Abstract
Hepatitis B virus (HBV) is one of the most common causes of liver cirrhosis and hepatocellular carcinoma. Despite recent strides in pharmacotherapy, complete cure of HBV infection still remains an enigma. The biggest obstacle in HBV therapy is clearance of covalently closed circular deoxyribonucleic acid (cccDNA). We discuss about the role of cccDNA in HBV life cycle, efficacy and shortcomings of currently available antivirals as well as promising novel targets to achieve ideal HBV cure.
Abbreviations: ccc, covalently closed circular; DNA, deoxyribonucleic acid; HBV, hepatitis B virus
Keywords: cccDNA, HBV, antivirals
Hepatitis B virus (HBV) infection has emerged as a major health concern for regions all over the world. Almost 2 billion people worldwide are estimated to be infected with HBV. Around 10% of this population, have chronic infection with HBV and are at risk of developing disastrous complications like liver cirrhosis and hepatocellular carcinoma.1 Prior to 21st century public health funding and research was mainly directed toward communicable diseases like tuberculosis, malaria and Human Immunodeficiency Virus (HIV) infections. A recent global burden of disease study has shown that unlike most other communicable diseases, viral hepatitis has been on the rise. The absolute burden and global rank of viral hepatitis in causing mortality worldwide has increased significantly in the last decade. Between 1990 and 2013 global viral hepatitis deaths, and disability adjusted life years have increased from 0.89 million to 1.45 million and 31.7 million to 42.5 million respectively.2
Present Antivirals and Their Limitations
The currently available anti-HBV therapy consists of Interferon (Standard and Pegylated) and Nucleos(t)ide agents Lamivudine, Adefovir, Telbuvidine, Tenofovir and Entecavir. Interferon acts predominantly by inhibition of HBV replication and clearance of infected hepatocytes through stimulation of cell mediated immunity. It also inhibits viral transcription independent of immune cell; through engagement of cell surface receptors and subsequent activation of pathways that lead to increased expression of intracellular genes which cause breakdown in viral RNAs.3 They have weaker antiviral action as compared to Nucleos(t)ides and an unfavorable side effect profile.4 The advent of oral Nucleos(t)ide analogues made treatment protocols easier with once daily oral dosing. Older agents (Lamivudine, Adefovir, Telbivudine) have fallen out of favor mainly due to high rates of resistance. This problem has been vastly overcome by Tenofovir and Entecavir which have high barrier to resistance as well high antiviral potency.5, 6
Currently available antivirals for HBV suffer from several limitations. At best they can achieve only a “functional cure” in HBV which is defined by absence of detectable HBV deoxyribonucleic acid (DNA) in peripheral blood and hepatitis B e antigen (HBeAg)/HBV surface antigen (HBsAg) loss with or without seroconversion in HBeAg +ve chronic hepatitis B or HBsAg loss with/without seroconversion in HBeAg −ve chronic hepatitis B. Highest efficacy of these antivirals is seen in suppressing the blood HBV DNA levels eventually making it undetectable on long-term therapy in up to 90% of both HBeAg +ve and HBeAg −ve patients treated with Nucleot(s)ide analogues.7, 8 The next most achievable target is loss of HBeAg and seroconversion to anti-HBe. The cumulative rates achieved with one year of Interferon therapy and long-term therapy with Tenofovir and Entecavir (5–7 years) is reported to be around 30–45%.8, 9, 10 These antivirals have even more dismal results when clearance of HBsAg is considered. After one year of IFN therapy, rates of HBsAg clearance in both HBeAg +ve and HBeAg −ve patients was 3–5% at 6 months post completion of therapy; increasing to 11% at 4 year follow up.9, 11 With Nucleos(t)ide therapies the success rate of HBsAg loss is much better in HBeAg +ve patients achieving rate of about 12% with 7 years of Tenofovir therapy.8 Extremely dismal HBsAg clearance rates of around 1.4% per annum are seen with these agents in HBeAg −ve patients10, 12 which is only as good as rate of spontaneous annual HBsAg clearance rate of 1.3%13 (Table 1).
Table 1.
Efficacy of Currently Available Antivirals for Treatment of HBV.
| HBeAg +ve CHB |
HBeAg −ve CHB |
cccDNA decline | |||||
|---|---|---|---|---|---|---|---|
| HBeAg loss |
HBsAg loss |
HBsAg loss |
|||||
| 1 year | >1 year | 1 year | 2 year | 1 year | >1 year | 1 year | |
| Pegylated interferon | 27 | NA | 3 | NA | 4 | 8 @ 3 years | ND |
| Lamivudine | 16–21 | 50 @ 5 years | ≤1 | 3 | ≤1 | NA | 1 log |
| Adefovir | 12 | 43 @ 3 years | 0 | NA | 0 | 5 @ 5 years | 0.8 log |
| Telbivudine | 22 | 30 @ 2 years | <1 | NA | <1 | NA | NA |
| Entecavir | 21 | 39 @ 3 years | 2 | 5 | <1 | NA | 1 log |
| Tenofovir | 21 | ND | 3 | 5 @ 64 weeks | 0 | NA | NA |
NA = data not available.
Unfortunately it has been observed that even after achieving “functional cure”; an individual is not guaranteed of complete protection from HBV related complications. Reactivation of HBV in previously treated patients who had achieved “functional cure” is a common phenomenon.14 This usually occurs in situations associated with change in immunological response to HBV as with immunosuppressive therapy (corticosteroids, cancer chemotherapy, antirejection drugs in organ transplantation, etc.). Also it is well known that occult HBV infection carriers (i.e. HBsAg −ve negative patients with positive HBV DNA in the liver with/without extremely low blood HBV DNA levels) may be a source of HBV transmission through blood transfusion and orthotopic liver transplantation.15 Even more concerning is the finding that hepatocellular carcinoma can still occur in patients who have even achieved HBsAg loss.16 Considering the fact that HBsAg loss is considered the zenith of achievable treatment goal with current antivirals; this fact is dispiriting and questions the achievable treatment benchmarks of current therapy.
Thanks to extensive research, it is now clear that in the dynamics of HBV life cycle, some amount of HBV DNA gets incorporated into the host DNA, while the rest gets converted to covalently closed circular DNA (cccDNA) which is virtually never cleared from the hepatocyte. Hence the concept of “complete cure” which incorporates above treatment goals with loss of cccDNA is a more desirable goal of HBV therapy.
Hepatitis B Virus Lifecycle and Role of Covalently Closed Circular Deoxyribonucleic Acid
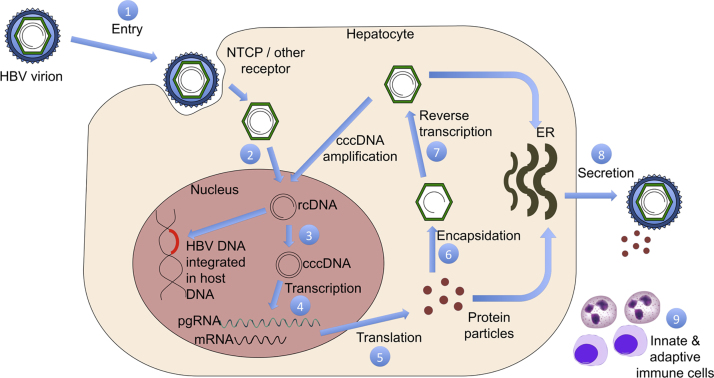
It is imperative to know the HBV lifecycle prior to understanding the lacunae with current antivirals and its solutions. HBV is a DNA virus, belonging to Hepadnaviridae family. An icosahedral capsid lodges the relaxed circular partially double stranded DNA (rcDNA) and viral polymerase. A surface envelope surrounds the nucleocapsid complex. The sodium taurocholate cotransporting polypeptide (NTCP) which is normally involved in bile acid transport has been identified as the major (but not the only one) target of HBV for infecting hepatocytes. After uncoating in cytoplasm of the hepatocyte, the naked nucleocapsid is transported to the nucleus. Here the HBV DNA can have two fates; it either gets integrated in the host genome, or gets converted into covalently closed circular DNA (cccDNA). The resulting cccDNA is the template for transcription of the pregenomic RNA (pgRNA) and several subgenomic RNAs. In turn, the pgRNA acts as the template for reverse transcription and translation of the core and polymerase proteins. A unique feature of HBV replication is the formation of nucleocapsid in the cytosol of hepatocyte; which during the process of formation also incorporates the pgRNA and viral polymerase (Pol). Reverse transcription occurs after this step in the nucleocapsid and negative stranded DNA is formed from pgRNA by the action of Pol. Subsequent steps are destruction of pgRNA and formation of positive stranded DNA by action of Pol. The mature nucleocapsid thus formed is either tagged for secretion and envelopment with various surface proteins; or is recycled to further replenish and amplify the cccDNA pool.17
The cccDNA exists as a minichromosome with a typical beads on string pattern consisting of histone and nucleosomal proteins.18 Epigenetic modifications like histone acetylation and DNA methylation play a role in transcriptional activity of ccDNA.19
Measurement of cccDNA is feasible with some assays which rely on amplification of cccDNA after action with deoxyribonuclease and cccDNA-specific primers.20, 21 However specificity of these assays is questionable as the quantitative PCR also detects the vast excess of relaxed circular DNA is present in infected hepatocytes giving false positive results. Presently, when ideal assays of cccDNA are awaited, HBsAg can be used as a surrogate marker to quantify cccDNA levels especially in HBeAg positive patients.22
Studies have shown that cccDNA pool can reach an average of 5–50 copies per cell through process of intracellular recycling.23 Levels of cccDNA have been found to correlate with markers of active viral replication, being higher HBeAg-positive patients compared to HBeAg-negative patients, inactive carriers, and HBsAg cleared patients.21 Also as compared to HBsAg positive patients, intrahepatic cccDNA levels are lower in HBsAg-negative patients.24 Patients with occult HBV infection also have demonstrable cccDNA. The occurrence of HBV reactivation leading to clinical flares proves the fact that cccDNA is replication competent.25
Role of Immunity in Hepatitis B Virus Infection
The magnitude of liver injury in HBV infection is primarily determined by the host immunity and not by the virus per se as HBV is non-cytopathic. Whereas innate immunity (predominantly natural killer cells and interferons) induces a non-specific response; the adaptive immune system (including antibodies as well as CD4+ and CD8+ lymphocytes) is responsible for a more specific and robust response. CD4+ T lymphocytes need antigen (i.e. infected hepatocyte) presentation in conjunction with class II major histocompatibility complex (MHC) molecules. Once activated, CD4+ cells help in producing cytokines and neutralizing antibodies to combat HBV. For CD8+ cytotoxic T cells (CTL) to act, infected hepatocytes must be processed and presented with class I MHC molecules. Once bound to infected hepatocyte, the CTLs bring about hepatocyte lysis. Cellular arm of the immune response is the more important mechanism of immunity in HBV infection.26
During acute HBV infection, a robust immune response is mediated initially by innate immunity cells which release various cytokines. Later CD8+ T cells, driven by type 1 helper T cells continue the immune attack and infiltrate the liver and are HBV specific. These cellular responses are HBV specific and very powerful yet self-limited. A contrastingly weak and poorly focused T cell response against HBV is seen in chronic HBV infections.27
Targeting Covalently Closed Circular Deoxyribonucleic Acid
The effect of presently available antivirals for HBV infection are not very encouraging. Adefovir, Entecavir or Lamivudine treatment for 48 weeks results in the reduction of intrahepatic cccDNA by 0.8–1.0 log21, 28 (Table 1). As Interferons act through immune modulation, they may be better placed in causing cccDNA reduction as compared to long term Nucleos(t)ide therapy. A combination treatment with pegylated IFN-a2b and adefovir or pegylated IFN-a2b plus entecavir has been shown to reduce cccDNA by 2.4 logs and 1.4 logs respectively.29, 30 The individual efficacy of Interferon in reducing cccDNA levels is as yet unknown though previous attempts have yielded exciting conclusions.31 In a recent study, Chuaypen et al.22 measured the levels of intrahepatic DNA in paired liver biopsy specimens before and after Interferon therapy.
The failure of Nucleos(t)ide analogues in clearing cccDNA could be partly explained through dynamics of viral and hepatocyte replication. It is estimated that the half-life of HBV virion is 1 day while that of a rapidly replicating infected hepatocyte is 10–100 days.32 During the treatment course of HBV, reduction in viral population occurs in a typical biphasic pattern.33, 34 The initial spike is due to rapid clearance of viral particles from the serum as they have shorter half-lives, while the delayed peak is attributable to destruction of infected hepatocytes with longer half-lives. Based on these dynamics, it is estimated to take around 15 years for complete eradication of cccDNA with Nucleos(t)ide therapy,25 and hence seems an unreasonable goal.
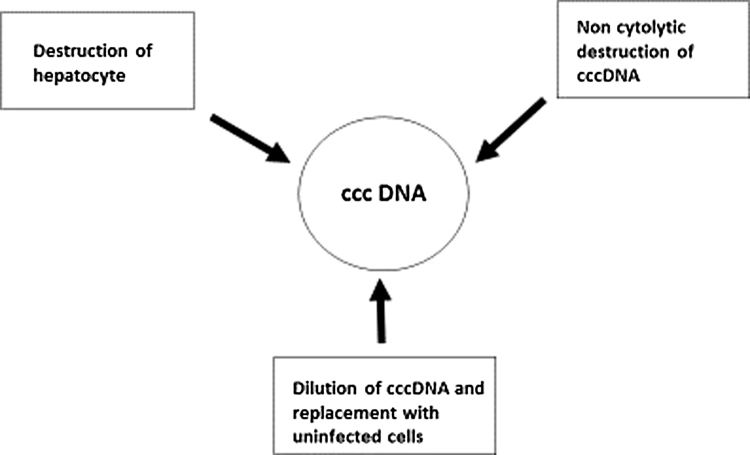
Due to these limitations, novel strategies have been proposed for elimination of cccDNA. Currently 3 methods of destruction of cccDNA have been identified based on host targeting or agents which directly act on cccDNA. Mechanisms of host targeting include destruction of the infected hepatocyte (cytolysis) and dilution of cccDNA by replacement with regenerating uninfected cells. Directly acting agents act through non cytolytic specific destruction of cccDNA (Figure 1, Figure 2).
Figure 1.
HBV life cycle and potential targets for drug action.
Figure 2.
Therapeutic methods for cccDNA elimination.
Novel Host Targeting Agents
The essential role played by cytolytic destruction of infected hepatocytes in clearing HBV DNA was classically demonstrated by Fourel et al.35 They demonstrated in HBV infected duck hepatocytes that rapid clearance of infected hepatocytes was caused not just by the inhibition of viral replication but also by an acceleration of the rate of hepatocyte turnover. Even in humans it is clear that hepatocyte turnover is much more rapid in HBV infected liver than in healthy liver, possibly as a protective mechanism.17 It has been established that CD8+ve T cells play a central role in destruction of HBV infected hepatocytes.36 Researchers have engineered T cells with chimeric receptors directed against HBV surface proteins which have demonstrated efficacy in destroying HBV infected hepatocytes both in vivo and in vitro.37, 38
After destruction of the HBV infected cells, it is postulated that regenerating uninfected hepatocytes proliferate and eventually replace the infected cell population.39, 40 Summers et al. demonstrated that in woodchucks infected chronically infected with woodchuck hepatitis virus which were treated with nucleoside analogue; the viral cccDNA declined 20- to 100-fold although the frequency of the integrated WHV remained relatively constant over the course of treatment. They thus concluded that the uninfected hepatocytes were derived from the infected hepatocyte population.39 Subsequently the same group also demonstrated clonal expansion of hepatocytes during chronic WHV infection which was responsible for high degree of hepatocyte proliferation and selection which occurred during the chronic period of WHV infection.41
Toll like receptors (TLR) are unique components of innate immune system having various antimicrobial actions. It has been shown that activation of TLR-7 has beneficial effects in chronic viral infections. It is capable of triggering both innate and adaptive immune responses for eliminating infection. GS-9620, a molecule devised to act as an agonist at TLR-7 has been shown to activate useful transcription factors involved in interferon regulation.42
Novel Directly Acting Antivirals
HBV entry locus is an attractive target for preventing initiation as well as spread of HBV infection to other hepatocytes. The process of viral entry involves two critical steps which include binding of HBV to heparin sulfate proteoglycans and then its interaction with sodium taurocholate co-transporting polypeptide (NTCP). A promising drug, Myrcludex-B which has been derived from L protein of HBV, has been shown to block de novo HBV infection by competing with viral pre-S1 motif to bind with NTCP. Theoretically, this drug would thus be able to prevent further amplification of cccDNA by inhibiting further infection of fresh hepatocytes. Other agents like Irbesartan, Cyclosporine, Ritonavir and Ezetimibe are also capable of playing a role in HBV entry inhibition. However, the effects of these drugs are only modest and not as efficacious as seen with Myrcludex-B.43 With improvement of our understanding in HBV dynamics, it is now realized that HBV entry into the hepatocyte is not limited only to NTCP and there are other portals as well. Thus Myrcludex-B may not be completely efficacious in preventing infection/re-infection.
Formation of cccDNA from relaxed circular DNA (rcDNA) is one of the critical steps in maintaining and propagating HBV infection. Several compounds were tested by researchers to find substances capable of inhibiting cccDNA formation. Disubstituted sulfonamides CCC-0975 and CCC-0346 have shown good efficacy in preventing cccDNA formation when tested in in vitro cell lines.44 Efficacy of these compounds when used in vivo is yet to be ascertained.
Non-cytolytic destruction of HBV DNA is a promising and probably the most feasible alternative. Specific, non-cytolytic destruction of cccDNA is primarily mediated by the pro-inflammatory cytokines IFN-γ, TNF-α and IFN-α45 which are involved in innate and adaptive immunity. It has been postulated that IFN-γ and TNF-α suppress viral replication by destabilizing viral RNA and also eliminating the HBV nucleocapsid particles,46 however this has not been conclusively proven in subsequent research.47 The recent discovery of protein family involved in innate immunity called apolipoprotein B mRNA editing enzyme catalytic polypeptide-like 3 (APOBEC3)-family proteins have renewed interest in this field. The mechanisms by which APOBEC3 family of proteins act include inhibition of packaging of pgRNA in the HBV capsid,48 foreign DNA degradation49 and hypermutation of HBV cccDNA.50 Also of interest is the fact that APOBEC3 can be upregulated by IFN-a and lymphotoxin β receptor activation.31 These findings have brought us close to determining the crucial elements involved in innate and adaptive immunity which can be harnessed to achieve complete HBV cure.
Non-cytolytic destruction of cccDNA can also be achieved using specific endonucleases. A wide range of DNA cleavage enzymes have been studied for this purpose including zincfinger nucleases (ZFNs), transcription activator-like effector nucleases (TALENs) and the RNA-guided clustered regulatory interspaced short palindromic repeats (CRISPR) and CRISPR associated (Cas) protein endonucleases.51 A study which utilized adeno associated virus vector for specifically delivering HBV specific ZFNs in infected hepatocytes found that total HBV DNA levels were reduced by 1000-fold including a reduction in cccDNA by 10-fold.52
In chronic HBV infection, a persistent production of HBsAg is observed. HBsAg is secreted in various forms, most abundant of which include empty non-infectious particles. These serve as a false decoy for engaging the inflammation, thus precluding an effective response against actual infectious particles. With the discovery of small interfering RNAs (si-RNA), it is now possible to target and cease the transcription of messenger RNA (mRNA) which is responsible for HBsAg production. Several si-RNA molecules are in various stages of evaluation at present. ARC-520; composed of two si-RNA sequences has been found to reduce HBV cccDNA across several genotypes.53 These findings have been seen in animal studies with better outcomes documented in HBeAg positive subjects. Validation in humans is still lacking. Moreover; use of si-RNA approach is limited by inability to direct specific delivery into hepatocytes in vivo, susceptibility of si-RNA to endosomal lysis and several undesirable side effects owing to actions on un-intended targets.43 Despite all these limitations, si-RNA directed approach seems highly promising as researchers have devised novel chemical modifications in these molecules which reduce cross-reactivity and allow efficient delivery of si-RNA to intended target.
The HBV nucleocapsid is a pivotal element in HBV life cycle responsible for the critical steps of genome packing and reverse transcription. Molecules like heteroarylpyrimidines and sulfamoylbenzamides target specific capsid protein sequences to cause disruption of capsid assembly. This has been shown to inhibit HBV replication both in vivo and vitro. Molecules like BAY 41-410954 and morphothiadine mesilate GLS455 have been based on this concept and shown promising results.
Therapeutic Vaccination
Vaccination in HBV is based on the principle of activation of adaptive CD4+ and CD8+ cells to neutralize HBV. The biggest hindrance in development of therapeutic vaccine is the inability to break immune tolerance and the phenomenon of immune exhaustion.56 Hence newer strategies using novel targets are now being attempted. A recombinant yeast derived vaccine expressing multitude of HBV antigens including HBV X, HBV core and HBV surface antigens has been studied in 49 healthy previously unvaccinated individuals. T cell responses were documented in most patients without any significant adverse events.57 A nasal vaccine combining HBV surface and core antigens was tested in a phase 1 trial involving 19 healthy men and reported no significant adverse events.58 In order to overcome immune exhaustion, combining therapeutic vaccines with nucleos(t)ide therapy seems to be a promising approach.
However most of the above therapies are still in preclinical stages of development (Table 2). Nonetheless a lot of research has been recently focused on developing novel agents in treating HBV. It is imperative that cccDNA remains the most sought-after target in these newer strategies.
Table 2.
Novel Drugs for HBV Treatment, Targets and Present Development Status.
| Sr. no. | Step in life cycle | Drug | Present status |
|---|---|---|---|
| Novel antiviral agents | |||
| 1 | Entry inhibitor | Myrcludex B | Phase 2 |
| 2 | cccDNA inhibitor | CCC-0975 | |
| CCC-0346 | |||
| 3, 4 | Epigenetic inhibitor, translation inhibitor | ARC 520 | Phase 2/3 |
| ARB-1467 | Phase 2 | ||
| BB-HB-331 | Phase 1 | ||
| 5 | Nucleocapsid inhibition | BAY-41-4109 | Phase 1 |
| Morphothiadine mesilate GLS4 | Phase 2 | ||
| NVR 3-778 | Phase 2 | ||
| 6 | Reverse transcription inhibitors | Tenofovir alafenamide | Phase 3 |
| Besifovir | Phase 2 | ||
| Lagociclovir | Phase 2 | ||
| CMX-157 | Phase 2 | ||
| 7 | Secretion inhibitors | Rep 2139-Ca | Phase 2 |
| Novel agents acting on host | |||
| 1 | Immunomodulators | GS-9620 | Phase 2 |
| RG7795 | Phase 2 | ||
| ARB-1598 | Phase 1 | ||
| CYT107 | Phase 2 | ||
| 2 | Therapeutic vaccine | ABX 203 | Phase 3 |
| GS-4774 | Phase 1 | ||
| INO-1800 | Phase 1 | ||
Conclusion
In conclusion, the currently available therapeutic armamentarium for treatment of chronic HBV infection is far from ideal. Apart from poor HBsAg clearance, the biggest hindrance with currently available therapies is persistence of cccDNA which has significant clinical consequences. Understanding the cellular and molecular mechanisms involving HBV replication and cccDNA dynamics is pivotal in devising more effective strategies. Exciting targets have been identified which definitely hint at better treatment outcomes including achieving the elusive “complete cure” in HBV infection.
Conflicts of Interest
The authors have none to declare.
References
- 1.Schweitzer A., Horn J., Mikolajczyk R.T., Krause G., Ott J.J. Estimations of worldwide prevalence of chronic hepatitis B virus infection: a systematic review of data published between 1965 and 2013. Lancet Lond Engl. 2015;386(10003):1546–1555. doi: 10.1016/S0140-6736(15)61412-X. [DOI] [PubMed] [Google Scholar]
- 2.Stanaway J.D., Flaxman A.D., Naghavi M. The global burden of viral hepatitis from 1990 to 2013: findings from the Global Burden of Disease Study 2013. Lancet Lond Engl. 2016 doi: 10.1016/S0140-6736(16)30579-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dianzani F., Antonelli G., Capobianchi M.R. The biological basis for clinical use of interferon. J Hepatol. 1990;11(suppl 1):S5–S10. doi: 10.1016/0168-8278(90)90156-l. [DOI] [PubMed] [Google Scholar]
- 4.Gish R.G., Given B.D., Lai C.-L. Chronic hepatitis B: virology, natural history, current management and a glimpse at future opportunities. Antiviral Res. 2015;121:47–58. doi: 10.1016/j.antiviral.2015.06.008. [DOI] [PubMed] [Google Scholar]
- 5.Ghany M.G., Doo E.C. Antiviral resistance and hepatitis B therapy. Hepatol Baltim Md. 2009;49(5 suppl):S174–S184. doi: 10.1002/hep.22900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ke W., Liu L., Zhang C. Comparison of efficacy and safety of tenofovir and entecavir in chronic hepatitis B virus infection: a systematic review and meta-analysis. PLOS ONE. 2014;9(6):e98865. doi: 10.1371/journal.pone.0098865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chang T.-T., Lai C.-L., Kew Yoon S. Entecavir treatment for up to 5 years in patients with hepatitis B e antigen-positive chronic hepatitis B. Hepatol Baltim Md. 2010;51(2):422–430. doi: 10.1002/hep.23327. [DOI] [PubMed] [Google Scholar]
- 8.Buti M., Tsai N., Petersen J. Seven-year efficacy and safety of treatment with tenofovir disoproxil fumarate for chronic hepatitis B virus infection. Dig Dis Sci. 2015;60(5):1457–1464. doi: 10.1007/s10620-014-3486-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lau G.K.K., Piratvisuth T., Luo K.X. Peginterferon Alfa-2a, lamivudine, and the combination for HBeAg-positive chronic hepatitis B. N Engl J Med. 2005;352(26):2682–2695. doi: 10.1056/NEJMoa043470. [DOI] [PubMed] [Google Scholar]
- 10.Idilman R., Gunsar F., Koruk M. Long-term entecavir or tenofovir disoproxil fumarate therapy in treatment-naïve chronic hepatitis B patients in the real-world setting. J Viral Hepat. 2015;22(5):504–510. doi: 10.1111/jvh.12358. [DOI] [PubMed] [Google Scholar]
- 11.Marcellin P., Lau G.K.K., Bonino F. Peginterferon alfa-2a alone, lamivudine alone, and the two in combination in patients with HBeAg-negative chronic hepatitis B. N Engl J Med. 2004;351(12):1206–1217. doi: 10.1056/NEJMoa040431. [DOI] [PubMed] [Google Scholar]
- 12.Nagata N., Kagawa T., Hirose S. Off-treatment durability of antiviral response to nucleoside analogues in patients with chronic hepatitis B. BMC Gastroenterol. 2016;16:38. doi: 10.1186/s12876-016-0454-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tseng T.-C., Liu C.-J., Chen C.-L. Higher lifetime chance of spontaneous surface antigen loss in hepatitis B carriers with genotype C infection. Aliment Pharmacol Ther. 2015;41(10):949–960. doi: 10.1111/apt.13170. [DOI] [PubMed] [Google Scholar]
- 14.Perrillo R.P. Acute flares in chronic hepatitis B: the natural and unnatural history of an immunologically mediated liver disease. Gastroenterology. 2001;120(4):1009–1022. doi: 10.1053/gast.2001.22461. [DOI] [PubMed] [Google Scholar]
- 15.Raimondo G., Pollicino T., Romanò L., Zanetti A.R. A 2010 update on occult hepatitis B infection. Pathol Biol (Paris) 2010;58(4):254–257. doi: 10.1016/j.patbio.2010.02.003. [DOI] [PubMed] [Google Scholar]
- 16.Liu J., Yang H.-I., Lee M.-H. Spontaneous seroclearance of hepatitis B seromarkers and subsequent risk of hepatocellular carcinoma. Gut. 2014;63(10):1648–1657. doi: 10.1136/gutjnl-2013-305785. [DOI] [PubMed] [Google Scholar]
- 17.Wang Y.-J., Yang L., Zuo J.-P. Recent developments in antivirals against hepatitis B virus. Virus Res. 2016;213:205–213. doi: 10.1016/j.virusres.2015.12.014. [DOI] [PubMed] [Google Scholar]
- 18.Bock C.T., Schranz P., Schröder C.H., Zentgraf H. Hepatitis B virus genome is organized into nucleosomes in the nucleus of the infected cell. Virus Genes. 1994;8(3):215–229. doi: 10.1007/BF01703079. [DOI] [PubMed] [Google Scholar]
- 19.Levrero M., Pollicino T., Petersen J., Belloni L., Raimondo G., Dandri M. Control of cccDNA function in hepatitis B virus infection. J Hepatol. 2009;51(3):581–592. doi: 10.1016/j.jhep.2009.05.022. [DOI] [PubMed] [Google Scholar]
- 20.Wong D.K.-H., Yuen M.-F., Yuan H. Quantitation of covalently closed circular hepatitis B virus DNA in chronic hepatitis B patients. Hepatol Baltim Md. 2004;40(3):727–737. doi: 10.1002/hep.20353. [DOI] [PubMed] [Google Scholar]
- 21.Werle-Lapostolle B., Bowden S., Locarnini S. Persistence of cccDNA during the natural history of chronic hepatitis B and decline during adefovir dipivoxil therapy. Gastroenterology. 2004;126(7):1750–1758. doi: 10.1053/j.gastro.2004.03.018. [DOI] [PubMed] [Google Scholar]
- 22.Chuaypen N., Sriprapun M., Praianantathavorn K. Kinetics of serum HBsAg and intrahepatic cccDNA during pegylated interferon therapy in patients with HBeAg-positive and HBeAg-negative chronic hepatitis B. J Med Virol. 2016 doi: 10.1002/jmv.24601. [DOI] [PubMed] [Google Scholar]
- 23.Zhang Y.-Y., Zhang B.-H., Theele D., Litwin S., Toll E., Summers J. Single-cell analysis of covalently closed circular DNA copy numbers in a hepadnavirus-infected liver. Proc Natl Acad Sci U S A. 2003;100(21):12372–12377. doi: 10.1073/pnas.2033898100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jones S.A., Boregowda R., Spratt T.E., Hu J. In vitro epsilon RNA-dependent protein priming activity of human hepatitis B virus polymerase. J Virol. 2012;86(9):5134–5150. doi: 10.1128/JVI.07137-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yang H.-C., Kao J.-H. Persistence of hepatitis B virus covalently closed circular DNA in hepatocytes: molecular mechanisms and clinical significance. Emerg Microbes Infect. 2014;3(9):e64. doi: 10.1038/emi.2014.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rehermann B. Immune responses in hepatitis B virus infection. Semin Liver Dis. 2003;23(1):21–38. doi: 10.1055/s-2003-37586. [DOI] [PubMed] [Google Scholar]
- 27.Curry M.P., Koziel M. The dynamics of the immune response in acute hepatitis B: new lessons using new techniques. Hepatol Baltim Md. 2000;32(5):1177–1179. doi: 10.1053/jhep.2000.20121. [DOI] [PubMed] [Google Scholar]
- 28.Wong D.K.-H., Yuen M.-F., Ngai V.W.-S., Fung J., Lai C.-L. One-year entecavir or lamivudine therapy results in reduction of hepatitis B virus intrahepatic covalently closed circular DNA levels. Antivir Ther. 2006;11(7):909–916. [PubMed] [Google Scholar]
- 29.Wursthorn K., Lutgehetmann M., Dandri M. Peginterferon alpha-2b plus adefovir induce strong cccDNA decline and HBsAg reduction in patients with chronic hepatitis B. Hepatol Baltim Md. 2006;44(3):675–684. doi: 10.1002/hep.21282. [DOI] [PubMed] [Google Scholar]
- 30.Hagiwara S., Kudo M., Osaki Y. Impact of peginterferon alpha-2b and entecavir hydrate combination therapy on persistent viral suppression in patients with chronic hepatitis B. J Med Virol. 2013;85(6):987–995. doi: 10.1002/jmv.23564. [DOI] [PubMed] [Google Scholar]
- 31.Lucifora J., Xia Y., Reisinger F. Specific and nonhepatotoxic degradation of nuclear hepatitis B virus cccDNA. Science. 2014;343(6176):1221–1228. doi: 10.1126/science.1243462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nowak M.A., Bonhoeffer S., Hill A.M., Boehme R., Thomas H.C., McDade H. Viral dynamics in hepatitis B virus infection. Proc Natl Acad Sci U S A. 1996;93(9):4398–4402. doi: 10.1073/pnas.93.9.4398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tsiang M., Rooney J.F., Toole J.J., Gibbs C.S. Biphasic clearance kinetics of hepatitis B virus from patients during adefovir dipivoxil therapy. Hepatol Baltim Md. 1999;29(6):1863–1869. doi: 10.1002/hep.510290626. [DOI] [PubMed] [Google Scholar]
- 34.Wolters L.M.M., Hansen B.E., Niesters H.G.M., DeHertogh D., de Man R.A. Viral dynamics during and after entecavir therapy in patients with chronic hepatitis B. J Hepatol. 2002;37(1):137–144. doi: 10.1016/s0168-8278(02)00115-0. [DOI] [PubMed] [Google Scholar]
- 35.Fourel I., Cullen J.M., Saputelli J. Evidence that hepatocyte turnover is required for rapid clearance of duck hepatitis B virus during antiviral therapy of chronically infected ducks. J Virol. 1994;68(12):8321–8330. doi: 10.1128/jvi.68.12.8321-8330.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Thimme R., Wieland S., Steiger C. CD8(+) T cells mediate viral clearance and disease pathogenesis during acute hepatitis B virus infection. J Virol. 2003;77(1):68–76. doi: 10.1128/JVI.77.1.68-76.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bohne F., Protzer U. Adoptive T-cell therapy as a therapeutic option for chronic hepatitis B. J Viral Hepat. 2007;14(suppl 1):45–50. doi: 10.1111/j.1365-2893.2007.00913.x. [DOI] [PubMed] [Google Scholar]
- 38.Krebs K., Böttinger N., Huang L.-R. T cells expressing a chimeric antigen receptor that binds hepatitis B virus envelope proteins control virus replication in mice. Gastroenterology. 2013;145(2):456–465. doi: 10.1053/j.gastro.2013.04.047. [DOI] [PubMed] [Google Scholar]
- 39.Summers J., Jilbert A.R., Yang W. Hepatocyte turnover during resolution of a transient hepadnaviral infection. Proc Natl Acad Sci U S A. 2003;100(20):11652–11659. doi: 10.1073/pnas.1635109100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Guo J.T., Zhou H., Liu C. Apoptosis and regeneration of hepatocytes during recovery from transient hepadnavirus infections. J Virol. 2000;74(3):1495–1505. doi: 10.1128/jvi.74.3.1495-1505.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mason W.S., Jilbert A.R., Summers J. Clonal expansion of hepatocytes during chronic woodchuck hepatitis virus infection. Proc Natl Acad Sci U S A. 2005;102(4):1139–1144. doi: 10.1073/pnas.0409332102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rebbapragada I., Birkus G., Perry J., Xing W., Kwon H., Pflanz S. Molecular determinants of GS-9620-dependent TLR7 activation. PLOS ONE. 2016;11(1):e0146835. doi: 10.1371/journal.pone.0146835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Boucle S., Bassit L., Ehteshami M., Schinazi R.F. Toward elimination of hepatitis B virus using novel drugs, approaches, and combined modalities. Clin Liver Dis. 2016;20(4):737–749. doi: 10.1016/j.cld.2016.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cai D., Mills C., Yu W. Identification of disubstituted sulfonamide compounds as specific inhibitors of hepatitis B virus covalently closed circular DNA formation. Antimicrob Agents Chemother. 2012;56(8):4277–4288. doi: 10.1128/AAC.00473-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.McClary H., Koch R., Chisari F.V., Guidotti L.G. Relative sensitivity of hepatitis B virus and other hepatotropic viruses to the antiviral effects of cytokines. J Virol. 2000;74(5):2255–2264. doi: 10.1128/jvi.74.5.2255-2264.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Guidotti L.G., Ishikawa T., Hobbs M.V., Matzke B., Schreiber R., Chisari F.V. Intracellular inactivation of the hepatitis B virus by cytotoxic T lymphocytes. Immunity. 1996;4(1):25–36. doi: 10.1016/s1074-7613(00)80295-2. [DOI] [PubMed] [Google Scholar]
- 47.Zhu Y., Cullen J.M., Aldrich C.E. Adenovirus-based gene therapy during clevudine treatment of woodchucks chronically infected with woodchuck hepatitis virus. Virology. 2004;327(1):26–40. doi: 10.1016/j.virol.2004.06.017. [DOI] [PubMed] [Google Scholar]
- 48.Turelli P., Mangeat B., Jost S., Vianin S., Trono D. Inhibition of hepatitis B virus replication by APOBEC3G. Science. 2004;303(5665):1829. doi: 10.1126/science.1092066. [DOI] [PubMed] [Google Scholar]
- 49.Stenglein M.D., Burns M.B., Li M., Lengyel J., Harris R.S. APOBEC3 proteins mediate the clearance of foreign DNA from human cells. Nat Struct Mol Biol. 2010;17(2):222–229. doi: 10.1038/nsmb.1744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kitamura K., Wang Z., Chowdhury S., Simadu M., Koura M., Muramatsu M. Uracil DNA glycosylase counteracts APOBEC3G-induced hypermutation of hepatitis B viral genomes: excision repair of covalently closed circular DNA. PLoS Pathog. 2013;9(5):e1003361. doi: 10.1371/journal.ppat.1003361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lin C.-L., Kao J.-H. Review article: novel therapies for hepatitis B virus cure – advances and perspectives. Aliment Pharmacol Ther. 2016;44(3):213–222. doi: 10.1111/apt.13694. [DOI] [PubMed] [Google Scholar]
- 52.White M.K., Hu W., Khalili K. The CRISPR/Cas9 genome editing methodology as a weapon against human viruses. Discov Med. 2015;19(105):255–262. [PMC free article] [PubMed] [Google Scholar]
- 53.Gish R.G., Yuen M.-F., Chan H.L.Y. Synthetic RNAi triggers and their use in chronic hepatitis B therapies with curative intent. Antiviral Res. 2015;121:97–108. doi: 10.1016/j.antiviral.2015.06.019. [DOI] [PubMed] [Google Scholar]
- 54.Weber O., Schlemmer K.-H., Hartmann E. Inhibition of human hepatitis B virus (HBV) by a novel non-nucleosidic compound in a transgenic mouse model. Antiviral Res. 2002;54(2):69–78. doi: 10.1016/s0166-3542(01)00216-9. [DOI] [PubMed] [Google Scholar]
- 55.Manzoor S., Saalim M., Imran M., Resham S., Ashraf J. Hepatitis B virus therapy: what's the future holding for us? World J Gastroenterol. 2015;21(44):12558–12575. doi: 10.3748/wjg.v21.i44.12558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Brahmania M., Feld J., Arif A., Janssen H.L.A. New therapeutic agents for chronic hepatitis B. Lancet Infect Dis. 2016;16(2):e10–e21. doi: 10.1016/S1473-3099(15)00436-3. [DOI] [PubMed] [Google Scholar]
- 57.Gaggar A., Coeshott C., Apelian D. Safety, tolerability and immunogenicity of GS-4774, a hepatitis B virus-specific therapeutic vaccine, in healthy subjects: a randomized study. Vaccine. 2014;32(39):4925–4931. doi: 10.1016/j.vaccine.2014.07.027. [DOI] [PubMed] [Google Scholar]
- 58.Lobaina Y., Hardtke S., Wedemeyer H., Aguilar J.C., Schlaphoff V. In vitro stimulation with HBV therapeutic vaccine candidate Nasvac activates B and T cells from chronic hepatitis B patients and healthy donors. Mol Immunol. 2015;63(2):320–327. doi: 10.1016/j.molimm.2014.08.003. [DOI] [PubMed] [Google Scholar]