Abstract
Natural killer (NK) cells therapy has the potential to prolong survival in patients with advanced non-small cell lung cancer (NSCLC). We conducted a clinical trial to investigate the safety and efficacy of cetuximab plus NK cells therapy in patients with advanced NSCLC. Between June 2015 and August 2016, 54 patients with advanced EGFR-expressing NSCLC were assigned randomly to the cetuximab plus NK cells therapy group (A; n = 27) or cetuximab alone group (B; n = 27). Patients in group A received two courses of NK cells therapy continuously. Cetuximab was administered intravenously and the weekly maintenance dose was continued until tumor progression. All adverse effects were manageable and no significant difference was noted between the two groups (P > 0.05). Levels of CEA, NSE and circulating tumor cells (CTCs) in group A were significantly lower than those before treatment (P < 0.05). Patients in group A had a significant improvement in immune function and quality of life (QOL) (P < 0.05). Patients in group A survived longer than those in group B (median PFS: 6 months vs 4.5 months; median OS: 9.5 months vs 7.5 months; P < 0.05). Combination therapy could be an alternative to chemoradiotherapy for patients with advanced NSCLC.
Keywords: Non-small cell lung cancer, natural killer cells, cetuximab, clinical trial, efficacy, safety
Introduction
Lung cancer has been the leading cancer diagnosis and cancer-related death worldwide [1]. Over half of patients diagnosed with lung cancer die within 1 year of the diagnosis and 5-year survival is around 17.8% [2]. About 80% of lung cancer cases are non-small cell lung cancer (NSCLC). Most of these patients are treated with surgery and chemotherapy. However, these strategies prolong survival only modestly and are associated with a wide range of undesirable side effects [3,4]. Therefore, efforts to develop more effective therapeutic modalities for advanced NSCLC are an area of active investigation.
The epidermal growth factor receptor (EGFR) is a tyrosine kinase receptor that plays an integral part in the signaling pathways that control the growth of normal and malignant cells [5,6]. EGFR overexpression is associated with adverse prognostic profiles, including a shortened time to disease progression and overall survival (OS) [7]. Cetuximab (Erbitux®; ImClone Systems Branchburg, MJ, USA) is a chimeric mouse human antibody. It targets the extracellular domain of the EGFR, thereby inhibiting the binding of activating ligands to the receptor. Clinical trials have shown that cetuximab combined with radiotherapy or chemotherapy in patients with NSCLC is associated with a higher response rate, longer time to disease progression, and a significant improvement in survival [8,9].
For patients who are not candidates for surgery, immunotherapy is a promising treatment option for long-lasting control of advanced NSCLC [10-12]. In recent years, interest in the use of the immune system to treat NSCLC has been boosted by the encouraging clinical results of use of adoptive natural killer (NK) cells to treat tumors of the breast [13], liver [14], and digestive tract [15]. NK cells are critical innate immune lymphocytes which primarily lyse viral tumor cells through the production of cytokines and chemokines [16,17].
On the basis of the promising results of the studies mentioned above, we conducted a randomized clinical trial with the aim of investigating the efficacy and safety of cetuximab in combination with NK cells therapy as second-line and third-line treatments in patients with EGFR-expressing advanced NSCLC.
Patients and methods
Patient eligibility
This clinical trial was registered on ClinicalTrials.gov (ID: NCT02845856; Ph1/Ph2) and was approved by the Ethics Committee of Guangzhou Fuda Cancer Hospital (Guangzhou, China). Written informed consent was obtained from all patients in accordance with the Declaration of Helsinki.
Patients with NSCLC proven by histology or cytology of stage IIIB or IV according to the 7th Union For Cancer Control (UICC) tumor-node-metastasis (TNM) staging system, and immunohistochemical evidence of EGFR expression in at least one positively stained tumor cell, were eligible for inclusion in our study. Other inclusion criteria were: age > 18 years, lifespan > 4 months; Karnofsky performance status (KPS) score ≥ 60; adequate function of bone marrow (neutrophil count > 2 × 109/L, platelets ≥ 75 × 109/L, and hemoglobin ≥ 90 g/L); adequate hepatic function (bilirubin < 30 μmol/L, aminotransferase < 60 U/L); adequate renal function (serum creatinine < 130 μmol/L, serum urea < 10 mmol/L) and adequate cardiac function (a shortening fraction of ≥ 27% by echocardiography).
Patients with proven or symptomatic brain metastasis as well as those who had prior chemotherapy, radio therapy, or other immunosuppressive/immunostimulatory therapy within 3 weeks of study entry were excluded.
Cell processing
NK cells were generated under Good Manufacturing Practice conditions by a method described previously [18]. Mononuclear cells (MNCs) were isolated from peripheral blood using LymphoprepTM (Haoyang Biologicals, Tianjin, China) density gradient centrifugation from buffy coats obtained from healthy allogenic blood donors. To ensure selection of appropriate donors, the killer cell immunoglobulin-like receptor (KIR) genotype should not match with the human leukocyte antigen (HLA) class I molecules of the patient [18-22].
The peripheral blood of donors and recipients was tested with a TIANamp Blood DNA kit (Tiangen Biotech, Beijing, China) and KIR/HLA-Cw Genotyping Low Resolution kit (PCR-SSP) (Super Biotechnology Developing, Tianjin, China). MNCs were activated using a Human HANK Cell In Vitro Preparation kit (Hank Bioengineering, Shenzhen, China), along with lethally radiated K562-mb15-41BBL (K562D2) stimulatory cells [23], plasma treatment fluid, lymphocyte culture fluid additives, serum-free medium additives, and cell infusion additives according to manufacturer instructions. This kit is used for the expansion and activation of NK cells in peripheral blood or umbilical cord blood MNCs in vitro, as well as for the preparation of NK cells in greater quantities and with higher purity and activity (i.e., HANK cells) [18]. According to the instructions, NK cells were maintained in two plastic flasks (T75; Corning Costa, Cambridge, MA, USA) in an incubator in an atmosphere of 5% carbon dioxide at 37°C and 95% humidity. After culture, 8-10 billion NK cells were harvested using NK Cell Serum-free Medium and a culture bag (Haoyang Biologicals, Tianjin, China). Final cell counting and quality control testing were done on day 9 of culture. The quality-control indicators were: proportion of living cells ≥ 90%; proportion of CD56+CD3- cells ≥ 85% (detection via flow cytometry, as described previously [18]); endotoxin content ≤ 1 EU/mL; cell viability ≥ 80% (K562 cells were used as target cells in the cytotoxicity assay, as described previously [18]); and absence of bacteria, fungi, and mycoplasma cultures. All cell preparation processes were undertaken by the same technician and were assessed by another technician. After 12 days of culture, NK cells were counted and washed with physiologic (0.9%) saline. The cell concentration was adjusted to 20 × 106/mL with a saline solution containing an immune cell infusion additive (HK-005), and then divided into three parts and infused intravenously over 30 min on days 13-15. After infusion, new sample of peripheral blood were obtained to begin the new course on day 16.
Treatment plan
Patients were divided randomly between group A and group B to receive cetuximab plus NK cells therapy or cetuximab alone, respectively. The treatment schedule is summarized in Figure 1.
Figure 1.
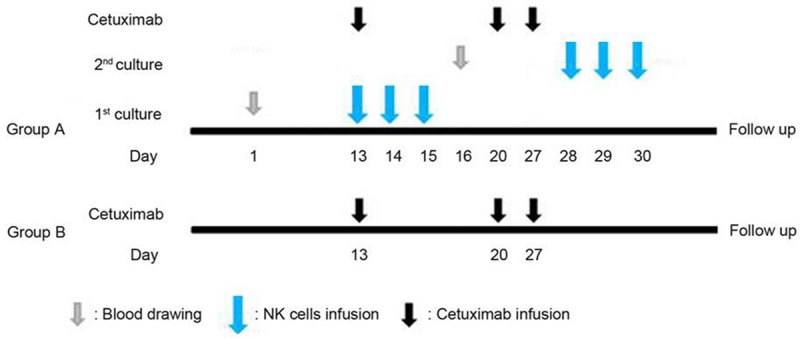
Treatment schedule. Peripheral blood from allogenic donor was drawn on day 1 and NK cells were infused intravenously on days 13 to 15. New sample of peripheral blood for the second course was drawn on day 16 and NK cells were infused intravenously on days 28-30. Cetuximab was administered intravenously on day 13 and the weekly maintenance dose was continued until tumor progression or unacceptable toxicity occurred.
Patients in group A received allogeneic NK cells therapy twice continuously. Cetuximab was infused intravenously at an initial dose of 400 mg/m2 (including a test dose of a 20 mg (10 mL) infusion administered over 10 min followed by an observation period of 30 min after administration for signs of anaphylaxis) over 2 h on day 13, and from day 20 onwards at 250 mg/m2 over 1 h per week. Premedication with an antihistamine was mandatory before the first infusion and was recommended for all further infusions. Cetuximab was infused before NK cells therapy on the days when both treatments were given, and was continued until disease progression or unacceptable toxicity occurred.
Assessment of immunologic parameters
Peripheral blood (2 mL) was obtained from patients on day 1 and day 90 for the detection of immune function by flow cytometry (FACSCantoTM II; BD Biosciences, San Jose, CA, USA). BD Multitest 6-color TBNK Reagent (BD Biosciences) was used to detect the number of CD3+CD4+ cells (95% confidence interval (CI), 441-2156 cells/μL), CD3+CD8+ cells (95% CI, 125-1312 cells/μL), total CD3+ cells (95% CI, 603-2990 cells/μL), CD3-CD19+ cells (95% CI, 107-698 cells/μL), and CD3-CD16+CD56+ cells (95% CI, 95-640 cells/μL). A BD Cytometric Bead Array (CBA) Human Th1/Th2 Cytokine Kit II (BD Biosciences) was used to detect expression of interleukin (IL)-2 (95% CI, 12.64-14.45 pg/mL), IL-4 (95% CI, 8.45-10.38 pg/mL), IL-6 (95% CI, 10-20.43 pg/mL), IL-10 (95% CI, 7.42-9.51 pg/mL), tumor necrosis factor (TNF)-β (95% CI, 4.18-5.50 pg/mL), and interferon (IFN)-γ (95% CI, 5.69-7.83 pg/mL). The tests were carried out according to the protocols given in the instruction manuals. Results above or within the reference range were considered to indicate normal immune function. Patients with one or more than one parameter exhibiting below-normal values were considered to have immune dysfunction.
Analyses of circulating tumor cell (CTC) levels
Peripheral blood (7 mL) was obtained from patients on day 1 and day 90 for the detection of CTC levels. MNCs from peripheral blood were separated using LymphoprepTM (Haoyang Biologicals) density gradient centrifugation from buffy coats and were washed twice with sterile Hank’s balanced salt solution (Life Technologies, Carlsbad, CA, USA). Isolated cells were enriched via binding to magnetic CD326 (EpCAM) MicroBeads (Miltenyi Biotech, Bergisch Gladbach, Germany) using magnetic activated cell sorting. Then, enriched isolated cells were labelled with monoclonal antibodies targeting the epithelial cell antigens CD45, CD326 as well as cytokeratins 8, 18 and 19 (Miltenyi Biotech) and incubated in the dark for 12 min at room temperature. The monoclonal antibodies (10 μL) anti-CD45-PE, Ep-CAM-APC, and cytokeratins 8, 18, and 19-FITC were added per 7.5 mL of whole blood. Cell pellets were resuspended in 500 μL PBS and enumerated by a FACSCantoTM II (Becton Dickinson, Franklin Lakes, NJ, USA) flow cytometer with a CD45-/CK+/CD326+ gating strategy. The absolute number of CD45-, CK+, and CD326+ cells on FACSCantoTM II was used to measure CTC levels [24,25].
Assessment of clinical safety and efficacy
Safety monitoring
Safety was determined based on regular interviews with patients, physical examination, and laboratory tests. All adverse effects were assessed and reported according to the National Cancer Institute Common Terminology Criteria for Adverse Events (v4.0).
Detection of levels of tumor markers
The serum concentration of carcino embryonic antigen (CEA) and neuron specific enolase (NSE) was detected using a chemiluminescent immunoassay in the laboratory of our hospital on day 1 and day 90.
Response to treatment
Tumor response was assessed by computed tomography (CT) in accordance with Response Evaluation Criteria in Solid Tumor (RECIST) v1.1 criteria [26] 3 months after treatment.
According to the degree of change in the largest transverse diameter, the therapeutic effect was classified as complete response (CR; arterial enhancement imaging indicated disappearance of all target lesions), partial response (PR; reduction in the sum of the diameter of target lesions by > 30%), stable disease (SD; tumor regression failed to reach PR status or tumor progression failed to reach PD status), and progressive disease (PD; the sum of the diameter of the tumors increased by > 20%). The response rate (RR) considered the CR and PR.
Evaluation of life quality
Evaluation of life quality was based on KPS criteria. “Improvement” denoted a KPS score that increased by ≥ 10 points after treatment. “Stability” denoted a KPS score that increased or decreased by < 10 points. “Decrease” denoted a KPS score that decreased by > 10 points. Responsive rate was calculated using the following equation: Responsive rate = (improved cases + stable cases)/total cases × 100%.
Progression-free survival (PFS) and OS
PFS was measured as the time from treatment initiation until radiologically confirmed disease progression was first noted or death from any cause occurred. OS was the time (calculated in months) from treatment initiation to the date of death.
Statistical analysis
The primary endpoint was OS. The secondary endpoints were PFS, overall response, QOL, immunologic parameters, and safety. All statistical tests for comparison of treatment groups were two-sided, and P < 0.05 was considered significant. Differences in frequencies of adverse effects were analyzed by Fisher’s exact test. The Kaplan-Meier method was used to estimate median PFS and OS and to draw survival curves. SPSS v17.0 (IBM, Armonk, NY, USA) was used for statistical analyses. Results were expressed as the mean ± standard deviation. Prism 5 (GraphPad, San Diego, CA, USA) was used to plot graphs.
Results
Patients
From June 2015 to August 2016, 54 patients with EGFR-overexpressing NSCLC were enrolled in our study and assigned randomly to two groups (n = 27). The clinical characteristics of the two groups were compared, and no significant difference was observed (P > 0.05) (Table 1).
Table 1.
Patient characteristics
| Characteristic | Group A (n = 27) | Group B (n = 27) | P value |
|---|---|---|---|
| Age (years) | P = 0.776 | ||
| < 55 | 9 | 10 | |
| ≥ 55 | 18 | 17 | |
| Sex | P = 0.735 | ||
| Male | 5 | 6 | |
| Female | 22 | 21 | |
| Tumor histology | P = 0.907 | ||
| Adenocarcinoma | 14 | 14 | |
| Squamous cell carcinoma | 10 | 9 | |
| Others | 3 | 4 | |
| EGFR-expressing cells | P = 0.859 | ||
| 1-10% | 4 | 6 | |
| 10-20% | 2 | 1 | |
| 20-35% | 1 | 1 | |
| > 35% | 20 | 19 | |
| KRAS genotyping | P = 0.715 | ||
| KRAS wild-type | 22 | 23 | |
| KRAS mutant | 5 | 4 | |
| Clinical stage (AJCC) | P = 0.551 | ||
| IIIB | 9 | 7 | |
| IV | 18 | 20 | |
| Karnofsky performance status | P = 0.743 | ||
| 60 | 3 | 5 | |
| 70 | 15 | 14 | |
| 80 | 9 | 8 | |
| Previous therapy | P = 0.521 | ||
| Surgery | 7 | 4 | |
| Chemotherapy | 22 | 22 | |
| Radiotherapy | 3 | 5 | |
| Tobacco use history | P = 0.784 | ||
| Never | 12 | 14 | |
| Current | 7 | 5 | |
| Previous | 8 | 8 |
AJCC, American Joint Committee on Cancer staging system.
Adverse effects
The treatment was well tolerated. After treatment, the white blood cell (WBC) count as well as levels of alanine aminotransferase (ALT), aspartate transaminase (AST) and creatinine (Cr) were not significantly different from those before treatment (P > 0.05) (Table 2). All treatment-related adverse effects experienced by patients during the study are shown in Figure 2. The incidence of adverse effects was compared, and no significant difference was noted between the two groups (P > 0.05). All the adverse effects were below grade 3 and most of them were grade 1. After symptomatic treatment, all symptoms were relieved and did not reappear.
Table 2.
Changes in laboratory index and tumor marker
| Item | Group | (n = 27) | Day 1 | Day 90 | P value |
|---|---|---|---|---|
| WBC (109/L) | A | 7.87 ± 4.90 | 8.22 ± 6.01 | P = 0.82 |
| B | 6.91 ± 2.96 | 6.94 ± 2.92 | P = 0.97 | |
| ALT (U/L) | A | 16.96 ± 9.04 | 18.40 ± 7.56 | P = 0.53 |
| B | 21.25 ± 11.29 | 21.72 ± 10.55 | P = 0.88 | |
| AST (U/L) | A | 25.33 ± 4.95 | 25.33 ± 8.98 | P = 0.99 |
| B | 28.54 ± 6.58 | 26.40 ± 7.58 | P = 0.30 | |
| Cr (μmol/L) | A | 73.59 ± 16.32 | 74.85 ± 20.98 | P = 0.81 |
| B | 63.12 ± 16.97 | 63.66 ± 16.89 | P = 0.91 | |
| CEA (ng/mL) | A | 36.90 ± 68.23 | 18.70 ± 33.77 | P = 0.02* |
| B | 37.42 ± 76.08 | 28.16 ± 69.80 | P = 0.66 | |
| NSE (ng/mL) | A | 33.04 ± 36.40 | 19.77 ± 6.93 | P = 0.04* |
| B | 27.45 ± 40.12 | 30.70 ± 23.2 | P = 0.57 |
P < 0.05 versus Group B.
Figure 2.
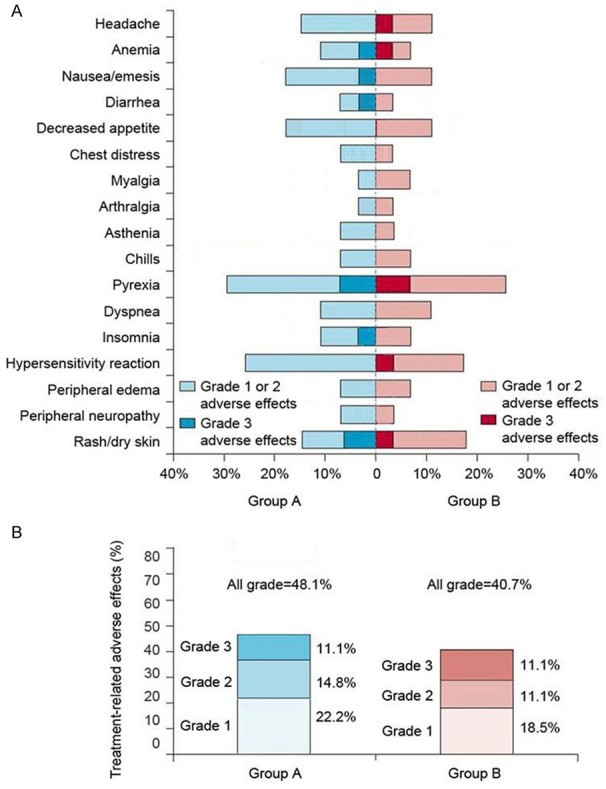
Treatment-related adverse effects. A. There was no significant difference between the two groups (P > 0.05). B. Proportions of patients having adverse effects, according to grade. There was no significant difference between the two group (P > 0.05).
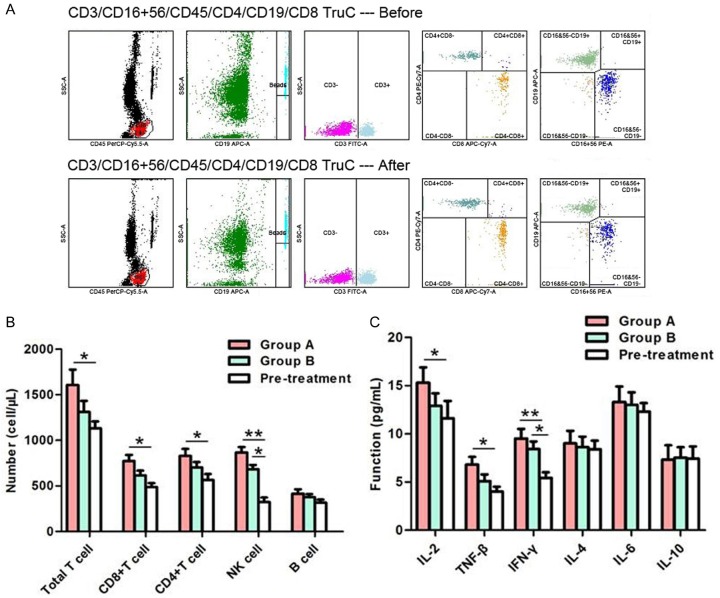
Immunological response
Immune function was compared before and after treatment (Figure 3). With regard to the lymphocyte number, the number of total T cells, CD8+ T cells and CD4+ T cells exhibited significant increases in group A (P < 0.05) and the number of NK cells increased in both groups (P < 0.05) after treatment. In terms of lymphocyte function, levels of IL-2 and TNF-β increased in group A (P < 0.05) and those of IFN-γ increased in both groups (P < 0.05) after treatment. However, changes in the levels of Th2-type cytokines were not significant (P > 0.05).
Figure 3.
Detection of the number and function of lymphocytes. A. Lymphocyte number in one of the patients in group A. Before treatment, the absolute number of total T cells, CD8+ T cells, CD4+ T cells, NK cells and B cells was 643.06, 192.49, 457.85, 128.31 and 323.65 cell/μL, respectively. After treatment, the absolute number of corresponding lymphocytes was 1170.76, 315.27, 858.36, 323.65 and 336.56 cell/μL, respectively. B. Comparison of lymphocyte number. After treatment, the number of total T cells, CD8+ T cells, and CD4+ T cells exhibited significant increases in group A (P < 0.05) and the number of NK cell increased in both groups (P < 0.05). C. Comparison of lymphocyte function. After treatment, levels of Th1-type cytokines increased in group A (P < 0.05) and those of IFN-γ increased in both groups (P < 0.05). *P < 0.05; **P < 0.01.
CTC levels
CTC levels were compared before and after treatment (Figure 4). The number of CTCs in group A decreased from 11.67 ± 5.60 to 8.56 ± 4.10/7.5 mL of blood after treatment (P = 0.02). However, the change of CTC levels in group B was not significant (P = 0.18).
Figure 4.
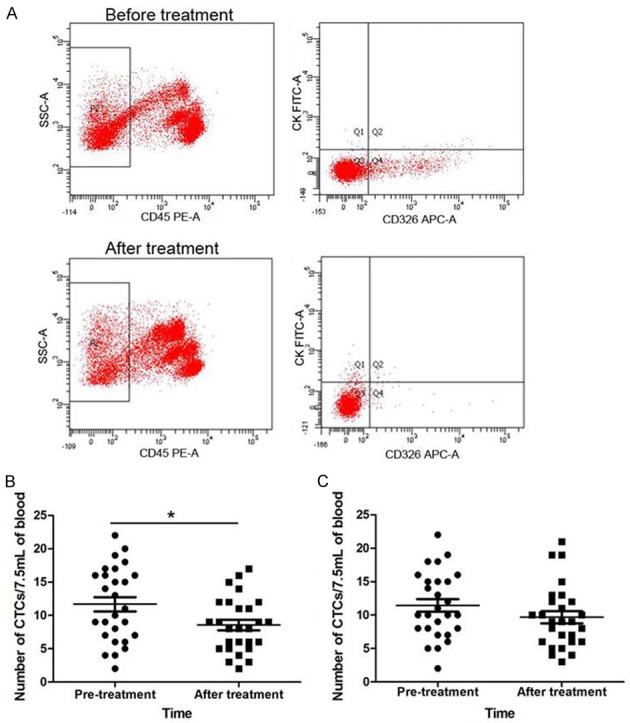
Detection of the number of CTCs. A. CTC detection in one of the patients in group A. Before treatment, the CTCs number was 19/7.5 mL of blood. After treatment, the CTC number was 13/7.5 mL of blood. B. The number of CTCs in group A decreased significantly after treatment (P = 0.02). C. Change in CTC levels in group B was not significant (P = 0.18). *P < 0.05.
Clinical efficacy
Tumor markers
Expression of CEA and NSE in the two groups was higher than normal 1 day before treatment. After treatment, expression of CEA and NSE in group A was significantly lower than that before treatment (P < 0.05) (Table 2). However, no significant difference was noted in group B after treatment (P > 0.05).
Treatment response
After 3 months follow-up, none of the 54 patients achieved CR (Table 3). Four patients (Figure 5) in group A achieved PR and 17 patients exhibited SD. Moreover, only 6 patients exhibited PD in group A. According to the RECIST evaluation, the clinical efficacy in group A was superior to that in group B (P < 0.05).
Table 3.
Clinical response
| Response | Overall | Group A | Group B | |||
|---|---|---|---|---|---|---|
|
| ||||||
| n | rate, % | n | rate, % | n | rate, % | |
| PR | 6 | 11.1 | 4 | 14.8 | 2 | 7.4* |
| SD | 31 | 57.4 | 17 | 63.0 | 14 | 51.9 |
| PD | 17 | 31.5 | 6 | 22.2 | 11 | 40.7* |
P < 0.05 versus Group B.
Figure 5.
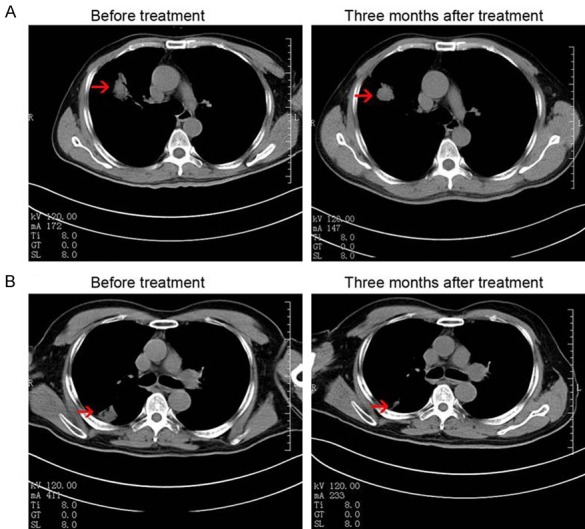
CT images of two representative cases who achieved PR 3 months after treatment in group A. A. Case 1: a 67-year-old male, stage IVA, a 3.9 × 3.5 cm tumor in the right lung before treatment, CT scan showed that the tumor decreased to 2.6 × 2.5 cm. B. Case 2. a 53-year-old male, stage IVA, a 3.5 × 3.5 cm tumor in the right lung before treatment, CT scan showed that the tumor decreased to 1.7 × 1.3 cm. (Red arrows indicate the tumor).
QOL
The KPS score before treatment in group A and B was 73.7 ± 6.4 and 72.8 ± 7.5, respectively. Three months after treatment, the KPS score was 82.96 ± 13.03 and 75.78 ± 14.83, respectively. The KPS scores increased in 11 patients, stabilized in 9 patients, and decreased in 7 patients in group A (Table 4); the corresponding value was 5, 9 and 13 in group B. After treatment, an increase in KPS score ≥ 10 was seen in 74.7% of patients in the group A and 51.9% of those in group B (P < 0.05).
Table 4.
Changes in quality of life
| Group | n | Improved | Stabilized | Decreased | Responsive rate % |
|---|---|---|---|---|---|
| Overall | 54 | 18 | 19 | 17 | 68.5% |
| A | 27 | 11* | 9 | 7* | 74.7%* |
| B | 27 | 5 | 9 | 13 | 51.9% |
P < 0.05 versus Group B.
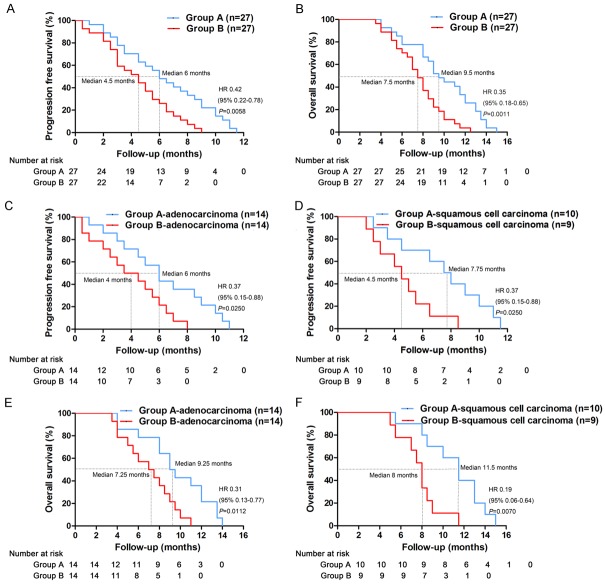
PFS and OS
Overall, the median PFS was 6 months in group A and 4.5 months in group B (Figure 6A). The median OS was 9.5 months in group A and 7.5 months in group B (Figure 6B). Separately, the median PFS was 6 months in patients with adenocarcinoma in group A and 4 months in group B (Figure 6C). The median PFS was 7.75 months in patients with squamous cell carcinoma in group A and 4.5 months in group B (Figure 6D). Moreover, the median OS was 9.25 months in patients with adenocarcinoma in group A and 7.25 months in group B (Figure 6E). The median PFS was 11.5 months in patients with squamous cell carcinoma in group A and 8 months in group B (Figure 6F). Treatment in group A resulted in a significant prolongation of PFS and OS compared with group B (P < 0.05).
Figure 6.
Kaplan-Meier estimates of PFS and OS. A. Comparison of PFS between the two groups. B. Comparison of OS between the two groups. C. Comparison of PFS between patients with adenocarcinoma in the two groups. D. Comparison of PFS between patients with squamous cell carcinoma in the two groups. E. Comparison of OS between patients with adenocarcinoma in the two groups. F. Comparison of OS between patients with squamous cell carcinoma in the two groups.
Discussion
Outcomes are poor for patients with EGFR-overexpression NSCLC, and systemic chemotherapy provides only modest benefits [27]. EGFR-directed tyrosine kinase inhibitors (such as erlotinib and gefitinib) are established treatment options for patients with advanced NSCLC who have been pretreated with platinum-based combinations [28,29], but their addition to first-line chemotherapy does not improve outcomes [30,31]. There is no difference in survival between conventional surgery and chemoradiotherapy [32], so there has been a marked shift in conventional therapy towards less radical and disfiguring treatments.
Overexpression of EGFR also leads to a poor prognosis, so a potential strategy to avoid the increased risk of recurrence is the use of cetuximab, which has been reported to prolong the survival time [33,34]. Cetuximab is a clinically approved anti-EGFR monoclonal antibody that binds to the extracellular domain of the EGFR to block EGFR dimerization, resulting in apoptosis and preventing tumor growth [35].
The relationship between cancer, cancer treatments and the immune system is extremely complex [36,37]. The recent success of immunotherapy has highlighted the potential of immune-based therapeutic approaches for NSCLC [38-40]. As part of the innate immune system, NK cells can kill tumor cells. NK cells comprise two subsets, from which the majority (about 90%) are phenotypically CD56dimCD16bright and exert mainly cytolytic functions, whereas the other subset, CD56brightCD16dim NK cells exert primarily immune regulatory functions [41]. These NK cells kill target cells that lack MHC molecules by activating receptors such as NKG2D, NKp30, NKp40, and NKp46. Tumor cells are more susceptible to NK cells due to their lack of MHC class-I molecules [42,43]. The KIRs present on NK cells prevent them from killing tumor cells that express similar MHC-I molecules. Hence, several studies have assessed the feasibility of NK cells allografts as an adoptive treatment for cancer [13,44].
It has been hypothesized that a combination of cetuximab and NK cells therapy may have a synergistic effect and enhance the efficacy of both treatments [45,46]. Here, we explore the potential of this novel treatment option for patients with advanced NSCLC. To our surprise, we found that combination therapy could improve the anti-tumor effect, and enhance immune function significantly (P < 0.05). Increases in the number of T cells and NK cells may be related to the improvement of cellular immunity and prevention of apoptosis of T cells [47]. Studies have shown that cytokines such as IL-12, IL-21 and TLR can stimulate NK cells to produce TNF-β and IFN-γ [48-50]. Therefore, increases in levels of Th1-type cytokines may be associated with cetuximab use, which can activate NK cells and potentiate antibody-dependent cell-mediated cytotoxicity (ADCC) [46,51]. A combination of NK cells therapy with cetuximab might not only reduce the tumor cell load but also neutralize tumor induced immunosuppression, thereby facilitating the effect of immunotherapy.
Moreover, we found that combination therapy could reduce levels of CTCs, CEA and NSE significantly (P < 0.05). Our previous studies have shown that the CTC level is a robust biomarker of the effects of immunotherapy and that its reduction may be related to tumor shrinkage [25,52]. Therefore, a reduction in CTC levels may reflect the fact that NK cells in the blood stream target CTCs to prevent metastasis and decrease or remove the residual tumor load [53]. An increased response rate and QOL reflect that combination therapy was superior to cetuximab alone for advanced NSCLC patients in our study. Furthermore, the prolongation of PFS and OS in the cetuximab plus NK cells therapy group was achieved with an acceptable safety profile. In this study, NK cells were activated K562-mb15-41BBL (K562D2) cells, which express IL-15 and 4-1BBL on the K562 cell surface. IL-15 trans-presentation mechanism operates in vivo to augment the tumor immune surveillance mechanism [54]. In the meantime, the activation of NK cells stimulated the production of IL-17A and Granzyme B which played an important role in preventing tumor development and metastasis [55,56]. Therefore, a prolongation of PFS and OS may be associated with the activation of NK cells.
In conclusion, the present study showed that combination therapy yielded better outcomes in patients with advanced EGFR-overexpressing NSCLC. This is the first clinical trial to investigate the safety and efficacy of a combination of cetuximab and NK cells therapy. Our study was not powered to adequately demonstrate its clinical benefit due to the small sample size. However, the encouraging survival results observed suggest that additional long-term follow-up on a larger cohort of patients merits consideration to further define the benefits of this combination therapy and to provide an alternative to chemoradiotherapy for patients with advanced EGFR-overexpressing NSCLC.
Acknowledgements
We would like to thank the native English speaking scientists of Elixigen Company for editing our manuscript. This work was supported by a grant from Tianhe Science and Technology project, China (201604KW008) and “Sanming Project of Medicine” in Shenzhen (SZSM201412009).
Disclosure of conflict of interest
None.
References
- 1.Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65:87–108. doi: 10.3322/caac.21262. [DOI] [PubMed] [Google Scholar]
- 2.Torre LA, Siegel RL, Jemal A. Lung cancer statistics. Adv Exp Med Biol. 2016;893:1–19. doi: 10.1007/978-3-319-24223-1_1. [DOI] [PubMed] [Google Scholar]
- 3.Tsao AS, Scagliotti GV, Bunn PA Jr, Carbone DP, Warren GW, Bai C, de Koning HJ, Yousaf-Khan AU, McWilliams A, Tsao MS, Adusumilli PS, RamiPorta R, Asamura H, Van Schil PE, Darling GE, Ramalingam SS, Gomez DR, Rosenzweig KE, Zimmermann S, Peters S, Ignatius Ou SH, Reungwetwattana T, Janne PA, Mok TS, Wakelee HA, Pirker R, Mazieres J, Brahmer JR, Zhou Y, Herbst RS, Papadimitrakopoulou VA, Redman MW, Wynes MW, Gandara DR, Kelly RJ, Hirsch FR, Pass HI. Scientific advances in lung cancer 2015. J Thorac Oncol. 2016;11:613–638. doi: 10.1016/j.jtho.2016.03.012. [DOI] [PubMed] [Google Scholar]
- 4.Zappa C, Mousa SA. Non-small cell lung cancer: current treatment and future advances. Transl Lung Cancer Res. 2016;5:288–300. doi: 10.21037/tlcr.2016.06.07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Arteaga CL. ErbB-targeted therapeutic approaches in human cancer. Exp Cell Res. 2003;284:122–130. doi: 10.1016/s0014-4827(02)00104-0. [DOI] [PubMed] [Google Scholar]
- 6.Hynes NE, Lane HA. ERBB receptors and cancer: the complexity of targeted inhibitors. Nat Rev Cancer. 2005;5:341–354. doi: 10.1038/nrc1609. [DOI] [PubMed] [Google Scholar]
- 7.Brabender J, Danenberg KD, Metzger R, Schneider PM, Park J, Salonga D, Holscher AH, Danenberg PV. Epidermal growth factor receptor and HER2-neu mRNA expression in non-small cell lung cancer Is correlated with survival. Clin Cancer Res. 2001;7:1850–1855. [PubMed] [Google Scholar]
- 8.Chen P, Wang L, Liu B, Zhang HZ, Liu HC, Zou Z. EGFR-targeted therapies combined with chemotherapy for treating advanced nonsmall-cell lung cancer: a meta-analysis. Eur J Clin Pharmacol. 2011;67:235–243. doi: 10.1007/s00228-010-0965-4. [DOI] [PubMed] [Google Scholar]
- 9.Nieder C, Pawinski A, Dalhaug A, Andratschke N. A review of clinical trials of cetuximab combined with radiotherapy for non-small cell lung cancer. Radiat Oncol. 2012;7:3. doi: 10.1186/1748-717X-7-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Spellman A, Tang SC. Immunotherapy for breast cancer: past, present, and future. Cancer Metastasis Rev. 2016;35:525–546. doi: 10.1007/s10555-016-9654-9. [DOI] [PubMed] [Google Scholar]
- 11.Hadden JW. The immunology and immunotherapy of breast cancer: an update. Int J Immunopharmacol. 1999;21:79–101. doi: 10.1016/s0192-0561(98)00077-0. [DOI] [PubMed] [Google Scholar]
- 12.Kubo M, Morisaki T, Kuroki H, Tasaki A, Yamanaka N, Matsumoto K, Nakamura K, Onishi H, Baba E, Katano M. Combination of adoptive immunotherapy with Herceptin for patients with HER2-expressing breast cancer. Anticancer Res. 2003;23:4443–4449. [PubMed] [Google Scholar]
- 13.Geller MA, Cooley S, Judson PL, Ghebre R, Carson LF, Argenta PA, Jonson AL, Panoskaltsis-Mortari A, Curtsinger J, McKenna D, Dusenbery K, Bliss R, Downs LS, Miller JS. A phase II study of allogeneic natural killer cell therapy to treat patients with recurrent ovarian and breast cancer. Cytotherapy. 2011;13:98–107. doi: 10.3109/14653249.2010.515582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sun C, Sun HY, Xiao WH, Zhang C, Tian ZG. Natural killer cell dysfunction in hepatocellular carcinoma and NK cell-based immunotherapy. Acta Pharmacol Sin. 2015;36:1191–1199. doi: 10.1038/aps.2015.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sakamoto N, Ishikawa T, Kokura S, Okayama T, Oka K, Ideno M, Sakai F, Kato A, Tanabe M, Enoki T, Mineno J, Naito Y, Itoh Y, Yoshikawa T. Phase I clinical trial of autologous NK cell therapy using novel expansion method in patients with advanced digestive cancer. J Transl Med. 2015;13:277. doi: 10.1186/s12967-015-0632-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhao Y, Hu J, Li R, Song J, Kang Y, Liu S, Zhang D. Enhanced NK cell adoptive antitumor effects against breast cancer in vitro via blockade of the transforming growth factor-beta signaling pathway. Onco Targets Ther. 2015;8:1553–1559. doi: 10.2147/OTT.S82616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cheng M, Chen Y, Xiao W, Sun R, Tian Z. NK cell-based immunotherapy for malignant diseases. Cell Mol Immunol. 2013;10:230–252. doi: 10.1038/cmi.2013.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhang M, Daniel S, Huang Y, Chancey C, Huang Q, Lei YF, Grinev A, Mostowski H, Rios M, Dayton A. Anti-West Nile virus activity of in vitro expanded human primary natural killer cells. BMC Immunol. 2010;11:3. doi: 10.1186/1471-2172-11-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Witt CS, Christiansen FT. The relevance of natural killer cell human leucocyte antigen epitopes and killer cell immunoglobulin-like receptors in bone marrow transplantation. Vox Sang. 2006;90:10–20. doi: 10.1111/j.1423-0410.2005.00712.x. [DOI] [PubMed] [Google Scholar]
- 20.Forte P, Baumann BC, Schneider MK, Seebach JD. HLA-Cw4 expression on porcine endothelial cells reduces cytotoxicity and adhesion mediated by CD158a+ human NK cells. Xenotransplantation. 2009;16:19–26. doi: 10.1111/j.1399-3089.2009.00510.x. [DOI] [PubMed] [Google Scholar]
- 21.Kunert K, Seiler M, Mashreghi MF, Klippert K, Schonemann C, Neumann K, Pratschke J, Reinke P, Volk HD, Kotsch K. KIR/HLA ligand incompatibility in kidney transplantation. Transplantation. 2007;84:1527–1533. doi: 10.1097/01.tp.0000290681.41859.41. [DOI] [PubMed] [Google Scholar]
- 22.Moretta L, Moretta A. Killer immunoglobulinlike receptors. Curr Opin Immunol. 2004;16:626–633. doi: 10.1016/j.coi.2004.07.010. [DOI] [PubMed] [Google Scholar]
- 23.Imai C, Iwamoto S, Campana D. Genetic modification of primary natural killer cells overcomes inhibitory signals and induces specific killing of leukemic cells. Blood. 2005;106:376–383. doi: 10.1182/blood-2004-12-4797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hristozova T, Konschak R, Budach V, Tinhofer I. A simple multicolor flow cytometry protocol for detection and molecular characterization of circulating tumor cells in epithelial cancers. Cytometry A. 2012;81:489–495. doi: 10.1002/cyto.a.22041. [DOI] [PubMed] [Google Scholar]
- 25.Shi J, Li Y, Liang S, Zeng J, Liu G, Mu F, Li H, Chen J, Liu T, Niu L. Analysis of circulating tumor cells in colorectal cancer liver metastasis patients before and after cryosurgery. Cancer Biol Ther. 2016;17:935–942. doi: 10.1080/15384047.2016.1210731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tsuchida Y, Therasse P. Response evaluation criteria in solid tumors (RECIST): new guidelines. Med Pediatr Oncol. 2001;37:1–3. doi: 10.1002/mpo.1154. [DOI] [PubMed] [Google Scholar]
- 27.Al-Farsi A, Ellis PM. Treatment paradigms for patients with metastatic non-small cell lung cancer, squamous lung cancer: first, second, and third-line. Front Oncol. 2014;4:157. doi: 10.3389/fonc.2014.00157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shepherd FA, Rodrigues Pereira J, Ciuleanu T, Tan EH, Hirsh V, Thongprasert S, Campos D, Maoleekoonpiroj S, Smylie M, Martins R, van Kooten M, Dediu M, Findlay B, Tu D, Johnston D, Bezjak A, Clark G, Santabarbara P, Seymour L. Erlotinib in previously treated non-small-cell lung cancer. N Engl J Med. 2005;353:123–132. doi: 10.1056/NEJMoa050753. [DOI] [PubMed] [Google Scholar]
- 29.Kim ES, Hirsh V, Mok T, Socinski MA, Gervais R, Wu YL, Li LY, Watkins CL, Sellers MV, Lowe ES, Sun Y, Liao ML, Osterlind K, Reck M, Armour AA, Shepherd FA, Lippman SM, Douillard JY. Gefitinib versus docetaxel in previously treated non-small-cell lung cancer (INTEREST): a randomised phase III trial. Lancet. 2008;372:1809–1818. doi: 10.1016/S0140-6736(08)61758-4. [DOI] [PubMed] [Google Scholar]
- 30.Herbst RS, Giaccone G, Schiller JH, Natale RB, Miller V, Manegold C, Scagliotti G, Rosell R, Oliff I, Reeves JA, Wolf MK, Krebs AD, Averbuch SD, Ochs JS, Grous J, Fandi A, Johnson DH. Gefitinib in combination with paclitaxel and carboplatin in advanced non-small-cell lung cancer: a phase III trial--INTACT 2. J. Clin. Oncol. 2004;22:785–794. doi: 10.1200/JCO.2004.07.215. [DOI] [PubMed] [Google Scholar]
- 31.Gatzemeier U, Pluzanska A, Szczesna A, Kaukel E, Roubec J, De Rosa F, Milanowski J, Karnicka-Mlodkowski H, Pesek M, Serwatowski P, Ramlau R, Janaskova T, Vansteenkiste J, Strausz J, Manikhas GM, Von Pawel J. Phase III study of erlotinib in combination with cisplatin and gemcitabine in advanced non-small-cell lung cancer: the Tarceva lung cancer investigation trial. J. Clin. Oncol. 2007;25:1545–1552. doi: 10.1200/JCO.2005.05.1474. [DOI] [PubMed] [Google Scholar]
- 32.Mirimanoff RO. Neoadjuvant chemoradiotherapy followed by surgery for stage IIIa and IIIb non-small-cell lung cancer (NSCLC): is it still justified? Chin Clin Oncol. 2015;4:49. doi: 10.3978/j.issn.2304-3865.2015.12.05. [DOI] [PubMed] [Google Scholar]
- 33.Stewart EL, Tan SZ, Liu G, Tsao MS. Known and putative mechanisms of resistance to EGFR targeted therapies in NSCLC patients with EGFR mutations-a review. Transl Lung Cancer Res. 2015;4:67–81. doi: 10.3978/j.issn.2218-6751.2014.11.06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hilbe W, Pall G, Kocher F, Pircher A, Zabernigg A, Schmid T, Schumacher M, Jamnig H, Fiegl M, Gachter A, Freund M, Kendler D, Manzl C, Zelger B, Popper H, Woll E. Multicenter Phase II study evaluating two cycles of docetaxel, cisplatin and cetuximab as induction regimen prior to surgery in chemotherapy-naive patients with NSCLC Stage IB-IIIA (INN06-Study) PLoS One. 2015;10:e0125364. doi: 10.1371/journal.pone.0125364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Capdevila J, Elez E, Macarulla T, Ramos FJ, Ruiz-Echarri M, Tabernero J. Anti-epidermal growth factor receptor monoclonal antibodies in cancer treatment. Cancer Treat Rev. 2009;35:354–363. doi: 10.1016/j.ctrv.2009.02.001. [DOI] [PubMed] [Google Scholar]
- 36.Nagalla S, Chou JW, Willingham MC, Ruiz J, Vaughn JP, Dubey P, Lash TL, Hamilton-Dutoit SJ, Bergh J, Sotiriou C, Black MA, Miller LD. Interactions between immunity, proliferation and molecular subtype in breast cancer prognosis. Genome Biol. 2013;14:R34. doi: 10.1186/gb-2013-14-4-r34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schmidt M, Bohm D, von Torne C, Steiner E, Puhl A, Pilch H, Lehr HA, Hengstler JG, Kolbl H, Gehrmann M. The humoral immune system has a key prognostic impact in node-negative breast cancer. Cancer Res. 2008;68:5405–5413. doi: 10.1158/0008-5472.CAN-07-5206. [DOI] [PubMed] [Google Scholar]
- 38.Manegold C, Dingemans AC, Gray JE, Nakagawa K, Nicolson M, Peters S, Reck M, Wu YL, Brustugun OT, Crino L, Felip E, Fennell D, Garrido P, Huber RM, Marabelle A, Moniuszko M, Mornex F, Novello S, Papotti M, Perol M, Smit EF, Syrigos K, van Meerbeeck JP, van Zandwijk N, Chih-Hsin Yang J, Zhou C, Vokes E. The potential of combined immunotherapy and antiangiogenesis for the synergistic treatment of advanced NSCLC. J Thorac Oncol. 2017;12:194–207. doi: 10.1016/j.jtho.2016.10.003. [DOI] [PubMed] [Google Scholar]
- 39.Bansal P, Osman D, Gan GN, Simon GR, Boumber Y. Recent advances in immunotherapy in metastatic NSCLC. Front Oncol. 2016;6:239. doi: 10.3389/fonc.2016.00239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Davies M. New modalities of cancer treatment for NSCLC: focus on immunotherapy. Cancer Manag Res. 2014;6:63–75. doi: 10.2147/CMAR.S57550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cooper MA, Fehniger TA, Caligiuri MA. The biology of human natural killer-cell subsets. Trends Immunol. 2001;22:633–640. doi: 10.1016/s1471-4906(01)02060-9. [DOI] [PubMed] [Google Scholar]
- 42.Cho D, Kim SK, Carson WE 3rd. NK cell-based immunotherapy for treating cancer: will it be promising? Korean J Hematol. 2011;46:3–5. doi: 10.5045/kjh.2011.46.1.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lanier LL, Phillips JH. Inhibitory MHC class I receptors on NK cells and T cells. Immunol Today. 1996;17:86–91. doi: 10.1016/0167-5699(96)80585-8. [DOI] [PubMed] [Google Scholar]
- 44.Re F, Staudacher C, Zamai L, Vecchio V, Bregni M. Killer cell Ig-like receptors ligand-mismatched, alloreactive natural killer cells lyse primary solid tumors. Cancer. 2006;107:640–648. doi: 10.1002/cncr.22002. [DOI] [PubMed] [Google Scholar]
- 45.Roberti MP, Rocca YS, Amat M, Pampena MB, Loza J, Colo F, Fabiano V, Loza CM, Arriaga JM, Bianchini M, Barrio MM, Bravo AI, Domenichini E, Chacon R, Mordoh J, Levy EM. IL-2- or IL-15-activated NK cells enhance Cetuximab-mediated activity against triple-negative breast cancer in xenografts and in breast cancer patients. Breast Cancer Res Treat. 2012;136:659–671. doi: 10.1007/s10549-012-2287-y. [DOI] [PubMed] [Google Scholar]
- 46.Veluchamy JP, Spanholtz J, Tordoir M, Thijssen VL, Heideman DA, Verheul HM, de Gruijl TD, van der Vliet HJ. Combination of NK cells and cetuximab to enhance anti-tumor responses in RAS mutant metastatic colorectal cancer. PLoS One. 2016;11:e0157830. doi: 10.1371/journal.pone.0157830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhang B, Sun T, Xue L, Han X, Lu N, Shi Y, Tan W, Zhou Y, Zhao D, Zhang X, Guo Y, Lin D. Functional polymorphisms in FAS and FASL contribute to increased apoptosis of tumor infiltration lymphocytes and risk of breast cancer. Carcinogenesis. 2007;28:1067–1073. doi: 10.1093/carcin/bgl250. [DOI] [PubMed] [Google Scholar]
- 48.Park YK, Shin DJ, Cho D, Kim SK, Lee JJ, Shin MG, Ryang DW, Lee JS, Park MH, Yoon JH, Jegal YJ. Interleukin-21 increases direct cytotoxicity and IFN-gamma production of ex vivo expanded NK cells towards breast cancer cells. Anticancer Res. 2012;32:839–846. [PubMed] [Google Scholar]
- 49.Lu H, Dietsch GN, Matthews MA, Yang Y, Ghanekar S, Inokuma M, Suni M, Maino VC, Henderson KE, Howbert JJ, Disis ML, Hershberg RM. VTX-2337 is a novel TLR8 agonist that activates NK cells and augments ADCC. Clin Cancer Res. 2012;18:499–509. doi: 10.1158/1078-0432.CCR-11-1625. [DOI] [PubMed] [Google Scholar]
- 50.Lu H, Yang Y, Gad E, Inatsuka C, Wenner CA, Disis ML, Standish LJ. TLR2 agonist PSK activates human NK cells and enhances the antitumor effect of HER2-targeted monoclonal antibody therapy. Clin Cancer Res. 2011;17:6742–6753. doi: 10.1158/1078-0432.CCR-11-1142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Chen S, Li X, Chen R, Yin M, Zheng Q. Cetuximab intensifies the ADCC activity of adoptive NK cells in a nude mouse colorectal cancer xenograft model. Oncol Lett. 2016;12:1868–1876. doi: 10.3892/ol.2016.4835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Shi J, Li Y, Liang S, Zeng J, Liu G, Mu F, Li H, Chen J, Lin M, Sheng S, Zhang H, Liu T, Niu L. Circulating tumour cells as biomarkers for evaluating cryosurgery on unresectable hepatocellular carcinoma. Oncol Rep. 2016;36:1845–1851. doi: 10.3892/or.2016.5050. [DOI] [PubMed] [Google Scholar]
- 53.Geller MA, Miller JS. Use of allogeneic NK cells for cancer immunotherapy. Immunotherapy. 2011;3:1445–1459. doi: 10.2217/imt.11.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kobayashi H, Dubois S, Sato N, Sabzevari H, Sakai Y, Waldmann TA, Tagaya Y. Role of transcellular IL-15 presentation in the activation of NK cell-mediated killing, which leads to enhanced tumor immunosurveillance. Blood. 2005;105:721–727. doi: 10.1182/blood-2003-12-4187. [DOI] [PubMed] [Google Scholar]
- 55.Kryczek I, Banerjee M, Cheng P, Vatan L, Szeliga W, Wei S, Huang E, Finlayson E, Simeone D, Welling TH, Chang A, Coukos G, Liu R, Zou W. Phenotype, distribution, generation, and functional and clinical relevance of Th17 cells in the human tumor environments. Blood. 2009;114:1141–1149. doi: 10.1182/blood-2009-03-208249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Oberoi P, Jabulowsky RA, Bahr-Mahmud H, Wels WS. EGFR-targeted granzyme B expressed in NK cells enhances natural cytotoxicity and mediates specific killing of tumor cells. PLoS One. 2013;8:e61267. doi: 10.1371/journal.pone.0061267. [DOI] [PMC free article] [PubMed] [Google Scholar]