Abstract
Obesity is an epidemic disease characterized by chronic low-grade inflammation associated with a dysfunctional fat mass. Adipose tissue is now considered an extremely active endocrine organ that secretes cytokine-like hormones, called adipokines, either pro- or anti-inflammatory factors bridging metabolism to the immune system. Leptin is historically one of most relevant adipokines, with important physiological roles in the central control of energy metabolism and in the regulation of metabolism-immune system interplay, being a cornerstone of the emerging field of immunometabolism. Indeed, leptin receptor is expressed throughout the immune system and leptin has been shown to regulate both innate and adaptive immune responses. This review discusses the latest data regarding the role of leptin as a mediator of immune system and metabolism, with particular emphasis on its effects on obesity-associated metabolic disorders and autoimmune and/or inflammatory rheumatic diseases.
Keywords: adipokines, adipose tissue, immunometabolism, leptin, metabolism, rheumatic diseases, rheumatoid arthritis, Type 2 diabetes mellitus (T2DM)
Introduction
Obesity, the greater public health problem in the western world, is associated with high-incident chronic autoimmune and inflammatory pathologies, such as T2DM, NAFLD, OA, and RA, thus having a huge social and economic impact (Zhang et al., 2014). Adipose tissue, initially considered as a simple energy storage tissue, is now recognized as an active endocrine organ and a bona fide immune organ, constituted not only by adipocytes but also by fibroblasts, endothelial cells and a wide array of immune cells (adipose tissue macrophages, neutrophils, mast cells, eosinophils, T and B cells that maintains tissue homeostasis in lean individuals (Huh et al., 2014; Vieira-Potter, 2014). The adipocyte expansion caused by positive energy balance leads to adipocyte hypoxia, apoptosis, and cell stress, ultimately resulting in the expression of chemoattractant molecules and infiltration of inflammatory cells (Vieira-Potter, 2014). The obese adipose tissue is also characterized by a markedly deregulated production of adipose tissue-derived factors, i.e., adipokines, a growing family of low molecular weight, biologically active proteins with pleiotropic functions (Al-Suhaimi and Shehzad, 2013). Adipokines are crucial players not only in energy metabolism but also in inflammation and immunity, most of them being increased in obesity and contributing to the associated ‘low-grade inflammatory state’ (Tilg and Moschen, 2006).
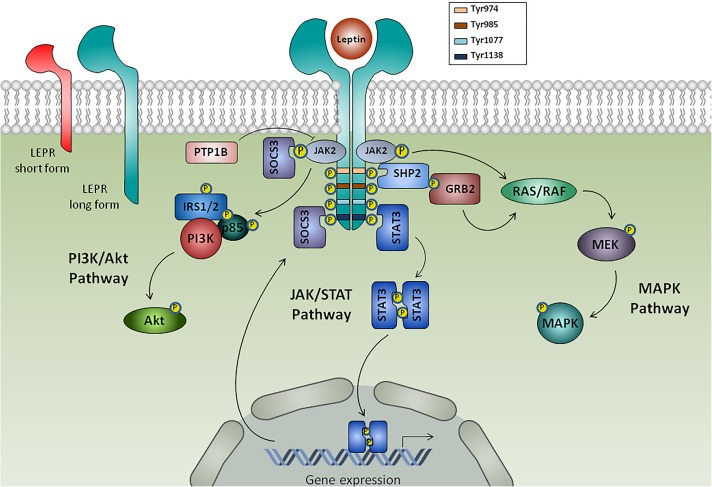
Leptin was discovered in 1994 by the group of Jeffrey Friedman (Zhang et al., 1994) and is the best-characterized member of adipokine family. Encoded by LEP gene (the human homolog of murine ob gene), leptin is a 16 kDa non-glycosylated protein mainly produced by adipocytes, but also by skeletal muscle, intestine, brain, joint tissues and bone (Scotece et al., 2014). This adipokine exerts its physiological activity through its receptor (LEPR or Ob-R), a class I cytokine receptor family from diabetes (db) gene (Münzberg and Morrison, 2015). There are at least six LEPR isoforms that differ in the length of the cytoplasmic domain: a soluble isoform, four short isoforms, and a long isoform, which has the full intracellular domain that allows the transduction of leptin signal via JAK and STAT signaling pathways (Frühbeck, 2006). Alternatively to canonical JAK/STAT pathway, LEPR could activate ERK 1/2, p38 MAPK, JNK, PKC, and PI3K/Akt pathways (Zhou and Rui, 2014) (Figure 1). This hormone, together with other regulatory molecules, has a central role in appetite and body weight homeostasis by inducing anorexigenic factors (as cocaine-amphetamine-related transcript) and suppressing orexigenic neuropeptides (as neuropeptide Y) on hypothalamus (Al-Suhaimi and Shehzad, 2013; Rosenbaum and Leibel, 2014). Therefore, central leptin resistance, caused by impairment of leptin transportation, leptin signaling and leptin target neural circuits, is considered the main risk factor for the obesity pathogenesis.(Al-Suhaimi and Shehzad, 2013; Rosenbaum and Leibel, 2014). Interestingly, leptin release is modulated in a circadian rhythm manner, which has been correlated with sweet taste recognition (Nakamura et al., 2008). Moreover, leptin also affects other physiological functions, namely bone metabolism, inflammation, infection and immune responses (Scotece et al., 2014) (Figure 2). Accordingly, LEPR is expressed in across the cells of innate and adaptive immune system, evoking leptin as a crucial linker of neuroendocrine and immune systems (Carlton et al., 2012; Procaccini et al., 2017).
FIGURE 1.
Leptin receptors and intracellular leptin signaling pathways. Leptin binds to its receptor (LEPR) isoforms: the soluble isoform (not shown), the short isoform and the long isoform. Binding of leptin to the long form of LEPR results in its dimerization and prompts Janus kinase 2 (JAK2) autophosphorylation, which phosphorylates cytoplasmatic domain of LEPR in tyrosine residues (Tyr974, Tyr985, Tyr1077, Tyr1138), each one functioning as docking sites for cytoplasmic adaptors. LEPR-phosphorylated Tyr1138 mediates the interaction with signaling transducer and activator of transcription 3 (STAT3), which dimerize and translocate to the nucleus to activate gene transcription of target genes, such as suppressor of cytokine signaling 3 (SOCS3) that acts as a negative feedback signaling. Additionally, leptin induces the activation of SHP2, which then recruits the adaptor protein Grb2 to prompt activation of Ras/Raf/MAPK signaling cascade. Leptin also mediated phosphatidylinositol-3-kinase (PI3K)/Akt activation via insulin receptor substrate 1/2 (IRS1/2) and protein tyrosine phosphatase 1B (PTP1B) acts as a negative regulator of leptin signaling through JAK2 dephosphorylation.
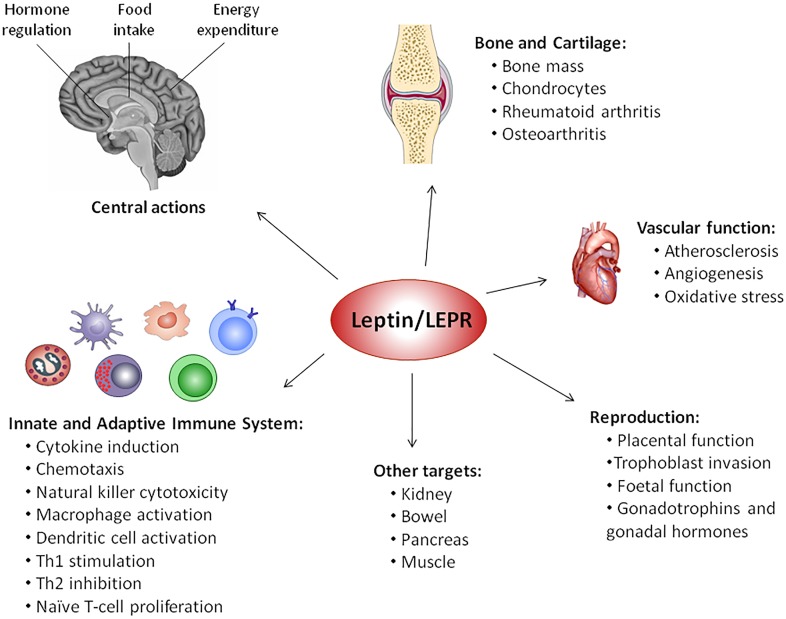
FIGURE 2.
Pleiotropic nature of leptin. Since its discovery in 1994, several physiological functions have been attributed to leptin, such as modulation of vascular function, reproduction, bone metabolism, inflammation, infection, and immune responses, behind central regulation of food intake, energy expenditure and hormone regulation, via activation of leptin receptor (LEPR).
This review summarizes the latest data regarding the role of leptin as a mediator of innate and adaptive immune cells activity, and its effects on obesity-associated metabolic disorders, namely T2DM and NAFLD, and autoimmune and/or inflammatory rheumatic diseases, such as OA and RA.
Leptin and Immunometabolism
The rising prevalence of obesity in western society is paralleled with a significant augment in autoimmune diseases. Accordingly, numerous association studies had demonstrated that overweight is implicated in a higher risk of developing multiple sclerosis (Kavak et al., 2015), RA (Ajeganova et al., 2013), and psoriasis (Duarte et al., 2013). On the other hand, malnutrition/starvation has been long related to increased susceptibility to infectious diseases (Taylor et al., 2013; Jones et al., 2014). These observations bring out immune response as a highly energy-dependent biological process that is dependent on an adequate food intake and metabolism. In fact, a recent article critically discuss the correlation between autoimmunity and overnutrition or metabolic pressure (De Rosa et al., 2017). At the interface of the historically distinct fields of immunology and metabolism, immunometabolism has emerged as a new research discipline (Mathis and Shoelson, 2011). In the last years, it has been evidenced that metabolic status of immune cells directly determines their function and differentiation, thus affecting immunity and tolerance, as well as the failure of the immune response in autoimmune pathologies (Gaber et al., 2017). In fact, innate and adaptive immune cells adapt to altered tissue microenvironment, characterized by hypoxia and nutrient competition, by reprogramming their metabolism (Gaber et al., 2017), and failure in this metabolic reconfiguration ultimately leads to a deregulated immune response and pathology (Gaber et al., 2017).
Leptin, the forerunner of adipokine family, is a key sensor of energy metabolism and a cornerstone in the regulation of metabolism-immune system interplay. Malnutrition results in hypoleptinemia, while obesity leads to hyperleptinemia, both conditions affecting the immune response in an opposite manner. In particular, obese subjects demonstrated decreased levels of Treg (central players in the control of peripheral immune tolerance), which are inversely correlated with leptin levels and BMI (Matarese et al., 2010). In malnutrition, altered T cell function and metabolism was associated with decreased leptin levels (Cohen et al., 2017). Leptin and LepR-deficient mouse models presented augmented number and activity of Treg cells together with a resistance to autoimmune diseases, and leptin replacement rescues Treg cell levels to wild-type mice values (Matarese et al., 2010). Accordingly, human T cell activation and production of cytokines can be induced after incubation with 10 ng/mL exogenous leptin following nutritional rehabilitation (Rodríguez et al., 2007). Furthermore, the central effect of leptin on the hypothalamus is mediated, at least in part, by inhibition of hypothalamic-pituitary-adrenal axis and activation of the sympatho-adrenal axis, having the sympathetic nervous system a function in the central control of the immune system (Pérez-Pérez et al., 2017). Moreover, most immune cells express LEPR at their surface, which evokes a straight action of leptin in the modulation of the immune response (Procaccini et al., 2017).
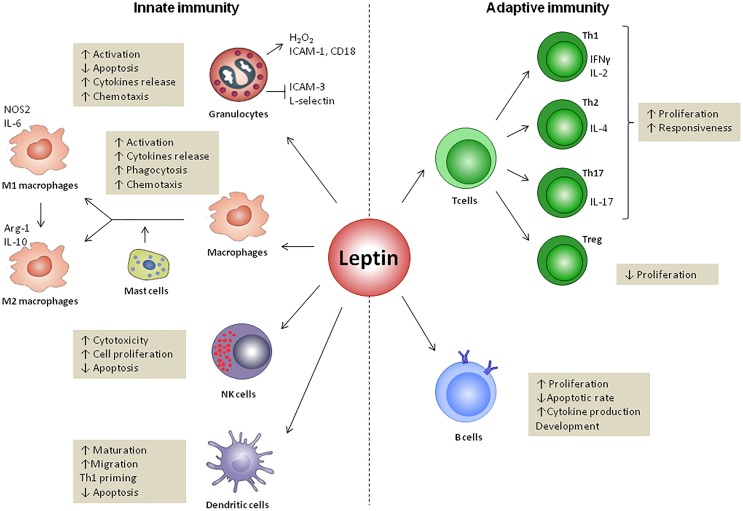
Leptin and Innate Immunity (Figure 3)
FIGURE 3.
Leptin effects on innate and adaptive immunity. Leptin regulates both innate and adaptive responses through modulation of immune cells survival and proliferation as well as its activity. In innate immunity, leptin increases the cytotoxicity of natural killer (NK) cells and promotes the activation of granulocytes, macrophages and DCs. Leptin also regulates the M1- or M2-phenotype polarization and modulates DCs, licensing them towards type 1 T helper cells (Th1) priming. In adaptive immunity, leptin increases the proliferation of naïve T cells and B cells while it reduces that of regulatory T cells (Treg). Leptin promotes the switch towards a pro-inflammatory Th1 (which secretes IFNγ) rather than anti-inflammatory Th2 (which secretes IL-4) phenotype, and facilitates Th17 responses. Finally, leptin activates B cells to secrete cytokines and modulates B cell development.
Granulocytes (Neutrophils, Eosinophils, and Basophils)
Human polymorphonuclear neutrophils express LEPR (Caldefie-Chezet et al., 2001), but only the short-form (Ob-Ra) was been detected (Zarkesh-Esfahani et al., 2004). Although the short-form of LEPR lacks most of the intracellular domain of the receptor, it is enough to signal through MAPK pathways, enhancing CD 11b expression and preventing apoptosis, but not through JAK-STAT pathways as long-form of LEPR (Bjorbaek et al., 1997; Zarkesh-Esfahani et al., 2004). Leptin is likely to act as a survival cytokine for neutrophils. At 500 nM, leptin delayed the cleavage of Bid and Bax, mitochondrial release of cytochrome C and second mitochondria-derived activator of caspase, as well as the activation of caspase-3 and caspase-8 (Bruno et al., 2005). PI3K, NF-κB, and MAPK pathways were involved in the anti-apoptotic activity of leptin in human neutrophils in vitro (Bruno et al., 2005; Sun et al., 2013). Additionally, leptin (250 ng/ml) stimulated the release of oxygen radicals, such as superoxide anion and hydrogen peroxide, by PMNs (Caldefie-Chezet et al., 2001, 2003).
There is strong evidence for an effect of leptin on neutrophil chemotaxis and infiltration. Leptin (50 ng/ml) mediated the migration of human neutrophils in vitro, through activation of p38 MAPK and Src kinases (Montecucco et al., 2006), and by indirect mechanisms via TNF-α released by monocytes (Caldefie-Chezet et al., 2001; Zarkesh-Esfahani et al., 2004), having no secretagogue properties (no detectable [Ca2+]i mobilization, oxidant production, or β2-integrin upregulation) (Montecucco et al., 2006). Otherwise, leptin inhibits neutrophil chemotaxis to classical chemoattractants, like interleukin (IL)-8 (Montecucco et al., 2006). Murine neutrophils with Q223R LEPR mutation had reduced chemotaxis toward leptin (Naylor et al., 2014), while neutrophils from human volunteers and wild-type C57BL/6 mice migrated toward leptin in a dose-dependent manner, requiring JAK2/PI3K signaling (Ubags et al., 2014). Nevertheless, one study found that physiological concentrations of leptin (1–100 ng/ml) do not affect human neutrophils, and high leptin concentrations induced survival and changes in neutrophils proteome, but no effect on chemotaxis was observed (Kamp et al., 2013). In vivo studies clarified the effect of leptin in neutrophils. It was observed that neutrophil populations were enhanced in rats with high-fat-diet induced obesity, compared with control diet rats (do Carmo et al., 2013), and neutrophils from obese subjects displayed elevated superoxide release and chemotactic activity (Brotfain et al., 2015). Furthermore, leptin administration (50 μg) increased pulmonary neutrophilia in Escherichia coli pneumonia murine model as well as in healthy mice (Ubags et al., 2014).
Alike neutrophils, both human eosinophils and basophils expressed LEPR on the cell surface (Bruno et al., 2005; Suzukawa et al., 2011). In eosinophils, leptin (50 ng/ml) enhanced the release of pro-inflammatory cytokines IL-1β and IL-6, and chemokines IL-8, growth-related oncogene-α and MCP-1 (Wong et al., 2007). It also modulated the surface expression of adhesion molecules; in particular, up-regulates ICAM-1 and CD18, and suppress ICAM-3 and L-selectin (Wong et al., 2007). Treatment of human eosinophils with recombinant leptin in vitro delayed apoptosis via JAK, NF-κB, and p38 MAPK signaling pathways, suggesting leptin as a survival cytokine (Wong et al., 2007), similar to neutrophils (Bruno et al., 2005). Furthermore, leptin also stimulated chemokinesis (Wong et al., 2007) and enhanced chemotactic migration of eosinophils isolated from human peripheral blood, in a dose-dependent manner, however, the underlying mechanisms remain unclear (Kato et al., 2011). In obese individuals, eosinophils demonstrated greater adhesion and chemotaxis toward eotaxin and RANTES (CCL5), compared with non-obese healthy volunteers (Grotta et al., 2013).
In human basophils, leptin treatment (10 nM) induced a strong migratory response, promoted the secretion of type 2 cytokines IL-4 and IL-13, and up-regulated the cell surface expression of CD63, which may have an exacerbating action on allergic inflammation (Suzukawa et al., 2011). Moreover, leptin is a survival-enhancing factor of human basophils, as aforementioned for eosinophils and neutrophils. Although leptin was a weak effect on direct induction of basophil degranulation, it potently primed basophils for enhanced degranulation in response to aggregation of IgE or its high-affinity receptor FcεRI (Suzukawa et al., 2011).
Altogether, these findings suggest leptin as a potent activator of neutrophils, eosinophils, and basophils through its positive action in cell survival, cytokines release and chemotaxis.
Monocytes and Macrophages
Both isoforms of LEPR are expressed in PBMCs, being lower in cells from obese individuals compared with lean subjects (Tsiotra et al., 2000). Functional LEPR was also expressed in macrophages (O’Rourke et al., 2001). The effect of leptin on monocytes and macrophages has been well-established since its first evidence in Santos-Alvarez et al. (1999). Leptin promoted the proliferation of human circulating monocytes in vitro as well as its activation through induction of TNF-α and IL-6 production, and stimulation of surface markers, namely CD25, HLA-DR, CD38, CD71, CD11b, CD11c, and CD16 (Santos-Alvarez et al., 1999; Cannon et al., 2014). Moreover, leptin potentiated the stimulatory effect of LPS or PMA on human monocytes (Santos-Alvarez et al., 1999), and increased CCLs in cultured murine macrophages, being JAK2-STAT3 signaling pathway involved (Kiguchi et al., 2009). Leptin (625 nM) also augmented the production of several inflammatory mediators in monocytes/macrophages, such as interleukin 1 receptor antagonist (IL-1Ra) (Gabay et al., 2001), interferon-c-inducible protein (Meier et al., 2003), leukotrienes (Mancuso, 2004), nitric oxide (Dixit et al., 2003), and pro-inflammatory cytokines, namely TNF-α, IL-6, IL-1β, and resistin (Tsiotra et al., 2013; Scotece et al., 2014; Inzaugarat et al., 2017). By contrast, it was reported that 1 μg/ml leptin had no effect on IL-1β secretion but enhanced IL-18 in the THP-1 murine monocytic cell line. These apparent discrepancies could be species-specific (human vs. murine cells) and/or leptin treatment-dependent (1 μg/ml for 24 h vs. 1 μg/ml for 3 h). Additionally, recombinant leptin increased the expression of TLR2, but not TLR4, in human monocytes (Jaedicke et al., 2013).
A dose-dependent effect of leptin as a trophic factor to prevent apoptosis was found in serum-deprived human monocytes, being this effect mediated by the p42/p44 MAPK pathways (Najib and Sánchez-Margalet, 2002). Leptin (2 nM) stimulated the oxidative burst in monocytes (Sánchez-Pozo et al., 2003), and increased LPL expression through oxidative stress- and PKC-dependent pathways (Maingrette and Renier, 2003). Moreover, leptin promoted the phagocytosis of apoptotic cells by macrophages from lupus mice, via modulation of cAMP levels (Amarilyo et al., 2014). Leptin also promoted a defensive environment against Leishmania donovani infection by induction of macrophage phagocytic activity and intracellular ROS generation (Dayakar et al., 2016). Accordingly, macrophages from knocked-out LepR Tyr 985 mice presented reduced phagocytosis and killing activity of Klebsiella pneumoniae that was associated with diminished ROS production (Mancuso et al., 2012). It has been demonstrated that leptin-mediated protein radical formation, tyrosine nitration and activation of KCs are caused by peroxynitrite formation, which exacerbated NASH in diet-induced obese mice (Chatterjee et al., 2013). Concerning to chemoattractive activity, it was verified that leptin induced in vitro chemotactic responses for monocytes and macrophages (Curat et al., 2004; Gruen et al., 2007), via intracellular calcium influx, JAK/STAT, MAPK and PI3K pathways (Gruen et al., 2007). However, it has been reported that hematopoietic LEPR deficiency in mice did not change macrophage accumulation in WAT after diet-induced obesity versus wild-type mice (Gutierrez and Hasty, 2012). Likely, compensatory in vivo effects of other cytokines (like IL-1 or TNF-α) present in WAT could occur in obese individuals and which are absent in in vitro assays.
Leptin treatment (50 ng/ml) of human macrophages in culture, induced ‘alternatively activated’ or M2-phenotype surface markers, but they were able to secrete M1-typical cytokines (TNF-α, IL-6, IL-1β, IL-1ra, IL-10, MCP-1, and macrophage inflammatory protein 1-alpha (MIP-1α)), suggesting a role for leptin in the phenotype of macrophages found in adipose tissue (Acedo et al., 2013). In macrophages, leptin also triggered catecholamine-dependent increases in cAMP-histone deacetylase 4 signaling pathway, that reduced inflammation in adipose tissue (Luan et al., 2014). Additionally, leptin increased the expression of LEPR in M2 macrophages and stimulated IL-8 expression via p38 and ERK signaling pathways (Cao et al., 2016). In tumor-associated macrophages, leptin induced the expression of IL-18 via NF-kB, possible contributing to tumor progression (Li K. et al., 2016).
Macrophages are indirectly regulated by leptin through mast cells (Zhou et al., 2015). In particular, leptin expression was reduced in both human and mouse mast cells from lean adipose tissue compared with obese individuals. Leptin deficiency led to the anti-inflammatory activity of mast cells and, consequently, to a shift in macrophage polarization from M1 to M2; in vitro co-cultures of mast cells with BMDM increased IL-4-mediated arginase-1 and IL-10 expression, and suppressed LPS-mediated iNOS and IL-6 expression (Zhou et al., 2015). Furthermore, reduction of mast cells in leptin-deficient ob/ob mice exacerbated obesity and diabetes, indicating an important role of mast cells in obesity-related inflammation through its reactivity to leptin levels (Zhou et al., 2015).
Biologic drugs used for the treatment of psoriatic arthritis, namely adalimumab (an anti-TNF-α monoclonal antibody) and ustekinumab (a monoclonal antibody against the p40 subunit of IL-12 and IL-23), augmented LEPR expression in THP-1 human macrophages (Voloshyna et al., 2016). However, only ustekinumab was able to increase the expression of leptin, suggesting a novel mechanism for this biological drug. Further mechanistic studies focused on the leptin pathway could have potential therapeutic action in common obesity-related complications of psoriasis (Voloshyna et al., 2016). The establishment of LepR-deficient macrophage cell line DB-1, derived from differentiated bone marrow cells of Lepr-knockout mice, provide a powerful tool to study the role of leptin and its receptor in obesity-associated inflammation and immune system deregulation (Dib et al., 2016).
NK Cells
The role of leptin in regulating NK cell development and activation was first verified in obese Lepr-deficient (db/db) mice, which showed decreased NK cell function (Tian et al., 2002). In this animal model, the population of NK cells in bone marrow was impaired through an increase in apoptotic rate, and recombinant leptin (200 ng/ml) significantly enhanced the survival of immature NK cells from wild-type mice via modulation of Bcl-2 and Bax gene expression (Lo et al., 2009). Furthermore, leptin administration (500 μg/kg) led to a higher activity of NK cells in lean animals (Nave et al., 2008). Consistently, human NK cells expressed functional long- and short-form of LEPR that influenced NK cell cytotoxicity through STAT3 activation and, consequently, transcription of genes encoding IL-2 and perforin (Zhao et al., 2003).
The above-mentioned results indicated that leptin signaling is required for normal NK cell immune function. However, there are some controversial findings concerning the time of leptin treatment in vitro. Short-term stimulation of human NK cells with leptin (50 nM) raised the secretion of IFNγ and cytotoxicity (Wrann et al., 2012; Laue et al., 2015). By contrast, long-term exposure to leptin decreased NK cell proliferation and immune function (Wrann et al., 2012). Obesity is partially characterized by a state of long-term, highly elevated leptin exposure, and NK cells from obese animals were significantly resistant to leptin stimulation (Nave et al., 2008), which could explain the functional desensitization of NK cells after long-term exposure. Accordingly, exposure of NK-92 human cell line to hyperleptinemia (similar to that observed in obese individuals) led to metabolic activation of NK-92 cells after 24 h, but there is a reduction of cell metabolism after 96 h (Lamas et al., 2013). Furthermore, obese individuals have lower NK function compared to lean individuals (Laue et al., 2015) and, after weight loss, the decrease of plasma leptin levels is accompanied by a restoration of IFNγ production by NK cell (Jahn et al., 2015; Bähr et al., 2017; Favreau et al., 2017).
Overall, leptin signaling seems to be necessary for normal NK cell immune function, increasing the immune activity and cell proliferation, and reducing the apoptotic rate of NK cells. Long-term exposure to hyperleptinemia, observed in obesity, has been associated with decreased NK immune activity possibly due to the development of leptin resistance. Further studies are needed to better understand the correlation between leptin levels and NK cell development and function, as well as the potential implications in obesity.
Dendritic Cells
Human DCs, both immature and mature DCs, present functional active LEPR with the capacity to signal STAT-3 phosphorylation (Mattioli et al., 2009). Leptin (10 nM) acted as an activator of human DCs, evidenced by up-regulation of IL-1β, IL-6, IL-12, TNF-α and MIP-1α production, improvement of immature DCs migration (Mattioli et al., 2008; Al-Hassi et al., 2013) and their chemotactic responsiveness, licensing them toward Th1 priming (Mattioli et al., 2008). Moreover, leptin treatment promoted DC survival through decreased apoptosis via activation of NF-κB and PI3K-Akt signaling pathways, with a parallel increase of bcl-2 and bcl-xL gene expression (Lam et al., 2006; Mattioli et al., 2009).
Lepr-deficient db/db mouse bone marrow culture displayed a reduced number of DCs, attributable to dysregulation of Bcl-2 genes and a consequent increase of apoptosis (Lam et al., 2006). Moreover, DCs from db/db mice possessed markedly reduced expression of co-stimulatory molecules and a Th 2-type cytokine profile, with a poor capacity to stimulate allogeneic T cell proliferation (Lam et al., 2006). Consistently, db/db DCs demonstrated down-regulation of PI3K/Akt and STAT-3 pathways (Lam et al., 2006). Lep-deficient ob/ob mice presented a reduced expression of DC maturation markers (CD40, CD80, and CD86), decreased production of inflammatory cytokines (IL-12, TNF-α, and IL-6), and augmented TGF-β production, but ob/ob mice-derived DCs were more efficient in inducing Treg or Th17 cells than wild-type animals (Moraes-Vieira et al., 2014). In DCs from ob/ob mice, leptin deficiency resulted in defective antigen presentation function toward Leishmania donovani, which was not reversed by leptin treatment (Maurya et al., 2016). Conversely, one report verified no changes in the phenotype, activation, antigen processing or presentation of DCs from leptin-knockout mice, but these cells showed an enhanced ability to activate T cells, suggesting that leptin may dampen T-cell responsiveness in the physiological context (Ramirez and Garza, 2014). Diet-induced obesity in mice fed with HFD results in an elevation of serum leptin levels and splenic CD11c+ DCs, with diminished DC cell stimulatory capacity, being these effects distinct from that caused by HFD alone in obese-resistant mice (Boi et al., 2016).
Altogether, these data demonstrated the important role of leptin in DC activation, chemoattraction, and survival, with possible implications in DC maturation and migration. Given the ability of DCs to orchestrate immune response and promote potent immunogenic responses through activation of T cell immunity, DCs-based immunotherapies to elicit immunity against cancer and infectious diseases are currently being developed. In particular, DCs can be differentiated ex vivo, exposed to antigens and induced to mature in the presence of adjuvants. Then, the mature DCs are injected into the patient and migrate to the lymph nodes to present antigens to T cells. Thus, the modulation of DCs maturation and activity by leptin is of most importance considering a potential application of leptin in immunotherapeutic approaches and as novel adjuvant immunopotentiator in vaccination protocols employing ex vivo generated autologous DCs.
Leptin and Adaptive Immunity (Figure 3)
The role of leptin in adaptive immunity has first evidenced working with ob/ob and db/db mice, which showed thymus atrophy, T-cell lymphopenia, and impaired delayed-type hypersensibility (Lord et al., 1998; Howard et al., 1999; Matarese, 2000). Moreover, chronic leptin administration (1 μg/g body weight) reversed immunosuppressive status and thymic atrophy of ob/ob mice (Lord et al., 1998, Nature; Howard et al., 1999, J. Clin. Inv.). Since then, the role of leptin in T and B cell populations have been extensively studied.
T Cells
T lymphocytes expressed the long form of LEPR (higher in peripheral CD4+ than in CD8+ T cells) (Lord et al., 1998; Kim et al., 2010), with signaling capacity to activate JAK-STAT pathway (Sanchez-Margalet and Martin-Romero, 2001). Consequently, leptin modulated cell proliferation, responsiveness, and polarization of T cells. Leptin dose-dependently promoted the proliferation of human naïve (CD45RA+) CD4+ T cells, whereas it minimally affected memory (CD45RO+) CD4+ T cells proliferation (Lord et al., 1998, 2002). Additionally, morbidly obese children, who were congenitally deficient in leptin, presented a decreased number of circulating CD4+ T cells, as well as impaired T cell proliferation and cytokine release, which were reversed by administration of recombinant human leptin (Farooqi et al., 2002). Moreover, leptin inhibited autophagy in human CD4+CD25- conventional T cells via mTOR pathway (Cassano et al., 2014), which emerged as the potential link between immunity and nutritional status (Procaccini et al., 2012).
T Helper Cells
Leptin also promoted CD4+ T cell polarization toward a Th1 response (which secretes IFNγ and IL-2) rather than Th2 response (which secretes IL-4) (Martín-Romero et al., 2000). Accordingly, under Th2-polarizing conditions, the in vitro leptin treatment decreased IL-4-producing T cells and inhibited T cell proliferation (Batra et al., 2010). However, it was recently reported that in vivo leptin-deficiency attenuated allergic airway inflammation and that high leptin levels associated with obesity promoted proliferation and survival of Th2 lymphocytes, as well as the production of type 2 cytokines, altogether contributing to allergic responses (Zheng et al., 2016). Besides that, leptin was involved in thymus morphology and functions (Lamas et al., 2016), particularly in thymocyte differentiation of double positive CD4+CD8+ T cells into single positive CD4+ T cells (Kim et al., 2010).
IL-17-producing Th cells (Th17) have a crucial role in the promotion and maintenance of inflammatory and autoimmune pathologies. Leptin was demonstrated to increase Th17 population and responsiveness in SLE, via retinoic acid-related orphan receptor (ROR)γt (Yu et al., 2013; Fujita et al., 2014; Reis et al., 2015). In collagen-induced arthritis mouse model, articular injection of leptin (5 μg) increased the number of Th17 in the joint tissue, resulting in exacerbating joint inflammation, and consequently early onset of arthritis and increased disease severity (Deng et al., 2012). Leptin, in concentrations similar to that found in blood during pregnancy, promoted the differentiation of peripheral blood CD4+ cells to Th17 cells, but suppressed the formation of Treg cells in vitro (Orlova and Shirshev, 2014). CD4+ T cell-derived leptin, but not plasma leptin, were positively correlated with the percentage of Th17 cells or RORγt levels in chronic lymphocytic thyroiditis, an organ-specific autoimmune disease (Wang et al., 2013). Furthermore, Lepr-deficient CD4+ T cells verified a reduced capacity for Th17 differentiation, via down-regulation of STAT3 activation (Reis et al., 2015).
T Regulatory Cells (Treg)
Leptin also regulated CD4+CD25+ Treg proliferation (Matarese et al., 2010). Treg lymphocytes play a critical role in controlling the inappropriate immune responses characteristic of autoimmune diseases and allergy. In humans, leptin negatively affected the proliferation of Foxp3+CD4+CD25+ Treg; in vitro leptin neutralization, during anti-CD3 and anti-CD28 stimulation, led to the proliferation of the isolated human Treg cell (De Rosa et al., 2007). Obese individuals presented a reduced number of CD4+CD25+CD127-Foxp3+ Treg cells, which was correlated with body weight, BMI, and plasma leptin levels (Wagner et al., 2013). Moreover, leptin-deficient mice presented an increased percentage of peripheral Treg, compared with wild-type mice, which is reversed after leptin administration (De Rosa et al., 2007). It was verified that leptin played an important role in Treg dysfunction in patients with pulmonary arterial hypertension (Huertas et al., 2016). Accordingly, Lepr-deficient rats developed less severe hypoxia-induced pulmonary hypertension and were protected against decreased Treg function after exposure to hypoxia (Huertas et al., 2016). In SLE, the disease-associated higher leptin serum levels were negatively correlated with disease severity and number of Treg cells (Ma et al., 2015; Margiotta et al., 2016; Wang et al., 2017), and fasting-induced hypoleptinaemia was related to Treg population recovery in lupus-prone mice (Liu et al., 2012). Leptin-deficient ob/ob mice and a mouse model of lupus with leptin deficiency demonstrated increased frequency of Tregs cells (Fujita et al., 2014; Lourenço et al., 2016). These data evidenced the potential of anti-leptin-based approaches for immune system-dysregulated pathologies associated with reduced Treg function, such as SLE, obesity, T2DM, and metabolic syndrome.
T cell metabolism is directly related to its function (MacIver et al., 2013); effector T cells, such as Th1 and Th17, demand a high glycolytic metabolism to fuel proliferation and function, while Treg cells require oxidative metabolism to fuel suppressive activity. Recently, leptin was found to directly promote T-cell glycolytic metabolism and consequently induce Th17 cell differentiation, being Treg cells unchanged, in a mouse model of experimental autoimmune encephalomyelitis (Gerriets et al., 2016). Leptin regulated glucose metabolism partly by up-regulation of glucose transporter Glut1 (Saucillo et al., 2014). Moreover, fasting led to decreased ability of T cells to secrete IL-2 and IFNγ, and inability to up-regulate glucose uptake and glycolytic flux (Saucillo et al., 2014), while Treg expansion was increased (Liu et al., 2012); leptin administration (1 μg/g body weight) rescued peripheral T cell function and metabolism in fasted mice (Saucillo et al., 2014). Likely, fasting was extensively reported to be associated with immune deficiency and increased susceptibility to infection (Gerriets and MacIver, 2014). Thus, leptin seems to provide a key link between nutritional status and inflammatory T cell responses (Gerriets et al., 2016; De Rosa et al., 2017).
Altogether, these data revealed the ability of leptin to increase immune activity by modulation of T cell number and function. Leptin can promote proliferation of naive T cells, as well as Th1 and Th17 proliferation and cytokine production. Moreover, leptin decreases Treg cell proliferation. Considering the regulatory effects of leptin on Th17 and Treg populations, revoking leptin signaling might be a potential therapeutic approach for inflammation and autoimmunity.
B Cells
In contrast to macrophages and T cells, little is known about the role of leptin in the B lymphocytes development and function. B cells expressed the long form of the LEPR, suggesting a direct effect of leptin on B cell function (Busso et al., 2002). Accordingly, db/db and ob/ob presented a reduced number of peripheral blood and bone marrow B lymphocytes, which was recovered after leptin treatment (Bennett et al., 1996; Claycombe et al., 2008). Conversely, db/db mice presented an increased absolute number of B cells in the peritoneal cavity (Jennbacken et al., 2013), and the increase of leptin was correlated with a decrease in B cells of mice with unbalanced diets (carbohydrate-rich and fat-rich) (Martínez-Carrillo et al., 2015). Thus, further investigation is needed to better clarify the role of leptin in lymphopoiesis. Leptin promoted B cell homeostasis through inhibition of apoptosis and induction of cell cycle entry via Bcl-2 and cyclin D1 activation (Lam et al., 2010). Furthermore, leptin dose-dependently activated human peripheral blood B cells, inducing the secretion of pro-inflammatory cytokines, namely TNF-α and IL-6, and the anti-inflammatory cytokine IL-10, via JAK-STAT and p38MAPK-ERK1/2 signaling pathways (Agrawal et al., 2011). Likewise, leptin (50 ng/ml) activated and induced the production of higher amounts of TNF-α, IL-6, and IL-10 by B cells from aged subjects compared to young individuals (Gupta et al., 2013), which is associated with leptin-mediated STAT3 phosphorylation (Gupta et al., 2013; Frasca et al., 2016).
Leptin can also modulate B cell development – decrease pro-B, pre-B and immature B cells and increase mature B cells- in bone marrow of fasted mice, characterized by low serum leptin levels (Tanaka et al., 2011). Leptin administration reversed the starvation-induced lymphopenia of bone marrow B cells, indicating an important role of central leptin in the immune system (Tanaka et al., 2011; Fujita et al., 2012). Moreover, leptin might regulate B cell activity in obesity (Nikolajczyk, 2010; Frasca et al., 2016). In particular, B cells were described to accumulate in murine VAT and to critically regulate T2DM-associated inflammation through activation of CD8+ and Th1 cells and release of pathogenic antibodies (Winer et al., 2011; DeFuria et al., 2013).
In summary, leptin can increase B cell population by augmenting proliferation and reducing the apoptotic rate, activate B cell to secrete pro-, anti- and regulatory cytokines, and also modulate B cell development.
Leptin and Immune-Metabolic Pathologies
Leptin and Obesity-Associated Metabolic Disorders
Obesity is associated with life-threatening co-morbidities, including insulin resistance, T2DM, NAFLD and steatohepatitis (NASH) (Lebovitz, 2003; Kamada et al., 2008; Klöting and Blüher, 2014; Fasshauer and Blüher, 2015). Adipokines, in particular leptin, mediate the crosstalk between adipose tissue and metabolic organs (especially liver, muscle, pancreas and central nervous system) (Cao, 2014). Thus, leptin has emerged as a significant pathological component in the development of metabolic disorders (DePaoli, 2014).
Type 2 Diabetes Mellitus
T2DM is the most significant obesity-associated metabolic disorder and their prevalence is increasing worldwide in parallel (Bhupathiraju and Hu, 2016). Leptin has been proposed as a therapeutic target of T2DM, for its impact on food intake and body weight as well as its potential to improve insulin action (Kalra, 2009). Interestingly, leptin-deficient mice (Pelleymounter et al., 1995) and human (Farooqi et al., 1999, 2002; Ozata et al., 1999) have diabetic features, which were reversed with leptin replacement. The anti-diabetic effect of leptin is mediated by activation of IRS-PI3K pathway that improved insulin sensitivity in peripheral tissues (Morton et al., 2005). Activation of JAK2/IRS/PI3K/Akt signaling pathway by leptin and insulin triggers the translocation of glucose transporter type 4 (GLUT4) from cytosol to cell surface, and glucose uptake (Benomar et al., 2006; Zhao and Keating, 2007). Moreover, in the liver, leptin deficiencies decrease AMPK activity (Namkoong et al., 2005), which is also involved in glucose homeostasis regulation (Schultze et al., 2012). Leptin has also been implicated in the regulation of insulin secretion by pancreatic β-cells (Kulkarni et al., 1997), as well as in peripheral insulin resistance (Silha et al., 2003; Yadav et al., 2011). However, clinical trials to evaluate the potential of leptin monotherapy in obese humans with T2DM failed to demonstrate therapeutic activity acutely or chronically, with no observation of important weight loss or metabolic improvements (insulin sensitization, amelioration of glucose and lipid metabolism) (Mittendorfer et al., 2011; Moon et al., 2011; Wolsk et al., 2011). In this context, unresponsiveness to leptin – leptin resistance, caused by hyperleptinemia observed in obese humans, should be considered (Frederich et al., 1995). Further understanding of leptin resistance mechanisms could enable new leptin targeted therapies for obesity and diabetes in specific subsets of patients. In fact, leptin therapy improved the diabetic condition in children (Farooqi et al., 1999, 2002) and adults (Licinio et al., 2004) with familial leptin deficiency, and in lipoatrophic diabetes (Oral et al., 2002).
Importantly, T2DM is associated with altered components of immune system, including modified levels of specific chemokines and cytokines, changed number and activation status of leukocytes populations and increased apoptosis and tissue fibrosis (Donath, 2014), all promoted by obesity-associated inflammation in adipose tissue (Donath and Shoelson, 2011). Given the modulator action of leptin in innate and adaptive immune system (deeply described above), it is rational to see leptin as a linker of T2DM development, not only with metabolism but also with inflammation. Indeed, in patients with newly diagnosed T2DM, leptin levels were correlated with CRP, an inflammatory marker broadly evaluated for its association with risk factors for T2DM pathology (Morteza et al., 2013).
Non-alcoholic Fatty Liver Disease
NAFLD, the major cause of chronic liver illness in developed countries, comprises a wide group of pathologies primary caused by a buildup of fat in the liver that spans from simple steatosis to NASH, liver fibrosis, cirrhosis, and hepatocellular carcinoma (Tiniakos et al., 2010). Given that NAFLD is increasing worldwide and it is associated with high-incident extra-hepatic complications such as obesity, T2DM, cardiovascular diseases and chronic kidney disease (Byrne and Targher, 2015; Polyzos et al., 2015), great efforts have been made in the last years to unravel the mechanisms underlying the disease pathophysiology and further development of effective NAFLD therapies.
Hepatic inflammation and hepatocyte injury and death are hallmarks of NAFLD/NASH. Fat overload by hepatocytes causes lipotoxicity and the release of DAMPs which activated Kupfer cells (KC; specialized liver macrophages) and HSC promoting inflammation and fibrosis, respectively. KC activation plays a central role in NAFLD pathophysiology through the production of pro-inflammatory cytokines and chemokines such as TNF-α, IL-1β, IL-6, CCL2 and CCL5, that contributed to leukocyte infiltration and inflammatory necrosis of hepatocytes, and fibrogenesis (Arrese et al., 2016). Dysbiosis of the gut microbiota may also conduct to KC activation through PAMPs, which originate in the gut and reach liver via portal circulation due to altered intestinal permeability. Other immune cells have been implicated in the NAFLD pathophysiology, although its role is less clear (Arrese et al., 2016). NK cells were impaired in experimental NASH, while natural killer T cells (unique immune cell subtype that expresses NK cells surface markers as well as T-cell antigen receptor) are depleted in steatosis but increased later during disease progression likely leading to inflammation and fibrosis in NASH via the production of IL-4, osteopontin, and IFN-γ (Tajiri and Shimizu, 2012; Tian et al., 2013). Neutrophils exacerbate the ongoing inflammation through macrophage recruitment and cell damage via the release of myeloperoxidase, ROS, and elastase (Xu R. et al., 2014). The role of DCs in NASH is complex and somehow controversial. DCs rapidly infiltrate into the liver in experimental NASH exhibiting an activated immune phenotype, but its depletion exacerbates hepatic inflammation (Tacke and Yoneyama, 2013; Arrese et al., 2016). B- and T-cells also contributed to hepatic inflammation via secretion of pro-inflammatory cytokines that stimulated KC activation (Arrese et al., 2016).
Considering the strong metabolic and inflammatory components of NAFLD, leptin is regarded as a key regulator of NAFLD physiopathology (Polyzos et al., 2015). Leptin seems to feature a dual activity in NAFLD experimental models by exerting an early protective anti-steatosis effect in the initial stages of the disease, and a late pro-inflammatory and pro-fibrogenic action, when the disease persists or progress (Polyzos et al., 2015). In leptin-resistant Zucker fa/fa diabetic fatty rats, the expression of SREBP-1c (master regulator of glucose metabolism, and lipid and fatty acid production) is increased in liver (Kakuma et al., 2000), and infusion of adenovirus-leptin decreased hepatic triglyceride synthesis and β-oxidation via SREBP-1c down-regulation and PPARα up-regulation (Lee et al., 2002), thus preventing hepatic lipid accumulation.
Through anti-steatotic effect, leptin can ultimately lead to hepatic detrimental effects. Leptin activated HSCs, leading to up-regulation of pro-inflammatory and pro-angiogenic factors expression (like angiopoietin-1 and vascular endothelial growth factor), as well as collagen α1 and tissue inhibitor of metalloproteinase-1, ultimately acting as hepatic fibrogenesis inducer (Polyzos et al., 2015). Activated HSCs were able to secrete leptin, thus establishing a vicious cycle that further promotes liver fibrosis (Polyzos et al., 2015). Moreover, leptin was reported as potent HSCs mitogen and to prevent HSCs apoptosis, hence promoting the pathogenesis of hepatic fibrosis. Leptin (200 nM) increased the expression of TGF-β1 in KCs and sinusoidal endothelial cells, and connective tissue growth factor in KCs (Ikejima et al., 2002), being KCs-HSCs cross-talk proposed for liver fibrosis (Wang et al., 2009). Additionally, leptin is involved in macrophage-mediated KCs activation via induction of oxidative stress in macrophages (Chatterjee et al., 2013). Compared to healthy subjects, NAFLD patients demonstrated an increased leptin-stimulated TNFα and ROS production in peripheral monocytes, as well as IFNγ production in circulating CD4+ cells (a marker of Th1 differentiation) (Inzaugarat et al., 2017). Altogether, these data elucidated the role of leptin in NAFLD by modulation of HSCs, KCs, and inflammatory cells response.
In ob/ob mice, the congenital absence of leptin abrogated the development of CCl4-induced hepatic fibrosis comparing to lean littermates, which is reverted by leptin treatment (100 ng/ml) (Saxena et al., 2002). Likewise, xenobiotics- or thioacetamide-induced hepatic fibrosis was prevented in Zucker fa/fa rats, being involved the activation of HSCs and expression of procollagen-I and TGF-β1 (Ikejima et al., 2005). In humans, the role of leptin is controversial. Although leptin serum levels were initially related with hepatic steatosis but not with necroinflammation or fibrosis (Chitturi et al., 2002), later studies failed to demonstrate any significant association (Tsochatzis et al., 2009). Recombinant leptin has been successfully used in the treatment of insulin resistance and hepatic steatosis in patients with lipodystrophy and NASH (Oral et al., 2002; Petersen et al., 2002; Javor et al., 2005). However, large-scale and well-designed prospective cohort studies are necessary to deeply elucidate the role of leptin in hepatic lipid handling, inflammation, and fibrosis along with the identification of NAFLD patients subsets that may benefit from therapies directed to leptin system.
Leptin in Rheumatic Diseases
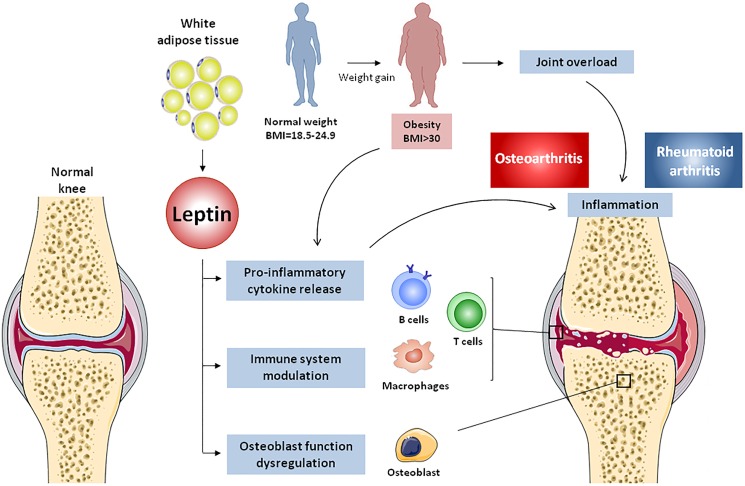
Leptin has been described as a key factor in the pathophysiology of rheumatic diseases due to its capability to modulate bone and cartilage metabolism and to influence innate and adaptive immune responses (Figure 4).
FIGURE 4.
Effects of adipose tissue-derived leptin on osteoarthritis and rheumatoid arthritis. Body weight gain, accompanied by white adipose tissue expansion, lead to obesity and subsequent increase of mechanical load, resulting in cartilage degradation and osteoarthritis onset. Adipose tissue-derived leptin causes osteoblast dysregulation in subchondral bone, thus promoting joint destruction. Additionally, leptin induces pro-inflammatory cytokine release from innate and adaptive immune cells, generating an inflammatory environment that prompts cartilage damage and rheumatoid arthritis.
Osteoarthritis
Osteoarthritis, the most common joint disease, is a painful and debilitating illness characterized by progressive degeneration of articular joints. Initially seen as simply “wear and tear” disease, OA is currently considered a complex and multifactorial pathology triggered by inflammatory and metabolic imbalances that affect the entire joint structure (articular cartilage, meniscus, ligaments, bone, and synovium) (Loeser et al., 2012). Leptin levels are increased in serum, infrapatellar fat pad (IPFP), synovial tissues, and cartilage of OA patients compared to healthy individuals (Dumond et al., 2003; de Boer et al., 2012; Conde et al., 2013). Accordingly, leptin-deficient or LepR-deficient mice developed extreme obese phenotype without increased incidence of knee OA, suggesting that leptin signaling is essential to the development and progression of obesity-associated OA (Griffin et al., 2009). Furthermore, long form of LEPR was found to be expressed in human cartilage cells – chondrocytes (Figenschau et al., 2001).
Some initial findings suggested an anabolic role of leptin in cartilage. In particular, exogenous leptin administration (30 μg) stimulated proteoglycan and growth factors (insulin-like growth factor-1 and TGF-β) synthesis in rat knee-joint cartilage (Dumond et al., 2003). However, most of the studies reported a catabolic role of leptin underlying OA pathogenesis. A recent study determining the gene expression profile of leptin-induced articular rat cartilage by microarray analysis, associated the up-regulation of matrix metalloproteinases (MMPs), inflammatory factors, growth factors and osteogenic genes with leptin-induced OA phenotype (Fan et al., 2018). Our group demonstrated that leptin (400 or 800 nM), in synergy with IFNγ or IL-1β, activated type 2 nitric oxide synthase (NOS2) via JAK2, PI3K and MAPK (MEK1 and p38) pathways, in cultured human and murine chondrocytes (Otero et al., 2003, 2005, 2007). Nitric oxide (NO), is a well-known pro-inflammatory mediator which lead to joint degradation through induction of chondrocyte phenotype loss, apoptosis and metalloproteinase (MMP) activity (Rahmati et al., 2016). Leptin (800 nM), alone or in combination with IL-1β, also induced the expression of COX-2 and the production of PGE2, IL-6, and IL-8 in cartilage explants of OA patients and human primary chondrocytes (Vuolteenaho et al., 2009; Gomez et al., 2011), revealing that leptin contributed to the pro-inflammatory environment of OA cartilage. It was also demonstrated that leptin (500 ng/ml) enhanced IL-6 production, mediated by chondrocyte-synovial fibroblast cross-talk, in OA patients (Pearson et al., 2017). Moreover, leptin modulated the production of inflammatory mediators by immune cells. In particular, the production of IL-6, IL-8, and CCL3 were increased by leptin in CD4+ T cells from OA patients, but not from healthy subjects (Scotece et al., 2017); thus, demonstrating new insights into the role of leptin in the immune system and OA pathophysiology.
Leptin can directly induce the expression of MMPs that are involved in OA-related joint destruction, like MMP-1 (also known as interstitial collagenase), MMP-3 (also known as stromelysin), and MMP-13 (also known as collagenase), via NF-κB, PKC, and MAPK pathways (Bao et al., 2010; Koskinen et al., 2011; Hui et al., 2012). MMP-2 (72 kDa type IV collagenase), MMP-9, and disintegrin and metalloproteinase with thrombospondin motifs (ADAMTS) 4 and ADAMTS5, were also increased by leptin, while fibroblast growth factor 2 and proteoglycan were down-regulated (Bao et al., 2010; Conde et al., 2011b). Leptin (800 nM) can perpetuate the cartilage-degradation processes due to induction of VCAM-1, an adhesion molecule responsible for leukocyte and monocyte chemotaxis and infiltration to inflamed joints, via JAK2 and PI3K pathways in chondrocytes (Conde et al., 2012; Vestweber, 2015). SOCS-3 was pointed as a regulator of leptin-induced expression of MMP-1, -3, and -13, and pro-inflammatory mediators IL-6, NO and COX-2 (Koskinen-kolasa et al., 2016). Additionally, leptin increased the production of other adipokines, namely lipocalin-2, by human cultured chondrocytes (Conde et al., 2011a).
MicroRNAs, small single-stranded non-coding segments of RNA, are increasingly recognized as regulatory molecules involved in disease processes, including OA, inflammation, and obesity (Marques-Rocha et al., 2015; Deiuliis, 2016; Nugent, 2016). miR-27 was found to be decreased in OA chondrocytes and to directly targeted the 3′-untranslated region of leptin (Zhou et al., 2017). Furthermore, the injection of OA rats with miR-27 lentiviral overexpression vector resulted in decreased levels of IL-6 and -8, as well as MMP-9 and -13, thus indicating the protective action of miR-27 in OA, possibly by targeting leptin.
Chondrogenic progenitor cells as cartilage seed cells are crucial to maintain cartilage homeostasis and replace damaged tissue (Seol et al., 2012). Leptin (50 ng/ml) can reduce CPCs migratory ability and their chondrogenic potential, and augment CPCs osteogenic transformation, hence changing CPC differentiation fate (Zhao et al., 2016). CPC cell cycle arrest and senescence are also induced by leptin (Zhao et al., 2016). Furthermore, leptin influenced the regulation of bone metabolism through induction of abnormal osteoblast function, which is associated to joint destruction in OA patients (Findlay and Atkins, 2014; Conde et al., 2015). The augmented production of leptin by OA subchondral osteoblasts is related with in vitro elevated levels of alkaline phosphatase, osteocalcin, collagen type 1, and TGF-β1, all being responsible for dysregulated osteoblast function (Mutabaruka et al., 2010). Additionally, bone morphogenic protein (BMP)-2 is increased in leptin-stimulated human primary chondrocytes (Chang et al., 2015). Leptin also suppressed bone formation in vivo (Ducy et al., 2000), but there are some discrepancies with in vitro results.
Taking together, this evidence indicated a key role of leptin in OA pathophysiology by influencing pro-inflammatory status, cartilage catabolic activity, as well as cartilage and bone remodeling. However, studies in a large cohort of patients are needed to better clarify the leptin significance in the development and progression of OA.
Rheumatoid Arthritis
Rheumatoid arthritis is a chronic inflammatory joint illness characterized by synovial membrane inflammation and hyperplasia (“swelling”), production of autoantibodies, namely rheumatoid factor and anti-citrullinated protein antibody – autoimmune disease, destruction of cartilage and bone (“deformity”), and systemic features including skeletal, cardiovascular, pulmonary, and psychological complications (McInnes, 2011; Smolen et al., 2016). Evidencing the crucial role of immune system in RA pathology, RA-associated synovitis comprises both innate immune cells (like monocytes, DCs, and mast cells) and adaptive immune cells (like Th1, Th17, and B cells) (McInnes, 2011; Smolen et al., 2016). As described above, leptin modulates neutrophils chemotaxis, activates proliferation and phagocytosis of monocytes and/or macrophages, regulates NK cytotoxicity, induces proliferation of naïve T cells, promotes Th1 cell immune response and down-regulates Th2 cell immune response. Moreover, leptin modulates the activity of Treg cells, which are potent inhibitors of autoimmunity, thus having a potential implication in RA pathophysiology (Toussirot et al., 2015).
Several studies have found a positive correlation between serum and synovial leptin levels and RA pathology (Otero et al., 2006; Targońska-Stępniak et al., 2008; Yoshino et al., 2011; Olama et al., 2012), but there are controversial results (Anders et al., 1999; Popa et al., 2005; Hizmetli et al., 2007; Oner et al., 2015). Differing results may be due to the relatively small sample size, inconsistency of the baseline characteristics of participants (age, race, disease duration, BMI, …), co-existence of other auto-immune diseases, employment of different methods to measure leptin levels in RA patients, or underlying patients treatments that intervene with the endocrine system. The present consensus is that leptin levels are elevated in RA patients, and serum and synovial fluid levels of leptin were associated with disease duration and parameters of RA activity (Olama et al., 2012; Lee and Bae, 2016), although large cohorts studies are necessary. Experimental animal models of arthritis had demonstrated the leptin action in joint inflammation. In particular, compared with control mice, leptin-deficient mice presented a less severe antigen-induced arthritis, decreased levels of TNF-α and IL-1β in knees synovium, and an impaired antigen-specific T cell proliferative response with lower IFN-γ and higher IL-10 production, which indicates a shift toward Th2 cell response (Busso et al., 2002). Accordingly, injection of leptin (5 μg) into the knee joint of collagen-immunized mice augmented arthritis severity, accompanied by elevated synovial hyperplasia and joint damage through enhancement of Th17 cell response (Deng et al., 2012). In fact, clinical trials using a humanized anti-IL-17 monoclonal antibody added to oral disease-modifying anti-rheumatic drugs, demonstrated improved signs and symptoms of RA, indicating the therapeutic potential of IL-17-directed strategies (Genovese et al., 2010).
Since leptin modulates the immune system, as well as insulin resistance and metabolic disorders like metabolic syndrome and obesity, all RA-associated conditions, this adipokine represents an attractive therapeutic target for RA. Accordingly, reducing leptin levels in RA patients by fasting improved the clinical symptoms of the disease (Fraser et al., 1999). Amidst the possible therapeutic approaches to antagonize leptin actions in RA, are leptin mutants with antagonist activity, and monoclonal antibodies against human LEPR or leptin itself (Tian et al., 2014). Interestingly, clinical studies evaluating the effect of drug modulators of insulin sensitivity (affected by leptin levels as described above), such as PPARγ agonists, are ongoing to provide new potential treatment to improve the inflammatory status and cardiovascular outcome in RA patients (Chimenti et al., 2015). Further understanding of leptin mechanisms would be of utmost importance for RA treatment.
Hence, leptin can be pointed as a link between immune tolerance, metabolic function, and autoimmunity, and leptin signaling-directed strategies could provide future innovative therapies for autoimmune disorders like RA.
Systemic Lupus Erythematosus
Systemic lupus erythematosus is a chronic autoimmune disorder of unclear etiology characterized by hyperactive T and B cells, autoantibody production, deposition of immune complex, elevated blood levels of pro-inflammatory cytokines and multisystem organ damage, encompassing from mild manifestations (non-erosive arthritis or skin rash) to life-threatening complications (lupus nephritis, neuropsychiatric disorders, cardiovascular disorders, and metabolic syndrome). Although the pathogenesis of SLE is poorly understood, genetic, hormonal and environmental factors have been implicated in the onset of this heterogeneous disease, which predominantly affects women of childbearing age (Gatto et al., 2013; Liu and La Cava, 2014).
Several studies suggest the implication of adipokines, namely leptin, in the pathogenesis of SLE. Although some reports found no statistical association between disease activity and leptin levels (Li H.M. et al., 2016), recently, a meta-analysis of eighteen studies determined that serum/plasma leptin levels were significantly elevated in SLE patients (Lee and Song, 2018). Furthermore, leptin has been suggested as a player that affects the cardiovascular risk in SLE patients. Accordingly, leptin and HFD induced proinflammatory high-density lipoproteins and atherosclerosis in BWF1 lupus-prone mice, and leptin levels were correlated with BMI, disease activity index (SLEDAI), as well as insulin and CRP levels, all CVD risk factors, in SLE patients (Xu W.D. et al., 2014).
The role of leptin in SLE development has been investigated using leptin-deficient (ob/ob) mice treated with lupus-inducing agent (Lourenço et al., 2016). Leptin deficiency protected mice from the development of autoantibodies as well as renal disease, and elevated the levels of Treg cells, compared with wild-type controls. Moreover, in (NZBxNZW)F1 lupus-prone mice, leptin administration accelerated the development of autoantibodies and renal disease, while leptin antagonism delayed disease progression (Lourenço et al., 2016). At cellular level, leptin promoted Th1 responses in human CD4+ T cells and in lupus-prone mice via RORγ transcription, whereas leptin neutralization inhibited Th17 responses in autoimmune-prone mice (Yu et al., 2013). Additionally, fasting induced hypoleptinemia or leptin-deficient mice demonstrated decreased levels of Th17 and elevated levels of Treg cells (Liu et al., 2012). In SLE, apoptotic cells represent the major source of self antigens that promote and fuel autoimmune responses. Leptin promoted T cell survival and proliferation of autoreactive T cells in mice with an autoreactive T cell repertoire, including (NZBxNZW)F1 lupus-prone mice (Amarilyo et al., 2013). Leptin also promoted phagocytosis of apoptotic cells by macrophages in lupus-prone mice, which increase the availability of apoptotic-derived antigens to T cells and subsequent development of self-antigen-reactive T cells (Amarilyo et al., 2014).
Altogether, these data support the involvement of leptin in the development of SLE. However, further investigations are needed to fully understand the role of leptin in SLE and thus, explore this adipokine as potential therapeutic target of SLE.
Conclusion and Future Outlook
Obesity and its comorbidities, such as T2DM, non-alcoholic fatty acid liver disease, OA and RA, reached epidemic proportions and are still rising in developing countries. Anti-obesity therapeutic options have provided only a limited long-term efficacy (lifestyle changes, physical activity, diet, and pharmacotherapies) or are not completely safe (bariatric surgery) (Zhang et al., 2014). Therefore, it has become increasingly relevant to disclose new clinical biomarkers and to develop innovative therapeutic strategies for obesity-associated pathologies and chronic inflammation.
The adipose tissue-derived factor leptin has been emerged as a key regulator of nutritional state and metabolism, as well as a modulator of immune system activation and innate-adaptive frontier; thus bridging obesity with metabolic disorders (T2DM and NAFLD) and inflammatory pathologies that affect bones and joints (OA and RA). Consequently, plasma leptin concentration could be a biological marker of the inflammatory status and the onset and evolution of pathologies associated with dysregulation of the immune system, and hereafter evaluations will be essential to establish leptin as clinical biomarker. Moreover, control of bioactive leptin levels by high-affinity leptin-binding molecules, miRNAs targeting leptin, LEPRs antagonists or monoclonal humanized antibodies against LEPR are likely to be feasible therapeutic approaches (Otvos et al., 2011). Recombinant leptin is already available for use in patients with leptin congenital deficiency while the synthetic leptin analog metreleptin has been approved for lipodystrophy treatment (Tchang et al., 2015). Importantly, the development of antibodies that could cross-react with endogenous leptin and cause an effective leptin-deficient state responsible for the loss of efficacy and infection has become a significant concern (DePaoli, 2014). However, given the pleiotropic action of leptin, a systematic approach to modulate their levels and thus prevent obesity-associated disorders might be, for the moment, unavailable. Instead, strategies targeting leptin’s actions precisely and in specific immune cell subpopulations, or targeting of specific receptor isoforms, could be a potential viable option to add novel therapeutic agents against immune-metabolic pathologies. It is now clear that leptin is an important regulator of metabolic status and influence inflammatory and immune responses in several diseases. Nonetheless, leptin network is complex and a lack of a full understanding of leptin’s immunomodulatory mechanisms in almost all of the cells of immune system and its potential side effects are still problems that need to be figured out in drug discovery. Further insights into the pathophysiological role of leptin in the immune system and in obesity-associated disorders will be of great importance for the development of novel therapeutic approaches for these diseases.
Author Contributions
VF and JP have made a substantial contribution to acquisition and analysis of data and critically revised it. VC-C, CR-F, AM, MG-G, and RG have been involved in drafting the manuscript and revising it critically for important intellectual content. OG made a substantial contribution to conception and design of the review article, drafting the manuscript, and critically revising it. All authors approved the final version to be published.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Abbreviations
- ADAMTS
a disintegrin and metalloproteinase with thrombospondin motifs
- AMPK
adenosine monophosphate-activated protein kinase
- Arg-1
arginase-1
- BMDM
bone marrow-derived macrophages
- BMI
body mass index
- cAMP
cyclic adenosine monophosphate
- CCL
CC-chemokine ligand
- CD
cluster of differentiation
- COX-2
cyclooxygenase-2
- CPCs
chondrogenic progenitor cells
- CRP
C-reactive protein
- DAMPs
damage-associated molecular patterns
- DCs
dendritic cells
- ERK
extracellular signal-regulated kinase
- GLUT
glucose transporter
- HFD
high-fat diet
- HLA-DR
human leukocyte antigen-antigen D related
- HSC
hepatic stellate cell
- ICAM
intercellular adhesion molecule
- IFNγ
interferon γ
- IL
interleukin
- iNOS
inducible nitric oxide synthase
- IPFP
intrapatellar fat pad
- IRS
insulin receptor substrate
- JAK
Janus kinase
- JNK
c-jun N-terminal kinase
- KC
kupffer cells
- LEPR
leptin receptor
- LPL
lipoprotein lipase
- LPS
lipopolysaccharide
- MAPK
mitogen-activated protein kinase
- MCP-1
monocyte chemoattractant protein 1
- MEK
mitogen-activated protein kinase kinase
- MIP-1α
macrophage inflammatory protein-1 alpha
- miRNA
microRNA
- MMPs
matrix metalloproteinases
- NAFLD
non-alcoholic liver disease
- NASH
non-alcoholic steatohepatitis
- NF-κB
nuclear factor- κB
- NO
nitric oxide
- NOS2
nitric oxide synthase 2
- OA
osteoarthritis
- PAMPs
pathogen-associated molecular patterns
- PGE2
prostaglandin E2
- PI3K
phosphoinositide 3-kinase
- PKC
protein kinase C
- PMA
phorbol myristate acetate
- PMNs
human polymorphonuclear neutrophils
- PPARα
peroxisome proliferator-activated receptor alpha
- RA
rheumatoid arthritis
- RORγt
retinoic acid-related orphan receptor gamma t
- ROS
reactive oxygen species
- SLE
systemic lupus erythematosus
- SREBP-1c
sterol regulatory element-binding protein-1c
- STAT
signal transducer and activator of transcription
- T2DM
type 2 diabetes mellitus
- TGF-β
transforming growth factor
- Th
T helper cells
- TLR
toll-like receptor
- TNF-α
tumour necrosis factor-α
- Treg
T regulatory cells
- VAT
visceral adipose tissue
- VCAM
vascular cell adhesion molecule
- WAT
white adipose tissue
Footnotes
Funding. OG is Staff Personnel of Xunta de Galicia (Servizo Galego de Saude, SERGAS) through a research-staff stabilization contract (ISCIII/SERGAS). VF is a “Sara Borrell” Researcher funded by ISCIII and FEDER. RG is a “Miguel Servet” Researcher funded by Instituto de Salud Carlos III (ISCIII) and FEDER. OG, MG-G, and RG are members of RETICS Program, RD16/0012/0014 (RIER: Red de Investigación en Inflamación y Enfermedades Reumáticas) via Instituto de Salud Carlos III (ISCIII) and FEDER. The work of OG and JP (PIE13/00024 and PI14/00016, PI17/00409), and RG (PI16/01870 and CP15/00007) was funded by Instituto de Salud Carlos III and FEDER. OG is a beneficiary of a project funded by Research Executive Agency of the European Union in the framework of MSCA-RISE Action of the H2020 Program (Project No. 734899). The funders had no role in study design, data collection, and analysis, decision to publish, or preparation of the manuscript.
References
- Acedo S. C., Gambero S., Cunha F. G., Lorand-Metze I., Gambero A. (2013). Participation of leptin in the determination of the macrophage phenotype: an additional role in adipocyte and macrophage crosstalk. In Vitro Cell. Dev. Biol. Anim. 49 473–478. 10.1007/s11626-013-9629-x [DOI] [PubMed] [Google Scholar]
- Agrawal S., Gollapudi S., Su H., Gupta S. (2011). Leptin activates human B cells to secrete TNF-α, IL-6, and IL-10 via JAK2/STAT3 and p38MAPK/ERK1/2 signaling pathway. J. Clin. Immunol. 31 472–478. 10.1007/s10875-010-9507-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ajeganova S., Andersson M. L., Hafström I. (2013). Association of obesity with worse disease severity in rheumatoid arthritis as well as with comorbidities: a long-term followup from disease onset. Arthritis Care Res. 65 78–87. 10.1002/acr.21710 [DOI] [PubMed] [Google Scholar]
- Al-Hassi H. O., Bernardo D., Murugananthan A. U., Mann E. R., English N. R., Jones A., et al. (2013). A mechanistic role for leptin in human dendritic cell migration: differences between ileum and colon in health and Crohn’s disease. Mucosal Immunol. 6 751–761. 10.1038/mi.2012.113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Suhaimi E. A., Shehzad A. (2013). Leptin, resistin and visfatin: the missing link between endocrine metabolic disorders and immunity. Eur. J. Med. Res. 18:12 10.1186/2047-783X-18-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amarilyo G., Iikuni N., Liu A., Matarese G., La Cava A. (2014). Leptin enhances availability of apoptotic cell-derived self-antigen in systemic lupus erythematosus. PLoS One 9:e112826 10.1371/journal.pone.0112826 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amarilyo G., Iikuni N., Shi F. D., Liu A., Matarese G., La Cava A. (2013). Leptin promotes lupus T-cell autoimmunity. Clin. Immunol. 149 530–533. 10.1016/j.clim.2013.09.002 [DOI] [PubMed] [Google Scholar]
- Anders H. J., Rihl M., Heufelder A., Loch O., Schattenkirchner M. (1999). Leptin serum levels are not correlated with disease activity in patients with rheumatoid arthritis. Metabolism 48 745–748. 10.1016/S0026-0495(99)90174-9 [DOI] [PubMed] [Google Scholar]
- Arrese M., Cabrera D., Kalergis A. M., Feldstein A. E. (2016). Innate immunity and inflammation in NAFLD/NASH. Dig. Dis. Sci. 61 1294–1303. 10.1007/s10620-016-4049-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bähr I., Goritz V., Doberstein H., Gesine G., Hiller R., Rosenstock P., et al. (2017). Diet-induced obesity is associated with an impaired NK cell function and an increased colon cancer incidence. J. Nutr. Metab. 2017:4297025 10.1155/2017/4297025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bao J. P., Chen W. P., Feng J., Hu P. F., Shi Z. L., Wu L. D. (2010). Leptin plays a catabolic role on articular cartilage. Mol. Biol. Rep. 37 3265–3272. 10.1007/s11033-009-9911-x [DOI] [PubMed] [Google Scholar]
- Batra A., Okur B., Glauben R., Erben U., Ihbe J., Stroh T., et al. (2010). Leptin: a critical regulator of CD4+ T-cell polarization in vitro and in vivo. Endocrinology 151 56–62. 10.1210/en.2009-0565 [DOI] [PubMed] [Google Scholar]
- Bennett B. D., Solar G. P., Yuan J. Q., Mathias J., Thomas G. R., Matthews W. (1996). A role for leptin and its cognate receptor in hematopoiesis. Curr. Biol. 6 1170–1180. 10.1016/S0960-9822(02)70684-2 [DOI] [PubMed] [Google Scholar]
- Benomar Y., Naour N., Aubourg A., Bailleux V., Gertler A., Djiane J., et al. (2006). Insulin and leptin induce Glut4 plasma membrane translocation and glucose uptake in a human neuronal cell line by a phosphatidylinositol 3-kinase-dependent mechanism. Endocrinology 147 2550–2556. 10.1210/en.2005-1464 [DOI] [PubMed] [Google Scholar]
- Bhupathiraju S., Hu F. (2016). Epidemiology of obesity and diabetes and their cardiovascular complications. Circ. Res. 118 1723–1735. 10.1161/CIRCRESAHA.115.306825 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjorbaek C., Uotani S., Da Silva B., Flier J. S., Bjørbæk C., Uotani S., et al. (1997). Divergent signaling capacities of the long and short isoforms of the leptin receptor. J. Biol. Chem. 272 32686–32695. 10.1074/jbc.272.51.32686 [DOI] [PubMed] [Google Scholar]
- Boi S. K., Buchta C. M., Pearson N. A., Francis M. B., Meyerholz D. K., Grobe J. L., et al. (2016). Obesity alters immune and metabolic profiles: new insight from obese-resistant mice on high-fat diet. Obesity 24 2140–2149. 10.1002/oby.21620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brotfain E., Hadad N., Shapira Y., Avinoah E., Zlotnik A., Raichel L., et al. (2015). Neutrophil functions in morbidly obese subjects. Clin. Exp. Immunol. 181 156–163. 10.1111/cei.12631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruno A., Conus S., Schmid I., Simon H.-U. (2005). Apoptotic pathways are inhibited by leptin receptor activation in neutrophils. J. Immunol. 174 8090–8096. 10.4049/jimmunol.174.12.8090 [DOI] [PubMed] [Google Scholar]
- Busso N., So A., Chobaz-Peclat V., Morard C., Martinez-Soria E., Talabot-Ayer D., et al. (2002). Leptin signaling deficiency impairs humoral and cellular immune responses and attenuates experimental arthritis. J. Immunol. 168 875–882. 10.4049/jimmunol.168.2.875 [DOI] [PubMed] [Google Scholar]
- Byrne C. D., Targher G. (2015). NAFLD: a multisystem disease. J. Hepatol. 62 S47–S64. 10.1016/j.jhep.2014.12.012 [DOI] [PubMed] [Google Scholar]
- Caldefie-Chezet F., Poulin A., Tridon A., Sion B., Vasson M. P. (2001). Leptin: a potential regulator of polymorphonuclear neutrophil bactericidal action? J. Leukoc. Biol. 69 414–418. [PubMed] [Google Scholar]
- Caldefie-Chezet F., Poulin A., Vasson M.-P. (2003). Leptin regulates functional capacities of polymorphonuclear neutrophils. Free Radic. Res. 37 809–814. 10.1080/1071576031000097526 [DOI] [PubMed] [Google Scholar]
- Cannon J. G., Sharma G., Sloan G., Dimitropoulou C., Baker R. R., Mazzoli A., et al. (2014). Leptin regulates CD16 expression on human monocytes in a sex-specific manner. Physiol. Rep. 2:e12177 10.14814/phy2.12177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao H. (2014). Adipocytokines in obesity and metabolic disease. J. Endocrinol. 220 T47–T59. 10.1530/JOE-13-0339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao H., Lin J., Chen W., Xu G., Sun C. (2016). Baseline adiponectin and leptin levels in predicting an increased risk of disease activity in rheumatoid arthritis: a meta-analysis and systematic review. Autoimmunity 49 1–7. 10.1080/08916934.2016.1230847 [DOI] [PubMed] [Google Scholar]
- Carlton E. D., Demas G. E., French S. S. (2012). Leptin, a neuroendocrine mediator of immune responses, inflammation, and sickness behaviors. Horm. Behav. 62 272–279. 10.1016/j.yhbeh.2012.04.010 [DOI] [PubMed] [Google Scholar]
- Cassano S., Pucino V., La Rocca C., Procaccini C., De Rosa V., Marone G., et al. (2014). Leptin modulates autophagy in human CD4+CD25- conventional T cells. Metabolism 63 1272–1279. 10.1016/j.metabol.2014.06.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang S. F., Hsieh R. Z., Huang K. C., Chang C. A., Chiu F. Y., Kuo H. C., et al. (2015). Upregulation of bone morphogenetic protein-2 synthesis and consequent collagen II expression in leptin-stimulated human chondrocytes. PLoS One 10:e0144252 10.1371/journal.pone.0144252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chatterjee S., Ganini D., Tokar E. J., Kumar A., Das S., Corbett J., et al. (2013). Leptin is key to peroxynitrite-mediated oxidative stress and Kupffer cell activation in experimental non-alcoholic steatohepatitis. J. Hepatol. 58 778–784. 10.1016/j.jhep.2012.11.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chimenti M. S., Triggianese P., Conigliaro P., Candi E., Melino G., Perricone R. (2015). The interplay between inflammation and metabolism in rheumatoid arthritis. Cell Death Dis. 6:e1887 10.1038/cddis.2015.246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chitturi S., Farrell G., Frost L., Kriketos A., Lin R., Fung C., et al. (2002). Serum leptin in NASH correlates with hepatic steatosis but not fibrosis: a manifestation of lipotoxicity? Hepatology 36 403–409. 10.1053/jhep.2002.34738 [DOI] [PubMed] [Google Scholar]
- Claycombe K., King L. E., Fraker P. J. (2008). A role for leptin in sustaining lymphopoiesis and myelopoiesis. Proc. Natl. Acad. Sci. U.S.A. 105 2017–2021. 10.1073/pnas.0712053105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen S., Danzaki K., MacIver N. J. (2017). Nutritional effects on T-cell immunometabolism. Eur. J. Immunol. 47 225–235. 10.1002/eji.201646423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conde J., Gomez R., Bianco G., Scotece M., Lear P., Dieguez C., et al. (2011a). Expanding the adipokine network in cartilage: identification and regulation of novel factors in human and murine chondrocytes. Ann. Rheum. Dis. 70 551–559. 10.1136/ard.2010.132399 [DOI] [PubMed] [Google Scholar]
- Conde J., Scotece M., Abella V., Gómez R., López V., Villar R., et al. (2015). Identification of novel adipokines in the joint. Differential expression in healthy and osteoarthritis tissues. PLoS One 10:e0123601 10.1371/journal.pone.0123601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conde J., Scotece M., Gomez R., Lopez V., Gomez-Reino J. J., Gualillo O. (2011b). Adipokines and osteoarthritis: novel molecules involved in the pathogenesis and progression of disease. Arthritis 2011:203901 10.1155/2011/203901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conde J., Scotece M., López V., Abella V., Hermida M., Pino J., et al. (2013). Differential expression of adipokines in infrapatellar fat pad (IPFP) and synovium of osteoarthritis patients and healthy individuals. Ann. Rheum. Dis. 73 631–633. 10.1136/annrheumdis-2013-204189 [DOI] [PubMed] [Google Scholar]
- Conde J., Scotece M., López V., Gómez R., Lago F., Pino J., et al. (2012). Adiponectin and leptin induce VCAM-1 expression in human and murine chondrocytes. PLoS One 7:e52533 10.1371/journal.pone.0052533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curat C. A., Miranville A., Sengenès C., Diehl M., Tonus C., Busse R., et al. (2004). From blood monocytes to adipose tissue-resident macrophages: induction of diapedesis by human mature adipocytes. Diabetes Metab. Res. Rev. 53 1285–1292. 10.2337/diabetes.53.5.1285 [DOI] [PubMed] [Google Scholar]
- Dayakar A., Chandrasekaran S., Veronica J., Maurya R. (2016). Leptin induces the phagocytosis and protective immune response in Leishmania donovani infected THP-1 cell line and human PBMCs. Exp. Parasitol. 160 54–59. 10.1016/j.exppara.2015.12.002 [DOI] [PubMed] [Google Scholar]
- de Boer T. N., van Spil W. E., Huisman A. M., Polak A. A., Bijlsma J. W., Lafeber F. P., et al. (2012). Serum adipokines in osteoarthritis; comparison with controls and relationship with local parameters of synovial inflammation and cartilage damage. Osteoarthritis Cartilage 20 846–853. 10.1016/j.joca.2012.05.002 [DOI] [PubMed] [Google Scholar]
- De Rosa V., La Cava A., Matarese G. (2017). Metabolic pressure and the breach of immunological self-tolerance. Nat. Immunol. 18 1190–1196. 10.1038/ni.3851 [DOI] [PubMed] [Google Scholar]
- De Rosa V., Procaccini C., Calì G., Pirozzi G., Fontana S., Zappacosta S., et al. (2007). A key role of leptin in the control of regulatory T cell proliferation. Immunity 26 241–255. 10.1016/j.immuni.2007.01.011 [DOI] [PubMed] [Google Scholar]
- DeFuria J., Belkina A. C., Jagannathan-Bogdan M., Snyder-Cappione J., Carr J. D., Nersesova Y. R., et al. (2013). B cells promote inflammation in obesity and type 2 diabetes through regulation of T-cell function and an inflammatory cytokine profile. Proc. Natl. Acad. Sci. U.S.A. 110 5133–5138. 10.1073/pnas.1215840110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deiuliis J. A. (2016). MicroRNAs as regulators of metabolic disease: pathophysiologic significance and emerging role as biomarkers and therapeutics. Int. J. Obes. 40 88–101. 10.1038/ijo.2015.170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng J., Liu Y., Yang M., Wang S., Zhang M., Wang X., et al. (2012). Leptin exacerbates collagen-induced arthritis via enhancement of Th17 cell response. Arthritis Rheum. 64 3564–3573. 10.1002/art.34637 [DOI] [PubMed] [Google Scholar]
- DePaoli A. M. (2014). Leptin in common obesity and associated disorders of metabolism. J. Endocrinol. 223 T71–T81. 10.1530/JOE-14-0258 [DOI] [PubMed] [Google Scholar]
- Dib L. H., Ortega M. T., Melgarejo T., Chapes S. K. (2016). Establishment and characterization of DB-1: a leptin receptor-deficient murine macrophage cell line. Cytotechnology 68 921–933. 10.1007/s10616-015-9843-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixit V. D., Mielenz M., Taub D. D., Parvizi N. (2003). Leptin induces growth hormone secretion from peripheral blood mononuclear cells via a protein kinase C- and nitric oxide-dependent mechanism. Endocrinology 144 5595–5603. 10.1210/en.2003-0600 [DOI] [PubMed] [Google Scholar]
- do Carmo L. S., Rogero M. M., Paredes-Gamero E. J., Nogueira-Pedro A., Xavier J. G., Cortez M., et al. (2013). A high-fat diet increases interleukin-3 and granulocyte colony-stimulating factor production by bone marrow cells and triggers bone marrow hyperplasia and neutrophilia in Wistar rats. Exp. Biol. Med. 238 375–384. 10.1177/1535370213477976 [DOI] [PubMed] [Google Scholar]
- Donath M. Y. (2014). Targeting inflammation in the treatment of type 2 diabetes: time to start. Nat. Rev. Drug Discov. 13 465–476. 10.1038/nrd4275 [DOI] [PubMed] [Google Scholar]
- Donath M. Y., Shoelson S. E. (2011). Type 2 diabetes as an inflammatory disease. Nat. Rev. Immunol. 11 98–107. 10.1038/nri2925 [DOI] [PubMed] [Google Scholar]
- Duarte G. V., Oliveira M., de F., Cardoso T. M., Follador I., Silva T. S., et al. (2013). Association between obesity measured by different parameters and severity of psoriasis. Int. J. Dermatol. 52 177–181. 10.1111/j.1365-4632.2011.05270.x [DOI] [PubMed] [Google Scholar]
- Ducy P., Amling M., Takeda S., Priemel M., Schilling A. F., Beil F. T., et al. (2000). Leptin inhibits bone formation through a hypothalamic relay: a central control of bone mass. Cell 100 197–207. 10.1016/S0092-8674(00)81558-5 [DOI] [PubMed] [Google Scholar]
- Dumond H., Presle N., Terlain B., Mainard D., Loeuille D., Netter P., et al. (2003). Evidence for a key role of leptin in osteoarthritis. Arthritis Rheum. 48 3118–3129. 10.1002/art.11303 [DOI] [PubMed] [Google Scholar]
- Fan Q., Liu Z., Shen C., Li H., Ding J., Jin F., et al. (2018). Microarray study of gene expression profile to identify new candidate genes involved in the molecular mechanism of leptin- induced knee joint osteoarthritis in rat. Hereditas 155:4 10.1186/s41065-017-0039-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farooqi I. S., Jebb S. A., Langmack G., Lawrence E., Cheetham C. H., Prentice A. M., et al. (1999). Effects of recombinant leptin therapy in a child with congenital leptin deficiency. N. Engl. J. Med. 341 879–884. 10.1056/NEJM199909163411204 [DOI] [PubMed] [Google Scholar]
- Farooqi I. S., Matarese G., Lord G. M., Keogh J. M., Lawrence E., Agwu C., et al. (2002). Beneficial effects of leptin on obesity, T cell hyporesponsiveness, and neuroendocrine/metabolic dysfunction of human congenital leptin deficiency. J. Clin. Invest. 110 1093–1103. 10.1172/JCI15693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fasshauer M., Blüher M. (2015). Adipokines in health and disease. Trends Pharmacol. Sci. 36 461–470. 10.1016/j.tips.2015.04.014 [DOI] [PubMed] [Google Scholar]
- Favreau M., Menu E., Gaublomme D., Vanderkerken K., Faict S., Maes K., et al. (2017). Leptin receptor antagonism of iNKT cell function: a novel strategy to combat multiple myeloma. Leukemia 31 2678–2685. 10.1038/leu.2017.146 [DOI] [PubMed] [Google Scholar]
- Figenschau Y., Knutsen G., Shahazeydi S., Johansen O., Sveinbjörnsson B. (2001). Human articular chondrocytes express functional leptin receptors. Biochem. Biophys. Res. Commun. 287 190–197. 10.1006/bbrc.2001.5543 [DOI] [PubMed] [Google Scholar]
- Findlay D. M., Atkins G. J. (2014). Osteoblast-chondrocyte interactions in osteoarthritis. Curr. Osteoporos. Rep. 12 127–134. 10.1007/s11914-014-0192-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frasca D., Ferracci F., Diaz A., Romero M., Lechner S., Blomberg B. B. (2016). Obesity decreases B cell responses in young and elderly individuals. Obesity 24 615–625. 10.1002/oby.21383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraser D. A., Thoen J., Reseland J. E., Førre O., Kjeldsen-Kragh J. (1999). Decreased CD4+ lymphocyte activation and increased interleukin-4 production in peripheral blood of rheumatoid arthritis patients after acute starvation. Clin. Rheumatol. 18 394–401. 10.1007/s100670050125 [DOI] [PubMed] [Google Scholar]
- Frederich R. C., Hamann A., Anderson S., Löllmann B., Lowell B. B., Flier J. S. (1995). Leptin levels reflect body lipid content in mice: evidence for diet-induced resistance to leptin action. Nat. Med. 1 1311–1314. 10.1038/nm1295-1311 [DOI] [PubMed] [Google Scholar]
- Frühbeck G. (2006). Intracellular signalling pathways activated by leptin. Biochem. J. 393 7–20. 10.1042/BJ20051578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujita Y., Fujii T., Mimori T., Sato T., Nakamura T., Iwao H., et al. (2014). Deficient leptin signaling ameliorates systemic lupus erythematosus lesions in MRL/Mp-Fas lpr mice. J. Immunol. 192 979–984. 10.4049/jimmunol.1301685 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujita Y., Yanagida H., Mimori T., Jin Z. X., Sakai T., Kawanami T., et al. (2012). Prevention of fasting-mediated bone marrow atrophy by leptin administration. Cell. Immunol. 273 52–58. 10.1016/j.cellimm.2011.11.007 [DOI] [PubMed] [Google Scholar]
- Gabay C., Dreyer M. G., Pellegrinelli N., Chicheportiche R., Meier C. A. (2001). Leptin directly induces the secretion of interleukin 1 receptor antagonist in human monocytes. J. Clin. Endocrinol. Metab. 86 783–791. 10.1210/jc.86.2.783 [DOI] [PubMed] [Google Scholar]
- Gaber T., Strehl C., Buttgereit F. (2017). Metabolic regulation of inflammation. Nat. Rev. Rheumatol. 13 267–279. 10.1038/nrrheum.2017.37 [DOI] [PubMed] [Google Scholar]
- Gatto M., Zen M., Ghirardello A., Bettio S., Bassi N., Iaccarino L., et al. (2013). Emerging and critical issues in the pathogenesis of lupus. Autoimmun. Rev. 12 523–536. 10.1016/j.autrev.2012.09.003 [DOI] [PubMed] [Google Scholar]
- Genovese M. C., Van den Bosch F., Roberson S. A., Bojin S., Biagini I. M., Ryan P., et al. (2010). LY2439821, a humanized anti-interleukin-17 monoclonal antibody, in the treatment of patients with rheumatoid arthritis: a phase I randomized, double-blind, placebo-controlled, proof-of-concept study. Arthritis Rheum. 62 929–939. 10.1002/art.27334 [DOI] [PubMed] [Google Scholar]
- Gerriets V. A., Danzaki K., Kishton R. J., Eisner W., Nichols A. G., Saucillo D. C., et al. (2016). Leptin directly promotes T-cell glycolytic metabolism to drive effector T-cell differentiation in a mouse model of autoimmunity. Eur. J. Immunol. 46 1970–1983. 10.1002/eji.201545861 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerriets V. A., MacIver N. J. (2014). Role of T cells in malnutrition and obesity. Front. Immunol. 5:379 10.3389/fimmu.2014.00379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez R., Scotece M., Conde J., Gomez-Reino J. J., Lago F., Gualillo O. (2011). Adiponectin and leptin increase IL-8 production in human chondrocytes. Ann. Rheum. Dis. 70 2052–2054. 10.1136/ard.2010.145672 [DOI] [PubMed] [Google Scholar]
- Griffin T. M., Huebner J. L., Kraus V. B., Guilak F. (2009). Extreme obesity due to impaired leptin signaling in mice does not cause knee osteoarthritis. Arthritis Rheum. 60 2935–2944. 10.1002/art.24854 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grotta M. B., Squebola-Cola D. M., Toro A. A., Ribeiro M. A., Mazon S. B., Ribeiro J. D., et al. (2013). Obesity increases eosinophil activity in asthmatic children and adolescents. BMC Pulm. Med. 13:39 10.1186/1471-2466-13-39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruen M. L., Hao M., Piston D. W., Hasty A. H. (2007). Leptin requires canonical migratory signaling pathways for induction of monocyte and macrophage chemotaxis. AJP Cell Physiol. 293 C1481–C1488. 10.1152/ajpcell.00062.2007 [DOI] [PubMed] [Google Scholar]
- Gupta S., Agrawal S., Gollapudi S. (2013). Increased activation and cytokine secretion in B cells stimulated with leptin in aged humans. Immun. Ageing 10:3 10.1186/1742-4933-10-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutierrez D. A., Hasty A. H. (2012). Haematopoietic leptin receptor deficiency does not affect macrophage accumulation in adipose tissue or systemic insulin sensitivity. J. Endocrinol. 212 343–351. 10.1530/JOE-11-0338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hizmetli S., Kisa M., Gokalp N., Bakici M. Z. (2007). Are plasma and synovial fluid leptin levels correlated with disease activity in rheumatoid arthritis? Rheumatol. Int. 27 335–338. 10.1007/s00296-006-0264-7 [DOI] [PubMed] [Google Scholar]
- Howard J. K., Lord G. M., Matarese G., Vendetti S., Ghatei M. A., Ritter M. A., et al. (1999). Leptin protects mice from starvation-induced lymphoid atrophy and increases thymic cellularity in ob/ob mice. J. Clin. Invest. 104 1051–1059. 10.1172/JCI6762 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huertas A., Phan C., Bordenave J., Tu L., Thuillet R., Le Hiress M., et al. (2016). Regulatory T cell dysfunction in idiopathic, heritable and connective tissue-associated pulmonary arterial hypertension. Chest 149 1482–1493. 10.1016/j.chest.2016.01.004 [DOI] [PubMed] [Google Scholar]
- Huh J. Y., Park Y. J., Ham M., Kim J. B. (2014). Crosstalk between adipocytes and immune cells in adipose tissue inflammation and metabolic dysregulation in obesity. Mol. Cells 37 365–371. 10.14348/molcells.2014.0074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hui W., Litherland G. J., Elias M. S., Kitson G. I., Cawston T. E., Rowan A. D., et al. (2012). Leptin produced by joint white adipose tissue induces cartilage degradation via upregulation and activation of matrix metalloproteinases. Ann. Rheum. Dis. 71 455–462. 10.1136/annrheumdis-2011-200372 [DOI] [PubMed] [Google Scholar]
- Ikejima K., Okumura K., Lang T., Honda H., Abe W., Yamashina S., et al. (2005). The role of leptin in progression of non-alcoholic fatty liver disease. Hepatol. Res. 33 151–154. 10.1016/j.hepres.2005.09.024 [DOI] [PubMed] [Google Scholar]
- Ikejima K., Takei Y., Honda H., Hirose M., Yoshikawa M., Zhang Y. J., et al. (2002). Leptin receptor-mediated signaling regulates hepatic fibrogenesis and remodeling of extracellular matrix in the rat. Gastroenterology 122 1399–1410. 10.1053/gast.2002.32995 [DOI] [PubMed] [Google Scholar]
- Inzaugarat M. E., De Matteo E., Baz P., Lucero D., Garcia C. C., Ballerga E. G., et al. (2017). New evidence for the therapeutic potential of curcumin to treat nonalcoholic fatty liver disease in humans. PLoS One 12:e0172900 10.1371/journal.pone.0172900 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaedicke K. M., Roythorne A., Padget K., Todryk S., Preshaw P. M., Taylor J. J. (2013). Leptin up-regulates TLR2 in human monocytes. J. Leukoc. Biol. 93 561–571. 10.1189/jlb.1211606 [DOI] [PubMed] [Google Scholar]
- Jahn J., Spielau M., Brandsch C., Stangl G. I., Delank K. S., Bähr I., et al. (2015). Decreased NK cell functions in obesity can be reactivated by fat mass reduction. Obesity 23 2233–2241. 10.1002/oby.21229 [DOI] [PubMed] [Google Scholar]
- Javor E. D., Ghany M. G., Cochran E. K., Oral E. A., DePaoli A. M., Premkumar A., et al. (2005). Leptin reverses nonalcoholic steatohepatitis in patients with severe lipodystrophy. Hepatology 41 753–760. 10.1002/hep.20672 [DOI] [PubMed] [Google Scholar]
- Jennbacken K., Ståhlman S., Grahnemo L., Wiklund O., Fogelstrand L. (2013). Glucose impairs B-1 cell function in diabetes. Clin. Exp. Immunol. 174 129–138. 10.1111/cei.12148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones K. D., Thitiri J., Ngari M., Berkley J. A. (2014). Childhood malnutrition: toward an understanding of infection, inflammation, and antimicrobials. Food Nutr. Bull. 35(Suppl. 2), S64–S70. 10.1001/jama.2009.1266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kakuma T., Lee Y., Higa M., Wang Z. W., Pan W., Shimomura I., et al. (2000). Leptin, troglitazone, and the expression of sterol regulatory element binding proteins in liver and pancreatic islets. Proc. Natl. Acad. Sci. U.S.A. 97 8536–8541. 10.1073/pnas.97.15.8536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalra S. P. (2009). Central leptin gene therapy ameliorates diabetes type 1 and 2 through two independent hypothalamic relays: a benefit beyond weight and appetite regulation. Peptides 30 1957–1963. 10.1016/j.peptides.2009.07.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamada Y., Takehara T., Hayashi N. (2008). Adipocytokines and liver disease. J. Gastroenterol. 43 811–822. 10.1007/s00535-008-2213-6 [DOI] [PubMed] [Google Scholar]
- Kamp V. M., Langereis J. D., van Aalst C. W., van der Linden J. A., Ulfman L. H., Koenderman L. (2013). Physiological concentrations of leptin do not affect human neutrophils. PLoS One 8:e73170 10.1371/journal.pone.0073170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato H., Ueki S., Kamada R., Kihara J., Yamauchi Y., Suzuki T., et al. (2011). Leptin has a priming effect on eotaxin-induced human eosinophil chemotaxis. Int. Arch. Allergy Immunol. 155 335–344. 10.1159/000321195 [DOI] [PubMed] [Google Scholar]
- Kavak K. S., Teter B. E., Hagemeier J., Zakalik K., Weinstock-Guttman B., Edwards K., et al. (2015). Higher weight in adolescence and young adulthood is associated with an earlier age at multiple sclerosis onset. Mult. Scler. 21 858–865. 10.1177/1352458514555787 [DOI] [PubMed] [Google Scholar]
- Kiguchi N., Maeda T., Kobayashi Y., Fukazawa Y., Kishioka S. (2009). Leptin enhances CC-chemokine ligand expression in cultured murine macrophage. Biochem. Biophys. Res. Commun. 384 311–315. 10.1016/j.bbrc.2009.04.121 [DOI] [PubMed] [Google Scholar]
- Kim S. Y., Lim J. H., Choi S. W., Kim M., Kim S. T., Kim M. S., et al. (2010). Preferential effects of leptin on CD4 T cells in central and peripheral immune system are critically linked to the expression of leptin receptor. Biochem. Biophys. Res. Commun. 394 562–568. 10.1016/j.bbrc.2010.03.019 [DOI] [PubMed] [Google Scholar]
- Klöting N., Blüher M. (2014). Adipocyte dysfunction, inflammation and metabolic syndrome. Rev. Endocr. Metab. Disord. 15 277–287. 10.1007/s11154-014-9301-0 [DOI] [PubMed] [Google Scholar]
- Koskinen A., Vuolteenaho K., Nieminen R., Moilanen T., Moilanen E. (2011). Leptin enhances MMP-1, MMP-3 and MMP-13 production in human osteoarthritic cartilage and correlates with MMP-1 and MMP-3 in synovial fluid from OA patients. Clin. Exp. Rheumatol. 29 57–64. [PubMed] [Google Scholar]
- Koskinen-kolasa A., Vuolteenaho K., Korhonen R., Moilanen T., Moilanen E. (2016). Catabolic and proinflammatory effects of leptin in chondrocytes are regulated by suppressor of cytokine signaling-3. Arthritis Res. Ther. 215 1–13. 10.1186/s13075-016-1112-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kulkarni R. N., Wang Z. L., Wang R. M., Hurley J. D., Smith D. M., Ghatei M. A., et al. (1997). Leptin rapidly suppresses insulin release from insulinoma cells, rat and human islets and, in vivo, in mice. J. Clin. Invest. 100 2729–2736. 10.1172/JCI119818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lam Q. L., Liu S., Cao X., Lu L. (2006). Involvement of leptin signaling in the survival and maturation of bone marrow-derived dendritic cells. Eur. J. Immunol. 36 3118–3130. 10.1002/eji.200636602 [DOI] [PubMed] [Google Scholar]
- Lam Q. L., Wang S., Ko O. K., Kincade P. W., Lu L. (2010). Leptin signaling maintains B-cell homeostasis via induction of Bcl-2 and Cyclin D1. Proc. Natl. Acad. Sci. U.S.A. 107 13812–13817. 10.1073/pnas.1004185107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamas A., Lopez E., Carrio R., Lopez D. M. (2016). Adipocyte and leptin accumulation in tumor-induced thymic involution. Int. J. Mol. Med. 37 133–138. 10.3892/ijmm.2015.2392 [DOI] [PubMed] [Google Scholar]
- Lamas B., Goncalves-Mendes N., Nachat-Kappes R., Rossary A., Caldefie-Chezet F., Vasson M. P., et al. (2013). Leptin modulates dose-dependently the metabolic and cytolytic activities of NK-92 cells. J. Cell. Physiol. 228 1202–1209. 10.1002/jcp.24273 [DOI] [PubMed] [Google Scholar]
- Laue T., Wrann C. D., Hoffmann-Castendiek B., Pietsch D., Hübner L., Kielstein H. (2015). Altered NK cell function in obese healthy humans. BMC Obes. 2:1 10.1186/s40608-014-0033-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebovitz H. E. (2003). The relationship of obesity to the metabolic syndrome. Int. J. Clin. Pract. Suppl. 134 18–27. [PubMed] [Google Scholar]
- Lee Y., Yu X., Gonzales F., Mangelsdorf D. J., Wang M.-Y., Richardson C., et al. (2002). PPAR alpha is necessary for the lipopenic action of hyperleptinemia on white adipose and liver tissue. Proc. Natl. Acad. Sci. U.S.A. 99 11848–11853. 10.1073/pnas.182420899 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee Y. H., Bae S. C. (2016). Circulating leptin level in rheumatoid arthritis and its correlation with disease activity: a meta-analysis. Z. Rheumatol. 75 1021–1027. 10.1007/s00393-016-0050-1 [DOI] [PubMed] [Google Scholar]
- Lee Y. H., Song G. G. (2018). Association between circulating leptin levels and systemic lupus erythematosus: an updated meta-analysis. Lupus 27 428–435. 10.1177/0961203317725587 [DOI] [PubMed] [Google Scholar]
- Li H. M., Zhang T. P., Leng R. X., Li X. P., Li X. M., Liu H. R., et al. (2016). Emerging role of adipokines in systemic lupus erythematosus. Immunol. Res. 64 820–830. 10.1007/s12026-016-8808-8 [DOI] [PubMed] [Google Scholar]
- Li K., Wei L., Huang Y., Wu Y., Su M., Pang X., et al. (2016). Leptin promotes breast cancer cell migration and invasion via IL-18 expression and secretion. Int. J. Oncol. 48 2479–2487. 10.3892/ijo.2016.3483 [DOI] [PubMed] [Google Scholar]
- Licinio J., Caglayan S., Ozata M., Yildiz B. O., de Miranda P. B., O’Kirwan F., et al. (2004). Phenotypic effects of leptin replacement on morbid obesity, diabetes mellitus, hypogonadism, and behavior in leptin-deficient adults. Proc. Natl. Acad. Sci. U.S.A. 101 4531–4536. 10.1073/pnas.0308767101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu A., La Cava A. (2014). Epigenetic dysregulation in systemic lupus erythematosus. Autoimmunity 47 215–219. 10.3109/08916934.2013.844794 [DOI] [PubMed] [Google Scholar]
- Liu Y., Yu Y., Matarese G., La Cava A. (2012). Cutting edge: fasting-induced hypoleptinemia expands functional regulatory T cells in systemic lupus erythematosus. J. Immunol. 188 2070–2073. 10.4049/jimmunol.1102835 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lo C., Lam Q. L., Yang M., Ko K.-H., Sun L., Ma R., et al. (2009). Leptin signaling protects NK cells from apoptosis during development in mouse bone marrow. Cell. Mol. Immunol. 6 353–360. 10.1038/cmi.2009.46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loeser R. F., Goldring S. R., Scanzello C. R., Goldring M. B. (2012). Osteoarthritis: a disease of the joint as an organ. Arthritis Rheum. 64 1697–1707. 10.1002/art.34453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lord G. M., Matarese G., Howard J. K., Baker R. J., Bloom S. R., Lechler R. I. (1998). Leptin modulates the T-cell immune response and reverses starvation-induced immunosuppression. Nature 394 897–901. 10.1038/29795 [DOI] [PubMed] [Google Scholar]
- Lord G. M., Matarese G., Howard J. K., Bloom S. R., Lechler R. I. (2002). Leptin inhibits the anti-CD3-driven proliferation of peripheral blood T cells but enhances the production of proinflammatory cytokines. J. Leukoc. Biol. 72 330–338. [PubMed] [Google Scholar]
- Lourenço E. V., Liu A., Matarese G., La Cava A. (2016). Leptin promotes systemic lupus erythematosus by increasing autoantibody production and inhibiting immune regulation. Proc. Natl. Acad. Sci. U.S.A. 113 10637–10642. 10.1073/pnas.1607101113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luan B., Goodarzi M. O., Phillips N. G., Guo X., Chen Y. D., Yao J., et al. (2014). Leptin-mediated increases in catecholamine signaling reduce adipose tissue inflammation via activation of macrophage HDAC4. Cell Metab. 19 1058–1065. 10.1016/j.cmet.2014.03.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma L., Li D., Sookha M. R., Fang M., Guan Y., Sun X., et al. (2015). Elevated serum leptin levels in patients with systemic lupus erythematosus. Pharmazie 70 720–723. 10.1691/ph.2015.5649 [DOI] [PubMed] [Google Scholar]
- MacIver N. J., Michalek R. D., Rathmell J. C. (2013). Metabolic regulation of T lymphocytes. Annu. Rev. Immunol. 31 259–283. 10.1146/annurev-immunol-032712-095956 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maingrette F., Renier G. (2003). Leptin increases lipoprotein lipase secretion by macrophages: involvement of oxidative stress and protein kinase C. Diabetes Metab. Res. Rev. 52 2121–2128. 10.2337/diabetes.52.8.2121 [DOI] [PubMed] [Google Scholar]
- Mancuso P. (2004). Leptin augments alveolar macrophage leukotriene synthesis by increasing phospholipase activity and enhancing group IVC iPLA2 (cPLA2gamma) protein expression. AJP Lung Cell. Mol. Physiol. 287 L497–L502. 10.1152/ajplung.00010.2004 [DOI] [PubMed] [Google Scholar]
- Mancuso P., Myers M. G., Goel D., Serezani C. H., O’Brien E., Goldberg J., et al. (2012). Ablation of leptin receptor-mediated ERK activation impairs host defense against Gram-negative pneumonia. J. Immunol. 189 867–875. 10.4049/jimmunol.1200465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margiotta D., Navarini L., Vadacca M., Basta F., Vullo M., Pignataro F., et al. (2016). Relationship between leptin and regulatory T cells in systemic lupus erythematosus?: preliminary results. Eur. Rev. Med. Pharmacol. Sci. 20 636–641. [PubMed] [Google Scholar]
- Marques-Rocha J. L., Samblas M., Milagro F. I., Bressan J., Martínez J. A., Marti A. (2015). Noncoding RNAs, cytokines, and inflammation-related diseases. FASEB J. 29 3595–3611. 10.1096/fj.14-260323 [DOI] [PubMed] [Google Scholar]
- Martínez-Carrillo B. E., Jarillo-Luna R. A., Campos-Rodríguez R., Valdés-Ramos R., Rivera-Aguilar V. (2015). Effect of diet and exercise on the peripheral immune system in young Balb/c mice. Biomed Res. Int. 2015:458470 10.1155/2015/458470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martín-Romero C., Santos-Alvarez J., Goberna R., Sánchez-Margalet V. (2000). Human leptin enhances activation and proliferation of human circulating monocytes. Cell. Immunol. 199 15–24. 10.1006/cimm.1999.1490 [DOI] [PubMed] [Google Scholar]
- Matarese G. (2000). Leptin and the immune system: how nutritional status influences the immune response. Eur. Cytokine Netw. 11 7–13. 10.1519/JSC.0b013e3181bab493 [DOI] [PubMed] [Google Scholar]
- Matarese G., Procaccini C., De Rosa V., Horvath T. L., La Cava A. (2010). Regulatory T cells in obesity: the leptin connection. Trends Mol. Med. 16 247–256. 10.1016/j.molmed.2010.04.002 [DOI] [PubMed] [Google Scholar]
- Mathis D., Shoelson S. E. (2011). Immunometabolism: an emerging frontier. Nat. Rev. Immunol. 11 81–83. 10.1038/nri2922 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattioli B., Giordani L., Quaranta M. G., Viora M. (2009). Leptin exerts an anti-apoptotic effect on human dendritic cells via the PI3K-Akt signaling pathway. FEBS Lett. 583 1102–1106. 10.1016/j.febslet.2009.02.029 [DOI] [PubMed] [Google Scholar]
- Mattioli B., Straface E., Matarrese P., Quaranta M. G., Giordani L., Malorni W., et al. (2008). Leptin as an immunological adjuvant: enhanced migratory and CD8+ T cell stimulatory capacity of human dendritic cells exposed to leptin. FASEB J. 22 2012–2022. 10.1096/fj.07-098095 [DOI] [PubMed] [Google Scholar]
- Maurya R., Bhattacharya P., Ismail N., Dagur P. K., Joshi A. B., Razdan K., et al. (2016). Differential role of leptin as an immunomodulator in controlling visceral Leishmaniasis in normal and leptin-deficient mice. Am. J. Trop. Med. Hyg. 95 109–119. 10.4269/ajtmh.15-0804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McInnes I. (2011). The pathogenesis of rheumatoid arthritis. N. Engl. J. Med. 365 2205–2219. 10.7748/phc2011.11.21.9.29.c8797 [DOI] [PubMed] [Google Scholar]
- Meier C. A., Chicheportiche R., Dreyer M., Dayer J. M. (2003). IP-10, but not RANTES, is upregulated by leptin in monocytic cells. Cytokine 21 43–47. 10.1016/S1043-4666(02)00491-X [DOI] [PubMed] [Google Scholar]
- Mittendorfer B., Horowitz J. F., DePaoli A. M., McCamish M. A., Patterson B. W., Klein S. (2011). Recombinant human leptin treatment does not improve insulin action in obese subjects with type 2 diabetes. Diabetes Metab. Res. Rev. 60 1474–1477. 10.2337/db10-1302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montecucco F., Bianchi G., Gnerre P., Bertolotto M., Dallegri F., Ottonello L. (2006). Induction of neutrophil chemotaxis by leptin: crucial role for p38 and Src kinases. Ann. N. Y. Acad. Sci. 1069 463–471. 10.1196/annals.1351.045 [DOI] [PubMed] [Google Scholar]
- Moon H.-S., Matarese G., Brennan A. M., Chamberland J. P., Liu X., Fiorenza C. G., et al. (2011). Efficacy of metreleptin in obese patients with type 2 diabetes: cellular and molecular pathways underlying leptin tolerance. Diabetes Metab. Res. Rev. 60 1647–1656. 10.2337/db10-1791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moraes-Vieira P. M., Larocca R. A., Bassi E. J., Peron J. P., Andrade-Oliveira V., Wasinski F., et al. (2014). Leptin deficiency impairs maturation of dendritic cells and enhances induction of regulatory T and Th17 cells. Eur. J. Immunol. 44 794–806. 10.1002/eji.201343592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morteza A., Nakhjavani M., Asgarani F., Ghaneei A., Esteghamati A., Mirmiranpour H. (2013). The lost correlation between leptin and CRP in type 2 diabetes. Eur. Cytokine Netw. 24 53–59. 10.1684/ecn.2013.0329 [DOI] [PubMed] [Google Scholar]
- Morton G. J., Gelling R. W., Niswender K. D., Morrison C. D., Rhodes C. J., Schwartz M. W. (2005). Leptin regulates insulin sensitivity via phosphatidylinositol-3-OH kinase signaling in mediobasal hypothalamic neurons. Cell Metab. 2 411–420. 10.1016/j.cmet.2005.10.009 [DOI] [PubMed] [Google Scholar]
- Münzberg H., Morrison C. D. (2015). Structure, production and signaling of leptin. Metabolism 64 13–23. 10.1016/j.metabol.2014.09.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mutabaruka M.-S., Aoulad Aissa M., Delalandre A., Lavigne M., Lajeunesse D. (2010). Local leptin production in osteoarthritis subchondral osteoblasts may be responsible for their abnormal phenotypic expression. Arthritis Res. Ther. 12:R20 10.1186/ar2925 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Najib S., Sánchez-Margalet V. (2002). Human leptin promotes survival of human circulating blood monocytes prone to apoptosis by activation of p42/44 MAPK pathway. Cell. Immunol. 220 143–149. 10.1016/S0008-8749(03)00027-3 [DOI] [PubMed] [Google Scholar]
- Nakamura Y., Sanematsu K., Ohta R., Shirosaki S., Koyano K., Nonaka K., et al. (2008). Diurnal variation of human sweet taste recognition thresholds is correlated with plasma leptin levels. Diabetes Metab. Res. Rev. 57 2661–2665. 10.2337/db07-1103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Namkoong C., Kim M. S., Jang P. G., Han S. M., Park H. S., Koh E. H., et al. (2005). Enhanced hypothalamic AMP-activated protein kinase activity contributes to hyperphagia in diabetic rats. Diabetes Metab. Res. Rev. 54 63–68. 10.2337/diabetes.54.1.63 [DOI] [PubMed] [Google Scholar]
- Nave H., Mueller G., Siegmund B., Jacobs R., Stroh T., Schueler U., et al. (2008). Resistance of Janus kinase-2 dependent leptin signaling in natural killer (NK) Cells: a novel mechanism of NK cell dysfunction in diet-induced obesity. Endocrinology 149 3370–3378. 10.1210/en.2007-1516 [DOI] [PubMed] [Google Scholar]
- Naylor C., Burgess S., Madan R., Buonomo E., Razzaq K., Ralston K., et al. (2014). Leptin receptor mutation results in defective neutrophil recruitment to the colon during Entamoeba histolytica infection. mBio 5:e02046–14 10.1128/mBio.02046-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikolajczyk B. S. (2010). B cells as under-appreciated mediators of non-auto-immune inflammatory disease. Cytokine 50 234–242. 10.1016/j.cyto.2010.02.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nugent M. (2016). MicroRNAs: exploring new horizons in osteoarthritis. Osteoarthritis Cartilage 24 573–580. 10.1016/j.joca.2015.10.018 [DOI] [PubMed] [Google Scholar]
- Olama S. M., Senna M. K., Elarman M. (2012). Synovial/Serum leptin ratio in rheumatoid arthritis: the association with activity and erosion. Rheumatol. Int. 32 683–690. 10.1007/s00296-010-1698-5 [DOI] [PubMed] [Google Scholar]
- Oner S., Volkan O., Oner C., Mengi A., Direskeneli H., Da T. (2015). Serum leptin levels do not correlate with disease activity in rheumatoid arthritis. Acta Reumatol. Port. 40 50–54. [PubMed] [Google Scholar]
- Oral E. A., Simha V., Ruiz E., Andewelt A., Premkumar A., Snell P., et al. (2002). Leptin-replacement therapy for lipodystrophy. N. Engl. J. Med. 346 570–578. 10.1056/NEJMoa012437 [DOI] [PubMed] [Google Scholar]
- Orlova E. G., Shirshev S. V. (2014). Role of leptin and ghrelin in induction of differentiation of IL-17-producing and T-regulatory cells. Bull. Exp. Biol. Med. 156 819–822. 10.1007/s10517-014-2459-3 [DOI] [PubMed] [Google Scholar]
- O’Rourke L., Yeaman S. J., Shepherd P. R. (2001). Insulin and leptin acutely regulate cholesterol ester metabolism in macrophages by novel signaling pathways. Diabetes Metab. Res. Rev. 50 955–961. 10.2337/diabetes.50.5.955 [DOI] [PubMed] [Google Scholar]
- Otero M., Gomez Reino J. J., Gualillo O. (2003). Synergistic induction of nitric oxide synthase type II: in vitro effect of leptin and interferon-gamma in human chondrocytes and ATDC5 chondrogenic cells. Arthritis Rheum. 48 404–409. 10.1002/art.10811 [DOI] [PubMed] [Google Scholar]
- Otero M., Lago R., Gomez R., Lago F., Dieguez C., Gómez-Reino J. J., et al. (2006). Changes in plasma levels of fat-derived hormones adiponectin, leptin, resistin and visfatin in patients with rheumatoid arthritis. Ann. Rheum. Dis. 65 1198–1201. 10.1136/ard.2005.046540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otero M., Lago R., Gómez R., Lago F., Gomez-Reino J. J., Gualillo O. (2007). Phosphatidylinositol 3-kinase, MEK-1 and p38 mediate leptin/interferon-gamma synergistic NOS type II induction in chondrocytes. Life Sci. 81 1452–1460. 10.1016/j.lfs.2007.09.007 [DOI] [PubMed] [Google Scholar]
- Otero M., Lago R., Lago F., Reino J. J., Gualillo O. (2005). Signalling pathway involved in nitric oxide synthase type II activation in chondrocytes: synergistic effect of leptin with interleukin-1. Arthritis Res. Ther. 7 R581–R591. 10.1186/ar1708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otvos L., Shao W.-H., Vanniasinghe A. S., Amon M. A., Csilla Holub M., Kovalszky I., et al. (2011). Toward understanding the role of leptin and leptin receptor antagonism in preclinical models of rheumatoid arthritis. Peptides 32 1567–1574. 10.1016/j.peptides.2011.06.015 [DOI] [PubMed] [Google Scholar]
- Ozata M., Uçkaya G., Beyhan Z., Ozdemir I. C. (1999). Plasma leptin levels in male patients with idiopathic central diabetes insipidus. J. Endocrinol. Invest. 22 451–454. 10.1007/BF03343589 [DOI] [PubMed] [Google Scholar]
- Pearson M. J., Herndler-Brandstetter D., Tariq M. A., Nicholson T. A., Philp A. M., Smith H. L., et al. (2017). IL-6 secretion in osteoarthritis patients is mediated by chondrocyte-synovial fibroblast cross-talk and is enhanced by obesity. Sci. Rep. 7:3451 10.1038/s41598-017-03759-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelleymounter M. A., Cullen M. J., Baker M. B., Hecht R., Winters D., Boone T., et al. (1995). Effects of the obese gene product on body weight regulation in ob/ob mice. Science 269 540–543. 10.1126/science.7624776 [DOI] [PubMed] [Google Scholar]
- Pérez-Pérez A., Vilariño-García T., Fernández-Riejos P., Martín-González J., Segura-Egea J. J., Sánchez-Margalet V. (2017). Role of leptin as a link between metabolism and the immune system. Cytokine Growth Factor Rev. 35 71–84. 10.1016/j.cytogfr.2017.03.001 [DOI] [PubMed] [Google Scholar]
- Petersen K. F., Oral E. A., Dufour S., Befroy D., Ariyan C., Yu C., et al. (2002). Leptin reverses insulin resistance and hepatic steatosis in patients with severe lipodystrophy. J. Clin. Invest. 109 1345–1350. 10.1172/JCI200215001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polyzos S. A., Kountouras J., Mantzoros C. S. (2015). Leptin in nonalcoholic fatty liver disease: a narrative review. Metabolism 64 60–78. 10.1016/j.metabol.2014.10.012 [DOI] [PubMed] [Google Scholar]
- Popa C., Netea M. G., Radstake T. R. D., van Riel P. L., Barrera P., van der Meer J. W. (2005). Markers of inflammation are negatively correlated with serum leptin in rheumatoid arthritis. Ann. Rheum. Dis. 64 1195–1198. 10.1136/ard.2004.032243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Procaccini C., De Rosa V., Galgani M., Carbone F., Cassano S., Greco D., et al. (2012). Leptin-induced mTOR activation defines a specific molecular and transcriptional signature controlling CD4+ effector T cell responses. J. Immunol. 189 2941–2953. 10.4049/jimmunol.1200935 [DOI] [PubMed] [Google Scholar]
- Procaccini C., La Rocca C., Carbone F., De Rosa V., Galgani M., Matarese G. (2017). Leptin as immune mediator: interaction between neuroendocrine and immune system. Dev. Comp. Immunol. 66 120–129. 10.1016/j.dci.2016.06.006 [DOI] [PubMed] [Google Scholar]
- Rahmati M., Mobasheri A., Mozafari M. (2016). Inflammatory mediators in osteoarthritis: a critical review of the state-of-the-art, current prospects, and future challenges. Bone 85 81–90. 10.1016/j.bone.2016.01.019 [DOI] [PubMed] [Google Scholar]
- Ramirez O., Garza K. M. (2014). Leptin deficiency in vivo enhances the ability of splenic dendritic cells to activate T cells. Int. Immunol. 26 627–636. 10.1093/intimm/dxu067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reis B. S., Lee K., Fanok M. H., Mascaraque C., Amoury M., Cohn L. B., et al. (2015). Leptin receptor signaling in T cells is required for Th17 differentiation. J. Immunol. 194 5253–5260. 10.4049/jimmunol.1402996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodríguez L., Graniel J., Ortiz R. (2007). Effect of leptin on activation and cytokine synthesis in peripheral blood lymphocytes of malnourished infected children. Clin. Exp. Immunol. 148 478–485. 10.1111/j.1365-2249.2007.03361.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenbaum M., Leibel R. L. (2014). Role of leptin in energy homeostasis in humans. J. Endocrinol. 223 T83–T96. 10.1530/JOE-14-0358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez-Margalet V., Martin-Romero C. (2001). Human leptin signaling in human peripheral blood mononuclear cells: activation of the JAK-STAT pathway. Cell. Immunol. 211 30–36. 10.1006/cimm.2001.1815 [DOI] [PubMed] [Google Scholar]
- Sánchez-Pozo C., Rodriguez-Baño J., Domínguez-Castellano A., Muniain M. A., Goberna R., Sánchez-Margalet V. (2003). Leptin stimulates the oxidative burst in control monocytes but attenuates the oxidative burst in monocytes from HIV-infected patients. Clin. Exp. Immunol. 134 464–469. 10.1111/j.1365-2249.2003.02321.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santos-Alvarez J., Goberna R., Sánchez-Margalet V. (1999). Human leptin stimulates proliferation and activation of human circulating monocytes. Cell. Immunol. 194 6–11. 10.1006/cimm.1999.1490 [DOI] [PubMed] [Google Scholar]
- Saucillo D. C., Gerriets V. A., Sheng J., Rathmell J. C., Maciver N. J., Stedman S. W. (2014). Leptin metabolically licenses T cells for activation to link nutrition and immunity. J. Immunol. 192 136–144. 10.4049/jimmunol.1301158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saxena N. K., Ikeda K., Rockey D. C., Friedman S. L. (2002). Leptin in hepatic fibrosis: evidence for increased collagen production in stellate cells and lean littermates of ob/ob mice. Hepatology 35 762–771. 10.1053/jhep.2002.32029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultze S. M., Hemmings B. A., Niessen M., Tschopp O. (2012). PI3K/AKT, MAPK and AMPK signalling: protein kinases in glucose homeostasis. Expert Rev. Mol. Med. 14:e1 10.1017/S1462399411002109 [DOI] [PubMed] [Google Scholar]
- Scotece M., Conde J., López V., Lago F., Pino J., Gómez-Reino J. J., et al. (2014). Adiponectin and leptin: new targets in inflammation. Basic Clin. Pharmacol. Toxicol. 114 97–102. 10.1111/bcpt.12109 [DOI] [PubMed] [Google Scholar]
- Scotece M., Pérez T., Conde J., Abella V., López V., Pino J., et al. (2017). Adipokines induce pro-inflammatory factors in activated Cd4+ T cells from osteoarthritis patient. J. Orthop. Res. 35 1299–1303. 10.1002/jor.23377 [DOI] [PubMed] [Google Scholar]
- Seol D., McCabe D. J., Choe H., Zheng H., Yu Y., Jang K., et al. (2012). Chondrogenic progenitor cells respond to cartilage injury. Arthritis Rheum. 64 3626–3637. 10.1002/art.34613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silha J. V., Krsek M., Skrha J. V., Sucharda P., Nyomba B. L., Murphy L. J. (2003). Plasma resistin, adiponectin and leptin levels in lean and obese subjects: correlations with insulin resistence. Eur. J. Endocrinol. 149 331–335. 10.1530/eje.0.1490331 [DOI] [PubMed] [Google Scholar]
- Smolen J. S., Aletaha D., McInnes I. B. (2016). Rheumatoid arthritis. Lancet 388 2023–2038. 10.1016/S0140-6736(16)30173-8 [DOI] [PubMed] [Google Scholar]
- Sun Z., Dragon S., Becker A., Gounni A. S. (2013). Leptin inhibits neutrophil apoptosis in children via ERK/NF-κB-dependent pathways. PLoS One 8:e55249 10.1371/journal.pone.0055249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzukawa M., Nagase H., Ogahara I., Han K., Tashimo H., Shibui A., et al. (2011). Leptin enhances survival and induces migration, degranulation, and cytokine synthesis of human basophils. J. Immunol. 186 5254–5260. 10.4049/jimmunol.1004054 [DOI] [PubMed] [Google Scholar]
- Tacke F., Yoneyama H. (2013). From NAFLD to NASH to fibrosis to HCC: role of dendritic cell populations in the liver. Hepatology 58 494–496. 10.1002/hep.26405 [DOI] [PubMed] [Google Scholar]
- Tajiri K., Shimizu Y. (2012). Role of NKT cells in the pathogenesis of NAFLD. Int. J. Hepatol. 2012:850836 10.1155/2012/850836 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka M., Suganami T., Kim-Saijo M., Toda C., Tsuiji M., Ochi K., et al. (2011). Role of central leptin signaling in the starvation-induced alteration of B-cell development. J. Neurosci. 31 8373–8380. 10.1523/JNEUROSCI.6562-10.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Targońska-Stępniak B., Majdan M., Dryglewska M. (2008). Leptin serum levels in rheumatoid arthritis patients: relation to disease duration and activity. Rheumatol. Int. 28 585–591. 10.1007/s00296-007-0480-9 [DOI] [PubMed] [Google Scholar]
- Taylor A. K., Cao W., Vora K. P., De La Cruz J., Shieh W. J., Zaki S. R., et al. (2013). Protein energy malnutrition decreases immunity and increases susceptibility to influenza infection in mice. J. Infect. Dis. 207 501–510. 10.1093/infdis/jis527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tchang B. G., Shukla A. P., Aronne L. J. (2015). Metreleptin and generalized lipodystrophy and evolving therapeutic perspectives. Expert Opin. Biol. Ther. 15 1061–1075. 10.1517/14712598.2015.1052789 [DOI] [PubMed] [Google Scholar]
- Tian G., Liang J.-N., Wang Z.-Y., Zhou D. (2014). Emerging role of leptin in rheumatoid arthritis. Clin. Exp. Immunol. 177 557–570. 10.1111/cei.12372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian Z., Chen Y., Gao B. (2013). Natural killer cells in liver disease. Hepatology 57 1654–1662. 10.1002/hep.26115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian Z., Sun R., Wei H., Gao B. (2002). Impaired natural killer (NK) cell activity in leptin receptor deficient mice: leptin as a critical regulator in NK cell development and activation. Biochem. Biophys. Res. Commun. 298 297–302. 10.1016/S0006-291X(02)02462-2 [DOI] [PubMed] [Google Scholar]
- Tilg H., Moschen A. R. (2006). Adipocytokines: mediators linking adipose tissue, inflammation and immunity. Nat. Rev. Immunol. 6 772–783. 10.1038/nri1937 [DOI] [PubMed] [Google Scholar]
- Tiniakos D. G., Vos M. B., Brunt E. M. (2010). Nonalcoholic fatty liver disease: pathology and pathogenesis. Annu. Rev. Pathol. Mech. Dis. 5 145–171. 10.1146/annurev-pathol-121808-102132 [DOI] [PubMed] [Google Scholar]
- Toussirot É., Michel F., Binda D., Dumoulin G. (2015). The role of leptin in the pathophysiology of rheumatoid arthritis. Life Sci. 140 29–36. 10.1016/j.lfs.2015.05.001 [DOI] [PubMed] [Google Scholar]
- Tsiotra P. C., Boutati E., Dimitriadis G., Raptis S. A. (2013). High insulin and leptin increase resistin and inflammatory cytokine production from human mononuclear cells. Biomed Res. Int. 2013:487081 10.1155/2013/487081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsiotra P. C., Pappa V., Raptis S. A., Tsigos C. (2000). Expression of the long and short leptin receptor isoforms in peripheral blood mononuclear cells: implications for leptin’s actions. Metabolism 49 1537–1541. 10.1053/meta.2000.18519 [DOI] [PubMed] [Google Scholar]
- Tsochatzis E. A., Papatheodoridis G. V., Archimandritis A. J. (2009). Adipokines in nonalcoholic steatohepatitis: from pathogenesis to implications in diagnosis and therapy. Mediators Inflamm. 2009:831670 10.1155/2009/831670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ubags N. D., Vernooy J. H., Burg E., Hayes C., Bement J., Dilli E., et al. (2014). The role of leptin in the development of pulmonary neutrophilia in infection and Acute Lung Injury. Crit. Care Med. 42 143–151. 10.1097/CCM.0000000000000048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vestweber D. (2015). How leukocytes cross the vascular endothelium. Nat. Rev. Immunol. 15 692–704. 10.1038/nri3908 [DOI] [PubMed] [Google Scholar]
- Vieira-Potter V. J. (2014). Inflammation and macrophage modulation in adipose tissues. Cell. Microbiol. 16 1484–1492. 10.1111/cmi.12336 [DOI] [PubMed] [Google Scholar]
- Voloshyna I., Mounessa J., Carsons S. E., Reiss A. B. (2016). Effect of inhibition of interleukin-12/23 by ustekinumab on the expression of leptin and leptin receptor in human THP-1 macrophages. Clin. Exp. Dermatol. 41 308–311. 10.1111/ced.12699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vuolteenaho K., Koskinen A., Kukkonen M., Nieminen R., Päivärinta U., Moilanen T., et al. (2009). Leptin enhances synthesis of proinflammatory mediators in human osteoarthritic cartilage–mediator role of NO in leptin-induced PGE2, IL-6, and IL-8 production. Mediators Inflamm. 2009:345838 10.1155/2009/345838 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner N. M., Brandhorst G., Czepluch F., Lankeit M., Eberle C., Herzberg S., et al. (2013). Circulating regulatory T cells are reduced in obesity and may identify subjects at increased metabolic and cardiovascular risk. Obesity 21 461–468. 10.1002/oby.20087 [DOI] [PubMed] [Google Scholar]
- Wang J., Leclercq I., Brymora J. M., Xu N., Ramezani-Moghadam M., London R. M., et al. (2009). Kupffer cells mediate leptin-induced liver fibrosis. Gastroenterology 137 713–723. 10.1053/j.gastro.2009.04.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S., Baidoo S. E., Liu Y., Zhu C., Tian J., Ma J., et al. (2013). T cell-derived leptin contributes to increased frequency of T helper type 17 cells in female patients with Hashimoto’s thyroiditis. Clin. Exp. Immunol. 171 63–68. 10.1111/j.1365-2249.2012.04670.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X., Qiao Y., Yang L., Song S., Han Y., Tian Y., et al. (2017). Leptin levels in patients with systemic lupus erythematosus inversely correlate with regulatory T cell frequency. Lupus 26 1401–1406. 10.1177/0961203317703497 [DOI] [PubMed] [Google Scholar]
- Winer D. A., Winer S., Shen L., Wadia P. P., Yantha J., Paltser G., et al. (2011). B cells promote insulin resistance through modulation of T cells and production of pathogenic IgG antibodies. Nat. Med. 17 610–617. 10.1038/nm.2353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolsk E., Mygind H., Grondahl T. S., Pedersen B. K., van Hall G. (2011). The role of leptin in human lipid and glucose metabolism: the effects of acute recombinant human leptin infusion in young healthy males. Am. J. Clin. Nutr. 94 1533–1544. 10.3945/ajcn.111.012260 [DOI] [PubMed] [Google Scholar]
- Wong C. K., Cheung P. F., Lam C. W. (2007). Leptin-mediated cytokine release and migration of eosinophils: implications for immunopathophysiology of allergic inflammation. Eur. J. Immunol. 37 2337–2348. 10.1002/eji.200636866 [DOI] [PubMed] [Google Scholar]
- Wrann C. D., Laue T., Hübner L., Kuhlmann S., Jacobs R., Goudeva L., et al. (2012). Short-term and long-term leptin exposure differentially affect human natural killer cell immune functions. Am. J. Physiol. Endocrinol. Metab. 302 E108–E116. 10.1152/ajpendo.00057.2011 [DOI] [PubMed] [Google Scholar]
- Xu R., Huang H., Zhang Z., Wang F.-S. (2014). The role of neutrophils in the development of liver diseases. Cell. Mol. Immunol. 11 224–231. 10.1038/cmi.2014.2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu W. D., Zhang M., Zhang Y. J., Liu S. S., Pan H. F., Ye D. Q. (2014). Association between leptin and systemic lupus erythematosus. Rheumatol. Int. 34 559–563. 10.1007/s00296-013-2774-4 [DOI] [PubMed] [Google Scholar]
- Yadav A., Jyoti P., Jain S. K., Bhattacharjee J. (2011). Correlation of adiponectin and leptin with insulin resistance: a pilot study in healthy north Indian population. Indian J. Clin. Biochem. 26 193–196. 10.1007/s12291-011-0119-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshino T., Kusunoki N., Tanaka N., Kaneko K., Kusunoki Y., Endo H., et al. (2011). Elevated serum levels of resistin, leptin, and adiponectin are associated with C-reactive protein and also other clinical conditions in rheumatoid arthritis. Intern. Med. 50 269–275. 10.2169/internalmedicine.50.4306 [DOI] [PubMed] [Google Scholar]
- Yu Y., Liu Y., Shi F.-D., Zou H., Matarese G., La Cava A. (2013). Cutting edge: leptin-induced RORγt expression in CD4+ T cells promotes Th17 responses in systemic lupus erythematosus. J. Immunol. 190 3054–3058. 10.4049/jimmunol.1203275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zarkesh-Esfahani H., Pockley A. G., Wu Z., Hellewell P. G., Weetman A. P., Ross R. J. (2004). Leptin indirectly activates human neutrophils via induction of TNF-α. J. Immunol. 172 1809–1814. 10.4049/jimmunol.172.3.1809 [DOI] [PubMed] [Google Scholar]
- Zhang Y., Liu J., Yao J., Ji G., Qian L., Wang J., et al. (2014). Obesity: pathophysiology and intervention. Nutrients 6 5153–5183. 10.3390/nu6115153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y., Proenca R., Maffei M., Barone M., Leopold L., Friedman J. M. (1994). Positional cloning of the mouse obese gene and its human homologue. Nature 372 425–432. 10.1038/372425a0 [DOI] [PubMed] [Google Scholar]
- Zhao F.-Q., Keating A. (2007). Functional properties and genomics of glucose transporters. Curr. Genomics 8 113–128. 10.2174/138920207780368187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao X., Dong Y., Zhang J., Li D., Hu G., Yao J., et al. (2016). Leptin changes differentiation fate and induces senescence in chondrogenic progenitor cells. Cell Death Dis. 7:e2188 10.1038/cddis.2016.68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Y., Sun R., You L., Gao C., Tian Z. (2003). Expression of leptin receptors and response to leptin stimulation of human natural killer cell lines. Biochem. Biophys. Res. Commun. 300 247–252. 10.1016/S0006-291X(02)02838-3 [DOI] [PubMed] [Google Scholar]
- Zheng C., Yang Q., Cao J., Xie N., Liu K., Shou P., et al. (2016). Local proliferation initiates macrophage accumulation in adipose tissue during obesity. Cell Death Dis. 7:e2167 10.1038/cddis.2016.54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou B., Li H., Shi J. (2017). miR-27 inhibits the NF-κB signaling pathway by targeting leptin in osteoarthritic chondrocytes. Int. J. Mol. Med. 40 523–530. 10.3892/ijmm.2017.3021 [DOI] [PubMed] [Google Scholar]
- Zhou Y., Rui L. (2014). Leptin signaling and leptin resistance. Front. Med. 7 207–222. 10.1007/s11684-013-0263-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y., Yu X., Chen H., Sjöberg S., Roux J., Zhang L., et al. (2015). Leptin deficiency shifts mast cells toward anti-inflammatory actions and protects mice from obesity and diabetes by polarizing M2 macrophages. Cell Metab. 22 1045–1058. 10.1016/j.cmet.2015.09.013 [DOI] [PMC free article] [PubMed] [Google Scholar]