Abstract
Background
Obesity is affecting children in epidemic proportions in the United States with nearly 25% of children being obese. Consequences of obesity including dyslipidemia, type 2 diabetes and cardiovascular disease are leading to morbidity at younger ages. Parallel to the obesity and diabetes epidemics, the prevalence of vitamin D deficiency has reached very high levels and has been associated with insulin resistance and dyslipidemia. Studies exploring the impact of vitamin D repletion on insulin sensitivity and dyslipidemia in children are sparse.
The aim of this study was to determine the impact of treatment with vitamin D (ergocalciferol) in obese African American (AA) children on vitamin D levels and insulin secretion and sensitivity.
Methods
This pilot study was conducted in a tertiary care Pediatric Emergency Department (ED). African American obese children (n = 29; 22 female) 13–17 y, with 25-hydroxy vitamin D level [25(OH)D] <20 ng/ml, were randomized to receive either 50,000 IU vitamin D2/week or a placebo for 12 weeks. Pre- and post- oral glucose tolerance testing with glucose and insulin levels drawn at 0, 30, 60, 90 and 120 min were performed. Pre/post intervention lipid profiles and calcium levels were also evaluated.
Results
There was no difference in serum 25(OH)D between groups at baseline. Follow-up 25(OH)D level was greater in the treatment vs. placebo group, and significantly increased from baseline in the treatment group only. However, there was no difference between groups in baseline vs. follow-up insulin- or lipid-related parameters. Follow-up serum 25(OH)D was positively correlated with fasting insulin and high-density lipoprotein (HDL) level in the vitamin D treated group only.
Conclusion
While serum 25(OH)D levels in obese AA teens increased adequately with vitamin treatment for 12 weeks and correlated with fasting insulin, it did not significantly impact insulin secretion or sensitivity. Larger studies are required over a longer period of time to confirm and explore the reasons for this finding.
Background
Obesity is affecting children in epidemic proportions in the United States at the rate of nearly 25% [1]. Consequences of obesity include type 2 diabetes and cardiovascular disease, morbidities which now are beginning to occur at younger ages [2]. In parallel, prevalence of vitamin D [25(OH)D] deficiency has increased. Studies have reported that 9% of children and adolescents are vitamin D-deficient [i.e., serum 25(OH)D concentration < 20 mg/ml] and 60% are vitamin D-insufficient [serum 25(OH) D concentration 20–30 ng/ml] [3]. The vitamin D metabolite 25(OH)D is a pro-hormone known to play a major role in calcium homeostasis. But in recent years, animal and in vitro studies have linked 25(OH)D deficiency to development of diabetes and cardiovascular disease [4], [5], [6]. Studies addressing the association between low serum 25(OH)D levels and insulin secretion and sensitivity are limited in high risk children [7]. To our knowledge, limited studies exist in children that have addressed the efficacy of vitamin D treatment on insulin secretion and sensitivity and parameters of cardiovascular health in obese, vitamin D deficient children [8], [9], [10], [11].
The objectives of this 12-week pilot study in obese, vitamin D-deficient AA children were to evaluate 1) the impact of vitamin D treatment on serum 25(OH) D levels and 2) the impact of vitamin D repletion on insulin sensitivity indices and insulin response to glucose challenge. We hypothesized that 25(OH)D level would increase to sufficient levels with treatment and be positively associated with insulin sensitivity and secretion in vitamin D-deficient obese (BMI ≥ 95th percentile) AA children.
Methods
This was a 12-week randomized, double-blinded placebo-controlled pilot trial that was performed in the Emergency Department (ED) of a tertiary care Children's Hospital from June 2013 to May 2014. This study was approved by the UAB Institutional Review Board.
Study population
This study included subjects from a convenience sample of AA children between the ages of 13 and 17 years with BMI ≥ 95th percentile and 25(OH)D level of ≤20 ng/ml. Exclusion criteria included: children who had pre-existing chronic diseases (including type 1 or type 2 diabetes), pregnancy, on medications influencing blood glucose [e.g., insulin, anabolic steroids, androgens or prolonged (>3 months) oral steroids], on vitamin D treatment, hemoglobin A1C (HbA1c) of ≥6.5%, a serum calcium level of ≥10.8 mg.dL [12].
Study protocol
Once enrolled, the study involved three visits.
Screening visit
Eligibility screening was done by reviewing height, weight and BMI percentiles of the study subjects [13]. Informed consent and assent were obtained from parents and from all children above the age of 13 years, respectively, prior to enrollment. During the screening visit, information on patient demographics, date of last menstrual period (when applicable), dietary history was obtained, and Tanner staging was performed. Screening labs included serum calcium, 25(OH)D, HbA1C, and a urine pregnancy test. Parents of children who met the above inclusion criteria were contacted the following day and detailed instructions regarding return visit were given. Participants were instructed to fast overnight (at least 10 h) prior to their baseline visit and were reminded by phone a day prior to the visit.
Baseline visit
Participants who were deemed eligible for inclusion returned directly to the outpatient laboratory area after the instructed overnight fast. Blood was drawn for primary and secondary outcomes measures (listed below), and a urine specimen was obtained for calcium and creatinine levels. Following this, the participants were given a standard flavored oral glucose dose of 1.75 g/kg (maximum of 75 g) to be consumed over a 5-minute period. Blood measurements of glucose and insulin were obtained at 30, 60, 90 and 120 min post-ingestion. All samples were drawn between 8 am and 12 pm. Participants were then randomized to either Vitamin D2 (50,000 IU orally once per week) treatment or placebo (also orally once per week) for 12 weeks.
The randomization was performed and maintained by our hospital pharmacist while all other study personnel were blinded to this randomization. The capsules were prepared and provided by the pharmacist for the study period to participants, and instruction was given to maintain a diary of doses taken. All participants were also given calcium carbonate in the form of Tums (500 mg/day). The participants were given periodic reminders via phone call and/or text message based on participant preference by the research assistant to ensure compliance. The participants were also scheduled a 12-week follow-up visit, with instructions.
12-Week follow-up visit
Participants arrived in the fasted state, with their completed drug diary and medication containers, in which a pill count was performed and documented for remaining medications. All baseline evaluations were repeated. At the end of the study procedure, the research assistant was unblinded to the randomization by the pharmacist and a pre-printed prescription for 12 weeks of vitamin D treatment at the same dose used for the treatment group was given to those patients who had been given the placebo for ethical reasons.
Primary and secondary outcome measures
The primary outcome measure was the change in 25(OH) D levels from baseline after 12-week of treatment with vitamin D or placebo.
Secondary outcome measures were insulin-glucose homeostasis measures assessed by calculation of the whole body insulin sensitivity index (WBISI) derived from the Matsuda index, calculation of Homeostasis Model Assessment of Insulin Resistance (HOMA–IR) and area under the curve (AUC) for insulin and glucose [14], [15]. Matsuda index (WBISI) has been validated in children and has been found to correlate with insulin sensitivity derived from insulin clamp studies, the gold standard assessment of insulin sensitivity. Post OGTT AUC and incremental AUC for glucose and insulin were calculated according to published guidelines [16].
HOMA-IR is a validated estimate of insulin resistance derived from product of fasting glucose (mmol/L) and fasting insulin (µU/mL) divided by a constant of 22.5 or when measured in mass units, it is the product of fasting glucose (mg/dL) and fasting insulin (mg/dL) divided by a constant of 405. Studies have shown that HOMA-IR levels of >3.19 in children can be used as a surrogate marker of insulin resistance in non-diabetic children [17].
Other secondary outcome measures were changes in the lipid parameters; i.e., high density lipoprotein (HDL), low density lipoprotein (LDL), triglycerides and total cholesterol. We also collected other variables including urine calcium and creatinine (to monitor for hypercalciuria associated with hypervitaminosis D) and HbA1c levels.
Laboratory assays
All the analytes were measured at the clinical laboratory of the Michigan Children’s Hospital. All the chemistry analytes were measured on Vista 1500. Plasma Insulin was measured using Siemens Immulite 2000. The Insulin assay is a two-site sandwich immunoassay using direct chemiluminescent technology which uses constant amounts of two antibodies. The first antibody, in the Lite Reagent, is a monoclonal mouse anti-insulin antibody labeled with acridinium ester. The second antibody, in the Solid Phase, is a monoclonal mouse anti-insulin antibody, which is covalently coupled to paramagnetic particles. The results were reported in micro-units/ml. Serum 25(OH)D was measured on Centaur XP using chemiluminescent immunoassay and reported in nanograms/ml.
HDL, LDL and total cholesterol were measured and reported in mg/dL. Calcium was measured using dye binding method using spectrophotometry technique and reported in mg/dL. Urine creatinine was measured using an enzymatic method.
Statistical procedures
Descriptive statistics were performed to characterize the study groups. The Kolmogorov-Smirnov test was performed and visual inspection of the distribution of the data was conducted for evaluating normality. Extreme insulin values were observed in 2 patients in the treatment group and 1 patient in the control group. Where indicated, separate analyses with inclusion and exclusion of subjects with extreme values were performed. Baseline fasting insulin, two-hour insulin, WBISI, HOMA-IR, and insulin AUC; post-intervention 25(OH)D, fasting insulin, two-hour insulin, insulin AUC, and triglycerides; and the pre-/post- difference variables, 25(OH)D, fasting insulin, HOMA-IR, total cholesterol, and triglycerides were not normally distributed, thus median and interquartile ranges are presented. Means and standard deviations (for normally distributed, continuous variables), median and interquartile range (for non-normally distributed continuous variables), and frequency (for the categorical variable, gender) were evaluated. The unpaired sample t-test (for normally distributed variables) and the Wilcoxon-Mann-Whitney test (for non-normally distributed data) were used to identify differences between study groups (treatment vs. placebo); the paired t-test was used to test for baseline versus follow-up differences within the same study groups. The effect of vitamin D replacement upon vitamin D status and the associations of 25OHD with lipids, HOMA-IR, fasting insulin, and fasting glucose were visualized by means of spaghetti plots. Partial Pearson correlation analysis was used to test the associations between follow-up 25(OH)D levels and the outcome variables across groups, controlling for the baseline outcome variable being tested. Based on prior studies, a sample size of 30 (15 in each group) would have a 80% power of detecting a difference between the two groups [18], [19]. To allow for a 15% attrition rate, 44 were enrolled. All statistical analyses were performed using SAS software (version 9.2, SAS Institute Inc., Cary NC) and a two-sided P-value of α = 0.05 was considered statistically significant.
Results
A total of 86 patients (100% non Hispanic African Americans) were enrolled in the ED. Of these, 15 patients were deemed ineligible due to normal 25(OH)D levels or high hemoglobin A1c levels, nine patients withdrew after enrollment in the ED and 32 patients were lost to follow-up (12 of which occurred after randomization). Thus only 30 patients completed the study entirely. However, one subject from the vitamin D treatment group did not improve serum 25(OH)D, which instead was decreased. This subject also did not return used medication vials, leading to speculation regarding adherence to study protocol, and was thus removed from the final analysis, leaving a final sample size of 29, with 14 in the placebo group and 15 in the treatment group. Of the 15 patients in the treatment group, two were enrolled in Winter (13%), three (20%) in Spring, four (27%) in Summer and six (40%) in Fall. Of the placebo group participants, two (14%) were enrolled in Winter, 2 (14%) in Spring, four in Summer (28%) and six (42%) in Fall.
The diary detailing the vitamin D intake including study drug, diet and other sources was recovered in entirety in 20 patients (11 in the study drug arm). Based on this, the average daily total intake of vitamin D in the study group was 5,360 ± 2,240 IU versus 125 ± 60.4 IU in the placebo group.
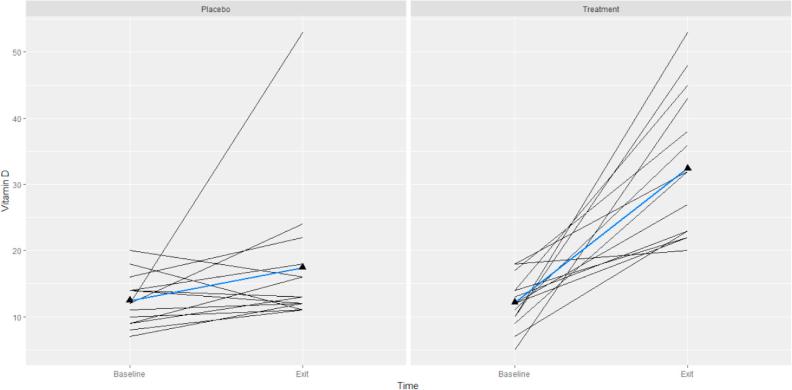
Baseline and follow-up descriptive characteristics by study group are shown in the Table 1. There was no statistical difference between baseline group characteristics. Fig. 1 shows the effect of vitamin D therapy over the course of the treatment. In both placebo and treatment groups, the baseline levels of 25(OH)D did not influence the magnitude of the responses to vitamin D supplementation. Changes of serum vitamin D was significant (p < 0.0001) in the treatment and non-significant (p < 0.126) in the placebo groups, while there was no significant difference in serum 25(OH)D between these two groups at baseline (p = 0.832).
Table 1.
Demographics, baseline and follow up outcome variables of study groups.
| Variable | Baseline |
p-value |
12-Week |
p-value |
p-value |
p-value |
||
|---|---|---|---|---|---|---|---|---|
| Vitamin D | Placebo | Between group | Vitamin D | Placebo | Between group | Vitamin D base/12wk |
Placebo base/12wk |
|
| Age (years) | 15.1 (1.4) | 15.3 (1.4) | 0.513 | n/a | n/a | |||
| Gender (% female) | 86.7 | 64.3 | 0.143 | n/a | n/a | |||
| Weight (kg) | 101.5 (19.0) | 110.5 (26.1) | 0.228 | n/a | n/a | |||
| Height (cm) | 168.9 (7.9) | 168.2 (9.4) | 0.974 | n/a | n/a | |||
| BMI (kg/m2) | 35.4 (5.1) | 38.2 (7.7) | 0.211 | n/a | n/a | |||
| HbA1c (%) | 5.5 (0.3) | 5.6 (0.3) | 0.142 | n/a | n/a | |||
| 25(OH)D (ng/ml) | 12.1 (3.8) | 12.4 (3.8) | 0.832 | 32.0 (22.0, 43.0) | 13.0 (12.0, 18.0) | 0.001 | <0.0001 | 0.126 |
| Fasting insulin (µU/ml) | 18.8 (15.5, 37.1) | 23.3 (17.4, 40.6) | 0.809 | 23.5 (15.7, 38.1) | 23.0 (18.4, 44.4) | 0.550 | 0.747 | 0.947 |
| Fasting glucose (mg/dl) | 82.8 (9.5) | 83.4 (8.2) | 0.900 | 84.5 (7.0) | 85.3 (9.4) | 0.687 | 0.435 | 0.567 |
| 2 h insulin (µU/ml) | 118.9 (52.0, 178.8) | 111.5 (44.7, 194.1) | 0.892 | 70.4 (67.3, 110.7) | 86.6 (73.0, 108.7) | 0.725 | 0.134 | 0.609 |
| WBISI (Matsuda Index) | 2.0 (1.4, 2.7) | 2.4 (1.2, 2.9) | 0.574 | 2.6 (1.3) | 2.8 (1.7) | 0.744 | 0.356 | 0.948 |
| HOMA-IR | 5.1 (3.2, 7.4) | 4.8 (3.8, 8.5) | 0.769 | 5.2 (2.9, 8.8) | 4.6 (3.7, 10.7) | 0.471 | 0.849 | 0.699 |
| Glucose AUC (mg/dl × 120 min) | 104.7 (24.6) | 101.5 (16.1) | 0.686 | 105.1 (22.5) | 102.5 (18.3) | 0.680 | 0.854 | 0.977 |
| Insulin AUC (µU/ml × 120 min) | 146.9 (96.4, 214.0) | 141.1 (72.0, 212.2) | 0.977 | 112.5 (75.2, 145.6) | 94.6 (71.1, 181.3) | 0.556 | 0.279 | 0.542 |
| Total cholesterol (mg/dl) | 154.1 (24.3) | 155.9 (38.5) | 0.978 | 151.4 (27.5) | 144.4 (38.4) | 0.469 | 0.278 | 0.433 |
| HDL (mg/dl) | 46.9 (10.2) | 41.9 (11.7) | 0.188 | 44.5 (11.0) | 41.4 (12.5) | 0.414 | 0.162 | 0.791 |
| LDL (mg/dl) | 203.3 (56.4) | 219.5 (60.0) | 0.639 | 215.3 (44.6) | 240.9 (77.6) | 0.274 | 0.531 | 0.094 |
| Triglycerides (mg/dl) | 72.9 (28.6) | 79.9 (22.3) | 0.434 | 69.0 (49.0, 73.0) | 68.0 (52.0, 98.0) | 0.462 | 0.408 | 0.950 |
Data are presented as frequency, mean (SD), or median (interquartile range). Bolded values indicate significant difference between groups (P ≤ 0.05).
Fig. 1.
Spaghetti plot of 25 (OH) D (ng/ml) over the course of the trial by study groups (placebo and treatment) for all subjects. Each subject shown as a separate line. The blue lines represent the average trend in our data (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
In the treatment group at the 12-week follow-up, eight patients were vitamin D sufficient with 25(OH)D levels >30 ng/ml and seven patients were insufficient with levels between 20 and 30 ng/ml. None of the participants had serum 25(OH)D in the toxic range. In the placebo group at the 12-week follow up, 11 study participants remained deficient with serum 25(OH)D levels of <20 ng/ml and three participants were insufficient with 25(OH)D levels of 20–30 ng/ml. None of the patients developed hypercalciuria and serum calcium levels remained normal in both groups pre- and post-treatment. It is important to note that serum 25(OH)D was not in the elevated range for any of the participants indicating the safety of the current treatment.
Participants had markedly elevated HOMA-IR at baseline and at follow-up in both groups (Table 1). There was no significant increase from baseline in the 12 week fasting insulin, HOMA IR, or lipid levels in either group.
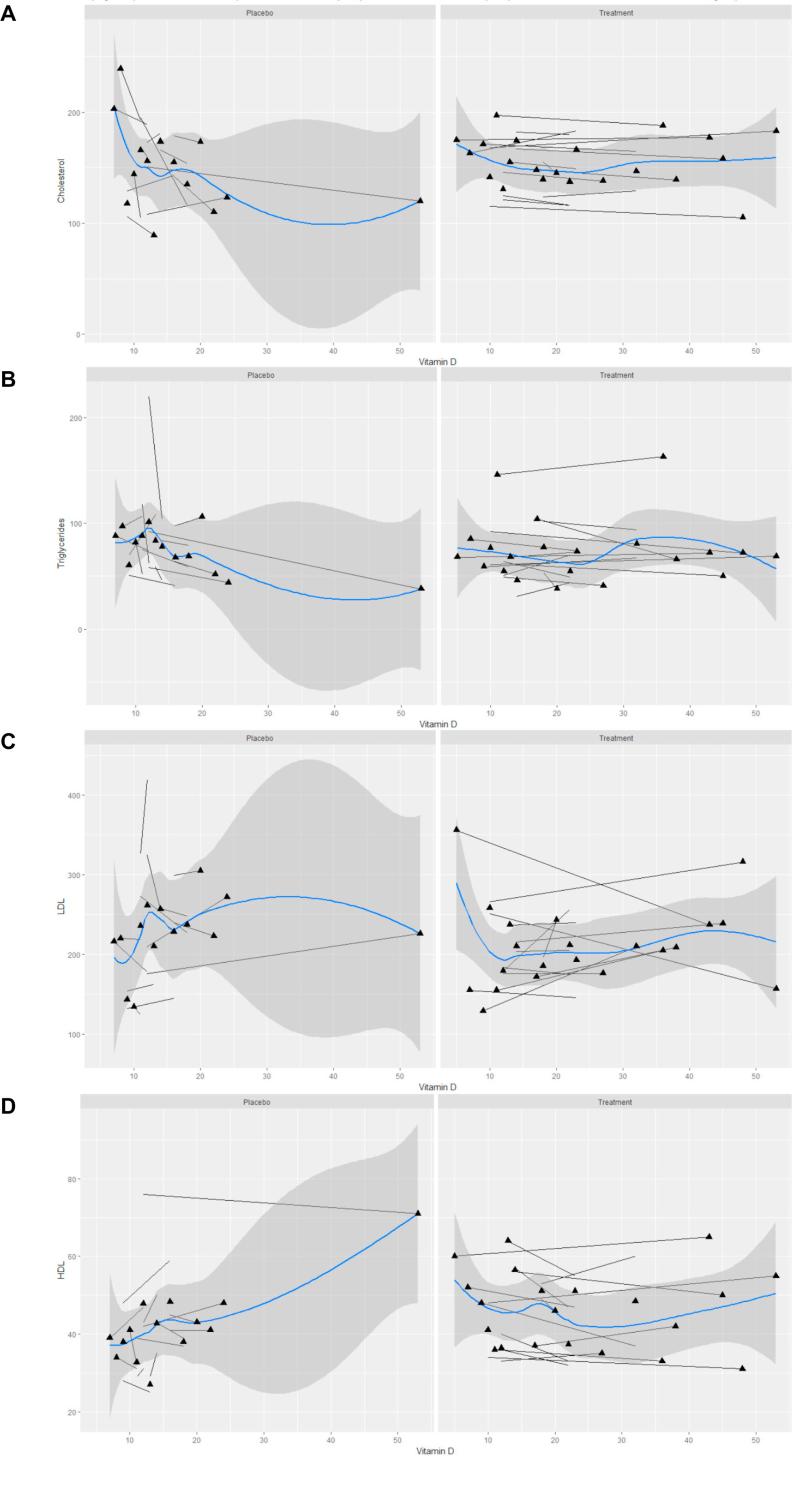
The associations between 25(OH)D levels and different serum lipids are shown in Fig. 2. For these measures, in placebo group, the variability between individuals was more than that in the study group over the course of the trial. Non-linear associations between these variables were observed. The effect of 25(OH)D upon lipid outcomes shows that vitamin D therapy improved serum lipids levels when considering the levels of all serum lipids as a whole. However, only changes in HDL were correlated with post-supplementation serum vitamin D status in our study (r = 0.57, p < 0.027).
Fig. 2.
Spaghetti plot for the associations of serum 25OHD (ng/ml) with lipids: cholesterol (A), Triglyceride (B), Low-density lipoprotein (LDL) (C) and High-density lipoprotein (HDL) (D) cholesterols by study groups (placebo and treatment). Each subject shown as a separate line. The blue line represents average trends in our data. The shaded area depicts the standard error (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
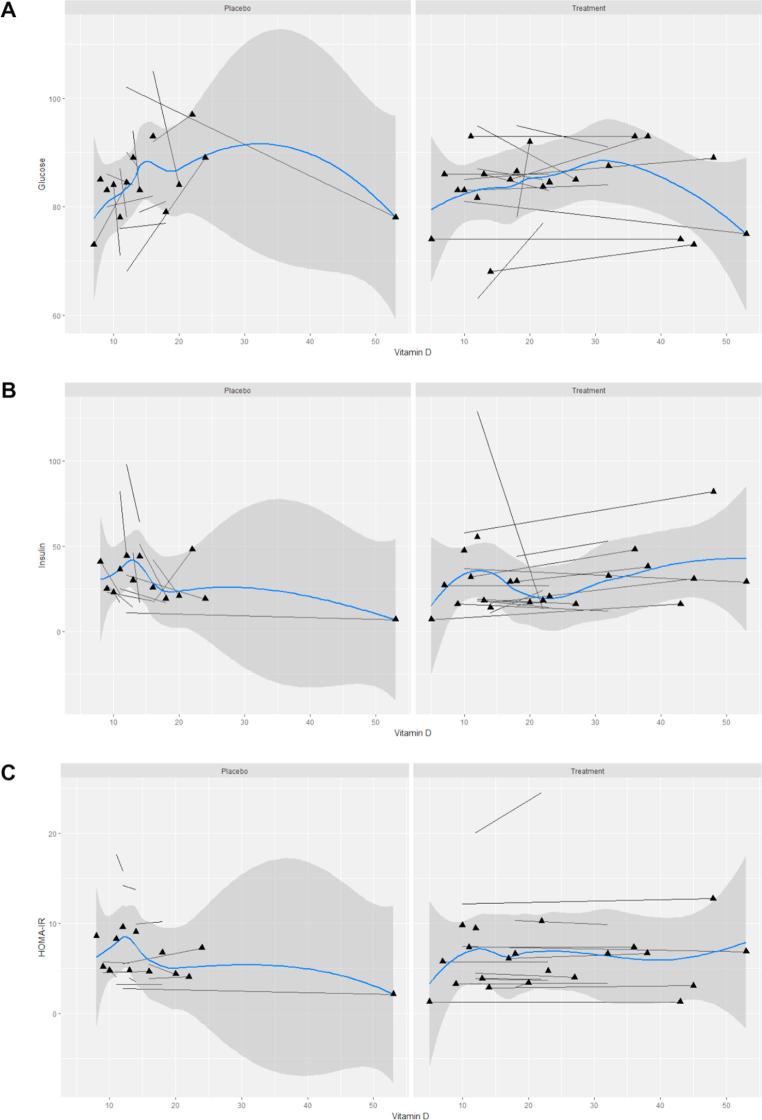
Fig. 3 demonstrates the associations of 25(OH)D levels with fasting glucose, fasting insulin and HOMA-IR. Again these variables were non-linearly correlated. Our findings revealed that any alteration in HOMA-IR, an index that includes fasting insulin and fasting glucose levels, was not significantly correlated with 25(OH)D concentrations following the trial (r = 0.38, p = 0.186). Consistently, in Fig. 3, no relationship was found between this index and serum vitamin D status.
Fig. 3.
Spaghetti plot for the associations of serum 25(OH) D (ng/ml) with fasting glucose (A), fasting insulin (B), Homeostatic model assessment for insulin resistance (HOMA-IR) (C) by study groups (placebo and treatment). Each subject shown as a separate line. The blue line represents average trend in our data. The shaded area depicts the standard error (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
In the Vitamin D-treated group, follow-up 25(OH)D was positively correlated with follow-up fasting insulin (r = 0.5, P < 0.05) and HDL (r = 0.6, P < 0.05) but not HOMA-IR (r = 0.5, P = 0.08). BMI did not influence these associations. These relationships were not observed for baseline measures (data not shown). In the placebo group, follow-up (or baseline) 25(OH)D was not correlated with any follow-up insulin- or lipid-related parameter. Exclusion of subjects with extreme insulin values did not alter these findings (data not shown).
Discussion
In vitamin D-deficient obese AA children, vitamin D treatment at 50,000 IU per week for 12 weeks significantly improved their serum 25(OH)D levels. However, significant changes in insulin secretion and sensitivity were not observed in either group. This study is unique in assessing whether vitamin D repletion may independently improve insulin- and lipid-related parameters short- term in obese AA children with vitamin D deficiency.
The effect of vitamin D repletion on diabetes risk has been equivocal across populations [18], [19], [20], [21], [22]. The discrepant findings may be explained by inherent population differences and/or the specific metabolic effect studied and interpreted. Vitamin D may be most effective for insulin secretion rather than insulin sensitivity in non-diabetic 25(OH)D deficient obese African Americans. Vitamin D has been shown to be required for maintenance of normal glucose tolerance and insulin release in animals and humans [21], [22], [23], [24]. Prolonged depletion leads to failure of insulin secretion which over time becomes irreversible [25]. Similar to the findings of Harris et al, while fasting insulin values increased with vitamin D treatment, in our study they remained within the normal range [19]. This finding could be viewed as adverse in terms of glucose homeostasis, but within physiological norms, raised insulin may lend beneficial metabolic effects, further evidenced by an observed decrease in HDL. Fasting insulin is important for utilization of peripheral tissues for fasting systemic glucose, largely produced and secreted by the liver.
Even though all the participants in our study had elevated baseline HOMA-IR, there was no significant improvement with vitamin D repletion. This study adds to the field by demonstrating the lack of effect of adequate serum 25(OH) D levels on insulin sensitivity. Boucher et al were the first to report a positive association between 25(OH)D and oral glucose tolerant test-induced insulin secretion (30 min post-OGTT) in an Asian adult population [21]. Another randomized trial including 61 pre-diabetics (35-55y) treated with two intramuscular injections of 300,000 units of vitamin D one month apart, showed an increase in mean HOMA-IR and a decrease in Matsuda index (WBISI) at two months’ follow-up [26]. Authors hypothesized that this finding was due to genetic predisposition to insulin resistance which could not be overcome vitamin D administration. Furthermore, Harris et al, noted worsening insulin resistance in adult African Americans after treatment with 4000 IU of vitamin D daily [18].
These reports, in addition to other equivocal studies across diverse populations varying by life stage, degree of glucose tolerance, and/or weight status, may explain the lack of perceived benefit of vitamin D replacement on glycemic control. Prophylaxis may be most important in certain populations, such as obese AA children with vitamin D deficiency. Change in metabolism associated with improving vitamin D status may rely on energy delivery and similar to the characteristic increase in insulin resistance with pubertal progression, perhaps a similar phenomenon occurs. Thus larger randomized controlled studies are required to determine optimal dose and duration of treatment of AA children with vitamin D deficiency and insulin resistance.
Although lipid parameters did not differ between groups or from baseline to follow-up, which is similar to the study by Ponda et al, our study observed a positive correlation between 25(OH)D and HDL in the vitamin D-treated group at follow-up [27]. While we did not observe an increase in triglycerides, there was a marginal correlation between triglyceride level and 25(OH)D in the placebo group only who did not increase 25(OH)D levels. It is possible that the duration of 12 weeks was insufficient to observe significant differences in lipid profile as suggested in some adult literature. Hence more studies may be required to determine if this trend persists with long-term monitoring in children.
It is important to note that in our study, treatment with high doses of vitamin D with supplemental calcium for 12 weeks did not result in hypercalciuria or other adverse effects in any of our patients thus suggesting safety of this treatment dose and duration in teens.
Limitations
Our study had several limitations. This study included a homogenous cohort of obese AA teens. While this lends for insight into this specific population, findings cannot be extrapolated to other populations. Further our study sample was limited by significant dropouts and lost to follow-up and it is possible that results may have been impacted by the lower numbers. In our study, there were significantly more numbers of females that were randomized than males which may have contributed to differences in insulin resistance and secretion as well. Another limitation of the study is lack of adequate daily calcium intake by the participants. Even though as part of the study participants consumed Tums 500 mg daily (equivalent to elemental calcium 200 mg daily), this is well below the recommended daily allowance for calcium. Further, we did not measure BMI at the 12 week follow up visit, and did not have a measure of pubertal status. It is possible that BMI and pubertal changes in 12 weeks may have contributed to insulin resistance and impacted our study results. However, given that all participants were both categorized as obese and AA, less variability in body habitus and maturation by age would be expected.
Conclusion
Improvement of vitamin D levels in obese AA teens led to a positive correlation between 25(OH)D and fasting insulin which remained within physiologically normal concentrations, and a positive correlation between 25(OH)D and HDL after 12-weeks. This was not observed in the placebo group who did not improve vitamin D status. Improvement in serum 25(OH)D levels may contribute to higher fasting insulin, but not insulin sensitivity, and seems to also have a beneficial impact on lipid metabolism. Larger studies are required over a longer period of time to confirm and explore these findings.
Acknowledgments
We thank the Blue Cross Blue Shield of Michigan foundation for their support of this study. This study was funded by the Blue Cross Blue Shield of Michigan Foundation Investigator Initiated Research Program.
We would also like to thank the following people for their help with the study: Courtney Cartrette, Research Assistant, Brigete Webb, Research Assistant, Dr.Victoria Popescu, Research Assistant, Latoya Jean Pierre, Research Assistant. We would like to thank our research coordinator, Elizabeth Duffy, for her invaluable help with IRB, study documents and oversight of the study progress.
Contributor Information
Usha Sethuraman, Email: usethu@dmc.org.
Lynae Hanks, Email: thanks@southernresearch.org.
Minoo Bagheri, Email: bagherim@uab.edu.
Ambika Ashraf, Email: AAshraf@peds.uab.edu.
References
- 1.Ogden C.L., Carroll M.D., Kit B.K. Prevalence of childhood and adult obesity in the United States, 2011–2012. J Am Med Assoc. 2014;311(8):806–814. doi: 10.1001/jama.2014.732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Daniels S.R., Arnett D.K., Eckel R.H. Overweight in children and adolescents: pathophysiology, consequences, prevention, and treatment. Circulation. 2005;111:1999–2002. doi: 10.1161/01.CIR.0000161369.71722.10. [DOI] [PubMed] [Google Scholar]
- 3.Kumar J., Muntner P., Kaskel F.J. Prevalence and associations of 25-hydroxyvitamin D deficiency in US children: NHANES 2001–2004. Pediatrics. 2009 Sep;124(3):e362–70. doi: 10.1542/peds.2009-0051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zeitz U., Weber K., Soegiarto D.W., Wolf E., Balling R., Erben R.G. Impaired insulin secretory capacity in mice lacking a functional vitamin D receptor. FASEB J. 2003;17:509–511. doi: 10.1096/fj.02-0424fje. [DOI] [PubMed] [Google Scholar]
- 5.Sokol S.I., Srinivas V., Crandall J.P. The effects of vitamin D repletion on endothelial function and inflammation in patients with coronary artery disease. Vasc Med. 2012;17:394–404. doi: 10.1177/1358863X12466709. [DOI] [PubMed] [Google Scholar]
- 6.Pirro M., Manfredelli M.R., Helou R.S. Association of parathyroid hormone and 25-OH-vitamin D levels with arterial stiffness in postmenopausal women with vitamin D insufficiency. J Atheroscler Thromb. 2012;19:924–931. doi: 10.5551/jat.13128. [DOI] [PubMed] [Google Scholar]
- 7.Andrea K., Brooks L.J., Dougherty S. A cross sectional study of vitamin D and insulin resistance in children. Arch Dis Child. 2011 doi: 10.1136/adc.2010.187591. [DOI] [PubMed] [Google Scholar]
- 8.Belenchia A.M., Tosh E.K., Hillman L.S. Correcting vitamin D insufficiency improves insulin sensitivity in obese adolescents: a randomized controlled trial. Am J Clin Nutr. 2013;97(4):774–781. doi: 10.3945/ajcn.112.050013. [DOI] [PubMed] [Google Scholar]
- 9.Poomthavorn P., Saowan S., Mahachoklertwattana P. Vitamin D status and glucose homeostasis in obese children and adolescents living in the tropics. Int J Obes (Lond) 2012 Jan 10;36(4):491–495. doi: 10.1038/ijo.2011.260. [DOI] [PubMed] [Google Scholar]
- 10.Alemzadeh R., Kichler J., Babar G. Hypovitaminosis D in obese children and adolescents: relationship with adiposity, insulin sensitivity, ethnicity, and season. Metabolism. 2008;57(2):183–191. doi: 10.1016/j.metabol.2007.08.023. [DOI] [PubMed] [Google Scholar]
- 11.Ashraf A., Alvarez J., Gower B. Associations of vitamin D and components of the metabolic syndrome in obese adolescent females. Obesity. 2011 Nov;19(11):2214–2221. doi: 10.1038/oby.2011.110. PMID: 21546933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Executive summary: standards of medical care in diabetes. Diabetes Care. 2012;35(1):S11–S63. doi: 10.2337/dc12-s004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.https://www.cdc.gov/growthcharts/2000growthchart-us.pdf.
- 14.Matsuda M., DeFronzo R.A. Insulin sensitivity indices obtained from oral glucose tolerance testing: comparison with the euglycemic insulin clamp. Diabetes Care. 1999 Sep;22(9):1462–1470. doi: 10.2337/diacare.22.9.1462. [DOI] [PubMed] [Google Scholar]
- 15.Matthews D.R., Hosker J.P., Rudenski A.S. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28:412–419. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- 16.Le Floch J.P., Escuyer P., Baudin E. Blood glucose area under the curve. Methodological aspects. Diabetes Care. 1990;13(2):172–175. doi: 10.2337/diacare.13.2.172. [DOI] [PubMed] [Google Scholar]
- 17.Keskin M., Kurtoglu S., Kendirci M. Pediatrics 2005;115;e500; Homeostasis Model Assessment is more reliable than the fasting Glucose/Insulin ratio and Quantitative Insulin sensitivity check index for assessing insulin resistance among obese children and adolescents. Pediatrics. 2005;115(4):e500–3. doi: 10.1542/peds.2004-1921. [DOI] [PubMed] [Google Scholar]
- 18.Boucher B.J., Mannan N., Noonan K. Glucose intolerance and impairment of insulin secretion in relation to vitamin D deficiency in east London Asians. Diabetologia. 1995;38(10):1239–1245. doi: 10.1007/BF00422375. [DOI] [PubMed] [Google Scholar]
- 19.Harris S.S., Pittas A.G., Palermo N.J. A randomized, placebo- controlled trial of vitamin D supplementation to improve glycemia in overweight and obese African Americans. Diabetes Obes Metab. 2012;14:789–794. doi: 10.1111/j.1463-1326.2012.01605.x. [DOI] [PubMed] [Google Scholar]
- 20.Kelishadi R., Salek S., Salek M., Hashemipour M., Movahedian M. Effects of vitamin D supplementation on insulin resistance and cardiometabolic risk factors in children with metabolic syndrome: a triple-masked controlled trial. J Pediatr. 2014;90:28–34. doi: 10.1016/j.jped.2013.06.006. [DOI] [PubMed] [Google Scholar]
- 21.Boucher B.J., Mannan N., Noonan K. Glucose intolerance and impairment of insulin secretion in relation to vitamin D deficiency in East London Asians. Diabetologia. 1995;38(10):1239–1245. doi: 10.1007/BF00422375. [DOI] [PubMed] [Google Scholar]
- 22.Borissova A.M., Tankova T., Kirilov G. The effect of vitamin D3 on insulin secretion and peripheral insulin sensitivity in type 2 diabetic patients. Int J Clin Pract. 2003;57(4):258–261. [PubMed] [Google Scholar]
- 23.Bickle D.D. Clinical counterpoint: vitamin D; new actions, new analogues, new therapeutic potential. Endocr Rev. 1992;13(4):765–784. doi: 10.1210/edrv-13-4-765. [DOI] [PubMed] [Google Scholar]
- 24.Reichel H., Koeffler H.P., Norman A.W. The role of vitamin D endocrine system in health and disease. N Engl J Med. 1989;320(15):980–991. doi: 10.1056/NEJM198904133201506. [DOI] [PubMed] [Google Scholar]
- 25.Gedik O., Akalin S. Effects of vitamin D deficiency and repletion on insulin and glucagon secretion in man. Diabetologia. 1986;29(3):142–145. doi: 10.1007/BF02427083. [DOI] [PubMed] [Google Scholar]
- 26.Iraj B., Aminoroayya A., Amini M. Does the intramuscular injection of vitamin D increase insulin resistance? J Res Pharm Pract. 2012;1(2):60–65. doi: 10.4103/2279-042X.108372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ponda M.P., Huang X., Odeh M.A. Vitamin D may not improve lipid levels: a serial clinical laboratory data study. Circulation. 2012;17(126):270–277. doi: 10.1161/CIRCULATIONAHA.111.077875. 3. [DOI] [PMC free article] [PubMed] [Google Scholar]