Abstract
Objective
Chronic heart failure with reduced ejection fraction (HF-REF) represents a major public health issue and is associated with considerable morbidity and mortality. We evaluated the cost-effectiveness of sacubitril/valsartan (formerly LCZ696) compared with an ACE inhibitor (ACEI) (enalapril) in the treatment of HF-REF from the perspective of healthcare providers in the UK, Denmark and Colombia.
Methods
A cost-utility analysis was performed based on data from a multinational, Phase III randomised controlled trial. A decision-analytic model was developed based on a series of regression models, which extrapolated health-related quality of life, hospitalisation rates and survival over a lifetime horizon. The primary outcome was the incremental cost-effectiveness ratio (ICER).
Results
In the UK, the cost per quality-adjusted life-year (QALY) gained for sacubitril/valsartan (using cardiovascular mortality) was £17 100 (€20 400) versus enalapril. In Denmark, the ICER for sacubitril/valsartan was Kr 174 000 (€22 600). In Colombia, the ICER was COP$39.5 million (€11 200) per QALY gained. Deterministic sensitivity analysis showed that results were most sensitive to the extrapolation of mortality, duration of treatment effect and time horizon, but were robust to other structural changes, with most scenarios associated with ICERs below the willingness-to-pay threshold for all three country settings. Probabilistic sensitivity analysis suggested the probability that sacubitril/valsartan was cost-effective at conventional willingness-to-pay thresholds was 68%–94% in the UK, 84% in Denmark and 95% in Colombia.
Conclusions
Our analysis suggests that, in all three countries, sacubitril/valsartan is likely to be cost-effective compared with an ACEI (the current standard of care) in patients with HF-REF.
Keywords: heart failure with reduced ejection fraction, health care economics, quality and outcomes of care
Introduction
Chronic heart failure with reduced ejection fraction (HF-REF) represents a major public health issue and is associated with considerable morbidity and mortality. Globally, heart failure (HF) affects an estimated 26 million people and is responsible for 1%–2% of hospitalisations in the USA and Europe.1 HF as a primary diagnosis accounts for approximately 2% of the UK National Health Service (NHS) annual budget,2 and up to 4% of healthcare expenditure if hospitalisations with HF as a secondary diagnosis and nursing home admissions are considered.2
The ACE inhibitor (ACEI) enalapril was the first treatment shown to reduce the risk of hospitalisation and death in patients with HF-REF; ACEIs remain the first-line therapeutic option in these patients.3–5 Despite this and several other therapeutic advances in the field, individuals with HF-REF remain at high risk of hospitalisation and death and experience poorer health-related quality of life (HRQL) than age-matched and gender-matched individuals in the general population.6–8
Sacubitril/valsartan (formerly LCZ696) is a first-in-class angiotensin receptor neprilysin inhibitor (ARNI). The Prospective comparison of ARNI with ACEI to Determine Impact on Global Mortality and morbidity in Heart Failure (PARADIGM-HF) trial was a multinational, Phase III, prospective, double-blind, randomised active-controlled trial, comparing the effects of sacubitril/valsartan and enalapril on mortality and morbidity, in addition to standard of care, in patients with chronic HF-REF.9 PARADIGM-HF demonstrated that, compared with enalapril, treatment with sacubitril/valsartan significantly reduced the composite primary outcome of cardiovascular (CV) death or HF hospitalisation, and both components of this composite, by approximately 20%.10
As the drug acquisition cost of sacubitril/valsartan is higher than that of an ACEI, reimbursement by national payers requires an estimation of expected costs and benefits in order to determine value for money. This study assesses the cost-effectiveness of sacubitril/valsartan versus enalapril from three perspectives: the UK, the Danish and the Colombian healthcare systems.
Methods
Consistent with UK and Danish guidance,1 11 the England and Wales National Heart Failure Audit (2013) found that 73% of patients discharged with HF-REF were treated with ACEI,11 while 18% were treated with an angiotensin receptor blocker (ARB). Therefore, enalapril was selected as the base case comparator in this economic analysis.
A systematic review of economic evaluations of chronic HF treatments was performed to inform the design of the economic evaluation. The health states most frequently employed were ‘alive’ and ‘dead’, with outcomes such as hospitalisation or New York Heart Association (NYHA) functional class distribution considered in the ‘alive’ state. Therefore, a decision-analytic model was developed with ‘alive’ and ‘dead’ health states to estimate costs and benefits over the population lifetime, with hospitalisation rates, HRQL (evaluated using the EuroQol-5 Dimension (EQ-5D) index score) and adverse event (AE) rates included in the ‘alive’ health states (figure 1).
Figure 1.
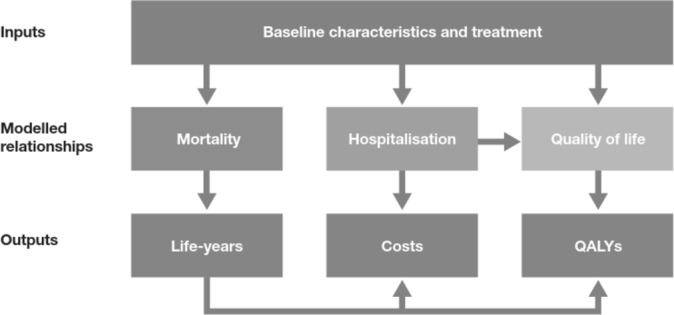
Conceptual model. QALY, quality-adjusted life-year.
A 1-month cycle length with half-cycle correction was employed. Costs and benefits beyond 1 year were discounted at rates of 3.5%, 3% and 5% for the UK, Danish and Colombian settings, respectively, according to local guidelines.11–13
The risks of events were estimated, dependent on patients’ baseline characteristics (reported previously)10 14 and treatment (sacubitril/valsartan or ACEI), through multivariable regression models. Outcomes were estimated for each patient in PARADIGM-HF and results presented as the expected costs and benefits across the patient cohort. Cost-effectiveness was estimated for all a priori defined subgroups in PARADIGM-HF by averaging across the members of the relevant subgroup. The primary model outcome was the incremental cost-effectiveness ratio (ICER), expressed as the cost per quality-adjusted life-year (QALY) gained, reported to the nearest 100 currency units. The ICERs for each setting were compared with country-specific cost-effectiveness thresholds, as listed in table 1.
Table 1.
ICER and cost-effectiveness thresholds in the settings considered in the cost-effectiveness model
| ICER comparison per setting | Cost-effectiveness threshold/QALY gained | Source |
| UK | £20 000 (EUR 23 862)* | NICE† |
| £30 000 (EUR 35 793)* | ||
| Denmark | Kr250 000 (EUR 33 624)‡ | National drug reimbursement committee |
| Colombia | COP$52.4 million (EUR 15 975)§ | Colombian HTA guidance12 |
*Exchange rate used: 1 GBP=1.19 EUR.
†National Institute for Health and Care Excellence. Guide to the methods of technology appraisal, 2013.
‡Exchange rate used: 1 DKK=0.13 EUR.
§Exchange rate used: COP$1=0.0003 EUR, equivalent to three times Colombian GDP.12
COP, Colombian peso; DKK, Danish kroner; EUR, Euro; GBP, British pound sterling; GDP, gross domestic product; HTA, health technology assessment; ICER, incremental cost-effectiveness ratio; Kr, Danish kroner; QALY, quality-adjusted life-year.
Deterministic and probabilistic sensitivity analyses were performed to test parameter uncertainty in the model; the methods are provided in the online Supplementary file 1.
heartjnl-2016-310661supp001.pdf (4.1MB, pdf)
All statistical analyses were performed using Stata V.13.15 The cost-effectiveness model was built in MS Excel.
Statistical analysis
Mortality
Multivariable parametric survival analysis (using the full analysis set of PARADIGM-HF) was used to model CV mortality over time using baseline characteristics and treatment allocation (sacubitril/valsartan or ACEI). The model was applied both during and beyond the duration of PARADIGM-HF. The baseline risk of CV mortality was assumed to follow a Gompertz distribution, selected from potential distributions by clinical experts following review of projected life expectancies. Details of model selection and the effects of using the Weibull and exponential distributions are provided in the online Supplementary file 1. The base case analysis was based on CV mortality from PARADIGM-HF, with non-CV mortality estimated using national life tables adjusted to remove the risk of CV mortality.16–18
Hospitalisation and AEs
The monthly risk of all-cause hospitalisation was estimated using a multivariable negative binomial regression model, allowing analysis of count data (in this case, the total number of hospitalisations during the double-blind phase of PARADIGM-HF for a patient). Additional details on candidate covariates and their selection procedure were the same as employed for the model of CV mortality and are provided in the online Supplementary file 1.
AEs used were those reported in the primary analysis of PARADIGM-HF. A constant rate of AEs was assumed over the model time horizon.
Health-related quality of life
The EQ-5D tariff published by Dolan19 was applied to EQ-5D responses collected in PARADIGM-HF to generate utility values for each patient for the UK setting. The tariff published by Wittrup-Jensen et al 20 was applied to the Danish setting. As there is no Colombian EQ-5D tariff, the Dolan tariff was applied in the Colombian setting.
A multilevel model of EQ-5D was developed to allow the prediction of EQ-5D dependent on baseline characteristics, hospitalisation, AEs and time since randomisation. A constant decline in EQ-5D common to all patients was assumed as a simplifying assumption, and sacubitril/valsartan was assumed to confer a constant effect at all time points on HRQL.
The effect of hospitalisation on HRQL was captured through the effect on EQ-5D at visits falling 0–30 days and 30–90 days post-hospitalisation to capture both acute and medium-term effects.
Cost-effectiveness model
Model structure
All patients started in the ‘alive’ health state and transitions to the ‘dead’ health state were governed by the mortality model. During each cycle, the probability of death was calculated based on the cohort’s baseline characteristics and the time since randomisation. In the ‘alive’ health state, patients were at risk of hospitalisations and AEs.
The model structure was constant across the UK, Denmark and Colombia, but the data used to inform the model differed by country. These differences are summarised in table 2. The UK and Colombian models used the PARADIGM-HF patients as the base case population in the model, whereas the Danish model reweighted the characteristics of the PARADIGM-HF patients to more closely match the Danish patient population in terms of age, gender, ejection fraction and NYHA class. A scenario analysis in which the characteristics of the PARADIGM-HF patients were reweighted to match the UK HF-REF population was also performed (see online Supplementary file 1).
Table 2.
Summary of differences between models
| Component | UK | Denmark | Colombia |
| Population | PARADIGM-HF | Reweighted PARADIGM-HF | PARADIGM-HF |
| Analysis type | Patient-level analysis | Patient-level analysis | Patient-level analysis |
| Mortality | CV mortality from PARADIGM-HF+life tables | CV mortality from PARADIGM-HF+life tables | CV mortality from PARADIGM-HF+life tables |
| EQ-5D tariff | Dolan | Wittrup-Jensen | Dolan |
| Discontinuation considered in the base case? | No | No | No |
CV, cardiovascular; PARADIGM-HF, Prospective comparison of ARNI with ACEI to Determine Impact on Global Mortality and morbidity in Heart Failure.
Resource use
Costs included pharmacological therapies, hospitalisations, AEs and background medical management, including general practitioner visits and other outpatient contacts (see online Supplementary file 1). The costs of the ACEI (enalapril) and sacubitril/valsartan were based on the average doses taken in PARADIGM-HF (enalapril, 18.9 mg/day and sacubitril/valsartan, 375 mg/day).10 Costs of background therapies were based on recommended doses and utilisation reported in PARADIGM-HF at baseline.10 The base year for costs was 2015.
The cost data used in the model are summarised in the online Supplementary file 1. The resource use for hospitalisations and AEs was assumed to be constant between model arms.
Results
Statistical analysis
Full details of statistical models included in the economic model are provided in the online Supplementary file 1.
The model of CV mortality estimated a HR for sacubitril/valsartan of 0.81 (P<0.001), which was consistent with the primary statistical analysis in PARADIGM-HF.10 Baseline EQ-5D was a highly significant predictor of CV mortality (P<0.001).
Similarly, the model of all-cause hospitalisation estimated a rate ratio for sacubitril/valsartan of 0.84 (P<0.001). Baseline EQ-5D was again observed to be a highly significant predictor (P<0.001). The predicted average annual rates of all-cause hospitalisation were 0.42 and 0.50 for sacubitril/valsartan and enalapril, respectively, congruent with the results of PARADIGM-HF.
The results varied slightly between Denmark and the UK. This was due to the differing EQ-5D tariffs applied, as baseline EQ-5D was included as an independent variable in all models. However, the treatment effects for sacubitril/valsartan were almost identical in all three countries (see the online Supplementary file 1).
In the UK, the model predicted a mean life expectancy of 9.27 and 8.36 years for sacubitril/valsartan and ACEI, respectively, indicating an additional 0.91 years of life with sacubitril/valsartan treatment. All-cause mortality at year 5 was estimated to be 33% and 38% for sacubitril/valsartan and ACEI, respectively (table 3).
Table 3.
Model predicted clinical outcomes over lifetime unless otherwise stated
| Component | ACEI | Sacubitril/valsartan | Incremental | ||||||
| UK | Denmark | Colombia | UK | Denmark | Colombia | UK | Denmark | Colombia | |
| Life expectancy, years | 8.36 | 7.34 | 7.95 | 9.27 | 8.07 | 8.78 | 0.91 | 0.73 | 0.83 |
| Number of HF hospitalisations per patient | 0.89 | 0.82 | 0.85 | 0.84 | 0.76 | 0.79 | −0.05 | −0.05 | −0.05 |
| Number of CV hospitalisations per patient | 2.18 | 2.01 | 2.08 | 2.06 | 1.88 | 1.95 | −0.13 | −0.13 | −0.13 |
| All-cause hospitalisations | 3.50 | 3.22 | 3.34 | 3.30 | 3.01 | 3.13 | −0.20 | −0.21 | −0.21 |
| All-cause mortality (%) at year 2 | 16% | 20% | 17% | 14% | 17% | 15% | −0.02 | −0.02 | −0.02 |
| All-cause mortality (%) at year 5 | 38% | 44% | 40% | 33% | 40% | 36% | −0.05 | −0.04 | −0.04 |
| All-cause mortality (%) at year 10 | 66% | 72% | 68% | 60% | 67% | 63% | −0.05 | −0.04 | −0.05 |
ACEI, angiotensin-converting-enzyme inhibitor; CV, cardiovascular; HF, heart failure.
In Denmark, the predicted mean life expectancy was 7.34 for the ACEI arm and 8.07 for the sacubitril/valsartan arm, a gain of 0.73 years of life. All-cause mortality at year 5 was estimated to be 40% and 44% in the sacubitril/valsartan and ACEI arms, respectively (table 3).
In Colombia, predicted mean life expectancy in the ACEI arm was 7.95 years and in the sacubitril/valsartan arm was 8.78, a gain of 0.83 years. All-cause mortality at year 5 was estimated to be 36% and 40% in the sacubitril/valsartan and ACEI arms, respectively (table 3).
A longitudinal analysis estimated that the EQ-5D scores declined at a rate of 0.008 per year across both arms when using the Dolan tariff and 0.006 per year when using the Wittrup-Jensen tariff. Sacubitril/valsartan was associated with a small but statistically significant positive effect on EQ-5D, compared with enalapril, after adjusting for baseline characteristics (including baseline EQ-5D), hospitalisation, AEs and time. The absolute mean difference in EQ-5D score between treatments, after randomisation, was 0.011 (P<0.001) using the Dolan tariff and 0.009 (P<0.001) using the Wittrup-Jensen tariff. Hospitalisation for any reason within the previous 30 days was associated with a reduction in EQ-5D of 0.105 (P<0.001), compared with those who were not hospitalised, and hospitalisation 30–90 days previously was associated with a reduction of 0.054 (P<0.001).
Cost-effectiveness and model outcomes
UK setting
Sacubitril/valsartan treatment led to an additional (discounted) lifetime cost of £8906 per patient. Incremental acquisition costs of sacubitril/valsartan (£8665 per patient) were partly offset by savings attributable to reduced hospitalisation costs (£598 per patient). Sacubitril/valsartan treatment was associated with a QALY gain of 0.52, with a cost per QALY gained of £17 100 (€20 393) (table 4).
Table 4.
Base case cost-effectiveness results (per patient) estimated over lifetime
| Component | ACEI | Sacubitril/valsartan | Incremental | ||||||
| UK (GBP) | Denmark (DKK) | Colombia (COP) | UK (GBP) | Denmark (DKK) | Colombia (COP) | UK (GBP) | Denmark (DKK) | Colombia (COP) | |
| Primary therapy costs | 170 | 1281 | 3 575 530 | 8836 | 89 821 | 21 000 189 | 8665 | 88 540 | 17 424 660 |
| Background therapy costs | 607 | 5729 | 4 138 229 | 662 | 6226 | 4 478 344 | 55 | 497 | 340 116 |
| Hospitalisation costs | 8296 | 132 368 | 17 679 700 | 7697 | 122 267 | 16 273 369 | −598 | −10 102 | −1 406 331 |
| HF management costs | 5639 | 5605 | 3 792 947 | 6153 | 6091 | 4 104 685 | 514 | 486 | 311 737 |
| Adverse events | 102 | 364 | 98 318 | 110 | 393 | 100 044 | 8 | 29 | 1725 |
| Titration | 0 | 0 | 0 | 262 | 1534 | 51 600 | 262 | 1534 | 51 600 |
| Total costs | 14 814 | 145 346 | 29 284 724 | 23 720 | 226 330 | 46 008 231 | 8906 | 80 984 | 16 723 507 |
| QALYs | 5.06 | 4.81 | 4.52 | 5.58 | 5.27 | 4.95 | 0.52 | 0.47 | 0.42 |
| ICER | 17 134 | 173 994 | 39 522 754 | ||||||
ACEI, angiotensin-converting-enzyme inhibitor; COP, Colombian peso; DKK, Danish kroner; GBP, British pound sterling; HF, heart failure; ICER, incremental cost-effectiveness ratio; QALY, quality-adjusted life-years.
Danish setting
Treatment with sacubitril/valsartan led to a lifetime incremental cost of Kr80 984 and an incremental QALY gain of 0.47, resulting in an ICER of Kr174 000 (€22 620, table 4).i The incremental acquisition costs of sacubitril/valsartan (Kr88 540 per patient) were partially offset by a reduction in hospitalisation costs (Kr10 102 per patient).
Colombian setting
Sacubitril/valsartan was associated with an incremental lifetime cost of COP$16 723 507 and an incremental QALY gain of 0.42, giving an ICER of COP$39.5 million (€11 200) per QALY gained (table 4). The incremental acquisition costs of sacubitril/valsartan (COP$17 424 660 per patient) were partially offset by a reduction in hospitalisation costs (COP$1 406 331 per patient).
Estimates of cost-effectiveness were consistent across subgroups (detail provided in the online Supplementary file 1).
Deterministic sensitivity analysis
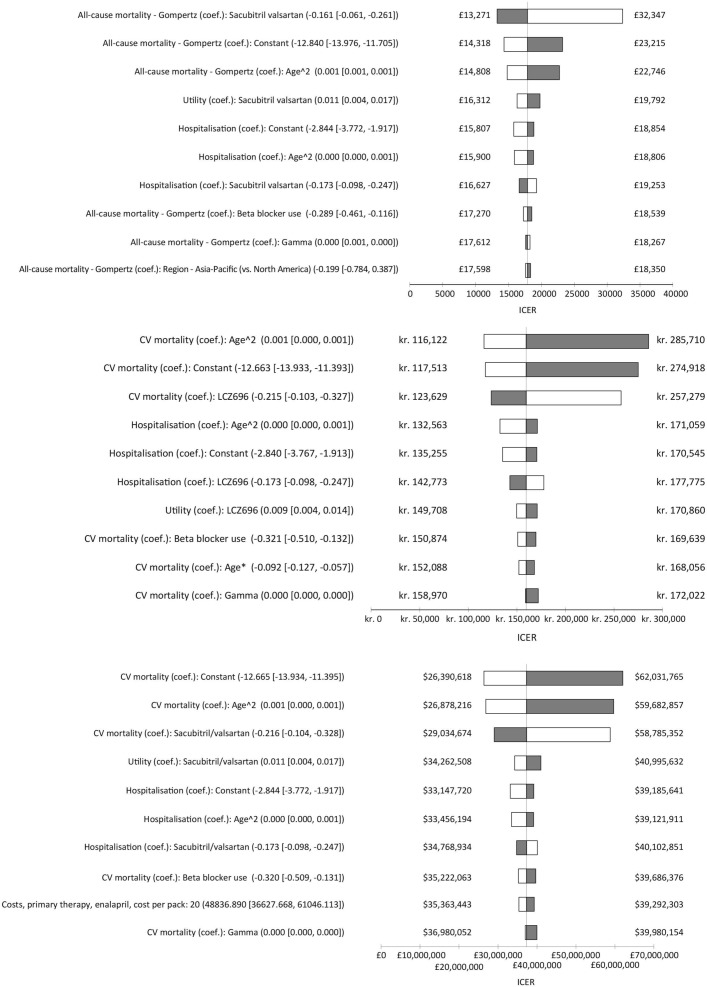
In all the three settings, the three most influential parameters were the coefficient for sacubitril/valsartan, the age-squared coefficient and the constant coefficient in the Gompertz model of CV mortality (figure 2).
Figure 2.
Tornado diagrams. ICER, incremental cost-effectiveness ratio.
In the UK setting, the most influential variable was the constant coefficient, which increased the ICER to £26 500 at the upper limit of the 95% CI and decreased it to £12 700 at the lower limit. The coefficient for sacubitril/valsartan, the age-squared coefficient and the constant coefficient in the Gompertz model of CV mortality were the only parameters to produce an ICER over £20 000 (figure 2).
In the Danish setting, the coefficient for sacubitril/valsartan, the age-squared coefficient and the constant coefficient in the Gompertz model of CV mortality were the only three parameters to generate an ICER over Kr250 000. The most influential parameter was the age-squared term in the regression model for CV mortality, which increased the ICER to Kr285 700 at the upper limit and reduced it to Kr116 100 at the lower limit (figure 2).
In the Colombian setting, the constant coefficient for CV mortality was the most influential parameter, which produced an ICER of COP$24.1 million at the lower limit of the 95% CI and an ICER of COP$55.0 million at the upper limit. No parameter produced an ICER above the willingness-to-pay (WTP) threshold of COP$52.4 million (figure 2).
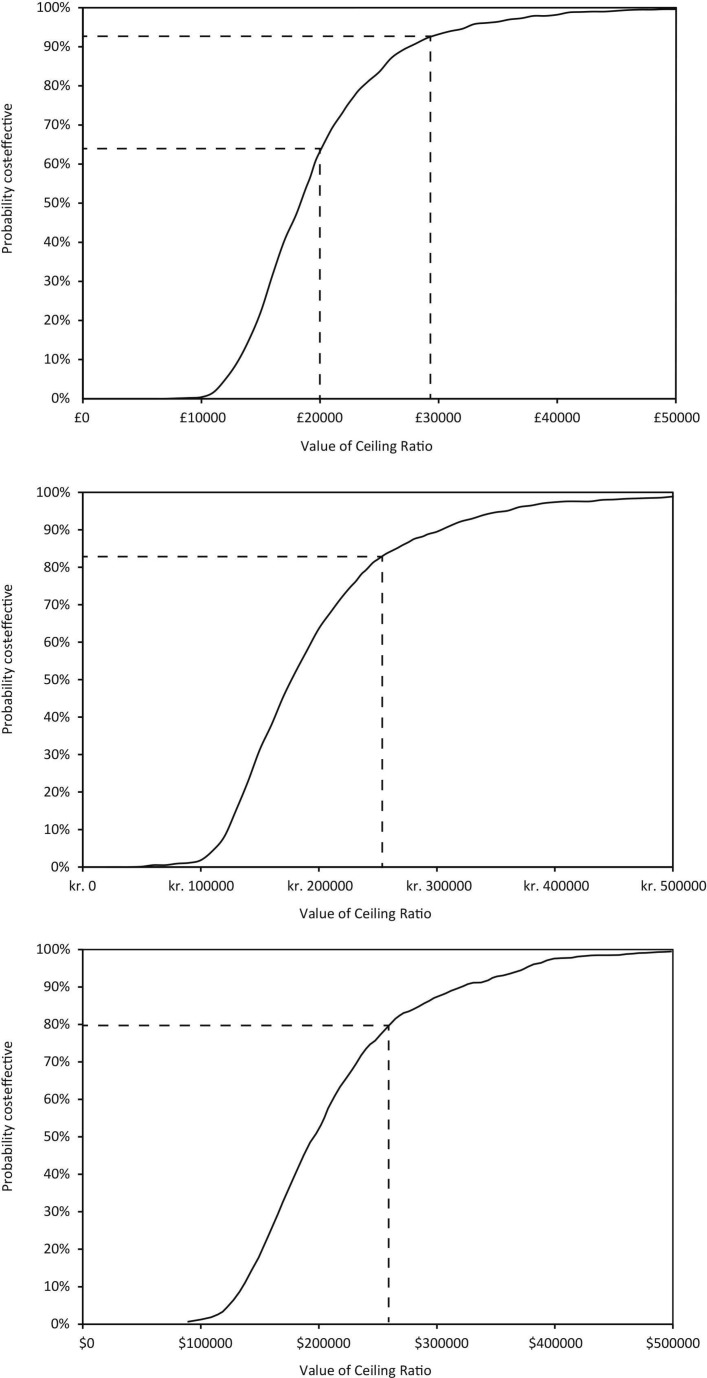
Probabilistic sensitivity analysis (PSA)
In the UK, the expected ICER from the PSA was £18 000 (95% CI £8900 to £34 700). At cost-effectiveness thresholds of £20 000 and £30 000 per QALY gained, the probabilities that sacubitril/valsartan was cost-effective were 68% and 94%, respectively (figure 3).
Figure 3.
Cost-effectiveness acceptability curves.
In Denmark, the expected ICER from PSA was Kr176 700 (95% CI Kr76 200 to Kr367 300). The probability that sacubitril/valsartan was cost-effective at Kr250 000 was 84% (figure 3).
In Colombia, the expected ICER from the PSA was COP$33.8 million. The probability of being cost-effective at a WTP threshold of COP$52.4 million (three times the average per capita income) was 95% (figure 3).
Discussion
The base-case analysis for the UK indicates that sacubitril/valsartan was cost-effective at a WTP threshold of £20 000 per QALY, compared with an ACEI, with an ICER of £17 100. This result was not only mainly driven by reductions in mortality but also by improvements in HRQL and reductions in hospitalisation. The findings of the analysis were robust to changes in most structural assumptions, and a PSA suggested that the probability that sacubitril/valsartan was cost-effective at a WTP threshold of £20 000 was 68%. Three analyses from the perspective of third-party healthcare payers in the USA have also suggested that sacubitril/valsartan is cost-effective at commonly accepted WTP thresholds.21–23
Our findings were consistent across all three countries. The base-case analysis for Denmark, another high-income country, found that sacubitril/valsartan was cost-effective at a WTP threshold of Kr250 000 per QALY, compared with an ACEI, with an ICER of Kr174 000. The base-case analysis for Colombia, a middle-income country, found that sacubitril/valsartan was cost-effective at a WTP threshold of COP$52.4 million per QALY, with an ICER of COP$39.5 million.
These results are in line with National Institute for Health and Care Excellence (NICE) guidance, which recommends sacubitril/valsartan as a cost-effective option for the treatment of HF-REF in patients with NYHA class II–IV, a left ventricular ejection fraction of ≤35% and who are already taking a stable dose of ACEI or ARB.24
The UK analysis predicted (discounted) gains in life years and QALYs with sacubitril/valsartan of 0.62 (9%) and 0.52 (10%), respectively. These are comparatively large benefits in terms of absolute and relative gains compared with the benefits of other treatments for HF-REF, especially as PARADIGM-HF was an active-controlled rather than placebo-controlled trial. The statistical models of mortality presented here were developed to align with the methods outlined by the NICE Decision Support Unit.25 This approach provides shorter estimates of survival in both arms of the model than have been estimated using non-parametric methods from PARADIGM-HF and correspondingly lower gains in life expectancy.26 Given the importance of mortality as a determinant of cost-effectiveness in the present analysis, such estimates may provide a conservative estimate of cost-effectiveness for sacubitril/valsartan.
The observation that cost-effectiveness in this analysis was mainly driven by improvements in mortality is consistent with the results of some, but not all, prior analyses.27 However, other model-based studies concluded that reductions in hospitalisation are the key determinant of cost-effectiveness.28 This difference may be a consequence of the different analytical approaches. This model considered independent statistical models of mortality, hospitalisation and long-term HRQL changes, and the relationships between these outcomes were contained implicitly in the analysis. In contrast, the model of Lee et al 28 considered mortality and long-term HRQL to be direct functions of the number of hospitalisations experienced by an individual, and therefore model outcomes were conditional on the rate of hospitalisation itself. Nevertheless, both this model and that of Lee et al predicted that the proportions of QALY gains attributable to life extension were of similar magnitude for their respective interventions (88% and 80% in this model and in Lee et al, respectively). The cost savings attributable to reduced hospitalisation were also of a similar magnitude (8% and 12% in this model and in Lee et al, respectively). These differences in the conclusions therefore appear to reflect decisions made in the conceptual design of the respective analyses.
The present evaluation used methods consistent with a recently published economic evaluation in HF,27 but incorporated a novel approach to the prediction of HRQL, in which EQ-5D was extrapolated based on time trends observed in PARADIGM-HF. A key strength of this analysis was that PARADIGM-HF collected EQ-5D at multiple time points, permitting this longitudinal analysis. This allowed the direct modelling of EQ-5D over time, rather than predicting HRQL based on intermediate clinical measures such as NYHA class.
The key limitation of this analysis was the extrapolation beyond the follow-up of PARADIGM-HF (with median follow-up of 27 months). This is a cause of uncertainty which cannot readily be characterised in sensitivity analysis, but is common to all modelling exercises, particularly in HRQL estimates. However, the assumption of an annual decline in EQ-5D of 0.008 appears consistent with data from other studies; 1-year longitudinal data presented by Berg et al in patients with chronic HF suggests an annual decrease in EQ-5D of 0.006.29
PARADIGM-HF was a geographically diverse study, and the patient population may not be generalisable to individual countries. Although there is no evidence that the treatment effect differed across subgroups in PARADIGM-HF,10 cost-effectiveness is driven by absolute benefit, which is dependent on patients’ absolute risk of events, reflecting their baseline clinical characteristics. If patient characteristics differ between PARADIGM-HF and the HF-REF population in the UK, Denmark or Colombia, then absolute benefit, and therefore cost-effectiveness, would be expected to differ too. However, the results of the subgroup and scenario analyses suggest that the conclusions of the base-case analysis would not change to any meaningful extent.
In conclusion, this analysis suggests that sacubitril/valsartan likely represents a cost-effective option in the treatment of HF-REF for the NHS in the UK, the Danish healthcare system and for the Colombian healthcare system.
Key messages.
What is already known on this subject?
Prospective comparison of ARNI with ACEI to Determine Impact on Global Mortality and morbidity in Heart Failure trial showed that sacubitril/valsartan, an angiotensin receptor neprilysin inhibitor, was superior to the ACE inhibitor enalapril in reducing the risks of death and heart failure hospitalisation in patients with heart failure with reduced ejection fraction. Published analyses from the perspective of third-party healthcare payers in the USA have suggested that sacubitril/valsartan is cost-effective at commonly accepted willingness-to-pay thresholds.
What might this study add?
We evaluated the cost-effectiveness of sacubitril/valsartan compared with enalapril in the treatment of heart failure with reduced ejection fraction from the perspectives of the UK National Health Service, the Danish healthcare system (high-income countries) and the Colombian healthcare system (middle-income country). We found sacubitril/valsartan to be cost-effective at conventional willingness-to-pay thresholds in all three country settings.
How might this impact on clinical practice?
This analysis, which shows sacubitril/valsartan to be a cost-effective use of resources, should aid decision-makers and may enhance availability of this efficacious treatment for patients with heart failure with reduced ejection fraction.
Acknowledgments
The authors are grateful to Morten Størling Hedegaard, Vera Gielen and Elizabeth Karpf of Novartis AG for providing inputs to the Danish, UK and Colombian model adaptations, respectively.
Footnotes
Results presented here use the reweighted population to remain in line with Danish HTA submissions. Costs and QALYs increase in both arms when using an unweighted population; however, the ICER remains similar at Kr170 000.
Contributors: JJVM, DT, EH, MRC, AB, MT, JMC, RH and CD contributed to the design of the evaluations. DT, EH, FW and ML were responsible for data collection, development of the economic model and performing the analysis. CD and RH were responsible for overseeing the content. All authors contributed to the writing of the manuscript.
Funding: This analysis and the PARADIGM-HF study were funded by Novartis AG.
Competing interests: CD and RH are permanent employees of Novartis AG. CD and RH oversaw the all stages of the economic evaluation and contributed to the design and reporting of the economic model. DRG Abacus received funding from Novartis AG to undertake the analysis and has supported EH, DT JJVM and FW through salary payments. York Health Economics Consortium received funding from Novartis AG to provide consulting input and has supported MT through salary payments. MRC and AB have received consulting fees from Novartis AG. JJVM’s employer, Glasgow University, has received consulting fees and research grant support from Novartis AG.
Provenance and peer review: Not commissioned; externally peer reviewed.
Correction notice: Since this paper has been published online an update has been made to the paragraph Colombian setting. The ICER value of €15 975 has been changed to € 11 200.
References
- 1. Ambrosy AP, Fonarow GC, Butler J, et al. . The global health and economic burden of hospitalizations for heart failure: lessons learned from hospitalized heart failure registries. J Am Coll Cardiol 2014;63:1123–33. 10.1016/j.jacc.2013.11.053 [DOI] [PubMed] [Google Scholar]
- 2. Stewart S, Jenkins A, Buchan S, et al. . The current cost of heart failure to the national health service in the UK. Eur J Heart Fail 2002;4:361–71. 10.1016/S1388-9842(01)00198-2 [DOI] [PubMed] [Google Scholar]
- 3. National Institute for Health and Care Excellence. Chronic heart failure (clinical guideline 108). 2010. http://www.nice.org.uk/guidance/CG108
- 4. European Society of Cardiology. ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. 2012. http://eurheartj.oxfordjournals.org/content/33/14/1787.full.pdf
- 5. Scottish Intercollegiate Guidelines Network. SIGN guideline 95: management of chronic heart failure. http://www.sign.ac.uk/guidelines/fulltext/95/section1.html (accessed 28 Aug 2014).
- 6. Göhler A, Geisler BP, Manne JM, et al. . Utility estimates for decision-analytic modeling in chronic heart failure-health states based on New York heart association classes and number of rehospitalizations. Value Health 2009;12:185–7. 10.1111/j.1524-4733.2008.00425.x [DOI] [PubMed] [Google Scholar]
- 7. Ardehali H, Qasim A, Cappola T, et al. . Endomyocardial biopsy plays a role in diagnosing patients with unexplained cardiomyopathy. Am Heart J 2004;147:919–23. 10.1016/j.ahj.2003.09.020 [DOI] [PubMed] [Google Scholar]
- 8. Pagley PR, Beller GA, Watson DD, et al. . Improved outcome after coronary bypass surgery in patients with ischemic cardiomyopathy and residual myocardial viability. Circulation 1997;96:793–800. 10.1161/01.CIR.96.3.793 [DOI] [PubMed] [Google Scholar]
- 9. McMurray JJ, Packer M, Desai AS, et al. . Dual angiotensin receptor and neprilysin inhibition as an alternative to angiotensin-converting enzyme inhibition in patients with chronic systolic heart failure: rationale for and design of the Prospective comparison of ARNI with ACEI to Determine Impact on Global Mortality and morbidity in Heart Failure trial (PARADIGM-HF). Eur J Heart Fail 2013;15:1062–73. 10.1093/eurjhf/hft052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. McMurray JJ, Packer M, Desai AS, et al. . Angiotensin-neprilysin inhibition versus enalapril in heart failure. N Engl J Med 2014;371:993–1004. 10.1056/NEJMoa1409077 [DOI] [PubMed] [Google Scholar]
- 11. British Society for Heart Failure. National heart failure audit. April 2012–March 2013. 2013. http://www.ucl.ac.uk/nicor/audits/heartfailure/documents/annualreports/hfannual12-13.pdf (accessed 28 Aug 2014).
- 12. Faria R, Mejía A. Guidelines for the economic evaluation of healthcare technologies in Colombia: technical support documents. Bogotá DC, Colombia: Instituto de Evaluación Tecnológica en Salud, 2014. [Google Scholar]
- 13. Nielsen OW, Egstrup K, Buyer L, et al. . 5. Kronisk hjertesvigt. 2016. http://nbv.cardio.dk/chf (accessed 13 Dec 2016).
- 14. McMurray JJV, Packer M, Desai AS, et al. . Baseline characteristics and treatment of patients in Prospective comparison of ARNI with ACEI to Determine Impact on Global Mortality and morbidity in Heart Failure trial (PARADIGM-HF). Eur J Heart Fail 2014;16:817–25. 10.1002/ejhf.115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. StataCorp. Stata Statistical Software: Release 13. College Station, TX: StataCorp LP, 2013. [Google Scholar]
- 16. World Heatlh Organisation. Download the raw data files of the WHO mortality database. 2015. http://www.who.int/healthinfo/statistics/mortality_rawdata/en/ (accessed 01 Oct 2017).
- 17. Office for National Statistics. Interim life tables, England & Wales 2010-12. Secondary interim life tables, England & Wales 2010-12. 2014. http://www.ons.gov.uk/ons/publications/re-reference-tables.html?edition=tcm%3A77-325699 (accessed 24 October 2013).
- 18. Denmark S. Table FOLK2. 2015. www.statistikbanken.dk (accessed 14 Apr 2016).
- 19. Dolan P. Modeling valuations for EuroQol health states. Med Care 1997;35:1095–108. 10.1097/00005650-199711000-00002 [DOI] [PubMed] [Google Scholar]
- 20. Wittrup-Jensen KU, Lauridsen J, Gudex C, et al. . Generation of a Danish TTO value set for EQ-5D health states. Scand J Public Health 2009;37:459–66. 10.1177/1403494809105287 [DOI] [PubMed] [Google Scholar]
- 21. Ollendorf DA, Tarlochan Sandhu A, Chapman R, et al. . CardioMEMSTM HF System (St. Jude Medical) and sacubitril/valsartan (EntrestoTM, Novartis) for management of congestive heart failure: effectiveness, value, and value-based price benchmarks: institute for clinical and economic review. 2015. https://icer-review.org/wp-content/uploads/2016/01/CHF_Draft_Report_091115.pdf
- 22. Gaziano TA, Fonarow GC, Claggett B, et al. . Cost-effectiveness analysis of sacubitril/valsartan vs enalapril in patients with heart failure and reduced ejection fraction. JAMA Cardiol 2016;1:666 10.1001/jamacardio.2016.1747 [DOI] [PubMed] [Google Scholar]
- 23. King JB, Shah RU, Bress AP, et al. . Cost-effectiveness of sacubitril-valsartan combination therapy compared with enalapril for the treatment of heart failure with reduced ejection fraction. JACC Heart Fail 2016;4:392–402. 10.1016/j.jchf.2016.02.007 [DOI] [PubMed] [Google Scholar]
- 24. NICE. Sacubitril valsartan for treating symptomatic chronic heart failure with reduced ejection fraction: NICE technology appraisal guidance 388. 2016. https://www.nice.org.uk/guidance/ta388
- 25. National Institute for Health and Care Excellence. Nice DSU technical support document 14: survival analysis for economic evaluations alongside clinical trials – extrapolation with patient-level data. 2013. http://www.nicedsu.org.uk/NICE_DSU_TSD_Survival_analysis.updated_March_2013.v2.pdf (accessed 09 Mar 2016). [PubMed]
- 26. Claggett B, Packer M, McMurray JJ, et al. . Estimating the long-term treatment benefits of sacubitril-valsartan. N Engl J Med 2015;373:2289–90. 10.1056/NEJMc1509753 [DOI] [PubMed] [Google Scholar]
- 27. Griffiths A, Paracha N, Davies A, et al. . The cost effectiveness of ivabradine in the treatment of chronic heart failure from the U.K. national health service perspective. Heart 2014;100:1031–6. 10.1136/heartjnl-2013-304598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Lee D, Wilson K, Akehurst R, et al. . Cost-effectiveness of eplerenone in patients with systolic heart failure and mild symptoms. Heart 2014;100:1681–7. 10.1136/heartjnl-2014-305673 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Berg J, Lindgren P, Mejhert M, et al. . Determinants of utility based on the EuroQol five-dimensional questionnaire in patients with chronic heart failure and their change over time: results from the swedish heart failure registry. Value Health 2015;18:439–48. 10.1016/j.jval.2015.02.003 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
heartjnl-2016-310661supp001.pdf (4.1MB, pdf)