Abstract
Hypertension is the most important risk factor for cardiovascular diseases worldwide. However, the underlying molecular mechanisms of hypertension are complex and remain largely elusive. Here, we described a novel, microRNA-dependent therapeutic strategy for hypertension. First, we found that plasma microRNA-21-3p (miR-21-3p) levels were significantly reduced both in hypertensive patients and spontaneously hypertensive rats (SHRs) when compared with normal controls. In a series of experiments to dissect the role of miR-21-3p in hypertension, we showed that intravenous delivery of recombinant adeno-associated virus (rAAV)-mediated miR-21-3p expression induced a persistent attenuation of hypertension, with marked amelioration of target organ damages, including cardiac hypertrophy and fibrosis and artery and kidney fibrosis in SHRs, whereas miR-21-3p tough decoys (TuDs) counteracted the above effects. Computational prediction coupled with biochemical experiments revealed that the miR-21-3p-mediated hypotensive reduction effect was accomplished by regulating phenotypic switch of vascular smooth muscle cells (VSMCs) via suppression of the adrenal α2B-adrenergic receptor (ADRA2B) in arteries. Furthermore, we observed that activation of transcription factor NF-κB and SRF significantly increased the expression of miR-21-3p in VSMCs. In summary, our study is the first to identify a novel role and mechanism of miR-21-3p in blood pressure control and provides a possible strategy for hypertension therapy using rAAV-miR-21-3p.
Keywords: rAAV, miR-21-3p, hypertension, ADRA2B, VSMC, vascular remodeling, α-SMA, OPN, NF-κB, SRF
Introduction
Hypertension, persistent elevation of blood pressure, is a common and multifactorial cardiovascular disease. Approximately one billion people worldwide are affected by hypertension, and their progress is affected by both genetic and environmental factors. The underlying mechanisms of hypertension are complex. Several mechanisms have been implicated in the pathogenesis mechanisms of hypertension, including over-activation of the renin-angiotensin aldosterone system,1 increased activity of the sympathetic nervous system,2, 3 oxidative stress,4 and vascular remodeling.5, 6 However, the causes and mechanisms of hypertension are still not fully elucidated. Despite continuous advancement in antihypertensive therapy, blood pressure in a considerable proportion of hypertensive patients fails to be effectively controlled.7, 8 Therefore, it is necessary to identify novel and powerful treatments for hypertension.
MicroRNAs (miRNAs) are endogenous, small non-coding RNAs that have crucial roles in the regulation of gene expression through binding to the 3′ UTR of target mRNA at the post-transcription processing steps.9, 10 During the past few years, miRNAs have been proven to play important roles in a variety of physiological and pathological processes, such as development, metabolism, cellular differentiation, proliferation, cell death, and stress response.11, 12, 13, 14, 15 In recent years, the implications of miRNAs in the cardiovascular system have gradually been recognized.16, 17, 18 Genetic gain- and loss-of-function studies have revealed the prominent roles of miRNAs in various cardiovascular diseases, such as cardiac hypertrophy19 and fibrosis,20, 21 heart failure,22, 23, 24 myocardial infarction,25, 26 angiogenesis,27 atherosclerosis,28 and pulmonary arterial hypertension.29
Recently, an increasing body of evidence showed that miRNAs are associated with hypertension.30 An ingenious study defined the role of aberrant miR-153 as a contributor to the hypertensive state via targeting of KCNQ4 in an animal model of hypertension.31 miRNA-429 has been reported to reduce blood pressure in Sprague-Dawley rats.32 In an early study of cardiac hypertrophy, we found that overexpression of miR-21-3p markedly ameliorated hypertrophic response in cardiomyocytes on the condition of high pressure load induced by transverse aortic constriction in mice.33 It is well known that miR-21-5p (a guide strand) and miR-21-3p (a passenger strand) are generated from the 5′ and 3′ arms of the pre-miR-21 precursor, respectively. Recent reports have indicated that miRNA-5p and -3p species often co-express and both are functional.34, 35, 36 In our previous study, we determined that rAAV-mediated delivery of miR-21-5p significantly reduced blood pressure and attenuated cardiac hypertrophy in hypertensive rats, and this anti-hypertensive effect was attributed to miR-21-5p-medicated positive modulation of mt-Cytb translation in mitochondria.37 However, the role of miR-21-3p in hypertension is still undefined. In the present study, therefore, our aim was to investigate the roles of miR-21-3p in blood pressure regulation.
Results
Expression Patterns of Circulating miR-21-3p in Hypertension Patients and Spontaneously Hypertensive Rats
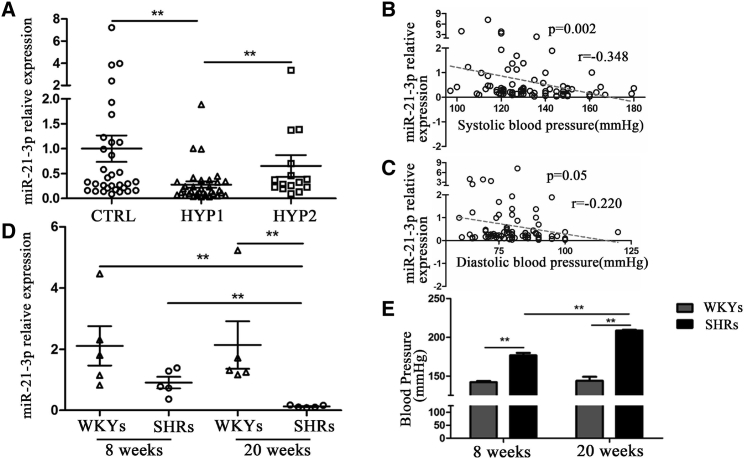
Forty-eight patients with hypertension, including 33 patients with resistant hypertension according to response to anti-hypertensive therapy and 15 with a good response to therapy, and 32 healthy volunteers were enrolled in the present study. The clinical characteristics are summarized in Table S1. There were no significant differences in age, sex, fasting glucose, BMI, and other biochemical parameters between hypertension patients and healthy volunteers. The results showed that hypertensive patients have a significantly lower level of circulating miR-21-3p than healthy controls. Interestingly, the patients with resistant hypertension have a lower miR-21-3p level than the patients with a good response to therapy (Figure 1A). Furthermore, results of a correlation analysis showed that the plasma levels of miR-21-3p were negatively correlated with the levels of either systolic or diastolic blood pressure (Figures 1B and 1C).
Figure 1.
The Expression Patterns of Circulating miR-21-3p in Patients with Hypertension and Spontaneously Hypertensive Rats
(A) The levels of circulating miR-21-3p in hypertension patients. (B and C) The correlation between circulating miR-21-3p levels and blood pressure (B) or diastolic blood pressure (C). (D) The expression of circulating miR-21-3p in WKYs and SHRs. (E) The levels of systolic pressure in WKYs and SHRs. CTRL, health control; HYP1, resistant hypertension group; HYP2, hypertension group with good response to antihypertensive therapy. Data are presented as mean ± SEM (n ≥ 3); *p < 0.05; **p < 0.01.
The level of circulating miR-21-3p was also examined in both spontaneously hypertensive rats (SHRs) and Wistar-Kyoto rats (WKYs) at two different ages (8 weeks old and 20 weeks old), respectively. There was no significant difference in miR-21-3p levels between 8-week-old WKYs and 20-week-old WKYs (Figure 1D), suggesting that the expression of miR-21-3p was not affected by age. Nonetheless, our results showed that plasma miR-21-3p level was significantly lower in SHRs than in WKYs, and that the plasma miR-21-3p level was remarkably decreased in SHRs at 20 weeks compared with 8 weeks (Figure 1D). In addition, systolic blood pressure of SHRs was also much higher at 20 weeks than at 8 weeks (Figure 1E). Together, these results implied that the concentration of plasma miR-21-3p might be associated with the level of blood pressure in vivo.
miR-21-3p Regulates Blood Pressure and the Functions of Arteries in Hypertension
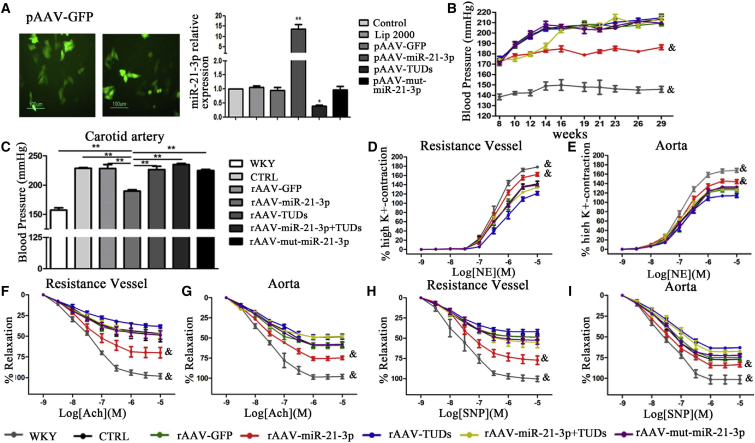
In order to investigate the role of miR-21-3p in hypertension, recombinant adeno-associated virus vectors (rAAVs) were constructed for mediating gain- and loss-of-function of miR-21-3p in a hypertensive animal model. The details of the synthetic sequences are summarized in Table S2. We investigated the impact of different rAAVs vectors on miR-21-3p expression in vitro in cell culture. The results showed that pAAV-D(+)-miR-21-3p transfection significantly increased the expression of miR-21-3p, whereas pAAV-D(+)-miR-21-3p-TuDs transfection remarkably reduced the miR-21-3p level (Figure 2A).
Figure 2.
Influence of miR-21-3p on Regulating Blood Pressure and Vascular Function in SHRs
(A) The expressions of miR-21-3p in HUVEC after being administered with various virus-expressing vectors. (B) The levels of systolic pressure in experimental rats. (C) Blood pressure of the carotid artery monitored by the Millar Pressure-Volume System. (D and E) The constrictions induced by NE in the resistance vessel (D) and aorta (E). (F and G) The endothelium-dependent relaxations induced by Ach in the resistance vessel (F) and aorta (G). (H and I) The endothelium-independent relaxations induced by SNP in the resistance vessel (H) and aorta (I). CTRL, SHR-Control (treated with saline). Data are presented as mean ± SEM (n ≥ 3); *p < 0.05; **p < 0.01; and &p < 0.05 versus SHR control.
Forty-eight 8-week-old male SHRs (180–220 g) were randomly allocated to several groups and respectively received saline, rAAV-GFP, rAAV-miR-21-3p, rAAV-miR-21-3p TuDs, and rAAV-mut-miR-21-3p (about 5 × 1011 viron particles) via intravenous injection. A subgroup of SHRs first treated with rAAV-miR-21-3p was further injected with rAAV-miR-21-3p-TuDs at 14 weeks to reverse the effect of miR-21-3p in hypertension. Six WKYs with the same age were injected with saline as normal control. We noted that systolic blood pressure of SHRs not treated with rAAV-miR-21-3p kept increasing from a baseline of ∼170 mmHg (at 8 weeks) to a peak of ∼210 mmHg (at 14 weeks) and remained at a high level, whereas the blood pressure of control WKYs was always maintained at a normal level of ∼140 mmHg. However, rAAV-miR-21-3p treatment significantly prevented elevation in the blood pressure of SHRs and the hypotensive effect was maintained stable until the end of the experiment at 30 weeks, whereas rAAV-mut-miR-21-3p did not affect blood pressure (Figure 2B). Interestingly, delivery of rAAV-miR-21-3p TuDs into SHRs treated with rAAV-miR-21-3p at 14 weeks fully reversed the anti-hypertensive effects of miR-21-3p in vivo (Figure 2B). These results suggest that rAAV-miR-21-3p treatment has a marked and long-term hypotensive effect in SHRs. Alternatively, the blood pressures of experimental rats were also measured by a catheter tip manometer advanced into the right carotid artery. Results showed that the blood pressures of the carotid artery were significantly reduced in rAAV-miR-21-3p-treated SHRs compared with other SHRs (Figure 2C). These data further suggest the finding that rAAV-miR-21-3p treatment significantly lowers blood pressure in SHRs.
Next, contractile responses to norepinephrine (NE) were analyzed to test the functional behavior of arteries. Cumulative addition of NE elicited a concentration-dependent contraction in the resistance vessel (mesenteric artery) and aorta from WKYs and SHRs with different treatments, respectively. A strong contractility was observed in resistance vessels from WKY rats, whereas vascular rings derived from SHRs exhibited a weaker response to NE. However, miR-21-3p markedly increased the vasoconstriction of arteries from SHRs treated with the highest concentration of NE (Figures 2D and 2E). Endothelium-dependent and -independent relaxation in the NE pre-contracted resistance vessel and aorta of experimental rats was also performed. The relaxant effect of acetylcholine (Ach) at the maximal concentration tested was significantly increased in the resistance vessel (Figure 2F) and aorta (Figure 2G) from SHRs treated with rAAV-miR-21-3p when compared with arteries from other groups. In addition, rAAV-miR-21-3p significantly increased the endothelium-independent vasodilation to sodium nitroprusside (SNP) both in the resistance vessel (Figure 2H) and aorta (Figure 2I) from SHRs, whereas the vessels from SHRs of other groups displayed weaker capacities of the vasodilator.
Tissue Distribution of rAAV-Medicated miR-21-3p Expression in SHRs
We examined the expression profile of miR-21-3p in WKYs and SHRs, and results showed that rAAV-miR-21-3p significantly elevated miR-21-3p levels in various tissues of SHRs, including resistance vessels, aorta, brain, heart, kidney, and liver, specifically in small resistance arteries that achieved an approximately 200-fold increase (Figures S1A–S1F). Furthermore, to accurately determine the location of rAAV-medicated miR-21-3p delivery in the arteries of SHRs, rAAV-GPF-miR-21-3p was constructed and intravenously injected into SHRs. Immunofluorescence staining confirmed that the expression of GFP was significantly increased in vascular smooth muscle cells (VSMCs) of vascular medial membrane (Figures S1G and S1H), whereas the level of miR-21-3p was also elevated there (Figures S1I and S1J). These results implied that the reduction of blood pressure induced by rAAV-miR-21-3p could be the result of increased miR-21-3p expression in VSMCs of arteries in SHRs.
miR-21-3p Protects Target Organs from Damage Induced by Hypertension
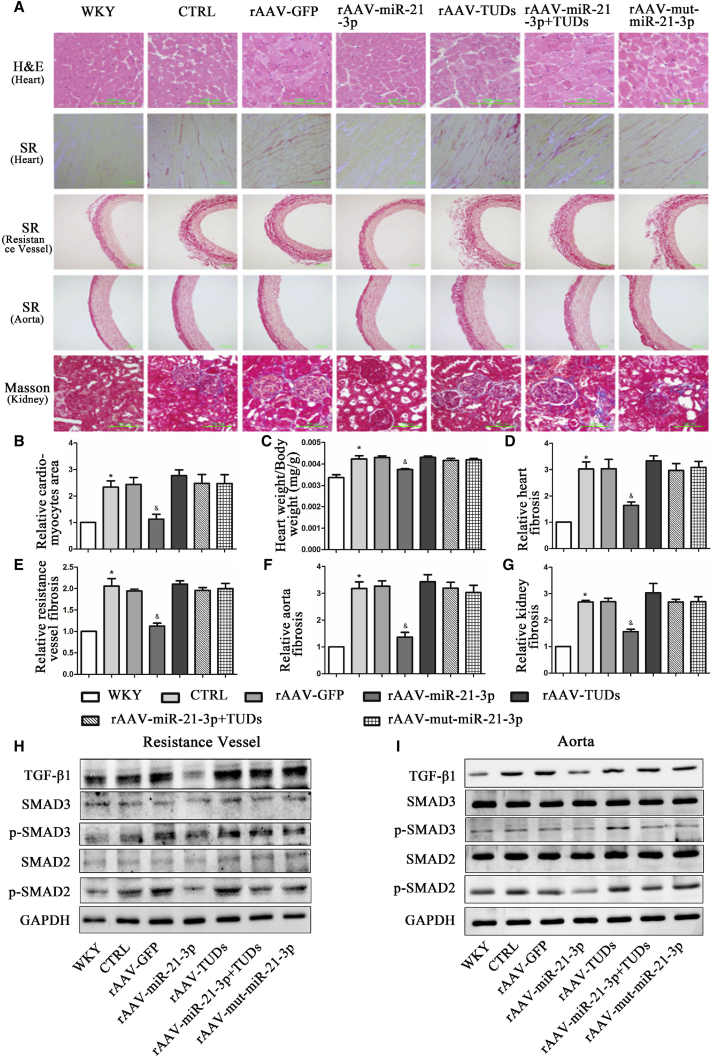
Myocardial hypertrophy and fibrosis are important parts of heart injury of long-term hypertension and independent predictors for cardiovascular events in clinical practice. In the present study, our results from echocardiography showed that rAAV-miR-21-3p treatment significantly ameliorated cardiac dysfunction and reduced interventricular septum thickness in SHRs (Table S3). H&E staining of myocardial tissue showed that miR-21-3p significantly reduced the sizes of the cardiac myocytes in SHRs (Figures 3A and 3B). In addition, the ratio of heart weight/body weight (HW/BW) was also decreased in miR-21-3p-treated SHRs compared with rAAV-GFP- or saline-treated SHRs (Figure 3C). Furthermore, Sirius Red staining showed that miR-21-3p treatment significantly decreased the red-stained area of cardiac tissues compared to rAAV-GFP-treated rats (Figures 3A and 3D). However, rAAV-miR-21-3p TuDs and rAAV-mut-miR-21-3p abolished this protective effect of rAAV-miR-21-3p in the heart. All these results suggested that administration of rAAV-miR-21-3p remarkably alleviated cardiac hypertrophy and fibrosis.
Figure 3.
Influence of miR-21-3p on the Protection of Target Organ Damage Induced by Hypertension in SHRs
(A) Representative images of morphological staining in various tissues from WKYs and SHRs. (B) Quantitative analysis of the cardiomyocyte area. (C) HW/ BW ratio. (D–G) Quantitative analysis of tissue fibrosis of the heart (D), resistance vessel (E), aorta (F), and kidney (G), respectively. (H and I) Protein expression of TGF-β1, SMAD2, p-SMAD2, SMAD3, and p-SMAD3 in resistance vessels (H) and aorta (I) from WKYs and SHRs with different treatments. CTRL, SHR-Control (treated with normal saline); SR, Sirius Red. Data are presented as mean ± SEM (n ≥ 3); *p < 0.05 versus WKYs; &p < 0.05 versus SHR control.
Sirius Red and Masson staining of tissue sections were also performed for assessing the levels of fibrosis in the resistance vessel, aorta, and kidney during hypertension, respectively. Compared with saline control and rAAV-GFP-treated SHRs, the fibrosis level of the resistance vessel (Figures 3A and 3E) and aorta (Figures 3A and 3F) in rAAV-miR-21-3p-treated SHRs was significantly decreased. However, rAAV-mut-miR-21-3p and rAAV-miR-21-3p TuDs could abrogate this anti-fibrotic effect of miR-21-3p in SHRs. As expected, miR-21-3p also exhibited a remarkable anti-fibrosis capacity in the kidney of SHRs (Figures 3A and 3G).
To further determine the effect of miR-21-3p in the amelioration of tissue fibrosis in SHRs, western blots were performed to examine the expressions of transforming growth factor β1 (TGF-β1), SMAD2, SMAD3, TIMP1, MMP9, and COL1 in different organs, respectively. The results showed that the protein levels of TGF-β1, phospho-SMAD2, phospho-SMAD3, TIMP1, and COL1 were significantly increased in the resistance vessel (Figures 3H and S2C), aorta (Figures 3I and S2D), heart (Figures S2A and S2E), and kidney (Figures S2B and S2F) in SHRs when compared with WKYs, whereas the level of MMP9 was markedly reduced. However, overexpression of miR-21-3p remarkably attenuated organ fibrosis in hypertension, as assessed by an increased level of MMP9 and reduced expressions of TGF-β1, phospho-SMAD2, phospho-SMAD3, TIMP1, and COL1 in the above tissues of SHRs. These observations suggested that rAAV-miR-21-3p significantly attenuated target organ fibrosis in SHRs.
ADRA2B Is a Physiological Target of miR-21-3p
Via bioinformatics analysis, α2B-adrenergic receptor (ADRA2B), which has been shown to play an important role in hypertension,38, 39 was found as a putative target of miR-21-3p in both humans and rats. As previously described, rAAV-miR-21-3p significantly increased the miR-21-3p level in VSMCs of arteries and remarkably ameliorated the function of resistance vessel in vivo; therefore, VSMC was chosen as the target cell.
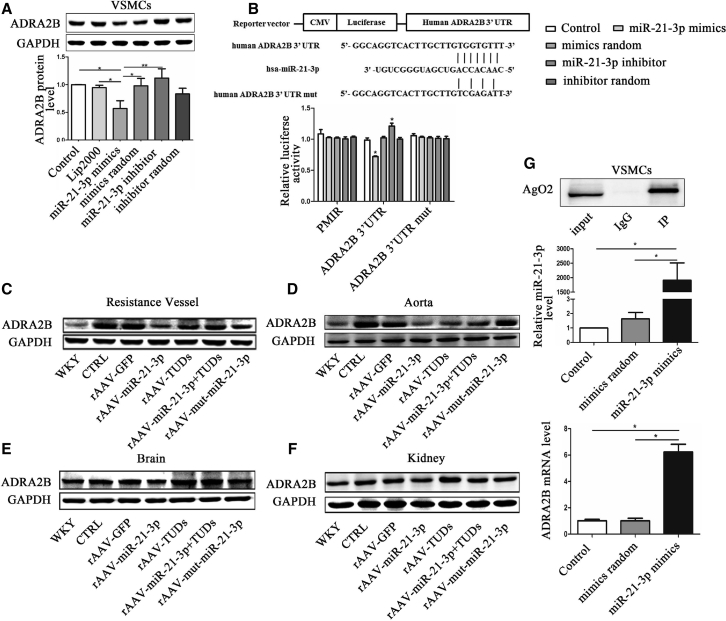
To validate the target, we performed miR-21-3p mimics and inhibitor in vitro experiments. Results showed that miR-21-3p mimics significantly reduced the expression of ADRA2B in VSMCs (Figure 4A). In addition, luciferase activity reporter assays showed that after co-transfection with miR-21-3p mimics, the relative luciferase activity of pMIR-ADRA2B 3′ UTR was significantly suppressed compared with negative or random control, whereas the miR-21-3p inhibitor slightly increased luciferase activity. However, no change occurred after co-transfection with the pMIR mutant-ADRA2B 3′ UTR vector (Figure 4B). Furthermore, we validated this result in experimental rats. It is recognized that ADRA2B is abundantly present in the vasculature, brain, and kidney. We therefore examined the protein levels of ADRA2B in resistance vessels, aorta, brain, and kidney from WKYs and SHRs, respectively. Western blots showed that miR-21-3p treatment significantly downregulated the expressions of ADRA2B in resistance vessels (Figure 4C), aorta (Figure 4D), brain (Figure 4E), and kidney (Figure 4F) in vivo. Moreover, to provide more evidence, we performed a binding assay by immunoprecipitated Ago2-containing complexes. Results showed that the RNA levels of miR-21-3p and ADRA2B, co-immunoprecipitated with Ago2 protein, were significantly increased compared with control (Figure 4G), suggesting that miR-21-3p targets ADRA2B by directly binding with its 3′ UTR. Collectively, these results strongly suggested that ADRA2B was an important physiological target of miR-21-3p.
Figure 4.
miR-21-3p Negatively Regulates the Expression of ADRA2B
(A) The expression of ADRA2B protein in VSMCs. (B) The luciferase activity of pMIR-ADRA2B 3′ UTR plasmid in the 239T cell after co-transfection with miR-21-3p mimics. (C–F) The expression of ADRA2B protein in the resistance vessel (C), aorta (D), brain (E), and kidney (F), respectively. (G) The level of ADRA2B mRNA captured by co-immunoprecipitation after administration with miR-21-3p mimics in VSMCs. CTRL, SHR-Control (treated with normal saline); PMIR, pMIR empty plasmid. Data are presented as mean ± SEM (n ≥ 3); *p < 0.05; **p < 0.01.
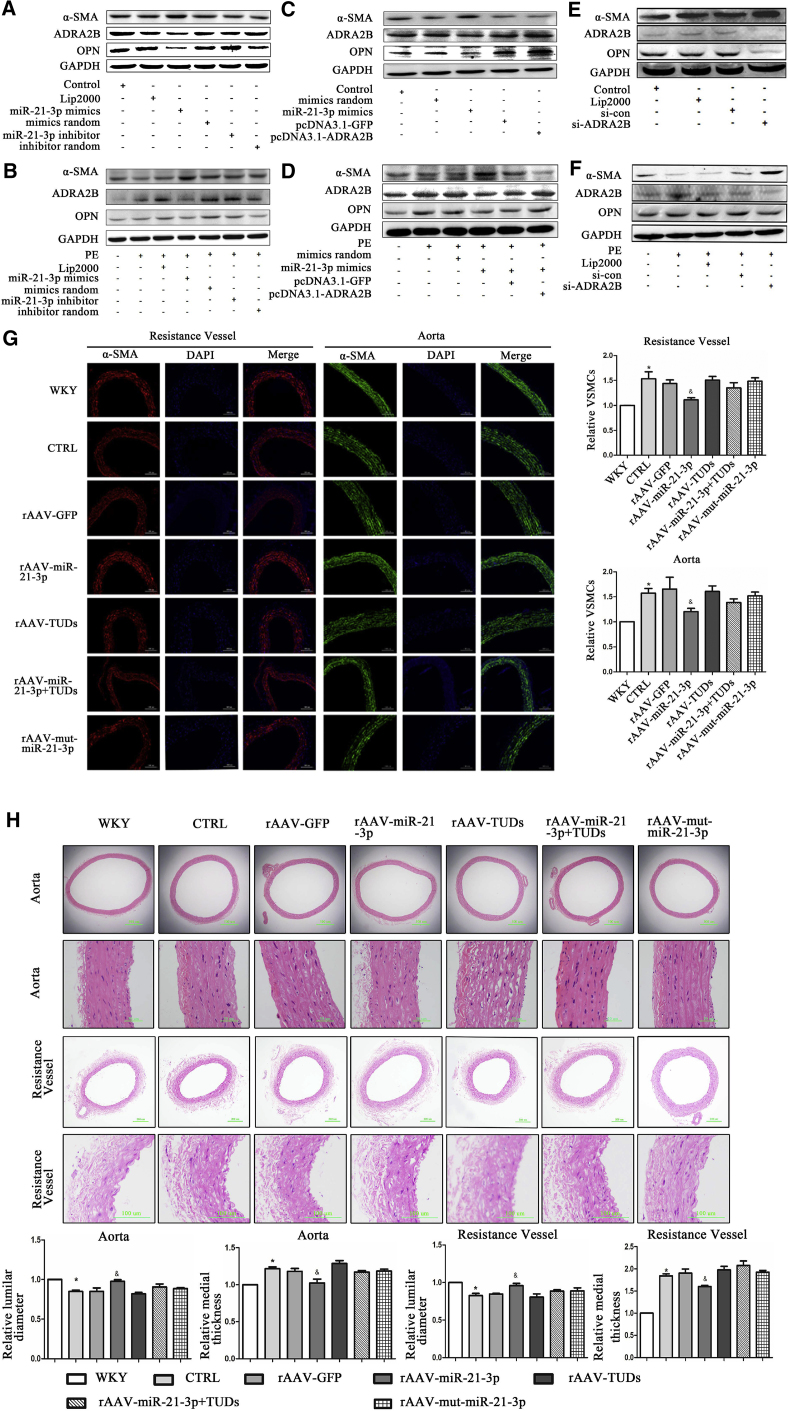
miR-21-3p Regulates the Phenotypic Switch of VSMCs via ADRA2B and Ameliorates Vascular Remodeling in Hypertension
Vascular remodeling contributes to increased peripheral resistance and plays a pivotal role in the development of hypertension.40 Recently, accumulating evidence revealed that miRNAs had abilities to control the proliferation, differentiation, and phenotypic switching of VSMCs.30, 41, 42 In the present study, to confirm whether miR-21-3p was able to regulate the phenotype switch of VSMCs in hypertension, a number of markers specific for contractile VSMC (α-SMA) and synthetic VSMC (OPN) were examined. Phenylephrine (PE) was used to mimic the environment of high pressure in vitro. Results showed that miR-21-3p mimics significantly stimulated the contractile type of VSMCs, as assessed by an increased level of α-SMA and reduced expression of OPN in VSMCs (Figure 5A). Furthermore, VSMCs were exposed to a high-pressure environment induced by PE. Interestingly, we found that PE markedly increased ADRA2B level in VSMCs and induced a switch from contractile VSMC to synthetic VSMC. However, miR-21-3p significantly attenuated this effect induced by PE in VSMCs (Figure 5B). Moreover, we investigated whether the effect of miR-21-3p in the switch of VSMC phenotype was mediated by ADRA2B. Western blots showed that overexpression of ADRA2B increased OPN in VSMCs, whereas the level of α-SMA was reduced (Figures 5C and 5D). However, silencing of ADRA2B by small interfering RNAs (siRNAs) significantly reduced OPN level and elevated the expression of α-SMA (Figures 5E and 5F). These results suggest that miR-21-3p may regulate phenotype switch of VSMCs via ADRA2B. It has been reported that the phenotype switch of VSMCs from contraction type to synthetic type may induce VSMC proliferation,41 and accelerated proliferation of VSMCs is closely linked with hypertension.43 Furthermore, to test the effects of miR-21-3p on proliferation of VSMCs in vivo, we analyzed the number of VSMCs in the resistance vessel and aorta from WKYs and SHRs by immunofluorescence staining. The relative quantitative morphometric analysis revealed that the VSMC numbers of the resistance vessel and aorta in SHRs were much higher than in WKYs (Figure 5G). However, miR-21-3p significantly suppressed the excessive proliferation of VSMCs in the arteries of SHRs (Figure 5G). Moreover, medial thickness and luminal diameter of the resistance vessel and aorta have been used for estimating arterial remodeling. Both in the resistance artery and aorta, we detected an obvious vascular remodeling in SHRs compared with WKYs, as shown by increased medial thickness and reduced luminal diameter, whereas miR-21-3p significantly decreased medial thickness and increased luminal diameter in the arteries of SHRs (Figure 5H). The above mentioned results suggested that miR-21-3p repressed phenotypic switching of VSMCs (from contractile type to synthetic type), inhibited VSMCs proliferation, and consequently ameliorated vascular remodeling in hypertension.
Figure 5.
miR-21-3p Regulates the Phenotypic Switch of VSMCs and Ameliorates Vascular Remodeling in Hypertension
(A and B) The expressions of α-SMA, ADRA2B, and OPN in VSMCs stimulated by miR-21-3p mimics treated with (B) or without (A) PE. (C and D) The expressions of α-SMA, ADRA2B, and OPN in VSMCs after administration with miR-21-3p mimics or pcDNA 3.1-ADRA2B plasmid treated with (D) or without (C) PE. (E and F) The expressions of α-SMA, ADRA2B, and OPN in VSMCs after co-transfection with ADRA2B-siRNA treated with (F) or without PE (E). (G) Immunofluorescence staining of the resistance vessel (α-SMA, red; nuclei, blue) and aorta (α-SMA, green; nuclei, blue) from WKYs and SHRs with different treatments. (H) H&E staining of the aorta and resistance vessel from WKYs and SHRs with different treatments; medial thickness and luminal diameter were quantified using morphometry. CTRL, SHR-Control (treated with normal saline); lip2000, lipofectamine 2000; PE, phenylephrine; siRNA-con, siRNA negative control. Data are presented as mean ± SEM (n ≥ 3); *p < 0.05 versus WKYs; &p < 0.05 versus SHR control.
AGTR1 and RHOB Are Potential Targets of miR-21-3p in Humans
Interestingly, besides ADRA2B, the genes of angiotensin II receptor type 1 (AGTR1) and Ras homolog family member B (RHOB), associated with arterial hypertension and pulmonary hypertension, were also predicted as potential targets of miR-21-3p only in humans. Western blot showed that miR-21-3p mimics significantly decreased the protein levels of AGTR1 and RHOB (Figure S3A) in human umbilical vein endothelial cells (HUVECs). Transfection of pMIR-AGTR1 3′ UTR or pMIR-RHOB 3′ UTR with miR-21-3p mimics into 293T cells resulted in a significant reduction of luciferase activity compared with control and the random group (Figure S3B and S3C). Ago2 co-immunoprecipitation further supported that AGTR1 and RHOB were potential targets of hsa-miR-21-3p (Figure S3D).
Expression of miR-21-3p Is Regulated by the Transcription Factor
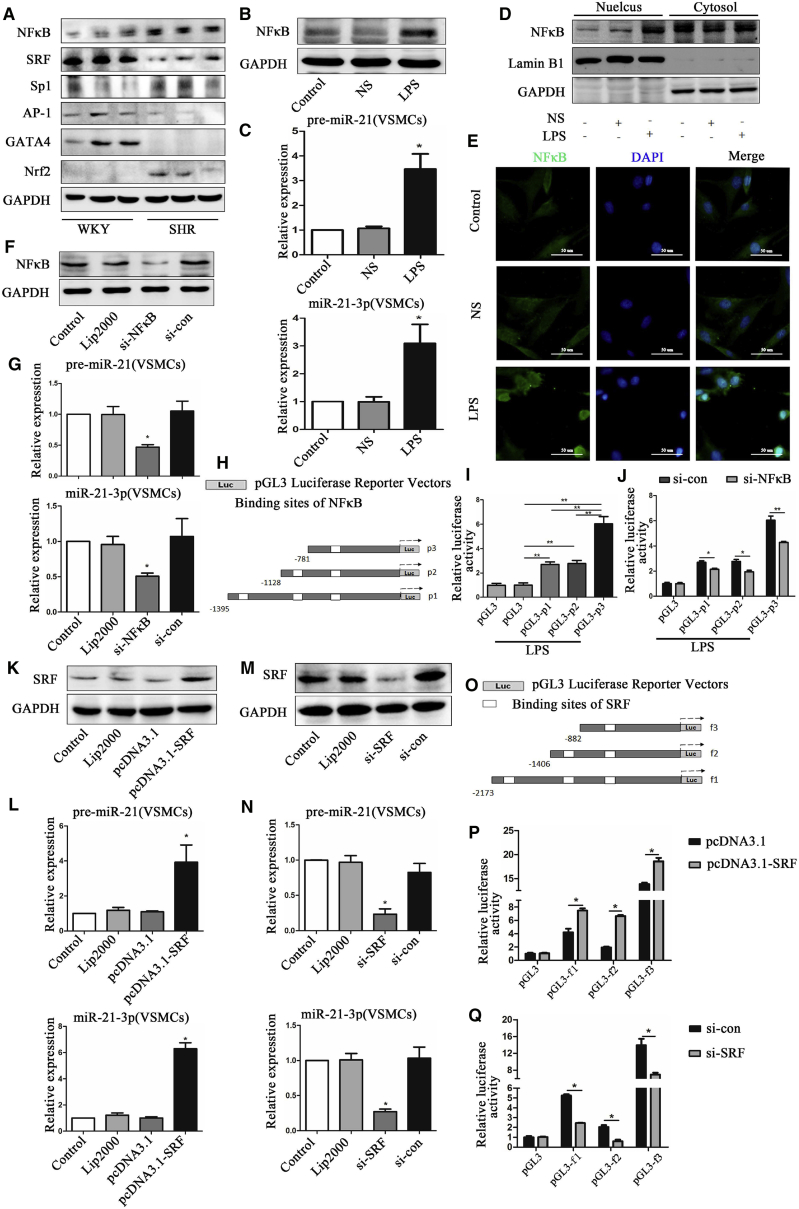
Ever increasing studies have demonstrated that the expressions of miRNAs are regulated by transcription factors.44, 45 We selected several transcription factors associated with cardiovascular diseases, such as nuclear factor κB (NF-κB), SRF, Sp1, AP-1, GATA-4, and Nrf2, and looked at their expressions in resistance vessels. Our results showed that the levels of expression of NF-κB, Sp1, and Nrf2 were significantly increased in SHRs than in WKYs, whereas the levels of SRF, AP-1, and GATA-4 were obviously reduced (Figure 6A). To further investigate whether the expression of miR-21-3p was regulated by the transcription factors mentioned above, a bioinformatics search (Alibaba 2.1) was performed to confirm the transcription factor binding sites in the upstream sequence (∼2,000 bp) of the miR-21-3p promoter. Interestingly, we found that NF-κB, SRF, Sp1, AP-1, and Nrf2 might have binding sites in the miR-21-3p promoter but not GATA4, so we selected NF-κB p65 and SRF, which were predicted with a higher score of binding in the miR-21-3p promoter by bioinformatics analyses as the representatives.
Figure 6.
Transcription Factor NF-κB and SRF Regulate the Expression of miR-21-3p in VSMCs
(A) The expression of transcription factors NF-κB, SRF, Sp1, AP-1, GATA-4, and Nrf2 in SHRs and WKYs. (B) The expression of NF-κB p65 protein in VSMCs after stimulation by LPS. (C) The expression of precursor-miR-21 and mature miR-21-3p in VSMCs after stimulation by LPS. (D) The nuclear translocation of p65 in VSMCs after stimulation by LPS. (E) Immunofluorescence staining of VSMCs treated with or without LPS (NF-κB, green; nuclei, blue). (F) The expression of NF-κB p65 protein in VSMCs after co-transfection with NF-κB siRNA. (G) The expression of precursor-miR-21 and mature miR-21-3p in VSMCs after co-transfection with NF-κB siRNA. (H) Construction of three promoter segments of the miR-21-3p transcript with different binding sites of NF-κB. (I) The luciferase activity of pGL3-p1, pGL3-p2, and pGL3-p3 reporter plasmids stimulated by LPS. (J) The luciferase activity of pGL3-p1, pGL3-p2, and pGL3-p3 reporter plasmids after co-transfection with NF-κB siRNA. (K) The expression of SRF protein in VSMCs after co-transfection with pcDNA3.1-SRF. (L) The expression of precursor-miR-21 and mature miR-21-3p in VSMCs after co-transfection with pcDNA3.1-SRF. (M) The expression of SRF protein in VSMCs after co-transfection with SRF siRNA. (N) The expression of precursor-miR-21 and mature miR-21-3p after co-transfection with SRF siRNA. (O) Construction of three promoter segments of the miR-21-3p transcript with different binding sites of SRF. (P) The luciferase activity of pGL3-f1, pGL3-f2, and pGL3-f3 reporter plasmids co-transfected with pcDNA3.1-SRF. (Q) The luciferase activity of pGL3-f1, pGL3-f2, and pGL3-f3 reporter plasmids after co-transfection with SRF siRNA. Lip2000, lipofectamine 2000; NS, saline; si-con, siRNA-negative control. Data are presented as mean ± SEM (n ≥ 3); *p < 0.05; **p < 0.01.
NF-κB p65
Western blot and qRT-PCR analysis showed that lipopolysaccharide (LPS) significantly increased the cellular levels of precursor-miR-21 and mature miR-21-3p by elevating p65 protein expression and activating NF-κB signaling via translocation of p65 to the nucleus in both VSMCs (Figures 6B–6E) and HUVEC (Figures S4A–S4C). However, in contrast, NF-κB siRNA dramatically reduced the cellular levels of precursor-miR-21 and mature miR-21-3p by suppressing the protein expression and nuclear translocation of p65 in VSMCs (Figures 6F and 6G) and HUVEC (Figure S4D). In addition, to exactly localize the binding sites of NF-κB in miR-21-3p upstream, three promoter segments of the miR-21-3p transcript with different lengths (−1,395 to −18, −1,128 to −18, and −781 to −18) confirmed by DNA sequencing were cloned into the pGL3 luciferase reporter vector, respectively (Figure 6H). Dual-luciferase assays showed that the relative luciferase activity of pGL3-p1, pGL3-p2, and pGL3-p3 was significantly increased after treatment of LPS compared with pGL3 control, and the highest luciferase activity was presented in pGL3-p3 (Figure 6I). Furthermore, transfection of NF-κB siRNA significantly reduced the elevated luciferase activity of pGL3-p1, pGL3-p2, and pGL3-p3 induced by LPS (Figure 6J). These data suggested that the major binding site of NF-κB was located in the region of −469 to −460 bp in the miR-21-3p gene upstream.
SRF
As shown in Figures 6K and 6L, overexpression of SRF by the pcDNA 3.1-SRF plasmid significantly increased the expressions of precursor-miR-21 and mature miR-21-3p in VSMCs. However, reduction of SRF gene expression by SRF siRNA resulted in decreased levels of precursor-miR-21 and mature miR-21-3p in VSMCs (Figures 6M and 6N). Bioinformatics analyses revealed that there are three different binding sites of SRF in miR-21-3p upstream (∼2,000 bp) as well as NF-κB. Moreover, to validate the binding sites of SRF in the miR-21-3p promoter, three promoter segments of miR-21-3p transcript with different lengths (−2,173 to +94, −1,406 to +94, and −882 to +94) were inserted into the pGL3 luciferase reporter vector and denoted by pGL3-f1, pGL3-f2, and pGL3-f3, respectively (Figure 6O). Our results revealed that the relative luciferase activity of pGL3-f1, pGL3-f2, and pGL3-f3 were obviously elevated after co-transfection with pcDNA 3.1-SRF plasmid, and the highest luciferase activity was presented in pGL3-f3 (Figure 6P). However, treatment of SRF siRNA significantly reduced the elevated luciferase activity of pGL3-f1, pGL3-f2, and pGL3-f3 compared with normal control (Figure 6Q). These data suggested that the major binding site of SRF was located in the region of −860 to −843 bp in the miR-21-3p gene upstream.
Discussion
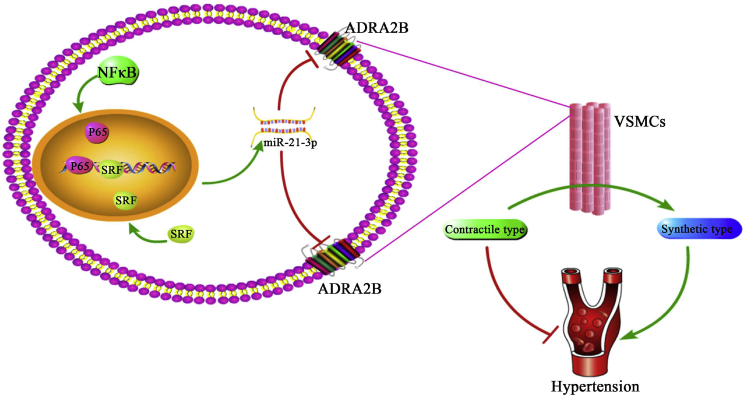
In the present study, the abnormal expression of miR-21-3p has been identified in hypertension patients, and its level negatively correlated with blood pressure level in vivo, suggesting miR-21-3p is involved in hypertension. rAAV-mediated miR-21-3p delivery significantly ameliorated hypertension via attenuating the expression of ADRA2B in VSMCs of arteries and alleviated target organ damages in a hypertensive animal model. Sponge absorption of miR-21-3p by miR-21-3p TuDs abolished this hypotensive effect. In addition, we revealed that transcription factor NF-κB and SRF directly regulated the expression of miR-21-3p via binding with the promoter of the miR-21-3p gene (Figure 7).
Figure 7.
The Role of miR-21-3p in Hypertension
Elevated miR-21-3p by rAAV-medicated delivery significantly decreases the blood pressure of SHRs by directly suppressing the expression of ADRA2B and protects target organ damages associated with hypertension. In addition, activation of NF-κB and SRF signaling remarkably increase the expression of miR-21-3p by directly binding with the promoter of the miR-21-3p gene.
In recent years, increasing studies clarified that miRNAs are perceived as novel therapeutic strategies in the cardiovascular diseases based on their crucial roles in various cardiovascular systems.46, 47, 48 miRNA mimics, synthetic double-stranded miRNA analogs, are used to regulate gene expression by simulating the miRNA-medicated gene silencing.49 However, miRNA mimics have not yet demonstrated efficacy in vivo, and their development has lagged far behind the anti-miRNA chemistries.46 Thus, some new and efficient approaches are needed for enhancing expressions of miRNAs in vivo. AAV vector, with low toxicity and antigenicity, is a promising vehicle for gene therapy. A previous study showed that overexpression of miRNA “tough decoys” (TuDs) medicated by rAAV can efficiently inhibit the function of miRNAs in mice long-term.50 In the present study, therefore, we constructed different rAAV vectors to investigate the roles and functions of differentially expressed miR-21-3p in hypertension. Importantly, we found that rAAV-miR-21-3p significantly increased the expressions of miR-21-3p in various tissues of SHRs, specifically in VSMCs of arteries. Moreover, rAAV-miR-21-3p treatment significantly reduced elevated blood pressure and ameliorated cardiac hypertrophy, vascular remodeling, and organ fibrosis in SHRs. However, these protective effects of miR-21-3p were abolished by injection of rAAV-miR-21-3p TuDs.
ADRA2B is an important member of the family of G-protein-coupled receptors that mediate the biological functions of endogenous catecholamines, epinephrine, and norepinephrine.51 Previous studies demonstrated that ADRA2B was highly expressed in the central nervous and peripheral micro-vascular systems, and was confirmed to play a crucial role in regulating blood pressure and controlling vascular tone.38, 52, 53 Long-term inhibition of the central ADRA2B gene by rAAV-medicated antisense significantly declined the blood pressure of hypertensive rats.54 In this study, ADRA2B was identified as an important target of miR-21-3p. Further, we confirmed that elevated miR-21-3p significantly reduced the level of ADRA2B in the brain in vivo, and that may be one of the potential mechanisms of miR-21-3p in lowering blood pressure. However, in the present study, the highest expression of miR-21-3p medicated by rAAV was located in the VSMCs of arteries; thus, VSMCs are the target cells in our study. We focused on identifying the roles of miR-21-3p in vascular function and remodeling by directly targeting ADRA2B in the arterial. It is well established that the contractile phenotype of VSMCs, which plays an important role in controlling the diameter of blood vessels, regulating organ blood flow, and keeping a stable blood pressure is essential in the development and maintenance of a normal and mature vasculature.55 The switch of VSMC from contractile type to synthetic type may contribute to vascular remodeling in hypertension.41 Importantly, in this study, we confirmed that miR-21-3p has the ability to control the phenotype switch of VSMCs by directly regulating the expression of ADRA2B, which may be the major mechanism of the miR-21-3p-medicated anti-hypertensive effect in arteries during hypertension.
Meanwhile, in the present study, we also demonstrated that AGTR1 and RHOB were the targets of miR-21-3p only in humans. These results may provide more evidence to support the conclusion that miR-21-3p plays an important role in regulating blood pressure.
In the current study, we discovered that overexpression of miR-21-3p medicated by rAAV vectors significantly reduced blood pressure in SHRs. However, at present, most applications of rAAV-vector-medicated gene delivery still stayed at pre-clinical. Some other approaches, which could be easily achieved, are needed to increase the level of miR-21-3p in vivo. Previous studies clarified that a statin has the ability to regulate the expressions of miRNAs.56, 57 Increasing studies determined that the levels of miRNAs are regulated by a transcription factor.11, 58 In the present study, we explored the transcriptional regulation of miR-21-3p upstream and hope to find that some drugs or transcription factors have the ability to activate endogenous miR-21-3p expression in vivo. Combining western blotting and bioinformatics analyses, we found that the expressions of six transcription factors (NF-κB, SRF, Sp1, AP-1, GATA-4, and Nrf2) were changed in hypertension, and most of them have been predicted to bind with the miR-21-3p promoter region. These results suggested that the above transcription factors might be involved in the regulation of miR-21-3p expression. It was reported that NF-κB was associated with hypertension,59 and previous studies demonstrated that SRF was involved in phenotypic switch of VSMCs.60, 61 Furthermore, both of them were predicted with a higher score of binding in the miR-21-3p promoter by a bioinformatics analysis. So NF-κB p65 and SRF were selected as the representative transcription factors of miR-21-3p in the subsequent study. Our results demonstrated the important roles of NF-κB and SRF in the regulation of miR-21-3p expression. Furthermore, using promoter deletion analysis, we finally confirmed that the major binding site of NF-κB and SRF was located in the region of −469 to −460 bp and −860 to −843 bp of the miR-21-3p transcript upstream, respectively. Together, we found that the regulation of miR-21-3p expression was very complex because various transcription factors may participate. Some of them may upregulate the expression of miR-21-3p, whereas others may downregulate. All these effects ultimately lead to the downregulation of miR-21-3p expression in SHRs.
In short, our data revealed, in part, the complex regulatory mechanisms of miR-21-3p expression and provided some potential theoretical basis for controlling blood pressure by regulating the level of miR-21-3p in vivo.
In summary, we showed that the level of circulating miR-21-3p was reduced both in hypertension patients and hypertensive rats. rAAV-mediated miR-21-3p overexpression significantly attenuated blood pressure in hypertension and weakened target organ damages associated with hypertension. Therefore, our findings provide a possibility of gene therapy for hypertension by rAAV-miR-21-3p.
Materials and Methods
Population
All the participants in this study were admitted to the Cardiovascular Division in Tongji Hospital between September 2012 and May 2013, and 5-mL blood samples were collected via venous puncture after written informed consent was obtained from them. After isolation by centrifugation, the plasma was transferred to RNase-free tubes and stored at −80°C until being further processed. This study was conducted in accordance with the Declaration of Helsinki and approved by the Ethics Committee of Tongji Hospital.
Construction and Preparation of rAAV
The rAAV-D(+) vector (double-stranded rAAV vector plasma), adenovirus helper plasmid phelper, and packaging plasmid pXX9 were provided kindly by Dr. Xiao Xiao and have been described previously.62 Packages and purification of rAAVs were performed as described previously.62, 63 The eluted rAAV was aliquoted and stored at −80°C for animal administration.
Experimental Animals
SHRs and WKYs were purchased from the Vital River Laboratory Animal Technology Company (supported by Charles River Laboratories) in Beijing. During the whole study period, the animals were housed at room temperature with 12-hr light/dark cycles and allowed free access to normal rat chow and water ad libitum. The animal experimental protocols complied with standards stated from NIH Guidelines for the Care and Use of Laboratory Animals and the Chinese Academy of Sciences and were approved by the Tongji Hospital Ethics Committee.
Measurement of Blood Pressure
The systolic blood pressure of rats was measured by a noninvasive sphygmomanometer (BP-2010A, Softron Biotechnology, Beijing, China) via cuffing the tails. The systolic blood pressures of WKYs and SHRs were measured every 2 weeks at the first 2 months after administration with rAAVs and were monitored every 2 or 3 weeks after 16 weeks of age while the level of blood pressure became stable. For each measurement, the systolic blood pressure was represented as the mean of at least 5 stable recordings.
The carotid arterial pressures of rats were also detected at the end of the experiment. A catheter tip manometer (AD Instruments, Bella Vista, NSW, Australia) was advanced into the right carotid artery for monitoring the blood pressure of the carotid artery.
Statistical Analysis
Relative expression levels of miRNAs were calculated by the 2-ΔΔct method. Data are expressed as mean ± SEM unless otherwise indicated. The data of blood pressure and function of the vascular system in all experimental rats over the time course were statistically analyzed by repeated-measured ANOVA. Concentration-dependent contractile response to NE was recorded as the percentage of the maximal contraction obtained with high K+ relaxation responses to cumulative concentrations of Ach and SNP and calculated as percentage inhibition of NE-induced peak contraction. Student’s t test (2 groups) and ANOVA (n groups) were used for analyzing normally distributed variables. Mann-Whitney U test (2 groups) and Kruskal-Wallis test (n groups) were performed as appropriate for assessing non-normally distributed variables. Categorical variables were analyzed by χ2 test. Correlation analysis was determined by Spearman correlation analysis. All statistical calculations were accomplished by SPSS 17.0 software, and p < 0.05 (two-sided) was considered a statistically significant difference.
Author Contributions
All authors have approved this manuscript and its contents and they are aware of the responsibilities connected with authorship. F.W. and Q.F. designed and performed the study and analyzed the data; C.C., L.Z., H.L., Y.Z., Y.W., C.X.Z., and X.X. participated in performing the study; D.W.W. designed and organized the study.
Conflicts of Interest
The authors declare no competing financial interests.
Acknowledgments
We thank Dr. Xiao Xiao for kind provision of recombinant adeno-associated virus. This work was supported by the National Natural Science Foundation of China (grants 91439203, 81630010, 81500327, and 81500328).
Footnotes
Supplemental Information includes Supplemental Materials and Methods, four figures, and four tables and can be found with this article online at https://doi.org/10.1016/j.omtn.2017.11.007.
Supplemental Information
References
- 1.Te Riet L., van Esch J.H., Roks A.J., van den Meiracker A.H., Danser A.H. Hypertension: renin-angiotensin-aldosterone system alterations. Circ. Res. 2015;116:960–975. doi: 10.1161/CIRCRESAHA.116.303587. [DOI] [PubMed] [Google Scholar]
- 2.Parati G., Esler M. The human sympathetic nervous system: its relevance in hypertension and heart failure. Eur. Heart J. 2012;33:1058–1066. doi: 10.1093/eurheartj/ehs041. [DOI] [PubMed] [Google Scholar]
- 3.DiBona G.F. Sympathetic nervous system and hypertension. Hypertension. 2013;61:556–560. doi: 10.1161/HYPERTENSIONAHA.111.00633. [DOI] [PubMed] [Google Scholar]
- 4.Paravicini T.M., Touyz R.M. Redox signaling in hypertension. Cardiovasc. Res. 2006;71:247–258. doi: 10.1016/j.cardiores.2006.05.001. [DOI] [PubMed] [Google Scholar]
- 5.Intengan H.D., Schiffrin E.L. Vascular remodeling in hypertension: roles of apoptosis, inflammation, and fibrosis. Hypertension. 2001;38:581–587. doi: 10.1161/hy09t1.096249. [DOI] [PubMed] [Google Scholar]
- 6.Feihl F., Liaudet L., Levy B.I., Waeber B. Hypertension and microvascular remodelling. Cardiovasc. Res. 2008;78:274–285. doi: 10.1093/cvr/cvn022. [DOI] [PubMed] [Google Scholar]
- 7.Egan B.M., Zhao Y., Axon R.N., Brzezinski W.A., Ferdinand K.C. Uncontrolled and apparent treatment resistant hypertension in the United States, 1988 to 2008. Circulation. 2011;124:1046–1058. doi: 10.1161/CIRCULATIONAHA.111.030189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Persell S.D. Prevalence of resistant hypertension in the United States, 2003-2008. Hypertension. 2011;57:1076–1080. doi: 10.1161/HYPERTENSIONAHA.111.170308. [DOI] [PubMed] [Google Scholar]
- 9.Bartel D.P. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell. 2004;116:281–297. doi: 10.1016/s0092-8674(04)00045-5. [DOI] [PubMed] [Google Scholar]
- 10.Winter J., Jung S., Keller S., Gregory R.I., Diederichs S. Many roads to maturity: microRNA biogenesis pathways and their regulation. Nat. Cell Biol. 2009;11:228–234. doi: 10.1038/ncb0309-228. [DOI] [PubMed] [Google Scholar]
- 11.Hermeking H. p53 enters the microRNA world. Cancer Cell. 2007;12:414–418. doi: 10.1016/j.ccr.2007.10.028. [DOI] [PubMed] [Google Scholar]
- 12.Carthew R.W., Sontheimer E.J. Origins and mechanisms of miRNAs and siRNAs. Cell. 2009;136:642–655. doi: 10.1016/j.cell.2009.01.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Takashima Y., Terada M., Udono M., Miura S., Yamamoto J., Suzuki A. Suppression of lethal-7b and miR-125a/b maturation by Lin28b enables maintenance of stem cell properties in hepatoblasts. Hepatology. 2016;64:245–260. doi: 10.1002/hep.28548. [DOI] [PubMed] [Google Scholar]
- 14.Nakagawa R., Leyland R., Meyer-Hermann M., Lu D., Turner M., Arbore G., Phan T.G., Brink R., Vigorito E. MicroRNA-155 controls affinity-based selection by protecting c-MYC+ B cells from apoptosis. J. Clin. Invest. 2016;126:377–388. doi: 10.1172/JCI82914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Murai K., Sun G., Ye P., Tian E., Yang S., Cui Q., Sun G., Trinh D., Sun O., Hong T. The TLX-miR-219 cascade regulates neural stem cell proliferation in neurodevelopment and schizophrenia iPSC model. Nat. Commun. 2016;7:10965. doi: 10.1038/ncomms10965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Small E.M., Frost R.J., Olson E.N. MicroRNAs add a new dimension to cardiovascular disease. Circulation. 2010;121:1022–1032. doi: 10.1161/CIRCULATIONAHA.109.889048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Thum T., Condorelli G. Long noncoding RNAs and microRNAs in cardiovascular pathophysiology. Circ. Res. 2015;116:751–762. doi: 10.1161/CIRCRESAHA.116.303549. [DOI] [PubMed] [Google Scholar]
- 18.Hodgkinson C.P., Kang M.H., Dal-Pra S., Mirotsou M., Dzau V.J. MicroRNAs and Cardiac Regeneration. Circ. Res. 2015;116:1700–1711. doi: 10.1161/CIRCRESAHA.116.304377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ganesan J., Ramanujam D., Sassi Y., Ahles A., Jentzsch C., Werfel S., Leierseder S., Loyer X., Giacca M., Zentilin L. MiR-378 controls cardiac hypertrophy by combined repression of mitogen-activated protein kinase pathway factors. Circulation. 2013;127:2097–2106. doi: 10.1161/CIRCULATIONAHA.112.000882. [DOI] [PubMed] [Google Scholar]
- 20.van Rooij E., Sutherland L.B., Thatcher J.E., DiMaio J.M., Naseem R.H., Marshall W.S., Hill J.A., Olson E.N. Dysregulation of microRNAs after myocardial infarction reveals a role of miR-29 in cardiac fibrosis. Proc. Natl. Acad. Sci. USA. 2008;105:13027–13032. doi: 10.1073/pnas.0805038105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nagpal V., Rai R., Place A.T., Murphy S.B., Verma S.K., Ghosh A.K., Vaughan D.E. MiR-125b is critical for fibroblast-to-myofibroblast transition and cardiac fibrosis. Circulation. 2016;133:291–301. doi: 10.1161/CIRCULATIONAHA.115.018174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Castaldi A., Zaglia T., Di Mauro V., Carullo P., Viggiani G., Borile G., Di Stefano B., Schiattarella G.G., Gualazzi M.G., Elia L. MicroRNA-133 modulates the β1-adrenergic receptor transduction cascade. Circ. Res. 2014;115:273–283. doi: 10.1161/CIRCRESAHA.115.303252. [DOI] [PubMed] [Google Scholar]
- 23.Flemming A. Heart failure: targeting miRNA pathology in heart disease. Nat. Rev. Drug Discov. 2014;13:336. doi: 10.1038/nrd4311. [DOI] [PubMed] [Google Scholar]
- 24.Wahlquist C., Jeong D., Rojas-Muñoz A., Kho C., Lee A., Mitsuyama S., van Mil A., Park W.J., Sluijter J.P., Doevendans P.A. Inhibition of miR-25 improves cardiac contractility in the failing heart. Nature. 2014;508:531–535. doi: 10.1038/nature13073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fiedler J., Jazbutyte V., Kirchmaier B.C., Gupta S.K., Lorenzen J., Hartmann D., Galuppo P., Kneitz S., Pena J.T., Sohn-Lee C. MicroRNA-24 regulates vascularity after myocardial infarction. Circulation. 2011;124:720–730. doi: 10.1161/CIRCULATIONAHA.111.039008. [DOI] [PubMed] [Google Scholar]
- 26.Wang K., Zhou L.Y., Wang J.X., Wang Y., Sun T., Zhao B., Yang Y.J., An T., Long B., Li N. E2F1-dependent miR-421 regulates mitochondrial fragmentation and myocardial infarction by targeting Pink1. Nat. Commun. 2015;6:7619. doi: 10.1038/ncomms8619. [DOI] [PubMed] [Google Scholar]
- 27.Fiedler J., Thum T. New insights into miR-17-92 cluster regulation and angiogenesis. Circ. Res. 2016;118:9–11. doi: 10.1161/CIRCRESAHA.115.307935. [DOI] [PubMed] [Google Scholar]
- 28.Feinberg M.W., Moore K.J. MicroRNA regulation of atherosclerosis. Circ. Res. 2016;118:703–720. doi: 10.1161/CIRCRESAHA.115.306300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Parikh V.N., Jin R.C., Rabello S., Gulbahce N., White K., Hale A., Cottrill K.A., Shaik R.S., Waxman A.B., Zhang Y.Y. MicroRNA-21 integrates pathogenic signaling to control pulmonary hypertension: results of a network bioinformatics approach. Circulation. 2012;125:1520–1532. doi: 10.1161/CIRCULATIONAHA.111.060269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shi L., Liao J., Liu B., Zeng F., Zhang L. Mechanisms and therapeutic potential of microRNAs in hypertension. Drug Discov. Today. 2015;20:1188–1204. doi: 10.1016/j.drudis.2015.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Carr G., Barrese V., Stott J.B., Povstyan O.V., Jepps T.A., Figueiredo H.B., Zheng D., Jamshidi Y., Greenwood I.A. MicroRNA-153 targeting of KCNQ4 contributes to vascular dysfunction in hypertension. Cardiovasc. Res. 2016 doi: 10.1093/cvr/cvw177. Published online July 7, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhu Q., Hu J., Wang L., Wang W., Wang Z., Li P.L., Boini K.M., Li N. Inhibition of microRNA-429 in the renal medulla increased salt sensitivity of blood pressure in Sprague Dawley rats. J. Hypertens. 2017;35:1872–1880. doi: 10.1097/HJH.0000000000001373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yan M., Chen C., Gong W., Yin Z., Zhou L., Chaugai S., Wang D.W. miR-21-3p regulates cardiac hypertrophic response by targeting histone deacetylase-8. Cardiovasc. Res. 2015;105:340–352. doi: 10.1093/cvr/cvu254. [DOI] [PubMed] [Google Scholar]
- 34.Ro S., Park C., Young D., Sanders K.M., Yan W. Tissue-dependent paired expression of miRNAs. Nucleic Acids Res. 2007;35:5944–5953. doi: 10.1093/nar/gkm641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Griffiths-Jones S., Hui J.H., Marco A., Ronshaugen M. MicroRNA evolution by arm switching. EMBO Rep. 2011;12:172–177. doi: 10.1038/embor.2010.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yang J.S., Phillips M.D., Betel D., Mu P., Ventura A., Siepel A.C., Chen K.C., Lai E.C. Widespread regulatory activity of vertebrate microRNA* species. RNA. 2011;17:312–326. doi: 10.1261/rna.2537911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Li H., Zhang X., Wang F., Zhou L., Yin Z., Fan J., Nie X., Wang P., Fu X.D., Chen C. MicroRNA-21 lowers blood pressure in spontaneous hypertensive rats by upregulating mitochondrial translation. Circulation. 2016;134:734–751. doi: 10.1161/CIRCULATIONAHA.116.023926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Moura E., Pinto C.E., Serrão M.P., Afonso J., Vieira-Coelho M.A. Adrenal α2-adrenergic receptors in the aging normotensive and spontaneously hypertensive rat. Neurobiol. Aging. 2012;33:969–978. doi: 10.1016/j.neurobiolaging.2010.06.021. [DOI] [PubMed] [Google Scholar]
- 39.Kintsurashvili E., Gavras I., Johns C., Gavras H. Effects of antisense oligodeoxynucleotide targeting of the alpha(2B)-adrenergic receptor messenger RNA in the central nervous system. Hypertension. 2001;38:1075–1080. doi: 10.1161/hy1101.093426. [DOI] [PubMed] [Google Scholar]
- 40.Owens E.A., Jie L., Reyes B.A.S., Van Bockstaele E.J., Osei-Owusu P. Elastin insufficiency causes hypertension, structural defects and abnormal remodeling of renal vascular signaling. Kidney Int. 2017;92:1100–1118. doi: 10.1016/j.kint.2017.04.044. [DOI] [PubMed] [Google Scholar]
- 41.Torella D., Iaconetti C., Catalucci D., Ellison G.M., Leone A., Waring C.D., Bochicchio A., Vicinanza C., Aquila I., Curcio A. MicroRNA-133 controls vascular smooth muscle cell phenotypic switch in vitro and vascular remodeling in vivo. Circ. Res. 2011;109:880–893. doi: 10.1161/CIRCRESAHA.111.240150. [DOI] [PubMed] [Google Scholar]
- 42.Shan Z., Qin S., Li W., Wu W., Yang J., Chu M., Li X., Huo Y., Schaer G.L., Wang S. An endocrine genetic signal between blood cells and vascular smooth muscle cells: role of microRNA-223 in smooth muscle function and atherogenesis. J. Am. Coll. Cardiol. 2015;65:2526–2537. doi: 10.1016/j.jacc.2015.03.570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhang M.J., Yin Y.W., Li B.H., Liu Y., Liao S.Q., Gao C.Y., Li J.C., Zhang L.L. The role of TRPV1 in improving VSMC function and attenuating hypertension. Prog. Biophys. Mol. Biol. 2015;117:212–216. doi: 10.1016/j.pbiomolbio.2015.03.004. [DOI] [PubMed] [Google Scholar]
- 44.Yang C.H., Yue J., Fan M., Pfeffer L.M. IFN induces miR-21 through a signal transducer and activator of transcription 3-dependent pathway as a suppressive negative feedback on IFN-induced apoptosis. Cancer Res. 2010;70:8108–8116. doi: 10.1158/0008-5472.CAN-10-2579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Degueurce G., D’Errico I., Pich C., Ibberson M., Schütz F., Montagner A., Sgandurra M., Mury L., Jafari P., Boda A. Identification of a novel PPARβ/δ/miR-21-3p axis in UV-induced skin inflammation. EMBO Mol. Med. 2016;8:919–936. doi: 10.15252/emmm.201505384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.van Rooij E., Olson E.N. MicroRNA therapeutics for cardiovascular disease: opportunities and obstacles. Nat. Rev. Drug Discov. 2012;11:860–872. doi: 10.1038/nrd3864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Melman Y.F., Shah R., Danielson K., Xiao J., Simonson B., Barth A., Chakir K., Lewis G.D., Lavender Z., Truong Q.A. Circulating MicroRNA-30d is associated with response to cardiac resynchronization therapy in heart failure and regulates cardiomyocyte apoptosis: a translational pilot study. Circulation. 2015;131:2202–2216. doi: 10.1161/CIRCULATIONAHA.114.013220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hinkel R., Penzkofer D., Zühlke S., Fischer A., Husada W., Xu Q.F., Baloch E., van Rooij E., Zeiher A.M., Kupatt C. Inhibition of microRNA-92a protects against ischemia/reperfusion injury in a large-animal model. Circulation. 2013;128:1066–1075. doi: 10.1161/CIRCULATIONAHA.113.001904. [DOI] [PubMed] [Google Scholar]
- 49.van Rooij E., Marshall W.S., Olson E.N. Toward microRNA-based therapeutics for heart disease: the sense in antisense. Circ. Res. 2008;103:919–928. doi: 10.1161/CIRCRESAHA.108.183426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Xie J., Ameres S.L., Friedline R., Hung J.H., Zhang Y., Xie Q., Zhong L., Su Q., He R., Li M. Long-term, efficient inhibition of microRNA function in mice using rAAV vectors. Nat. Methods. 2012;9:403–409. doi: 10.1038/nmeth.1903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bylund D.B., Eikenberg D.C., Hieble J.P., Langer S.Z., Lefkowitz R.J., Minneman K.P., Molinoff P.B., Ruffolo R.R., Jr., Trendelenburg U. International Union of Pharmacology nomenclature of adrenoceptors. Pharmacol. Rev. 1994;46:121–136. [PubMed] [Google Scholar]
- 52.Makaritsis K.P., Johns C., Gavras I., Gavras H. Role of alpha(2)-adrenergic receptor subtypes in the acute hypertensive response to hypertonic saline infusion in anephric mice. Hypertension. 2000;35:609–613. doi: 10.1161/01.hyp.35.2.609. [DOI] [PubMed] [Google Scholar]
- 53.Kopp U.C., Cicha M.Z., Smith L.A. Impaired interaction between efferent and afferent renal nerve activity in SHR involves increased activation of alpha2-adrenoceptors. Hypertension. 2011;57:640–647. doi: 10.1161/HYPERTENSIONAHA.110.166595. [DOI] [PubMed] [Google Scholar]
- 54.Shenouda S.M., Johns C., Kintsurashvili E., Gavras I., Gavras H. Long-term inhibition of the central alpha(2B)-adrenergic receptor gene via recombinant AAV-delivered antisense in hypertensive rats. Am. J. Hypertens. 2006;19:1135–1143. doi: 10.1016/j.amjhyper.2006.04.001. [DOI] [PubMed] [Google Scholar]
- 55.Owens G.K., Kumar M.S., Wamhoff B.R. Molecular regulation of vascular smooth muscle cell differentiation in development and disease. Physiol. Rev. 2004;84:767–801. doi: 10.1152/physrev.00041.2003. [DOI] [PubMed] [Google Scholar]
- 56.Takwi A.A., Li Y., Becker Buscaglia L.E., Zhang J., Choudhury S., Park A.K., Liu M., Young K.H., Park W.Y., Martin R.C. A statin-regulated microRNA represses human c-Myc expression and function. EMBO Mol. Med. 2012;4:896–909. doi: 10.1002/emmm.201101045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Li P., Yin Y.L., Guo T., Sun X.Y., Ma H., Zhu M.L., Zhao F.R., Xu P., Chen Y., Wan G.R. Inhibition of aberrant microRNA-133a expression in endothelial cells by statin prevents endothelial dysfunction by targeting GTP cyclohydrolase 1 in vivo. Circulation. 2016;134:1752–1765. doi: 10.1161/CIRCULATIONAHA.116.017949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Galardi S., Mercatelli N., Farace M.G., Ciafrè S.A. NF-kB and c-Jun induce the expression of the oncogenic miR-221 and miR-222 in prostate carcinoma and glioblastoma cells. Nucleic Acids Res. 2011;39:3892–3902. doi: 10.1093/nar/gkr006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ma F., Lin F., Chen C., Cheng J., Zeldin D.C., Wang Y., Wang D.W. Indapamide lowers blood pressure by increasing production of epoxyeicosatrienoic acids in the kidney. Mol. Pharmacol. 2013;84:286–295. doi: 10.1124/mol.113.085878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.McDonald O.G., Wamhoff B.R., Hoofnagle M.H., Owens G.K. Control of SRF binding to CArG box chromatin regulates smooth muscle gene expression in vivo. J Clin Invest. 2006;116:36–48. doi: 10.1172/JCI26505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Bell R.D., Deane R., Chow N., Long X., Sagare A., Singh I., Streb J.W., Guo H., Rubio A., Van Nostrand W. SRF and myocardin regulate LRP-mediated amyloid-beta clearance in brain vascular cells. Nat Cell Biol. 2009;11:143–153. doi: 10.1038/ncb1819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Xiao X., Li J., Samulski R.J. Production of high-titer recombinant adeno-associated virus vectors in the absence of helper adenovirus. J. Virol. 1998;72:2224–2232. doi: 10.1128/jvi.72.3.2224-2232.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Zhang F., Chen C.L., Qian J.Q., Yan J.T., Cianflone K., Xiao X., Wang D.W. Long-term modifications of blood pressure in normotensive and spontaneously hypertensive rats by gene delivery of rAAV-mediated cytochrome P450 arachidonic acid hydroxylase. Cell Res. 2005;15:717–724. doi: 10.1038/sj.cr.7290341. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.