Abstract
The technique of targeted expression of interesting genes, including distinct delivery systems and specific gene promoter-operating expression, is an important strategy for gene therapy against cancers. Up to now, extensive literature documented the efficacy of distinct delivery systems, such as the liposome system, nano-particle system, polyetherimide (PEI) system, and so on, in cancer gene therapy. However, a related document on the potential value of using a specific gene promoter, such as a tumor suppressor, in cancer gene therapy was still scary. The main obstacle might be that the selection of an ideal gene promoter to operate interesting gene expression in cancer gene therapy is still not fully understood. Therefore, many efforts need to be done in order to make it a real power tool for the human clinical treatment of cancer patients. The purpose of this review is to clarify the current state and some problematics in development of promoter-operating targeted expression of interesting genes and highlight its potential in cancer gene therapy.
Keywords: targeted expression, promoter, microRNAs, cancer
Main Text
Over time, the incidence of life-threatening tumors gradually increased; however, the mechanisms involved in tumor development have not been fully elucidated. The overall 5-year survival rate for cancer patients with surgery, chemotherapy, radiation, and other conventional treatments is still very low. Thus, the development of safe and effective alternative treatment strategies has become a major goal for researchers worldwide to improve cancer clinical outcomes.
Gene therapy, which has developed with the maturity of DNA recombination technology and gene cloning technology, is one of the most revolutionary medical technologies. It is to change the human genetic-material-based biomedical treatment and shows a unique advantage in the treatment of major diseases.1, 2 The history of gene therapy dates back to 1968; Rogers and Pfuderer3 first put forward the concept of gene therapy in Nature, which was documented as “use of viruses as carriers of added genetic information.” Up to now, gene therapy mainly has referred to the use of molecular biology methods to transfer human normal genes or therapeutically interesting genes through a certain way into human target cells or tissues, to correct gene defects or play a role in treatment, so as to achieve ideal outcome of modern biomedical treatment of diseases.4 With the development of biotechnology, gene therapy has been extended to treat malignant tumors, cardiovascular diseases, autoimmune diseases, metabolic diseases, and other critical diseases from the treatment of single-gene inherited diseases. Among them, the clinical trials of gene therapy for various malignancies accounted for two-thirds of the total. In 2004, the world’s first listed gene therapy drug was also restrictedly applied at cancer therapy, showing that tumor gene therapy was the most active and important research field of gene therapy.5
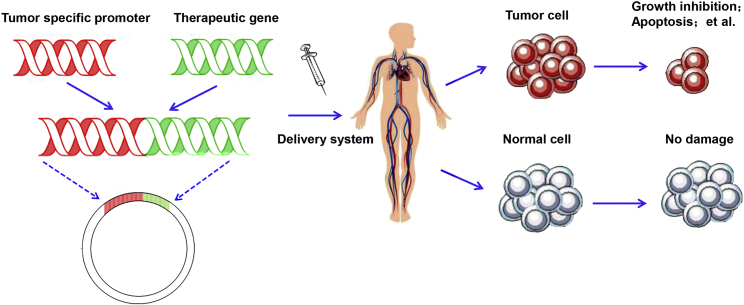
With the deep study of the mechanism of tumor development, people have realized that the tumor is a kind of genetic disease. Therefore, people have high expectations for gene therapy against tumors. After decades of development, gene-therapy-related technologies have matured, and several key technologies have been breakthroughs. Therefore, the next few years will be the key period of the development of cancer gene therapy. However, there are still many problems that would be effectively solved for the application of gene therapy in cancer. Among them, the key factors in cancer gene therapy are the targeting efficiency and the safety, that is to say the new therapeutic agents should specifically act on tumor cells, meanwhile reducing the damage to the normal cells. More recently, evidence suggested that the technique of targeted gene expression, including specific gene promoter-operating expression and diverse delivery systems, could better resolve targeting efficiency and safety problems, which are the key issues for the application of gene therapy for various cancers (Figure 1).
Figure 1.
A Sketch of Targeted Expression of Genes Operated by Tumor-Specific Promoter
In this review, we summarize current improvements made in promoter-operating targeted expression of gene therapy in cancer. Moreover, at the end of the article, we discuss challenges and future perspectives of promoter-operating targeted gene expression in cancer gene therapy.
The Principle of Promoter-Operating Targeted Expression of Gene Therapy in Cancer
The promoter is the upstream regulatory unit of the genetic region. Each gene has a promoter sequence at the 5′ phosphate terminus as a transcription start site, which is one of the important conditions for mRNA transcription to be initiated with the binding of RNA polymerase.6 Current studies have demonstrated that the promoter is activated by some specific transcription factors, and the promoter has tissue-specific regulatory function.7 Moreover, to tumor cells, there were some specific promoters that had specific transcriptional activity in tumor cells and no transcriptional activity or very low transcriptional activity in normal cells. For the moment, we termed these specific promoters as tumor-specific promoter (TSP). Thus, for cancer gene therapy, tumor-specific expression of interesting genes can be achieved by using TSPs to control these genes’ expression at the transcriptional level. In detail, these TSPs were constructed into a eukaryotic vector or virus vector to operate the expression of therapeutically interesting genes, such as tumor suppressor genes, suicide genes, antiangiogenic genes, and so on, and to archive valuable effects.
Therefore, the critical distinct principle of promoter-operating targeted expression of gene therapy in cancer is the optimal selection of TSPs that have specific activity in target cancer cells and subsequently operated the expression of interesting genes specifically in cancer cells, accordingly avoiding the damage to normal cells (Figure 2).
Figure 2.
The Principle of Promoter-Operating Targeted Expression in Cancer Gene Therapy
The Current Selection of Tumor-Specific Promoter in Cancer Gene Therapy
Promoter of Cholecystokinin Type A Receptor
Pancreatic cancer is an aggressive malignancy with morbidity rates almost equal to mortality rates because of the current lack of effective treatment options. In 2006, Li et al.8 reported that they modified and used the promoter of cholecystokinin type A receptor (CCKAR), which possessed relatively high activity in pancreatic cancer cells as compared with normal cells, to drive BikDD expression in a nude mouse xenograft mode of pancreatic cancer. They demonstrated an effective treatment by using the promoter of CCKAR, suggesting the feasibility of pancreatic-cancer-specific promoter-based gene therapy in pancreatic cancer treatment. Interestingly, in 2007, this research group further described a targeted approach to treat pancreatic cancer, in which they developed a versatile expression vector “VISA” operated by pancreatic-cancer-specific promoter (CCKAR-VISA), in which VP16-GAL4-WPRE was integrated with a systemic amplifier and a promoter gene of CCKAR, to target BikDD expression, a potent proapoptotic gene, in pancreatic tumors in vivo.9 Their data showed that this VISA system exhibited significant antitumor effects on pancreatic cancer and prolonged survival time of mice in multiple xenograft and syngeneic orthotopic murine models of pancreatic tumors, including the enhanced average tumor-specific CCKAR promoter activity of 600–700 times and the increased gene therapy specificity index, as well as prolonged expression time of 3–4 times. In 2014, they tried to develop human telomerase reverse transcriptase (hTERT)-VP16-Gal4-WPRE integrated systemic amplifier composite (T-VISA) vector and observed that the systemic administration of AT-VISA-BikDD encapsulated in liposomes could significantly repress the growth of prostate tumor and prolong the survival time of mice in orthotopic murine models, as well as in the murine transgenic adenocarcinoma prostate model, indicating the therapeutic potential of AT-VISA-BikDD in treatment of androgen-dependent prostate cancer (ADPC) and castration-resistant prostate cancer (CRPC) in the preclinical setting.10 These series of interesting works suggested the value of the usage of remodeled promoter of tumor-specific surface membrane molecules in increasing the efficiency of gene therapy.
Promoter of Vascular Endothelial Growth Factor Receptor
Tumor cell growth depends on vascular nutrition and oxygen supply. Then, the blocking on angiogenesis in tumor tissues is one of the effective ways to kill tumor or prevent tumor metastasis. Vascular endothelial growth factor (VEGF) is highly surface expressed in various cancer cells and is the most important angiogenesis-promoting factor, which can act on the vascular endothelial cells and induce the formation of tumor blood vessels, successively supplying oxygen for tumor growth. Kinase domain insert containing receptor (KDR), one of the two high-affinity tyrosine kinase receptors of VEGF, is lowly expressed in normal vascular endothelial cells and highly expressed in tumor vascular endothelial cells.11 Moreover, gene-directed enzyme pro-drug systems such as herpes simplex virus thymidine kinase (HSV-TK), in combination with the pro-drug ganciclovir (GCV), are reported as the most commonly used anti-cancer gene therapy system both in experimental models and clinical trials.12 Interestingly, Wang et al.13 used the differential expression of KDR in vascular endothelial cells between tumor tissues and normal tissues, and designed a KDR promoter-based HSV-TK expression adenovirus-mediated transfer system (AdKDR-tk), which only targeted expression of HSV-TK in tumor vascular endothelial cells. Then, recombinant adenovirus AdKDR-tk was used to infect KDR-expressing vascular endothelial cells human umbilical vein endothelial cells (HUVECs) in vitro. Their data showed that the survival rate of HUVECs infected with AdKDR-tk decreased significantly in the presence of GCV, a pro-drug for inducing cell death. While the survival rate of HepG2 cells, without KDR expressing, infected with AdKDR-tk was not significantly affected, indicating that KDR promoter could effectively regulate the specific expression of HSV-TK in KDR expression vascular endothelial cells, which might be a potential selection for targeted gene therapy in various cancers.
Promoter of hTERT
Telomerase is a reverse transcriptase that can reverse the synthesis of deoxyribonucleic acid by using an RNA sequence as its template. Its main function is to add a sequence of particles at the end of the chromosome to keep the telomere length stable. The activation of telomerase leads to unlimited proliferation of cells. Telomerase activity is evidently detected in the vast majority of tumors (in >90% of human cancers), but not in most normal cells.14, 15 Moreover, activation of telomerase is tightly regulated at the transcriptional level of the hTERT. Therefore, the use of the hTERT promoter-driven vector system could restrict the expression of therapeutically interesting genes to telomerase-positive tumors.
In 2001, Koga et al.16 first constructed an hTERT promoter-driven vector system that restricted the expression of fas-associating protein with a novel death domain (FADD) in telomerase-positive tumor cells. Their data showed that this system (hTERT/FADD) could effectively induce the apoptosis of tumor cells and inhibit the growth of tumor cells in vivo.16 At the same time, Komata et al.17 also reported that the hTERT promoter-operated expression of caspase-8 could significantly induce the apoptosis in hTERT-positive malignant glioma cells, but not in hTERT-negative astrocytes, fibroblasts, and alternative lengthening of telomeres cells, and significantly suppressed the growth of malignant glioma cells in the in vivo experimental setting. These works strongly suggested that the telomerase-specific transfer of distinct genes under the hTERT promoter is a novel targeting approach for the treatment of cancers.
Promoter of Thyroid Transcription Factor-1
Thyroid transcription factor (TTF)-1 is a member of the homeodomain-containing Nkx2 family of transcription factors. Recent evidence showed that TTF-1, as a lineage-specific oncogene, was dominantly expressed in lung cancer, but not other types of cancers, and its expression level was closely correlated with the prognosis of lung cancer patients.18, 19, 20 MicroRNA-7 (miR-7) is a unique member of miRNAs and plays an important role in the progression of various tumors.21, 22 Our previous works showed that miR-7 overexpression could obviously reduce the growth and metastasis of human lung cancer cells in vitro and in vivo.23 Moreover, the reduced expression of miR-7 was associated with the site’s mutation of its promoter region in lung cancer tissues.24 These data suggested that miR-7 may be a promising candidate for gene therapy against lung cancer.25 Most recently, our group first tried to construct a eukaryotic vector of promoter of TTF-1 gene-operating expression of miR-7 (termed p-T-miR-7) and observed its effects on the growth and migration of human lung cancer cells in vivo. Interestingly, we found that the growth and metastasis of human lung cancer cells in vivo was significantly reduced in remote hypodermic injection of the p-T-miR-7 group, accompanied by increased expression of miR-7 and altered transduction of the Akt and Erk pathway in situ in a lung cancer xenograft model in nude mice.26 Thus, these data for the first time indicate that TTF-1 promoter-operating distinct miRNA molecule expression also might be an ideal strategy for targeted expression of distinct miRNAs in lung cancer and were helpful for the development of gene therapy against clinical lung cancer.
Challenge and Future Perspectives
Efficiency
A large number of reports on TSPs have shown that TSPs have much lower activity than the commonly used (conventional) strong cytomegalovirus (CMV) enhancer/promoter, which is ubiquitously active without tumor specificity. Moreover, the efficiency of gene therapy depends greatly on the efficiency of interesting gene expression after systemic delivery. Therefore, one of the researchers’ goals in the current period is to develop a cancer-specific expression vector that would not only maintain cancer specificity, but also produce robust activity stronger than or comparable to that of the CMV promoter-driven expression vector in cancer cells, but much lower activity in normal cells. Notably, in 2012, Xie et al.27 developed a versatile T-based breast-cancer-specific promoter VISA composite (T-VISA) to target transgene expression in breast tumors and found that T-VISA has stronger activity comparable with or higher than that of the CMV promoter in cancer cells. They further found that the targeted expression of therapeutic gene BikDD driven by the T-VISA promoter could inhibit tumor cell growth at least as effectively as CMV-BikDD in vitro and in a dose-dependent manner. They further compared the therapeutic effects of T-VISA-BikDD and CMV-BikDD in an in vivo experimental setting, and confirmed that T-VISA-BikDD nanoparticles could more obviously inhibit tumor growth and prolong the survival rate of mice than CMV-BikDD nanoparticles did in the syngeneic orthotopic murine model.27 This interesting research suggested that optimal modification of an artificial promoter may be a useful approach to improve the target efficiency of targeted gene therapy against cancer.
Safety
Currently, the safety of gene therapy strategy, mainly including functional change of important organs and distribution of exogenous DNA, is another critical challenge for the potential application of promoter-operated gene expression therapy in cancer.28, 29, 30
In the aspect of distribution of exogenous DNA, Mahato et al.31 investigated the disposition characteristics of pDNA complexed with cationic liposomes after intravenous injection in mice, and found that liposomal pDNA encoding gene expression enriched in lung, heart, kidney, and spleen. Thanaketpaisarn et al.32 further reported that naked plasmid DNA (pDNA) encoding firefly luciferase was directly injected into the tail vein of mice, and found that the plasmid mostly enriched in liver, spleen, and kidney. Besides, some literature documented naked pDNA enriched in the liver in vivo after hydrodynamic injection via tail vein.33 Different from these works, in our recent research,26 we analyzed the distribution of naked plasmid p-T-miR-7 after remote hypodermic injection and found that the plasmid dominantly enriched in lung tissue and tumor mass in vivo. To the diverse phenomenon, we proposed two factors may be closely related to the different distribution of pDNA in vivo. The first factor was the different experimental setting including entry route and way, as well as the dosage, of naked pDNA. The second factor was the different hemodynamics and perfusion in distinct organs and tissues.
To the functional change of important organs, Xie et al.27 treated 4T1 tumor-bearing mice with the pDNA/nanoparticle complexes carrying either CMV-Luc or T-VISA-Luc via tail vein. Their data showed that T-VISA-BikDD induced remarkable cell apoptosis in tumor tissues, but not in the normal tissues such as the lung and the liver, whereas CMV-BikDD led to substantial apoptosis both in tumor tissues and in surrounding organs. To further evaluate whether treatment with T-VISA-BikDD was safer than CMV-BikDD, they used a single dose of 50 or 100 μg of pDNA to treat BALB/c mice by tail-veil injection and analyzed serum levels of alanine transaminase (ALT) and aspartate transaminase (AST). Their data showed that serum levels of ALT and AST increased on day 2 after injection in the CMV-BikDD treatment group, whereas they were within the reference range for the control in T-VISA-BikDD treatment groups at any measured time points. Similar to their finding, our recent work also showed that, after plasmid p-miR-7 subcutaneous injection treatment, there were not any significant pathological changes on both local skin tissue and some other important organs, including liver and heart, in the nude mouse model of human lung cancer.26 Combining these results, at least, indicated the potential safety of tumor-specific promoter-targeted expression of distinct genes in cancer gene therapy. However, the exact safety of promoter-operating targeted gene therapy, including gene integration and degradation time course, in cancer gene therapy still remains to be fully elucidated in the future.
Future Perspectives
In the last 20 years, there was outstanding progression on the research on promoter-operating targeted expression of gene therapy in cancer. In the future, there are still some core issues that should be further addressed, which will much benefit the ultimate application of promoter-operating targeted expression of gene therapy approach in clinical cancer patients. The first is still the optimal selection of the TSPs, which is a critical factor for specificity, efficiency, and safety problems of target gene therapy in cancer. Up to now, there have been some other alternative candidate TSPs used in different experimental studies (Table 1). However, different TSPs have their distinct advantages and disadvantages (Table 2). Therefore, it is particularly important to select the appropriate TSPs for a specific tumor. Just as we mentioned above, it is believed that the remold of artificial promoter might be a promising approach in the future, especially further verification, optimization, and modification of promoters. For example, researchers found that the adjustment of the length of promoter nucleic acid sequence is one of the optimization methods, including the verification of the core sequence of promoter.34 The second is the selection of more valuable interesting genes. Besides these documented interesting genes (Table 3), accumulating evidence further suggested that non-coding RNA sequences such as miRNA,26 short hairpin RNA (shRNA),35 and RNAi36 may be optional choices for the ideal genes in cancer gene therapy. Moreover, the identification of other new tumor-associated genes also may be helpful for the choice of interesting genes. The third is the delivery pathways and delivery system. The rational selection of drug delivery pathway will further improve the implementation of gene therapy. Moreover, the adoption of some delivery systems such as Nano carriers, with the characteristics of low toxicity, low immunogenicity, long metabolic cycle, and easy modification, are very valuable for enhancing the efficacy and reducing the potential side effect of gene therapy.37, 38 In addition, the exploration of promoter-related characteristic transcription factors, promoter epigenetic alterations, and its integrity are also helpful for the rapid development of promoter-targeted gene therapy research.
Table 1.
The Application of Promoter in Cancer Gene Therapy
| Promoters | Tumor Therapy |
|---|---|
| Promoters Targeting Non-specific Tumor Cells | |
| hTERT10, 16, 17, 34, 64 | lung cancer, liver cancer, gastrointestinal cancer, breast cancer, etc. |
| KDR12, 13 | lung cancer, liver cancer, gastrointestinal cancer, breast cancer, etc. |
| Bmi-136 | gastric cancer, prostate cancer, glioma cancer, etc. |
| Survivin39, 40, 60, 61, 63 | liver cancer, gastrointestinal cancer, gallbladder cancer, etc. |
| HER-240, 41 | prostate cancer, breast cancer, pancreatic cancer, etc. |
| uPAR42, 43 | colorectal cancer, colon cancer, pancreatic cancer, breast cancer, etc. |
| A3344, 45 | colorectal cancer, gastric cancer, etc. |
| COX-244, 46, 47 | colorectal cancer, endometrial cancer, breast cancer, etc. |
| FGF44, 48 | colorectal cancer, ovarian cancer, prostate cancer, etc. |
| Rad5149, 50 | lung cancer, breast cancer, cervix cancer, pancreatic cancer, etc. |
| Promoters Targeting Specific Tumor Cells | |
| CCKAR8, 9 | pancreatic cancer |
| TTF-126 | lung cancer |
| AFP51, 52 | liver cancer |
| CEA53, 54, 55 | gastrointestinal cancer |
| PSA56, 57 | prostate cancer |
| PB58, 59, 62 | prostate cancer |
A33, glycoprotein A33; AFP, alpha fetal protein; Bmi-1, B cell-specific Moloney leukemia virus insertion site 1; CCKAR, cholecystokinin type A receptor; CEA, carcinoma embryonic antigen; COX-2, cyclooxygenase-2; FGF, fibroblast growth factor; HER-2, human epidermal growth factor receptor 2; hTERT, human telomerase reverse transcriptase; KDR, kinase domain insert containing receptor; PB, probasin; PSA, prostate-specific antigen; Rad51, rad51 recombinase; TTF-1, thyroid transcription factor-1; uPAR, urokinase-type plasminogen activator receptor.
Table 2.
Key Features of Different Tumor-Specific Promoters in Cancer Gene Therapy
| Promoters | Character | Advantages | Disadvantages |
|---|---|---|---|
| Promoter Targeting Non-specific Tumor Cells | |||
| KDR | high activity in gastrointestinal cancer, lung cancer, liver cancer, breast cancer, etc. | tumor non-specificity, widely used for various tumors |
the effect on distinct tumors may be different |
| hTERT | high activity in gastrointestinal cancer, lung cancer, liver cancer, breast cancer, etc. | ||
| Bmi-1 | high activity in gastric cancer, prostate cancer, glioma cancer, etc. | ||
| Survivin | high activity in gastrointestinal cancer, lung cancer, liver cancer, breast cancer, etc. | ||
| HER-2 | high activity in prostate cancer, breast cancer, pancreatic cancer, etc. | ||
| uPAR | high activity in colorectal cancer, pancreatic cancer, colon cancer, breast cancer, etc. | ||
| A33 | high activity in colorectal cancer, gastric cancer, etc. | ||
| COX-2 | high activity in colorectal cancer, endometrial cancer, breast cancer, etc. | ||
| FGF | high activity in colorectal cancer, ovarian cancer, prostate cancer, etc. | ||
| Rad51 | high activity in lung cancer, breast cancer, cervix cancer, pancreatic cancer, etc. | ||
| Promoters Targeting Specific Tumor Cells | |||
| CCKAR | high activity in pancreatic cancer | tumor specificity, the effect on a specific tumor is relatively certain | only used for specific tumor |
| TTF-1 | high activity in lung cancer | ||
| AFP | high activity in liver cancer | ||
| CEA | high activity in gastrointestinal cancer | ||
| PSA | high activity in prostate cancer | ||
| PB | high activity in prostate cancer | ||
A33, glycoprotein A33; AFP, alpha fetal protein; Bmi-1, B cell-specific Moloney leukemia virus insertion site 1; CCKAR, cholecystokinin type A receptor; CEA, carcinoma embryonic antigen; COX-2, cyclooxygenase-2; FGF, fibroblast growth factor; HER-2, human epidermal growth factor receptor 2; hTERT, human telomerase reverse transcriptase; KDR, kinase domain insert containing receptor; PB, probasin; PSA, prostate-specific antigen; Rad51, rad51 recombinase; TTF-1, thyroid transcription factor-1; uPAR, urokinase-type plasminogen activator receptor.
Table 3.
The Application of Candidate Genes in Cancer Gene Therapy
| Gene-Based Therapeutic Strategies | Candidate Genes |
|---|---|
| Suicide gene | Bik,8, 9, 10 HSV-TK/GCV,12, 13, 41, 43, 46, 48, 59 FADD,16 caspase-8,17 caspase-3,40 CD/5-FC,41, 54 NfsB/CB 1954,41, 56 DTA,49, 50, 57 tBid,51 TRAIL,52 TK/CD,55 Bax,60 etc. |
| Tumor suppressor gene | miR-7,26 E1A,34, 45 15-PGDH,47 Gel,61 p202,62 P53,63 etc. |
| Antiangiogenic gene | CD105,35 Bmi-1,36 alpha3(IV)NC164 |
alpha3(IV)NC1, noncollagenous domain of alpha3(IV) collagen; Bax, BCL2-associated X protein; Bik, Bcl-2 interacting killer; Bmi-1, B cell-specific Moloney leukemia virus insertion site 1; CD105, Endoglin; CD/5-FC, cytosine deaminase/5-fluorocytosine; DTA, diphtheria toxin A; E1A, adenoviral type 5 transcription factor that possesses anticancer properties; FADD, fas-associating protein with a novel death domain; Gel, Gelonin; p202, interferon-activated gene 202B; HSV-TK/GCV, herpes simplex virus thymidine kinase gene/ganciclovir; miR-7, microRNA-7; NfsB/CB1954, nitroreductase/5-(aziridin-1-yl)-2,4-dinitrobenzamide; 15-PGDH, 15-hydroxyprostaglandin dehydrogenase; tBid, truncated BH3 interacting domain death agonist; TRAIL, tumor necrosis factor-related apoptosis-inducing ligand.
In all, successive research on the exploration of more ideal target genes, effective promoter optimization schemes, and rational route of delivery, as well as delivery system, will undoubtedly provide a better prospect of clinical application for promoter-operating targeted expression of gene therapy in cancer.
Author Contributions
C.C. designed and wrote the paper; D.Y. and H.W. wrote the paper; L.L., J.L., Y.Z., S.L., T.D., and M.G. analyzed the related information; L.X. conceived, designed, and wrote the paper; and all authors reviewed the paper.
Conflicts of Interest
All authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
This work was supported by the Program for High Level Innovative Talents in Guizhou Province (QKH-RC-2016-4031), the National Natural Science Foundation of China (31760258), the Project of Guizhou Provincial Department of Science and Technology (QKH-LH-2015-7542), the Program for New Century Excellent Talents in University, Ministry of Education of China (NCET-12-0661), the Program for Excellent Young Talents of Zunyi Medical University (15ZY-001), and the Project of Guizhou Provincial Department of Science and Technology (2009C491).
References
- 1.Hacein-Bey-Abina S., Pai S.Y., Gaspar H.B., Armant M., Berry C.C., Blanche S., Bleesing J., Blondeau J., de Boer H., Buckland K.F. A modified γ-retrovirus vector for X-linked severe combined immunodeficiency. N. Engl. J. Med. 2014;371:1407–1417. doi: 10.1056/NEJMoa1404588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Greene C.S., Krishnan A., Wong A.K., Ricciotti E., Zelaya R.A., Himmelstein D.S., Zhang R., Hartmann B.M., Zaslavsky E., Sealfon S.C. Understanding multicellular function and disease with human tissue-specific networks. Nat. Genet. 2015;47:569–576. doi: 10.1038/ng.3259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rogers S., Pfuderer P. Use of viruses as carriers of added genetic information. Nature. 1968;219:749–751. doi: 10.1038/219749a0. [DOI] [PubMed] [Google Scholar]
- 4.Luo J., Luo Y., Sun J., Zhou Y., Zhang Y., Yang X. Adeno-associated virus-mediated cancer gene therapy: current status. Cancer Lett. 2015;356(2 Pt B):347–356. doi: 10.1016/j.canlet.2014.10.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pearson S., Jia H., Kandachi K. China approves first gene therapy. Nat. Biotechnol. 2004;22:3–4. doi: 10.1038/nbt0104-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lo H.W., Day C.P., Hung M.C. Cancer-specific gene therapy. Adv. Genet. 2005;54:235–255. doi: 10.1016/S0065-2660(05)54010-0. [DOI] [PubMed] [Google Scholar]
- 7.Perera D., Poulos R.C., Shah A., Beck D., Pimanda J.E., Wong J.W. Differential DNA repair underlies mutation hotspots at active promoters in cancer genomes. Nature. 2016;532:259–263. doi: 10.1038/nature17437. [DOI] [PubMed] [Google Scholar]
- 8.Li Z., Ding Q., Li Y., Miller S.A., Abbruzzese J.L., Hung M.C. Suppression of pancreatic tumor progression by systemic delivery of a pancreatic-cancer-specific promoter driven Bik mutant. Cancer Lett. 2006;236:58–63. doi: 10.1016/j.canlet.2005.05.001. [DOI] [PubMed] [Google Scholar]
- 9.Xie X., Xia W., Li Z., Kuo H.P., Liu Y., Li Z., Ding Q., Zhang S., Spohn B., Yang Y. Targeted expression of BikDD eradicates pancreatic tumors in noninvasive imaging models. Cancer Cell. 2007;12:52–65. doi: 10.1016/j.ccr.2007.05.009. [DOI] [PubMed] [Google Scholar]
- 10.Xie X., Kong Y., Tang H., Yang L., Hsu J.L., Hung M.C. Targeted BikDD expression kills androgen-dependent and castration-resistant prostate cancer cells. Mol. Cancer Ther. 2014;13:1813–1825. doi: 10.1158/1535-7163.MCT-13-1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shibuya M. Differential roles of vascular endothelial growth factor receptor-1 and receptor-2 in angiogenesis. J. Biochem. Mol. Biol. 2006;39:469–478. doi: 10.5483/bmbrep.2006.39.5.469. [DOI] [PubMed] [Google Scholar]
- 12.Mesnil M., Yamasaki H. Bystander effect in herpes simplex virus-thymidine kinase/ganciclovir cancer gene therapy: role of gap-junctional intercellular communication. Cancer Res. 2000;60:3989–3999. [PubMed] [Google Scholar]
- 13.Wang Y., Xu H.X., Lu M.D., Tang Q. Expression of thymidine kinase mediated by a novel non-viral delivery system under the control of vascular endothelial growth factor receptor 2 promoter selectively kills human umbilical vein endothelial cells. World J. Gastroenterol. 2008;14:224–230. doi: 10.3748/wjg.14.224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang X., Mar V., Zhou W., Harrington L., Robinson M.O. Telomere shortening and apoptosis in telomerase-inhibited human tumor cells. Genes Dev. 1999;13:2388–2399. doi: 10.1101/gad.13.18.2388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Patel P.L., Suram A., Mirani N., Bischof O., Herbig U. Derepression of hTERT gene expression promotes escape from oncogene-induced cellular senescence. Proc. Natl. Acad. Sci. USA. 2016;113:E5024–E5033. doi: 10.1073/pnas.1602379113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Koga S., Hirohata S., Kondo Y., Komata T., Takakura M., Inoue M., Kyo S., Kondo S. FADD gene therapy using the human telomerase catalytic subunit (hTERT) gene promoter to restrict induction of apoptosis to tumors in vitro and in vivo. Anticancer Res. 2001;21(3B):1937–1943. [PubMed] [Google Scholar]
- 17.Komata T., Kondo Y., Kanzawa T., Ito H., Hirohata S., Koga S., Sumiyoshi H., Takakura M., Inoue M., Barna B.P. Caspase-8 gene therapy using the human telomerase reverse transcriptase promoter for malignant glioma cells. Hum. Gene Ther. 2002;13:1015–1025. doi: 10.1089/104303402753812421. [DOI] [PubMed] [Google Scholar]
- 18.Puglisi F., Barbone F., Damante G., Bruckbauer M., Di Lauro V., Beltrami C.A., Di Loreto C. Prognostic value of thyroid transcription factor-1 in primary, resected, non-small cell lung carcinoma. Mod. Pathol. 1999;12:318–324. [PubMed] [Google Scholar]
- 19.Barletta J.A., Perner S., Iafrate A.J., Yeap B.Y., Weir B.A., Johnson L.A., Johnson B.E., Meyerson M., Rubin M.A., Travis W.D. Clinical significance of TTF-1 protein expression and TTF-1 gene amplification in lung adenocarcinoma. J. Cell. Mol. Med. 2009;13(8B):1977–1986. doi: 10.1111/j.1582-4934.2008.00594.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhao Q., Xu S., Liu J., Li Y., Fan Y., Shi T., Wei S., Tang S.C., Liu H., Chen J. Thyroid transcription factor-1 expression is significantly associated with mutations in exon 21 of the epidermal growth factor receptor gene in Chinese patients with lung adenocarcinoma. OncoTargets Ther. 2015;8:2469–2478. doi: 10.2147/OTT.S90602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Luo J., Li H., Zhang C. MicroRNA-7 inhibits the malignant phenotypes of non-small cell lung cancer in vitro by targeting Pax6. Mol. Med. Rep. 2015;12:5443–5448. doi: 10.3892/mmr.2015.4032. [DOI] [PubMed] [Google Scholar]
- 22.Zeng C.Y., Zhan Y.S., Huang J., Chen Y.X. MicroRNA-7 suppresses human colon cancer invasion and proliferation by targeting the expression of focal adhesion kinase. Mol. Med. Rep. 2016;13:1297–1303. doi: 10.3892/mmr.2015.4643. [DOI] [PubMed] [Google Scholar]
- 23.Xu L., Wen Z., Zhou Y., Liu Z., Li Q., Fei G., Luo J., Ren T. MicroRNA-7-regulated TLR9 signaling-enhanced growth and metastatic potential of human lung cancer cells by altering the phosphoinositide-3-kinase, regulatory subunit 3/Akt pathway. Mol. Biol. Cell. 2013;24:42–55. doi: 10.1091/mbc.E12-07-0519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhao J., Wang K., Liao Z., Li Y., Yang H., Chen C., Zhou Y.A., Tao Y., Guo M., Ren T., Xu L. Promoter mutation of tumor suppressor microRNA-7 is associated with poor prognosis of lung cancer. Mol. Clin. Oncol. 2015;3:1329–1336. doi: 10.3892/mco.2015.648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhao J., Tao Y., Zhou Y., Qin N., Chen C., Tian D., Xu L. MicroRNA-7: a promising new target in cancer therapy. Cancer Cell Int. 2015;15:103. doi: 10.1186/s12935-015-0259-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lei L., Chen C., Zhao J., Wang H., Guo M., Zhou Y., Luo J., Zhang J., Xu L. Targeted expression of miR-7 operated by TTF-1 promoter inhibited the growth of human lung cancer through the NDUFA4 Pathway. Mol. Ther. Nucleic Acids. 2017;6:183–197. doi: 10.1016/j.omtn.2016.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Xie X., Li L., Xiao X., Guo J., Kong Y., Wu M., Liu W., Gao G., Hsu J.L., Wei W. Targeted expression of BikDD eliminates breast cancer with virtually no toxicity in noninvasive imaging models. Mol. Cancer Ther. 2012;11:1915–1924. doi: 10.1158/1535-7163.MCT-12-0191. [DOI] [PubMed] [Google Scholar]
- 28.Chandra D., Jahangir A., Cornelis F., Rombauts K., Meheus L., Jorcyk C.L., Gravekamp C. Cryoablation and Meriva have strong therapeutic effect on triple-negative breast cancer. OncoImmunology. 2015;5:e1049802. doi: 10.1080/2162402X.2015.1049802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Galanis E. Cancer: tumour-fighting virus homes in. Nature. 2011;477:40–41. doi: 10.1038/477040a. [DOI] [PubMed] [Google Scholar]
- 30.Breitbach C.J., Burke J., Jonker D., Stephenson J., Haas A.R., Chow L.Q., Nieva J., Hwang T.H., Moon A., Patt R. Intravenous delivery of a multi-mechanistic cancer-targeted oncolytic poxvirus in humans. Nature. 2011;477:99–102. doi: 10.1038/nature10358. [DOI] [PubMed] [Google Scholar]
- 31.Mahato R.I., Kawabata K., Takakura Y., Hashida M. In vivo disposition characteristics of plasmid DNA complexed with cationic liposomes. J. Drug Target. 1995;3:149–157. doi: 10.3109/10611869509059214. [DOI] [PubMed] [Google Scholar]
- 32.Thanaketpaisarn O., Nishikawa M., Yamashita F., Hashida M. Tissue-specific characteristics of in vivo electric gene: transfer by tissue and intravenous injection of plasmid DNA. Pharm. Res. 2005;22:883–891. doi: 10.1007/s11095-005-4583-2. [DOI] [PubMed] [Google Scholar]
- 33.Tada M., Hatano E., Taura K., Nitta T., Koizumi N., Ikai I., Shimahara Y. High volume hydrodynamic injection of plasmid DNA via the hepatic artery results in a high level of gene expression in rat hepatocellular carcinoma induced by diethylnitrosamine. J. Gene Med. 2006;8:1018–1026. doi: 10.1002/jgm.930. [DOI] [PubMed] [Google Scholar]
- 34.Xie X., Hsu J.L., Choi M.G., Xia W., Yamaguchi H., Chen C.T., Trinh B.Q., Lu Z., Ueno N.T., Wolf J.K. A novel hTERT promoter-driven E1A therapeutic for ovarian cancer. Mol. Cancer Ther. 2009;8:2375–2382. doi: 10.1158/1535-7163.MCT-09-0056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tesic N., Kamensek U., Sersa G., Kranjc S., Stimac M., Lampreht U., Preat V., Vandermeulen G., Butinar M., Turk B., Cemazar M. Endoglin (CD105) silencing mediated by shRNA under the control of Endothelin-1 promoter for targeted gene therapy of melanoma. Mol. Ther. Nucleic Acids. 2015;4:e239. doi: 10.1038/mtna.2015.12. [DOI] [PubMed] [Google Scholar]
- 36.Zhang X., Guo W., Wang X., Liu X., Huang M., Gan L., Cheng Y., Li J. Antitumor activity and inhibitory effects on cancer stem cell-like properties of Adeno-associated virus (AAV)-mediated Bmi-1 interference driven by Bmi-1 promoter for gastric cancer. Oncotarget. 2016;7:22733–22745. doi: 10.18632/oncotarget.8174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Venditto V.J., Szoka F.C., Jr. Cancer nanomedicines: so many papers and so few drugs! Adv. Drug Deliv. Rev. 2013;65:80–88. doi: 10.1016/j.addr.2012.09.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wicki A., Witzigmann D., Balasubramanian V., Huwyler J. Nanomedicine in cancer therapy: challenges, opportunities, and clinical applications. J. Control. Release. 2015;200:138–157. doi: 10.1016/j.jconrel.2014.12.030. [DOI] [PubMed] [Google Scholar]
- 39.Wang W., Ji W., Hu H., Ma J., Li X., Mei W., Xu Y., Hu H., Yan Y., Song Q. Survivin promoter-regulated oncolytic adenovirus with Hsp70 gene exerts effective antitumor efficacy in gastric cancer immunotherapy. Oncotarget. 2014;5:150–160. doi: 10.18632/oncotarget.1430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yang L., Cao Z., Li F., Post D.E., Van Meir E.G., Zhong H., Wood W.C. Tumor-specific gene expression using the survivin promoter is further increased by hypoxia. Gene Ther. 2004;11:1215–1223. doi: 10.1038/sj.gt.3302280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhang K.X., Jia W., Rennie P.S. Bioengineered viral vectors for targeting and killing prostate cancer cells. Bioeng. Bugs. 2010;1:92–96. doi: 10.4161/bbug.1.2.11102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Schewe D.M., Biller T., Maurer G., Asangani I.A., Leupold J.H., Lengyel E.R., Post S., Allgayer H. Combination analysis of activator protein-1 family members, Sp1 and an activator protein-2alpha-related factor binding to different regions of the urokinase receptor gene in resected colorectal cancers. Clin. Cancer Res. 2005;11:8538–8548. doi: 10.1158/1078-0432.CCR-05-0786. [DOI] [PubMed] [Google Scholar]
- 43.Teimoori-Toolabi L., Azadmanesh K., Amanzadeh A., Zeinali S. Selective suicide gene therapy of colon cancer exploiting the urokinase plasminogen activator receptor promoter. BioDrugs. 2010;24:131–146. doi: 10.2165/11530840-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 44.Rama A.R., Aguilera A., Melguizo C., Caba O., Prados J. Tissue specific promoters in colorectal cancer. Dis. Markers. 2015;2015:390161. doi: 10.1155/2015/390161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cafferata E.G., Macció D.R., Lopez M.V., Viale D.L., Carbone C., Mazzolini G., Podhajcer O.L. A novel A33 promoter-based conditionally replicative adenovirus suppresses tumor growth and eradicates hepatic metastases in human colon cancer models. Clin. Cancer Res. 2009;15:3037–3049. doi: 10.1158/1078-0432.CCR-08-1161. [DOI] [PubMed] [Google Scholar]
- 46.Wang Z.X., Bian H.B., Yang J.S., De W., Ji X.H. Adenovirus-mediated suicide gene therapy under the control of Cox-2 promoter for colorectal cancer. Cancer Biol. Ther. 2009;8:1480–1488. doi: 10.4161/cbt.8.15.8940. [DOI] [PubMed] [Google Scholar]
- 47.Kaliberova L.N., Kusmartsev S.A., Krendelchtchikova V., Stockard C.R., Grizzle W.E., Buchsbaum D.J., Kaliberov S.A. Experimental cancer therapy using restoration of NAD+-linked 15-hydroxyprostaglandin dehydrogenase expression. Mol. Cancer Ther. 2009;8:3130–3139. doi: 10.1158/1535-7163.MCT-09-0270. [DOI] [PubMed] [Google Scholar]
- 48.Teimoori-Toolabi L., Azadmanesh K., Zeinali S. Selective suicide gene therapy of colon cancer cell lines exploiting fibroblast growth factor 18 promoter. Cancer Biother. Radiopharm. 2010;25:105–116. doi: 10.1089/cbr.2009.0643. [DOI] [PubMed] [Google Scholar]
- 49.Hine C.M., Seluanov A., Gorbunova V. Use of the Rad51 promoter for targeted anti-cancer therapy. Proc. Natl. Acad. Sci. USA. 2008;105:20810–20815. doi: 10.1073/pnas.0807990106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hine C.M., Seluanov A., Gorbunova V. Rad51 promoter-targeted gene therapy is effective for in vivo visualization and treatment of cancer. Mol. Ther. 2012;20:347–355. doi: 10.1038/mt.2011.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ma S.H., Chen G.G., Yip J., Lai P.B.S. Therapeutic effect of alpha-fetoprotein promoter-mediated tBid and chemotherapeutic agents on orthotopic liver tumor in mice. Gene Ther. 2010;17:905–912. doi: 10.1038/gt.2010.34. [DOI] [PubMed] [Google Scholar]
- 52.Cao X., Yang M., Wei R.C., Zeng Y., Gu J.F., Huang W.D., Yang D.Q., Li H.L., Ding M., Wei N. Cancer targeting Gene-Viro-Therapy of liver carcinoma by dual-regulated oncolytic adenovirus armed with TRAIL gene. Gene Ther. 2011;18:765–777. doi: 10.1038/gt.2011.16. [DOI] [PubMed] [Google Scholar]
- 53.Xu C., Sun Y., Wang Y., Yan Y., Shi Z., Chen L., Lin H., Lü S., Zhu M., Su C., Li Z. CEA promoter-regulated oncolytic adenovirus-mediated Hsp70 expression in immune gene therapy for pancreatic cancer. Cancer Lett. 2012;319:154–163. doi: 10.1016/j.canlet.2012.01.009. [DOI] [PubMed] [Google Scholar]
- 54.Zhang G., Liu T., Chen Y.H., Chen Y., Xu M., Peng J., Yu S., Yuan J., Zhang X. Tissue specific cytotoxicity of colon cancer cells mediated by nanoparticle-delivered suicide gene in vitro and in vivo. Clin. Cancer Res. 2009;15:201–207. doi: 10.1158/1078-0432.CCR-08-1094. [DOI] [PubMed] [Google Scholar]
- 55.Qiu Y., Peng G.L., Liu Q.C., Li F.L., Zou X.S., He J.X. Selective killing of lung cancer cells using carcinoembryonic antigen promoter and double suicide genes, thymidine kinase and cytosine deaminase (pCEA-TK/CD) Cancer Lett. 2012;316:31–38. doi: 10.1016/j.canlet.2011.10.015. [DOI] [PubMed] [Google Scholar]
- 56.Latham J.P., Searle P.F., Mautner V., James N.D. Prostate-specific antigen promoter/enhancer driven gene therapy for prostate cancer: construction and testing of a tissue-specific adenovirus vector. Cancer Res. 2000;60:334–341. [PubMed] [Google Scholar]
- 57.Anderson D.G., Peng W., Akinc A., Hossain N., Kohn A., Padera R., Langer R., Sawicki J.A. A polymer library approach to suicide gene therapy for cancer. Proc. Natl. Acad. Sci. USA. 2004;101:16028–16033. doi: 10.1073/pnas.0407218101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kakinuma H., Bergert E.R., Spitzweg C., Cheville J.C., Lieber M.M., Morris J.C. Probasin promoter (ARR(2)PB)-driven, prostate-specific expression of the human sodium iodide symporter (h-NIS) for targeted radioiodine therapy of prostate cancer. Cancer Res. 2003;63:7840–7844. [PubMed] [Google Scholar]
- 59.Yu D., Scott C., Jia W.W., De Benedetti A., Williams B.J., Fazli L., Wen Y., Gleave M., Nelson C., Rennie P.S. Targeting and killing of prostate cancer cells using lentiviral constructs containing a sequence recognized by translation factor eIF4E and a prostate-specific promoter. Cancer Gene Ther. 2006;13:32–43. doi: 10.1038/sj.cgt.7700885. [DOI] [PubMed] [Google Scholar]
- 60.Garg H., Salcedo R., Trinchieri G., Blumenthal R. Improved nonviral cancer suicide gene therapy using survivin promoter-driven mutant Bax. Cancer Gene Ther. 2010;17:155–163. doi: 10.1038/cgt.2009.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wang Z., Zhou X., Li J., Liu X., Chen Z., Shen G., Guan T., Ye N., Wei X., Huang N. Suppression of hepatoma tumor growth by systemic administration of the phytotoxin gelonin driven by the survivin promoter. Neoplasma. 2013;60:469–479. doi: 10.4149/neo_2013_061. [DOI] [PubMed] [Google Scholar]
- 62.Wen Y., Giri D., Yan D.H., Spohn B., Zinner R.G., Xia W., Thompson T.C., Matusik R.J., Hung M.C. Prostate-specific antitumor activity by probasin promoter-directed p202 expression. Mol. Carcinog. 2003;37:130–137. doi: 10.1002/mc.10129. [DOI] [PubMed] [Google Scholar]
- 63.Liu C., Sun B., An N., Tan W., Cao L., Luo X., Yu Y., Feng F., Li B., Wu M. Inhibitory effect of Survivin promoter-regulated oncolytic adenovirus carrying P53 gene against gallbladder cancer. Mol. Oncol. 2011;5:545–554. doi: 10.1016/j.molonc.2011.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Miyoshi T., Hirohata S., Ogawa H., Doi M., Obika M., Yonezawa T., Sado Y., Kusachi S., Kyo S., Kondo S. Tumor-specific expression of the RGD-alpha3(IV)NC1 domain suppresses endothelial tube formation and tumor growth in mice. FASEB J. 2006;20:1904–1906. doi: 10.1096/fj.05-5565fje. [DOI] [PubMed] [Google Scholar]