doi: 10.1038/ajg.2017.213. [DOI] [PubMed] [Google Scholar]
- 24.Worthington R.P., Gogne A. Cultural aspects of primary healthcare in India: a case-based analysis. Asia Pac Fam Med. 2011;10:8. doi: 10.1186/1447-056X-10-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kaba R., Sooriakumaran P. The evolution of the doctor–patient relationship. Int J Surg. 2007;5(1):57–65. doi: 10.1016/j.ijsu.2006.01.005. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.