Abstract
Elastin is one of the most important and abundant extracellular matrix (ECM) proteins that provide elasticity and resilience to tissues and organs, including vascular walls, ligaments, skin, and lung. Besides hereditary diseases, such as Williams-Beuren syndrome (WBS), which results in reduced elastin synthesis, injuries, aging, or acquired diseases can lead to the degradation of existing elastin fibers. Thus, the de novo synthesis of elastin is required in several medical conditions to restore the elasticity of affected tissues. Here, we applied synthetic modified mRNA encoding tropoelastin (TE) for the de novo synthesis of elastin and determined the mRNA-mediated elastin synthesis in cells, as well as ex vivo in porcine skin. EA.hy926 cells, human fibroblasts, and mesenchymal stem cells (MSCs) isolated from a patient with WBS were transfected with 2.5 μg TE mRNA. After 24 hr, the production of elastin was analyzed by Fastin assay and dot blot analyses. Compared with untreated cells, significantly enhanced elastin amounts were detected in TE mRNA transfected cells. The delivered synthetic TE mRNA was even able to significantly increase the elastin production in elastin-deficient MSCs. In porcine skin, approximately 20% higher elastin amount was detected after the intradermal delivery of synthetic mRNA by microinjection. In this study, we demonstrated the successful applicability of synthetic TE encoding mRNA to produce elastin in elastin-deficient cells as well as in skin. Thus, this auspicious mRNA-based integration-free method has a huge potential in the field of regenerative medicine to induce de novo elastin synthesis, e.g., in skin, blood vessels, or alveoli.
Keywords: elastin, synthetic mRNA, Williams-Beuren syndrome, intradermal delivery, skin
Introduction
Elastin is an extracellular matrix (ECM) protein that provides elasticity and resilience to tissues and organs.1 Thus, this protein is most abundant in organs where elasticity and flexibility are of major importance. Elastin constitutes about 28%–32% of major blood vessels, 30%–57% of the aorta, 50% of elastic ligaments, 3%–7% of lung, and 2%–3% of the dry weight of skin.2, 3, 4 The expression of elastin primarily occurs during late and early neonatal periods,5 and after the first few years of life. From a young age, elastin synthesis decreases and ceases in adults.6 During development, elastin is produced and secreted by different types of cells, such as smooth muscle cells (SMCs), fibroblasts, and endothelial cells.7
Although the half-life of elastin is about 74 years8 and it is one of the most stable proteins known, the persistence of elastin, which is produced during development, is important for proper functioning of elastic connective tissues. Abnormalities in the elastin synthesis due to genetic disorders such as Williams-Beuren syndrome (WBS), also called elfin facies syndrome, autosomal dominant cutis laxa (ADCL), or supravalvular aortic stenosis (SVAS), result in several elastinopathies. Furthermore, in the skin of adults, aging, injuries, or sun damage also leads to the loss of elasticity, because the low level of elastin synthesis by the cells is not sufficient to efficiently repair the damage.
WBS occurs in 1 in 7,500–20,000 births9, 10 and is caused by the hemizygous microdeletion of approximately 26 to 28 genes on chromosome 7q11.23, which includes the elastin gene, resulting in less than 50% of normal elastin expression.11, 12 The condition is associated with connective tissue abnormalities and cardiovascular diseases, specifically SVAS and supravalvular pulmonary stenosis.13 Homozygous elastin null mutants (ELN−/−) result in increased proliferation of arterial SMCs, and that leads to thickening of the arterial wall, narrowing of the arteries, and death within a few days of birth.14 Cutis laxa is caused by mutations of elastin or fibulin-5 (FBLN5). It occurs in less than 1 in 1,000,000 births (http://www.orpha.net) and results in wrinkled, redundant, and sagging inelastic skin.15 It is associated with hernias, cardiac valve anomalies, and cardiovascular diseases, e.g., pulmonary stenosis, aortic and arterial dilatation, and emphysema.
Elastin is synthetized as a soluble monomeric precursor, called tropoelastin (TE).16 After the translation, TE is transported in association with elastin binding protein (EBP) to the extracellular space. Subsequently, TE is assembled on microfibrils and covalently cross-linked by lysyl oxidase (LOX) to form mature elastic fibers.
In recent years, the use of synthetic mRNAs as a new therapeutic drug for the production of desired proteins in cells gained in importance.17, 18, 19, 20, 21 Synthetic mRNAs with or without modified nucleotides are generated by in vitro transcription (IVT) using RNA polymerases. Furthermore, a poly A-tail is added at the 3′ end and a cap analog is incorporated at the 5′ end of the mRNA to improve the stability and the translation of the generated synthetic mRNA. Compared with viral vectors and DNA plasmids, the application of synthetic mRNAs has several advantages: Synthetic mRNAs do not need to enter the cell nucleus, thereby insertional mutagenesis is prevented and non-dividing cells can be transfected to produce the desired protein. Additionally, mRNAs are smaller than plasmids and viral vectors, which allows improved delivery of synthetic mRNAs into the cells. After the release of mRNA into the cytosol, the mRNA is immediately translated by ribosomes into proteins. Furthermore, permanent protein overexpression-related complications are prevented, because the synthetic mRNA is transiently present due to natural degradation in cells.
Here, we generated synthetic modified TE encoding mRNA and analyzed the ability to produce elastin in EA.hy926 cells, human fibroblasts, and mesenchymal stem cells (MSCs) isolated from a patient with WBS. Afterward, the synthetic mRNA-mediated production of elastin in skin was analyzed using an ex vivo porcine skin model.
Results
Synthesis of Modified TE mRNA and Analysis of Transfection Efficiency in EA.hy926 Cells and Fibroblasts
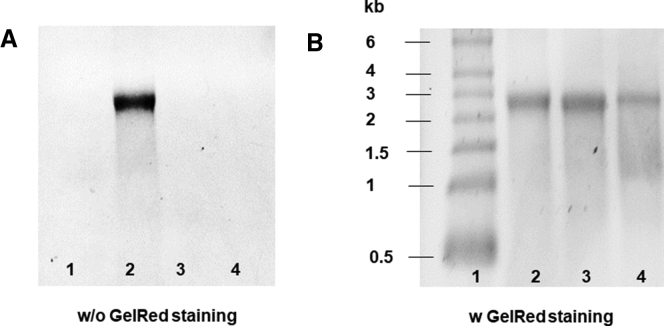
Modified TE mRNA containing 5mCTP and Ψ instead of cytidine triphosphate (CTP) and uridine triphosphate (UTP) was obtained after the IVT. The agarose gel electrophoresis showed that the generated mRNA has the expected length of approximately 2,500 nt (Figure 1). Additionally, an unmodified TE mRNA was generated and the product was also analyzed. Due to Cy3 conjugation, only the specific band for Cy3-labeled TE mRNA could be detected using UV-transilluminator (Figure 1A). After staining with GelRed, all mRNAs could be detected, thereby successful labeling of TE mRNA with Cy3 was demonstrated.
Figure 1.
Analysis of the Generated Synthetic TE mRNA and Cy3-Labeled TE mRNA by 1% Agarose Gel Electrophoresis
(Lane 1) RNA marker, 400 ng of (lane 2) Cy3-labeled TE mRNA, (lane 3) modified, or (lane 4) unmodified TE mRNA was loaded on 1% agarose gel. A single band at around 2,500 bases confirmed the purity and specific length of the synthetic mRNA. First, (A) Cy3-labeled TE mRNA was detected using an UV-transilluminator, and then (B) all nucleic acids were detected by GelRed staining.
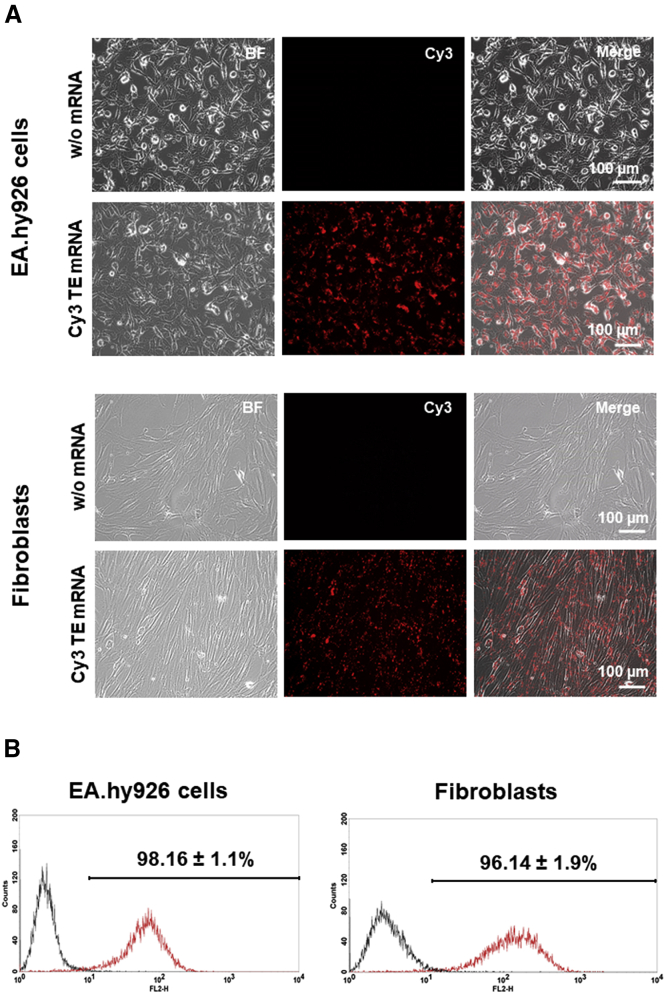
The generated Cy3-labeled TE mRNA was used to analyze the transfection efficiency. Therefore, 3 × 105 EA.hy926 cells or fibroblasts were transfected with lipoplexes containing 2.5 μg Cy3-labeled TE mRNA. The fluorescence microscopy analyses revealed a high transfection efficiency, which was also confirmed by flow cytometry measurements (Figure 2). After the incubation of cells for 4 hr at 37°C with lipoplexes, 98.16% ± 1.1% of EA.hy926 cells and 96.14% ± 1.9% of human fibroblasts were transfected with Cy3-labeled TE mRNA.
Figure 2.
Analysis of TE mRNA Transfection Efficiency Using Fluorescence Microscopy and Flow Cytometry
3 × 105 EA.hy926 cells and human fibroblasts were transfected with 2.5 μg Cy3-labeled TE mRNA using Lipofectamine 2000. Cells incubated only with the transfection reagent were used as negative control. (A) Fluorescence microscopy and (B) flow cytometry analysis were performed 4 hr after the incubation of cells with lipoplexes. Black line represents cells treated only with the transfection reagent, and red line represents cells treated with Cy3 TE mRNA. BF, bright field.
Characterization of WBS_MSCs
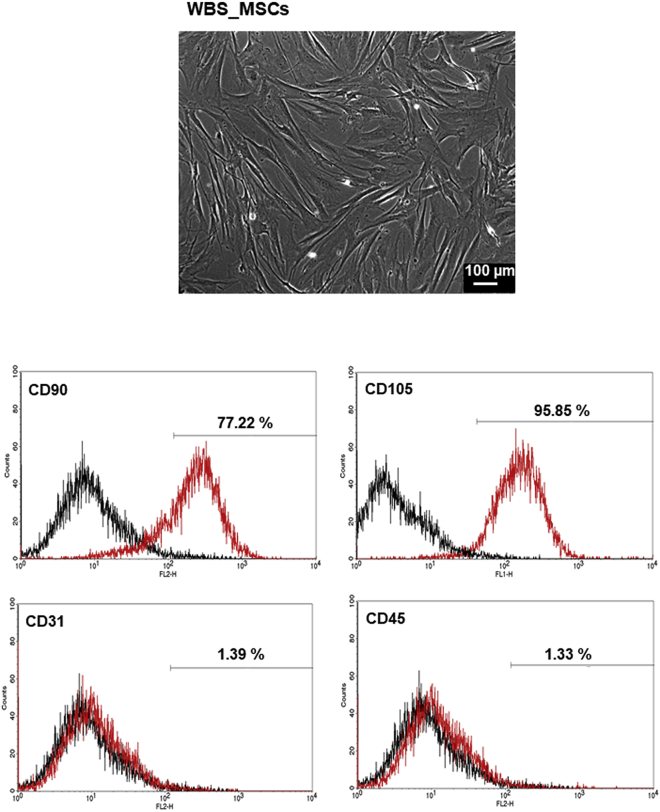
The isolated MSCs from a patient with WBS (WBS_MSCs) were characterized by staining with antibodies and performing of flow cytometry. The WBS_MSCs were negative for the expression of CD31 and CD45, but expressed the characteristic marker of MSCs, CD90, and CD105 (Figure 3).
Figure 3.
Characterization of MSCs Isolated from the Thymus of a WBS Patient
(Top panel) Phase-contrast micrograph of MSCs at passage 1. (Bottom panels) Flow cytometry analysis of WBS_MSCs after the staining with mouse anti-human antibodies against CD90, CD105, CD31, and CD45. Black line: negative control; red line: WBS_MSCs stained with specific antibodies.
Analysis of Cell Viability after the Delivery of Synthetic TE mRNA in EA.hy926 Cells
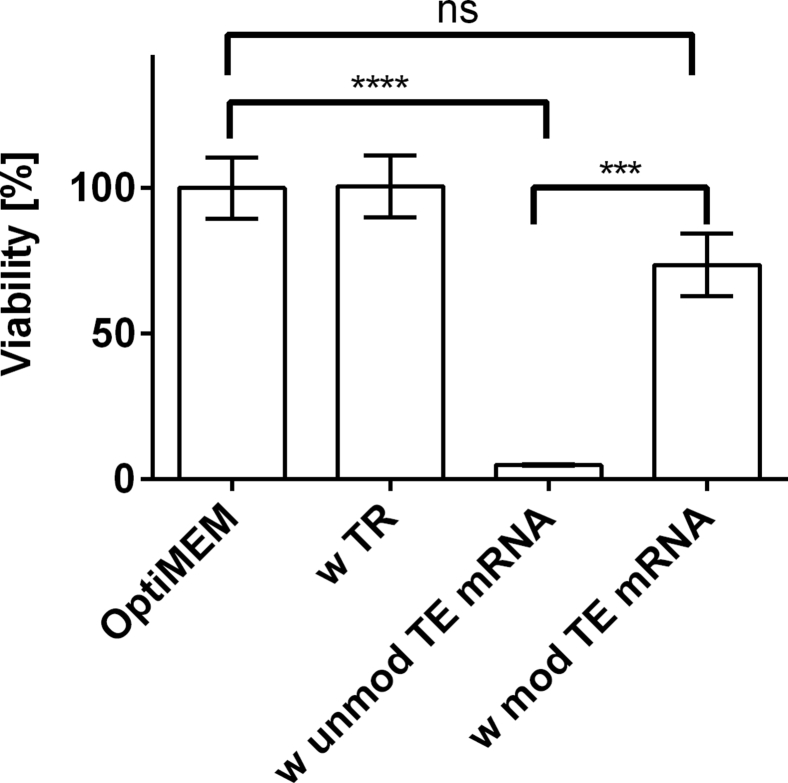
EA.hy926 cells were transfected with 2.5 μg of modified or unmodified TE mRNA. After 24 hr, the cell viability was determined using PrestoBlue assay. As shown in Figure 4, the transfection with unmodified TE mRNA led to a strong cytotoxicity. In contrast, the viability of cells after the transfection with modified mRNA was not significantly different from the cells incubated with OptiMEM or only with transfection reagent. The modified TE mRNA treatment revealed with a detected cell viability of 74% ± 11%, a good cytocompatibility.
Figure 4.
Analysis of the Influence of TE mRNA Transfection on the Viability of EA.hy293 Cells Using PrestoBlue Assay
4 × 105 EA.hy293 cells were transfected with 2.5 μg unmodified TE mRNA (unmod TE mRNA) or 2.5 μg modified TE mRNA (mod TE mRNA). Furthermore, cells were incubated only with OptiMEM or with transfection reagent (TR) Lipofectamine 2000. PrestoBlue cell viability assay was performed 24 hr after the transfection of cells. The viability of cells incubated with OptiMEM was set to 100%, and the viability relative to these cells was expressed. Data are shown as mean ± SEM (n = 3). Statistical differences were determined using one-way ANOVA with Bonferroni’s multiple comparisons test. ***p < 0.001; ****p < 0.0001.
Production of Elastin after the Transfection of Cells with Synthetic Modified TE mRNA
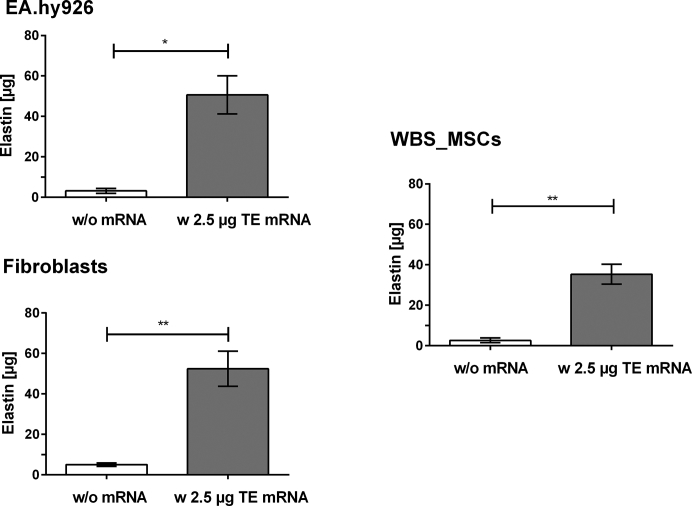
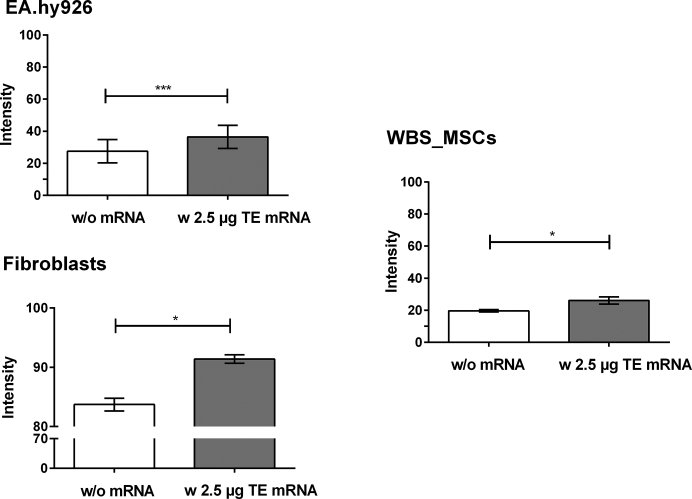
The production of elastin after the transfection of EA.hy926 cells, human fibroblasts, and WBS_MSCs with 2.5 μg synthetic modified TE mRNA was analyzed by using two independent methods, Fastin assay and dot blot analysis. Fastin assay detects elastin by binding to the basic and non-polar amino acid sequences, and dot blot analysis detects the elastin by using a specific antibody. Compared with control cells treated with OptiMEM containing Lipofectamine 2000, a significantly increased amount of elastin was detected after the delivery of synthetic modified TE mRNA into the cells using Fastin assay (Figure 5), as well as dot blot assay (Figure 6). In Table 1, the determined elastin amounts are compared.
Figure 5.
Detection of Elastin Amount after the Transfection of EA.hy293 Cells, Human Fibroblasts, and MSCs Derived from WBS Patient with Synthetic Modified TE mRNA Using Fastin Assay
3 × 105 EA.hy926 cells, human fibroblasts, or WBS_MSCs were transfected with 2.5 μg synthetic modified TE mRNA. After 24 hr, the elastin amount was detected using Fastin assay. As a control, cells were incubated with OptiMEM containing Lipofectamine 2000 without TE mRNA. Results are shown as mean ± SEM (EA.hy926 cells: n = 4, fibroblasts: n = 6, WBS_MSCs: n = 4). Statistical differences were determined using paired t test. *p < 0.05; **p < 0.01.
Figure 6.
Detection of Elastin Amount after the Transfection of EA.hy293 Cells, Human Fibroblasts, and MSCs Derived from WBS Patient with Synthetic Modified TE mRNA Using Dot Blot Assay
3 × 105 EA.hy926 cells, human fibroblasts, or WBS_MSCs were transfected with 2.5 μg synthetic modified TE mRNA. As a control, cells were incubated with OptiMEM containing Lipofectamine 2000 without TE mRNA. After 24 hr, 500 μL supernatant was blotted on nitrocellulose membrane, and elastin was detected using polyclonal antibody to human aortic elastin and the subsequent detection of alkaline-phosphatase-conjugated secondary antibody. Results are shown as mean ± SEM (EA.hy926 cells: n = 3, fibroblasts: n = 3, WBS_MSCs: n = 4). Statistical differences were determined using paired t test. *p < 0.05; ***p < 0.001.
Table 1.
Comparison of the Detected Elastin Amount Using Fastin Assay and Dot Blot Assay
| Cell Type | Fastin Elastin Assay |
Dot Blot Assay |
||||
|---|---|---|---|---|---|---|
| Elastin Amount (μg) |
Fold-Change | Intensity of Antibody Staining |
Fold-Change | |||
| w/o mRNA | w TE mRNA | w/o mRNA | w TE mRNA | |||
| EA.hy926 cells | 3.17 ± 1.18 | 50.66 ± 9.40 | 16 | 27.52 ± 7.29 | 36.45 ± 7.24 | 1.3 |
| Fibroblasts | 5.02 ± 0.88 | 52.46 ± 8.67 | 10.5 | 83.72 ± 1.07 | 91.39 ± 0.71 | 1.1 |
| WBS_MSCs | 2.66 ± 1.15 | 35.32 ± 4.9 | 13.3 | 19.6 ± 0.74 | 26.09 ± 2.31 | 1.3 |
w, with; w/o, without.
Using Fastin assay, an approximately 16-fold higher elastin amount was detected (Figure 5) after the transfection of 2.5 μg modified TE mRNA in EA.hy926 cells compared with the cells without mRNA treatment. In human fibroblasts about a 10-fold and in WBS-MSCs about a 13-fold higher amount of elastin was detected. Although statistically significant, the detected increase of elastin amount in dot blot assay (Figure S1) was less than the detected amounts in Fastin assay. Here, a 1.3-fold higher elastin amount was detected in TE mRNA transfected EA.hy926 cells (Figure 6) compared with control cells. In human fibroblasts, a 1.1-fold higher and 1.33-fold higher elastin amount was detected.
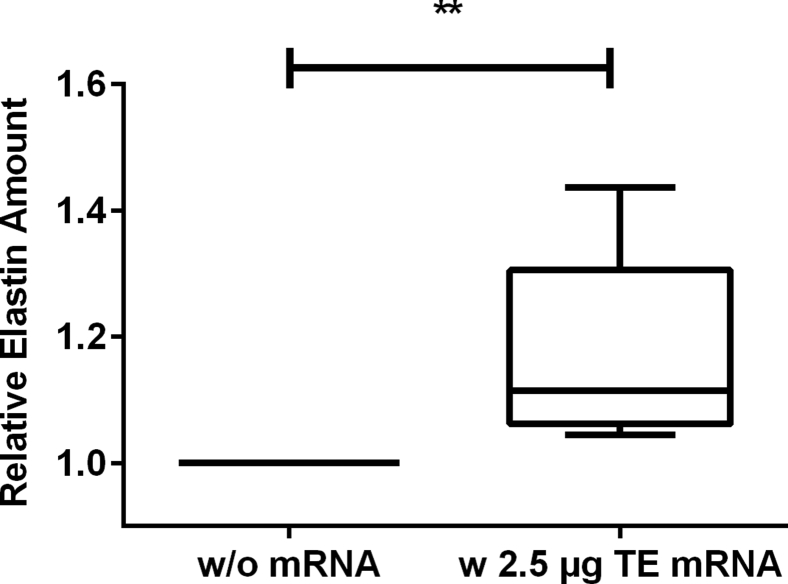
Intradermal Delivery of Synthetic Modified TE mRNA by Microinjection Results in Increased Elastin Synthesis
The ability of synthetic TE mRNA to mediate production of elastin was analyzed by microinjection of 2.5 μg synthetic modified TE mRNA complexed with Lipofectamine 2000 in a total volume of 35 μL into porcine skin. Using Fastin assay, we detected a 1.2-fold increased amount of elastin in the skin treated with synthetic modified TE mRNA compared with the skin without mRNA treatment (Figure 7).
Figure 7.
Detection of Elastin Amount after the Microinjection of Synthetic Modified TE mRNA into Pig Skin
2.5 μg synthetic modified TE mRNA was complexed with Lipofectamine 2000 and injected using hollow microneedles into the pig skin. After 72 hr, elastin amount in the skin was detected using Fastin assay. The elastin amount is presented relative to the skin samples treated only with the transfection reagent without mRNA. Data are presented as box plot, median with 25–75 percentiles (box) and 5–95 percentiles (whiskers) (n = 7). Statistical differences were determined using paired t test. **p < 0.01.
Discussion
Elastin is one of the most important components of the blood vessels, lungs, and skin. It provides elasticity and resilience to skin and has major effects on elastic properties of the lungs. Loss of elastin fibers in the aortic wall due to aging leads to functional and morphologic changes as observed in the impairment of the “Windkessel” function and aneurysm formation.22 Degradation of elastin in the skin due to aging, UV light exposure, or injury leads to impairment of elasticity and results in formation of wrinkles and scarring. Furthermore, diseases caused by mutations or deletion of the elastin gene, such as cutis laxa or WBS, can affect the functionality of various organs and especially blood vessels.
In this study, for the first time, we successfully demonstrated that the elastin synthesis can be significantly increased by the delivery of synthetic modified TE mRNA into cells as well as in porcine skin via microinjection. This approach is a new and promising method for counteracting pathological changes caused by loss of elastin in adult dermis. Previous studies with transforming growth factor β1 (TGF-β1) demonstrated positive effects on elastin synthesis in human fibroblasts,23, 24, 25 rat aortic SMCs,26 and transgenic mice.27, 28 In further studies, Mizuno et al.29 transfected rat endothelial cells with plasmids to express recombinant elastin. The transplantation of these cells after a myocardial infarction into the myocardial infarct scar reduced scar expansion and prevented left ventricular enlargement. Later, Xiong and colleagues30 demonstrated that aortic vascular SMCs can be efficiently transfected in vitro and in vivo with an adenoviral vector carrying a recombinant TE gene. The application of this vector in elastase-induced abdominal aortic aneurysm in rats resulted in accumulation of elastic fibers leading to a reduction of aortic diameter in the medial layer in vivo, which indicates that the expression of recombinant elastin and the reconstruction of elastic fibers within the aneurysmal tissue could be used to prevent or reverse the aneurysm dilatation. In a recent study, Xie and colleagues31 subcutaneously injected recombinant human TE into wound bed of a severe burn injury and demonstrated formation of new elastin fibers in a swine model. Despite the ability of TGF-β1 to induce elastin synthesis, it should be also mentioned that TGF-β1 can act as a significant stimulator of tumor progression, invasion, and metastasis.32 Welch et al.33 demonstrated that the in vitro pretreatment of adenocarcinoma cells with TGF-β1 enhanced the metastatic ability of these cells. Furthermore, the use of adenovirus-based vectors has the disadvantage that these vectors are highly immunogenic and often have high levels of pre-existing immune responses in the host, which can restrict the delivery of vectors into desired cells.34 The delivery of genes by using retroviral vectors can lead to the permanent expression of proteins, which can have adverse effects over time. Furthermore, these vectors transduce only replicating cells and they randomly incorporate into the genome of host cells, and this can result in insertional mutagenesis.35 In contrast, synthetic mRNA is shorter than plasmid DNA and does not need to enter the nucleus. After the delivery of mRNA into the cytosol, it is immediately translated by ribosomes into desired protein. Thus, compared with other gene delivery methods, the use of synthetic TE mRNA has huge potential in the field of regenerative medicine.
The successful detection of elastin after the transfection of cells with modified TE mRNA in the supernatant indicates that the synthetized TE could successfully bind to EBP and be transported to the cell surface. After the delivery of TE to the cell surface, the binding of galactosugars to EBP leads to the dramatic decrease of the affinity of the elastin site for its ligands and results in dissociation of TE from EBP.36 EBP is a spliced variant of lysosomal β-galactosidase, and it is also a part of the elastin receptor complex (ERC), which is a heterotrimer composed of EBP and two membrane-associated proteins, protective protein/cathepsin A and neuraminidase-1 (Neu-1). Especially, vascular SMCs express ERC, which directly interacts with elastin and mediates elastin-induced cellular activities.37, 38 Thus, in a previous study, the incorporation of α-elastin into type 1 collagen gel resulted in significant inhibition of SMC proliferation and migration.39
Using the transfection reagent Lipofectamine 2000, a highly efficient transfection of TE mRNA could be achieved. After 4 hr of transfection, 98% of EA.hy926 cells and 96% of human fibroblasts were transfected with the Cy3-labeled TE mRNA. Furthermore, the transfection of EAHy.926 cells with modified TE mRNA demonstrated no significant influence on cell viability. In contrast, the unmodified TE mRNA led to significantly reduced cell viability. We additionally analyzed the quality of the unmodified and modified TE mRNA by Agilent Bioanalyzer and because the quality of the generated mRNAs was high and similar in both samples, high-performance liquid chromatography (HPLC) purification, which is recommended by Karikó et al.40 and Weissman et al.41 to reduce immune reactions to synthetic mRNAs, was not performed in this study. Therefore, we assume that the use of unmodified nucleotides led to a stronger immune activation than modified nucleotides. In a previous study, Thess and colleagues42 successfully demonstrated that sequence engineering and the purification of synthetic mRNA with HPLC are able to avoid immune activation and to produce high levels of encoded protein in vitro and in vivo. Thus, we suggest that sequence engineering (codon optimization) of the unmodified mRNA could further reduce immune stimulation.
In a supplementary experiment, we analyzed using ELISA whether the translation of TE mRNA can be increased using m1Ψ or m1Ψ/5mCTP compared with the used modification Ψ/5mCTP (Figure S2). Thereby, we could show that by using m1Ψ or m1Ψ/5mCTP modifications, the translation of the TE mRNA could be significantly increased in EA.hy293 cells. The highest elastin concentration was detected after the transfection of cells with m1Ψ-modified TE mRNA.
Using dot blot assay, the detected elastin amount differences were less than using Fastin assay. The reason, therefore, could be the quick coverage of the nitrocellulose membrane with other proteins, such as albumin, that are present in high amounts in the medium. The detected differences of elastin amount by using Fastin assay were approximately 10-fold higher than in the dot blot assay. However, using the dot blot assay, the detected elastin amount in the synthetic modified TE mRNA transfected samples was also significantly higher than in the controls.
Importantly, we also demonstrated that synthetic modified TE mRNA can be locally delivered into the skin by using hollow microneedles. Using this method, the consistency and reliability of intradermal injections, which are difficult to conduct with standard needles, can be improved.43 Thus, this method can warrant a reliable intradermal delivery of mRNA and a targeted localization of mRNA in the skin. In our study, the microinjected modified synthetic TE mRNA was able to mediate elastin synthesis in the skin. The detected increase in elastin amount was approximately 1.2-fold, which corresponds to an increase of 20%.
In an additional experiment, human fibroblasts transfected with modified TE mRNA were seeded on gelatin scaffolds and after 21 days, the production of elastin fibers was analyzed using two-photon laser scanning microscopy (LSM). The scaffolds seeded with TE mRNA transfected cells showed at an excitation wavelength of 760 nm an increased autofluorescence signal compared with the control scaffolds seeded with cells without TE mRNA transfection (Figure S3). Elastin emits autofluorescent light from 403–531 nm when excited at 760 nm.44 Besides promising ex vivo skin results, these results indicate the generation of elastin fibers. However, particularly, extensive in vivo studies will allow us to obtain detailed information regarding the generation of functional elastic fibers, and currently, pig in vivo experiments are in progress.
Conclusions
The ECM protein elastin gives connective tissues its elasticity and thereby enables the proper functioning of several organs and tissues, such as the blood vessels, elastic ligaments, lung, and skin. Because this protein is physiologically synthesized only to a limited extent after the neonatal period, elastin deficiencies caused by degenerative changes or genetic defects lead to significant functional limitations in the affected organs or tissues. So far, these deficiencies can be only treated symptomatically. Thus, the presented approach, using synthetic modified TE mRNA for de novo synthesis of elastin, represents an enormous potential for the therapy of congenital or acquired elastin deficiencies to restore the lost elasticity. Furthermore, because elastin has a long half-life, a few treatments or even only one treatment could be enough to obtain an improvement of elasticity in the affected tissues.
Materials and Methods
Ethics Statement
The study was performed in accordance with the Federation of European Laboratory Animal Science Associations (FELASA) and American Association for Laboratory Animal Science (AALAS) recommendations on the care and use of laboratory animals. The Animal Care and Welfare Commissioner of the University of Tübingen approved the protocols and procedures.
MSCs were isolated from the thymus tissue of a 1-year-old child suffering from WBS who had undergone cardiovascular surgery. The guardians of the patient gave informed consent to participate and for the publication of this report. The study was approved by the Ethical Committee of the Faculty of Medicine of the University of Tübingen.
Animals
German Landrace pigs (70 ± 10 kg, female, 4–6 months old) were obtained from a local, specific pathogen-free breeding facility (Benz, Germany). After the euthanasia of pigs by intravenous injection of a lethal dose of potassium chloride (1 mmol kg−1; B. Braun Melsungen AG, Melsungen, Germany), the ears were explanted to obtain pig skin from the outer side of the ear.
Synthesis of TE mRNA
The plasmid pcDNA 3.3-containing human TE encoding sequence was produced by Aldevron (Fargo, ND, USA). The synthesis of TE mRNA was performed by IVT according to our previously established protocol.45 In brief, the HotStar HiFidelity Polymerase Kit (QIAGEN, Hilden, Germany) and 0.7 μM of each forward (5′-TTGGACCCTCGTACAGAAGCTAATACG-3′) and reverse primer (5′-T120-CTTCCTACTCAGGCTTTATTCAAAGACCA-3′) were used to amplify the plasmid insert by PCR. Primers were purchased from ELLA Biotech (Martinsried, Germany) in HPLC grade. PCR was run using the following cycling protocol: initial activation step at 94°C for 3 min, followed by 25 cycles of denaturation at 94°C for 45 s, annealing at 60°C for 1 min, extension at 72°C for 1 min, and final extension at 72°C for 5 min. During the PCR, a poly T-tail of 120 thymidines (T) was added to the insert. PCR product was purified using the QIAquick PCR purification kit (QIAGEN, Hilden, Germany) according to the manufacturer’s instructions.
The DNA is then in vitro transcribed into mRNA using the MEGAscript T7 Kit (Life Technologies, Darmstadt, Germany). To obtain modified TE mRNA, we performed the IVT reaction at 37°C for 4 hr, and the mixture contained 7.5 mM ATP, 1.875 mM GTP (both from MEGAscript T7 Kit), 7.5 mM 5-methylcytidine (5mCTP), 7.5 mM pseudouridine (Ψ) (both from TriLink BioTechnologies, San Diego, CA, USA), 2.5 mM 3′-O-Me-m7G(5′)ppp(5′)G RNA cap structure analog (New England Biolabs, Frankfurt am Main, Germany), 40 U RiboLock RNase inhibitor (Thermo Scientific, Waltham, MA, USA), and 1.5 μg PCR product. In contrast with modified TE mRNA, unmodified TE mRNA was synthetized by using 7.5 mM ATP, 1.875 mM GTP, 7.5 mM CTP, and 7.5 mM UTP instead of modified nucleotides Ψ and 5mCTP. Afterward, template DNA was removed by adding 1 μL TURBO DNase (from MEGAscript T7 Kit) and incubation at 37°C for 15 min. Then the reaction mixture was purified using the RNeasy Mini Kit (QIAGEN, Hilden, Germany) according to manufacturer’s instructions. The mRNA was treated for 30 min at 37°C with 15 U Antarctic phosphatase (New England Biolabs, Frankfurt am Main, Germany) and purified again using the RNeasy Mini Kit. The concentration of mRNA was adjusted to 100 ng/μL by adding nuclease-free water. The quality and purity of the PCR product and the synthetic mRNA were assessed by 1% agarose gel electrophoresis and staining with 1× GelRed (Biotium, Fremont, CA, USA) in 1× Tris-borate-EDTA (TBE) for 30 min at room temperature (RT). The obtained TE mRNA was stored at −80°C.
Cy3 Labeling of Synthetic TE mRNA
Cu(I)-free azide-(dibenzocyclooctyne [DBCO]) click chemistry was used to perform Cy3 labeling of synthetic TE mRNA. Therefore, 1.9 mM 5-azido-C3-UTP (Jena Bioscience, Jena, Germany) and 5.6 mM Ψ were used during the IVT instead of 7.5 mM Ψ. After the IVT, the reaction mixture was purified using the RNeasy MinElute Cleanup Kit (QIAGEN) according to the manufacturer’s instructions. Afterward, Cy3 was conjugated to 5-azido-C3-UTP-modified mRNA by incubation with 5-fold molar excess of DBCO-sulfo-Cy3 (Jena Bioscience) in a total amount of 40 μL nuclease-free water for 1 hr at 37°C. The molar amount of DBCO-sulfo-Cy3 in relation to the amount of azide-labeled mRNA was calculated using the manufacturer’s data sheet. Then the reaction mixture was purified using the RNeasy MinElute Cleanup Kit, and the quality and purity of the mRNA were verified by 1% agarose gel electrophoresis and staining with 1× GelRed in 1× TBE for 30 min at RT. The concentration was determined using a photometer, and the mRNA was stored at −80°C.
Isolation of MSCs from a Patient with WBS
To isolate MSCs from a patient with WBS (WBS_MSCs), we rinsed the thymus tissue of a WBS patient with Dulbecco’s PBS (DPBS; Life Technologies, Darmstadt, Germany) and cut it into small pieces. The tissue pieces were then incubated with 5 mL of 0.1% collagenase II solution (Merck Millipore, Darmstadt, Germany) in DMEM low glucose (Thermo Fisher Scientific, Waltham, MA, USA) at 37°C. The solution containing tissue pieces was briefly mixed every 30–60 min. After 3 hr, the tissue suspension was filtered through a 100-μm tissue strainer and the filtrate was centrifuged for 5 min at 300 × g. After centrifugation, the supernatant was aspirated and the cell pellet was resuspended in 30 mL of MSC medium and seeded in three T175 cell culture flasks. The cells were then incubated in 25 mL medium at 37°C and 5% CO2. The obtained cells are called WBS_MSCs.
Characterization of Isolated WBS_MSCs Using Flow Cytometry
Flow cytometry analysis was performed to determine the expression of surface markers on the cultured WBS_MSCs. To perform the antibody staining, we first washed 5 × 105 cells with 500 μL DPBS and then with 1 mL DPBS containing 0.5% BSA (Sigma-Aldrich). Subsequently, the cells were incubated for 30 min at 37°C in 500 μL DPBS/0.5% BSA containing antibodies (1:100 dilution). PE-conjugated mouse anti-human CD90/Thy-1 or CD45 antibodies (both from R&D Systems, Minneapolis, MN, USA), Alexa 488-conjugated mouse anti-human CD105 (Bio-Rad, Hercules, CA, USA), and PE-conjugated mouse anti-human CD31 (BD Biosciences, Heidelberg, Germany) were used for the staining. Afterward, cells were centrifuged at 300 × g for 5 min, washed, fixed in 1× CellFIX solution (10×; Becton Dickinson, Heidelberg, Germany), and measured immediately using a FACScan flow cytometer (Becton Dickinson). A total of 10,000 cells was counted and analyzed using CellQuest Pro Software (Becton Dickinson).
Cultivation of Cells
EA.hy926 cells were cultivated in DMEM with high glucose (Lonza, Basel, Switzerland) supplemented with 10% heat-inactivated FBS (Life Technologies), 1% L-glutamine (Life Technologies), and 1% penicillin/streptomycin (Life Technologies). Human BJ fibroblasts were cultivated in DMEM with high glucose containing 10% heat-inactivated FBS, 3% HEPES (Life Technologies), 1% L-glutamine, and 1% penicillin/streptomycin. WBS_MSCs were cultivated in DMEM/Ham’s F12 (1:1) medium (Biochrom, Berlin, Germany) containing 10% heat-inactivated FBS, 1% L-glutamine, and 1% penicillin/streptomycin. Cells were cultivated at 37°C and 5% CO2. Medium was changed every 3–4 days. After reaching 80% confluency, cells were washed once with DPBS and detached using 0.05% Trypsin-EDTA (Life Technologies). Subsequently, trypsin was inactivated by adding trypsin neutralization solution (TNS) 0.05% in 0.1% BSA (PromoCell, Heidelberg, Germany). Cell amount was measured using CASY Cell Counter (Schärfe System, Reutlingen, Germany).
Transfection of Cells with Synthetic TE mRNA
For the transfection, 3 × 105 EA.hy926 cells, BJ fibroblasts, or WB_MSCs were seeded per well of a six-well plate, and the cells were cultivated overnight at 37°C in a 5% CO2 atmosphere. To generate transfection complexes, called lipoplexes, we incubated 2.5 μg TE mRNA with 4 μL Lipofectamine 2000 in 500 μL OptiMEM I reduced serum-free media (Invitrogen, Carlsbad, CA, USA) for 20 min at RT. Cell medium was discarded and cells were washed once with 1 mL DPBS. Afterward, 500 μL OptiMEM was added to each well of the six-well plate. Subsequently, 500 μL OptiMEM containing lipoplexes was added to the wells. Additionally, 500 μL OptiMEM containing only 4 μL Lipofectamine 2000 was added as a control. After 4 hr of incubation at 37°C, transfection mixtures were removed and 1 mL cell culture medium without phenol red was added to each well. Cells were cultivated at 37°C and 5% CO2 overnight. The supernatant was collected and stored at −80°C. Cells were detached using 0.05% Trypsin-EDTA (Life Technologies) and 0.05% TNS in 0.1% BSA (PromoCell, Heidelberg, Germany).
Analysis of Transfection Efficiency Using Cy3-Labeled TE mRNA
To assess the uptake of the Cy3 TE mRNA into EA.hy926 cells and human fibroblasts, we transfected 3 × 105 cells as described above and 4 hr post-transfection, cells were washed twice with 1 mL DPBS. The Cy3 TE mRNA uptake into the cells was examined using fluorescence microscopy (Axiovert135; Carl Zeiss, Oberkochen, Germany). Additionally, the cells were detached, washed with 1 mL DPBS, and resuspended in 500 μL 1× CellFIX solution to perform flow cytometry analysis. Flow cytometry measurements were performed using a FACScan flow cytometer. A total of 10,000 cells was counted and analyzed using CellQuest Pro Software.
Analysis of Cell Viability after the Transfection of EA.hy926 Cells with Modified and Unmodified TE mRNA
To analyze the cell viability after the transfection of EA.hy926 cells with modified and unmodified TE mRNA, we seeded 4 × 105 cells per well of a six-well plate, cultivated them overnight, and transfected for 4 hr at 37°C with lipoplexes containing 2.5 μg modified or unmodified TE mRNA. As negative controls, cells were treated only with OptiMEM or OptiMEM containing transfection reagent. After 24 hr, the cell viability was measured using PrestoBlue assay (Invitrogen, Carlsbad, CA, USA). Therefore, cells were washed with 1 mL DPBS, and 50 μL PrestoBlue cell viability reagent was added to 450 μL cell culture medium per well and incubated for 1.5 hr at 37°C. The fluorescence intensity of 100 μL supernatant was measured in triplicates at an excitation wavelength of 530 nm and emission wavelength of 600 nm using a multimode microplate reader (Mithras LB 940; Berthold Technologies).
Analysis of Elastin Synthesis in Cells
Fastin Assay
Fastin Elastin Kit (Biocolor, Carrickfergus, UK) was used to detect soluble TE and α-elastin polypeptides by first binding a dye label (5,10,15,20-tetraphenyl-21H,23H-porphine tetra-sulfonate [TPPS]) to the basic and non-polar amino acid sequences in mammalian elastin. By adding the Dye Dissociation Reagent, the elastin-bound dye is released into solution and measured at 513 nm using a microplate reader (EON Microplate Spectrophotometer; BioTek, Bad Friedrichshall, Germany). Fastin assay was performed according to the manufacturer’s instructions. An α-elastin standard from 1.25 to 80 μg was used to detect the elastin quantity in the samples.
Dot Blot Analysis
To detect the produced elastin by dot blot analysis, we blotted 500 μL supernatant using the Bio-Dot SF device (Bio-Rad, Hercules, CA, USA) on nitrocellulose membrane according to the manufacturer’s instructions. The membrane was incubated on a tilting shaker overnight at 4°C in TBST (20 mM Tris [pH 7.5], 500 mM NaCl, 0.05% Tween 20) containing 5% milk powder (Carl Roth, Karlsruhe, Germany). The following day, the membrane was incubated for 1 hr at RT with polyclonal antibody to human aortic elastin (EPC, Owensville, MO, USA) in TBST (1:200 dilution). Then the membrane was washed 3× for 5 min with TBST. Afterward, the membrane was incubated for 45 min at RT with alkaline-phosphatase-conjugated anti-rabbit IgG (Sigma-Aldrich, Munich, Germany) 1:10,000 diluted in TBST. Subsequently, the membranes were washed 3× for 5 min with TBST and incubated with the BCIP/NBT (5-bromo-4-chloro-3-indolyl phosphate/nitro blue tetrazolium) solution (Sigma-Aldrich, Munich, Germany) for colorimetric detection of alkaline-phosphatase-conjugated antibodies. The intensities of dot blots were analyzed using ImageJ software.
Delivery of TE mRNA into Pig Skin
Preparation of Porcine Skin
The skin was separated postmortem from the pig’s ears, washed with 0.9% NaCl solution (Fresenius Kabi, Bad Homburg, Germany), and trimmed to 1-mm thickness using a dermatome (Acculan 3Ti dermatome; B. Braun, Melsungen, Germany). Afterward, round pieces with a diameter of 15 mm were punched out. Using an iodine solution (0.5 M I2; Fluca Analytical, Seelze, Germany), we disinfected the surface of the punched skin pieces and then rinsed one to two times with DPBS. Furthermore, the skin was incubated for 30 min in an antibiotic solution composed of 250 μg/mL gentamicin (Sigma-Aldrich, St. Louis, MO, USA) and 1,250 μg/mL amphotericin B (PromoCell, Heidelberg, Germany) in DMEM (Thermo Fisher Scientific, Waltham, MA, USA) and then washed one to two times with DPBS. The excessive DPBS was removed using a sterile swab from the surface before performing intradermal injection.
Intradermal Delivery of Synthetic TE mRNA into Ex Vivo Porcine Skin Model
To perform the microinjections, 2.5 μg of synthetic TE mRNA was complexed with 4 μL Lipofectamine 2000 in OptiMEM in a total volume of 35 μL. The appropriate volume of OptiMEM containing Lipofectamine 2000 served as control. The intradermal injection of synthetic mRNA was performed using the hollow microinjection needles MicronJet600 from NanoPass Technologies (Nes Ziona, Israel). MicronJet600 is composed of three hollow microneedles of 600-μm length that are cleared for intradermal injections. After the injection of synthetic mRNA, each skin piece was washed 1× with 1 mL DPBS and transferred into ThinCert cell culture inserts with a pore size of 1 μm (Greiner Bio-One, Frickenhausen, Germany). The inserts containing the skin were placed in one well of a 12-well plate, which contained 1.5 mL human endothelial cell culture medium (VascuLife EnGS-Mv Microvascular Endothelial Kit; without hydrocortisone hemisuccinate; CellSystems, Troisdorf, Germany). The skin was collected after 72 hr to determine the elastin amount by using Fastin assay.
Detection of TE mRNA-Mediated Elastin Synthesis in Pig Skin Using Fastin Assay
Preparation of Skin
The medium on skin samples was swabbed, and the transfected region of the skin was cut from the skin piece using a scalpel. Samples were dried for 15 min at RT and then transferred into a reaction tube and immediately closed to prevent weight fluctuations due to evaporation. The weight of the individual skin samples was on average at 17 ± 3 mg.
Fastin Assay
Skin samples were treated for 1 hr at 100°C with 750 μL 0.25 M oxalic acid and then centrifuged at 10,000 × g for 10 min. The supernatant was collected and transferred into new reaction tubes. An α-elastin standard from 1.25 to 80 μg was used to detect the elastin quantity in the samples. To each sample, 500 μL elastin precipitating reagent was added, incubated for 15 min, and then centrifuged at 16,000 × g for 10 min. The supernatant was discarded, 1 mL of dye reagent was added to each tube, mixed, and incubated for 90 min at RT and 550 rpm. Then the samples were centrifuged at 12,000 × g for 10 min. The supernatant was discarded and 400 μL of dye dissociation reagent was added, mixed, and incubated 10 min at RT until the pellet is dissolved. Afterward, 100 μL of this solution was transferred into one well of a 96-well plate in triplicates, and the absorption was measured at 513 nm using a microplate reader (EON Microplate Spectrophotometer).
Statistical Analysis
Data are shown as mean ± SEM or median with 25–75 percentiles (box) and 5–95 percentiles (whiskers). One-way ANOVA followed by Bonferroni’s multiple comparison test was performed to compare the means of more than two groups. Student’s two-sample t test was performed for comparison of means between two groups. All statistical analyses were performed double-tailed using GraphPad Prism version 6.01. Differences of p < 0.05 were considered significant.
Author Contributions
M.L., R.M.P., and M.A.-A. conceived and designed the experiments. R.M.P., S.G., M.P., L.H., M.Y., and F.G. performed the experiments with support from M.L., A.B., T.K., A.N., M.A.-A., and H.P.W. and analyzed the data. E.K., Y.L., H.P.W., and C.S. contributed reagents/materials/analysis tools. M.A.-A. wrote the paper and supervised the project.
Acknowledgments
This study was funded by the German Heart Foundation/German Foundation of Heart Research through Grant F/38/13 and the European Social Funds in Baden-Wuerttemberg, Germany, and the Ministry of Science, Research, and the Arts of the State of Baden-Wuerttemberg (MWK-BW). Furthermore, we acknowledge support by Deutsche Forschungsgemeinschaft and Open Access Publishing Fund of University of Tübingen.
Footnotes
Supplemental Information includes three figures and can be found with this article online at https://doi.org/10.1016/j.omtn.2018.03.013.
Supplemental Information
References
- 1.Baldwin A.K., Simpson A., Steer R., Cain S.A., Kielty C.M. Elastic fibres in health and disease. Expert Rev. Mol. Med. 2013;15:e8. doi: 10.1017/erm.2013.9. [DOI] [PubMed] [Google Scholar]
- 2.Uitto J. Biochemistry of the elastic fibers in normal connective tissues and its alterations in diseases. J. Invest. Dermatol. 1979;72:1–10. doi: 10.1111/1523-1747.ep12530093. [DOI] [PubMed] [Google Scholar]
- 3.Vrhovski B., Weiss A.S. Biochemistry of tropoelastin. Eur. J. Biochem. 1998;258:1–18. doi: 10.1046/j.1432-1327.1998.2580001.x. [DOI] [PubMed] [Google Scholar]
- 4.Annabi N., Mithieux S.M., Camci-Unal G., Dokmeci M.R., Weiss A.S., Khademhosseini A. Elastomeric recombinant protein-based biomaterials. Biochem. Eng. J. 2013;77:110–118. doi: 10.1016/j.bej.2013.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Swee M.H., Parks W.C., Pierce R.A. Developmental regulation of elastin production. Expression of tropoelastin pre-mRNA persists after down-regulation of steady-state mRNA levels. J. Biol. Chem. 1995;270:14899–14906. doi: 10.1074/jbc.270.25.14899. [DOI] [PubMed] [Google Scholar]
- 6.Chin K., Wieslander C., Shi H., Balgobin S., Montoya T.I., Yanagisawa H., Word R.A. Pelvic organ support in animals with partial loss of fibulin-5 in the vaginal wall. PLoS ONE. 2016;11:e0152793. doi: 10.1371/journal.pone.0152793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Moore J., Thibeault S. Insights into the role of elastin in vocal fold health and disease. J. Voice. 2012;26:269–275. doi: 10.1016/j.jvoice.2011.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shapiro S.D., Endicott S.K., Province M.A., Pierce J.A., Campbell E.J. Marked longevity of human lung parenchymal elastic fibers deduced from prevalence of D-aspartate and nuclear weapons-related radiocarbon. J. Clin. Invest. 1991;87:1828–1834. doi: 10.1172/JCI115204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dayal D., Giri D., Senniappan S. A rare association of central hypothyroidism and adrenal insufficiency in a boy with Williams-Beuren syndrome. Ann. Pediatr. Endocrinol. Metab. 2017;22:65–67. doi: 10.6065/apem.2017.22.1.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Strømme P., Bjørnstad P.G., Ramstad K. Prevalence estimation of Williams syndrome. J. Child Neurol. 2002;17:269–271. doi: 10.1177/088307380201700406. [DOI] [PubMed] [Google Scholar]
- 11.Urbán Z., Riazi S., Seidl T.L., Katahira J., Smoot L.B., Chitayat D., Boyd C.D., Hinek A. Connection between elastin haploinsufficiency and increased cell proliferation in patients with supravalvular aortic stenosis and Williams-Beuren syndrome. Am. J. Hum. Genet. 2002;71:30–44. doi: 10.1086/341035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhang P., Huang A., Ferruzzi J., Mecham R.P., Starcher B.C., Tellides G., Humphrey J.D., Giordano F.J., Niklason L.E., Sessa W.C. Inhibition of microRNA-29 enhances elastin levels in cells haploinsufficient for elastin and in bioengineered vessels—brief report. Arterioscler. Thromb. Vasc. Biol. 2012;32:756–759. doi: 10.1161/ATVBAHA.111.238113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pober B.R. Williams-Beuren syndrome. N. Engl. J. Med. 2010;362:239–252. doi: 10.1056/NEJMra0903074. [DOI] [PubMed] [Google Scholar]
- 14.Li D.Y., Brooke B., Davis E.C., Mecham R.P., Sorensen L.K., Boak B.B., Eichwald E., Keating M.T. Elastin is an essential determinant of arterial morphogenesis. Nature. 1998;393:276–280. doi: 10.1038/30522. [DOI] [PubMed] [Google Scholar]
- 15.Milewicz D.M., Urbán Z., Boyd C. Genetic disorders of the elastic fiber system. Matrix Biol. 2000;19:471–480. doi: 10.1016/s0945-053x(00)00099-8. [DOI] [PubMed] [Google Scholar]
- 16.Wise S.G., Weiss A.S. Tropoelastin. Int. J. Biochem. Cell Biol. 2009;41:494–497. doi: 10.1016/j.biocel.2008.03.017. [DOI] [PubMed] [Google Scholar]
- 17.Warren L., Manos P.D., Ahfeldt T., Loh Y.H., Li H., Lau F., Ebina W., Mandal P.K., Smith Z.D., Meissner A. Highly efficient reprogramming to pluripotency and directed differentiation of human cells with synthetic modified mRNA. Cell Stem Cell. 2010;7:618–630. doi: 10.1016/j.stem.2010.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zangi L., Lui K.O., von Gise A., Ma Q., Ebina W., Ptaszek L.M., Später D., Xu H., Tabebordbar M., Gorbatov R. Modified mRNA directs the fate of heart progenitor cells and induces vascular regeneration after myocardial infarction. Nat. Biotechnol. 2013;31:898–907. doi: 10.1038/nbt.2682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kormann M.S., Hasenpusch G., Aneja M.K., Nica G., Flemmer A.W., Herber-Jonat S., Huppmann M., Mays L.E., Illenyi M., Schams A. Expression of therapeutic proteins after delivery of chemically modified mRNA in mice. Nat. Biotechnol. 2011;29:154–157. doi: 10.1038/nbt.1733. [DOI] [PubMed] [Google Scholar]
- 20.Stadler C.R., Bähr-Mahmud H., Celik L., Hebich B., Roth A.S., Roth R.P., Karikó K., Türeci Ö., Sahin U. Elimination of large tumors in mice by mRNA-encoded bispecific antibodies. Nat. Med. 2017;23:815–817. doi: 10.1038/nm.4356. [DOI] [PubMed] [Google Scholar]
- 21.Steinle H., Behring A., Schlensak C., Wendel H.P., Avci-Adali M. Concise review: application of in vitro transcribed messenger RNA for cellular engineering and reprogramming: progress and challenges. Stem Cells. 2017;35:68–79. doi: 10.1002/stem.2402. [DOI] [PubMed] [Google Scholar]
- 22.Tsamis A., Krawiec J.T., Vorp D.A. Elastin and collagen fibre microstructure of the human aorta in ageing and disease: a review. J. R. Soc. Interface. 2013;10:20121004. doi: 10.1098/rsif.2012.1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Choi W.S., Mitsumoto A., Kochevar I.E. Involvement of reactive oxygen species in TGF-beta1-induced tropoelastin expression by human dermal fibroblasts. Photochem. Photobiol. 2009;85:1425–1433. doi: 10.1111/j.1751-1097.2009.00611.x. [DOI] [PubMed] [Google Scholar]
- 24.Kucich U., Rosenbloom J.C., Abrams W.R., Rosenbloom J. Transforming growth factor-beta stabilizes elastin mRNA by a pathway requiring active Smads, protein kinase C-delta, and p38. Am. J. Respir. Cell Mol. Biol. 2002;26:183–188. doi: 10.1165/ajrcmb.26.2.4666. [DOI] [PubMed] [Google Scholar]
- 25.Kähäri V.M., Olsen D.R., Rhudy R.W., Carrillo P., Chen Y.Q., Uitto J. Transforming growth factor-beta up-regulates elastin gene expression in human skin fibroblasts. Evidence for post-transcriptional modulation. Lab. Invest. 1992;66:580–588. [PubMed] [Google Scholar]
- 26.Kothapalli C.R., Ramamurthi A. Induced elastin regeneration by chronically activated smooth muscle cells for targeted aneurysm repair. Acta Biomater. 2010;6:170–178. doi: 10.1016/j.actbio.2009.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Uitto J., Hsu-Wong S., Katchman S.D., Bashir M.M., Rosenbloom J. Skin elastic fibres: regulation of human elastin promoter activity in transgenic mice. Ciba Found. Symp. 1995;192:237–253. doi: 10.1002/9780470514771.ch13. discussion 253–258. [DOI] [PubMed] [Google Scholar]
- 28.Katchman S.D., Hsu-Wong S., Ledo I., Wu M., Uitto J. Transforming growth factor-beta up-regulates human elastin promoter activity in transgenic mice. Biochem. Biophys. Res. Commun. 1994;203:485–490. doi: 10.1006/bbrc.1994.2208. [DOI] [PubMed] [Google Scholar]
- 29.Mizuno T., Yau T.M., Weisel R.D., Kiani C.G., Li R.K. Elastin stabilizes an infarct and preserves ventricular function. Circulation. 2005;112(Suppl 9):I81–I88. doi: 10.1161/01.CIRCULATIONAHA.105.523795. [DOI] [PubMed] [Google Scholar]
- 30.Xiong J., Wang S.M., Chen L.H., Lin Y., Zhu Y.F., Ye C.S. Elastic fibers reconstructed using adenovirus-mediated expression of tropoelastin and tested in the elastase model of abdominal aortic aneurysm in rats. J. Vasc. Surg. 2008;48:965–973. doi: 10.1016/j.jvs.2008.04.016. [DOI] [PubMed] [Google Scholar]
- 31.Xie H., Lucchesi L., Zheng B., Ladich E., Pineda T., Merten R., Gregory C., Rutten M., Gregory K. Treatment of burn and surgical wounds with recombinant human tropoelastin produces new elastin fibers in scars. J. Burn Care Res. 2017;38:e859–e867. doi: 10.1097/BCR.0000000000000507. [DOI] [PubMed] [Google Scholar]
- 32.Akhurst R.J., Derynck R. TGF-beta signaling in cancer—a double-edged sword. Trends Cell Biol. 2001;11:S44–S51. doi: 10.1016/s0962-8924(01)02130-4. [DOI] [PubMed] [Google Scholar]
- 33.Welch D.R., Fabra A., Nakajima M. Transforming growth factor beta stimulates mammary adenocarcinoma cell invasion and metastatic potential. Proc. Natl. Acad. Sci. USA. 1990;87:7678–7682. doi: 10.1073/pnas.87.19.7678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Saxena M., Van T.T., Baird F.J., Coloe P.J., Smooker P.M. Pre-existing immunity against vaccine vectors—friend or foe? Microbiology. 2013;159:1–11. doi: 10.1099/mic.0.049601-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Vannucci L., Lai M., Chiuppesi F., Ceccherini-Nelli L., Pistello M. Viral vectors: a look back and ahead on gene transfer technology. New Microbiol. 2013;36:1–22. [PubMed] [Google Scholar]
- 36.Blanchevoye C., Floquet N., Scandolera A., Baud S., Maurice P., Bocquet O., Blaise S., Ghoneim C., Cantarelli B., Delacoux F. Interaction between the elastin peptide VGVAPG and human elastin binding protein. J. Biol. Chem. 2013;288:1317–1328. doi: 10.1074/jbc.M112.419929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hinek A. Nature and the multiple functions of the 67-kD elastin-/laminin binding protein. Cell Adhes. Commun. 1994;2:185–193. doi: 10.3109/15419069409004436. [DOI] [PubMed] [Google Scholar]
- 38.Xu J., Shi G.-P. Vascular wall extracellular matrix proteins and vascular diseases. Biochim. Biophys. Acta. 2014;1842:2106–2119. doi: 10.1016/j.bbadis.2014.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ito S., Ishimaru S., Wilson S.E. Inhibitory effect of type 1 collagen gel containing α-elastin on proliferation and migration of vascular smooth muscle and endothelial cells. Cardiovasc. Surg. 1997;5:176–183. doi: 10.1016/s0967-2109(97)00004-5. [DOI] [PubMed] [Google Scholar]
- 40.Karikó K., Muramatsu H., Ludwig J., Weissman D. Generating the optimal mRNA for therapy: HPLC purification eliminates immune activation and improves translation of nucleoside-modified, protein-encoding mRNA. Nucleic Acids Res. 2011;39:e142. doi: 10.1093/nar/gkr695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Weissman D., Pardi N., Muramatsu H., Karikó K. HPLC purification of in vitro transcribed long RNA. In: Rabinovich P.M., editor. Synthetic Messenger RNA and Cell Metabolism Modulation. Springer; 2013. pp. 43–54. [DOI] [PubMed] [Google Scholar]
- 42.Thess A., Grund S., Mui B.L., Hope M.J., Baumhof P., Fotin-Mleczek M., Schlake T. Sequence-engineered mRNA without chemical nucleoside modifications enables an effective protein therapy in large animals. Mol. Ther. 2015;23:1456–1464. doi: 10.1038/mt.2015.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Flynn P.M., Shenep J.L., Mao L., Crawford R., Williams B.F., Williams B.G. Influence of needle gauge in Mantoux skin testing. Chest. 1994;106:1463–1465. doi: 10.1378/chest.106.5.1463. [DOI] [PubMed] [Google Scholar]
- 44.Fritze O., Schleicher M., König K., Schenke-Layland K., Stock U., Harasztosi C. Facilitated noninvasive visualization of collagen and elastin in blood vessels. Tissue Eng. Part C Methods. 2010;16:705–710. doi: 10.1089/ten.TEC.2009.0309. [DOI] [PubMed] [Google Scholar]
- 45.Avci-Adali M., Behring A., Steinle H., Keller T., Krajeweski S., Schlensak C., Wendel H.P. In vitro synthesis of modified mRNA for induction of protein expression in human cells. J. Vis. Exp. 2014;(93):e51943. doi: 10.3791/51943. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.