Abstract
Aging is associated with progressive decline of skeletal muscle mass and function. This condition, termed sarcopenia, is associated with several adverse outcomes, including loss of autonomy and mortality. Due to the high prevalence of sarcopenia, a deeper understanding of its pathophysiology and possible remedies represents a high public health priority. Evidence suggests the existence of a relationship between declining growth hormone (GH) and insulin-like growth factor-1 (IGF-1) levels and age-related changes in body composition and physical function. Therefore, the age-dependent decline of GH and IGF-1 serum levels may promote frailty by contributing to the loss of muscle mass and strength. Preclinical studies showed that infusion of angiotensin II produced a marked reduction in body weight, accompanied by decreased serum and muscle levels of IGF-1. Conversely, overexpression of muscle-specific isoform of IGF-1 mitigates angiotensin II-induced muscle loss. Moreover, IGF-1 serum levels have been shown to increase following angiotensin converting enzyme inhibitors (ACEIs) treatment. Here we will review the most recent evidence regarding age-related changes of the GH/IGF-1 axis and its modulation by several interventions, including ACEIs which might represent a potential novel strategy to delay the onset and impede the progression of sarcopenia.
Keywords: Aging, Sarcopenia, GH/IGF-1 axis, Angiotensin, ACE-inhibitors
1. Introduction
Frailty is a common pathophysiological condition in older adults characterized by diminished reserve capacity and increased risk of disability, institutionalization and mortality. Poor muscle strength is a central feature of frailty, and sarcopenia has been identified as a major modifiable risk factor for this syndrome (Roubenoff, 2000). Multiple factors have been evoked in the etiology of sarcopenia. Among them, atrophy of skeletal muscle fibers secondary to loss of a-motor neurons (Vandervoort, 2002) appears to represent a major causative factor. Other mechanisms are also involved, such as physical inactivity (Szulc et al., 2004), increased levels of pro-inflammatory cytokines (e.g., tumor necrosis factor-a, interleukin-1b, interleukin-6, etc.) (Visser et al., 2002), increased production of free radicals and/or diminished antioxidant defense systems (Fulle et al., 2004), malnutrition (Dreyer and Volpi, 2005), and low anabolic hormone output (e.g., testosterone, growth hormone, etc.) (Szulc et al., 2004). Regarding the latter, attention has been recently focused on the growth hormone (GH)/insulin-like growth factor-1 (IGF-1) axis, which is regarded as an important regulator of body composition. Notably, local as well as systemic isoforms of IGF-1 have been described. Skeletal muscle expresses at least two distinct splicing variants of IGF-1, namely IGF-1Ea, which is similar to the systemic form, and the mechano growth factor (MGF), which is released in response to physical activity (Yang et al., 1996). These two muscle-derived variants of IGF-1 have different actions, with IGF-1Ea being a potent stimulator of protein synthesis, while MGF promotes satellite cells proliferation.
Serum levels of GH as well as those of its systemic mediators decline with advancing age, and this has been associated with detrimental changes in body composition (i.e., reduction of lean body mass and increased adiposity). Besides the dysfunction of GH/IGF-1 axis, alteration of other humoral factors may be involved in the onset and progression of muscle loss and physical disability at old age. In this regard, angiotensin II has been shown to enhance protein degradation and reduce the autocrine production of IGF-1 in rat muscle (Brink et al., 1996, 2001). In contrast, overexpression of muscle-specific IGF-1 (both splicing variants) almost completely prevented angiotensin II-induced muscle loss in mice (Song et al., 2005). Recent evidence suggests that angiotensin converting enzyme inhibitors (ACEIs) may induce positive changes on body composition and physical function in older populations (Onder et al., 2002). It is also documented that ACEIs increase blood flow to muscles (Frisbee and Lombard, 2000), raise skeletal muscle glucose uptake (Kudoh and Matsuki, 2000), and reduce systemic secretion of inflammatory cytokines (Egido and Ruiz-Ortega, 2007). These effects are attributed primarily, but not exclusively, to the inhibition of the renin–angiotensin–aldosterone system.
Here, we will review the most recent findings regarding the modulation of GH/IGF-1 axis by systemic and/or autocrine up-regulation of IGF-1 and ACEIs as potential strategies to counteract the age-associated muscle loss.
2. Biological actions of IGF-1 in skeletal muscle
IGF-1 is perhaps the most important mediator of muscle growth and repair (Goldspink, 2007) and is produced in several ways. In response to GH, the liver produces IGF-1 for systemic release. Skeletal muscle also produces and secretes IGF-1 that possesses autocrine and paracrine actions (Daughaday, 2000). Muscle IGF-1 production may occur in response to GH (Sadowski et al., 2001), testosterone (Bhasin et al., 2001), and muscle overload and stretch (Goldspink et al., 2002). DeVol et al. (1990) were among the first to demonstrate local production of IGF-1 in skeletal muscle. These investigators used a rat model of hypertrophy of the soleus and plantaris muscles following severing of the gastrocnemius tendon. They showed that muscle hypertrophy and IGF-1 production occurred independent of GH, as it was not blunted in hypophesectomized rats that were virtually devoid of circulating GH (DeVol et al., 1990).
Goldspink et al. (2002) have shown that IGF-1 exists in at least two isoforms as a result of alternative splicing of the IGF-1 gene. IGF-1Ea, which is produced in both the liver and muscle, was the first form discovered and it is often referred to as liver-type or systemic IGF-1.
IGF-1Eb (rodent form) and IGF-1Ec (human form) are produced mainly by the muscle and are usually referred to as mechano growth factor (MGF). Unlike MGF, liver-type IGF-1 is glycosylated, which protects it from proteolysis, conferring a relatively long half-life. In muscle, the two forms of IGF-1 are produced in response to different stimuli, have different actions in muscle and probably interact with different receptors. MGF is specifically produced in response to muscle overload, stretch or damage. IGF-1 is both hyperplastic and hypertrophic in skeletal muscle. The hyperplastic effect results in the proliferation of muscle satellite cells, which donate their nuclei to the multinucleated myofiber. The hypertrophic effect results in increased synthesis of contractile proteins by existing myonuclei. In vitro studies with cultured C2C12 mouse myoblasts have provided preliminary evidence that liver-like IGF-1 and MGF may support different aspects of these processes (Yang and Goldspink, 2002). Besides stimulating muscle protein synthesis, IGF-1 also suppresses proteolysis (Ballard and Francis, 1983; Ewton et al., 1987; Hembree et al., 1991). Additionally, IGF-1 may promote the delivery of amino acids and glucose to myocytes (Laager et al., 1993), and stimulate myoblast proliferation and differentiation (Foulstone et al., 2004).
Furthermore, systemic IGF-1 administration increases the rate of skeletal muscle functional recovery after injury (Schertzer and Lynch, 2006), reduces the susceptibility to contraction-induced damage (Schertzer et al., 2006), and improvinges endurance (Gregorevic et al., 2004) and contractile function (Lynch et al., 2001; Gregorevic et al., 2002).
The muscle anabolic properties of autocrine IGF-1 have been confirmed by models of overexpression of locally acting IGF-1 (Barton-Davis et al., 1998, 1999; Coleman et al., 1995; Musaro et al., 2001; Barton et al., 2002). Recently, it has been demonstrated that up-regulation of muscle IGF-1 accelerates the regenerative process in injured skeletal muscle by modulating the inflammatory response and limiting fibrosis (Pelosi et al., 2007). Additionally, muscle IGF-1 may act as a potent regenerative agent, by increasing the recruitment of transplanted bone marrow stem cells into sites of muscle injury (Musaro et al., 2004).
Interestingly, administration of muscle IGF-1 has been recently shown as a promising strategy to improve cardiac function and reduce infarct size after acute myocardial injury (Santini et al., 2007; Carpenter et al., 2008). Mechanisms hypothesized for this effect include the reduction of inflammatory response (e.g., IL-6 and IL-1b) and the severity of cardiomyocyte apoptosis. Furthermore, mice overexpressing muscle IGF-1 are protected against heart failure-induced sarcopenia possibly via inhibition of the ubiquitin-proteasome pathway of protein degradation. (Schulze et al., 2005).
3. Age-related changes in IGF-1 actions
Several studies suggest that IGF-1 is an important modulator of muscle mass, muscle strength and function, not only during development, but also across the entire life span (Ballard and Francis, 1983; Borst and Lowenthal, 1997). In a recent study, Grounds (2002) has concluded that loss of muscle mass occurring with age is mainly a result of atrophy and subsequent reduction in myofiber number (particularly fast-twitch type 2B), whereas impaired muscle regeneration may be only marginally involved. Grounds further hypothesizes that reduction of IGF-1 signaling may play a prominent role in motor neurons loss (Grounds, 2002). In support of this hypothesis, it has been demonstrated that IGF-1 administration has positive effects on neuronal function by preventing apoptotic death, and by stimulating axonal sprouting and repair of damaged axons (Lewis et al., 1993; Festoff et al., 1995; Vergani et al., 1999).
GH is secreted in a pulsatile manner, and pulse amplitude and frequency are markedly reduced with age (Goya et al., 1999). The main actions of GH are to stimulate the synthesis of IGF-1 by the liver for systemic release and to induce local IGF-1 production in skeletal muscle (Fig. 1). Owino et al. (2001) reported that overload-and stretch-dependent MGF production in muscle was impaired in aged mice. A similar phenomenon has been shown by Hameed and coworkers in older human subjects (Hameed et al., 2003). In addition, advanced age is accompanied by impaired signaling through the IGF type 1 receptor, as both receptor density (Martineau et al., 1999) and receptor affinity for IGF-1 (Arvat et al., 2000) are reduced with age.
Fig. 1.
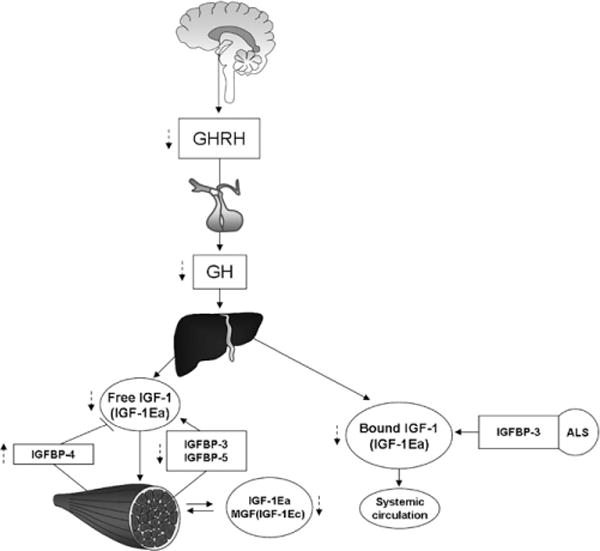
Synoptic view of the GH/IGF-1 axis. GH is released from the anterior pituitary gland in response to hypothalamus stimulus. Growth hormone releasing hormone (GHRH) initiates the pulse, which is self-limiting. IGF-1 circulates in both a “free” and “bound” state. The main circulating form of IGF-1 is IGF-1 + IGFBP-3 + ALS (acid labile subunit). Both bound and free circulating IGF-1 is represented by the IGF-1Ea form. There are also GH-independent, autocrine/paracrine isoforms of IGF-1 produced by skeletal muscle: IGF-1Ea and MGF or IGF-1Ec in humans; IGF-1Ea and IGF-1Eb in rodents. Dashed lines indicate age-related changes.
Aging is also associated with reduced insulin sensitivity which, in turn, may contribute to the impairment of IGF-1 activity. For instance, insulin directly stimulates hepatic IGF-1 production even in the absence of GH, as indicated by the increase of IGF-1 mRNA abundance in primary cultures of hepatocytes exposed to insulin (Boni-Schnetzler et al., 1991). Insulin may also modulate peripheral IGF-1 activity via regulation of IGF-binding proteins (IGFBPs). In particular, insulin reduces IGFBP-1 levels (Powell et al., 1991), which possesses inhibitory effect on IGF-1 by reducing IGF-1-related glucose consumption (Okajima et al., 1993). In addition, insulin may stimulate the production of IGFBP-3 (Nyomba et al., 1997), the main carrier of IGF-1 in serum. It may therefore be hypothesized that amelioration of insulin sensitivity at old age might contribute to restoring IGF-1 systemic levels and function.
Studies conducted in older adults have reported contrasting findings regarding the relation between systemic levels of IGF-1 and muscle strength and physical function. Indeed, several studies failed to demonstrate a significant relationship between plasma IGF-1 levels and measures of body composition and muscle strength in elderly individuals (Papadakis et al., 1995; Boonen et al., 1998; Kiel et al., 1998). Moreover, Janssen et al. (1998) showed that free and total IGF-1, IGFBP-1 and IGFBP-3 levels were not different among elderly persons with various levels of self-reported disability. In contrast, other studies have identified a significant association between low plasma IGF-1 levels, poor muscle strength, impaired physical performance and self-reported difficulties with mobility tasks (Kostka et al., 2000; Cappola et al., 2001). Moreover, a negative correlation between serum IGF-1 and levels of physical activity has also been reported (Landin-Wilhelmsen et al., 1994).
Interestingly, it was demonstrated in vitro that IL-6 possesses inhibitory effects on IGF-1 secretion and activity (Lazarus et al., 1993). IL-6 is a pro-inflammatory cytokine up-regulated with age (Ershler and Keller, 2000), that has been associated with increased mortality (Harris et al., 1999) and disability (Ferrucci et al., 1999) in the elderly. Of note, in a cross-sectional study conducted in older adults, it was reported that higher plasma IL-6 levels and lower systemic IGF-1 levels were associated with lower muscle strength and power (Barbieri et al., 2003).
As previously discussed, while systemic administration of IGF-1 results in controversial findings on muscle strength and function, transgenic overexpression of IGF-1 has been shown to exert beneficial effects on skeletal muscle (Musaro et al., 2001; Barton et al., 2002; Gregorevic et al., 2002, 2004; Schertzer et al., 2006). In this regard, it is important to remark that these two approaches act via different mechanisms. More specifically, in the systemic pathway of the IGF-1 system, IGFBPs play an important role in the systemic delivery of bound (biologically inactive) IGF-1 (Clemmons, 1997). The IGFBP family consists of six structurally related high-affinity binding proteins (IGFBP-1 through 6) distributed throughout the vascular and interstitial space. These proteins can modulate IGF-1 bioavailability either positively or negatively according to their relative abundances. IGFBPs are able to prolong systemic IGF-1 half-life, protecting it from degradation, and are also responsible for the delivery of IGF-1 to target tissues. In addition, IGFBPs prevent GH-related side effects such as hypoglycemia. In this regard, it has been reported that co-administration of IGF-1 and IGFBP-3 to patients with type 2 diabetes is associated with a lower incidence of side effects than the administration of IGF-1 alone (Clemmons et al., 2005). Skeletal muscle produces IGFBP-3, 4, 5 and 6, with IGFBP-3 and 5 being the most abundant (Shimasaki and Ling, 1991). IGFBP-3 and 5 can either potentiate or inhibit IGF-1 actions. In fact, soluble IGFBP-3 and 5 inhibit IGF-1-actions (Jones et al., 1993), whereas their membrane-bound isoforms potentiate IGF-1 effects (Rees and Clemmons, 1998). IGFBP-4 is generally thought to be inhibitory to IGF-1 and has been shown to prevent binding of IGF-1 to IGF-1 receptor (Qin et al., 1998). Interestingly, advancing age has been associated with increases in serum levels of IGFBP-4 (Honda et al., 1996) and declines in IGFBP-3 (Raynaud-Simon, 2003) and, in women, IGFBP-5 (Mohan et al., 1995), which may result in a reduction of IGF-1 action. Studies in healthy older persons have documented dose-dependent increases in serum levels of IGFBP-3 after GH administration (Munzer et al., 2006). In addition, GH replacement has been reported to increase IGFBP-4 and 5 levels in GH-deficient older adults (Fernholm et al., 2000). However, whether GH or IGF-1 administration alters local tissue production of IGFBPs and whether this influences autocrine/paracrine actions of IGF-1 remain to be determined.
4. Effects of GH supplementation on skeletal muscle in the elderly
The effects of GH administration on muscle mass, strength and physical performance are still under debate (Tables 1 and 2). In animal models, GH supplementation appears to be more effective in states of GH deficiency or reduced GH secretion (Table 1) than in normal hormonal state. In fact, in hypophysectomized rats, GH replacement has been shown to restore muscle mass and improve muscle fiber size and/or composition (Grindeland et al., 1994; Roy et al., 1996; Everitt et al., 1996), with an additive effect if administered together with exercise (Grindeland et al., 1994; Roy et al., 1996; Grossman et al., 1997). Several groups reported that GH administration restored skeletal muscle mass and reduced muscle protein degradation in conditions of reduced GH secretion and acute muscle atrophy such as suspension or immobilization of hind limb muscles (Carmeli et al., 1993; Grindeland et al., 1994; Roy et al., 1996; Fares et al., 1996; Grossman et al., 1997). However, other groups, using the same experimental model, found a positive effect of GH on muscle mass only when combined with resistance training (Allen et al., 1997), whereas others reported no effect of GH either alone or in combination with exercise (Linderman et al., 1994). The effects of GH administration in non-GH-deficient old animals are controversial. Some studies detected improvement in muscle mass (Cartee et al., 1996) and protein synthesis (Sonntag et al., 1985) following GH replacement, whereas others reported increased muscle mass and improved muscle endurance only when GH was combined with exercise training (Andersen et al., 2000) or administered at high dose (Dalla Libera et al., 2004). Conversely, in studies published by other groups, including ours (Ullman and Oldfors, 1989; Marzetti et al., 2008), GH supplementation failed to improve muscle mass, strength and physical performance in aged rats.
Table 1.
Principal studies on GH administration in rats
| Reference | Age (months) | Intervention | Duration | Outcomes |
|---|---|---|---|---|
| Linderman | Young* | HS+GH HS+GH+EX |
5 days | ↔ muscle mass and protein content |
| Marzetti | 28 | GH | 4 weeks | ↔muscle mass, strength and physical performance |
| Allen | 3 | HS+GH/IGF HS+GH/IGF1+EX |
14 days | ↑ muscle mass, CSA, myonuclear number in HS+GH/IGF-1+EX |
| Andersen | 18 | GH GH+EX |
73 days | ↑ muscle mass and endurance in GH+EX |
| Dalla Libera | * | LGH HGH |
2 weeks | ↑ CSA in HGH ↑ muscle mass in HGH ↔strength |
| Sonntag | 19-21 | GH | 8 days | ↑ skeletal muscle protein synthesis |
| Cartee | 9 20 31 |
GH | 10 days | ↑ muscle mass ↔ fiber type composition |
| Carmeli | 26 | GH GH+I |
3 weeks | ↑ muscle mass in GH+I compared to l |
| Fares | 26 | GH GH+I |
28 days | ↑ muscle mass in GH+I compared to I ↓ muscle protein degradation in GH+I compared to I |
| Everitt | 3 | Hypophysectomy GH |
42 days | ↑ muscle mass and slow fibers CSA |
| Grindeland | 2 | Hypophysectomy GH HS+GH HS+GH+EX |
7 days | ↑ muscle mass in GH, HS + GH, HS+GH+EX ↑ fiber CSA in HS+GH+EX |
| Roy | 2 | Hypophysectomy GH; IGF-1 HS+GH; HS+GH+EX HS+ IGF-1 ;HS+IGF1+EX |
10 days | ↑ muscle mass in GH, HS + GH, HS+GH+EX. HS+IGF-1+EX |
| Grossman | 2 | Hypophysectomy GH; IGF-1 HS+GH:HS+GH+EX HS+IGF-1; HS+IGF1+EX |
10 days | ↑ muscle mass in GH, Amb+IGF-1, GH+EX, HS+GH, HS+IGF-1, HS+GH+EX ↔ fiber type composition ↔ fiber CSA |
References are arranged in three groups based on the effect of GH administration. White identifies studies where no effects were detected; light grey indicates studies where partial effects were observed; dark grey indicates studies with significant beneficial effects. ↑: Increase; ↓: reduction; ↔: no effect. CSA: cross-sectional area; EX: exercise; GH: growth hormone; HGH: high dose GH; HS: hind limb suspension; I: immobilization; IGF-1: insulin-like growth factor-1; LGH: low dose GH.
Age not specified.
Table 2.
Trials on GH administration and lifestyle intervention in older persons
| Reference | Age | Sex | Intervention | Participants | Duration | Outcomes |
|---|---|---|---|---|---|---|
| Papadakis | 75 | M | GH | 52 | 6 months | ↔ strength, VO2 max, physical performance |
| Taaffe | 65-82 | M | GH + EX | 18 | 10 weeks | ↔ strength |
| Yarasheski | 67 | M | GH + EX | 23 | 16 weeks | ↔ muscle mass and strength |
| Thomson | 67 | F | GH+EX+Diet IGF-1+EX+Diet GH+IGF-1+EX+Diet |
33 | 12 weeks | ↔ strength, VO2max, physical performance |
| Hennessey | 71 | M/F | GH GH+EX EX |
31 | 6 months | ↑ strength in GH+EX and EX ↑ type II fibers in GH and GH+EX ↔ fiber cross-sectional area |
| Lange | 74 | M | GH GH+EX EX |
31 | 12 weeks | ↑ strength, muscle power, fiber size and CSA in EX |
| Brill | 68 | M | GH Te GH+Te |
10 | 1 month | ↔ muscle strength and flexibility ↑ physical performance test |
| Blackman | 65-88 | M/F | GH Sex Steroids GH+ Sex Steroids |
131 | 26 weeks | ↔ strength ↑ VO2 max (men only) |
| Giannoulis | 65-80 | M | GH Te GH+Te |
80 | 6 months | ↑ strength and VO2 max in GH+Te |
| Welle | 62-74 | M/F M |
GH GH |
16 10 |
Single injection 3 months |
↑ muscle mass and strength |
| Cuttica | 71-86 | M | GH | 5 | 6 months | ↑ strength |
| Sugimoto | 68-75 | F | GH | 8 | 48 weeks | ↑ strength |
References are arranged in three groups based on the effects of GH administration. White identifies studies where no effects were detected; light grey indicates studies where partial effects were observed; dark grey indicates studies with significant beneficial effects. ↑: Increase; ↔: no effect. CSA: cross-sectional area; EX: exercise; GH: growth hormone; IGF-1: insulin-like growth factor-1; Te: testosterone.
Similarly to what observed in animal models, in humans with GH deficiency, muscle mass and strength can be effectively improved by GH replacement (Jorgensen et al., 1989, 1994), whereas the discontinuation of the supplementation regimen leads to a reduction in muscle strength and size (Rutherford et al., 1991). However, controversial findings have been reported in healthy or moderately frail, non-GH-deficient older adults following GH administration. Several groups reported that in these persons GH administration did not affect strength, endurance or physical ability, either alone or in combination with resistance exercise training (Yarasheski et al., 1993, 1995; Deyssig et al., 1993; Taaffe et al., 1994; Papadakis et al., 1996; Thompson et al., 1998; Hennessey et al., 2001; Lange et al., 2002). The failure of GH to enhance the effects of resistance training in the elderly was best shown by Taaffe et al. (1994), who demonstrated that administration of recombinant human GH to exercising elderly men did not augment muscle fiber hypertrophy or tissue IGF-1 expression. Other groups reported only marginal effects of GH supplementation. For instance, Brill et al. (2002) showed that one month of GH administration improved balance and, in combination with testosterone, ameliorated physical function (30-m walk test), but was unable to enhance muscle strength. Blackman et al. (2002) found no significant increase in muscle strength in healthy aged women after supplementation with GH, either alone or with sex steroid, and reported only a marginal, yet significant increase of muscle strength in men in the group treated with GH and testosterone. Similar findings have emerged from a study by Giannoulis et al. (2006). In contrast, other groups reported significant improvements in muscle strength in older adults after GH supplementation. For instance, Welle et al. (1996) showed an increase of muscle mass and thigh strength in healthy older persons after three months of GH treatment. Other two small studies reported improved muscle strength after GH supplementation in healthy elderly (Cuttica et al., 1997) and in osteoporotic old women (Sugimoto et al., 1999). These contrasting findings may be explained by methodological differences between the studies and by the small number of subjects enrolled.
Several reasons may underlie the ineffectiveness of GH supplementation to improve muscle mass and strength, such as the failure of exogenous GH administration to mimic the pulsatile pattern of natural GH secretion or the induction of GH-related insulin resistance. It should also be considered that the majority of the trials conducted on GH supplementation have reported high incidence of side effects, including soft tissue edema, carpal tunnel syndrome, arthralgias, gynecomastia, glucose-metabolism-related adverse events, which pose serious concerns especially in older adults.
In conclusion, based on the current evidence, GH supplementation does not appear effective in improving muscle strength and physical performance at old age, either in humans or in animal models. Therefore, considering the potential for adverse reactions, GH administration should not be advised as a strategy to improve body composition and functionality in healthy older individuals.
5. ACE-inhibitors as a novel strategy to counteract sarcopenia by modulating the GH/IGF-1 axis
Angiotensin II (Ang II), besides its well-known haemodynamic effects, has been shown to produce a marked reduction in body weight in animal models as a result of increased protein degradation (Brink et al., 1996, 2001). This effect has been attributed to a reduction of local production of IGF-1 (Brink et al., 1996, 2001) and impaired insulin signaling at the muscle level (Folli et al., 1997). On the other hand, overexpression of muscle-specific IGF-1 almost completely prevented the Ang II-induced muscle loss and the up-regulation of the ubiquitin ligases atrogin-1 and MuRF-1 (Song et al., 2005). Atrogin-1 and MuRF-1 are muscle-specific proteins of the ubiquitin-proteasome pathway, which are dramatically induced in various models of muscle atrophy (Lecker et al., 2004) via the phosphatidylinositol 3-kinase (PI3K)/Akt pathway (Stitt et al., 2004). IGF-1 once bound to its skeletal muscle receptor is able to activate Akt leading to the inactivation of Forkhead box O (Foxo) proteins (Stitt et al., 2004), a subgroup of the Forkhead family of transcription factors. Akt-dependent phosphorylation of Foxo, in turn, blocks the expression of atrogin-1 and MuRF-1, thus providing one mechanistic explanation for the anti-catabolic property of IGF-1 in skeletal muscle. It was also reported that daily infusions of Ang II increased the expression of caspase-3 in murine skeletal muscle, resulting in enhanced proteolysis and apoptosis (Song et al., 2005). In contrast, overexpression of muscle-specific IGF-1 reduced apoptosis in skeletal muscle by activating the PI3K/Akt pathway, which resulted in the inactivation of Bad, a pro-apoptotic Bcl-2 family protein (Fig. 2).
Fig. 2.
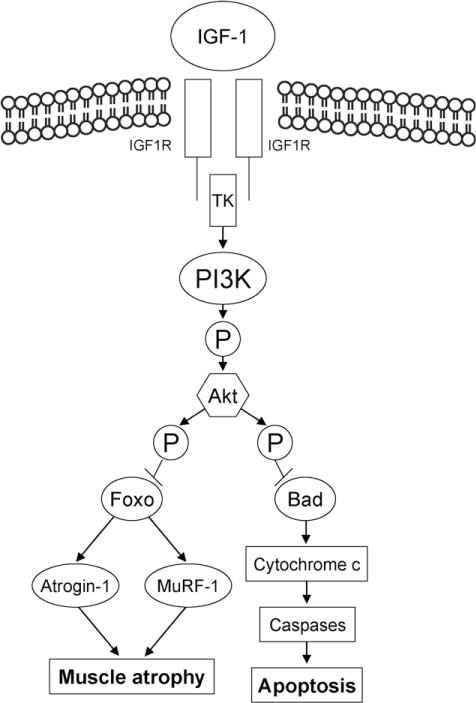
Actions of IGF-1 and angiotensin II in skeletal muscle. Binding of muscle IGF-1 to IGF-1 receptors via intracellular signaling pathways involving tyrosine kinase (TK) activity may exert antiapoptotic effect and reduce muscle atrophy via phosphatidylinositol 3-kinase (PI3K)-dependent Akt Kinase (Akt)-dependent phosphorylation. On the other hand, Ang II down-regulates IGF-1, IGFBP-3 and IGFBP-5 genes, thus negatively affecting the autocrine IGF-1 system. Moreover, Ang II can also stimulate the ubiquitin-proteasome pathway as a result of increased expression of atrophy-related ubiquitin ligases (atrogin-1 and MuRF-1).
In consideration of the catabolic effects of Ang II on skeletal muscle, ACEIs may represent a promising strategy to counteract sarcopenia. ACEIs are widely used in the treatment of haemodynamic disorders in geriatric populations (Grady, 2006). Recent evidence suggests that ACEIs may induce positive changes in body composition and physical function (Onder et al., 2002; Strazzullo et al., 2003; Di Bari et al., 2004). In particular, in a cohort study of 641 hypertensive women free of congestive heart failure (CHF), continued ACEIs users had significantly higher muscle strength and walking speed compared to those taking other antihyperten-sive drugs or not on antihypertensive treatment (Onder et al., 2002). Similar findings were observed in the Health ABC study, in which ACEIs users had greater leg muscle mass compared to other antihypertensive drugs users (Di Bari et al., 2004). Moreover, studies have demonstrated that ACEIs are able to delay the decline of skeletal muscle strength by increasing muscle blood flow (Frisbee and Lombard, 2000), reducing secretion of inflammatory cytokines (Egido and Ruiz-Ortega, 2007) and enhancing insulin sensitivity and glucose uptake by skeletal myocytes (Kudoh and Matsuki, 2000). In addition, a positive modulation of ACEIs on the IGF-1 system has been suggested, which may further explain the favorable effects of ACEIs on muscle strength and physical function (Corbalan et al., 1998; Maggio et al., 2006; Onder et al., 2007). Interestingly, chronic ACE inhibition therapy with enalapril completely restored IGF-1 plasma levels in patients with CHF (Corbalan et al., 1998). Of note, low levels of IGF-1 in the presence of CHF are predictive of decreased skeletal muscle cross-sectional area and strength (Niebauer et al., 1998). In the case of CHF, ACEIs increase physical performance primarily via amelioration of the haemodynamics. However, at least other two mechanisms need to be taken into consideration as a possible way for ACEIs to positively affect skeletal muscle impairment in patients with CHF. Firstly, CHF is associated with low skeletal muscle calcium levels which can be restored by captopril (Sylven et al., 1991). Secondly, it was recently reported that ACEIs completely prevented the impairment of skeletal muscle mitochondrial function observed four months after myocardial infarction-induced heart failure (Zoll et al., 2006). In fact, ACEIs are able to prevent the down-regulation of gene expression of several mitochondrial transcriptional factors, such as peroxisome proliferator activated receptor gamma coactivator 1 a, nuclear respiratory factor 2 DNA-binding subunit a and mitochondrial transcription factor A (Zoll et al., 2006). Onder et al. (2007) found that ACEIs use in older hypertensive adults was associated with significantly increased serum levels of IGFBP-3, possibly resulting in increased bioavailability of IGF-1. Similarly, Maggio et al. (2006) reported a positive association between ACEIs therapy and total IGF-1 serum levels.
Ignjatovic et al. (2004) indicated that ACE-inhibitors may directly activate the bradykinin (BK) B1 receptors, thus contributing to increase skeletal muscle blood flow and glucose metabolism. Interestingly, recent genotype studies suggest that BK induced by ACE inhibition may have longer half-life (Brown et al., 1998) and higher receptor kinase activity in individuals homozygous for the I allele of the ACE gene and with a (−)9 variant of the BK B2 receptor (Williams et al., 2004). The ACEIs-related increase of systemic BK levels could also positively influence muscle function by increasing nitric oxide (NO) production. In fact, several groups found that BK infusion to skeletal muscle tissue led to an increased activation of NO synthase (NOS) and NO production in humans and rats (Dietze et al., 1996; Mitchell and Tyml, 1996). Interestingly, two studies conducted on rats found reduced sarcomere numbers, muscle weight, fiber cross-sectional area and walking speed after treatment with NOS inhibitors (Koh and Tidball, 1999; Wang et al., 2001). Moreover, NO derived from the neuronal isoform of NOS was found to positively modulate sarcomere addition in rat soleus muscle (Blackman et al., 2002). Interestingly, enalapril was found to activate endothelial NOS, which is primarily responsible for NO generation in vasodilation (Ignjatovic et al., 2004).
It should however be considered that long-term use of ACEIs may lead to an up-regulation of ACE signaling, which in turn may increase Ang II production. In fact, Fleming’s group (Kohlstedt et al., 2004) showed that binding of an ACEI to ACE increased ACE phosphorylation and the ACE-associated activity of protein kinase CK2, leading to enhanced generation of Ang II in endothelial cells. Increased production of Ang II may reduce the effectiveness of ACE inhibition on sarcopenia by decreasing systemic and/or autocrine IGF-1 levels.
Future studies will have to better elucidate the impact of ACEIs on physical performance through the modulation of GH/IGF-1 axis at old age, especially in long-term users.
6. Conclusions
The current literature does not provide uniform evidence on the effectiveness of the modulation of GH/IGF-1 axis as a strategy to improve muscle strength and function in older adults. In particular, the effectiveness of GH supplementation, the most direct approach tested, in ameliorating muscle strength and physical performance at old age is not supported by scientific evidence either in humans or in animal models (Tables 1 and 2). Modulation of the paracrine/autocrine IGF-1 system may be more promising, but this approach has not yet been tested in humans. An attractive alternative approach is represented by ACEIs, which may improve physical function in older subjects by positively affecting body composition, at least partly via a favorable modulation of the GH/IGF-1 axis. Future studies will have to investigate the effects of ACEIs on both systemic and local IGF-1 in older individuals and the impact of chronic ACEIs therapy on body composition and physical performance at old age.
Acknowledgments
The authors would like to thank Ms. Hazel Lees for the editing of the manuscript. This research was supported by grants to C.L. (NIA R01-AG17994 and AG21042) and S.B. (Department of Veterans Affairs Merit Award). S.G. is partly supported by the Department of Gerontology, Geriatrics and Physiatrics of the Catholic University of the Sacred Heart of Rome, Italy. E.M. is supported by the University of Florida Institute on Aging and Claude D. Pepper Older Americans Independence Center (1 P30 AG028740).
References
- Allen DL, Linderman JK, Roy RR, Grindeland RE, Mukku V, Edgerton VR. Growth hormone/IGF-I and/or resistive exercise maintains myonuclear number in hindlimb unweighted muscles. J Appl Physiol. 1997;83:1857–1861. doi: 10.1152/jappl.1997.83.6.1857. [DOI] [PubMed] [Google Scholar]
- Andersen NB, Andreassen TT, Orskov H, Oxlund H. Growth hormone and mild exercise in combination increases markedly muscle mass and tetanic tension in old rats. Eur J Endocrinol. 2000;143:409–418. doi: 10.1530/eje.0.1430409. [DOI] [PubMed] [Google Scholar]
- Arvat E, Broglio F, Ghigo E. Insulin-Like growth factor I: implications in aging. Drugs Aging. 2000;16:29–40. doi: 10.2165/00002512-200016010-00003. [DOI] [PubMed] [Google Scholar]
- Ballard FJ, Francis GL. Effects of anabolic agents on protein breakdown in L6 myoblasts. Biochem J. 1983;210:243–249. doi: 10.1042/bj2100243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbieri M, Ferrucci L, Ragno E, Corsi A, Bandinelli S, Bonafe M, Olivieri F, Giovagnetti S, Franceschi C, Guralnik JM, Paolisso G. Chronic inflammation and the effect of IGF-I on muscle strength and power in older persons. Am J Physiol Endocrinol Metab. 2003;284:E481–E487. doi: 10.1152/ajpendo.00319.2002. [DOI] [PubMed] [Google Scholar]
- Barton ER, Morris L, Musaro A, Rosenthal N, Sweeney HL. Muscle-specific expression of insulin-like growth factor I counters muscle decline in mdx mice. J Cell Biol. 2002;157:137–148. doi: 10.1083/jcb.200108071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barton-Davis ER, Shoturma DI, Musaro A, Rosenthal N, Sweeney HL. Viral mediated expression of insulin-like growth factor I blocks the aging-related loss of skeletal muscle function. Proc Natl Acad Sci U S A. 1998;95:15603–15607. doi: 10.1073/pnas.95.26.15603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barton-Davis ER, Shoturma DI, Sweeney HL. Contribution of satellite cells to IGF-I induced hypertrophy of skeletal muscle. Acta Physiol Scand. 1999;167:301–305. doi: 10.1046/j.1365-201x.1999.00618.x. [DOI] [PubMed] [Google Scholar]
- Bhasin S, Woodhouse L, Casaburi R, Singh AB, Bhasin D, Berman N, Chen X, Yarasheski KE, Magliano L, Dzekov C, Dzekov J, Bross R, Phillips J, Sinha-Hikim I, Shen R, Storer TW. Testosterone dose–response relationships in healthy young men. Am J Physiol Endocrinol Metab. 2001;281:E1172–E1181. doi: 10.1152/ajpendo.2001.281.6.E1172. [DOI] [PubMed] [Google Scholar]
- Blackman MR, Sorkin JD, Munzer T, Bellantoni MF, Busby-Whitehead J, Stevens TE, Jayme J, O’Connor KG, Christmas C, Tobin JD, Stewart KJ, Cottrell E, St CC, Pabst KM, Harman SM. Growth hormone and sex steroid administration in healthy aged women and men: a randomized controlled trial. JAMA. 2002;288:2282–2292. doi: 10.1001/jama.288.18.2282. [DOI] [PubMed] [Google Scholar]
- Boni-Schnetzler M, Schmid C, Meier PJ, Froesch ER. Insulin regulates insulin-like growth factor I mRNA in rat hepatocytes. Am J Physiol. 1991;260:E846–E851. doi: 10.1152/ajpendo.1991.260.6.E846. [DOI] [PubMed] [Google Scholar]
- Boonen S, Lysens R, Verbeke G, Joosten E, Dejaeger E, Pelemans W, Flamaing J, Bouillon R. Relationship between age-associated endocrine deficiencies and muscle function in elderly women: a cross-sectional study. Age Ageing. 1998;27:449–454. doi: 10.1093/ageing/27.4.449. [DOI] [PubMed] [Google Scholar]
- Borst SE, Lowenthal DT. Role of IGF-I in muscular atrophy of aging. Endocrine. 1997;7:61–63. doi: 10.1007/BF02778065. [DOI] [PubMed] [Google Scholar]
- Brill KT, Weltman AL, Gentili A, Patrie JT, Fryburg DA, Hanks JB, Urban RJ, Veldhuis JD. Single and combined effects of growth hormone and testosterone administration on measures of body composition, physical performance, mood, sexual function, bone turnover, and muscle gene expression in healthy older men. J Clin Endocrinol Metab. 2002;87:5649–5657. doi: 10.1210/jc.2002-020098. [DOI] [PubMed] [Google Scholar]
- Brink M, Price SR, Chrast J, Bailey JL, Anwar A, Mitch WE, Delafontaine P. Angiotensin II induces skeletal muscle wasting through enhanced protein degradation and down-regulates autocrine insulin-like growth factor I. Endocrinology. 2001;142:1489–1496. doi: 10.1210/endo.142.4.8082. [DOI] [PubMed] [Google Scholar]
- Brink M, Wellen J, Delafontaine P. Angiotensin II causes weight loss and decreases circulating insulin-like growth factor I in rats through a pressor-independent mechanism. J Clin Invest. 1996;97:2509–2516. doi: 10.1172/JCI118698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown NJ, Blais C, Jr, Gandhi SK, Adam A. ACE insertion/deletion genotype affects bradykinin metabolism. J Cardiovasc Pharmacol. 1998;32:373–377. doi: 10.1097/00005344-199809000-00006. [DOI] [PubMed] [Google Scholar]
- Cappola AR, Bandeen-Roche K, Wand GS, Volpato S, Fried LP. Association of IGF-I levels with muscle strength and mobility in older women. J Clin Endocrinol Metab. 2001;86:4139–4146. doi: 10.1210/jcem.86.9.7868. [DOI] [PubMed] [Google Scholar]
- Carmeli E, Hochberg Z, Livne E, Lichtenstein I, Kestelboim C, Silbermann M, Reznick AZ. Effect of growth hormone on gastrocnemius muscle of aged rats after immobilization: biochemistry and morphology. J Appl Physiol. 1993;75:1529–1535. doi: 10.1152/jappl.1993.75.4.1529. [DOI] [PubMed] [Google Scholar]
- Carpenter V, Matthews K, Devlin G, Stuart S, Jensen J, Conaglen J, Jeanplong F, Goldspink P, Yang SY, Goldspink G, Bass J, McMahon C. Mechano-growth factor reduces loss of cardiac function in acute myocardial infarction. Heart Lung Circ. 2008;17:33–39. doi: 10.1016/j.hlc.2007.04.013. [DOI] [PubMed] [Google Scholar]
- Cartee GD, Bohn EE, Gibson BT, Farrar RP. Growth hormone supplementation increases skeletal muscle mass of old male Fischer 344/brown Norway rats. J Gerontol A Biol Sci Med Sci. 1996;51:B214–B219. doi: 10.1093/gerona/51a.3.b214. [DOI] [PubMed] [Google Scholar]
- Clemmons DR. Insulin-like growth factor binding proteins and their role in controlling IGF actions. Cytokine Growth Factor Rev. 1997;8:45–62. doi: 10.1016/s1359-6101(96)00053-6. [DOI] [PubMed] [Google Scholar]
- Clemmons DR, Moses AC, Sommer A, Jacobson W, Rogol AD, Sleevi MR, Allan G. Rh/IGF-I/rhIGFBP-3 administration to patients with type 2 diabetes mellitus reduces insulin requirements while also lowering fasting glucose. Growth Horm IGF Res. 2005;15:265–274. doi: 10.1016/j.ghir.2005.05.002. [DOI] [PubMed] [Google Scholar]
- Coleman ME, DeMayo F, Yin KC, Lee HM, Geske R, Montgomery C, Schwartz RJ. Myogenic vector expression of insulin-like growth factor I stimulates muscle cell differentiation and myofiber hypertrophy in transgenic mice. J Biol Chem. 1995;270:12109–12116. doi: 10.1074/jbc.270.20.12109. [DOI] [PubMed] [Google Scholar]
- Corbalan R, Acevedo M, Godoy I, Jalil J, Campusano C, Klassen J. Enalapril restores depressed circulating insulin-like growth factor 1 in patients with chronic heart failure. J Card Fail. 1998;4:115–119. doi: 10.1016/s1071-9164(98)90251-2. [DOI] [PubMed] [Google Scholar]
- Cuttica CM, Castoldi L, Gorrini GP, Peluffo F, Delitala G, Filippa P, Fanciulli G, Giusti M. Effects of six-month administration of recombinant human growth hormone to healthy elderly subjects. Aging (Milano) 1997;9:193–197. doi: 10.1007/BF03340149. [DOI] [PubMed] [Google Scholar]
- Dalla Libera L, Ravara B, Volterrani M, Gobbo V, Della BM, Angelini A, Danieli BD, Germinario E, Vescovo G. Beneficial effects of GH/IGF-1 on skeletal muscle atrophy and function in experimental heart failure. Am J Physiol Cell Physiol. 2004;286:C138–C144. doi: 10.1152/ajpcell.00114.2003. [DOI] [PubMed] [Google Scholar]
- Daughaday WH. Growth hormone axis overview—somatomedin hypothesis. Pediatr Nephrol. 2000;14:537–540. doi: 10.1007/s004670000334. [DOI] [PubMed] [Google Scholar]
- DeVol DL, Rotwein P, Sadow JL, Novakofski J, Bechtel PJ. Activation of insulin-like growth factor gene expression during work-induced skeletal muscle growth. Am J Physiol. 1990;259:E89–E95. doi: 10.1152/ajpendo.1990.259.1.E89. [DOI] [PubMed] [Google Scholar]
- Deyssig R, Frisch H, Blum WF, Waldhor T. Effect of growth hormone treatment on hormonal parameters, body composition and strength in athletes. Acta Endocrinol (Copenh) 1993;128:313–318. doi: 10.1530/acta.0.1280313. [DOI] [PubMed] [Google Scholar]
- Di Bari M, van de Poll-Franse LV, Onder G, Kritchevsky SB, Newman A, Harris TB, Williamson JD, Marchionni N, Pahor M. Antihypertensive medications and differences in muscle mass in older persons: the Health, Aging and Body Composition Study. J Am Geriatr Soc. 2004;52:961–966. doi: 10.1111/j.1532-5415.2004.52265.x. [DOI] [PubMed] [Google Scholar]
- Dietze GJ, Wicklmayr M, Rett K, Jacob S, Henriksen EJ. Potential role of bradykinin in forearm muscle metabolism in humans. Diabetes. 1996;45(Suppl 1):S110–S114. doi: 10.2337/diab.45.1.s110. [DOI] [PubMed] [Google Scholar]
- Dreyer HC, Volpi E. Role of protein and amino acids in the pathophysiology and treatment of sarcopenia. J Am Coll Nutr. 2005;24:140S–145S. doi: 10.1080/07315724.2005.10719455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egido J, Ruiz-Ortega M. Anti-inflammatory actions of quinapril. Cardiovasc Drugs Ther. 2007;21:211–220. doi: 10.1007/s10557-007-6019-1. [DOI] [PubMed] [Google Scholar]
- Ershler WB, Keller ET. Age-associated increased interleukin-6 gene expression, late-life diseases, and frailty. Annu Rev Med. 2000;51:245–270. doi: 10.1146/annurev.med.51.1.245. [DOI] [PubMed] [Google Scholar]
- Everitt AV, Terry V, Phillips MJ, Kerry HM, Shorey CD. Morphometric analysis of gastrocnemius muscle fiber size and fiber proportions in the hypophysectomized rat after prolonged administration of growth hormone or thyroxine. Growth Dev Aging. 1996;60:85–93. [PubMed] [Google Scholar]
- Ewton DZ, Falen SL, Florini JR. The type II insulin-like growth factor (IGF) receptor has low affinity for IGF-I analogs: pleiotypic actions of IGFs on myoblasts are apparently mediated by the type I receptor. Endocrinology. 1987;120:115–123. doi: 10.1210/endo-120-1-115. [DOI] [PubMed] [Google Scholar]
- Fares FA, Gruener N, Carmeli E, Reznick AZ. Growth hormone (GH) retardation of muscle damage due to immobilization in old rats. Possible intervention with a new long-acting recombinant GH. Ann N Y Acad Sci. 1996;786:430–443. doi: 10.1111/j.1749-6632.1996.tb39082.x. [DOI] [PubMed] [Google Scholar]
- Fernholm R, Bramnert M, Hagg E, Hilding A, Baylink DJ, Mohan S, Thoren M. Growth hormone replacement therapy improves body composition and increases bone metabolism in elderly patients with pituitary disease. J Clin Endocrinol Metab. 2000;85:4104–4112. doi: 10.1210/jcem.85.11.6949. [DOI] [PubMed] [Google Scholar]
- Ferrucci L, Harris TB, Guralnik JM, Tracy RP, Corti MC, Cohen HJ, Penninx B, Pahor M, Wallace R, Havlik RJ. Serum IL-6 level and the development of disability in older persons. J Am Geriatr Soc. 1999;47:639–646. doi: 10.1111/j.1532-5415.1999.tb01583.x. [DOI] [PubMed] [Google Scholar]
- Festoff BW, Yang SX, Vaught J, Bryan C, Ma JY. The insulin-like growth factor signaling system and ALS neurotrophic factor treatment strategies. J Neurol Sci. 1995;(129 Suppl):114–121. doi: 10.1016/0022-510x(95)00080-l. [DOI] [PubMed] [Google Scholar]
- Folli F, Kahn CR, Hansen H, Bouchie JL, Feener EP. Angiotensin II inhibits insulin signaling in aortic smooth muscle cells at multiple levels. A potential role for serine phosphorylation in insulin/angiotensin II crosstalk. J Clin Invest. 1997;100:2158–2169. doi: 10.1172/JCI119752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foulstone EJ, Huser C, Crown AL, Holly JM, Stewart CE. Differential signalling mechanisms predisposing primary human skeletal muscle cells to altered proliferation and differentiation: roles of IGF-I and TNFalpha. Exp Cell Res. 2004;294:223–235. doi: 10.1016/j.yexcr.2003.10.034. [DOI] [PubMed] [Google Scholar]
- Frisbee JC, Lombard JH. Short-term angiotensin converting enzyme inhibition reduces basal tone and dilator reactivity in skeletal muscle arterioles. Am J Hypertens. 2000;13:389–395. doi: 10.1016/s0895-7061(99)00204-6. [DOI] [PubMed] [Google Scholar]
- Fulle S, Protasi F, Di TG, Pietrangelo T, Beltramin A, Boncompagni S, Vecchiet L, Fano G. The contribution of reactive oxygen species to sarcopenia and muscle ageing. Exp Gerontol. 2004;39:17–24. doi: 10.1016/j.exger.2003.09.012. [DOI] [PubMed] [Google Scholar]
- Giannoulis MG, Sonksen PH, Umpleby M, Breen L, Pentecost C, Whyte M, McMillan CV, Bradley C, Martin FC. The effects of growth hormone and/or testosterone in healthy elderly men: a randomized controlled trial. J Clin Endocrinol Metab. 2006;91:477–484. doi: 10.1210/jc.2005-0957. [DOI] [PubMed] [Google Scholar]
- Goldspink G. Loss of muscle strength during aging studied at the gene level. Rejuvenat Res. 2007;10:397–405. doi: 10.1089/rej.2007.0597. [DOI] [PubMed] [Google Scholar]
- Goldspink G, Williams P, Simpson H. Gene expression in response to muscle stretch. Clin Orthop Relat Res. 2002:S146–S152. doi: 10.1097/00003086-200210001-00017. [DOI] [PubMed] [Google Scholar]
- Goya RG, Brown OA, Bolognani F. The thymus-pituitary axis and its changes during aging. Neuroimmunomodulation. 1999;6:137–142. doi: 10.1159/000026373. [DOI] [PubMed] [Google Scholar]
- Grady KL. Management of heart failure in older adults. J Cardiovasc Nurs. 2006;21:S10–S14. doi: 10.1097/00005082-200609001-00004. [DOI] [PubMed] [Google Scholar]
- Gregorevic P, Plant DR, Leeding KS, Bach LA, Lynch GS. Improved contractile function of the mdx dystrophic mouse diaphragm muscle after insulin-like growth factor-I administration. Am J Pathol. 2002;161:2263–2272. doi: 10.1016/S0002-9440(10)64502-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregorevic P, Plant DR, Lynch GS. Administration of insulin-like growth factor-I improves fatigue resistance of skeletal muscles from dystrophic mdx mice. Muscle Nerve. 2004;30:295–304. doi: 10.1002/mus.20082. [DOI] [PubMed] [Google Scholar]
- Grindeland RE, Roy RR, Edgerton VR, Grossman EJ, Mukku VR, Jiang B, Pierotti DJ, Rudolph I. Interactive effects of growth hormone and exercise on muscle mass in suspended rats. Am J Physiol. 1994;267:R316–R322. doi: 10.1152/ajpregu.1994.267.1.R316. [DOI] [PubMed] [Google Scholar]
- Grossman EJ, Grindeland RE, Roy RR, Talmadge RJ, Evans J, Edgerton VR. Growth hormone, IGF-I, and exercise effects on non-weight-bearing fast muscles of hypophysectomized rats. J Appl Physiol. 1997;83:1522–1530. doi: 10.1152/jappl.1997.83.5.1522. [DOI] [PubMed] [Google Scholar]
- Grounds MD. Reasons for the degeneration of ageing skeletal muscle: a central role for IGF-1 signalling. Biogerontology. 2002;3:19–24. doi: 10.1023/a:1015234709314. [DOI] [PubMed] [Google Scholar]
- Hameed M, Orrell RW, Cobbold M, Goldspink G, Harridge SD. Expression of IGF-I splice variants in young and old human skeletal muscle after high resistance exercise. J Physiol. 2003;547:247–254. doi: 10.1113/jphysiol.2002.032136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris TB, Ferrucci L, Tracy RP, Corti MC, Wacholder S, Ettinger WH, Jr, Heimovitz H, Cohen HJ, Wallace R. Associations of elevated interleukin-6 and C-reactive protein levels with mortality in the elderly. Am J Med. 1999;106:506–512. doi: 10.1016/s0002-9343(99)00066-2. [DOI] [PubMed] [Google Scholar]
- Hembree JR, Hathaway MR, Dayton WR. Isolation and culture of fetal porcine myogenic cells and the effect of insulin, IGF-I, and sera on protein turnover in porcine myotube cultures. J Anim Sci. 1991;69:3241–3250. doi: 10.2527/1991.6983241x. [DOI] [PubMed] [Google Scholar]
- Hennessey JV, Chromiak JA, DellaVentura S, Reinert SE, Puhl J, Kiel DP, Rosen CJ, Vandenburgh H, MacLean DB. Growth hormone administration and exercise effects on muscle fiber type and diameter in moderately frail older people. J Am Geriatr Soc. 2001;49:852–858. doi: 10.1046/j.1532-5415.2001.49173.x. [DOI] [PubMed] [Google Scholar]
- Honda Y, Landale EC, Strong DD, Baylink DJ, Mohan S. Recombinant synthesis of insulin-like growth factor-binding protein-4 (IGFBP-4): development, validation, and application of a radioimmunoassay for IGFBP-4 in human serum and other biological fluids. J Clin Endocrinol Metab. 1996;81:1389–1396. doi: 10.1210/jcem.81.4.8636339. [DOI] [PubMed] [Google Scholar]
- Ignjatovic T, Stanisavljevic S, Brovkovych V, Skidgel RA, Erdos EG. Kinin B1 receptors stimulate nitric oxide production in endothelial cells: signaling pathways activated by angiotensin I-converting enzyme inhibitors and peptide ligands. Mol Pharmacol. 2004;66:1310–1316. doi: 10.1124/mol.104.001990. [DOI] [PubMed] [Google Scholar]
- Janssen JA, Stolk RP, Pols HA, Grobbee DE, Lamberts SW. Serum free and total insulin-like growth factor-I, insulin-like growth factor binding protein-1 and insulin-like growth factor binding protein-3 Levels in healthy elderly individuals. Relation to self-reported quality of health and disability. Gerontology. 1998;44:277–280. doi: 10.1159/000022026. [DOI] [PubMed] [Google Scholar]
- Jones JI, Gockerman A, Busby WH, Jr, Camacho-Hubner C, Clemmons DR. Extracellular matrix contains insulin-like growth factor binding protein-5: potentiation of the effects of IGF-I. J Cell Biol. 1993;121:679–687. doi: 10.1083/jcb.121.3.679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jorgensen JO, Pedersen SA, Thuesen L, Jorgensen J, Ingemann-Hansen T, Skakkebaek NE, Christiansen JS. Beneficial effects of growth hormone treatment in GH-deficient adults. Lancet. 1989;1:1221–1225. doi: 10.1016/s0140-6736(89)92328-3. [DOI] [PubMed] [Google Scholar]
- Jorgensen JO, Thuesen L, Muller J, Ovesen P, Skakkebaek NE, Christiansen JS. Three years of growth hormone treatment in growth hormone-deficient adults: near normalization of body composition and physical performance. Eur J Endocrinol. 1994;130:224–228. doi: 10.1530/eje.0.1300224. [DOI] [PubMed] [Google Scholar]
- Kiel DP, Puhl J, Rosen CJ, Berg K, Murphy JB, MacLean DB. Lack of an association between insulin-like growth factor-I and body composition, muscle strength, physical performance or self-reported mobility among older persons with functional limitations. J Am Geriatr Soc. 1998;46:822–828. doi: 10.1111/j.1532-5415.1998.tb02714.x. [DOI] [PubMed] [Google Scholar]
- Koh TJ, Tidball JG. Nitric oxide synthase inhibitors reduce sarcomere addition in rat skeletal muscle. J Physiol. 1999;519(Pt 1):189–196. doi: 10.1111/j.1469-7793.1999.0189o.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohlstedt K, Brandes RP, Muller-Esterl W, Busse R, Fleming I. Angiotensin-converting enzyme is involved in outside-in signaling in endothelial cells. Circ Res. 2004;94:60–67. doi: 10.1161/01.RES.0000107195.13573.E4. [DOI] [PubMed] [Google Scholar]
- Kostka T, Arsac LM, Patricot MC, Berthouze SE, Lacour JR, Bonnefoy M. Leg extensor power and dehydroepiandrosterone sulfate, insulin-like growth factor-I and testosterone in healthy active elderly people. Eur J Appl Physiol. 2000;82:83–90. doi: 10.1007/s004210050655. [DOI] [PubMed] [Google Scholar]
- Kudoh A, Matsuki A. Effects of angiotensin-converting enzyme inhibitors on glucose uptake. Hypertension. 2000;36:239–244. doi: 10.1161/01.hyp.36.2.239. [DOI] [PubMed] [Google Scholar]
- Laager R, Ninnis R, Keller U. Comparison of the effects of recombinant human insulin-like growth factor-I and insulin on glucose and leucine kinetics in humans. J Clin Invest. 1993;92:1903–1909. doi: 10.1172/JCI116783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landin-Wilhelmsen K, Wilhelmsen L, Lappas G, Rosen T, Lindstedt G, Lund-berg PA, Bengtsson BA. Serum insulin-like growth factor I in a random population sample of men and women: relation to age, sex, smoking habits, coffee consumption and physical activity, blood pressure and concentrations of plasma lipids, fibrinogen, parathyroid hormone and osteocalcin. Clin Endocrinol (Oxf) 1994;41:351–357. doi: 10.1111/j.1365-2265.1994.tb02556.x. [DOI] [PubMed] [Google Scholar]
- Lange KH, Andersen JL, Beyer N, Isaksson F, Larsson B, Rasmussen MH, Juul A, Bulow J, Kjaer M. GH administration changes myosin heavy chain isoforms in skeletal muscle but does not augment muscle strength or hypertrophy, either alone or combined with resistance exercise training in healthy elderly men. J Clin Endocrinol Metab. 2002;87:513–523. doi: 10.1210/jcem.87.2.8206. [DOI] [PubMed] [Google Scholar]
- Lazarus DD, Moldawer LL, Lowry SF. Insulin-like growth factor-1 activity is inhibited by interleukin-1 alpha, tumor necrosis factor-alpha, and interleukin-6. Lymphokine Cytokine Res. 1993;12:219–223. [PubMed] [Google Scholar]
- Lecker SH, Jagoe RT, Gilbert A, Gomes M, Baracos V, Bailey J, Price SR, Mitch WE, Goldberg AL. Multiple types of skeletal muscle atrophy involve a common program of changes in gene expression. FASEB J. 2004;18:39–51. doi: 10.1096/fj.03-0610com. [DOI] [PubMed] [Google Scholar]
- Lewis ME, Neff NT, Contreras PC, Stong DB, Oppenheim RW, Grebow PE, Vaught JL. Insulin-like growth factor-I: potential for treatment of motor neuronal disorders. Exp Neurol. 1993;124:73–88. doi: 10.1006/exnr.1993.1177. [DOI] [PubMed] [Google Scholar]
- Linderman JK, Gosselink KL, Booth FW, Mukku VR, Grindeland RE. Resistance exercise and growth hormone as countermeasures for skeletal muscle atrophy in hindlimb-suspended rats. Am J Physiol. 1994;267:R365–R371. doi: 10.1152/ajpregu.1994.267.2.R365. [DOI] [PubMed] [Google Scholar]
- Lynch GS, Cuffe SA, Plant DR, Gregorevic P. IGF-I treatment improves the functional properties of fast- and slow-twitch skeletal muscles from dystrophic mice. Neuromuscul Disord. 2001;11:260–268. doi: 10.1016/s0960-8966(00)00192-9. [DOI] [PubMed] [Google Scholar]
- Maggio M, Ceda GP, Lauretani F, Pahor M, Bandinelli S, Najjar SS, Ling SM, Basaria S, Ruggiero C, Valenti G, Ferrucci L. Relation of angiotensin-converting enzyme inhibitor treatment to insulin-like growth factor-1 serum levels in subjects >65 years of age (the InCHIANTI study) Am J Cardiol. 2006;97:1525–1529. doi: 10.1016/j.amjcard.2005.11.089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martineau LC, Chadan SG, Parkhouse WS. Age-associated alterations in cardiac and skeletal muscle glucose transporters, insulin and IGF-1 receptors, and PI3-kinase protein contents in the C57BL/6 mouse. Mech Ageing Dev. 1999;106:217–232. doi: 10.1016/s0047-6374(98)00106-7. [DOI] [PubMed] [Google Scholar]
- Marzetti E, Groban L, Wohlgemuth SE, Lees HA, Lin M, Jobe H, Giovannini S, Leeuwenburgh C, Carter CS. Effects of short-term GH supplementation and treadmill exercise training on physical performance and skeletal muscle apoptosis in old rats. Am J Physiol Regul Integr Comp Physiol. 2008;294:R558–R567. doi: 10.1152/ajpregu.00620.2007. [DOI] [PubMed] [Google Scholar]
- Mitchell D, Tyml K. Nitric oxide release in rat skeletal muscle capillary. Am J Physiol. 1996;270:H1696–H1703. doi: 10.1152/ajpheart.1996.270.5.H1696. [DOI] [PubMed] [Google Scholar]
- Mohan S, Libanati C, Dony C, Lang K, Srinivasan N, Baylink DJ. Development, validation, and application of a radioimmunoassay for insulin-like growth factor binding protein-5 in human serum and other biological fluids. J Clin Endocrinol Metab. 1995;80:2638–2645. doi: 10.1210/jcem.80.9.7545694. [DOI] [PubMed] [Google Scholar]
- Munzer T, Rosen CJ, Harman SM, Pabst KM, St CC, Sorkin JD, Blackman MR. Effects of GH and/or sex steroids on circulating IGF-I and IGFBPs in healthy, aged women and men. Am J Physiol Endocrinol Metab. 2006;290:E1006–E1013. doi: 10.1152/ajpendo.00166.2005. [DOI] [PubMed] [Google Scholar]
- Musaro A, Giacinti C, Borsellino G, Dobrowolny G, Pelosi L, Cairns L, Otto-lenghi S, Cossu G, Bernardi G, Battistini L, Molinaro M, Rosenthal N. Stem cell-mediated muscle regeneration is enhanced by local isoform of insulin-like growth factor 1. Proc Natl Acad Sci U S A. 2004;101:1206–1210. doi: 10.1073/pnas.0303792101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musaro A, McCullagh K, Paul A, Houghton L, Dobrowolny G, Molinaro M, Barton ER, Sweeney HL, Rosenthal N. Localized Igf-1 transgene expression sustains hypertrophy and regeneration in senescent skeletal muscle. Nat Genet. 2001;27:195–200. doi: 10.1038/84839. [DOI] [PubMed] [Google Scholar]
- Niebauer J, Pflaum CD, Clark AL, Strasburger CJ, Hooper J, Poole-Wilson PA, Coats AJ, Anker SD. Deficient insulin-like growth factor I in chronic heart failure predicts altered body composition, anabolic deficiency, cytokine and neurohormonal activation. J Am Coll Cardiol. 1998;32:393–397. doi: 10.1016/s0735-1097(98)00226-5. [DOI] [PubMed] [Google Scholar]
- Nyomba BL, Berard L, Murphy LJ. Free insulin-like growth factor I (IGF-I) in healthy subjects: relationship with IGF-binding proteins and insulin sensitivity. J Clin Endocrinol Metab. 1997;82:2177–2181. doi: 10.1210/jcem.82.7.4070. [DOI] [PubMed] [Google Scholar]
- Okajima T, Iwashita M, Takeda Y, Sakamoto S, Tanabe T, Yasuda T, Rosenfeld RG. Inhibitory effects of insulin-like growth factor (IGF)-binding proteins-1 and -3 on IGF-activated glucose consumption in mouse BALB/c 3T3 fibroblasts. J Endocrinol. 1993;136:457–470. doi: 10.1677/joe.0.1360457. [DOI] [PubMed] [Google Scholar]
- Onder G, Liperoti R, Russo A, Capoluongo E, Minucci A, Lulli P, Cesari M, Maggio M, Bernabei R, Landi F. Use of ACE inhibitors is associated with elevated levels of IGFBP-3 among hypertensive older adults: results from the IlSIRENTE study. Eur J Clin Pharmacol. 2007;63:389–395. doi: 10.1007/s00228-007-0262-z. [DOI] [PubMed] [Google Scholar]
- Onder G, Penninx BW, Balkrishnan R, Fried LP, Chaves PH, Williamson J, Carter C, Di BM, Guralnik JM, Pahor M. Relation between use of angiotensin-converting enzyme inhibitors and muscle strength and physical function in older women: an observational study. Lancet. 2002;359:926–930. doi: 10.1016/s0140-6736(02)08024-8. [DOI] [PubMed] [Google Scholar]
- Owino V, Yang SY, Goldspink G. Age-related loss of skeletal muscle function and the inability to express the autocrine form of insulin-like growth factor-1 (MGF) in response to mechanical overload. FEBS Lett. 2001;505:259–263. doi: 10.1016/s0014-5793(01)02825-3. [DOI] [PubMed] [Google Scholar]
- Papadakis MA, Grady D, Black D, Tierney MJ, Gooding GA, Schambelan M, Grunfeld C. Growth hormone replacement in healthy older men improves body composition but not functional ability. Ann Intern Med. 1996;124:708–716. doi: 10.7326/0003-4819-124-8-199604150-00002. [DOI] [PubMed] [Google Scholar]
- Papadakis MA, Grady D, Tierney MJ, Black D, Wells L, Grunfeld C. Insulin-like growth factor 1 and functional status in healthy older men. J Am Geriatr Soc. 1995;43:1350–1355. doi: 10.1111/j.1532-5415.1995.tb06613.x. [DOI] [PubMed] [Google Scholar]
- Pelosi L, Giacinti C, Nardis C, Borsellino G, Rizzuto E, Nicoletti C, Wannenes F, Battistini L, Rosenthal N, Molinaro M, Musaro A. Local expression of IGF-1 accelerates muscle regeneration by rapidly modulating inflammatory cytokines and chemokines. FASEB J. 2007;21:1393–1402. doi: 10.1096/fj.06-7690com. [DOI] [PubMed] [Google Scholar]
- Powell DR, Suwanichkul A, Cubbage ML, DePaolis LA, Snuggs MB, Lee PD. Insulin inhibits transcription of the human gene for insulin-like growth factor-binding protein-1. J Biol Chem. 1991;266:18868–18876. [PubMed] [Google Scholar]
- Qin X, Strong DD, Baylink DJ, Mohan S. Structure-function analysis of the human insulin-like growth factor binding protein-4. J Biol Chem. 1998;273:23509–23516. doi: 10.1074/jbc.273.36.23509. [DOI] [PubMed] [Google Scholar]
- Raynaud-Simon A. Levels of plasma insulin-like growth factor I (IGF I), IGF II, IGF binding proteins, type 1 IGF receptor and growth hormone binding protein in community-dwelling elderly subjects with no malnutrition and no inflammation. J Nutr Health Aging. 2003;7:267–273. [PubMed] [Google Scholar]
- Rees C, Clemmons DR. Inhibition of IGFBP-5 binding to extracellular matrix and IGF-I-stimulated DNA synthesis by a peptide fragment of IGFBP-5. J Cell Biochem. 1998;71:375–381. [PubMed] [Google Scholar]
- Roubenoff R. Sarcopenia: a major modifiable cause of frailty in the elderly. J Nutr Health Aging. 2000;4:140–142. [PubMed] [Google Scholar]
- Roy RR, Tri C, Grossman EJ, Talmadge RJ, Grindeland RE, Mukku VR, Edgerton VR. IGF-I, growth hormone, and/or exercise effects on non-weight-bearing soleus of hypophysectomized rats. J Appl Physiol. 1996;81:302–311. doi: 10.1152/jappl.1996.81.1.302. [DOI] [PubMed] [Google Scholar]
- Rutherford OM, Jones DA, Round JM, Buchanan CR, Preece MA. Changes in skeletal muscle and body composition after discontinuation of growth hormone treatment in growth hormone deficient young adults. Clin Endocrinol (Oxf) 1991;34:469–475. doi: 10.1111/j.1365-2265.1991.tb00327.x. [DOI] [PubMed] [Google Scholar]
- Sadowski CL, Wheeler TT, Wang LH, Sadowski HB. GH regulation of IGF-I and suppressor of cytokine signaling gene expression in C2C12 skeletal muscle cells. Endocrinology. 2001;142:3890–3900. doi: 10.1210/endo.142.9.8365. [DOI] [PubMed] [Google Scholar]
- Santini MP, Tsao L, Monassier L, Theodoropoulos C, Carter J, Lara-Pezzi E, Slonimsky E, Salimova E, Delafontaine P, Song YH, Bergmann M, Freund C, Suzuki K, Rosenthal N. Enhancing repair of the mammalian heart. Circ Res. 2007;100:1732–1740. doi: 10.1161/CIRCRESAHA.107.148791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schertzer JD, Lynch GS. Comparative evaluation of IGF-I gene transfer and IGF-I protein administration for enhancing skeletal muscle regeneration after injury. Gene Ther. 2006;13:1657–1664. doi: 10.1038/sj.gt.3302817. [DOI] [PubMed] [Google Scholar]
- Schertzer JD, Ryall JG, Lynch GS. Systemic administration of IGF-I enhances oxidative status and reduces contraction-induced injury in skeletal muscles of mdx dystrophic mice. Am J Physiol Endocrinol Metab. 2006;291:E499–E505. doi: 10.1152/ajpendo.00101.2006. [DOI] [PubMed] [Google Scholar]
- Schulze PC, Fang J, Kassik KA, Gannon J, Cupesi M, MacGillivray C, Lee RT, Rosenthal N. Transgenic overexpression of locally acting insulin-like growth factor-1 inhibits ubiquitin-mediated muscle atrophy in chronic left-ventricular dysfunction. Circ Res. 2005;97:418–426. doi: 10.1161/01.RES.0000179580.72375.c2. [DOI] [PubMed] [Google Scholar]
- Shimasaki S, Ling N. Identification and molecular characterization of insulin-like growth factor binding proteins (IGFBP-1, -2, -3, -4, -5 and -6) Prog Growth Factor Res. 1991;3:243–266. doi: 10.1016/0955-2235(91)90003-m. [DOI] [PubMed] [Google Scholar]
- Song YH, Li Y, Du J, Mitch WE, Rosenthal N, Delafontaine P. Muscle-specific expression of IGF-1 blocks angiotensin II-induced skeletal muscle wasting. J Clin Invest. 2005;115:451–458. doi: 10.1172/JCI22324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonntag WE, Hylka VW, Meites J. Growth hormone restores protein synthesis in skeletal muscle of old male rats. J Gerontol. 1985;40:689–694. doi: 10.1093/geronj/40.6.689. [DOI] [PubMed] [Google Scholar]
- Stitt TN, Drujan D, Clarke BA, Panaro F, Timofeyva Y, Kline WO, Gonzalez M, Yancopoulos GD, Glass DJ. The IGF-1/PI3K/Akt pathway prevents expression of muscle atrophy-induced ubiquitin ligases by inhibiting FOXO transcription factors. Mol Cell. 2004;14:395–403. doi: 10.1016/s1097-2765(04)00211-4. [DOI] [PubMed] [Google Scholar]
- Strazzullo P, Iacone R, Iacoviello L, Russo O, Barba G, Russo P, D’Orazio A, Barbato A, Cappuccio FP, Farinaro E, Siani A. Genetic variation in the renin-angiotensin system and abdominal adiposity in men: the Olivetti Prospective Heart Study. Ann Intern Med. 2003;138:17–23. doi: 10.7326/0003-4819-138-1-200301070-00007. [DOI] [PubMed] [Google Scholar]
- Sugimoto T, Nakaoka D, Nasu M, Kanzawa M, Sugishita T, Chihara K. Effect of recombinant human growth hormone in elderly osteoporotic women. Clin Endocrinol (Oxf) 1999;51:715–724. doi: 10.1046/j.1365-2265.1999.00867.x. [DOI] [PubMed] [Google Scholar]
- Sylven C, Jansson E, Cederholm T, Hildebrand IL, Beermann B. Skeletal muscle depressed calcium and phosphofructokinase in chronic heart failure are upregulated by captopril—a double-blind, placebo-controlled study. J Intern Med. 1991;229:171–174. doi: 10.1111/j.1365-2796.1991.tb00326.x. [DOI] [PubMed] [Google Scholar]
- Szulc P, Duboeuf F, Marchand F, Delmas PD. Hormonal and lifestyle determinants of appendicular skeletal muscle mass in men: the MINOS study. Am J Clin Nutr. 2004;80:496–503. doi: 10.1093/ajcn/80.2.496. [DOI] [PubMed] [Google Scholar]
- Taaffe DR, Pruitt L, Reim J, Hintz RL, Butterfield G, Hoffman AR, Marcus R. Effect of recombinant human growth hormone on the muscle strength response to resistance exercise in elderly men. J Clin Endocrinol Metab. 1994;79:1361–1366. doi: 10.1210/jcem.79.5.7525633. [DOI] [PubMed] [Google Scholar]
- Ullman M, Oldfors A. Effects of growth hormone on skeletal muscle. I Studies on normal adult rats. Acta Physiol Scand. 1989;135:531–536. doi: 10.1111/j.1748-1716.1989.tb08612.x. [DOI] [PubMed] [Google Scholar]
- Thompson JL, Butterfield GE, Gylfadottir UK, Yesavage J, Marcus R, Hintz RL, Pearman A, Hoffman AR. Effects of human growth hormone, insulin-like growth factor I, and diet and exercise on body composition of obese postmenopausal women. J Clin Endocrinol Metab. 1998;83:1477–1484. doi: 10.1210/jcem.83.5.4826. [DOI] [PubMed] [Google Scholar]
- Vandervoort AA. Aging of the human neuromuscular system. Muscle Nerve. 2002;25:17–25. doi: 10.1002/mus.1215. [DOI] [PubMed] [Google Scholar]
- Vergani L, Losa M, Lesma E, Di Giulio AM, Torsello A, Muller EE, Gorio A. Glycosaminoglycans boost insulin-like growth factor-I-promoted neuroprotection: blockade of motor neuron death in the wobbler mouse. Neuroscience. 1999;93:565–572. doi: 10.1016/s0306-4522(99)00095-0. [DOI] [PubMed] [Google Scholar]
- Visser M, Pahor M, Taaffe DR, Goodpaster BH, Simonsick EM, Newman AB, Nevitt M, Harris TB. Relationship of interleukin-6 and tumor necrosis factor-alpha with muscle mass and muscle strength in elderly men and women: the Health ABC Study. J Gerontol A Biol Sci Med Sci. 2002;57:M326–M332. doi: 10.1093/gerona/57.5.m326. [DOI] [PubMed] [Google Scholar]
- Wang MX, Murrell DF, Szabo C, Warren RF, Sarris M, Murrell GA. Nitric oxide in skeletal muscle: inhibition of nitric oxide synthase inhibits walking speed in rats. Nitric Oxide. 2001;5:219–232. doi: 10.1006/niox.2001.0348. [DOI] [PubMed] [Google Scholar]
- Welle S, Thornton C, Statt M, McHenry B. Growth hormone increases muscle mass and strength but does not rejuvenate myofibrillar protein synthesis in healthy subjects over 60 years old. J Clin Endocrinol Metab. 1996;81:3239–3243. doi: 10.1210/jcem.81.9.8784075. [DOI] [PubMed] [Google Scholar]
- Williams AG, Dhamrait SS, Wootton PT, Day SH, Hawe E, Payne JR, Myerson SG, World M, Budgett R, Humphries SE, Montgomery HE. Bradykinin receptor gene variant and human physical performance. J Appl Physiol. 2004;96:938–942. doi: 10.1152/japplphysiol.00865.2003. [DOI] [PubMed] [Google Scholar]
- Yang S, Alnaqeeb M, Simpson H, Goldspink G. Cloning and characterization of an IGF-1 isoform expressed in skeletal muscle subjected to stretch. J Muscle Res Cell Motil. 1996;17:487–495. doi: 10.1007/BF00123364. [DOI] [PubMed] [Google Scholar]
- Yang SY, Goldspink G. Different roles of the IGF-I Ec peptide (MGF) and mature IGF-I in myoblast proliferation and differentiation. FEBS Lett. 2002;522:156–160. doi: 10.1016/s0014-5793(02)02918-6. [DOI] [PubMed] [Google Scholar]
- Yarasheski KE, Zachweija JJ, Angelopoulos TJ, Bier DM. Short-term growth hormone treatment does not increase muscle protein synthesis in experienced weight lifters. J Appl Physiol. 1993;74:3073–3076. doi: 10.1152/jappl.1993.74.6.3073. [DOI] [PubMed] [Google Scholar]
- Yarasheski KE, Zachwieja JJ, Campbell JA, Bier DM. Effect of growth hormone and resistance exercise on muscle growth and strength in older men. Am J Physiol. 1995;268:E268–E276. doi: 10.1152/ajpendo.1995.268.2.E268. [DOI] [PubMed] [Google Scholar]
- Zoll J, Monassier L, Garnier A, N’Guessan B, Mettauer B, Veksler V, Piquard F, Ventura-Clapier R, Geny B. ACE inhibition prevents myocardial infarction-induced skeletal muscle mitochondrial dysfunction. J Appl Physiol. 2006;101:385–391. doi: 10.1152/japplphysiol.01486.2005. [DOI] [PubMed] [Google Scholar]


