Abstract
Objective
We examined changes in prevalence of diabetic microvascular/macrovascular complications and diabetes care indicators for adults in Japan with type 2 and type 1 diabetes over one decade.
Research design and methods
Two independent cohorts were recruited with the same inclusion criteria in 2004 (cohort 1: 3319 with type 2 and 286 with type 1 diabetes) and in 2014 (cohort 2: 3932 with type 2 and 308 with type 1 diabetes). Prevalence of complications and care indicators including achieving treatment targets for glycemia, blood pressure, lipid control, body mass index (BMI), and smoking were compared. In addition, patients in cohort 1 were re-examined in 2014 and their data were compared with the baseline data of each cohort.
Results
In type 2 diabetes, the prevalence of nephropathy, retinopathy, neuropathy, chronic kidney disease, current smoking and stroke significantly decreased, with improvements in achieving treatment target rates in cohort 2 two as compared with cohort 1. In type 1 diabetes, the prevalence of nephropathy, retinopathy, chronic kidney disease, and hemoglobin A1Cvalues significantly decreased. Decreases in prevalence of microvascular complications in type 2 diabetes were similarly found in each age-matched and sex-matched group, whereas younger patients exhibited marked increase in BMI and lower treatment target achieving rates compared with elderly patients. Regarding normoalbuminuric renal impairment, only a slight increase in the prevalence was observed both in type 2 and type 1 diabetes. In cohort 1, re-examined in 2014, care indicators were significantly improved from 2004, while complications increased with getting 10 years older.
Conclusions
We observed declining trends of diabetic microvascular complications with improvement in diabetes care indicators in type 2 and type 1 diabetes. Younger patients with type 2 diabetes exhibited marked increase in BMI and lower rates of achieving treatment targets compared with elderly patients, which remains a concern.
Keywords: microvascular complications, type 2 diabetes, type 1 diabetes, nephropathy, diabetes care indicators
Significance of this study.
What is already known about this subject?
Diabetic microvascular complications increase risk of cardiovascular disease, end-stage renal disease and blindness, and its burden may increase with the increasing population of diabetes. While some studies indicated the improvement of diabetes care indicators over time, no studies have revealed the improvement in prevalence of diabetic microvascular complications.
The question is whether the prevalence of microvascular complications and diabetes care indicators in patients with type 2 and type 1 diabetes have been changed over time in real-world practice.
What are the new findings?
Comparing the two different cohorts recruited in 2004 and 2014 with similar age indicated that the prevalence of microvascular complications, chronic kidney disease, current smokers and stroke significantly decreased over time, with increased rates of achieving treatment targets for glycated hemoglobin A1C (HbA1c), blood pressure and lipid in type 2 diabetes. In type 1 diabetes, the prevalence of nephropathy, retinopathy, chronic kidney disease, and HbA1c values significantly decreased.
However, younger patients with type 2 diabetes exhibited increasing body mass index and lower rates of achieving therapeutic goals.
How might these results change the focus of research or clinical practice?
The improvement in prevalence of vascular complications and care indicators may lead to longevity in diabetic populations, which could increase ageing-related problems.
Introduction
Type 2 diabetes is pandemic and represents a major threat to public health in many countries of the world. With the expected increase in the prevalence of diabetes due to increasing rates of obesity and decreased physical activity, the burden of diabetic microvascular complications, known as diabetic nephropathy, retinopathy, or neuropathy, may also increase.1–3 These three microvascular complications are life-threatening because they themselves are the risk factors for cardiovascular disease (CVD) and premature death, and could lead to end-stage renal disease, blindness and autonomic neuropathy.1–7 In order to prevent microvascular complications and CVD, individualized glycemic control and multifactorial risk reduction are the cornerstones of high-quality diabetes care, as demonstrated in many clinical trials.8–13
Optimal individualized diabetes management has been promoted until now and the achievement of diabetes care indicators has been assessed. Secular changes in diabetes care indicators have been reported in the USA, Canada, Germany, and China. In these reports, care for diabetes has been generally improving, although it depended on the area and the time examined.14–20 However, secular changes in the prevalence of microvascular complications have not been adequately assessed and those in type 1 diabetes have not yet been reported.
In Japan, approximately 10 million individuals among the total population of 125 million have diabetes. Treatment goals for patients with diabetes have been set by national guidelines from the Japan Diabetes Society (JDS) since 2002, while most of the patients have been treated in primary care settings.21 Tracking the changes in prevalence of complications and the quality of care indicators at the population level is essential to help understand successes and failures and to direct quality improvement initiatives and health policies. To date, no studies have comprehensively evaluated the prevalence of complications and the quality of diabetes care over time at the national level.
In this study, we examined changes in prevalence of complications and quality of care indicators for adults in Japan with type 2 and type 1 diabetes collected in 2004 and 2014. Complications included both microvascular and macrovascular diseases, and diabetes care indicators included rate of achieving treatment targets for glycemia, blood pressure (BP), lipid control, body weight, and smoking.
Research design and methods
Study population
JDDM Study group
This study was a nationwide multicenter study conducted by the Japan Diabetes Clinical Data Management (JDDM) Study group. This study group was organized by general practitioners voluntarily gathering from all over Japan in order to elucidate the actual status of Japanese diabetes care and promote clinical diabetes research based on daily clinical practice in 2001 because there was no nationwide registry system in Japan. The majority of physicians in this study were the practitioners conducting daily general practice while specializing in or being particularly interested in diabetes care (see appendix). In this study group, all medical records on daily clinical practice, such as patient information, clinical data, medical precipitation history and so on, were accumulated over time at the central office using the same software so as to collect these data.
Cohort recruitment
Two independent cohorts were recruited in 2004 as cohort 1 and in 2014 as cohort 2 for the purpose of evaluating the risk of CVD and death in patients with diabetes in a prospective fashion. The inclusion criteria for the two cohorts were the same; age from 20 years to 70 years, being regularly treated for diabetes for more than 1 year prior to the baseline, and having type 2 or type 1 diabetes. Those who were pregnant or had gestational diabetes were not included. A patient who participated in cohort 1 was not allowed to enter cohort 2, thus no patients overlapped. Seventeen and 28 clinics participated in cohort 1 and cohort 2, respectively, of which 10 clinics participated in both cohorts.
The present study was primarily designed to investigate the trend of the prevalence of microvascular/macrovascular disease and diabetes care indicators by comparing the baseline clinical features of two different 10-year cohorts. In addition, patients in cohort 1 were re-examined in 2014 and their data were compared with the baseline data of each cohort to ensure the trend. Patients in our cohorts were treated with the aim of achieving the targets recommended by JDS: a glycated hemoglobin A1C (HbA1c) value of <7.0% (53 mmol/mol), BP <130/80 mm Hg, serum concentrations of low-density lipoprotein cholesterol <3.1 mmol/L (120 mg/dL), high-density lipoprotein (HDL) cholesterol ≥1.0 mmol/L (40 mg/dL), and non-HDL cholesterol <3.8 mmol/L (150 mg/dL), and body mass index (BMI) of 20–24 kg/m2.21 Lipid on target was defined as meeting all three levels. All participants provided written informed consent.
Measurements
Diabetes was diagnosed according to the JDS criteria. Briefly, type 1 diabetes was defined as a definitive requirement for insulin treatment in less than 1 year following diagnosis. Type 2 diabetes was defined by absence of ketoacidosis and glycemic control without insulin treatment for at least 2 years after diagnosis, and patients were divided into treatment groups by diet alone, hypoglycemic tablets, or insulin with or without using tablets. BMI was calculated as the ratio of body weight (kg) and height (m) squared. BP was measured with an appropriately sized cuff in the sitting position using an automated standardized BP device. Non-fasting blood samples were drawn and analyzed to measure serum creatinine (Cr) and lipids at local laboratories. HbA1c levels were measured at each clinic by high performance liquid chromatography and presented as National Glycohemoglobin Standardization Program values, according to the recommendations of the JDS.22 Serum and urinary concentrations of Cr and urinary albumin were measured by enzymatic methods and turbidimetric immunoassay, respectively. The urinary albumin excretion rate was recorded as the albumin-to-creatinine ratio (ACR). Normoalbuminuria, microalbuminuria and macroalbuminuria were defined as an ACR <30 mg/g Cr, ACR ≥30 mg/g Cr and <300 mg/g Cr, and ACR ≥300 mg/g Cr, respectively, in two of three spot urine specimens. The glomerular filtration rate (GFR) was estimated using the following equation by the Japanese Society of Nephrology: eGFR (ml/min/1.73 m2)=194×Scr−1.094×Age−0.287×0.739 (if female). According to the classification defined by Kidney Disease Improving Global Outcomes (KDIGO), patients were divided by the ACR (normoalbuminuria, microalbuminuria, and macroalbuminuria) and eGFR (≥90, 60–89, and <60) levels.23
Diabetic microvascular complications included nephropathy, retinopathy and neuropathy. Diabetic nephropathy was defined as ACR ≥30 mg/g Cr. Chronic kidney disease (CKD) was defined as ACR ≥30 mg/g Cr or eGFR <60 mL/min/1.73 m2. Diabetic retinopathy was diagnosed by ophthalmologists after pupillary dilatation with fundus photography, which was defined as presence of any retinopathy. Neuropathy was diagnosed in patients with two or more of three components, as recommended in the simplified diagnostic criteria proposed by the Diabetic Neuropathy Study Group in Japan:24 (1) Subjective symptoms in the bilateral lower limbs or feet. (2) Loss of or decreased ankle jerk reflex. (3) Decreased vibration perception, assessed using a C128 tuning fork and bilaterally measured at the medial malleoli. Neuropathic symptoms were defined as bilateral spontaneous pain, hypoesthesia including decreased perception to pinprick and temperature (cold tuning fork), or paresthesia of the legs. Macrovascular complications, that is, CVD, consisted of coronary artery disease (CAD), ischemic stroke, and peripheral artery disease (PAD). CAD included myocardial infarction, angina pectoris, and coronary interventions. Ischemic stroke included symptomatic brain infarction and carotid revascularization and did not include silent brain infarction, transient ischemic attack or brain hemorrhage. PAD was diagnosed when intermittent claudication occurred, with the confirmation of an ankle-brachial pressure index <0.9 that was measured automatically by volume-plethysmographic apparatus or significant peripheral artery stenosis by angiography, or leg amputation above the ankle as a result of diabetes. Smoking was defined as never/past/current.
Statistical analysis
The differences in clinical characteristics between cohort 1 and cohort 2 were analyzed, in which the significance of differences between groups was assessed by χ2 tests for categorical variables and the Student’s t-test for continuous variables. BMI, smoking, percentage of achieving treatment target for HbA1c, BP and lipid were compared according to the age-matched and sex-matched groups. The data of cohort 1 re-examined in 2014 were compared with their data in 2004 and with data of cohort 2, in which comparison of continuous variables of the same individuals between 2004 and 2014 was performed by paired t-test. Data were expressed as mean±SD if normally distributed. A p value of less than 5% (two-tailed) was considered significant. All analyses were performed with the statistical software package SPSS (SPSS Japan, Tokyo, Japan).
Results
Table 1 shows the clinical characteristics of patients with type 2 and type 1 diabetes compared between cohort 1 and cohort 2. In type 2 diabetes, the age was similar and cohort 2 had more percentage of male, longer duration, higher BMI, higher percentage of using tablets with lower percentages of diet only and insulin-use, and lower percentage of current smokers. The rates of achieving treatment targets for HbA1c, BP and lipid were all significantly higher in cohort 2, in which HbA1c improved in all groups of diet/tablets/insulin, and BP and lipid improved only in users of antihypertensive and lipid-lowering drugs, respectively. Regarding the comparison of the drugs for diabetes, hypertension and dyslipidemia between the two cohorts, cohort 2 was characterized by increased use of metformin and glinide and decreased use of sulfonylureas; increased use of ultra-long acting and decreased use of NPH, Mix, and short-acting insulin; and increased use of angiotensin receptor blockers, calcium channel blockers, diuretics, β-blockers, and statins and decreased use of ACE inhibitors and α-blockers. In type 1 diabetes, gender, age, and duration were the same, and the value for HbA1c was significantly lower in cohort 2. The trend of insulin use in type 1 diabetes was similar to that in type 2 diabetes and the use of ultra-short acting insulin increased.
Table 1.
Comparison between cohort 1 and cohort 2 for patients with type 2 and type 1 diabetes with respect to clinical characteristics, controlled levels of blood glucose, BP, and lipid, and treatments for diabetes, hypertension, and dyslipidemia. Cohorts 1 and 2 were recruited in 2004 and 2014, respectively, with the same inclusion criteria without any overlapping patients between the cohorts
| Type 2 diabetes | Type 1 diabetes | Data availability (%) | |||
| Cohort 1 | Cohort 2 | Cohort 1 | Cohort 2 | ||
| n=3319 | n=3932 | n=286 | n=308 | ||
| Male (%) | 64.2 | 68.8* | 42.0 | 40.9 | 100.0 |
| Age (years) | 58.3±8.3 | 58.6±8.7 | 45.2±13.0 | 43.6±13.5 | 100.0 |
| Known duration (years) | 11±8 | 12±8* | 14±9 | 15±10 | 100.0 |
| BMI (kg/m2) | 24.7±3.8 | 25.4±4.2* | 22.5±3.2 | 22.9±3.1 | 100.0 |
| Diet/tablets/insulin (%) | 13.9/64.8/21.3 | 9.7/70.8/19.5* | – | – | 100.0 |
| Smoking Never/past/current (%) | 47.9/20.9/31.2 | 41.7/33.3/25.0* | 52.4/17.1/30.4 | 58.8/16.2/25.0 | 99.7 |
| HbA1c, mmol/mol (%) | 58±8 (7.46±1.09) | 52±7 (7.00±0.95)* | 62±9 (7.91±1.22) | 60±8 (7.68±1.04)‡ | 100.0 |
| HbA1c on target (%) | 33.6 | 57.1* | 23.4 | 26.1 | 100.0 |
| with diet alone (%) | 66.5 | 80.6* | – | – | 100.0 |
| with oral hypoglycemic tablets (%) | 31.8 | 61.4* | – | – | 100.0 |
| with insulin | 17.6 | 29.9* | – | – | 100.0 |
| Systolic BP (mm Hg) | 129±14 | 127±14* | 122±16 | 122±15 | 100.0 |
| Diastolic BP (mm Hg) | 75±9 | 75±10 | 72±9 | 72±10 | 100.0 |
| Use of antihypertensive drugs (%) | 38.7 | 48.0* | 15.0 | 19.8 | 100.0 |
| BP on target (%) | 42.2 | 46.2† | 62.6 | 62.3 | 100.0 |
| without antihypertensive drugs (%) | 51.2 | 57.8 | 68.7 | 68.4 | 100.0 |
| with antihypertensive drugs (%) | 27.9 | 40.7* | 27.9 | 37.7 | 100.0 |
| LDL (mg/dl) | 116±29 | 108±28* | 102±27 | 107±28 | 98.0 |
| HDL (mg/dl) | 55±15 | 55±15 | 70±19 | 72±18 | 99.7 |
| non-HDL (mg/dl) | 145±33 | 132±33* | 123±29 | 124±31 | 95.5 |
| Use of lipid-lowering drugs (%) | 23.7 | 45.3* | 11.9 | 13.0 | 100.0 |
| Lipid on target (%) | 42.5 | 54.4* | 68.7 | 66.6 | 97.1 |
| without lipid-lowering drugs | 42.7 | 45.5 | 71.3 | 65.3 | 97.2 |
| with lipid-lowering drugs | 42.0 | 54.4* | 50.0 | 75.0 | 97.0 |
| All of HbA1c, BP and lipids on targets (%) | 7.3 | 15.5* | 9.5 | 12.1 | 99.0 |
| Non-insulin antidiabetic tablets | 100.0 | ||||
| Sulfonylureas | 57.4 | 34.1* | – | – | |
| Metformin | 42.3 | 54.7* | 10.1 | 8.1 | |
| Pioglitazone | 11.7 | 12.5 | – | – | |
| Glinide | 5 | 6.3* | – | – | |
| α-glucosidase inhibitors | 13.7 | 13.7 | 3.8 | 3.6 | |
| Dipeptidyl peptidase-4 inhibitors | – | 55.0 | – | – | |
| SGLT-2 inhibitors | – | – | |||
| total number of tablets per user | 1.7±0.8 | 2.1±1.0* | 1.3±0.5 | 1.4±0.7 | |
| GLP-1 agonist | – | 3.3 | – | – | 100.0 |
| Insulin | 100.0 | ||||
| Ultra-long-acting | 3.9 | 12.5* | 46.2 | 86.4* | |
| NPH | 6.7 | 0.3* | 34.3 | 1.0* | |
| Mix | 9.5 | 5.2* | 14.7 | 5.8* | |
| Short-acting | 2.3 | 0.2* | 23.8 | 2.9* | |
| Ultra-short-acting | 8.9 | 9.0 | 68.2 | 89.9* | |
| Antihypertensive drugs | 100.0 | ||||
| Angiotensin receptor blockers | 22.6 | 40.4* | 8.0 | 16.6† | |
| ACE inhibitors | 4.5 | 2.9* | 3.1 | 1.0 | |
| Calcium channel blockers | 23.9 | 27.1† | 7.0 | 12.3‡ | |
| Diuretics | 5.9 | 9.0* | 1.0 | 2.9 | |
| β-blockers | 4.6 | 6.0† | 2.1 | 1.3 | |
| α-blockers | 2.9 | 1.1* | 0 | 0.6 | |
| Others | 0.8 | 1.2 | 0 | 0.3 | |
| total number of drugs per user | 1.7±0.9 | 1.8±0.9* | 1.4±0.7 | 1.8±0.8‡ | |
| Antihyperlipidemic drugs | 97.1 | ||||
| Statins | 18.1 | 39.3* | 10.5 | 13.0 | |
| Fibrates | 4.9 | 4.6 | 1.7 | 0 | |
| total number of drugs per user | 1.0±0.2 | 1.1±0.2‡ | 1.0±1.2 | 1.1±0.2 | |
*p<0.001.
†p<0.01.
‡p<0.05 versus cohort 1.
BMI, body mass index; BP, blood pressure; HbA1c, hemoglobin A1C; HDL, high-density lipoprotein; LDL, low-density lipoprotein; SGLT-2, sodium glucose cotransporter-2.
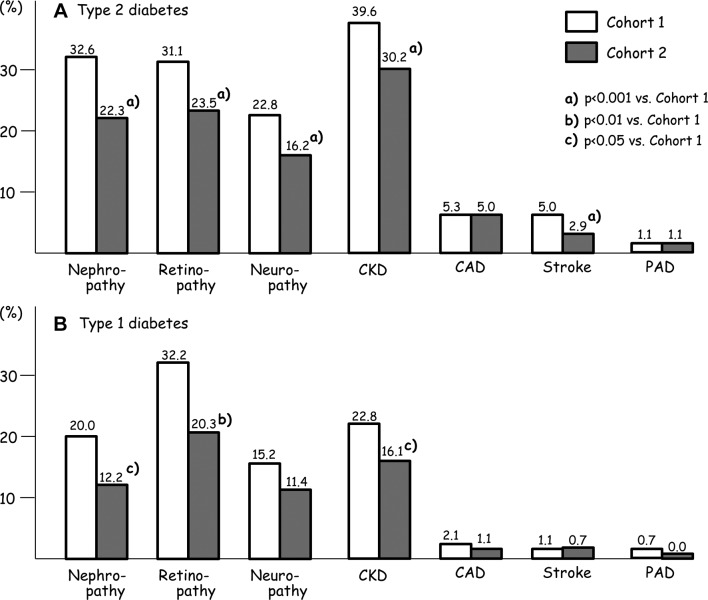
Prevalence of microvascular and macrovascular complications compared between cohort 1 and cohort 2 for patients with type 2 and type 1 diabetes is shown in figure 1. In type 2 diabetes, the prevalence of three microvascular complications, CKD, and ischemic stroke were significantly lower in cohort 2, whereas that of CAD and PAD did not differ between the cohorts. In type 1 diabetes, prevalence of nephropathy, retinopathy, and CKD were significantly lower in cohort 2. Rates of having all three microvascular complications in cohort 1 versus cohort 2 were 7.8% versus 4.7% (p<0.001) in type 2 diabetes, and 4.6% versus 3.9% in type 1 diabetes.
Figure 1.
Prevalence of microvascular and macrovascular complications compared between cohort 1 and cohort 2 for subjects with type 2 (A, upper panel) and type 1 (B, lower panel) diabetes. CAD, coronary artery disease; CKD, chronic kidney disease; PAD, peripheral artery disease.
Because different clinics participated in the two cohorts, the effect of interclinic differences on the above results cannot be excluded. Therefore, the analysis limited to 10 clinics that participated in both cohorts was performed (online supplementary table 1), and it indicated the same trend as table 1 and figure 1.
bmjdrc-2018-000521supp001.docx (40.6KB, docx)
Table 2 shows the differences between the two cohorts in type 2 diabetes regarding BMI, smoking, rates of achieving targets for HbA1c, BP, lipid, prevalence of microvascular and macrovascular complications according to the groups by sex and age. In male patients, BMI values for ages <54 years, 54–59 years and 60–64 years were significantly higher in cohort 2 than in cohort 1, and the same trend was observed in female patients. The percentage of current smokers decreased in all age groups of cohort 2 for male patients, whereas it increased in all age groups of cohort 2 for female patients. The rate on HbA1c target was significantly higher in cohort 2 in both male and female patients and in all age groups. The improvement in achieving HbA1c targets from 2004 to 2014 was more prominent in the higher age groups. In cohort 2, the rate on HbA1c target increased significantly with increasing age in both male and female patients (χ2 score 27.0, p<0.0001; χ2 score 10.7, p<0.05, respectively). The rates on BP target and that on lipid target were both higher in the higher age groups in cohort 2 than in cohort 1, whereas in the lower age groups no significant improvements were observed. Prevalence of microvascular and macrovascular complications was generally higher in the elderly groups. Prevalence of nephropathy, retinopathy, and neuropathy was lower in cohort 2 than cohort 1 in both sexes and all age groups, while the statistical significance was dependent on the number of patients in the group.
Table 2.
Comparison of BMI, smoking, and percentage of HbA1c on target, BP on target, lipid on target, and prevalence of microvascular and macrovascular complications between the two cohorts in patients with type 2 diabetes stratified by age and sex. Changes of rates of achieving the targets and prevalence from cohort 1 to cohort 2 were given in parentheses
| Age | Male | P values | Female | P values | ||
| Cohort 1 | Cohort 2 | Cohort 1 | Cohort 2 | |||
| N | ||||||
| <54 years | 591 | 768 | 236 | 235 | ||
| 54–59 years | 586 | 509 | 334 | 215 | ||
| 60–64 years | 526 | 693 | 290 | 333 | ||
| ≥65 years - | 428 | 734 | 328 | 445 | ||
| BMI kg/m2 | ||||||
| <54 years | 25.9±4.1 | 27.2±4.6 | <0.001 | 26.0±4.5 | 27.0±5.8 | <0.05 |
| 54–59 years | 24.5±3.1 | 25.7±3.9 | <0.001 | 24.6±4.1 | 25.7±4.9 | <0.01 |
| 60–64 years | 23.8±2.8 | 24.7±3.4 | <0.001 | 24.5±4.2 | 24.7±4.3 | NS |
| ≥65 years - | 24.2±3.2 | 24.2±3.0 | NS | 24.9±4.1 | 24.3±4.1 | NS |
| Smoking Never/past/current (%) |
||||||
| <54 years | 25.5/22.3/52.1 | 31.5/28.9/39.6 | <0.001 | 77.1/6.8/16.1 | 64.8/14.6/20.6 | <0.01 |
| 54–59 years | 25.6/25.6/48.7 | 19.6/45.7/34.8 | <0.001 | 83.2/6.6/10.2 | 73.4/14.0/12.6 | <0.01 |
| 60–64 years | 28.5/33.7/37.8 | 24.6/46.7/28.7 | <0.001 | 84.8/6.9/8.3 | 78.8/12.7/8.5 | NS |
| ≥65 years - | 33.9/35.0/31.1 | 24.5/51.8/23.7 | <0.001 | 87.5/7.9/4.6 | 85.1/9.8/5.0 | NS |
| HbA1c on target (%) | ||||||
| <54 years | 30.3 | 50.5 (+20.2) | <0.001 | 30.1 | 49.4 (+19.3) | <0.001 |
| 54–59 years | 33.8 | 58.7 (+24.9) | <0.001 | 29.9 | 58.6 (+28.7) | <0.001 |
| 60–64 years | 40.5 | 60.2 (+19.7) | <0.001 | 28.6 | 51.1 (+22.5) | <0.001 |
| ≥65 years - | 39.7 | 62.9 (+23.2) | <0.001 | 30.8 | 60.1 (+29.3) | <0.001 |
| BP on target (%) | ||||||
| <54 years | 40.9 | 38.9 (–2.0) | NS | 57.2 | 54.0 (-3.2) | NS |
| 54–59 years | 43.3 | 41.1 (–2.2) | NS | 41.6 | 51.2 (+9.6) | <0.05 |
| 60–64 years | 40.3 | 47.3 (+7.0) | <0.05 | 42.4 | 51.7 (+9.3) | <0.05 |
| ≥65 years - | 38.8 | 45.0 (+6.2) | <0.05 | 39.3 | 54.6 (+15.3) | <0.001 |
| Lipid on target (%) | ||||||
| <54 years | 32.7 | 41.8 (+9.1) | <0.01 | 44 | 48.7 (+4.7) | NS |
| 54–59 years | 45.1 | 58.4 (+13.3) | <0.001 | 38.2 | 55.4 (+17.2) | <0.001 |
| 60–64 years | 49.8 | 58.1 (+8.3) | <0.01 | 39.2 | 57.8 (+18.6) | <0.001 |
| ≥65 years - | 45.5 | 58.9 (+13.4) | <0.001 | 46.0 | 58.8 (+12.8) | <0.01 |
| Nephropathy (%) | ||||||
| <54 years | 30.6 | 23.2 (–7.4) | <0.01 | 33.2 | 19.2 (–14.0) | <0.01 |
| 54–59 years | 32.8 | 25.0 (–7.8) | <0.01 | 26.3 | 18.6 (–7.7) | <0.05 |
| 60–64 years | 35.4 | 25.6 (–9.8) | <0.001 | 28.6 | 16.2 (–12.4) | <0.001 |
| ≥65 years - | 35.7 | 24.3 (–11.4) | <0.001 | 36.9 | 17.1 (–19.8) | <0.001 |
| Retinopathy (%) | ||||||
| <54 years | 25.9 | 18.8 (–7.1) | <0.01 | 30.6 | 23.3 (–7.3) | NS |
| 54–59 years | 29.2 | 20.9 (–8.3) | <0.01 | 34.2 | 26.9 (–7.3) | NS |
| 60–64 years | 33.7 | 23.9 (–9.8) | <0.001 | 33.7 | 29.1 (–4.6) | NS |
| ≥65 years - | 32.1 | 24.6 (–7.5) | <0.01 | 33.5 | 26.8 (–6.7) | <0.05 |
| Neuropathy (%) | ||||||
| <54 years | 16.0 | 8.4 (–7.6) | <0.001 | 19.6 | 12.5 (–7.1) | <0.05 |
| 54–59 years | 20.4 | 14.0 (–6.4) | <0.01 | 21.8 | 15.3 (–6.5) | NS |
| 60–64 years | 25.5 | 16.4 (–9.1) | <0.001 | 30.2 | 18.4 (–11.8) | <0.01 |
| ≥65 years - | 27.0 | 21.3 (–5.7) | <0.05 | 26.8 | 24.4 (–2.4) | NS |
| CAD (%) | ||||||
| <54 years | 2.2 | 2.8 (+0.6) | NS | 3.0 | 0.4 (–2.6) | <0.05 |
| 54–59 years | 4.8 | 5.8 (+1.0) | NS | 3.0 | 2.0 (–1.0) | NS |
| 60–64 years | 6.5 | 7.0 (+0.5) | NS | 2.4 | 2.7 (+0.3) | NS |
| ≥65 years - | 11.9 | 8.5 (-3.4) | NS | 7.6 | 4.9 (–2.7) | NS |
| Stroke (%) | ||||||
| <54 years | 2.2 | 0.8 (–1.4) | <0.05 | 0.9 | 1.3 (+0.4) | NS |
| 54–59 years | 3.8 | 2.2 (–1.6) | NS | 3.0 | 2.3 (–0.7) | NS |
| 60–64 years | 6.9 | 3.6 (–3.3) | <0.05 | 4.6 | 2.1 (–2.5) | NS |
| ≥65 years - | 10.8 | 6.1 (–4.7) | <0.01 | 6.8 | 2.9 (–3.9) | <0.05 |
BMI, body mass index; BP, blood pressure; CAD, coronary artery disease; HbA1c, hemoglobin A1C; NS, not significant.
The rates of achieving treatment targets for HbA1c, BP and lipid in association with sex, duration, BMI and complications in patients with type 2 diabetes are shown in online supplementary table 2. The rates of achieving targets tended to be higher in those with shorter duration, BMI <25.0 and no complications. With respect to the analyses performed in table 2 and online supplementary table 2 for type 1 diabetes, BMI and the rates of achieving the three targets according to the median age (<45 years vs ≥45 years) or complication status were not different between the two cohorts (data not shown).
Distribution of patients according to KDIGO classification, in which patients were grouped by eGFR and albuminuria levels, is compared between the two cohorts in table 3. For both type 2 and type 1 diabetes, percentages of normoalbuminuria increased, with decreased percentages of microalbuminuria and macroalbuminuria in cohort 2 (p<0.001 and p<0.05, respectively). The distribution of eGFR categories were the same in the two cohorts in type 2 diabetes. In type 1 diabetes, proportion of eGFR categories were significantly different (p<0.05), in which percentage of eGFR ≥90 were higher in cohort 2. Proportion of normoalbuminuria with eGFR <60, that is, normoalbuminuric CKD, showed a slight increase from 6.9% to 7.9% in type 2 diabetes, and from 2.8% to 3.3% in type 1 diabetes.
Table 3.
Distribution of patients according to KDIGO classification compared between the two cohorts in type 2 and type 1 diabetes
| Type 2 diabetes | Type 1 diabetes | |||||||||||||||
| Cohort 1 | Cohort 2 | Cohort 1 | Cohort 2 | |||||||||||||
| Normo | Micro | Macro | Total | Normo | Micro | Macro | Total | Normo | Micro | Macro | Total | Normo | Micro | Macro | Total | |
| GFR ≥90 | 15.4 | 6.5 | 1.2 | 23.1 | 17.8 | 3.9 | 0.4 | 22.1 | 27.0 | 4.9 | 0.4 | 32.3 | 39.6 | 3.0 | 0.3 | 42.9* |
| GFR 60–89 | 45.1 | 13.7 | 3.3 | 62.1 | 52.1 | 10.3 | 2.0 | 64.4 | 50.2 | 7.0 | 3.5 | 60.7 | 44.9 | 5.6 | 0.0 | 50.5 |
| GFR <60 | 6.9 | 3.9 | 4.0 | 14.8 | 7.9 | 3.2 | 2.5 | 13.6 | 2.8 | 1.4 | 2.8 | 7.0 | 3.3 | 0.7 | 2.6 | 6.6 |
| Total | 67.4 | 24.1 | 8.5 | 100.0 | 77.8† | 17.4 | 4.9 | 100.0 | 80.0 | 13.3 | 6.7 | 100.0 | 87.8‡ | 9.2 | 3.0 | 100.0 |
Percentages are given.
*p<0.001,
†p<0.05 by χ2 test (3×2) to analyze the distribution of albuminuria categories (three groups) between cohort 1 and cohort 2 in type 2 and type 1 diabetes, respectively.
cp<0.05 by χ2 test (3×2) to analyze the distribution of GFR categories (three groups) between cohort 1 and cohort 2 in type 1 diabetes.
GFR, glomerular filtration rate; KDIGO, Kidney Disease Improving Global Outcomes.
In cohort 1, 2824 patients with type 2 diabetes (85.1%) and 218 patients with type 1 diabetes (76.2%) were continuously followed until 2014 and their clinical characteristics in 2014 were compared with their baseline data (in 2004) or those in cohort 2 (in 2014), as shown in table 4. Baseline data of these patients in 2004 were similar to those of the 3319 patients with type 2 diabetes and 286 patients with type 1 diabetes, respectively. In type 2 diabetes, the rates on target HbA1c, BP and lipid were significantly higher in 2014 than baseline data in 2004, but did not reach the levels of cohort 2. Regarding complications, the significant decrease of nephropathy was observed, while the prevalence of retinopathy, neuropathy, CKD, CAD and stroke significantly increased in 2014. In type 1 diabetes, HbA1c values and the rate on BP target were significantly improved. The increase in prevalence of CAD from 2004 to 2014 reached a statistical significance.
Table 4.
Clinical characteristics, controlled levels of blood glucose, BP and lipid, and prevalence of microvascular and macrovascular complications in patients with type 2 and type 1 diabetes in cohort 1, which were re-examined in 2014. Ascertainment rate was 85.1% (2824/3319) for type 2 diabetes and 76.2% (218/286) for type 1 diabetes, respectively
| Type 2 diabetes | P value versus cohort 1 in 2004 | P value versus cohort 2 in 2014 | Type 1 diabetes | P value versus cohort 1 in 2004 | P value versus cohort 2 in 2014 | |
| Cohort 1 in 2014 | Cohort 1 in 2014 | |||||
| n=2824 | n=218 | |||||
| Male (%) | 63.4 | NS | <0.001 | 43.1 | NS | NS |
| Age (years) | 67.7±8.2 | <0.001 | <0.001 | 55.0±12.3 | <0.001 | <0.001 |
| Known duration (years) | 21±8 | <0.001 | <0.001 | 24±9 | <0.001 | <0.001 |
| BMI (kg/m2) | 24.2±4.0 | <0.001 | <0.001 | 22.9±4.0 | <0.05 | NS |
| Diet/tablets/insulin (%) | 8.1/63.2/28.7 | <0.001 | <0.001 | – | – | – |
| HbA1c, mmol/mol (%) | 56±7 (7.17±0.93) | <0.001 | <0.001 | 60±8 (7.66±1.07) | <0.01 | NS |
| HbA1c on target (%) | 45.2 | <0.001 | <0.001 | 26.6 | NS | NS |
| with diet alone (%) | 63.0 | NS | <0.001 | – | – | – |
| with oral hypoglycemic tablets (%) | 49.8 | <0.001 | <0.001 | – | – | – |
| with insulin | 30.1 | <0.001 | NS | – | – | – |
| Systolic BP (mm Hg) | 128±14 | <0.05 | NS | 125±16 | <0.05 | NS |
| Diastolic BP (mm Hg) | 71±10 | <0.001 | <0.001 | 71±11 | NS | NS |
| Use of antihypertensive drugs (%) | 49.4 | <0.001 | <0.001 | 32.6 | <0.001 | <0.01 |
| BP on target (%) | 44.9 | <0.05 | <0.001 | 50.0 | <0.01 | <0.01 |
| without antihypertensive drugs (%) | 46.8 | <0.05 | <0.05 | 55.5 | <0.01 | <0.05 |
| with antihypertensive drugs (%) | 43.0 | <0.001 | NS | 38.6 | NS | NS |
| LDL (mg/dl) | 106±26 | <0.001 | <0.05 | 105±27 | NS | NS |
| HDL (mg/dl) | 56±16 | <0.001 | NS | 68±18 | <0.01 | <0.05 |
| non-HDL (mg/dl) | 130±29 | <0.001 | <0.05 | 125±29 | NS | NS |
| Use of lipid-lowering drugs (%) | 38.2 | <0.001 | <0.001 | 25.7 | <0.001 | <0.01 |
| Lipid on target (%) | 54.4 | <0.001 | NS | 61.8 | NS | <0.01 |
| without lipid-lowering drugs | 47.0 | <0.05 | NS | 61.9 | NS | <0.05 |
| with lipid-lowering drugs | 65.8 | <0.001 | NS | 61.7 | NS | NS |
| All of A1c, BP and lipids on targets (%) | 12.1 | <0.001 | <0.001 | 9.0 | NS | NS |
| Normoalbuminuria/microalbuminuria /macroalbuminuria (%) | 70.9/24.0/5.1 | <0.001 | <0.001 | 84.6/11.8/3.6 | NS | NS |
| CKD (%) | 50.5 | <0.001 | <0.001 | 28.2 | NS | <0.01 |
| Retinopathy (%) | 35.5 | <0.001 | <0.001 | 40.6 | NS | <0.001 |
| Neuropathy (%) | 28.8 | <0.001 | <0.001 | 20.9 | NS | <0.01 |
| CAD (%) | 12.6 | <0.001 | <0.001 | 5.7 | <0.05 | NS |
| Ischemic stroke (%) | 6.8 | <0.01 | <0.001 | 2.2 | NS | NS |
Analysis between continuous values in 2004 and in 2014 of cohort 1 patients was performed by paired t-test.
ACR, albumin-to-creatinine ratio; BMI, body mass index; BP, blood pressure; CAD, coronary artery disease; CKD; chronic kidney disease (ACR ≥30 or eGFR <60); eGFR, estimated glomerular filtration rate; HbA1c, hemoglobin A1C; HDL, high-density lipoprotein; LDL, low-density lipoprotein; NS, not significant.
Discussion
In this study, we examined secular trends in prevalence of microvascular/macrovascular complications and diabetes care indicators in patients with type 2 and type 1 diabetes. In type 2 diabetes, we found decreases in prevalence of nephropathy, retinopathy, neuropathy, CKD, and stroke, and improving trends of care indicators from the two independent cohorts and re-examined data of patients enrolled in cohort 1. These trends were similarly observed in subgroups stratified according to age, gender, diabetes duration or BMI. In addition, the subanalysis limited to 10 clinics that participated in both cohorts indicated the same trend observed in the whole cohorts. These findings may suggest that the difference in the two cohorts is a function of time rather than of difference in the composition of the cohorts. Also in type 1 diabetes, we found for the first time that the prevalence of nephropathy, retinopathy and CKD was significantly decreased from 2004 to 2014.
Although randomized controlled trials have suggested benefits for inhibiting the development of diabetic microvascular complications,8–13 such decreases in the prevalence during a decade dealing with a large number of patients have not been adequately demonstrated in real world practice. To our knowledge, no studies have shown the prevalence of all three microvascular complications on the same patients and the differences of prevalence over time. Few studies showed secular trends of diabetic neuropathy over time, partly because the diagnostic criteria vary among the studies. For retinopathy, a large study from a UK community-based diabetic retinopathy screening program reported a reduction in sight-threatening retinopathy from 1990 to 2006, but prevalence of any retinopathy increased from 23.2% to 25.6%.25 Luk et al noted a decreasing incidence of CHD, stroke, end-stage renal disease, and death, and corresponding improvements in care indicators over a 13-year period from 2000 to 2012 using the Hong Kong national registry system; however, they failed to observe changes in the prevalence of albuminuria or retinopathy.18
The improvements in achieving targets for care indicators demonstrated in patients with type 2 diabetes were consistent with other studies employing the same target levels; HbA1c from 45% to 52% and BP from 38% to 47% in the USA,15 and HbA1c from 32% to 65% and BP from 32% to 47% in Germany.17 These trends may largely attribute to more frequent use of drugs including new agents. We assume that the decreases in prevalence of complications are likely reflections of a long-term improvement of care indicators, though it remains unclear. In the present study, the re-examined data of patients enrolled in cohort 1 were significantly improved from 2004 to 2014, whereas the prevalence of microvascular/macrovascular complications increased according to the increasing duration of diabetes with getting 10 years older, except for nephropathy. Thus, further studies are required to elucidate this critical issue.
Regarding albuminuria and eGFR, the trend we found was a significant decrease of ACR ≥30 mg/g Cr without parallel improvements in CKD both in patients with type 2 and type 1 diabetes of similar age. The National Health and Nutrition Examination Survey reported a decrease of ACR ≥30 mg/g from 33.5% in 1998 to 23.9% in 2009 in US adults with diabetes aged <65 years, and did not observe any decrease of eGFR <60 mL/min/1.73 m2.26 In the JDDM follow-up study that observed patients with type 2 diabetes from 2004 to 2008, the regression rate from microalbuminuria to normoalbuminuria (28% per 4 year) was higher than the progression from normoalbuminuria to microalbuminuria (9% per 4 year), but the faster decline in eGFR was shown depending on the albuminuria levels.27 These may indicate that recent treatment advances still failed to improve GFR, and several studies have recently demonstrated the presence of normoalbuminuric renal impairment (CKD) in both type 2 and type 1 diabetes.28–32 The percentages of normoalbuminuric CKD in the present study, 7.9% in type 2 diabetes and 3.3% in type 1 diabetes, as demonstrated in table 4, were similar to those in other studies, in which the clinical features of patients with normoalbuminuric CKD were almost the same among the studies; they were older and predominant in women and non-smokers, and had less diabetic microvascular complications.28–32 The number of patients with normoalbuminuric CKD must further increase in the future because of increasing rate of regression of albuminuria as a consequence of increasing use of angiotensin receptor blockers, increasing age of the population and/or the longevity.30 33 We found, however, the prevalence itself was not prominently increasing over time at the same age range of patients with both type 2 and type 1 diabetes. Whether elderly patients with normoalbuminuric CKD are at a high risk of end-stage renal disease or CVD and need intensive intervention should be explored in the future.
To our knowledge, few studies have reported decreases in the prevalence of albuminuria, CKD or retinopathy over time for patients with type 1 diabetes until now. Management advances in insulin therapy as shown in table 1 have likely contributed to the improvement in HbA1c values with reducing glycemic variability. Even in patients with type 1 diabetes, slight increases of using antihypertensive and lipid-lowering drugs were observed. These have likely led to the decreases in microvascular complications. The decreased incidence of proteinuria, end-stage renal disease, and mortality in patients with type 1 diabetes appears to be in line with these findings.34–38
BMI values in the younger male and female patients with type 2 diabetes significantly increased from 2004 to 2014. Concomitantly, the rates for achieving treatment targets for HbA1c, BP and lipid in the younger patients were lower than in the elderly patients in 2014, and it may be alarming that the latter two values did not improve over time. The increase in BMI values and obesity was observed in our study and in other studies.14 17 39 This is important because obesity causes higher medical expenditure and poorer controls for blood glucose, BP, and lipid, and recently steatohepatitis is becoming a topic in such patients leading to liver cirrhosis, CVD, and cancer.38–41 These concerns may warrant weight reduction and strict control for younger adults with type 2 diabetes since they have more to gain from risk factor control because their life expectancy is longer and the potential for complications increases with the duration of diabetes. Their lower adherence and motivation to their lifestyle and medication remain a problem. Furthermore, it becomes more complicated if the cause of increased BMI is due to the more aggressive therapy of diabetes.
The strength of this study is that the prevalence of microvascular/macrovascular complications and assessment of care indicators were simultaneously evaluated with a larger number of patients than in other studies,14–17 and the trend over time was ensured by the re-examination as well as age-matched and sex-matched groups. In addition, the present results were based on medical records of daily clinical practice, whereas in most other studies, information regarding the diagnosis of diabetes, medications and compilations were self-reported.14–17 These facts have firmly contributed to investigating the secular trend in the present study. It was of interest that the percentage of current smokers decreased in men and increased in women with type 2 diabetes; the effect of smoking trend on prevalence of complications should be explored in future studies.
There are some limitations that should be described. Although the study was performed in primary care settings, a selection bias may have been caused by physicians voluntarily participating in this study. They were general practitioners specializing or being interested in diabetes care. Thus, the control levels of diabetes care in patients treated by them might be better than those by other general practitioners, because they might take care of patients more aggressively than the others. Conversely, they might take care of more patients with poor glycemic controls or severe complications than the others. These points might influence the results of this study. Also, this study was basically performed to compare the two independent cohorts over a decade. Thus, we cannot completely exclude a possibility that the findings in this study were due to a difference in the composition of the cohorts, although the trends were similarly observed in subgroups stratified according to age, gender, diabetes duration or BMI. Next, there was a lack of information on dietary and exercise behavior, adherence to medication, and socioeconomic status. These factors are likely to affect the outcome of complications and care indicators.42 43 Finally, with respect to comparison between baseline and re-examined data in cohort 1, the re-examined data were limited to those who continuously visited the participating clinics. Thus, we should acknowledge that the current data of those lost to follow-up were not available.
In conclusion, we observed declining trends in diabetic microvascular complications with improvement of diabetes care indicators, such as therapeutic goal attainment and habit of smoking in patients with type 2 and type 1 diabetes. Increasing BMI levels with lower rates for achieving therapeutic goals in younger patients with type 2 diabetes remains a concern. Importantly, although the improvement in prevalence of vascular complications and care indicators may lead to longevity in the diabetic population, this could increase ageing-related problems.
Acknowledgments
The authors thank Sanae Horiguchi-Watanabe, Yukari Mizutani, Risa Sasajima-Yamashita, Suguho Takahashi (Jiyugaoka Medical Clinic, Obihiro, Japan) and Daisuke Mutou (JDDM Data Center, Tsukuba, Japan) for their skillful data collection and analyses.
Footnotes
Contributors: HY, SA and HM designed the study. HY, KK, KY, OT and SS generated data. HY and SA performed the statistical analysis. All authors contributed to the interpretation of the data. HY, SA and HM drafted the manuscript. HY takes responsibility for the statistical methods and organised the multivariate analysis. All authors critically reviewed the manuscript. HY is the guarantor of this work and as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Funding: The present study was supported by research programme grant from the Japan Diabetes Society and the Manpei Suzuki Diabetes Foundation.
Competing interests: None declared.
Patient consent: Obtained.
Ethics approval: Ethical committee of the Japan Diabetes Clinical Data Management Study and each participating clinic.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data sharing statement: No additional data are available.
Collaborators: The following clinics from the JDDM study group contributed to the present study. Both cohorts; Fukumoto Clinic (Ibusuki), HEC Science clinic (Yokohama), Iwasaki Naika Clinic (Iwakuni), Jiyugaoka Medical Clinic (Obihiro), Kawai Clinic (Tsukuba), Sugimoto Clinic (Kitakyusyu), Minami Masae Clinic (Fukuoka), Oishi Naika Clinic (Kyoto), Takamura Naika Clinic (Fussa), Miyazawa Naika Clinic (Hokkaido) Cohort 1; Akasaka Chuo Clinic (Tokyo), Doi Clinic (Uji), Fuji Oyama Hospital (Gotenba), Kudo Clinic (Aomori), Takai Clinic (Ofuna), Takeda Clinic (Sagamihara), Toyo Kohan Clinic (Kure), Cohort 2; Abe Clinic (Oita), Hasegawwa Naika Clinic (Chitose), Hikari Clinic (Nara), Hotaruno Central Clinic (Kisarazu), Iwamoto Clinic (Zenkoji), JCHO Takaoka Fushiki Hospital (Takaoka), Kurihara Naika Clinic (Hokkaido), Misaki Naika Clinic (Funabashi), Okada Naika Clinic (Fukuoka), Okuguchi Naika Clinic (Sendai), Takaki Naika Clinic (Niigata), Tama Center Clinic Mirai (Tama), Tanaka Naika Clinic (Kurume), Tomonaga Clinic (Tokyo), Wakamatsu Kinen Hospital (Kagoshima), Wakamatsu Naika Clinic (Yurihonjo), Yagi Clinic (Naha), Yamada Naika Clinic (Obihiro).
Correction notice: Please insert (JDDM 46) at the end of the title, as follows “Declining trends of diabetic nephropathy, retinopathy, and neuropathy with improving diabetes care indicators in Japanese patients with type 2 and type 1 diabetes (JDDM 46)”.
References
- 1. NCD Risk Factor Collaboration (NCD-RisC). Worldwide trends in diabetes since 1980: a pooled analysis of 751 population-based studies with 4.4 million participants. Lancet 2016;387:1513–30. 10.1016/S0140-6736(16)00618-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Saaddine JB, Honeycutt AA, Narayan KM, et al. Projection of diabetic retinopathy and other major eye diseases among people with diabetes mellitus: United States, 2005-2050. Arch Ophthalmol 2008;126:1740–7. 10.1001/archopht.126.12.1740 [DOI] [PubMed] [Google Scholar]
- 3. Selvin E, Parrinello CM, Sacks DB, et al. Trends in prevalence and control of diabetes in the United States, 1988-1994 and 1999-2010. Ann Intern Med 2014;160:517–25. 10.7326/M13-2411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Brownrigg JR, Hughes CO, Burleigh D, et al. Microvascular disease and risk of cardiovascular events among individuals with type 2 diabetes: a population-level cohort study. Lancet Diabetes Endocrinol 2016;4:588–97. 10.1016/S2213-8587(16)30057-2 [DOI] [PubMed] [Google Scholar]
- 5. Molitch ME, DeFronzo RA, Franz MJ, et al. Nephropathy in diabetes. Diabetes Care 2004;27(Suppl 1):S79–83. [DOI] [PubMed] [Google Scholar]
- 6. Klein BE. Overview of epidemiologic studies of diabetic retinopathy. Ophthalmic Epidemiol 2007;14:179–83. 10.1080/09286580701396720 [DOI] [PubMed] [Google Scholar]
- 7. Tesfaye S. Recent advances in the management of diabetic distal symmetrical polyneuropathy. J Diabetes Investig 2011;2:33–42. 10.1111/j.2040-1124.2010.00083.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Nathan DM, Genuth S, Lachin J, et al. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med 1993;329:977–86. 10.1056/NEJM199309303291401 [DOI] [PubMed] [Google Scholar]
- 9. The UK Prospective Diabetes Study (UKPDS) Group. Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). UK Prospective Diabetes Study (UKPDS) Group. Lancet 1998;352:837–53. [PubMed] [Google Scholar]
- 10. Holman RR, Paul SK, Bethel MA, et al. 10-year follow-up of intensive glucose control in type 2 diabetes. N Engl J Med 2008;359:1577–89. 10.1056/NEJMoa0806470 [DOI] [PubMed] [Google Scholar]
- 11. Nathan DM, Cleary PA, Backlund JY, et al. Intensive diabetes treatment and cardiovascular disease in patients with type 1 diabetes. N Engl J Med 2005;353:2643–53. 10.1056/NEJMoa052187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kearney PM, Blackwell L, Collins R, et al. Efficacy of cholesterol-lowering therapy in 18,686 people with diabetes in 14 randomised trials of statins: a meta-analysis. Lancet 2008;371:117–25. 10.1016/S0140-6736(08)60104-X [DOI] [PubMed] [Google Scholar]
- 13. The UK Prospective Diabetes Study Group. Tight blood pressure control and risk of macrovascular and microvascular complications in type 2 diabetes: UKPDS 38. UK Prospective Diabetes Study Group. BMJ 1998;317:703–13. [PMC free article] [PubMed] [Google Scholar]
- 14. Ali MK, Bullard KM, Saaddine JB, et al. Achievement of goals in U.S. diabetes care, 1999-2010. N Engl J Med 2013;368:1613–24. 10.1056/NEJMsa1213829 [DOI] [PubMed] [Google Scholar]
- 15. Stark Casagrande S, Fradkin JE, Saydah SH, et al. The prevalence of meeting A1C, blood pressure, and LDL goals among people with diabetes, 1988-2010. Diabetes Care 2013;36:2271–9. 10.2337/dc12-2258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kuznik A, Mardekian J. Trends in utilization of lipid- and blood pressure-lowering agents and goal attainment among the U.S. diabetic population, 1999-2008. Cardiovasc Diabetol 2011;10:31 10.1186/1475-2840-10-31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Du Y, Heidemann C, Schaffrath Rosario A, et al. Changes in diabetes care indicators: findings from German National Health Interview and Examination Surveys 1997-1999 and 2008-2011. BMJ Open Diabetes Res Care 2015;3:e000135 10.1136/bmjdrc-2015-000135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Luk AOY, Hui EMT, Sin MC, et al. Declining Trends of Cardiovascular-Renal Complications and Mortality in Type 2 Diabetes: The Hong Kong Diabetes Database. Diabetes Care 2017;40:928–35. 10.2337/dc16-2354 [DOI] [PubMed] [Google Scholar]
- 19. Yu NC, Su HY, Chiou ST, et al. Trends of ABC control 2006-2011: a National Survey of Diabetes Health Promotion Institutes in Taiwan. Diabetes Res Clin Pract 2013;99:112–9. 10.1016/j.diabres.2012.11.018 [DOI] [PubMed] [Google Scholar]
- 20. Rathmann W, Scheerer M, Rohwedder K, et al. Changes in patient characteristics, glucose lowering treatment, glycemic control and complications in type 2 diabetes in general practices (Disease Analyzer, Germany: 2008-2016). Postgrad Med 2018;130:244–50. 10.1080/00325481.2018.1421842 [DOI] [PubMed] [Google Scholar]
- 21. Tajima N, Noda M, Origasa H, et al. Evidence-based practice guideline for the treatment for diabetes in Japan 2013. Diabetol Int 2015;6:151–87. 10.1007/s13340-015-0206-2 [DOI] [Google Scholar]
- 22. Seino Y, Nanjo K, Tajima N, et al. Report of the committee on the classification and diagnostic criteria of diabetes mellitus. J Diabetes Investig 2010;1:212–28. 10.1111/j.2040-1124.2010.00074.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Levey AS, Eckardt KU, Tsukamoto Y, et al. Definition and classification of chronic kidney disease: a position statement from Kidney Disease: Improving Global Outcomes (KDIGO). Kidney Int 2005;67:2089–100. 10.1111/j.1523-1755.2005.00365.x [DOI] [PubMed] [Google Scholar]
- 24. Yasuda H, Sanada M, Kitada K, et al. Rationale and usefulness of newly devised abbreviated diagnostic criteria and staging for diabetic polyneuropathy. Diabetes Res Clin Pract 2007;77(Suppl 1):S178–83. 10.1016/j.diabres.2007.01.053 [DOI] [PubMed] [Google Scholar]
- 25. Misra A, Bachmann MO, Greenwood RH, et al. Trends in yield and effects of screening intervals during 17 years of a large UK community-based diabetic retinopathy screening programme. Diabet Med 2009;26:1040–7. 10.1111/j.1464-5491.2009.02820.x [DOI] [PubMed] [Google Scholar]
- 26. Afkarian M, Zelnick LR, Hall YN, et al. Clinical Manifestations of Kidney Disease Among US Adults With Diabetes, 1988-2014. JAMA 2016;316:602–10. 10.1001/jama.2016.10924 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Yokoyama H, Araki S, Haneda M, et al. Chronic kidney disease categories and renal-cardiovascular outcomes in type 2 diabetes without prevalent cardiovascular disease: a prospective cohort study (JDDM25). Diabetologia 2012;55:1911–8. 10.1007/s00125-012-2536-y [DOI] [PubMed] [Google Scholar]
- 28. Yokoyama H, Sone H, Oishi M, et al. Prevalence of albuminuria and renal insufficiency and associated clinical factors in type 2 diabetes: the Japan Diabetes Clinical Data Management study (JDDM15). Nephrol Dial Transplant 2009;24:1212–9. 10.1093/ndt/gfn603 [DOI] [PubMed] [Google Scholar]
- 29. Penno G, Solini A, Bonora E, et al. Clinical significance of nonalbuminuric renal impairment in type 2 diabetes. J Hypertens 2011;29:1802–9. 10.1097/HJH.0b013e3283495cd6 [DOI] [PubMed] [Google Scholar]
- 30. Pugliese G. Updating the natural history of diabetic nephropathy. Acta Diabetol 2014;51:905–15. 10.1007/s00592-014-0650-7 [DOI] [PubMed] [Google Scholar]
- 31. Thorn LM, Gordin D, Harjutsalo V, et al. The Presence and Consequence of Nonalbuminuric Chronic Kidney Disease in Patients With Type 1 Diabetes. Diabetes Care 2015;38:2128–33. 10.2337/dc15-0641 [DOI] [PubMed] [Google Scholar]
- 32. Penno G, Russo E, Garofolo M, et al. Evidence for two distinct phenotypes of chronic kidney disease in individuals with type 1 diabetes mellitus. Diabetologia 2017;60:1102–13. 10.1007/s00125-017-4251-1 [DOI] [PubMed] [Google Scholar]
- 33. de Boer IH, Rue TC, Hall YN, et al. Temporal trends in the prevalence of diabetic kidney disease in the United States. JAMA 2011;305:2532–9. 10.1001/jama.2011.861 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Skupien J, Warram JH, Smiles A, et al. Improved glycemic control and risk of ESRD in patients with type 1 diabetes and proteinuria. J Am Soc Nephrol 2014;25:2916–25. 10.1681/ASN.2013091002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Andrésdóttir G, Jensen ML, Carstensen B, et al. Improved prognosis of diabetic nephropathy in type 1 diabetes. Kidney Int 2015;87:417–26. 10.1038/ki.2014.206 [DOI] [PubMed] [Google Scholar]
- 36. Otani T, Yokoyama H, Ohashi Y, et al. Improved incidence of end-stage renal disease of type 1 diabetes in Japan, from a hospital-based survey. BMJ Open Diabetes Res Care 2016;4:e000177 10.1136/bmjdrc-2015-000177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Bojestig M, Arnqvist HJ, Hermansson G, et al. Declining incidence of nephropathy in insulin-dependent diabetes mellitus. N Engl J Med 1994;330:15–18. 10.1056/NEJM199401063300103 [DOI] [PubMed] [Google Scholar]
- 38. de Boer IH, Sun W, Cleary PA, et al. DCCT/EDIC Research Group. Intensive diabetes therapy and glomerular filtration rate in type 1 diabetes. N Engl J Med 2011;365:2366–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Kusunoki-Tsuji C, Araki SI, Kume S, et al. Impact of obesity on annual medical expenditures and diabetes care in Japanese patients with type 2 diabetes mellitus. J Diabetes Investig 2017. 10.1111/jdi.12766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Haslam DW, James WP. Obesity. Lancet 2005;366:1197–209. 10.1016/S0140-6736(05)67483-1 [DOI] [PubMed] [Google Scholar]
- 41. Byrne CD, Targher G. EASL-EASD-EASO Clinical Practice Guidelines for the management of non-alcoholic fatty liver disease: is universal screening appropriate? Diabetologia 2016;59:1141–4. 10.1007/s00125-016-3910-y [DOI] [PubMed] [Google Scholar]
- 42. Scott A, Chambers D, Goyder E, et al. Socioeconomic inequalities in mortality, morbidity and diabetes management for adults with type 1 diabetes: A systematic review. PLoS One 2017;12:e0177210 10.1371/journal.pone.0177210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Grintsova O, Maier W, Mielck A. Inequalities in health care among patients with type 2 diabetes by individual socio-economic status (SES) and regional deprivation: a systematic literature review. Int J Equity Health 2014;13:43 10.1186/1475-9276-13-43 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjdrc-2018-000521supp001.docx (40.6KB, docx)