Abstract
We explored causal mediation of sleep quality and perceived stress in development of painful temporomandibular disorder (TMD). Sleep quality and perceived stress were assessed at baseline and quarterly intervals thereafter in 2,737 initially TMD-free adults in the Orofacial Pain Prospective Evaluation and Risk Assessment study (OPPERA) prospective cohort study. During follow-up, incident TMD cases were classified using research diagnostic criteria. Mediation analysis was conducted using a weighted Cox proportional hazards regression model that estimated hazard ratios (HRs) and 95% confidence limits (CL) of first-onset TMD. Models determined whether: 1) poor sleep quality during follow-up mediated the effect of baseline perceived stress on first-onset TMD, and 2) perceived stress during follow-up mediated the effect of baseline poor sleep quality on first-onset TMD. In both analyses, the total effect was decomposed into natural direct and indirect effects. Poor baseline sleep quality led to heightened perceived stress that then contributed to TMD development. When the total effect of poor sleep quality (HR = 2.10, CL = 1.76, 2.50) was decomposed, 34% of its effect was mediated by perceived stress (indirect effect HR = 1.29, CL = 1.06, 1.58). The effect of perceived stress on first-onset TMD was not mediated by sleep quality. Improving sleep may avert escalation of stress, limiting effects of both factors on TMD development.
Keywords: Epidemiology, mediation analysis, Cox models, temporomandibular disorder, sleep quality, perceived stress
Sleep is an active process, so critical to multiple physiologic systems that the sleep/wake cycle is closely regulated by circadian and homeostatic drives. Studies in animal models42 and humans55 have elucidated physiological and behavioral consequences of sleep deprivation. Experimental laboratory studies and epidemiologic studies show that sleep deprivation leads to dysregulation across multiple stress, metabolic, immune, and inflammatory systems.4,36 Taken together, findings imply an important homeostatic function of sleep. Nonetheless, evidence of consequences of sleep deprivation does not translate well into knowledge of sleep function.41,51 Current views of sleep function include the allocation of energy to optimize biological activities,48 consolidation of newly acquired information in memory,18,23 and the regulation of emotional processing24,56 (see Siegel51 and Krueger et al31 for reviews). Until sleep function is better understood, knowledge of how sleep disturbance transmits its effects to other disorders, such as pain, also remains incomplete.
Sleep disturbance is highly prevalent in people with pain. Delayed sleep onset, frequent awakening after sleep onset, low sleep efficiency, and poor-quality sleep affect 67% to 88% of people with chronic pain.37,54 Apart from problems with sleep initiation and maintenance, sleep-disordered breathing,2 restless legs syndrome,27 and narcolepsy,17 are also more common among people with pain disorders than in pain-free individuals, although there is little evidence that sleep architecture differs in people with pain.6,57 Longitudinal studies show that although disturbed sleep and pain have bidirectional relationships, the effect of sleep disturbance on pain development is greater than the effect of pain on sleep disturbance.21 Moreover, the extent of sleep disturbance predicts sensitivity to experimental pain52 and intensity of clinical pain1 in a dose-response manner.
Psychological distress commonly overlaps with sleep disturbance and chronic pain, forming a triad of disorders with shared pathophysiological features.10,13,39,58 Longitudinal studies show psychological distress is often a precursor of chronic pain,25,26,30 and stress and poor sleep are related. According to cognitive appraisal theory,34 psychological distress arises when an individual first appraises an event as threatening and secondarily appraises the threat to exceed their coping resources. Exposure to psychological distress induces change in pain processing pathways that can result in hyperalgesia.16,59
The next challenge is to explore how differences in these risk factors correspond to change in intermediary factors along the causal pathway to pain development. The current study builds on findings from the Orofacial Pain Prospective Evaluation and Risk Assessment study (OPPERA) prospective cohort study showing that perceived stress20 and poor sleep quality46 contribute to development of painful first-onset temporomandibular disorder (TMD). The aim of this analysis was to examine causal mediation of sleep quality and perceived stress in development of painful first-onset TMD.
Methods
This study was approved by the institutional review boards of the University of Maryland-Baltimore, University at Buffalo, University of North Carolina at Chapel Hill, University of Florida, and the data coordinating center Battelle Memorial Institute. Written informed consent was obtained from all study participants and the research was conducted in accordance with the Declaration of the World Medical Association.
Design, Setting, Study Participants, and Enrollment
The OPPERA study is a prospective investigation of the etiology and persistence of first-onset painful TMD. From May 2006 to November 2008, OPPERA recruited community-based volunteers at 4 study sites located at Baltimore, Maryland; Buffalo, New York; Chapel Hill, North Carolina; and Gainesville, Florida. Telephone screening identified eligible participants aged from 18 to 44 years, with no significant history of TMD symptoms, no significant medical illnesses or recent history of facial injury or surgery, not pregnant or nursing, ≤4 headaches per month within the preceding 3 months, not receiving orthodontic treatment, never diagnosed with TMD, and no use of a night guard or occlusal splint. These volunteers attended a research clinic where they were clinically examined using research diagnostic criteria for TMD.19 Those confirmed as TMD-free (n = 3,263) were enrolled, completed baseline questionnaires, and were followed for up to 5.2 years (median follow-up = 2.8 years).
Baseline Assessment of Subjective Sleep Quality and Perceived Stress
Among the self-administered questionnaires in OP-PERA, 2 relevant to this analysis are the 19-item Pitts-burgh Sleep Quality Index (PSQI)9 and the 10-item Perceived Stress Scale (PSS).15 The PSQI assesses habitual sleep quality and sleep disturbance over the previous month. A global score is obtained from the sum of its 7 component scores that ranges from 0 to 21, with higher scores denoting worse sleep quality. A global score of >5 has diagnostic sensitivity of 89.6% and specificity of 86.5% in distinguishing poor from good sleep.9 The PSQI was reported to be a unidimensional construct in people with painful TMD,43 and therefore this analysis used the PSQI global score. The PSQI was administered at baseline only. For brevity the Sleep Quality Numeric Rating Scale (SQ-NRS) was administered at each quarterly follow-up.
The original 14-item PSS14 was developed to assess stress on the basis of cognitive appraisal theory.34 We used the 10-item version of this instrument, for which the summary score has a potential range of 0 to 40.15 The PSS score is a global measure of the degree to which situations in one’s life are appraised as stressful. Items were designed to evaluate and estimate the degree that respondents find their lives unpredictable, uncontrollable, and overloaded. Response categories measure the frequency of perceived stress and higher scores denote greater psychological stress.
Follow-up Assessment of Subjective Sleep Quality and Psychological Stress
Every 3 months after enrollment, sleep quality and perceived stress were monitored in the Quarterly Health Update questionnaire that was self-completed by study participants. As a more parsimonious measure than the PSQI, the SQ-NRS asked participants to rate their sleep quality over the preceding 3 months. An anchor value of 0 represented “worst sleep imaginable” whereas the anchor value of 10 represented “best sleep imaginable.” As well as its brevity, the SQ-NRS is simple to administer and easy to complete.11,35 Its psychometric properties of reproducibility, convergent validity, and responsiveness to treatment are established. During analysis, the SQ-NRS values were reverse-coded for directional consistency with the PSQI in which higher scores denote worse sleep quality. Psychological stress was evaluated with the same 10-item PSS used at baseline. The number of Quarterly Health Update questionnaires that participants completed varied with their time in study. For each participant a mean score was obtained for stress and sleep quality.
Incident TMD Case Ascertainment
The classification of first-onset TMD was on the basis of the research diagnostic criteria for temporomandibular disorder. Study participants who reported TMD pain symptoms in the Quarterly Health Update questionnaires were invited to study clinics for a follow-up examination to verify first-onset TMD using the same clinical criteria used in the baseline examination. The 2 essential criteria were: symptoms of orofacial pain reported for ≥5 days in the previous 30 days and examiner findings of TMD myalgia, arthralgia, or both. Myalgia was on the basis of pain during jaw maneuver or digital palpation in ≥3 of 8 muscle groups (temporalis, masseter, lateral pterygoid, and submandibular and postmandibular areas), each assessed bilaterally. Arthralgia was on the basis of pain in temporomandibular joint(s) during jaw maneuver or digital palpation.
OPPERA’s sample size of 3,200 enrolled study participants was sufficient to yield 196 first-onset TMD cases during follow-up, assuming 30% loss to follow-up. The Quarterly Health Update questionnaire monitored development of painful TMD by asking about orofacial pain symptoms noticed in the past 3 months. Participants who reported experiencing these symptoms were asked to return to research clinics where the baseline examination was repeated to determine the presence or absence of TMD. Follow-up continued until clinically determined TMD developed, the participant was lost to follow-up, or the study ended in May 2011.
Potential Confounders
We considered the covariates of study site and demographic variables of age, sex, and race/ethnicity as the minimum set of potential cofounders of associations and included them in all analytic models. Conditional on these covariates, analysis makes assumptions that there was no confounding between: the predictor (eg, perceived stress) and TMD; the predictor and the mediator (eg, sleep quality); the mediator and TMD; and no confounding of the predictor on the relationship between the mediator and TMD. In analyses, sleep quality and perceived stress were separately modeled as the predictor and the mediator to help clarify the causal sequence in painful TMD development.
Statistical Approach
The average annual incidence rate of first-onset TMD in the complete cohort was calculated for descriptive purposes and Poisson regression was used to estimate covariate-adjusted incidence rates. Descriptive statistics showing patterns of sleep quality and psychological stress during follow-up were generated by creating a nested case-control subgroup of the complete cohort, specifically for this analysis. For each first-onset TMD case with follow-up data on sleep quality and perceived stress (n = 258), 1 noncase who had not developed TMD was selected. Cases were matched to a noncase according to study site, length of time in study, and baseline PSS score (±2). We compared these 258 pairs of cases and noncases at multiple time points throughout follow-up to examine their respective trajectories in perceived stress and sleep quality. This informed us about the reactivity of these known TMD risk factors before TMD onset.
Next we explored whether baseline sleep quality predicted change in perceived stress. To do this, baseline sleep quality was plotted against perceived stress at baseline, first quarter after enrollment, and last quarter before the second clinic visit, at which time first-onset cases were identified. This comparison enabled us to determine whether the cross-sectional association at baseline persisted when perceived stress was measured at follow-up.
Hypothesis tests for the study’s main aim were evaluated using Cox proportional hazards regression models. The models estimate hazard ratios (HRs) and their 95% confidence limits (CL), which approximate the incidence rate ratio for a selected predictor characteristic.
Baseline measures of the predictors (ie, sleep quality; PSQI global score) and perceived stress (PSS score), were modeled as binary variables. The normally distributed PSQI scores were dichotomized at the established cut point of ≤5 (good sleep quality) versus >5 (poor sleep quality). The rationale for selecting the cut point for the normally distributed PSS scores was to produce a distributional balance with poor sleep quality. This put the cut point at the 69th percentile, corresponding to ≤17 (low perceived stress) versus >17 (high perceived stress).
Cox models accounted for the time-varying nature of sleep quality and perceived stress because values for these variables can change with repeated measurement over the duration of follow-up.
Repeated measures of sleep quality (SQ-NRS) and perceived stress (PSS) obtained during follow-up were modeled as the mediators. The continuous raw scores for these scales were standardized (ie, z-scores were computed [z = ([value mean]/SD)]). This allowed direct comparisons in interpreting the magnitude of the mediated (ie, natural indirect) effects. Differences were considered statistically significant if the null value of unity did not fall between the 95% CL of the HR.
Causal mediation analysis was conducted using Cox proportional hazards regression within a counterfactual framework using the method described by Lange and Hansen for censored time to event data.33 All models adjusted for the set of confounders. The method allows the total effect of changing a predictor (eg, from good to poor sleep quality), on first-onset TMD to be measured as the number of incident first-onset TMD cases per unit of time. The method decomposed this total direct effect into a part directly attributable to the predictor (natural direct effect) and a part mediated through another factor (natural indirect effect). Two separate models were specified: one in which sleep quality was first the predictor and perceived stress was the potential mediator; and the second in which perceived stress was the predictor and sleep quality the potential mediator.
Natural direct and indirect effects within a counterfactual framework have been described in detail by Robins and Greenland.44 In brief, understand the meaning of a natural effect is helped when controlled direct and indirect effects are first understood. The controlled direct effect (eg, of poor sleep quality relative to good sleep quality) is a measure of the expected increase in the rate of first-onset TMD, when the mediator (eg, perceived stress) is set at a fixed value for all study participants. In contrast, the natural direct effect then is a measure of the expected increase in the rate of TMD, when the mediator variable is allowed to vary between participants, which is a more realistic proposition. A participant’s natural value of the mediator is taken to be the counterfactual value it would have taken if he/she were not exposed (eg, good sleep quality). The objective is to estimate 2 quantities. 1) The natural direct effect on the HR scale defined as the effect on first-onset TMD of predictor value = a, versus value = a* (the counterfactual), conditioned on the set of potential confounders if the mediator was set to its value when the predictor value = a*. 2) The natural indirect effect on the HR scale is the effect on first-onset TMD when predictor value = a, and the mediator is set to what it would have been when predictor valued = a, versus value = a* (the counterfactual). Controlled effects cannot be decomposed into direct and indirect effects. A sensitivity analysis explored potential bias because of unmeasured confounding. This was tested by adding cigarette smoking to the set of confounders and repeating mediation analyses. The rationale for selecting cigarette smoking is that smoking is of the strongest predictors of TMD development. The mediation proportion was calculated as the natural log of indirect effect divided the total effect and expressed as a percentage.
Because the degree of bias introduced by missing data was shown to be minimal in OPPERA,3 multivariable analysis used complete-case analysis without imputation. All analyses were conducted in SAS 9.4 (SAS Institute Inc, Cary, NC).
Results
In up to 5.2 years of follow-up (median = 2.8 years), the incidence rate of first-onset TMD was 3.91% of subjects per annum (Table 1). As reported previously,53 the incidence rate was marginally higher among women than men, although the difference was not statistically significant.
Table 1.
Description of Subjective Sleep Quality and Perceived Stress at Baseline for All Participants, OPPERA Prospective Cohort Study, 2006 to 2011 (N = 2,722)
| CHARACTERISTICS | N (%)* | TMD INCIDENCE RATE (%) PER ANNUM (95% CL)† |
SITE-ADJUSTED HR (95% CL)‡ |
MEAN PSQI§ (95% CL) | MEAN PSS (95% CL) |
|---|---|---|---|---|---|
| All participants | 2,722 (100.0) | 3.91 (3.10, 4.94) | NA | 4.71 (4.60, 4.82) | 14.4 (14.2, 14.7) |
| Sex | |||||
| Male | 1,099 (40.4) | 3.36 (2.53, 4.48) | Referent | 4.68 (4.51, 4.86) | 13.9 (13.5, 14.3) |
| Female | 1,623 (59.6) | 4.28 (3.34, 5.49) | 1.28 (.99, 1.65) | 4.73 (4.59, 4.87) | 14.8 (14.5, 15.1) |
| Age, y | |||||
| 18–24 | 1,416 (52.0) | 2.95 (2.22, 3.93) | Referent | 4.37 (4.22, 4.52) | 14.4 (14.1, 14.7) |
| 25–34 | 732 (26.9) | 4.45 (3.32, 5.97) | 1.54 (1.15, 2.08) | 4.74 (4.53, 4.95) | 14.3 (13.9, 14.8) |
| 35–44 | 574 (21.1) | 5.33 (3.92, 7.26) | 1.86 (1.34, 2.59) | 5.55 (5.31, 5.79) | 14.7 (14.2, 15.2) |
| Race/ethnicity | |||||
| White | 1,441 (52.9) | 3.91 (3.04, 5.02) | Referent | 4.46 (4.31, 4.61) | 13.7 (13.3, 14.0) |
| African American | 758 (27.9) | 6.01 (4.35, 8.30) | 1.54 (1.13, 2.08) | 5.55 (5.34, 5.76) | 15.7 (15.3, 16.2) |
| Asian | 256 (9.40) | 1.50 (.77, 2.95) | .39 (.20, .76) | 4.22 (3.87, 4.57) | 15.9 (15.2, 16.7) |
| Hispanic | 178 (6.54) | 3.73 (2.21, 6.29) | .95 (.59, 1.55) | 4.09 (3.67, 4.51) | 14.1 (13.2, 15.0) |
| Other | 89 (3.27) | 3.36 (1.54, 7.30) | .90 (.42, 1.94) | 4.61 (4.00, 5.21) | 13.0 (11.7, 14.3) |
| PSQI¶ | |||||
| Good sleep (≤5) | 1,839 (68.9) | 2.83 (2.17, 3.69) | Referent | NA | 12.9 (12.6, 13.1) |
| Poor sleep (>5) | 829 (31.1) | 6.36 (4.88, 8.27) | 2.22 (1.73, 2.85) | NA | 17.9 (17.5, 18.3) |
| Perceived stress‖ | |||||
| Low (≤17) | 1,869 (68.9) | 3.21 (2.48, 4.15) | Referent | 4.05 (3.92, 4.17) | NA |
| High (>17) | 987 (31.1) | 5.46 (4.17, 7.17) | 1.69 (1.32, 2.17) | 6.22 (6.03, 6.41) | NA |
Abbreviation: NA, not applicable.
Subgroup numbers for some characteristics may sum to less than 2,722 because of missing data for that characteristic.
Site- adjusted, rate per 100 person-years.
Ratio of the hazard rates of first-onset TMD.
PSQI global score in which higher scores denote worse sleep quality.
PSQI I dichotomized at the threshold for poor sleep quality.
PSS score dichotomized at a cut-point to produce a distributional balance with poor sleep quality.
Compared with white individuals, African American individuals developed TMD at a greater rate and Asian participants developed it at a lower rate. Highest incidence rates were observed among the approximate one-third of participants who had poor sleep quality and the one-third with highest scores for perceived stress. Groups with poorer sleep likewise tended to have higher levels of perceived stress.
In the 258 pairs of subjects in the nested case-control group matched for baseline stress, sleep quality (SQ-NRS with potential range: 0–10) began to diverge in the final 6 months before TMD onset or censoring. Divergence was characterized as worsening of mean sleep quality ratings among cases, relative to noncases, in the weeks preceding TMD onset (Fig 1A). There was a similar temporal pattern in mean perceived stress (PSS; potential range: 0–40), although divergence between the 2 groups occurred only in the final 3 months before censoring, just before TMD onset (Fig 1B). Hence, deterioration in sleep quality in cases relative to control participants preceded the cases’ escalation of perceived stress.
Figure 1.
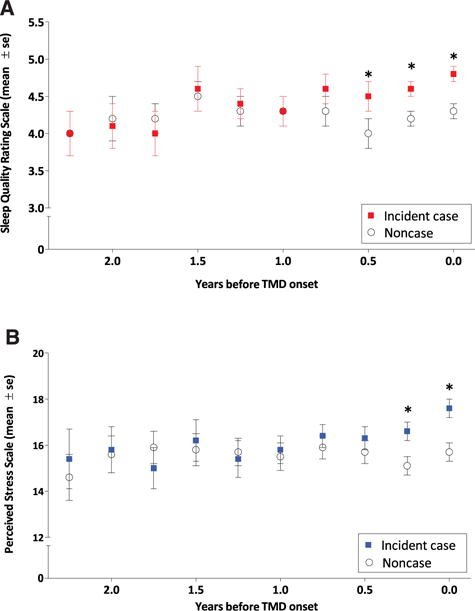
In the months before TMD onset, sleep quality (A) and perceived stress (B) worsened for participants who became incident cases, relative to noncases. Plotted values are mean (± standard error; se) Sleep Quality Numeric Rating score, which has a potential range of 0 to 10 (A) and mean (± se) PSS score, which has a potential range of 0 to 40 (B). Data are from a nested case-control study of 258 future cases and noncase pairs matched according to study site, baseline PSS score, and number of completed Quarterly Health Update questionnaires. Statistically significant differences between future incident cases and noncases in perceived stress and sleep quality were evident in the 2 or 3 quarters before censoring as indicated by asterisk (*) symbols.
We examined the time-lagged effect setting baseline sleep quality as the predictor and inspecting its relationship with perceived stress over time. Poorer sleep quality at baseline was positively associated with mean perceived psychological stress at baseline (Fig 2). It was likewise associated with psychological stress in the first quarter of follow-up (Fig 2) and the final quarter of follow-up (Fig 2). As indicated by the degree overlap of the confidence intervals, the relationship did not differ throughout follow-up. These associations between sleep and stress persisted when the data were examined separately for cases and noncases.
Figure 2.
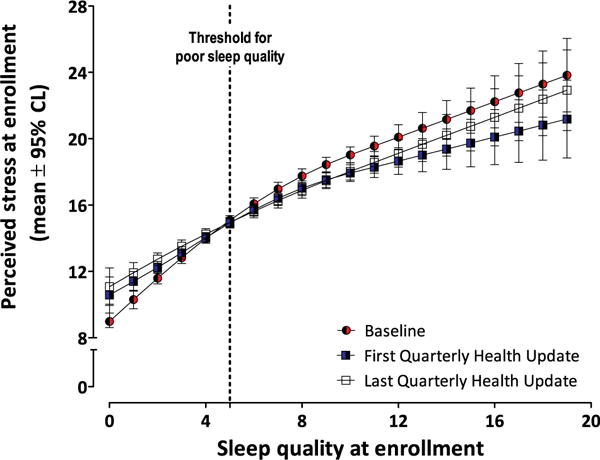
The positive association between poor sleep quality (higher X-axis scores on the PSQI) and perceived stress persisted throughout follow-up. Values on the Y axis are mean (95% confidence limits) PSS scores. The 3 plotted series are data from baseline, at first Quarterly Health Update, and final Quarterly Health Update. The dotted vertical line at the value “5” on the X axis indicates the threshold for poor sleep quality.
Decomposition of natural direct and indirect effects is depicted graphically in Fig 3. Poor sleep quality led to heightened perceived stress that in turn contributed to development of first-onset TMD (Fig 3A). When the total effect of poor sleep quality (HR = 2.10, CL = 1.76, 2.50) was decomposed, 34% of its effect was mediated by perceived stress (indirect effect HR = 1.29, CL = 1.06, 1.58). There was a significant total effect of perceived stress on first-onset TMD (HR = 1.70, 95% CL = 1.42, 2.04; Fig 3B). Formally, the effect was not transmitted via poor sleep quality, because the null value of unity fell within the CL for the estimate of a natural indirect effect (HR = 1.15, 95% CL: = .96, 1.38). It is possible that sleep quality may have a weak mediating effect, because the null value was only just enclosed with the CL. In sensitivity analysis, cigarette smoking status was added to the set of confounders and mediation analysis was repeated. Because smoking did not change the estimates by more than 5% and did not alter the interpretation of findings, the models without smoking (reported in this analysis) were robust to the effect of unmeasured confounding by smoking.
Figure 3.
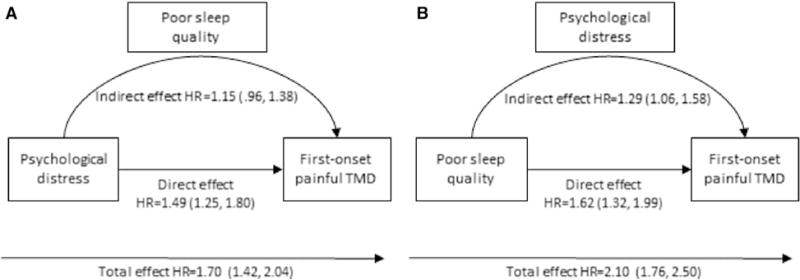
(A) The association of perceived stress and first-onset painful TMD mediated by poor sleep quality. The mediation model is adjusted for study site, age, sex, and race/ethnicity. The predictor is perceived stress (PSS score reported by each participant at baseline) dichotomized at the 69th percentile corresponding to ≤17 = low stress versus >17 = high stress. The mediator is the continuous score on SQ-NRS (z-score transformed mean SQ-NRS reported by each participant at quarterly follow-up visits). Values are covariate-adjusted HRs and 95% CL (N = 2,711). (B) Mediational model of first-onset TMD. On the left side, the effect of stress on first-onset TMD is mediated by poor sleep quality. On the right side, the effect of poor sleep quality and first-onset TMD is mediated by perceived stress. Both mediation models are adjusted for study site, age, sex, and race/ethnicity. Poor sleep quality (PSQI global score at baseline) was dichotomized at ≤5 = good sleep quality versus >5 = poor sleep quality. Stress is the continuous score on the PSS (z-score transformed mean PSS score reported by each participant at quarterly follow-up visits). Values are covariate-adjusted HRs and 95% CL (N = 2,650).
Discussion
Not only did poor sleep quality contribute directly to development of first-onset TMD in this study, it also exacerbated the perception of psychological stress. Perceived stress played a critical role in transmitting the effect of poor sleep quality to TMD onset. Among subjects destined to develop TMD, sleep deteriorated approximately 3 months before increases in psychological stress, both of which preceded TMD onset. This pattern was confirmed quantitatively with mediation analysis showing that approximately one-third of the total effect of poor sleep quality on TMD development was mediated by heightened perceived stress. Observed HRs represent substantial effect sizes, exceeding estimates for many other psychological risk factors measured in the study.20 The effect was not reciprocal: sleep quality did not significantly mediate the effect of perceived stress in first-onset TMD, although sleep and stress themselves were strongly associated. Although poor sleep and psychological stress were positively associated (Fig 2), consistent with a recent review47 and consistent with perceived stress exerting an adverse effect on sleep quality, the potential bivariate causal relationships between stress and sleep appear to yield to further complexity where the relationship of stress > sleep > TMD was not a significant causal pathway to TMD development in this analysis. The use of mediation analysis in this prospective cohort study avoids temporal ambiguity between sleep quality and perceived stress, both of which predict first-onset TMD. The identification of a mediator decreases the probability that the relationship is spurious, while improving the causal plausibility. Longitudinal study designs that assess variables only at baseline and end of study are insufficient to draw inferences about mediation. A certain amount of time is required to have lapsed between baseline predictor assessment and measurement of the mediator, before the outcome has developed. Although other longitudinal analyses have confirmed these factors as predictive of TMD onset, this is the first study to show the direction of effect and mediation. Although the indirect effect (ie, mediated portion) was not statistically significant (HR = 1.15, 95% CL = .96, 1.38), it approached statistical significance. It is quite likely that other studies will report a significant mediation effect.
This analysis extends findings of Buenaver et al,8 who showed that the effect of pain catastrophizing—the tendency to magnify pain and ruminate upon it—among chronic myofascial TMD patients was mediated through poor sleep quality. Although the bootstrapping technique used in that analysis is intended to estimate indirect effects, the cross-sectional study design precluded causal testing and lacked the rigor of a prospective design.8 Evidence of mediation in this analysis adds to OPPERA’s time-lagged analysis that revealed a progressive worsening of sleep quality during follow-up before the emergence of pain symptoms. In contrast, sleep quality remained stable among participants who did not develop TMD.45 That analysis had investigated whether the putative effect of sleep quality was mediated by increased sensitivity to experimental pain, but found no evidence of such mediation.45 Because of the overlap and temporal ambiguity between sleep disturbance, perceived stress, and pain, it is not surprisingly that different causal configurations have been proposed. For example, in a study of persistent pain nested within the prospective North Staffordshire Osteoarthritis Project, investigators reported that pain interference mediated the effect of sleep disturbance on probable depression, as well as the effect of probable depression on sleep disturbance.10 The is an upturn (worsening) in the sleep and stress relationship during the last few follow-up periods, before TMD onset. It is reasonable to expect that stress becomes substantially worse, because of life events for example, and aggravates sleep. However, stress would still be observed to mediate sleep (and not the reverse).
It is also not unexpected that these clustered disorders share pathophysiological features and that they operate through similar pain mechanisms.7 Perceived stress has been proposed as a unifying factor for this clustering.49 Several areas of the central nervous system involved in pain processing—the thalamus, limbic system, and pre-frontal cortex—are also engaged in sleep regulation and the stress response. Exposure to perceived stress also induces change in pain processing pathways that can result in hyperalgesia.16,59 Stress disrupts mechanisms of neuroplasticity in brain structures that are functionally abnormal in depression.38,40 The effect of stress on depression is amplified in carriers of the short allele of the serotonin transporter gene promoter region. Compared with individuals who are homozygous for the long allele, those with 1 or 2 copies of the short allele of the serotonin transporter gene promoter polymorphism have increased perceived stress, depression, and greater norepinephrine secretion.12
A heightened processing of noxious input common to psychological stress, sleep disturbance, and pain. Atrophy of the hippocampus and increased limbic area activation is also common to each.29,50 Likewise, all show dysregulation of the hypothalamic-pituitary-adrenal (HPA) axis and similar changes in levels of serotonin, brain-derived neurotrophic factor, and proinflammatory cytokines including tumor necrosis factor, interleukin-1, and interlukin-6.7 An altered dopaminergic function in symptoms of these disorders prompted Finan and Smith22 to propose the mesolimbic dopamine system as a putative mechanism underlying the comorbidity of these disorders.
Sleep disturbance is associated with activation of the HPA pathway5 and dysregulation of cortisol activity. A recent study, in which baseline sleep disturbance (PSQI) predicted increased depressive symptoms 4 months later, examined diurnal cortisol patterns in a mediation analysis.28 The idea was that disturbance of sleep dysregulates HPA activity serving as a possible neurobiological mediator in its relationship with heightened depressive symptoms. Findings showed that worse sleep quality in these participants was associated with less overall cortisol output and a flatter diurnal cortisol slope, indicative of higher evening values. Both of these cortisol indices were related to heightened depression symptoms and both had significant indirect effects.28 The partici pants in this study were prostate cancer survivors, so although the findings may not generalize to chronic pain, the relationship shows biological plausibility. Our own pilot case control study of chronic TMD found that TMD cases reported greater perceived stress than control participants, but had lower hair cortisol concentration than control participants, suggestive of a dysregulated HPA pathway.32
The major strengths of this analysis include OPPERA’s population-based prospective cohort design with repeated assessments during a long follow-up and examiner-verification of TMD by experienced clinicians. OPPERA’s design established the necessary temporal sequence and chain of relations to show the transmission of a predictor to an outcome and observance of time-varying exposures.
The major limitation is that the validity of estimates of direct and indirect effect depends upon the assumption of no unmeasured confounding of the associations between: sleep quality and first-onset TMD, sleep quality and perceived stress, perceived stress and first-onset TMD, and that perceived stress and first-onset TMD were not confounded by sleep quality. If there is an uncontrolled common cause of the mediator and outcome affected by exposure, estimates of direct and indirect effects will be biased and cannot be interpreted causally. The length of follow-up was determined by 4 key dates: the date of enrollment into OPPERA, the date that TMD developed, the date at which participants were lost to follow-up, and the date that the study closed. This variability poses interpretive ambiguities if this study were to estimate the relative risk of developing TMD. However, that potential pitfall was avoided by the use of Cox proportional hazards regression. Because of its probability density function, these models take account of the cumulative probability of developing TMD. This allows estimates to be accurate, irrespective of varying lengths of follow-up times between participants.
Conclusions
This analysis shows that the association between poor sleep quality and first-onset TMD is partially mediated by perceived stress. Implicit in this finding is the possibility that interventions to improve sleep quality will benefit patients in 2 ways: first, by reducing stress; and second, by reducing risk of developing painful TMD. Also implicit is that stress processes exert unique effects on first-onset TMD and that stress itself requires clinical attention. Further work is warranted to determine whether poor sleep quality contributes to the transition to persistent TMD and to elucidate the biological mechanisms involved.
Perspective.
Causal mediation analysis highlights mechanisms by which poor sleep quality promotes development of TMD. First, poor sleep quality exerts a direct effect on pain. Second, it triggers a heightened perception of stress, which acts as an intermediate factor in the causal pathway between poor sleep quality and first-onset TMD pain.
Acknowledgments
This work was supported by the National Institutes of Health and National Institute of Dental and Cranial Research (grant numbers U01-DE17018 and R03-DE022595).
Footnotes
Roger B. Fillingim is a consultant and equity stock holder, and William Maixner is a founder and equity stock holder in Algynomics, a company providing research services in personalized pain medication and diagnostics. The remaining authors have no conflicts of interest to declare.
References
- 1.Affleck G, Urrows S, Tennen H, Higgins P, Abeles M. Sequential daily relations of sleep, pain intensity, and attention to pain among women with fibromyalgia. Pain. 1996;68:363–368. doi: 10.1016/s0304-3959(96)03226-5. [DOI] [PubMed] [Google Scholar]
- 2.Aytekin E, Demir SE, Komut EA, Okur SC, Burnaz O, Caglar NS, Demiryontar DY. Chronic widespread musculoskeletal pain in patients with obstructive sleep apnea syndrome and the relationship between sleep disorder and pain level, quality of life, and disability. J Phys Ther Sci. 2015;27:2951–2954. doi: 10.1589/jpts.27.2951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bair E, Brownstein NC, Ohrbach R, Greenspan JD, Dubner R, Fillingim RB, Maixner W, Smith SB, Diatchenko L, Gonzalez Y, Gordon SM, Lim PF, Ribeiro-Dasilva M, Dampier D, Knott C, Slade GD. Study protocol, sample characteristics, and loss to follow-up: The OPPERA prospective cohort study. J Pain. 2013;14:T2–T19. doi: 10.1016/j.jpain.2013.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Banks S, Dinges DF. Behavioral and physiological consequences of sleep restriction. J Clin Sleep Med. 2007;3:519–528. [PMC free article] [PubMed] [Google Scholar]
- 5.Besedovsky L, Lange T, Born J. Sleep and immune function. Pflugers Arch. 2012;463:121–137. doi: 10.1007/s00424-011-1044-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bjurstrom MF, Irwin MR. Polysomnographic characteristics in nonmalignant chronic pain populations: A review of controlled studies. Sleep Med Rev. 2016;26:74–86. doi: 10.1016/j.smrv.2015.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Boakye PA, Olechowski C, Rashiq S, Verrier MJ, Kerr B, Witmans M, Baker G, Joyce A, Dick BD. A critical review of neurobiological factors involved in the interactions between chronic pain, depression, and sleep disruption. Clin J Pain. 2016;32:327–336. doi: 10.1097/AJP.0000000000000260. [DOI] [PubMed] [Google Scholar]
- 8.Buenaver LF, Quartana PJ, Grace EG, Sarlani E, Simango M, Edwards RR, Haythornthwaite JA, Smith MT. Evidence for indirect effects of pain catastrophizing on clinical pain among myofascial temporomandibular disorder participants: The mediating role of sleep disturbance. Pain. 2012;153:1159–1166. doi: 10.1016/j.pain.2012.01.023. [DOI] [PubMed] [Google Scholar]
- 9.Buysse DJ, Reynolds CF, 3rd, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh Sleep Quality Index: A new instrument for psychiatric practice and research. Psychiatry Res. 1989;28:193–213. doi: 10.1016/0165-1781(89)90047-4. [DOI] [PubMed] [Google Scholar]
- 10.Campbell P, Tang N, McBeth J, Lewis M, Main CJ, Croft PR, Morphy H, Dunn KM. The role of sleep problems in the development of depression in those with persistent pain: A prospective cohort study. Sleep. 2013;36:1693–1698. doi: 10.5665/sleep.3130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cappelleri JC, Bushmakin AG, McDermott AM, Sadosky AB, Petrie CD, Martin S. Psychometric properties of a single-item scale to assess sleep quality among individuals with fibromyalgia. Health Qual Life Outcomes. 2009;7:54. doi: 10.1186/1477-7525-7-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Caspi A, Sugden K, Moffitt TE, Taylor A, Craig IW, Harrington H, McClay J, Mill J, Martin J, Braithwaite A, Poulton R. Influence of life stress on depression: Moderation by a polymorphism in the 5-HTT gene. Science. 2003;301:386–389. doi: 10.1126/science.1083968. [DOI] [PubMed] [Google Scholar]
- 13.Chiu YH, Silman AJ, Macfarlane GJ, Ray D, Gupta A, Dickens C, Morriss R, McBeth J. Poor sleep and depression are independently associated with a reduced pain threshold. Results of a population based study. Pain. 2005;115:316–321. doi: 10.1016/j.pain.2005.03.009. [DOI] [PubMed] [Google Scholar]
- 14.Cohen S, Kamarck T, Mermelstein R. A global measure of perceived stress. J Health Soc Behav. 1983;24:385–396. [PubMed] [Google Scholar]
- 15.Cohen S, Williamson G. Perceived stress in a probability sample of the United States. In: Spacapan S, Oskamp S, editors. The Social Psychology of Health: Claremont Symposium on Applied Social Psychology. Newbury Park, CA: Sage; 1988. [Google Scholar]
- 16.Crettaz B, Marziniak M, Willeke P, Young P, Hellhammer D, Stumpf A, Burgmer M. Stress-induced allodynia–evidence of increased pain sensitivity in healthy humans and patients with chronic pain after experimentally induced psychosocial stress. PLoS One. 2013;8:e69460. doi: 10.1371/journal.pone.0069460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dauvilliers Y, Bayard S, Shneerson JM, Plazzi G, Myers AJ, Garcia-Borreguero D. High pain frequency in narcolepsy with cataplexy. Sleep Med. 2011;12:572–577. doi: 10.1016/j.sleep.2011.01.010. [DOI] [PubMed] [Google Scholar]
- 18.Diekelmann S, Born J. The memory function of sleep. Nat Rev Neurosci. 2010;11:114–126. doi: 10.1038/nrn2762. [DOI] [PubMed] [Google Scholar]
- 19.Dworkin SF, LeResche L. Research diagnostic criteria for temporomandibular disorders: Review, criteria, examinations and specifications, critique. J Craniomandib Disord. 1992;6:301–355. [PubMed] [Google Scholar]
- 20.Fillingim RB, Ohrbach R, Greenspan JD, Knott C, Diatchenko L, Dubner R, Bair E, Baraian C, Mack N, Slade GD, Maixner W. Psychological factors associated with development of TMD: The OPPERA prospective cohort study. J Pain. 2013;14:T75–T90. doi: 10.1016/j.jpain.2013.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Finan PH, Goodin BR, Smith MT. The association of sleep and pain: An update and a path forward. J Pain. 2013;14:1539–1552. doi: 10.1016/j.jpain.2013.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Finan PH, Smith MT. The comorbidity of insomnia, chronic pain, and depression: Dopamine as a putative mechanism. Sleep Med Rev. 2013;17:173–183. doi: 10.1016/j.smrv.2012.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gais S, Rasch B, Dahmen JC, Sara S, Born J. The memory function of noradrenergic activity in non-REM sleep. J Cogn Neurosci. 2011;23:2582–2592. doi: 10.1162/jocn.2011.21622. [DOI] [PubMed] [Google Scholar]
- 24.Goldstein AN, Walker MP. The role of sleep in emotional brain function. Annu Rev Clin Psychol. 2014;10:679–708. doi: 10.1146/annurev-clinpsy-032813-153716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hammen C. Stress and depression. Annu Rev Clin Psychol. 2005;1:293–319. doi: 10.1146/annurev.clinpsy.1.102803.143938. [DOI] [PubMed] [Google Scholar]
- 26.Hasin DS, Goodwin RD, Stinson FS, Grant BF. Epidemiology of major depressive disorder: Results from the National Epidemiologic Survey on Alcoholism and Related Conditions. Arch Gen Psychiatry. 2005;62:1097–1106. doi: 10.1001/archpsyc.62.10.1097. [DOI] [PubMed] [Google Scholar]
- 27.Hoogwout SJ, Paananen MV, Smith AJ, Beales DJ, O’Sullivan PB, Straker LM, Eastwood PR, McArdle N, Champion D. Musculoskeletal pain is associated with restless legs syndrome in young adults. BMC Musculoskelet Disord. 2015;16:294. doi: 10.1186/s12891-015-0765-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hoyt MA, Bower JE, Irwin MR, Weierich MR, Stanton AL. Sleep quality and depressive symptoms after prostate cancer: The mechanistic role of cortisol. Behav Neurosci. 2016;130:351–356. doi: 10.1037/bne0000107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Joo EY, Kim H, Suh S, Hong SB. Hippocampal substructural vulnerability to sleep disturbance and cognitive impairment in patients with chronic primary insomnia: Magnetic resonance imaging morphometry. Sleep. 2014;37:1189–1198. doi: 10.5665/sleep.3836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kendler KS, Karkowski LM, Prescott CA. Stressful life events and major depression: Risk period, long-term contextual threat, and diagnostic specificity. J Nerv Ment Dis. 1998;186:661–669. doi: 10.1097/00005053-199811000-00001. [DOI] [PubMed] [Google Scholar]
- 31.Krueger JM, Frank MG, Wisor JP, Roy S. Sleep function: Toward elucidating an enigma. Sleep Med Rev. 2016;28:46–54. doi: 10.1016/j.smrv.2015.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lambert CA, Sanders A, Wilder RS, Slade GD, Van Uum S, Russell E, Koren G, Maixner W. Chronic HPA axis response to stress in temporomandibular disorder. J Dent Hyg. 2013;87:73–81. [PMC free article] [PubMed] [Google Scholar]
- 33.Lange T, Hansen JV. Direct and indirect effects in a survival context. Epidemiology. 2011;22:575–581. doi: 10.1097/EDE.0b013e31821c680c. [DOI] [PubMed] [Google Scholar]
- 34.Lazarus R, Folkman S. Stress, Appraisal, and Coping. New York: Springer Publishing Company; 1984. [Google Scholar]
- 35.Martin S, Chandran A, Zografos L, Zlateva G. Evaluation of the impact of fibromyalgia on patients’ sleep and the 436 The Journal of Pain content validity of two sleep scales. Health Qual Life Outcomes. 2009;7:64. doi: 10.1186/1477-7525-7-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Meerlo P, Sgoifo A, Suchecki D. Restricted and disrupted sleep: Effects on autonomic function, neuroendocrine stress systems and stress responsivity. Sleep Med Rev. 2008;12:197–210. doi: 10.1016/j.smrv.2007.07.007. [DOI] [PubMed] [Google Scholar]
- 37.Morin CM, LeBlanc M, Daley M, Gregoire JP, Merette C. Epidemiology of insomnia: Prevalence, self-help treatments, consultations, and determinants of help-seeking behaviors. Sleep Med. 2006;7:123–130. doi: 10.1016/j.sleep.2005.08.008. [DOI] [PubMed] [Google Scholar]
- 38.Nekovarova T, Yamamotova A, Vales K, Stuchlik A, Fricova J, Rokyta R. Common mechanisms of pain and depression: Are antidepressants also analgesics? Front Behav Neurosci. 2014;8:99. doi: 10.3389/fnbeh.2014.00099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nicassio PM, Ormseth SR, Kay M, Custodio M, Irwin MR, Olmstead R, Weisman MH. The contribution of pain and depression to self-reported sleep disturbance in patients with rheumatoid arthritis. Pain. 2012;153:107–112. doi: 10.1016/j.pain.2011.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pittenger C, Duman RS. Stress, depression, and neuroplasticity: A convergence of mechanisms. Neuropsychopharmacology. 2008;33:88–109. doi: 10.1038/sj.npp.1301574. [DOI] [PubMed] [Google Scholar]
- 41.Rechtschaffen A, Bergmann BM. Sleep deprivation in the rat: An update of the 1989 paper. Sleep. 2002;25:18–24. doi: 10.1093/sleep/25.1.18. [DOI] [PubMed] [Google Scholar]
- 42.Rechtschaffen A, Gilliland MA, Bergmann BM, Winter JB. Physiological correlates of prolonged sleep deprivation in rats. Science. 1983;221:182–184. doi: 10.1126/science.6857280. [DOI] [PubMed] [Google Scholar]
- 43.Rener-Sitar K, John MT, Bandyopadhyay D, Howell MJ, Schiffman EL. Exploration of dimensionality and psychometric properties of the Pittsburgh Sleep Quality Index in cases with temporomandibular disorders. Health Qual Life Outcomes. 2014;12:10. doi: 10.1186/1477-7525-12-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Robins JM, Greenland S. Identifiability and exchange-ability for direct and indirect effects. Epidemiology. 1992;3:143–155. doi: 10.1097/00001648-199203000-00013. [DOI] [PubMed] [Google Scholar]
- 45.Sanders AE, Akinkugbe AA, Bair E, Fillingim RB, Greenspan JD, Ohrbach R, Dubner R, Maixner W, Slade GD. Subjective sleep quality deteriorates before development of painful temporomandibular disorder. J Pain. 2016;17:669–677. doi: 10.1016/j.jpain.2016.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sanders AE, Slade GD, Bair E, Fillingim RB, Knott C, Dubner R, Greenspan JD, Maixner W, Ohrbach R. General health status and incidence of first-onset temporomandibular disorder: The OPPERA prospective cohort study. J Pain. 2013;14:T51–T62. doi: 10.1016/j.jpain.2013.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sanford LD, Suchecki D, Meerlo P. Stress, arousal, and sleep. Curr Top Behav Neurosci. 2015;25:379–410. doi: 10.1007/7854_2014_314. [DOI] [PubMed] [Google Scholar]
- 48.Schmidt MH. The energy allocation function of sleep: A unifying theory of sleep, torpor, and continuous wakefulness. Neurosci Biobehav Rev. 2014;47:122–153. doi: 10.1016/j.neubiorev.2014.08.001. [DOI] [PubMed] [Google Scholar]
- 49.Senba E. A key to dissect the triad of insomnia, chronic pain, and depression. Neurosci Lett. 2015;589:197–199. doi: 10.1016/j.neulet.2015.01.012. [DOI] [PubMed] [Google Scholar]
- 50.Sheline YI, Wang PW, Gado MH, Csernansky JG, Vannier MW. Hippocampal atrophy in recurrent major depression. Proc Natl Acad Sci U S A. 1996;93:3908–3913. doi: 10.1073/pnas.93.9.3908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Siegel JM. Clues to the functions of mammalian sleep. Nature. 2005;437:1264–1271. doi: 10.1038/nature04285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sivertsen B, Lallukka T, Petrie KJ, Steingrimsdottir OA, Stubhaug A, Nielsen CS. Sleep and pain sensitivity in adults. Pain. 2015;156:1433–1439. doi: 10.1097/j.pain.0000000000000131. [DOI] [PubMed] [Google Scholar]
- 53.Slade GD, Bair E, Greenspan JD, Dubner R, Fillingim RB, Diatchenko L, Maixner W, Knott C, Ohrbach R. Signs and symptoms of first-onset TMD and sociodemographic predictors of its development: The OPPERA prospective cohort study. J Pain. 2013;14:T20–T32. e1–e3. doi: 10.1016/j.jpain.2013.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Smith MT, Haythornthwaite JA. How do sleep disturbance and chronic pain inter-relate? Insights from the longitudinal and cognitive-behavioral clinical trials literature. Sleep Med Rev. 2004;8:119–132. doi: 10.1016/S1087-0792(03)00044-3. [DOI] [PubMed] [Google Scholar]
- 55.Van Dongen HP, Maislin G, Mullington JM, Dinges DF. The cumulative cost of additional wakefulness: Dose-response effects on neurobehavioral functions and sleep physiology from chronic sleep restriction and total sleep deprivation. Sleep. 2003;26:117–126. doi: 10.1093/sleep/26.2.117. [DOI] [PubMed] [Google Scholar]
- 56.Walker MP, van der Helm E. Overnight therapy? The role of sleep in emotional brain processing. Psychol Bull. 2009;135:731–748. doi: 10.1037/a0016570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Weingarten JA, Dubrovsky B, Basner RC, Redline S, George L, Lederer DJ. Polysomnographic measurement of sleep duration and bodily pain perception in the Sleep Heart Health Study. Sleep. 2016;39:1583–1589. doi: 10.5665/sleep.6026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wilson KG, Eriksson MY, D’Eon JL, Mikail SF, Emery PC. Major depression and insomnia in chronic pain. Clin J Pain. 2002;18:77–83. doi: 10.1097/00002508-200203000-00002. [DOI] [PubMed] [Google Scholar]
- 59.Zheng G, Hong S, Hayes JM, Wiley JW. Chronic stress and peripheral pain: Evidence for distinct, region-specific changes in visceral and somatosensory pain regulatory pathways. Exp Neurol. 2015;273:301–311. doi: 10.1016/j.expneurol.2015.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]


