Erysipelothrix rhusiopathiae is the causative agent of pig erysipelas and can be associated with sporadic cases or larger outbreaks of septicaemia with characteristic skin lesions or chronic polyarthritis.1 Within the genus Erysipelothrix, at least 6 species (Erysipelothrix rhusiopathiae, Erysipelothrix tonsillarum, Erysipelothrix species strain 1, Erysipelothrix species strain 2, Erysipelothrix species strain 3 and Erysipelothrix inopinata) and 28 serotypes (1a, 1b, 2–26 and N) have been recognised.1 E rhusiopathiae serotypes 1 and 2 are frequently isolated from clinically affected pigs, although other E rhusiopathiae serotypes have been sporadically associated with clinical disease.1 2 While there is no experimental evidence that Erysipelothrix species other than E rhusiopathiae cause disease in pigs,3 certain Erysipelothrix species strains have been isolated from clinical cases4 5 and from condemned carcases in abattoirs.2 6
Pig erysipelas is generally seen in adults and grow-finish pigs after the decline of maternal antibodies.1 Humoural immunity is considered most important for disease prevention and vaccines containing live or inactivated E rhusiopathiae serotype 1 or 2 isolates are commonly used.7 In the UK, there are two E rhusiopathiae bacterins available commercially based on serotype 2 or serotypes 1 and 2.8
In recent years, the incidence of E rhusiopathiae infection in pigs appears to have increased worldwide2 9–11 and is also increasing in European poultry production systems.12 This study summarises the findings associated with chronic E rhusiopathiae infection in a commercial wean-finish pig herd in the UK (farm A) that received piglets from an E rhusiopathiae vaccinated high health breeding herd free of porcine reproductive and respiratory syndrome virus (PRRSV) and Mycoplasma hyopneumoniae as monitored by serological testing in three-month intervals. Specifically, the breeding farm used a commercial E rhusiopathiae serotype 2-based bacterin (Porcillis Ery, Intervet UK) which was administered to gilts twice before first service and to sows once at each weaning.
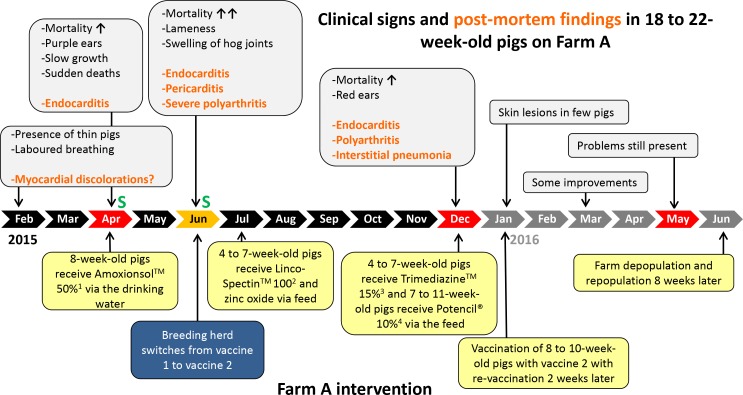
Farm A, a continuous flow farm with routine PCV2 vaccination at 4 weeks of age (Ingelvac CircoFLEX, Boehringer Ingelheim), experienced clinical signs of pig erysipelas of pigs aged 18–22 weeks from February 2015 through August 2016 characterised by delayed growth and a high incidence of lameness and ear discolourations (Fig 1). Morbidity was approximately 8–12 per cent during this time.
FIG 1:
Timeline of the E rhusiopathiae infection dynamics, including clinical signs, postmortem findings and intervention strategies on farm A. E rhusiopathiae serotype 15 was isolated in April and December 2015 and in May 2016 (red-coloured chevrons), an untypable E rhusiopathiae was isolated in June 2015 from pigs aged 18–22 (orange-coloured chevron) and S Typhimurium was isolated in April and June 2015 from pigs aged 6–10 weeks (green S). Vaccine 1 contains an E rhusiopathiae serotype 2 stain (Porcillis Ery, Intervet UK) and vaccine 2 contains serotype 1 and 2 strains (Eryseng Parvo, Hipra, Spain). 1Vetoquinol, active ingredient amoxcycillin. 2Zoetis, active ingredients lincomycin and spectinomycin. 3Vetoquinol, active ingredients sulfadiazine and trimethoprim. 4Elanco, active ingredient phenoxymethyl penicillin potassium.
A total of 10 animals with representative clinical signs observed in different age groups in farm A were selected by the veterinarian and euthanased for postmortem examination as specified below. Three 18-week-old pigs that were in the hospital pen due to losing body condition showed interstitial pneumonia, valvular endocarditis with dilated hearts and enlarged joints with turbid fluid at necropsy. Four 22-week-old pigs, from a pen with severe lameness in 8 per cent of the pigs, showed turbid joint liquid in several articulations but had no cardiac lesions. E rhusiopathiae was isolated on several occasions from the spleen, heart and joint swabs from the affected animals aged 18–22 weeks in farm A (Fig 1). Additionally, three small pigs aged 6–10 weeks showed necrotic colitis without pulmonary or heart lesions, and Salmonella enterica serovar Typhimurium was isolated from caecum swabs from these animals. This finding was unrelated to the E rhusiopathiae cases described in the pigs aged 18– 22 weeks. The S Typhimurium could have contributed to an overall immunocompromised condition of the pig herd.
The E rhusiopathiae isolates were serotyped as previously described.2 Serotype 15 was identified on three different occasions (Fig 1). After initial onset of clinical signs, medicated feed containing 44 ppm lincomycin, 44 ppm spectinomycin and 1250–2500 ppm zinc oxide was administered to the farm A pigs aged 4–7 weeks. In addition, amoxicillin was administered via the drinking water at eight weeks of age. At the same time, the breeding herd moved to a different vaccine supplier/vaccine product and now used a commercial E rhusiopathiae serotypes 1 and 2-based bacterin (Eryseng Parvo, Hipra, Spain) in gilts and sows according to the manufacturer’s instructions. Because of lack of clinical improvement, 14-week-old farm A pigs started to be medicated with penicillin via feed at a concentration of 200 ppm in December 2015 (Fig 1). Skin lesions typical of pig erysipelas were first observed in January 2016 in a few pigs at which time off-label vaccination with Eryseng was initiated in pigs aged 8–10 weeks with revaccination 2 weeks later. However, no obvious impact of the intervention strategies was observed.
In December 2015, 25 serum samples were collected from poor-doing farm A pigs aged 6–22 weeks. Ten serum samples were obtained from farm B pigs aged 17–22 weeks in March 2016 for comparison purposes. Serum samples were tested for the presence of antibodies against E rhusiopathiae surface protective antigen (Spa)A by an in-house fluorescent microsphere immunoassay.13 The positive cut-off was established at a mean fluorescence intensity value of 1800. On the clinically affected farm A, seropositive animals were first detected at 14 weeks of age and the mean anti-E rhusiopathiae antibody levels increased with age, being higher in pigs aged 18–22 weeks (Fig 2). In contrast, healthy pigs from farm B aged 17–20 weeks, which received piglets from the same breeding herd as farm A and operated in an all-in-all-out system, were negative for anti-E rhusiopathiae antibodies (Fig 2).
Fig 2:
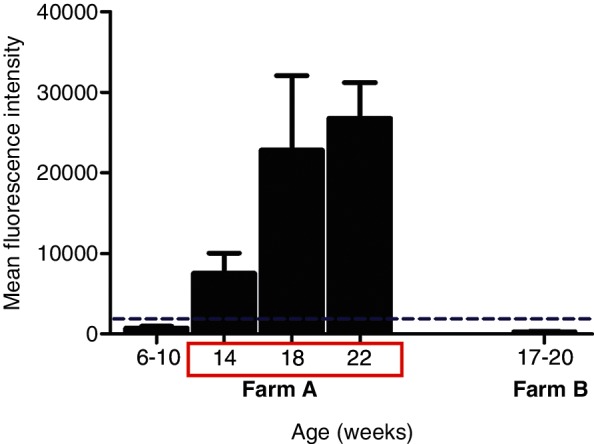
Antibodies against E rhusiopathiae SpaA were measured in serum samples obtained in December 2016 before implementation of E rhusiopathiae vaccination in growing pigs, from pigs of different ages from the clinically affected farm A and the unaffected farm B. Clinical signs were seen in pigs between 14 and 22 weeks of age and included discoloration of the ears and lameness (red box). The dashed line indicates the assay cut-off.
The recurring E rhusiopathiae outbreaks on vaccinated farm A could indicate issues with vaccine handling and administration or vaccine failure. E rhusiopathiae strains express Spa, classified in SpaA or SpaB based on phylogenetic analysis, which are associated with protection.14 In mouse challenge studies, recombinant Spa protected against virulent E rhusiopathiae strains containing the homologous Spa but protection varied against strains possessing a heterologous Spa.14 15 Serotypes 1 and 2 (present in vaccines) and serotype 15 (field isolate) all contain SpaA (data not shown) and cross-protection should have occurred. Vaccination with E rhusiopathiae serotype 2 provided protection against serotype 15 challenge in mice and pigs.16 Recent studies have also shown that commercially available vaccines protected mice and pigs against challenge with highly pathogenic serotype 1a and untypeable strains containing mutations in the spa region.11 However, most cross-protection studies evaluate only short-term protection and acute erysipelas and the conclusions may not always be applicable for long-term protection and/or chronic erysipelas.17 The breeding herd was vaccinated against E rhusiopathiae on a regular basis. A change of the vaccine type/supplier had no effect on the clinical outcomes in farm A pigs.
As clinical signs on farm A were only seen after 12–14 weeks of age, from January 2016 onwards the pigs were vaccinated at 8–10 weeks of age using an inactivated vaccine and revaccinated two weeks later. This two-dose off-label vaccination protocol however also failed to provide protection under the high-infectious pressure conditions. Alternatively, vaccine handling and administration issues could have occurred. As seroconversion due to vaccination or natural infection cannot be differentiated and antibody responses after vaccination were not assessed, the reason for the lack of clinical improvement after implementation of vaccination in the growing pigs in farm A is unknown. It has been shown that, in the presence of E rhusiopathiae passively derived antibodies, pigs vaccinated with a live E rhusiopathiae vaccine at 8–10 weeks of age had improved antibody responses compared with pigs vaccinated at six weeks.18 In piglets without passively derived antibodies, seroconversion occurred regardless of the age at vaccination.18 Passively derived antibodies could potentially have decreased the efficacy of the inactivated vaccine used in farm A, although piglets aged 6–10 weeks were seronegative before implementing the vaccination programme (Fig 2) and the breeding herd vaccination protocol remained unchanged. Furthermore, seroconversion against E rhusiopathiae was seen at 14 weeks of age in farm A (Fig 2), suggesting active infection or recirculation at 12–13 weeks of age.13
Antimicrobial therapy can also impact the host immune system.19 Specifically, after E rhusiopathiae vaccination and compared with an untreated control group, the antibody response was lower in pigs receiving intramuscular doses of ceftiofur, doxycycline and tiamulin and higher in pigs treated intramuscularly with amoxicillin or tulathromycin.19 The effects of lincomycin and spectinomycin administered in farm A on the response to E rhusiopathiae vaccination are unknown. In addition, pathogens such as PRRSV and PCV2 may also impair the immune system, interfere with vaccinations and contribute to bacterial infections. Serum samples from affected animals tested negative for the presence of antibodies against PRRSV (IDEXX PRRS X3 Ab test; IDEXX Laboratories) in March 2015. In December 2015, PCV2 DNA20 was identified in one of 25 serum samples. Combining these results suggests that PRRSV and PCV2 were unlikely to have contributed to the persistence of the outbreak.
On farm A, E rhusiopathiae infection persisted after multiple antimicrobial treatments and vaccination of the growing pigs. Clinical signs associated with the E rhusiopathiae serotype 15 infection consisted of lameness of various degrees and slow growth with presence of stunted pigs. Macroscopic lesions were limited to joints and the heart. Classical skin lesions1 were only observed in few pigs approximately 10 months after the initial problems had started. The reasons for the lack of improvement after implementing or changing antimicrobial treatments and vaccinations are unknown, but the high stocking rate and poor hygiene in the continuous flow of pigs likely contributed. Because of the inability to control the clinical disease signs, farm A was depopulated in July 2016, washed, disinfected, left empty for eight weeks and then repopulated with implementation of an All-In/All-Out production system. Clinical signs consistent with pig erysipelas were not observed after repopulation. Appropriate medication and vaccination protocols may be inefficient to control chronic erysipelas under high-infectious pressure settings.
Footnotes
Funding: Funding for this study was provided by the Biotechnology and Biological Sciences Research Council (BBSRC) Institute Strategic Programme Grant awarded to the Roslin Institute (BB/J004324/1; BBS/E/D/20241864).
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Opriessnig T, Wood RL et al. Erysipelas : Zimmerman JJ, Karricker LA, Ramirez A, Schwartz KJ, Stevenson GW, Diseases of Swine: Wiley-Blackwell, Ames, IA, 2012:750–9. [Google Scholar]
- 2.Bender JS, Irwin CK, Shen HG, et al. Erysipelothrix spp. genotypes, serotypes, and surface protective antigen types associated with abattoir condemnations. J Vet Diagn Invest 2011;23:139–42. 10.1177/104063871102300126 [DOI] [PubMed] [Google Scholar]
- 3.Harada K, Muramatsu M, Suzuki S, et al. Evaluation on the pathogenicity of Erysipelothrix tonsillarum for pigs by immunosuppression with cyclophosphamide or dexamethasone. Res Vet Sci 2011;90:20–2. 10.1016/j.rvsc.2010.05.009 [DOI] [PubMed] [Google Scholar]
- 4.Copes J, Nievas V, Vigo G, et al. Aislamiento e identificación serológica de Erysipelothrix rhusiopathiae de cerdos con lesiones sistémicas compatibles con las del mal rojo en la República Argentina. Rev. Biomed 2001;12:244–8. [Google Scholar]
- 5.Imada Y, Takase A, Kikuma R, et al. Serotyping of 800 strains of Erysipelothrix isolated from pigs affected with erysipelas and discrimination of attenuated live vaccine strain by genotyping. J Clin Microbiol 2004;42:2121–6. 10.1128/JCM.42.5.2121-2126.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang Q, Fidalgo S, Chang BJ, et al. The detection and recovery of Erysipelothrix spp. in meat and abattoir samples in Western Australia. J Appl Microbiol 2002;92:844–50. 10.1046/j.1365-2672.2002.01578.x [DOI] [PubMed] [Google Scholar]
- 7.Haesebrouck F, Pasmans F, Chiers K, et al. Efficacy of vaccines against bacterial diseases in swine: what can we expect? Vet Microbiol 2004;100:255–68. 10.1016/j.vetmic.2004.03.002 [DOI] [PubMed] [Google Scholar]
- 8.McNeil M, Gerber PF, Thomson J, et al. Serotypes and Spa types of Erysipelothrix rhusiopathiae isolates from British pigs (1987 to 2015). Vet J 2017;225:13–15. 10.1016/j.tvjl.2017.04.012 [DOI] [PubMed] [Google Scholar]
- 9.Ding Y, Zhu D, Zhang J, et al. Virulence determinants, antimicrobial susceptibility, and molecular profiles of Erysipelothrix rhusiopathiae strains isolated from China. Emerg Microbes Infect 2015;4:e69 10.1038/emi.2015.69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.To H, Sato H, Tazumi A, et al. Characterization of Erysipelothrix rhusiopathiae strains isolated from recent swine erysipelas outbreaks in Japan. J Vet Med Sci 2012;74:949–53. 10.1292/jvms.11-0533 [DOI] [PubMed] [Google Scholar]
- 11.Uchiyama M, Yamamoto K, Ochiai M, et al. Prevalence of Met-203 type spaA variant in Erysipelothrix rhusiopathiae isolates and the efficacy of swine erysipelas vaccines in Japan. Biologicals 2014;42:109–13. 10.1016/j.biologicals.2013.12.002 [DOI] [PubMed] [Google Scholar]
- 12.Eriksson H, Nyman AK, Fellström C, et al. Erysipelas in laying hens is associated with housing system. Vet Rec 2013;173:18 10.1136/vr.101388 [DOI] [PubMed] [Google Scholar]
- 13.Giménez-Lirola LG, Xiao CT, Halbur PG, et al. Development of a novel fluorescent microbead-based immunoassay and comparison with three enzyme-linked immunoassays for detection of anti-Erysipelothrix spp. IgG antibodies in pigs with known and unknown exposure. J Microbiol Methods 2012;91:73–9. 10.1016/j.mimet.2012.07.014 [DOI] [PubMed] [Google Scholar]
- 14.To H, Nagai S. Genetic and antigenic diversity of the surface protective antigen proteins of Erysipelothrix rhusiopathiae. Clin Vaccine Immunol 2007;14:813–20. 10.1128/CVI.00099-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ingebritson AL, Roth JA, Hauer PJ. Erysipelothrix rhusiopathiae: association of Spa-type with serotype and role in protective immunity. Vaccine 2010;28:2490–6. 10.1016/j.vaccine.2010.01.041 [DOI] [PubMed] [Google Scholar]
- 16.Sawada T, Takahashi T. Cross protection of mice and swine given live-organism vaccine against challenge exposure with strains of Erysipelothrix rhusiopathiae representing ten serovars. Am J Vet Res 1987;48:81–4. [PubMed] [Google Scholar]
- 17.Lacave G, Cox E, Hermans J, et al. Induction of cross-protection in mice against dolphin Erysipelothrix rhusiopathiae isolates with a swine commercial vaccine. Vet Microbiol 2001;80:247–53. 10.1016/S0378-1135(01)00311-X [DOI] [PubMed] [Google Scholar]
- 18.Pomorska-Mól M, Markowska-Daniel I, Pejsak Z. Effect of age and maternally-derived antibody status on humoral and cellular immune responses to vaccination of pigs against Erysipelothrix rhusiopathiae. Vet J 2012;194:128–30. 10.1016/j.tvjl.2012.03.009 [DOI] [PubMed] [Google Scholar]
- 19.Pomorska-Mól M, Kwit K, Wierzchosławski K, et al. Effects of amoxicillin, ceftiofur, doxycycline, tiamulin and tulathromycin on pig humoral immune responses induced by erysipelas vaccination. Vet Rec 2016;178:559 10.1136/vr.103533 [DOI] [PubMed] [Google Scholar]
- 20.Opriessnig T, Yu S, Gallup JM, et al. Effect of vaccination with selective bacterins on conventional pigs infected with type 2 porcine circovirus. Vet Pathol 2003;40:521–9. 10.1354/vp.40-5-521 [DOI] [PubMed] [Google Scholar]