Abstract
Background and objectives
Histopathological distinction of various nodular lesions in liver with sufficient sensitivity and specificity is a challenge even in an expert set up. The panel of immunohistochemical markers composed of glutamine synthetase (GS), Glypican3 (GPC3) and heat shock protein 70 (HSP70) was recommended by the International Consensus Group for Hepatocellular Neoplasia group for the differentiation of high grade dysplastic nodule and early hepatocellular carcinoma (HCC). The panel has been extensively validated in the western population. This study aims to test this panel on Indian population on resected, explanted and autopsy cirrhotic and non-cirrhotic liver specimens of HCC.
Methodology
This study was conducted on 39 such liver specimens (12 cirrhotic, 12 pre-cirrhotic and 11 non-cirrhotic, non-fibrotic livers), including 35 cases of HCC over a period of 12 years. Immunohistochemistry was performed with antibodies against GS, GPC3 and HSP70 on the sections containing both malignant and dysplastic nodules.
Results
The diagnostic yield depended upon the nature of background liver pathology and was found to be high for only those HCCs arising in cirrhotic background, when positivity of any two markers was taken to be in favor of HCC (sensitivity—58.33%; specificity—100%). GS had a sensitivity and Negative predictive value of 100% for HCCs arising in cirrhotic livers.
Conclusions
Strong positivity for GS is a highly sensitive marker for HCC in a cirrhotic background regardless of the differentiation of the tumor in Indian population. This may be due to preferential activation of Wnt pathway in Indian patients with cirrhosis. The sensitivity of the panel was too low for detecting HCCs arising in non-cirrhotic livers, even in the pre-cirrhotic chronically inflamed livers, even though the specificity was high. GPC3 and HSP70 appear to be useful as individual markers for HCCs arising in non-cirrhotic livers.
Abbreviations: C, cirrhotic; GPC3, Glypican3; GS, glutamine synthetase; HCC, hepatocellular carcinoma; HGDN, high grade dysplastic nodule; HSP70, heat shock protein 70; ICGHN, International Consensus Group for Hepatocellular Neoplasia; LGDN, low grade dysplastic nodules; NCNF, non-cirrhotic, non-fibrotic livers; PC, pre-cirrhotic
Keywords: hepatocellular carcinoma, dysplastic nodules, glutamine synthetase, heat shock protein 70, Glypican3
Pathological diagnosis of the various nodular lesions of liver is a challenge, especially on small biopsies. A panel of immuno-histochemical markers composed of glutamine synthetase (GS), Glypican3 (GPC3) and heat shock protein 70 (HSP70), where positivity of any two markers was taken to be in favor of hepatocellular carcinoma (HCC), was recommended by the International Consensus Group for Hepatocellular Neoplasia (ICGHN) for the differentiation of high grade dysplastic nodule (HGDN) and early HCC.1 These antigens were selected based on various detailed studies on the multistep hepatocarcinogenesis in cirrhotic livers both on molecular and protein expression levels.2, 3, 4, 5, 6, 7, 8, 9 This panel was tested by Di Tommasso et al. on resection and biopsy specimens of HCC10, 11 and has been validated by other studies.12 All these studies have reported moderate sensitivity and high specificity for the histopathological diagnosis of HCC in cirrhotic livers.10, 11, 12 This panel has created considerable interest in the histopathological diagnosis of HCC in general especially in small biopsies.
HCCs arising in a background of chronic inflammation without cirrhosis is not uncommon and is well recognized.13, 14, 15 and so are the HCCs arising in a non-cirrhotic and non-fibrotic livers.16 There are no studies regarding the usefulness of this panel in HCCs arising in non-cirrhotic livers which have a definitive difference in the clinico-pathological characteristics when compared with cirrhotic livers.16, 17, 18 This study aims to test the diagnostic utility of this panel in HCCs arising in non-cirrhotic livers.
Materials and Methods
All cases of surgically resected specimens of HCC including two explanted cirrhotic livers received during 12-year period in the department of histopathology along with cases of HCC and cirrhosis from recent autopsy archives were studied. Nodular or non-nodular lesions from the adjoining areas of HCCs and from the explanted livers were sampled when possible and studied in detail. The cases with extensive tumor necrosis or non availability of tissue in the blocks and nodular lesions like hepatocellular adenoma, focal and diffuse nodular regenerative hyperplasia and small biopsies were excluded from this study.
After exclusions, this study was conducted on 39 liver specimens of which 34 were surgically resected specimens and one was an autopsy specimen of HCC. The others were cirrhotic explanted livers and an autopsy specimen of macronodular cirrhosis secondary to hereditary tyrosenemia in a four-year old child.
Morphology
The hematoxylin and eosin stained sections were studied and the nodular lesions identified were classified according to International Working Party (1995) classification of nodular lesions of cirrhotic liver.19 A diagnosis of HCC was made based on standard histopathologic criteria and supportive evidence based on reticulin stain and IHC for CD34 and hepatocytic markers when appropriate. The HCCs were classified as well (WD), moderately (MD) and poorly differentiated (PD) tumors based on morphology.
The adjacent liver parenchyma of the cases of HCC were extensively studied and were classified as cirrhotic (C) if there was nodule formation and fibrosis stage five or six according to modified Ishak staging system.20 Chronically inflamed livers with fibrosis stage two to four were considered to be pre-cirrhotic (PC) and other cases with essentially normal adjacent liver parenchyma were classified as non-cirrhotic, non-fibrotic livers (NCNF).
Immunohistochemistry
Formalin-fixed, paraffin-embedded tissue sections were consecutively cut and immunostained with antibodies against GS (0.50 mg/ml, mouse monoclonal, Abcam, England {ab64613}) HSP70 (0.05 mg/ml, mouse monoclonal, Abcam, England {ab2787}) and GPC3 (1.00 mg/ml, rabbit polyclonal, Abcam, England {ab66596}). At least two sections containing the tumor and the adjacent liver were stained in addition to the sections containing the pre-neoplastic lesions and the adjacent liver parenchyma. Sections were hydrated and treated with hydrogen peroxide/methanol for 10 min at room temperature to quench endogenous peroxidase activity. To perform antigen retrieval, sections were placed in citrate buffer (pH 6.0, 0.01 M) and boiled in a pressure cooker for 10 min and allowed to cool for 20 min at room temperature. Incubation with GPC3 was carried out overnight (8 h) at 4 °C while HSP70 and GS were incubated at room temperature for 1 h, followed by 40 min incubation with secondary antibody. Diamino benzidine (DAB) detection was followed by Mayer's hematoxylin for nuclei counterstaining.
Interpretation
Staining for GPC3 (cytoplasmic/membranous staining) and HSP70 (nucleocytoplasmic staining) were classified as positive in samples with more than 10% immunoreactive lesional hepatocytes. Control sections were stained and evaluated with each batch. GS was considered positive when staining was diffuse and unrelated to perivenular areas in more than 50% of tumor cells. Non-lesional immunoreactive perivenular hepatocytes were used as an internal control. The patterns of staining were also documented (Table 3). Positivity for at least two markers was taken to be in favor of HCC (Figure 1, Figure 2).
Table 3.
Patterns of Staining of HSP70, Glutamine Synthetase and Glypican3 in HCC.
| Antibody | Pattern of staining | Interpretation (positive/negative) |
|---|---|---|
| Glutamine synthetase (GS) | Diffuse strong cytoplasmic positivity unrelated to vascular structures/septae | Positive (19/35) |
| Patchy strong positivity along the septae | Negative (11/35) | |
| HSP70 | Diffuse strong cytoplasmic with nuclear positivity | Positive (17/35) |
| Isolated cytoplasmic positivity | Negative (4/35) | |
| Glypican3 (GPC3) | Cytoplasmic positivity/strong membranous positivity in >10% of cells | Positive (10/35) |
| Occasional cells positive/weak cytoplasmic staining | Negative (8/35) |
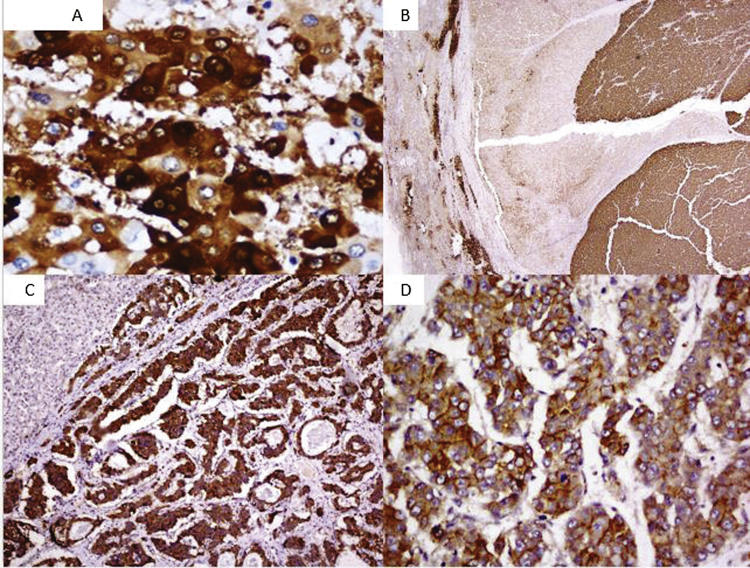
Figure 1.
(A) Strong cytoplasmic staining of glutamine synthetase (magnification 400×). (B) Differential positivity of glutamine synthetase within the same tumor, adjacent liver parenchyma shows positivity in the perivenular hepatocytes (magnification 40×). (C) Diffuse and strong cytoplasmic of Glypican3 staining in the tumor cells. The adjacent liver parenchyma is negative. (magnification 100×). (D) Strong membranous positivity of Glypican3 in more than 10% tumor cells (magnification 400×).
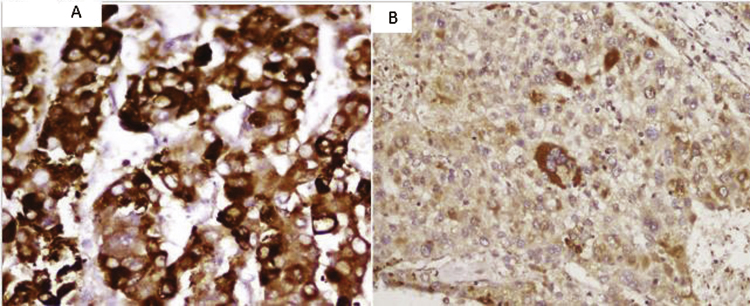
Figure 2.
(A) Strong nucleocytoplasmic staining of heat shock protein 70 (HSP70) in the tumor cells (magnification 400×). (B) Cytoplasmic positivity with no nuclear positivity, considered to be negative (magnification 100×).
Statistical Analysis
Data were described as number and percentage. Chi-square test was used to compare categorical data. P value less than 0.05 was considered statistically significant. Sensitivity was calculated as the proportion of tumors resulting in positive tests. Specificity was calculated as the proportion of non-HCC lesions resulting in negative tests. The positive predictive value was calculated as the proportion of positive tests that correctly identified HCC lesions. The negative predictive value was calculated as the proportion of negative tests that correctly identified non-HCC nodules. Receiver operator characteristic analysis (ROC) were attempted when appropriate. Calculations were done using Statistical Package for Social Sciences (SPSS Inc, Chicago, IL Version 17.0 for Windows).
Results
Characteristics of Patients
Twelve cases of HCC had background cirrhosis and 12 cases had irregular portal tract fibrosis (stage two to four) along with mild to moderate portal tract inflammation indicating an ongoing chronic hepatitis and were considered to be pre-cirrhotic. A total of 11 patients (seven cases with cirrhosis and four cases with chronic hepatitis) were positive for Hepatitis B, five cases (two cases with cirrhosis and three cases with chronic hepatitis) were positive for Hepatitis C and two cases were positive for autoantibodies (one case with cirrhosis and one case with chronic hepatitis). The serological status of the rest of the patients (two patients with cirrhosis and four patients with chronic hepatitis) were unknown. The remaining 11 cases did not show any evidence of fibrosis, steatosis or inflammation in the non-tumorous parenchyma and were essentially normal. The characteristics of these patients are given in Table 1.
Table 1.
Histological Characteristics of the Cases of Hepatocellular Carcinoma.
| Background liver | Cirrhotic (C) | Non-cirrhotic non-fibrotic (NCNF) | Pre-cirrhotic (PC) |
|---|---|---|---|
| No. of HCC cases | 12 (2WD+9 MD+1PD) | 11 (4WD+5MD+2PD) | 12 (2WD+6MD+4PD) |
| Tumor size | 4.93 ± 2.53 cm | 10.98 ± 5.89 cm | 7.31 ± 2.07 cm |
| Age | 50.17 ± 14.76 | 54.82 ± 12.96 | 58.08 ± 9.50 |
| Sex ratio (M:F) | 12:0 | 9:2 | 12:0 |
WD, well differentiated; MD, moderately differentiated; PD, poorly differentiated.
Histological Features
Eight of the tumors were WD, 20 were MD and seven were PD. No early HCCs could be identified from the specimens. A number of non-malignant lesions were identified on studying the adjacent liver of the HCC cases and the explant livers. The lesions are given in Table 2.
Table 2.
Non-Malignant Lesions Identified in the Adjacent Liver Parenchyma.
| Lesion | Number of nodules | Size |
|---|---|---|
| Low grade dysplastic nodules (LGDN)/large regenerative nodules (LRN) | 7/2 | 0.61 ± 0.17/1.75 ± 0.35 cm |
| High grade dysplastic nodule (HGDN) | 1 | 1.6 cm |
| Foci of altered hepatocytes (FAH) | 2 | <1 mm |
Only one HGDN was identified among all cases, in an explanted liver of a 61 year old female with NASH related cirrhosis. The diagnosis of HGDN was made on morphological basis by the absence of stromal invasion and preserved reticulin pattern as in accordance to the consensus nomenclature. The low grade dysplastic nodules (LGDNs) and large regenerative nodules (LRNs), identified in seven different cases of HCC were considered together in the same group for further comparisons. The liver specimen of the patient with hereditary tyrosinemia showed only macroregenerative nodules/LGDNs.
Immunohistochemistry
Overall Staining Pattern of Glutamine Synthetase, Glypican3 and Heat Shock Protein 70
Among the non-malignant lesions, none of the cirrhotic nodules, LRNs or LGDNs, foci of altered hepatocytes or benign hepatocytes showed any positivity for any of the three antibodies. However the single HGDN showed focal strong positivity to GS in a small group of cells.
As regards to the malignant lesions, GS had the highest sensitivity in our study being positive in 19/35 (54.28%) cases followed by HSP70, 17/35 (48.57%) and GPC3, 10/35 (28.57%) of cases of HCC. Positivity with all three antibodies was observed in only 3/35 (8.5%) cases. 12/35 cases (34.28%) showed positivity for at least two markers and 28/35 (80%) tumors were positive for at least one marker. Seven (20%) cases were negative for all three antibodies.
Diagnostic Yield
The overall sensitivity of at least two antibodies being positive for the diagnosis of HCC was 34.28% and specificity was 100%. The negative predictive value was 59.5%. Differentiation of the tumors did not appear to affect the positivity for these markers, however tumors arising in cirrhotic, pre-cirrhotic and normal liver background had specific patterns of positivity with different antibodies in the panel.
Of the 12 cases of HCC arising in a cirrhotic background, all cases (100%) showed diffuse cytoplasmic positivity unrelated to the vascular structures for GS. The staining characters of GS in the tumor cells were strong and diffuse when compared to the granular character of that of the perivenular normal hepatocytes. Six cases (50%) showed convincing nucleocytoplasmic staining with HSP 70. However two cases (one MD and one WD) showed strong cytoplasmic staining with very occasional nuclear positivity which was considered negative in the present study. Only three cases (25%) showed convincing granular cytoplasmic staining in >10% tumor cells with GPC3. Two cases (one WD and one PD) showed positivity with all three antibodies. None of the tumors arising in a cirrhotic background was negative with all three antibodies. The sensitivity and specificity pattern of the IHC panel in tumors arising in a cirrhotic background is depicted in Table 4.
Table 4.
Usefulness of the IHC Panel in Diagnosis of HCC Arising in Cirrhotic Liver.
| NM (n = 10) |
M (n = 12) |
Sensitivity | Specificity | PPV | NPV | |
|---|---|---|---|---|---|---|
| All 3 positive | 0 | 2 | 16.67% | 100% | 100% | 50% |
| At least 2 positive | 0 | 7 | 58.33% | 100% | 100% | 66.67% |
| At least 1 positive | 1 | 12 | 100% | 90.00% | 92.31% | 100% |
| HSP70 + GS | 0 | 6 | 50% | 100% | 100% | 62.50% |
| GS + GPC3 | 0 | 3 | 25% | 100% | 100% | 52.63% |
| HSP70 + GPC3 | 0 | 2 | 16.67% | 100% | 100% | 50% |
| HSP70 | 0 | 6 | 50% | 100% | 100% | 62.50% |
| GPC3 | 0 | 3 | 25% | 100% | 100% | 52.63% |
| GS | 1 | 12 | 100% | 90.00% | 92.31% | 100% |
NM, non-malignant lesions; M, malignant lesions; PPV, positive predictive value; NPV; negative predictive value; HSP70, heat shock protein 70; GS, glutamine synthetase; GPC3, Glypican3.
The sensitivity of two markers being positive was 58.33% while the specificity was 100% in a cirrhotic setting. The most useful combination appeared to be HSP and GS (50% sensitivity). The sensitivity of GS was 100% in case of tumors arising in a cirrhotic background while the specificity was 90.00%, if the focal strong staining of the HGDN is also taken into account.
Among the HCCs arising in pre-cirrhotic livers, only 4 (33.33%) (1 WD, 1 MD and 2 PD) cases showed positivity with two antibodies. Ten (83.33%) cases showed positivity for at least one immune-histochemical marker while two (16.67%) cases (1 WD and 1 MD) were negative for all three markers. GPC3 and HSP70 were the antibodies with higher sensitivity (50%). However, GS showed a dramatic drop in sensitivity to 25%. Similarly, HCC arising in NCNF showed poor diagnostic yield by this panel of antibodies. Five cases (45.45%) were negative for all three antibodies and only one (9.09%) case showed positivity for two antibodies. HSP70 was found more consistently positive than the other antibodies (5/11 cases) with lesser variability of the quality of the staining. The sensitivity of HSP70 remained about the same as the other two groups (45.45%). The sensitivity patterns are depicted in Table 5.
Table 5.
Sensitivity of the Immunohistochemical Panel for Hepatocellular Carcinomas Arising in Pre-Cirrhotic and Non-Cirrhotic, Non-Fibrotic Livers.
| Pre-cirrhotic livers | Non-cirrhotic non-fibrotic livers | |
|---|---|---|
| N = 12 (sensitivity) | N = 11 (sensitivity) | |
| All 3 positive | 1 (8.33%) | 0 |
| At least 2 positive | 4 (33.33%) | 1 (9.09%) |
| At least 1 positive | 10 (83.33%) | 6 (54.55%) |
| HSP + GS | 3 (25%) | 0 |
| GS + G3 | 2 (16.67%) | 0 |
| HSP + G3 | 1 (8.33%) | 1 (9.09%) |
| HSP | 6 (50%) | 5 (45.45%) |
| GPC3 | 6 (50%) | 1 (9.09%) |
| GS | 3 (25%) | 1 (9.09%) |
HSP70, heat shock protein 70; GS, glutamine synthetase; GPC3, Glypican3.
Based on the above observations, it was inferred that the likelihood of a HCC becoming positive with two or more antibodies was the highest when the background liver was cirrhotic (P < 0.05).
Discussion
Though the specificity of the panel appeared to be 100%, when positivity for two antibodies is taken to be in favor of HCC, the overall sensitivity (34.28%) was too low for routine use of this panel for the histopathological diagnosis of HCC in the wide sense.
However, the diagnostic yield of this panel was found to be maximum for HCCs arising in a cirrhotic background (sensitivity—58.33%; specificity—100%). All the twelve cases included showed convincing positivity for at least one marker. The striking observation in the present study is regarding GS, whose sensitivity was 100% for HCCs arising in a cirrhotic background while the specificity was 90.00%. Moreover, the negative predictive value for GS is 100% in the present study. This is in contrast to the original observations by Tommaso et al. as well as in other studies that followed.11, 12, 13 A combination of two antibodies appear to have decreased the sensitivity (from 100% to GS alone to 58.33% for a combination of 2 antibodies) without affecting the specificity much. Another observation in this study is the sensitivity of GPC3 which was 25% in comparison to the reported sensitivity of around 60% in HCCs arising in cirrhotic livers.10, 11, 12 None of the non-malignant nodules or benign hepatocytes qualified to enter into differential diagnosis of early HCC by staining positive with two antibodies indicating the strong specificity of this panel for malignant lesions. Area under the curve is almost one in ROC analysis when plotted for single, two and all three antibodies being positive for classifying a lesion as HCC, again indicating the strong specificity of the panel. A comparison of reported sensitivity/specificity patterns of the panel in HCCs arising in cirrhotic livers is represented in Table 6.
Table 6.
Comparison of Available Studies on the Panel in HCCs Arising in Cirrhotic Livers:.
| GS Sensitivity & specificity |
HSP Sensitivity & specificity |
GPC3 Sensitivity & specificity |
Most useful combination | |
|---|---|---|---|---|
| Present study (resection specimens) | 100% & 90% | 50% & 100% | 25% & 100% | HSP70 + GS |
| Di Tommaso et al. (resection specimens)11 | 59.38% & 86.36% | 78.13% & 95.45% | 68.75%& 90.91% | HSP70 + GPC3 |
| Di Tommaso et al. (small biopsies)12 | 57.9% & 96% | 40.4% & 90% | 61.4%&92% | GPC3 + GS |
| Tremosini et al. (small biopsies)13 | 50.0% & 90.0% | 57.5% & 85.0% | 57.5% & 95.0% | GPC3 + HSP70 |
GS is a well-recognised target of Wnt/β catenin pathway and it catalyzes the synthesis of glutamine, which is the major energy source of tumor cells.20 GS immunostaining is suggested to identify a subgroup of HCCs with specific epidemiologic and genetic profiles with improved overall survival.21 The consistent and strong reactivity with GS suggests more prevalent activation of Wnt signaling pathway in Indian patients with cirrhosis. In addition, it can be hypothesized that Wnt signaling pathway activation could be the key transforming event in our study population. One of the factors explaining this phenomena might be the fact that most of the available literature on HCC are from Europe, USA or Japan where the brunt of the HCC load is due to HCV.22 This is in contrast to the Indian scenario where HBV is the major etiological agent in HCC development. The genotypes of HBV (A and D) and HCV (2, 3, 5 and 6) are the most prevalent in India which are less virulent and are less likely to progress to HCC probably accounting for the low incidence status of India for HCC.23, 24 Unfortunately, we do not possess the genotype information for many of the patients in our study to support this notion. The possible association of GS positivity, and thereby Wnt signaling pathway and infection with these less virulent forms of Hepatitis B virus also merits further exploration in our population, especially in an era of targeted therapy.25, 26 GS immunostaining appears to have the potential of identifying candidates for such targeted therapy in future.
Apart from the fall in sensitivity, the staining characteristics also changed dramatically with GS in pre-cirrhotic (25%) and non-cirrhotic livers (9.09%), with increasing cases showing differential positivity within the tumor area, which are more likely to be misrepresented in small biopsies.
GPC3 appears to be expressed more often in HCCs arising in pre-cirrhotic livers (50%) and rarely in those of NCNF in our material. Apart from its diagnostic value, mechanistic insight into the roles of GPC3 and of c-Myc in the development of HCC are being investigated recently.27 Moreover the expression of GPC3 and its mRNA in the tumor tissue has been shown to correlate with worse prognosis.28, 29 The rise in GPC3 sensitivity in pre-cirrhotic livers might extrapolated to the activation of c-myc oncogene pathway, which could be serving as an alternative pathway of hepatocarcinogenesis in pre-cirrhotic livers.27
HSP70 is found to be one of the most abundantly upregulated gene in early HCC.6 The sensitivity of HSP remained constant (50%) irrespective of the background pathology and the specificity was 100%.
Though the data for comparison for staining patterns in HCC arising from pre-cirrhotic and normal liver backgrounds is not available in the available literature, the difference in the staining pattern between HCCs arising in a cirrhotic background and non-cirrhotic background can be explained by the possible differences in the hepatocarcinogenesis. As reported by Trekova et al., the genomic profiles of HCCs arising in a non-cirrhotic background is different from the one that develops in a cirrhotic background.17Alteration of the p53 pathway was found to be playing a more important role in the pathogenesis of HCC associated with cirrhosis, whereas alterations in cell cycle regulators p21waf1/cip1 and p27Kip1 play a more important role in the pathogenesis of non-cirrhotic HCC.17 The results of our studies also supports a notion of an alternate molecular pathway of hepatocarcinogenesis in non-cirrhotic livers on the basis of differences in staining pattern. However, it also makes this panel unsuitable for diagnosis of HCCs arising in a non-cirrhotic background, even in pre-cirrhotic livers.
In contrast to the west, where the screening protocols for cirrhotic patients are well established and followed and thereby resulting in detection of early lesions, the Indian scenario is different where patients often present in advanced stages with large tumor sizes,23, 24 which might be one of the factors explaining non-availability of early HCCs in the present study. This is true for many malignancies in the Indian set up, where many patients present with multiple liver metastasis. In addition, the prevalence of HCCs among the non-cirrhotic livers also appears to be high when compared to literature. With increasing number of small biopsies and FNACs being done for space occupying lesions diagnosed on imaging, the need of the hour is a robust panel of antibodies which would effectively confirm the diagnosis of HCC and rule out benign hepatocellular lesions as well as other metastatic malignancies.
Two tissue microarray based studies by Lagana et al. have tested this panel of antibodies in the context of hepatocellular adenomas, metastatic tumors and cholangiocarcinomas.30, 31 They have reported that only GPC3 shows a reliable degree of specificity to HCC, i.e., it effectively indicated malignancy as well as of hepatocellular origin. While HSP-70 immunoreactivity was noted in around 88% of intrahepatic cholangiocarcinomas and metastatic tumors, GS was positive in around 70% of the same.30, 31 indicating that the reactivity does not confirm hepatocellular differentiation.
In conclusion our study suggests that a diagnosis of HCC should largely depend on morphology. Regardless of the tumor differentiation, a diffuse and strong reactivity for GS strongly supports a diagnosis of HCC if the background liver is cirrhotic or shows an advanced fibrosis by radiology in Indian scenario. Negativity for this marker helps to rule out HCC in an expert set up. Positivity for two of the markers is highly specific for HCC. However, in non-cirrhotic livers, including the pre-cirrhotic ones, i.e., with histological fibrosis stage four or less, this panel appears to have too low sensitivity for routine diagnosis of HCC. However, HSP70 and GPC3 are useful as single markers, in supporting a diagnosis of HCC. The positivity with these antibodies should be interpreted in context as they probably indicate a pathomechanism rather than hepatocellular origin of the lesion thus an additional marker of hepatocellular differentiation should be used when appropriate.
Authors’ Contribution
Dr. Preithy Uthamalingam: Conception and design of the work, data acquisition, interpretation and analysis, drafting the article.
Prof. Ashim Das: Conception and design of the work, data interpretation and analysis, drafting and revising the article.
Prof. Arunanshu Behra: Data interpretation and revision of drafts.
Dr. Naveen Kalra: Data interpretation and revision of drafts.
Prof. Yogesh Chawla: Data interpretation and revision of drafts.
Conflicts of Interest
The authors have none to declare.
References
- 1.Pathologic diagnosis of early hepatocellular carcinoma: a report of the International Consensus Group for Hepatocellular Neoplasia. Hepatology. 2009;49:658–664. doi: 10.1002/hep.22709. [DOI] [PubMed] [Google Scholar]
- 2.Capurro M., Wanless I.R., Sherman M. Glypican-3: a novel serum and histochemical marker for hepatocellular carcinoma. Gastroenterology. 2003;125:89–97. doi: 10.1016/s0016-5085(03)00689-9. [DOI] [PubMed] [Google Scholar]
- 3.Midorikawa Y., Ishikawa S., Iwanari H. Glypican-3, overexpressed in hepatocellular carcinoma, modulates FGF2 and BMP-7 signaling. Int J Cancer. 2003;103:455–465. doi: 10.1002/ijc.10856. [DOI] [PubMed] [Google Scholar]
- 4.Nakatsura T., Yoshitake Y., Senju S. Glypican-3, overexpressed specifically in human hepatocellular carcinoma, is a novel tumor marker. Biochem Biophys Res Commun. 2003;306:16–25. doi: 10.1016/s0006-291x(03)00908-2. [DOI] [PubMed] [Google Scholar]
- 5.Sung Y.K., Hwang S.Y., Park Y.N. Glypican-3 is overexpressed in human hepatocellular carcinoma. Cancer Sci. 2003;94:259–262. doi: 10.1111/j.1349-7006.2003.tb01430.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chuma M., Sakamoto M., Yamazaki K. Expression profiling in multistage hepatocarcinogenesis: identification of HSP70 as a molecular marker of early hepatocellular carcinoma. Hepatology. 2003;37:198–207. doi: 10.1053/jhep.2003.50022. [DOI] [PubMed] [Google Scholar]
- 7.Christa L., Simon M.T., Flinois J.P., Gebhardt R., Brechot C., Lasserre C. Overexpression of glutamine synthetase in human primary liver cancer. Gastroenterology. 1994;106:1312–1320. doi: 10.1016/0016-5085(94)90024-8. [DOI] [PubMed] [Google Scholar]
- 8.Osada T., Sakamoto M., Nagawa H. Acquisition of glutamine synthetase expression in human hepatocarcinogenesis. Relation to disease recurrence and possible regulation by ubiquitin-dependent proteolysis. Cancer. 1999;85:819–831. doi: 10.1002/(sici)1097-0142(19990215)85:4<819::aid-cncr9>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 9.Llovet J.M., Chen Y., Wurmbach E. A molecular signature to discriminate dysplastic nodules from early hepatocellular carcinoma in HCV cirrhosis. Gastroenterology. 2006;131:1758–1767. doi: 10.1053/j.gastro.2006.09.014. [DOI] [PubMed] [Google Scholar]
- 10.Di Tommaso L., Franchi G., Park Y.N. Diagnostic value of HSP70, glypican3, and glutamine synthetase in hepatocellular nodules in cirrhosis. Hepatology. 2007;45:725e34. doi: 10.1002/hep.21531. [DOI] [PubMed] [Google Scholar]
- 11.Di Tommaso L., Destro A., Seok J.Y. The application of markers (HSP70, GPC3 and GS) in liver biopsies is useful for detection of hepatocellular carcinoma. J Hepatol. 2009;50:746e54. doi: 10.1016/j.jhep.2008.11.014. [DOI] [PubMed] [Google Scholar]
- 12.Tremosini S., Forner A., Boix L. Prospective validation of an immunohistochemical panel (glypican3, heat shock protein 70 and glutamine synthetase) in liver biopsies for diagnosis of very early hepatocellular carcinoma. Gut. 2012;61:1481–1487. doi: 10.1136/gutjnl-2011-301862. [DOI] [PubMed] [Google Scholar]
- 13.Albeldawi M., Soliman M., Lopez R., Zein N.N. Hepatitis C virus-associated primary hepatocellular carcinoma in non-cirrhotic patients. Dig Dis Sci. 2012;57:3265–3270. doi: 10.1007/s10620-012-2260-y. [DOI] [PubMed] [Google Scholar]
- 14.Kawada N., Imanaka K., Kawaguchi T. Hepatocellular carcinoma arising from non-cirrhotic nonalcoholic steatohepatitis. J Gastroenterol. 2009;44:1190–1194. doi: 10.1007/s00535-009-0112-0. [DOI] [PubMed] [Google Scholar]
- 15.Lee A.T., Lee C.G. Oncogenesis and transforming viruses: the hepatitis B virus and hepatocellularcarcinoma – the etiopathogenic link. Front Biosci. 2007;12:234–245. doi: 10.2741/2061. [DOI] [PubMed] [Google Scholar]
- 16.Young AL1, Adair R., Prasad K.R. Hepatocellular carcinoma within a noncirrhotic, nonfibrotic, seronegative liver: surgical approaches and outcomes. J Am Coll Surg. 2012;214(2):174–183. doi: 10.1016/j.jamcollsurg.2011.10.005. [DOI] [PubMed] [Google Scholar]
- 17.Tretiakova M.S., Shabani-Rad M.T., Guggisberg K., Hart J., Anders R.A., Gao Z.H. Genomic and immunophenotypical differences between hepatocellular carcinoma with and without cirrhosis. Histopathology. 2010;56:683–693. doi: 10.1111/j.1365-2559.2010.03554.x. [DOI] [PubMed] [Google Scholar]
- 18.Lubrano J., Huet E., Tsilividis B. Long-term outcome of liver resection for hepatocellular carcinoma in noncirrhotic nonfibrotic liver with no viral hepatitis or alcohol abuse. World J Surg. 2008;32:104–109. doi: 10.1007/s00268-007-9291-0. [DOI] [PubMed] [Google Scholar]; International Working Party Terminology of nodular hepatocellular lesions. Hepatology. 1995;22:983–993. doi: 10.1016/0270-9139(95)90324-0. [DOI] [PubMed] [Google Scholar]
- 19.Ishak K., Baptista A., Bianchi L. Histological grading and staging of chronic hepatitis. J Hepatol. 1995;22:696–699. doi: 10.1016/0168-8278(95)80226-6. [DOI] [PubMed] [Google Scholar]
- 20.Cadoret A., Ovejero C., Terris B. New targets of beta-catenin signaling in the liver are involved in the glutamine metabolism. Oncogene. 2002;21:8293–8301. doi: 10.1038/sj.onc.1206118. [DOI] [PubMed] [Google Scholar]
- 21.Dal Bello B., Rosa L., Campanini N. Glutamine synthetase immunostaining correlates with pathologic features of hepatocellular carcinoma and better survival after radiofrequency thermal ablation. Clin Cancer Res. 2010;16:2157–2166. doi: 10.1158/1078-0432.CCR-09-1978. [DOI] [PubMed] [Google Scholar]
- 22.Bosch F.X., Ribes J., Diaz M., Cleries R. Primary liver cancer: worldwide incidence and trends. Gastroenterology. 2004;127(5 suppl 1):S5–S16. doi: 10.1053/j.gastro.2004.09.011. [DOI] [PubMed] [Google Scholar]
- 23.Kumar R., Saraswat M.K., Sharma B.C., Sakhuja P., Sarin S.K. Characteristics of hepatocellular carcinoma in India: a retrospective analysis of 191 cases. QJM. 2008;101:479–485. doi: 10.1093/qjmed/hcn033. [DOI] [PubMed] [Google Scholar]
- 24.Paul S.B., Sreenivas V., Gulati M.S. Incidence of hepatocellular carcinoma among Indian patients with cirrhosis of liver: an experience from a tertiary care center in northern India. Indian J Gastroenterol. 2007;26:274–278. [PubMed] [Google Scholar]
- 25.Lachenmayer A., Alsinet C., Savic R. Wnt-pathway activation in two molecular classes of hepatocellular carcinoma and experimental modulation by sorafenib. Clin Cancer Res. 2012;18:4997–5007. doi: 10.1158/1078-0432.CCR-11-2322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pez F., Lopez A., Kim M., Wands J.R., Caron de Fromentel C., Merle P. Wnt signaling and hepatocarcinogenesis: molecular targets for the development of innovative anticancer drugs. J Hepatol. 2013;59:1107–1117. doi: 10.1016/j.jhep.2013.07.001. [DOI] [PubMed] [Google Scholar]
- 27.Li L., Jin R., Zhang X. Oncogenic activation of glypican-3 by c-Myc in human hepatocellular carcinoma. Hepatology. 2012;56:1380–1390. doi: 10.1002/hep.25891. [DOI] [PubMed] [Google Scholar]
- 28.Du J.L., Wang Y.L., Shi H.Y., Guo A.T., Wei L.X. Expression of glypican-3, hepatocyte antigen, alpha-fetoprotein, CD34 and CD10 in hepatocellular carcinoma: a clinicopathologic analysis of 375 cases. Zhonghua Bing Li Xue Za Zhi. 2012;41:309–313. doi: 10.3760/cma.j.issn.0529-5807.2012.05.006. [DOI] [PubMed] [Google Scholar]
- 29.Wang Y.L., Zhu Z.J., Teng D.H., Yao Z., Gao W., Shen Z.Y. Glypican-3 expression and its relationship with recurrence of HCC after liver transplantation. World J Gastroenterol. 2012;18:2408–2414. doi: 10.3748/wjg.v18.i19.2408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lagana S.M., Moreira R.K., Remotti H.E., Bao F. Glutamine synthetase, heat shock protein-70, and glypican-3 in intrahepatic cholangiocarcinoma and tumors metastatic to liver. Appl Immunohistochem Mol Morphol. 2013;21:254–257. doi: 10.1097/PAI.0b013e3182642c9c. [DOI] [PubMed] [Google Scholar]
- 31.Lagana S.M., Salomao M., Bao F., Moreira R.K., Lefkowitch J.H., Remotti H.E. Utility of an immunohistochemical panel consisting of glypican-3, heat-shock protein-70, and glutamine synthetase in the distinction of low-grade hepatocellular carcinoma from hepatocellular adenoma. Appl Immunohistochem Mol Morphol. 2013;21:170–176. doi: 10.1097/PAI.0b013e31825d527f. [DOI] [PubMed] [Google Scholar]