Highlights
-
•
Chronic use of metformin 1500 mg/day may exceed the recommended therapeutic level.
-
•
It is necessary to consider combination therapy to avoid metformin accumulation.
-
•
Obese patients with T2DM are recommended to use metformin with a shorter interval.
Keywords: Metformin, Steady-state level, Patient-factors, Therapeutic dosage, Type-2 diabetes mellitus
Abstract
Aims
This prospective study aimed to analyze metformin steady-state concentration in repeated constant dosage and the influencing patient-factors as well as to correlate them with glycemic control.
Methods
The validated HPLC-UV method was used to examine metformin steady-state concentration, while FBG and glycated albumin were used as the parameters of glycemic control during metformin administration.
Results
A total of 82 type-2 diabetes patients were involved with 32.1% of them having metformin Cssmin and 84.1% having Cssmax of metformin within the recommended therapeutic range. One patient had metformin Css that exceeded minimum toxic concentration despite his normal renal function and administered therapeutic dosage of metformin. Higher Cssmax was found in patients with metformin monotherapy, while patients with longer duration of metformin use had significantly higher Cssmin.
Conclusions
Along with initial hyperglycemia and eGFR, metformin Cssmin became the only parameter that influenced FBG level (P < 0.05). Duration of previous metformin use should be considered in the strategy of optimizing metformin dosage. The type-2 diabetes patients with obesity are more suggested to take shorter interval of metformin administration (or possibly with sustained-release formulation) to keep Cssmin within the therapeutic range.
Introduction
Diabetes mellitus is a chronic metabolic condition affecting 10 million of Indonesian population mostly with type 2 diabetes mellitus (T2DM) in 2015 [1]. While metformin should be prescribed routinely for patients whose blood glucose cannot be controlled merely by lifestyle modification, the plasma steady-state concentration (PSSC) of metformin often seems to be neglected in clinical setting because it is not a priority in therapeutic drug monitoring.
Metformin PSSC could estimate optimum doses, particularly in elderly patients, patients with renal impairment, or even in specific conditions such as pregnancy with PCOS or obesity, due to changes in its pharmacokinetic profiles. In addition, PSSC could confirm inadequacy of metformin doses as well as metformin incompliance [2]. PSSC is achieved after repetitive administrations of metformin at a constant frequency which is expected within the therapeutic range [3]. However, the proposed upper limit of metformin concentration, which is 5 µg/ml to avoid lactic acidosis, should be considered in the metformin therapy management.
To date, studies of metformin PSSC have merely focused on minimum steady-state concentration (Cssmin) in relation to genetic factors [4], [5], [6] despite the widely-acknowledged fact that Cssmax is more recommended for measuring the safety of long-term drug use [3]. Although lactic acidosis in some patients with a higher plasma metformin concentration remains debatable, several studies have found such condition [7], [8], [9], making measuring this level become important to optimize the dosage and prevent metformin-associated lactic acidosis (MALA) [3]. However, most of those studies were conducted retrospectively with unidentified variety of doses, duration, and last metformin dose administered. Therefore, this present prospective study attempts to determine the trough and peak PSSC of metformin with constant dosage and identical time interval (τ) as well as to identify patient-factors associated with metformin PSSC and glycemic control to provide a new approach to clinical recommendation for metformin administration in T2DM.
Subjects, materials and methods
Study design
T2DM patients aged 30–60 years originating prospectively in six primary healthcare centers of Yogyakarta Province, Indonesia were involved. The study included patients administrated with metformin 500 mg twice daily for at least 2 weeks in either single or combined with other antihyperglycemic agents. There were restrictions on the metformin daily dose, time interval, and other medications. Patients taking cimetidine, nifedipine, or furosemide were excluded due to potential interaction that might cause changes in metformin pharmacokinetic [10]. Exception also applied to patients under systemic steroid treatment [11]. Patients with serum creatinine level ≥1.5 mg/dL in men and ≥1.4 mg/dL in women [12], history of thyroid dysfunction [13], and chronic liver disease [14], as well as patients not adhering to metformin therapy could not participate in this research. The study was approved by the Ethics Committee of the Faculty of Medicine of Gadjah Mada University (No. KE/FK/648/EC) and conducted in accordance with the Declaration of Helsinki. A written informed consent was obtained from all the subjects.
Steady-state pharmacokinetics of metformin assay
To measure trough PSSC, blood samples were taken immediately before administration of the next dose (pre-dose). Subsequently, blood samples taken 3.5 ± 0.5 h after metformin ingestion were used to measure peak PSSC (post-dose). Determination of metformin plasma concentrations was done using validated RP-HPLC assay with Sunfire® C-18 column 4.6 × 150 mm × 5 µm from Waters and SM7 injector with UV detector at 233 nm. All parameters for the bioanalytical method have fulfilled the Guidance for Bioanalytical Method Validation by FDA. The linearity of standard curve (r) was 0.9999 with <15% accuracy value (% diff) and <15% precision value (CV). The obtained selectivity value (CV) was <15% while the CV of recovery ranged from 1.22% to 1.89% (in review). Metformin PSSC was used to estimate the elimination rate followed by calculation of metformin half-life.
Statistical analysis
The obtained PSSCs were each presented as the mean ± SD. Independent t-test and one-way ANOVA were performed to compare PSSCs for each group of patient characteristics. Spearman-test was used to investigate patient factors in metformin PSSC and to analyze the influence of metformin PSSC on FBG and GA. Linear regression was used to analyze patient-factors affecting the glycemic control. A p value ≤0.05 was considered statistically significant.
Results
PSSC examination was performed for 83 out of 86 patients, while three of them did not follow the procedure. Meanwhile, one patient had a history of routine use of 1500 mg/day metformin before participating in this study, given therefore a separate discussion.
Steady-state pharmacokinetics of metformin
There were 82 T2DM patients with prescribed 500 mg metformin twice daily. One patient participated in blood sampling only for Cssmax because of his time constraint. Therefore, 81 plasma concentrations for Cssmin and 82 for Cssmax were collected. (Table 1).
Table 1.
Peak concentrations of metformin at steady-state for multiple doses of 500 mg metformin per 12 h.
| Patient Grouping | Frequency (%) | Cssmin (µg/mL) (P Value) | Cssmax (µg/mL) (P Value) |
|---|---|---|---|
| Sex | |||
| Male | 16 (19.5) | 0.706 ± 0.546 | 1.698 ± 1.017 |
| Female | 66 (80.5) | 0.575 ± 0.429 (0.396) |
1.845 ± 0.921 (0.577) |
| Age (years) | |||
| <50 | 33 (40.2) | 0.616 ± 0.430 | 1.815 ± 1.117 |
| ≥50 | 49 (59.8) | 0.589 ± 0.470 (0.792) |
1.817 ± 0.804 (0.992) |
| BMI (kg/m2) | |||
| <30 | 69 (84.1) | 0.623 ± 0.472 | 1.822 ± 0.944 |
| ≥30 | 13 (15.9) | 0.475 ± 0.321 (0.282) |
1.787 ± 0.931 (0.903) |
| eGFR (mL/min/1.73 m2)c | |||
| 40–60 | 6 (7.3) | 0.957 ± 0.546 | 2.244 ± 0.529 |
| >60–120 | 72 (87.8) | 0.586 ± 0.439 | 1.832 ± 0.954 |
| >120 | 4 (4.9) | 0.298 ± 0.275 (0.059) |
0.896 ± 0.416 (0.075) |
| Clcr (mL/min)d | |||
| 40–60 | 9 (11.0) | 0.638 ± 0.563 | 1.907 ± 0.812 |
| >60–120 | 65 (79.3) | 0.607 ± 0.373 | 1.899 ± 0.931 |
| >120 | 8 (9.8) | 0.500 ± 0.844 (0.796) |
1.136 ± 0.900 (0.068) |
| Duration of T2DM (years) | |||
| <5 | 29 (35.4) | 0.593 ± 0.475 | 1.824 ± 1.118 |
| ≥5 | 53 (64.6) | 0.603 ± 0.444 (0.923) |
1.812 ± 0.832 (0.955) |
| Duration of routine use of 500 mg metformin twice daily (weeks) | |||
| 2–6 | 18 (22.0) | 0.415 ± 0.365 | 1.584 ± 0.946 |
| >6 | 64 (78.0) | 0.652 ± 0.463 (0.049)a |
1.882 ± 0.930 (0.235) |
| Antidiabetics Regimenb | |||
| Metformin Monotherapy | 35 (43.2) | 0.611 ± 0.415 | 2.168 ± 0.927 |
| Combination with Sulfonylureas | 46 (56.8) | 0.587 ± 0.488 (0.819) | 1.543 ± 0.868 (0.003)a |
Significance level < 0.05.
One patient with insulin combination therapy was excluded.
CKD-EPI formula.
Cockcroft-Gault formula using IBW for the obese patients.
Table 1 shows that metformin use >6 weeks had 1.57 higher Cssmin than that in patients with 2–6 week administration (P < 0.05). In the monotherapy group, Cssmax was also more significant, reaching 1.44-fold, compared to that in metformin-sulfonylurea patients (glibenclamide or glimepiride). It was also found that metformin t1/2 in patients using it for 2–6 weeks and >6 weeks was each 4.01 h and 6.27 h (not displayed). There was a positive correlation between age and Cssmax as well as Cssmin while increased BMI caused a decrease in metformin Cssmin.
Steady-state pharmacokinetics of metformin in patient with history of 1500 mg/day use
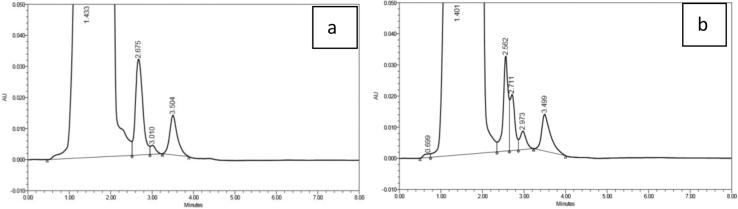
A 58.67-year-old male patient with BMI 20.81 kg/m2 has been diagnosed as having T2DM for ± 6 years with a history of taking metformin 1500 mg/day. The patient had a stroke in February 2015, with no history of liver disorder, COPD and asthma, alcohol consumption, intravenous contract medium use, or sepsis. His eGFR was normal, 94 mL/min/1.73 m2, and he was given valsartan and aspirin. After routine taken of metformin 1500 mg/day since 2014, his dose has been reduced to 500 mg twice daily since January 2015. His PSSC measurement procedure was identical to that of the other 82 patients. His metformin chromatogram is presented in Fig. 1.
Fig. 1.
Chromatogram of plasma metformin in a patient with history of taking 1500 mg metformin per day: (a) Cssmin, (b) Cssmax.
The AUC in chromatogram shows that the metformin Cssmin and Cssmax reached 15.175 µg/mL and 16.198 µg/mL, respectively (or 117.487 µmol/l and 125.407 µmol/l). The patient was then given a blood gas analysis (Table 2).
Table 2.
Biochemical parameters of a patient with T2DM whose metformin Css exceeded MTC.
| Parameter | Reference | Result |
|---|---|---|
| Creatinine | 0.7–1.2 mg/dL | 0.89 |
| eGFR | >60 mL/min/1.73 m2 | 94 |
| Clcr | >60 mL/min | 109.24 |
| Initial FBG | <100 mg/dl | 148 |
| Initial GA | 11–16% | 25.81 |
| Final FBG | <100 mg/dl | 136 |
| Final GA | 11–16% | 27.46 |
| Arterial pH | 7.35–7.45 | 7.479 |
| pCO2 | 35.0–45.0 mmHg | 28.9 |
| pO2 | 80–100 mmHg | 153.80 |
| O2 Saturation | 75.0–99.0% | 98.50 |
| Bicarbonate (HCO3−) | 22–26 mmol/L | 21.7 |
| Total CO2 | 23.0–27.0 mmol/L | 22.5 |
Correlation between metformin PSSC and glycemic control
Among 35 patients, 32 were examined for FBG, two patients were not fasting, while one patient did not take metformin on day 39. Since glycated albumin (GA) has been well known for its accuracy on non-fasting subjects, the two patients remained eligible for GA test [15]. In total, 34 patients were given GA examination. The patient-related factors influencing FBG and GA are described in Table 3.
Table 3.
Patient factors affecting glycemic control after routine administration of 500 mg metformin twice daily for 6 weeks.
| Dependent Variable | Independent Variable | Coefficient | Correlation Coefficient | P Value |
|---|---|---|---|---|
| Final FBG | Duration of previous metformin therapy | −33.335 | −0.415 | 0.010* |
| Initial GA | 3.428 | 0.513 | 0.002* | |
| Cssmin | −27.215 | −0.296 | 0.160 | |
| Cssmax | 13.275 | 0.321 | 0.126 | |
| FBG Changes | eGFR | 1.202 | 0.425 | 0.005* |
| Initial FBG | −1.327 | −1.277 | 0.000* | |
| Initial GA | 4.491 | 0.497 | 0.012* | |
| Cssmin | −40.719 | −0.328 | 0.042* | |
| Cssmax | 24.444 | 0.438 | 0.015* | |
| Final GA | eGFR | 0.075 | 0.266 | 0.037* |
| Initial FBG | −0.059 | −0.575 | 0.003* | |
| Initial GA | 1.036 | 1.156 | 0.000* | |
| Cssmax | 1.608 | 0.290 | 0.014* | |
| GA Changes | eGFR | 0.074 | 0.381 | 0.035* |
| Initial FBG | −0.056 | −0.780 | 0.000* | |
| Cssmax | 1.591 | 0.414 | 0.012* |
Significance value < 0.05; Css steady-state concentration; FBG fasting blood glucose; GA glycated albumin; eGFR estimated glomerulus filtration rate.
Both Cssmax and Cssmin insignificantly affected final FBG with medium correlation. The finding not in line with the prediction was shown by the positive correlation between metformin Cssmax and glycemic response. Different from Cssmax, an expected correlation appeared between Cssmin and final FBG as well as FBG changes. However, this study found that there was no influence of Cssmin on GA. The patient factors that significantly influenced final GA were eGFR, initial GA, and initial FBG as well as metformin Cssmax (P < 0.05). In addition, a stronger negative correlation appeared between initial FBG and GA changes (r −0.780; P 0.000) compared to when final GA became a dependent variable.
Discussion
Pharmacokinetics of metformin steady-state
This present study was the first to demonstrate the trough as well as peak PSSCs of metformin with a fixed interval between doses of 500 mg metformin in 82 T2DM patients. The obtained Cssmin in this study was relatively lower than the peak concentration in single dose of 500 mg metformin administered to 6 healthy subjects, which was 1.02 ± 0.34 µg/mL [16], as well as to 24 healthy subjects with Cpmax 1.75 ± 0.11 µg/mL (19). Since it has been acknowledged that the half-life of metformin elimination is ±5 h in T2DM patients with a normal renal function [17], the administration of metformin per-12 h (2.4-fold t1/2) led to lower Cssmin compared to the peak concentration in single dose of 500 mg metformin. In this study, administration of 500 mg metformin twice daily did not form accumulation, proven by the obtained R (drug accumulation) of 1.04. This finding strengthened the previous result, in which the obtained metformin t1/2 was relatively similar to t1/2 in literature (6.2 h) based on the pharmacokinetic profile of 500 mg metformin in single dose [18], [19].
This research also found an enormous variety of metformin Cssmin (>100-fold) and Cssmax (15-fold). The patients were given an identical formulation of metformin, which was the generic dosage form produced by national pharmaceutical industry; therefore, the effect of formulation could be neglected.
This present study became the first to indicate that the duration of prior metformin use could significantly influence Cssmin in a chronic therapy involving T2DM patients; this has never been found in previous studies. Deep compartment is known to be responsible for metformin Cssmin variation due to a difference in the duration of multiple dose that cannot be identified in single dose administration [16]. Higher Vd.F represents the degree of equilibrium and Vd that could only be identified in a study involving metformin multiple doses. The findings of this study could be caused by inter-compartmental equilibrium in metformin Vd to erythrocytes [20]. The study of 9 T2DM patients found lower concentration of metformin on day 1 compared to that on either day 5 or day 6 [20]. Therefore, this study confirmed the deceleration of average metformin elimination, reaching 1.57-fold, due to distribution in the deep compartment into erythrocytes among patients with a history of metformin use >6 weeks, which caused a significant difference in metformin t1/2 (P < 0.05). Therefore, the use of blood as a biological sample is not recommended for metformin TDM [21], [22] based on the rapid elimination of metformin and a potential delay in examining plasma metformin concentration (since metformin is a non-priority drug in TDM), which makes its level lower than the actual result [23]. However, MTC in erythrocytes is yet unknown, making plasma the most selected bio-fluid samples. Therefore, this study emphasized that not only should the dose, administration and sampling time be controlled, but the previous duration use should also be considered when conducting a study of metformin trough concentration although the time to reach steady-state has been estimated based on metformin t1/2. This study recommends further investigations into metformin Cssmin currently used as an independent variable for the effect of genetic variations especially on metformin-transporter coding genes [24], [4].
Metformin concentration has been widely acknowledged to have a correlation with lactate metabolism. If plasma metformin concentration exceeds MTC, metabolic disorder will have a serious effect. To date, the possible interaction between metformin and sulfonylureas at pharmacokinetic level has yet to be identified. This study found that patients with monotherapy were at relatively greater risk of lactic acidosis due to the higher obtained Cssmax compared to the combination therapy group (P < 0.05). This significant difference was possibly caused by two conditions. The first was potential interaction between metformin and food. A study found an extended tmax median up to 1.5 h when metformin was co-administered with food [25]. Furthermore, the time delay to achieve Cpmax was shorter, only 37 min, shown by subjects who took metformin along with food [26]. Another study also found relatively similar extended tmax (30 min) in a group consuming high-fat diet. In addition, 1.18-fold Cpmax was found in a fasting group though with insignificantly different metformin AUC [27]. However, a RCT study showed that high standardized-fat breakfast did not influence the bioequivalence parameters between fasting group and breakfast group [25]. Another study investigated metformin interaction with four diet types on pharmacokinetic parameters of metformin. Compared to the fasting condition, AUC and Cpmax showed a bioequivalent result except with high-carbohydrate diet that showed a slight decrease in those parameters [28]. Meanwhile, the procedure of this present study determined that patients with combination therapy took sulfonylurea together or maximum 15 min prior to breakfast at 6 a.m., while the metformin-monotherapy group was also suggested to have breakfast at 6 a.m. Prior to Cssmax examination, 500 mg metformin was administered along with non-standardized snacks for all subjects. Consequently, possible metformin-food interaction that might cause differences in Cssmax could not be neglected.
The second likely reason was the obtained average Cssmin that was higher in the metformin-monotherapy group (1.09-fold) compared to that of the other group though statistically they were insignificantly different (P > 0.05). This was also indicated by the significantly strong correlation (r = 0.648) between Cssmin and Cssmax.
Gender insignificantly correlated with metformin PSSC. However, the study found higher metformin Cssmin in male subjects and lower Cssmax compared to female subjects (P > 0.05). Analysis of 47 data sets from 26 studies of bioequivalence reported to FDA revealed that ± 75% of the data found higher Cssmax in female subjects [29]. In general, intestinal transit time of solid dosage form is longer in women than in men, which may lead to higher metformin Cssmax [30]. Therefore, women are more susceptible to higher bioavailability [31], including that of metformin. Meanwhile, metformin Cssmin was found to be lower in female possibly due to their higher BMI (26.16 kg/m2 compared to 22.38 kg/m2). Since metformin is hydrophilic, BMI does not affect its Vd [32]. The difference was possibly caused by increased excretion of metformin due to higher eGFR and more effective tubular secretion, resulting in lower Cssmin in women than in men.
A positive correlation was found between age and metformin PSSC. This is in line with a study of 36 respondents which found that age was a predictive factor for metformin pharmacokinetic profile [33]. Older age leads to a decrease in the Vd of hydrophilic drugs, such as metformin. Along with reduced renal function in older age, metformin could experience reduced renal excretion and increased concentration in plasma [34]. Since this present study excluded geriatric patients (>60 years old), the correlation was very weak (r < 0.2).
In addition, increased IMT means reduced metformin PSSC. Different PSSCs were shown more clearly in Cssmin than in Cssmax (1.31-fold compared to 1.02-fold). As predicted, Cssmin could better reflect metformin disposition, particularly the renal elimination affected by BMI, as opposed to Cssmax. In obese patients, metformin elimination is higher than in subjects with normal BMI or likely lean body due to increased eGFR and tubular secretion, while increasing mass of fat in obese patients does not affect its Vd [35], [36].
Based on these findings, two recommendations in clinical setting are provided. First, not only age and renal function, duration of previous metformin use should also be considered in the strategy of optimizing metformin dose, or when the glycemic response of metformin 1000 mg/day has yet to be achieved, it is better to combine metformin with other antihyperglycemic agents (if necessary) to prevent lactic acidosis due to metformin accumulation in chronic therapy. Although the recommended maximum dose of metformin is not affected by sex, BMI, and T2DM duration, obese T2DM patients are more suggested to take shorter interval of metformin administration (or possibly with sustained-release formulation) to keep Cssmin within the therapeutic range.
This study also found one patient with a history of taking 1500 mg/day metformin and Css exceeding MTC. High concentration of metformin is a risk factor for lactic acidosis as shown by a study involving 7 patients suffering from lactic acidosis with plasma metformin concentration reaching 256–682 µmol/l [37]. Although his PSSCs were higher than MTC, he did not have lactic acidosis. The absence of nausea, vomiting, and abnormal pH (<7.37) [38] in the patient was similar to another finding that showed no correlation between plasma metformin concentration and prognosis of MALA [39], [40], [41] as well as between metformin concentration and lactic acidosis. This was also indicated by 52 patients with Clcr <60 mL/min; no correlation was found between Cssav and lactate concentration in both metformin-monotherapy and metformin-sulfonylurea groups [18]. In general, the risk of MALA can increase in hypoxia, such as in myocardial infarction, acute heart failure, or septicemia as well as liver and renal disorders [38], [42]. Therefore, metformin accumulation does not have such a serious effect as lactic acidosis if it is not accompanied by particular pathological conditions [43].
The patient was found to have taken ECG test, and the result was normal heart function. Since he did not have such risk factors, the high metformin PSSC would possibly have no clinical effects. However, the database of pharmacovigilance studies from 1985 to October 2013 found a significantly positive correlation among plasma metformin concentration, lactate concentration, and creatinine level (r > 0.5). In addition, there was a significantly strong negative correlation between metformin concentration and blood pH (r 0.65) [8]. Therefore, although the patient experienced no clinical effects, extended elimination that could possibly occur in plasma metformin accumulation (>5 µg/mL) needs monitoring. Furthermore, in such case, metformin use combined with other antihyperglycemic drugs is recommended (as opposed to increasing metformin dose) when the glycemic target is not yet reached to prevent possible lactic acidosis.
Correlation between metformin steady-state concentration and glycemic control
When the obtained metformin Css was compared to the recommended therapeutic range, only 32.1% patients had Cssmin within the therapeutic range, while those having Cssmax within the therapeutic range reached 84.1%. Although this study excluded patients aged >60 years and involved only 6 patients (17.65%) having BMI ≥ 30 kg/m2, the findings had been in line with another study involving 1856 T2DM patients which showed that both age and BMI were not the covariates influencing the efficacy of antihyperglycemic therapy [44]. Therefore, obese and non-obese T2DM patients would derive an equal benefit of metformin monotherapy. This is in accordance with a phase IV clinical trial in 371 T2DM patients which found that glycemic response after metformin-monotherapy were insignificantly different among patients with various BMI [45]. Along with initial hyperglycemia and Css, eGFR was correlated with FBG changes, final GA, and GA changes with a strong positive correlation. This means that the higher the eGFR, the better the metformin renal excretion, causing reduced bioavailability of metformin and thus decreased glycemic response.
As predicted, previously longer duration of metformin administration would result in lower final FBG. The average final FBGs in patients who formerly took metformin for 2–6 weeks and in patients using metformin for >6 weeks were each 162.36 ± 46.82 mg/dL and 138.90 ± 46.63 mg/dL. Meanwhile, both Cssmax and Cssmin insignificantly influenced final FBG (P 0.160 and P 0.126, respectively) with a moderate correlation. A finding different from the prediction was shown by the positive correlation between Cssmax and final FBG, FBG changes, final GA, and GA changes. It was unexpected due to the various hyperglycemic baselines. Glycemic response of antidiabetics has been known to be influenced by glycemic control baseline [44]; therefore, in nearly controlled FBG, antidiabetics are administered for maintaining glycemic control. A similar finding was shown by a RCT study involving 529 newly-diagnosed T2DM patients, in which rapid FBG decrease occurred one week after metformin therapy and lasted for 8 weeks of use, and it functioned as maintenance therapy until the study ended in week 24 [46].
As expected, a correlation appeared between Cssmin and FBG as well as FBG changes. However, this study found no influence of Cssmin on final GA or GA changes. Meanwhile, a RCT study involving 451 T2DM patients resulted in a significant FBG reduction after administration of 1000 mg/day metformin compared to the placebo. More decrease in FBG occurred along with the increasing dose of up to 2000 mg/day [47]. In addition, glycemic response is more obvious in patients with high baseline of hyperglycemia, such as in 1856 patients of 3 RCT studies showing that the best antidiabetic response occurred to patients with HbA1c ≥ 8% [44]. The differences in average FBG baseline and involvement of patients with good glycemic control might cause the discrepancy between research findings and predicted results. A study involving 18 T2DM patients showed up to 8.1% reduced GA after metformin administration that was monitored every 4 weeks [48]. The lower response in this study, 4.85%, was caused by differences in metformin doses. Unachieved glycemic control, particularly with metformin Cssmin (67.9% respondents) might also contribute to the metformin response in this study. The baseline GA, which was 12.65% higher than the average initial GA of this present study, had resulted in lower GA reduction.
In general, this study found that metformin Cssmax showed an unexpected correlation with glycemic response. Besides limited number of patients, the high variations of hyperglycemic baseline caused the analysis of metformin Cssmax to show some contradictive results. Although with weak negative correlation, Cssmin influenced FBG. This finding confirmed the importance of calculating the dose and interval of metformin administration, such as in obese patients, to maintain Cssmin within the recommended therapeutic range. A long-term study involving far more respondents and using equal initial hyperglycemia, completed with more-frequent assessment of glycemic control, would produce a more comprehensive analysis relating to metformin steady-state concentration and its glycemic response. After being treated with metformin 1000 mg/day, the percentage of patients with metformin Cssmin was 32.1% while 84.1% had metformin Cssmax within the recommended therapeutic range. Patients with metformin monotherapy possessed higher Cssmax, but patients having a longer duration of metformin administration possessed significantly higher Cssmin. Metformin Cssmin, together with initial hyperglycemia, was the only factor that influenced FBG.
Funding
The authors would like to thank the Ministry of Research, Technology, and Higher Education of Indonesia for funding part of this research through the Doctoral Research Grant (No. 008/DirDPPM/70/Disertasi Doktor – Kemenristekdikti/IV/2017). The funder had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Declarations of interest
None.
Footnotes
Supplementary data associated with this article can be found, in the online version, at https://doi.org/10.1016/j.jcte.2018.05.001.
Appendix A. Supplementary data
References
- 1.International Diabetes Federation. Indonesia VS World Prevalence of Diabetes.
- 2.Kang J.-S., Lee M.-H. Overview of therapeutic drug monitoring. Korean J Intern Med. 2009;24:1–10. doi: 10.3904/kjim.2009.24.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shargel L., Wu-Pong S., Yu A.B.C. Appleton & Lange Reviews. Mc Graw Hill; New York: 2005. Applied Biopharmaceutics and Pharmacokinetics. [Google Scholar]
- 4.Christensen M.M.H., Brasch-Andersen C., Green H. The pharmacogenetics of metformin and its impact on plasma metformin steady-state levels and glycosylated hemoglobin A1c. Pharmacogenet Genomics. 2011;21:837–850. doi: 10.1097/FPC.0b013e32834c0010. [DOI] [PubMed] [Google Scholar]
- 5.Stage T.B., Damkier P., Pedersen R.S. A Twin study of the trough plasma steady state concentration of Metformin. Clin Ther. 2015;37:e116–e117. doi: 10.1097/FPC.0000000000000133. [DOI] [PubMed] [Google Scholar]
- 6.Frid A., Sterner G.N., Löndahl M. Novel assay of metformin levels in patients with type 2 diabetes and varying levels of renal function: clinical recommendations. Diabetes Care. 2010;33:1291–1293. doi: 10.2337/dc09-1284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dell’Aglio D.M., Perino L.J., Kazzi Z. Acute metformin overdose: examining serum pH, lactate level, and metformin concentrations in survivors versus nonsurvivors: a systematic review of the literature. Ann Emerg Med. 2009;54:818–823. doi: 10.1016/j.annemergmed.2009.04.023. [DOI] [PubMed] [Google Scholar]
- 8.Boucaud-Maitre D., Ropers J., Porokhov B. Lactic acidosis: relationship between metformin levels, lactate concentration and mortality. Diabet Med J Br Diabet Assoc. 2016;33:1536–1543. doi: 10.1111/dme.13098. [DOI] [PubMed] [Google Scholar]
- 9.Duong J.K., Furlong T.J., Roberts D.M. The role of metformin in metformin-associated lactic acidosis (MALA): case series and formulation of a model of pathogenesis. Drug Saf. 2013;36:733–746. doi: 10.1007/s40264-013-0038-6. [DOI] [PubMed] [Google Scholar]
- 10.Rojas L.B.A., Gomes M.B. Metformin: an old but still the best treatment for type 2 diabetes. Diabetol Metab Syndr. 2013;5:6. doi: 10.1186/1758-5996-5-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.American Pharmacist Association . 20th ed. Lexi-comp; Ohio, USA: 2012. Drug information handbook. [Google Scholar]
- 12.Bakris G.L., Molitch M.E. Should restrictions be relaxed for metformin use in chronic kidney disease? Yes, they should be relaxed! What’s the fuss? Diabetes Care. 2016;39:1287–1291. doi: 10.2337/dc15-2534. [DOI] [PubMed] [Google Scholar]
- 13.Koga M., Murai J., Saito H. Effects of thyroid hormone on serum glycated albumin levels: Study on non-diabetic subjects. Diabetes Res Clin Pract. 2009;84:163–167. doi: 10.1016/j.diabres.2009.01.013. [DOI] [PubMed] [Google Scholar]
- 14.Koga M., Kasayama S. Clinical impact of glycated albumin as another glycemic control marker. Endocr J. 2010;57:751–762. doi: 10.1507/endocrj.k10e-138. [DOI] [PubMed] [Google Scholar]
- 15.Wu W.-C., Ma W.-Y., Wei J.-N. Serum glycated albumin to guide the diagnosis of diabetes mellitus. PLoS One. 2016;11:e0146780. doi: 10.1371/journal.pone.0146780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tucker G.T., Casey C., Phillips P.J. Metformin kinetics in healthy subjects and in patients with diabetes mellitus. Br J Clin Pharmacol. 1981;12:235–246. doi: 10.1111/j.1365-2125.1981.tb01206.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Graham G.G., Punt J., Arora M. Clin Pharmacokinet Metformin. 2011;50:81–98. doi: 10.2165/11534750-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 18.Duong J.K., Kumar S.S., Kirkpatrick C.M. Population pharmacokinetics of metformin in healthy subjects and patients with type 2 diabetes mellitus: simulation of doses according to renal function. Clin Pharmacokinet. 2013;52:373–384. doi: 10.1007/s40262-013-0046-9. [DOI] [PubMed] [Google Scholar]
- 19.Ritscel W., Kearns G. sixth ed. American Pharmacist Association; Washington DC: 2004. Handbook of basic pharmacokinetics including clinical applications. [Google Scholar]
- 20.Sambol N.C., Chiang J., O’Conner M. Pharmacokinetics and pharmacodynamics of metformin in healthy subjects and patients with noninsulin-dependent diabetes mellitus. J Clin Pharmacol. 1996;36:1012–1021. doi: 10.1177/009127009603601105. [DOI] [PubMed] [Google Scholar]
- 21.Robert F., Fendri S., Hary L. Kinetics of plasma and erythrocyte metformin after acute administration in healthy subjects. Diabetes Metab. 2003;29:279–283. doi: 10.1016/s1262-3636(07)70037-x. [DOI] [PubMed] [Google Scholar]
- 22.Lalau J.-D., Lacroix C. Measurement of metformin concentration in erythrocytes: clinical implications. Diabetes Obes Metab. 2003;5:93–98. doi: 10.1046/j.1463-1326.2003.00241.x. [DOI] [PubMed] [Google Scholar]
- 23.Lalau J.-D., Kajbaf F., Lalau J.-D. Interpreting the consequences of metformin accumulation in an emergency context: impact of the time frame on the blood metformin levels, interpreting the consequences of metformin accumulation in an emergency context: impact of the time frame on the blood metformin levels. Int J Endocrinol. 2014;2014(2014):e717198. doi: 10.1155/2014/717198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Christensen M.M.H., Højlund K., Hother-Nielsen O. Steady-state pharmacokinetics of metformin is independent of the OCT1 genotype in healthy volunteers. Eur J Clin Pharmacol. 2015;71:691–697. doi: 10.1007/s00228-015-1853-8. [DOI] [PubMed] [Google Scholar]
- 25.Karim A., Slater M., Bradford D. Oral antidiabetic drugs: effect of food on absorption of pioglitazone and metformin from a fixed-dose combination tablet. J Clin Pharmacol. 2007;47:48–55. doi: 10.1177/0091270006293756. [DOI] [PubMed] [Google Scholar]
- 26.Sambol N.C., Brookes L.G., Chiang J. Food intake and dosage level, but not tablet vs solution dosage form, affect the absorption of metformin HCl in man. Br J Clin Pharmacol. 1996;42:510–512. doi: 10.1111/j.1365-2125.1996.tb00017.x. [DOI] [PubMed] [Google Scholar]
- 27.Marathe P.H., Arnold M.E., Meeker J. Pharmacokinetics and bioavailability of a metformin/glyburide tablet administered alone and with food. J Clin Pharmacol. 2000;40:1494–1502. [PubMed] [Google Scholar]
- 28.Saffar F., Aiache J.M., Andre P. Influence of food on the disposition of the antidiabetic drug metformin in diabetic patients at steady-state. Methods Find Exp Clin Pharmacol. 1995;17:483–487. [PubMed] [Google Scholar]
- 29.Chen M.L., Lee S.C., Ng M.J. Pharmacokinetic analysis of bioequivalence trials: implications for sex-related issues in clinical pharmacology and biopharmaceutics. Clin Pharmacol Ther. 2000;68:510–521. doi: 10.1067/mcp.2000.111184. [DOI] [PubMed] [Google Scholar]
- 30.Freire A.C., Basit A.W., Choudhary R. Does sex matter? The influence of gender on gastrointestinal physiology and drug delivery. Int J Pharm. 2011;415:15–28. doi: 10.1016/j.ijpharm.2011.04.069. [DOI] [PubMed] [Google Scholar]
- 31.Soldin O., Mattison D. Sex differences in pharmacokinetics and pharmacodynamics. Clin Pharmacokinet. 2009;48:143–157. doi: 10.2165/00003088-200948030-00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Meibohm B., Beierle I., Derendorf H. How important are gender differences in pharmacokinetics? Clin Pharmacokinet. 2002;41:329–342. doi: 10.2165/00003088-200241050-00002. [DOI] [PubMed] [Google Scholar]
- 33.Sambol N.C., Chiang J., Lin E.T. Kidney function and age are both predictors of pharmacokinetics of metformin. J Clin Pharmacol. 1995;35:1094–1102. doi: 10.1002/j.1552-4604.1995.tb04033.x. [DOI] [PubMed] [Google Scholar]
- 34.Mangoni A.A., Jackson S.H.D. Age-related changes in pharmacokinetics and pharmacodynamics: basic principles and practical applications. Br J Clin Pharmacol. 2004;57:6–14. doi: 10.1046/j.1365-2125.2003.02007.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bardin C., Nobecourt E., Larger E. Population pharmacokinetics of metformin in obese and non-obese patients with type 2 diabetes mellitus. Eur J Clin Pharmacol. 2012;68:961–968. doi: 10.1007/s00228-011-1207-0. [DOI] [PubMed] [Google Scholar]
- 36.Ghobadi C., Johnson T.N., Aarabi M. Application of a systems approach to the bottom-up assessment of pharmacokinetics in obese patients: expected variations in clearance. Clin Pharmacokinet. 2011;50:809–822. doi: 10.2165/11594420-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 37.Frid A, Sterner GN, Londahl M, et al. Novel assay of metformin levels in patients wth type 2 diabetes and varying levels of renal function. Diabetes Care; 33. [DOI] [PMC free article] [PubMed]
- 38.Tahrani A.A., Varughese G.I., Scarpello J.H. Metformin, heart failure, and lactic acidosis: is metformin absolutely contraindicated? BMJ. 2007;335:508–512. doi: 10.1136/bmj.39255.669444.AE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kajbaf F., Lalau J.-D. The prognostic value of blood pH and lactate and metformin concentrations in severe metformin-associated lactic acidosis. BMC Pharmacol Toxicol. 2013;14:22. doi: 10.1186/2050-6511-14-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lalau J.D., Race J.M. Lactic acidosis in metformin-treated patients. Prognostic value of arterial lactate levels and plasma metformin concentrations. Drug Saf. 1999;20:377–384. doi: 10.2165/00002018-199920040-00006. [DOI] [PubMed] [Google Scholar]
- 41.Stades A.M.E., Heikens J.T., Erkelens D.W. Metformin and lactic acidosis: cause or coincidence? A review of case reports. J Intern Med. 2004;255:179–187. doi: 10.1046/j.1365-2796.2003.01271.x. [DOI] [PubMed] [Google Scholar]
- 42.Jones G.C., Macklin J.P., Alexander W.D. Contraindications to the use of metformin. BMJ. 2003;326:4–5. doi: 10.1136/bmj.326.7379.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lalau J.-D. Lactic acidosis induced by metformin: incidence, management and prevention. Drug Saf. 2010;33:727–740. doi: 10.2165/11536790-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 44.Garber A., Marre M., Blonde L. Influence of initial hyperglycaemia, weight and age on the blood glucose lowering efficacy and incidence of hypoglycaemic symptoms with a single-tablet metformin-glibenclamide therapy (Glucovance) in type 2 diabetes. Diabetes Obes Metab. 2003;5:171–179. doi: 10.1046/j.1463-1326.2003.00259.x. [DOI] [PubMed] [Google Scholar]
- 45.Ji L, Li H, Guo X, et al. Impact of baseline BMI on glycemic control and weight change with metformin monotherapy in Chinese type 2 diabetes patients: phase IV open-label trial. PLoS One; 8. Epub ahead of print 28 February 2013. DOI: 10.1371/journal.pone.0057222. [DOI] [PMC free article] [PubMed]
- 46.Schwartz S., Fonseca V., Berner B. Efficacy, tolerability, and safety of a novel once-daily extended-release metformin in patients with type 2 diabetes. Diabetes Care. 2006;29:759–764. doi: 10.2337/diacare.29.04.06.dc05-1967. [DOI] [PubMed] [Google Scholar]
- 47.Garber A.J., Duncan T.G., Goodman A.M. Efficacy of metformin in type II Diabetes. Am J Med. 1997;103:491–497. doi: 10.1016/s0002-9343(97)00254-4. [DOI] [PubMed] [Google Scholar]
- 48.Sumitani S., Morita S., Deguchi R. Metformin decreases glycated albumin to glycated haemoglobin ratio in patients with newly diagnosed type 2 diabetes. Ann Clin Biochem. 2015;52:76–81. doi: 10.1177/0004563214522984. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.