Abstract
The disorder of lipid metabolism is pathologically linked to hyperlipidemia, lipid storage disease, obesity and other related diseases. Intriguingly, recent studies have revealed that lipid metabolism disorders play an important role in carcinogenesis and development as well, since they cause abnormal expression of various genes, proteins, and dysregulation of cytokines and signaling pathways. More importantly, lipid-lowering drugs and anti-lipid per-oxidation treatment have been showing their advantages in clinic, in comparison with other anti-cancer drugs with high toxicity. Thus, further elucidation of molecular mechanism between lipid metabolism and cancer is essential in developing novel diagnostic biomarkers and therapeutic targets of human cancers.
Keywords: Lipid, lipid metabolism, carcinogenesis, cancer development, cancer treatment
Introduction
Disorder of lipid metabolism is characterized by abnormalities of lipids and lipid metabolites existed predominantly in plasma as well as in other tissues, which is caused by congenital or acquired factors. Classically, lipids are mainly classified into two types: lipoid (e.g. phospholipids, glycolipids and sterols, etc.) and fat, such as triglycerides (TG), and sterols also include cholesterol, sex hormones and vitamin D, etc. These lipids are digested, absorbed through small intestine, transformed by liver, stored in adipose tissue, subsequently, served to other tissues when they are in need. TG as important energy storage substance plays a major role in energy supply, thermoregulation, and organism protection combined with skin, bone, muscle and other tissues, as well as assistance in absorption of fat-soluble vitamins and so on. Additionally, phospholipids as the main components of biofilm structures, prevent cell membrane from damage, and promote TG metabolism and degradation of deposited cholesterol. Cholesterol is also the basic component of the cell membrane, which maintains the stability of phospholipids bilayer in cell membrane. Furthermore, it serves as the precursor of steroid hormones, such as gonadal hormone and adrenal cortex hormones. Therefore, the level of lipids is regulated by various related genes, hormones and enzymes. Abnormalities of related genes, hormones and enzymes lead to lipids metabolism disorders, resulting in cardiovascular disorders (CVD), metabolic diseases and cancers, etc.
In addition to energy supply, lipids also promote cell growth and proliferation. Douglas Hanahan et al. proposed that the disorder of energy metabolism might induce malignancies [1]. Recently, the frontier of cancer research has shown that energy metabolism, especially lipid metabolism, is significantly elevated during carcinogenesis. Furthermore, abnormality of lipid metabolism promotes cancer development, invasion and metastasis via multiple signaling pathways which implies that targeting lipid metabolism could be a novel strategy for cancer prevention and treatment.
Lipid metabolism and diseases
It is well known that lipid metabolism is a complex physiological process, involving lipid intake, synthesis and transportation, and so on. Lipids are mainly derived from food intake and de novo synthesis of acetyl coenzyme A in vivo. Consistently, high expression of fatty acid synthase (FASN) has been observed in cancer cells [2,3]. In mammals, FASN is an important enzyme with six catalytic domains, which catalyzes the synthesis of endogenous long chain fatty acid. FASN converts dietary carbohydrates to long chain saturated fatty acids through acetyl-CoA, malonylCoA and nicotinamide adenine dinucleotide phosphate (NADPH) [4]. Abnormally elevated cholesterol levels may be attributed to sterol-regulatory element binding proteins (SREBPs) mediated by 3-hydroxy-3-methyl glutaryl coenzyme A reductase (HMGR) in cancer cells. Upon cholesterol depletion, nascent SREBPs embedded in the endoplasmic reticulum (ER), are transported to the Golgi apparatus, where nascent SREBPs undergo intramembrane proteolysis under the control of Insulin-induced gene (Insig) and SREBP cleavage-activating protein (SCAP). Then up-regulated HMGR regulates cholesterol synthesis [5] (Figure 2). The mevalonate pathway catalyzed by HMGR is one of the main pathways to produce sterols as well [6]. Conversely, excessive accumulation of intracellular oxysterols results in up-regulation of liver X receptors (LXRs), and thus stimulates SREBPs (Figure 2). LXRs belong to nuclear receptor subfamily of ligand-activated transcription factors, including LXRα and LXRβ. The activation of LXRα and LXRβ up-regulates the expression of the related proteins, such as SREBP-1C (a subtype of SREBP) and ATP-binding cassette transporter A1 (ABCA1), thereby regulating lipid synthesis, transportation and so on. LXRs belong to nuclear receptor subfamily of ligand-activated transcription factors, including LXRα and LXRβ. The activation of LXRα and LXRβ up-regulates the expression of the related proteins, such as SREBP-1C (a subtype of SREBP) and ABCA1, thereby regulating lipid synthesis, transportation and so on. LXRs are also the regulatory hub of multiple metabolic pathways, including fatty acid, cholesterol and energy metabolism [7,8]. For example, up-regulated expression of SREBPs promotes lipid synthesis, while as, up-regulated expression of ABCA1 accelerates intracellular cholesterol efflux.
Figure 2.
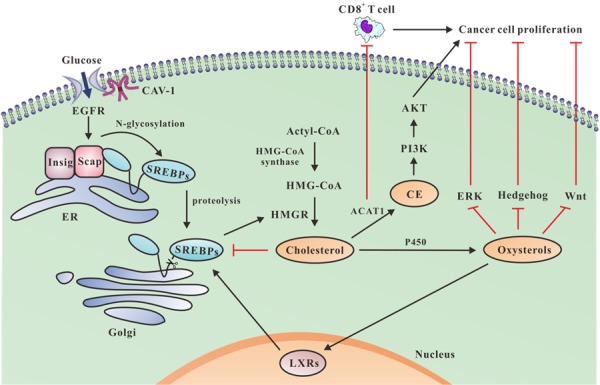
The effects of cholesterol synthesis on cancer cell proliferation. Glucose uptake via CAV-1 promotes EGFR signaling pathway, induces N-glycosylation of SCAP and consequent activation of SREBPs. Upon cholesterol depletion, the nascent SREBPs, embedded in the ER, are transported to the Golgi. SREBPs undergo intramembrane proteolysis under the control of Insig and SCAP to be the hydrolysis products of SREBPs, which up-regulate HMGR to promote cholesterol synthesis. Oxysterols produced by cytochrome P450 enzyme-mediated cholesterol oxidation reaction, lead to up-regulation of SREBPs since they up-regulate LXRs, and thereby stimulate HMGR. Oxysterols slow down proliferation by interfering with ERK, Hedgehog and Wnt pathways. ACAT1 catalyzes cholesterol esterification leads to activation of PI3K/AKT pathway, thus, significantly promotes cancer proliferation. Moreover, ACAT1 inhibits proliferation of CD8+ T cells, promoting cancer growth.
The key to maintain lipid homeostasis is whether excessive intracellular uptake or synthesis of lipids can be metabolized or transported to be outside of the cell membrane. Similarly, lipid efflux also involves multiple proteins, including ABCA1, Apolipoprotein (Apo) A-1, ApoE, peroxisome proliferators-activated receptors (PPARs), Scavenger receptor class B type 1 (SR-B1) and Caveolin-1 (Cav-1), etc. ABCA1 is mainly involved in the regulation of reverse cholesterol transport and prevention of type II diabetes mellitus (T2DM) and atherosclerosis (AS) [9]. Binding of ApoA-1 to ABCA1 may also enhance the activity of lipid-transport pathway. In addition to the known role of ApoA-1 as the key carrier of high density lipoprotein (HDL) and cholesterol receptor, it also enhances HDL influx and cholesterol efflux. Furthermore, it promotes excessive cholesterol excretion from peripheral liver tissue. It is reported that higher levels of plasma cholesterol are associated with lower risk of Parkinson’s disease, indicating that abnormal lipid metabolism may be closely correlated with neurodegenerative diseases [10]. Moreover, ApoA-1 up-regulation can promote glioblastoma cell apoptosis and necrosis [11]. Furthermore, PPARs induce over-expression of HDL receptors, such as SR-B1 and ABCA1 which accelerate cellular cholesterol efflux. The nuclear receptors PPARs include three isotypes, α, β/δ and γ, which orchestrate lipid synthesis and cholesterol metabolism, reducing the incidence of nonalcoholic fatty liver disease and liver cancer [12].
Dyslipidemia, in particular, elevated levels of serum lipid and up-regulation of lipoproteins, have been considered to be the common pathology of various diseases, such as obesity, T2DM and hepatitis [13]. Increased levels of low density lipoprotein (LDL) and serum total cholesterol (TC) and decreased level of HDL not only result in AS, but also lead to coronary heart diseases and brain infarction [14]. Taken together, lipid metabolic disorders are involved in pathologies of various diseases directly or indirectly.
Abnormal levels of lipid and carcinogenesis, cancer development
Numerous of studies have demonstrated that abnormal levels of lipids are intimately related to carcinogenesis and cancer metastasis. Malignant transformation and accelerated of cancer cell proliferation is in high demand of energy, which induces alterations in lipid metabolism to allow the survival of cancer cells [15]. Based on clinic pathological study of breast cancer, researchers have found that plasma levels of TC, TG, HDL and LDL of breast cancer patients were significantly higher than that of control group. In addition, TC and TG levels of patients with metastasis were significantly higher than that of patients without lymphatic metastasis [16]. Further study has showed that cholesterol ester (CE) accumulation promotes breast cancer cell proliferation. These results highlight intratumoral CE accumulation as a potential indicator in diagnosis of human breast cancer [17]. Moreover, increased free fatty acids (FFAs) not only induce the expression of plasminogen activator inhibitor-1 (PAI-1) in breast cancer cells, but also inhibit the degradation of fibrin, facilitating breast cancer invasion and metastasis [18]. Meanwhile, LDL and very low-density lipoprotein (VLDL) is in favor of breast cancer progression and metastasis through protein kinase B (AKT)-induced epithelial-mesenchymal transition (EMT) and cancer angiogenesis [19]. Experiments in vivo confirmed the link between cholesterol, androgen levels and prostate cancer [20]. In addition to being a necessary precursor of androgen, cholesterol activates signaling pathways of cancer proliferation, promoting prostate cancer progression. Biochemical study showed that CE accumulation was a consequence of phosphoinositide-3-kinase (PI3K)/AKT pathway activation in cancer cells, thus, promoting prostate cancer progression (Figure 2). Metastasis to the bone is one of important features of prostate cancer with poor prognosis [21]. Elin Thysell et al. pointed out that the mean levels of cholesterol of prostate cancer patients with bone metastases was 127.30 mg/g as compared to 81.06 in that of patients with bone metastases from different origin and 35.85 mg/g in that of normal bone [22]. These data suggested that high levels of cholesterol might contribute to the metastases of prostate cancer into bone.
Previous studies have reported that higher levels of TC are associated with higher prevalence of colorectal cancer [23]. Consistently, the level of TC decreased by absorbance of polyunsaturated fatty acids from dietary led to lower risk of colorectal cancer [24]. In some prospective studies, TC level was positively associated with breast, prostate and colon cancers. On the other hand, TC levels were negatively associated with liver, stomach and lung cancer [25]. Studies reported that nearly two-fifths of the patients (41.3%) of colorectal cancer exhibited elevated LDL level while most patients (88.3%) showed normal HDL levels [26,27]. In addition, declined HDL and elevated LDL were associated with poor prognostic outcomes in metastatic colorectal cancer patient. In consistent, HDL prevented the development of metastatic colorectal cancer while LDL promoted its development. Ting et al. demonstrated that reduced levels of LDL and LDL receptor (LDLR) prevented malignant development of small-cell lung cancer [28]. Both LDL and LDLR are favorable prognostic factors for the survival of patients with small-cell lung cancer. 50% of the children who had acute lymphoblastic leukemia displayed dyslipidemia, were the characteristic with raised serum TG and LDL, as well as decreased HDL and TC [29]. Moreover, ApoE has been shown to be associated with development and metastasis of lung adenocarcinoma and gastric cancer [30,31]. A recent study showed that ApoE levels were three times higher in cells with lymph node metastases (LNM) than that of cells without LNM, which indicated that ApoE was a candidate biomarker for lung adenocarcinoma metastasis [30]. Additionally, ApoE is also predominantly expressed in gastric cancer. Cancers with high ApoE expression are able to invade deeply into the muscle layer, the serosal layer, or show more positive lymph node metastasis [31]. Therefore, abnormal lipid levels are intimately associated with the occurrence, development and metastasis of many types of cancers. The quantities of lipids and lipoprotein are associated with the risk or prognosis of cancer (Table 1).
Table 1.
Abnormal lipids levels and carcinogenesis, cancer development
| Lipid/Lipoprotein | Level | The types of cancers | Effect | References |
|---|---|---|---|---|
| TC | ↑ | Prostate cancer, colorectal cancer, breast cancer | Carcinogenesis risk↑ | [16,24] |
| ↑ | Gastric cancer, liver cancer, lung cancer | Prognosis risk↓ | [25] | |
| TG | ↑ | Small cell lung cancer, pancreatic cancer, ovarian cancer, breast cancer, gastric cancer | Carcinogenesis risk↑ | [16,29] |
| CE | ↑ | Breast cancer, prostate cancer | Cell Proliferation↑ | [17,21] |
| LDL | ↑ | Non-small cell lung cancer, breast cancer, colorectal cancer | Prognosis risk↑ | [28,29] |
| VLDL | ↑ | Breast cancer | Angiogenesis↑ | [19] |
| HDL | ↑ | Colorectal cancer | Carcinogenesis risk↑ | [16] |
| FFAs | ↑ | Breast cancer | Metastasis↑ | [18] |
| ApoE | ↑ | Lung adenocarcinoma, stomach cancer | Metastasis↑ | [28-30] |
| ApoA-1 | ↑ | Glioblastoma, leukemia | Carcinogenesis risk↓ | [11,29] |
↑-up-regulation, ↓-down-regulation.
Lipids metabolism and carcinogenesis, cancer development
Lipid intake and carcinogenesis, cancer development
Generally, human cells intake fatty acids from two sources: diet intake or endogenous intake. Based on previous studies, excessive fatty acids intake from diet was considered to be the main factor causing carcinogenesis and cancer development. Han et al. found that high-fat diet (HFD) increased the incidence of poorly differentiated carcinoma in transgenic adenocarcinoma mouse prostate model, which was accompanied by up-regulation of proteins associated with cell proliferation and angiogenesis, such as vascular endothelial growth factor (VEGF), thereby reducing the survival rate of the mice [32]. Recent reports showed that HFD accelerated growth and proliferation of prostate cancer cells through activation of monocyte chemoattractant protein (MCP)-1/CC chemokine receptor 2 (CCR2) signaling [33]. HFD increased the level of insulin-like growth factors (IGF), inducing prostate cancer [34]. Nevertheless, microRNA (miR)-130a was attenuated in HFD-induced prostate cancer proliferation [35].
Moreover, excessive intake of lipids promotes cancer development by inducing inflammatory response. HFD promotes progression of prostate cancer attributing to increased levels of pro-inflammatory cytokines, such as interleukin (IL)-1α, IL-1β, IL-6, and tumor necrosis fact (TNF)-α [36]. In addition, increased macrophage markers were observed during consumption of HFD, such as EGF-like module-containing mucin-like hormone receptor-like 1 (EMR1), complement receptor 4 (CR4) and toll-like receptor (TLR)-4, and inflammatory cytokines in the adipose tissue, which provided evidence for the link between HFD consumption and increased risk of colon cancer [37]. M2 macrophages are used for various forms of alternatively activated macrophages resulting from monocyte exposure to IL-4 or IL-13, immune complexes/TLR ligands, IL-10 or glucocorticoids [38]. HFD reduced cancer cell apoptosis and increased recruitment of total and M2 macrophages into epithelial tumors, which promoted cell proliferation, angiogenesis, thereby leading to increased incidence of breast cancer [15].
Consistently, a study published in Nature reported that excessive lipid intake increased the expression of B-scavenger receptor (CD36), a member of cell surface fatty acid receptors. Clinically, inhibition of CD36 reduces the metastasis of human melanoma and breast cancer [39] (Figure 1). Recently, lysophosphatidic acid (LPA) has attracted attention as a key regulator of colorectal cancer, which has diverse pathophysiological functions such as proliferation and infiltration of mammalian cancerous cells mechanically due to the decreased expression of mRNA encoding LPA5, one of G protein-coupled receptors [40]. Dietary feeding of ω-3 polyunsaturated fatty acids (PUFAs) significantly increases levels of epoxydocosapentaenoic acids (EDPs), metabolites of ω-3PUFA produced by cytochrome P450 enzymes in plasma and tumor tissue, with reduced expressions of pro-oncogenic genes such as C-myc, Axin2, and C-jun in cancer tissues, inhibiting colorectal cancer growth [41]. Taken together, lipid intake has significant effects on cancer cell proliferation (Figure 1). However, the underlying mechanisms need to be further elucidated.
Figure 1.
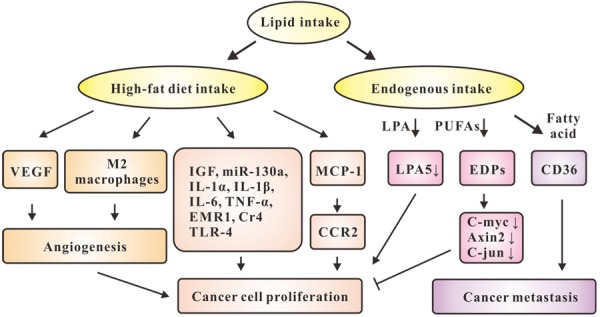
The effects of lipid uptake on cancer cell proliferation and metastasis. Lipid intake includes high-fat diet intake or endogenous intake. Intake of high-fat up-regulates VEGF and promotes the recruitment of M2 macrophages, which promote angiogenesis, thereby accelerate cancer cell proliferation. HFD increases levels of IGF, miR-130a, IL-1α, IL-1β, IL-6, TNF-α, EMR1, CR4, and TLR-4, promoting cancer cell proliferation. HFD also accelerates cancer cell proliferation through activation of MCP-1/CCR2 signaling. Endogenous intake includes LPA, PUFAs and fatty acid. Decreased expression of LPA5 accelerates proliferation. PUFAs significantly increase levels of EDPs, which results in reduced expressions of C-myc, Axin2, and C-jun, and inhibition of cancer growth. Intake of fatty acid increases the expression of CD36, which impairs cancer metastasis.
Lipids synthesis and carcinogenesis, cancer development
In healthy adults, most types of tissues take advantages of dietary fatty acids to function properly, except liver cells and adipose tissue as well as specific physiologic processes, such as endometrial cell proliferation, which use de novo synthesis to generate fatty acids [42]. De novo lipids synthesis provides a constant energy supply, which is essential for cancer cell growth and proliferation [43]. Cholesterol synthesis starts with acetyl-CoA, which takes two steps to convert to 3-hydroxy-3 methyl glutaryl CoA (HMG-CoA) via HMG-CoA synthase, and then transformed into mevalonate via rate-limiting enzyme HMGR. Cholesterol is easily oxidized when it is exposed to the air. However, whether auto-oxidation of cholesterol exists in vivo is controversial. Oxysterols are thought to be generated in the rate in proportion to that of cholesterol synthesis. Oxysterols is the oxidized products of the oxidation reaction mediated by enzyme cytochrome P450. Oxysterols interfere with the process of cell proliferation, resulting in cell death of various cancers, such as leukemia, glioblastoma, colon, breast and prostate cancer, while they have little or no effect on senescent cells. Oxysterols regulate the transcription of the key enzyme HMGR in cholesterol synthesis by binding to Insig-1, Insig-2 and LXRs. Oxysterols slow down cell proliferation as well as stimulate cell death by interfering with ERK, Hedgehog and Wnt pathways [44]. Glucose metabolites, which are normally used for ATP production, are redirected to be used in lipid synthesis via the pentose pathways. Both the metabolic rate and glucose uptake are increased to maintain the faster proliferation of cancer cells. This atypical metabolic process is known as the Warburg effect [45]. Cheng et al. documented that glucose uptake via CAV-1 enhanced epidermal growth factor receptor (EGFR) signaling pathway, which induced N-glycosylation of SCAP and consequent activation of SREBPs. SCAP is the key glucose-responsive protein serving as the oncogenic signaling hub and fueling for SREBP-dependent lipogenesis. Blockade of SCAP N-glycosylation ameliorates EGFR-driven glioblastoma growth [46] (Figure 2). The available evidences suggested that SREBP-1 not only enhanced cholesterol biosynthesis, but also promoted invasion and metastasis of breast cancer [47] and glioblastoma [48]. Previous study by Pizer et al. showed that fatty acid synthesis was heavily inhibited in human acute promyelocytic leukemia HL60 cells upon culture condition of serum-free and fatty acid-free medium [49]. However, compared with normal cells, the expression of FASN is higher in breast cancer, colon cancer, prostate cancer, melanoma, lung cancer, bladder cancer, ovarian cancer, gastric cancer, endometrial cancer, kidney cancer, skin cancer, pancreatic cancer, head and neck cancer and tongue cancer [50-52]. FASN plays a crucial role in EMT, a process that the epithelial cells transited to mesenchymal cell type. The close relationship between EMT and cancer development and metastasis has been well documented. It was corroborated by study that the expression of FASN during EMT, while silence of FASN might reverse EMT [50]. In addition, Acetyl CoA carboxylase (ACC) is the rate-limiting enzyme in fatty acid synthesis, which also plays a pivotal role in carcinogenesis and development. In preclinical models, ACC is required for the de novo fatty acid synthesis for the growth and viability of non-small-cell lung cancer cells [53]. Down-regulation of CE in T cells by genetic ablation or pharmacological inhibition of Acetyl-Coenzyme A acetyl transferase 1 (ACAT1), a key cholesterol esterification enzyme5, led to potentiated effector function and enhanced proliferation of CD8+T cells and increased cholesterol level, resulting in the inhibition of the growth and metastasis of melanoma [54] (Figure 2).
Lipid transport and carcinogenesis, cancer development
Our group proposed a novel model of cholesterol efflux from lipid-loaded cells including four subsystems and one center coupled to each other [55]. The novel model consists of intracellular trafficking subsystem of CAV-1 complex, transmembrane transporting subsystem of ABCA1 complex, transmembrane transporting subsystem of SR-B1 complex, extracellular trafficking subsystem of HDL/Apo-A1 and CAV transporting center. In brief, the CAV-1 system transports cholesterol from intracellular compartments to caveolae. Subsequently, both ABCA1 and SR-B1 complex systems transfer cholesterol from caveolae to extracellular HDL/Apo-A1. These four subsystems are orchestra together as a functional overall system of cholesterol efflux (Figure 3). Our group for the first time puzzled together the network of various key proteins responsible for cholesterol transportation. The transmembrane subtransport system with ABCA1 is the core of overall lipid transportation. The deficiency of ABCA1 allows for accumulation of mitochondrial cholesterol, inhibiting the release of mitochondrial cell death-promoting molecules, such as cytochrome C, and thus facilitating cancer cell survival [56]. MiR-183, targeting the 3’UTR of ABCA1 mRNA, increases intracellular cholesterol. MiR-183 both promotes the proliferation and inhibits the apoptosis of colon cancer cells by degrading ABCA1 [57]. Likewise, the reduced expression of ABCA1 due to promoter hypermethylation increases cholesterol levels, promoting cell growth of ovarian cancer cell lines A2780 and CP70 [58]. ABC transporters are widely expressed in epithelial cells of normal mammary gland, but ABCA1 is hardly expressed in breast cancer cells [59]. Meanwhile, several studies have described a closely link between ABCA1 and prostate cancer. Intracellular cholesterol is a substrate for de novo androgen synthesis under regulation of AKT signaling, promoting prostate cancer progression. Silence of ABCA1 due to hypermethylation directly leads to high intracellular cholesterol levels, contributing to prostate cancer progression [60]. Remarkable, previous study highlighted that the ABCA1-accelerated cholesterol efflux was critical for LXRs inhibition in human oral squamous cell carcinoma cells [8]. In particular, glioblastoma, a type of highly lethal brain cancer, requires cholesterol for its survival. However, LXR-623, a LXRα-partial/LXRβ-full agonist with brain-penetration, selectively kills glioblastoma cells in an LXRβ and cholesterol-dependent manner, resulting in tumor regression and prolonged patient survival [61]. Meanwhile, previous study has identified that the synthetic LXR agonist GW3965 potently suppresses glioblastoma growth in glioblastoma xenograft model in vivo. Targeting LDLR by using the LXR agonist GW3965 causes inducible degradation of LDLR and increases ABCA1-mediated cholesterol efflux, potently promoting cancer cell death [62]. Moreover, down-regulation of PPARγ resulted in an obvious decrease in lipid accumulation, thereby inhibiting the growth of hepatocellular carcinoma in xenograft model in nude mice [63]. Interestingly, SR-B1 is observed to bind to HDL with high affinity to mediate selective cellular uptake and efflux of CE from the lipoprotein core. SR-B1 is also implicated in cholesterol metabolism of cancer cells, whereby over-expression of SR-B1 has been observed in a number of cancer cell lines, including breast and prostate cancers [64]. Cell endocytosis of CAV-1 has been thought to be associated with membrane proteins, extracellular matrix tissue, cholesterol distribution and cell signaling. CAV-1 regulates cell metabolism mainly targeting glycolysis, mitochondrial bioenergetics, glutaminolysis, fatty acid metabolism, and autophagy, which is tightly linked to carcinogenesis and development [65]. Epigenetic profile analysis showed that promoter methylation of CAV-1 gene led to CAV-1 silence, thereby promoting colon cancer cells growth (Figure 3) [66]. How the involved proteins, enzymes or genes during the process of lipid metabolism coordinate together to contribute to carcinogenesis, and caner development are summarized in Table 2.
Figure 3.
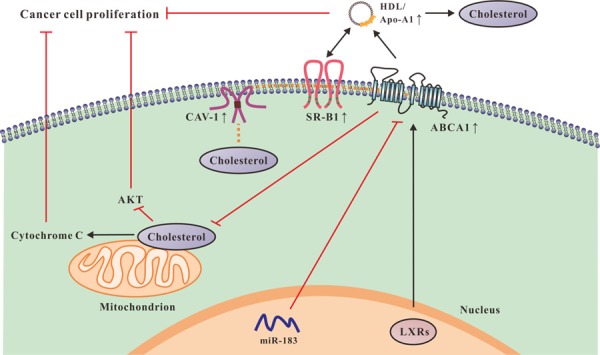
The effects of cholesterol transportation on cancer cell proliferation. ABCA1 inhibits accumulation of mitochondrial cholesterol, which leads to increased release of cytochrome C and inhibition of AKT signaling, impairing cancer cell proliferation. MiR-183 promotes proliferation by degrading ABCA1 in cancer cells. LXRs increase ABCA1-mediated cholesterol efflux, resulting in cancer regression. In addition, CAV-1 complex subsystem transports cholesterol from intracellular compartments to caveolae, both subsystems of SR-B1 and ABCA1 transfer cholesterol from CAV-1 to extracellular HDL/ApoA-1. A network regulates the four systems and the entire transportation process. The elevated expressions of CAV-1, SR-B1, ABCA1 and HDL/ApoA-1 accelerate cholesterol efflux, which lead to inhibition of cancer cell proliferation.
Table 2.
The relationships between lipid metabolism and carcinogenesis, cancer development
| Lipid metabolism process | Factors | Effect | References |
|---|---|---|---|
| Lipid intake | CD36↓ | (–) Melanoma and breast cancer growth and metastasis | [39] |
| LPA↑ | (–) Colorectal cancer proliferation | [40] | |
| C-myc↓Axin2↓C-jun↓ | (–) Colorectal cancer growth | [41] | |
| Lipid synthesis | SREBP↓ | (–) Breast cancer and glioblastoma metastasis | [45-47] |
| ACAT1↑ | (–) Melanoma growth | [53] | |
| ACC↑ | (–) Non-small cell lung cancer growth | [50] | |
| FASN↓ | (–) Colon, prostate, breast, lung, kidney, ovary, bladder, endometrium, skin, pancreas, tongue, head and neck cancer invasion and metastasis | [48-51] | |
| Lipid transportation | ABCA1↑ | (–) Colon, ovary, prostate, oral cancer growth | [8,55-57,59] |
| CAV-1↑ | (–) Colon cancer growth | [65] | |
| SR-B1↑ | (–) Prostate, ovarian cancer growth and metastasis | [63] | |
| PPAR↑ | (–) Liver cancer growth | [62] | |
| LXRs↑ | (–) Oral cancer, hepatocellular carcinoma and glioblastoma growth | [8,60,61] |
↑-up-regulation, ↓-down-regulation, (–) –inhibition.
The application of drugs targeting lipid metabolism in cancers
Lipid-lowering drugs in the treatment of the cancers
Recently, Lipid-lowering drugs such as statins used alone or in combination with therapeutic agents have been being studied more.
Statins are HMGR inhibitors that inhibit mevalonate in cells through competitive inhibition of endogenous cholesterol synthesis rate-limiting enzyme, thereby resulting in reduction of intracellular cholesterol synthesis. Then, the feedback from cholesterol improves the number and activity of LDLR, predominantly in liver cells [67]. The application of the synthetic statins in the treatment of various human malignancies has shown promising outcome based on numerous in vitro and in vivo studies. Statins disturb lipid metabolism and subsequently interfere with signaling pathways of cell proliferation and cell survival, leading to cell apoptosis [68]. Statins reduce serum TC levels and activate liver tissue LDLR by interfering with intracellular lipid oxidative stress, effectively inhibiting cell proliferation of breast cancer [69]. A previous study found that statins induced accumulation of cytosolic lipid droplets as well as up-regulation of ABCA7 and triacylglycerol and phospholipids synthesis (such as 1-acylglycerol-3-phosphate O-acyltransferase 2) during the apoptotic process of pancreatic cancer cells in vitro [70]. Furthermore, statins decreases cancer cell proliferation by inhibiting the synthesis of cholesterol, which is essential for new membrane formation in rapidly proliferating cells [71].
Most strikingly, statins in combination with other agents has been clinically applied. For instance, the combination of prostate-restricted replication competent adenovirus-mediated TRAIL with lovastatin, as a potential treatment for advanced prostate cancer, enhances TRAIL-induced apoptosis by depleting cholesterol of lipid rafts and influencing the expression of death receptor, coxsackievirus receptor and adenovirus receptor in prostate cancer cells [72]. Additionally, the combined treatment of eicosapentaenoic acid and lovastatin enhances the regulatory effect on gene expression of HMGR and LDLR, thereby inhibits hepatocarcinoma cell proliferation [73]. The combination of tamoxifen and lovastatin results in a synergistic stimulation of the LDL receptor activity, which probably blocks sterol synthesis. Based on these results, the simultaneous inhibition of sterol biosynthesis and intracellular cholesterol transportation appears to be an efficient way to treat breast cancer [74]. In addition, a preclinical study demonstrates that the cholesterol-uptake inhibitor ezetimibe reduces serum cholesterol levels, which may prevent prostate cancer growth by inhibiting tumor angiogenesis [75].
Anti-lipid per-oxidation drugs in the application of the cancers
Lipid per-oxidation is a free radical chain reaction process of the oxidative degradation of unsaturated fatty acids. The reaction consists of three major steps: initiation, propagation and termination. And in the initiative step, a fatty acid radical is produced. In the step of propagation numerous free radicals are produced, such as lipid peroxyl radicals, lipid oxygen free radicals and lipid free radicals, etc. More importantly, during the termination step various small molecules are produced, which induce oncogenic mutations and activate oncogenic pathways, promoting carcinogenesis [76]. Therefore, agents with antioxidant activity are considered as an important strategy for cancer prevention and treatment [77]. Probucol, as an antioxidant has long been used for the treatment of hypercholesterolemia and anti-lipid per-oxidation in clinic. Probucol inhibits cholesterol efflux from normal human skin fibroblasts without interference with SR-B1-mediated efflux, as well as inhibits ABCA1 translocation to the plasma membrane [78]. Further, probucol is considered to protect lipids and LDL from oxidation, which potentially inhibits angiogenesis, which has an anti-carcinogenic effect on human head and neck squamous carcinoma cells [79]. Probucol not only decreases plasma levels of LDL and HDL, but also increases selective uptake of CE, thereby inhibiting hepatoma cells growth [80]. In particularly, high concentration of probucol significantly inhibits the metastasis of breast cancer into lung [81].
Nowadays, various active substances from traditional Chinese medicine have showed excellence antioxidant effects, such as celastrol, curcumin, quercetin, berberine, etc. They all have effect on inhibition of carcinogenesis and development through anti-lipid per-oxidation activities. Celastrol isolated from the traditional Chinese medicinal herb Tripterygium wilfordii Hook.f (Thunder God’s Vine), has shown its anti-invasive and anti-metastatic activities in preclinical models of prostate cancer, breast cancer, colon cancer and pancreatic cancer [82]. Celastrol as a very potent inhibitor of lipid per-oxidation effectively reduces lipid accumulation and serum LDL levels [83]. Moreover, celastrol is able to effectively cause weight loss and attenuate high fat mediated oxidative injury by up-regulating ABCA1 expression, reducing the levels of TC, TG, LDL and ApoB in plasma, increasing antioxidant enzymes activities and inhibiting NADPH oxidize activity, furthermore, decreasing the serum levels of malondialdehyde (MDA) and reactive oxygen species (ROS) in dose-dependent way [84].
Besides, curcumin is a diketone active ingredient isolated from turmeric in Chinese medicine. Because of its excellent anti-proliferative and anti-lipid per-oxidation activity, it has been widely used in the treatment of cancer and related disease caused by the damage of ROS and reactive nitrogen species (RNS). Curcumin has been studied in numerous cancers, including colorectal, cervical, uterus, ovary, prostate, breast, lung, stomach, pancreas, bladder, oral, esophagus cancer and osteocarcinoma [85]. Curcumin enhances the effects of chemotherapeutic agents against glioblastoma multiforme through its inhibition of lipid droplet accumulation [86]. Curcumin also inhibits occurrence and development of multiple cancers by provoking the PPARγ-LXR-ABCA1 pathway-mediated cholesterol efflux from adipocytes [87].
Quercetin is a polyphenolic flavonoid compound, whose anti-lipid oxidative activity has been taken advantage in cancer treatment. Quercetin effectively decreases the expression of FASN, inhibiting proliferation of nasopharyngeal carcinoma cells, of human gastric cancer cells and of human leukemia T-cells [88]. In addition, quercetin significantly improves the plasma nonenzymatic antioxidant capacity and reduces lipid peroxidation, protecting from oxidative damage evoked by doxorubicin and docetaxel during the treatment of breast cancer [89]. Quercetin also elevates lipid peroxides and thus reduces the tumor size and the cumulative number of papillomas [90]. The study from Sharmila specifically indicated that quercetin treatment prevented the lipid peroxidation and maintains H2O2 level, thereby inhibiting the development of prostate cancer [91].
Berberine, alkaloid extracted from berberine, cork and three needles, has been used for cancer treatment. Berberine inhibits lipid accumulation by up-regulating LDLR, resulting in inhibition of human hepatoma cells growth [92]. Berberine regulates lipid metabolism through the inhibition of AMP activated protein kinase (AMPK), FASN, and 5-tetradecyloxy-2-furoic acid (TOFA), inducing breast cancer cells apoptosis [93]. Berberine significantly attenuates lipid per-oxidation and enhances the anti-oxidative capabilities. Thus, berberine inhibits neoplastic transformation by the induction of antioxidant defense system and then induces apoptosis [94].
Conclusion and prospect
Preclinical cancer studies and clinical trials have revealed the crucial role of lipid metabolism in tumor growth and metastasis. Lipid metabolism and cell survival or proliferation of cancers shares certain common pathways involving numerus proteins as well as various cells, tissues and organelles. Abnormalities in these pathways lead to tumor growth. Based on these findings, many drugs targeting lipid metabolism have been developed for cancer treatment. However, some inhibitors are able to inhibit cancer cell proliferation and tumor growth but they induce cytotoxicity of normal cells as well. Thus, it is particularly important to develop a number of drugs with high specificities, thus decreasing toxicities. However, there are existing challenges: 1) Specific rodent cancer models with hyperlipidemia need to be further established; 2) The molecular mechanism of lipids and lipid oxidation underlying tumor development has not been fully understood; 3) The clinical studies of lipid metabolism and carcinogenesis need further epidemiological analysis. More and more novel molecular targets of lipid metabolism would be identified upon further effort, improving the efficacy on cancer prevention and treatment. In conclusion, further studies are required to understand lipid metabolism of cancer cells both big picture and detail oriented, which will provide effective clinical therapeutic strategies targeting against cancers, the top human health threat.
Acknowledgements
This work is supported by grants from the National Natural Sciences Foundation of China (No. 81270359 and No. 81774130 to Li Qin, No. 81670268 to Duan-Fang Liao), projects of Hunan Provincial Education Department (No. 15A138 to Li Qin, No. 16C1210 to Neng Zhu and No. 15A141 to Ke Du), Key projects of Hunan traditional Chinese Medicine Administration (No. 201614 to Li Qin), and the Natural Science Foundation of Guangxi Province (No. 2015GXNSFEA139003 to Duan-Fang Liao).
Disclosure of conflict of interest
None.
References
- 1.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 2.Duan J, Chen L, Zhou M, Zhang J, Sun L, Huang N, Bin J, Liao Y, Liao W. MACC1 decreases the chemosensitivity of gastric cancer cells to oxaliplatin by regulating FASN expression. Oncol Rep. 2017;37:2583–2592. doi: 10.3892/or.2017.5519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Menendez JA, Lupu R. Fatty acid synthase regulates estrogen receptor-α signaling in breast cancer cells. Oncogenesis. 2017;6:e299. doi: 10.1038/oncsis.2017.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wu X, Qin L, Fako V. Molecular mechanisms of fatty acid synthase (FASN)-mediated resistance to anti-cancer treatments. Adv Biol Regul. 2014;4:214–221. doi: 10.1016/j.jbior.2013.09.004. [DOI] [PubMed] [Google Scholar]
- 5.Chandrahasa R, Yellaturu Xiong D, Raghow R, Elam MB. Insulin enhances the biogenesis of nuclear sterol regulatory element-binding protein (SREBP)-1c by posttranscriptional down-regulation of Insig-2A and its dissociation from SREBP cleavage-activating protein (SCAP). SREBP-1c complex. J Biol Chem. 2009;284:31726–1734. doi: 10.1074/jbc.M109.050914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jekson R, Keiko K, Masashi S, Muranaka T. AKIN10, a representative arabidopsis SNF1related protein kinase 1 (SnRK1), phosphorylates and downregulates plant HMG-CoA reductase. FEBS Lett. 2017;591:1159–1166. doi: 10.1002/1873-3468.12618. [DOI] [PubMed] [Google Scholar]
- 7.Pinto CL, Kalasekar SM, McCollum CW, Riu A, Jonsson P, Lopez J, Swindell EC, Bouhlatouf A, Balaguer P, Bondesson M, Gustafsson JÅ. Lxr regulates lipid metabolic and visual perception pathways during zebrafish development. Mol Cell Endocrinol. 2016;419:29–43. doi: 10.1016/j.mce.2015.09.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kaneko T, Chihiro K, Naoki I, Kashiwagi K, Yaginuma N, Ohkoshi C, Tanaka M, Sugino T, Imura T, Hasegawa H, Chiba H. Liver X receptor reduces proliferation of human oral cancer cells by promoting cholesterol efflux via up-regulation of ABCA1 expression. Oncotarget. 2015;6:33345–33357. doi: 10.18632/oncotarget.5428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yassine HN, Belopolskaya A, Schall C, Stump CS, Lau SS, Reaven PD. Enhanced cholesterol efflux to HDL through the ABCA1 transporter in hypertriglyceridemia of type 2 diabetes. Metabolism. 2014;63:727–734. doi: 10.1016/j.metabol.2014.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rahmani F, Aabi MH. Does apolipoprotein A1 predict microstructural changes in subgenual cingulum in early Parkinson? J Neurol. 2017;264:684–693. doi: 10.1007/s00415-017-8403-5. [DOI] [PubMed] [Google Scholar]
- 11.Carbone F, Satta N, Montecucco F, Virzi J, Burger F, Roth A, Roversi G, Tamborino C, Casetta I, Seraceni S, Trentini A, Padroni M, Dallegri F, Lalive PH, Mach F, Fainardi E, Vuilleumier N. Anti-ApoA-1 IgG serum levels predict worse post-stroke outcomes. Eur J Clin Invest. 2016;46:805–817. doi: 10.1111/eci.12664. [DOI] [PubMed] [Google Scholar]
- 12.Mello T, Materozzi M, Galli A. PPARs and mitochondrial metabolism: from NAFLD to HCC. PPAR Res. 2016;2016:7403230. doi: 10.1155/2016/7403230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sheth J, Shah A, Sheth F, Trivedi S, Nabar N, Shah N, Thakor P, Vaidya R. The association of dyslipidemia and obesity with glycated hemoglobin. Clin Diabetes Endocrinol. 2015;1:1–6. doi: 10.1186/s40842-015-0004-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bora K, Pathak SM, Borah P, Das D. Association of decreased high-density lipoprotein cholesterol (HDL-C) with obesity and risk estimates for decreased HDL-C attributable to obesity: preliminary findings from a hospitalbased study in a city from northeast India. J Prim Care Community Health. 2016;8:26–30. doi: 10.1177/2150131916664706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhu YR, Aupperlee MD, Zhao Y, Tan YS, Kirk EL, Sun X, Troester MA, Schwartz RC, Haslam SZ. Pubertal and adult windows of susceptibility to a high animal fat diet in Trp53-null mammary tumorigenesis. Oncotarget. 2016;7:83409–83423. doi: 10.18632/oncotarget.13112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wei LJ, Zhang C, Zhang H, Wei X, Li SX, Liu JT, Ren XB. A case-control study on the association between serum lipid level and the risk of breast cancer. Chinese Journal of Preventive Medicine. 2016;50:1091–1095. doi: 10.3760/cma.j.issn.0253-9624.2016.12.013. [DOI] [PubMed] [Google Scholar]
- 17.Calvo GD, Vilaró LL, Nasarre L, Perez-Olabarria M, Vázquez T, Escuin D, Badimon L, Barnadas A, Lerma E, Llorente-Cortés V. Intratumor cholesteryl ester accumulation is associated with human breast cancer proliferation and aggressive potential: a molecular and clinicopathological study. BMC Cancer. 2015;15:460. doi: 10.1186/s12885-015-1469-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Byon CH, Hardy RW, Ren CC, Ponnazhagan S, Welch DR, McDonald JM, Chen Y. Free fatty acids enhance breast cancer cell migration through plasminogen activator inhibitor-1 and SMAD4. Lab Invest. 2009;89:1221–1228. doi: 10.1038/labinvest.2009.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lu CW, Lo YH, Chen CH, Lin CY, Tsai CH, Chen PJ, Yang YF, Wang CH, Tan CH, Hou MF, Yuan SF. VLDL and LDL, but not HDL, promote breast cancer cell proliferation, metastasis and angiogenesis. Cancer Lett. 2017;388:130–138. doi: 10.1016/j.canlet.2016.11.033. [DOI] [PubMed] [Google Scholar]
- 20.Raza S, Meyer M, Goodyear C, Hammer KDP, Guo B, Ghribi O. The cholesterol metabolite 27-hydroxycholesterol stimulates cell proliferation via ERβ in prostate cancer cells. Cancer Cell Int. 2017;17:52. doi: 10.1186/s12935-017-0422-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yue S, Li J, Lee SY, Lee HJ, Shao T, Song B, Cheng L, Masterson TA, Liu X, Ratliff TL, Cheng JX. Cholesteryl ester accumulation induced by PTEN loss and PI3K/AKT activation underlies human prostate cancer aggressiveness. Cell Metab. 2014;19:393–406. doi: 10.1016/j.cmet.2014.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Thysell E, Surowiec I, Hörnberg E, Crnalic S, Widmark A, Johansson AI, Stattin P, Bergh A, Moritz T, Antti H, Wikström P. Metabolomic characterization of human prostate cancer bone metastases reveals increased levels of cholesterol. PLoS One. 2010;5:e14175. doi: 10.1371/journal.pone.0014175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yan G, Li L, Zhu B. Lipidome in colorectal cancer. Oncotarget. 2016;7:33429–33439. doi: 10.18632/oncotarget.7960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Muka T, Kraja B, Ruiter R, de Keyser CE, Hofman A, Stricker BH, Kiefte-de Jong JC, Franco OH. Dietary polyunsaturated fatty acids intake modifies the positive association between serum total cholesterol and colorectal cancer risk: the rotterdam study. J Epidemiol Community Health. 2016;70:881–887. doi: 10.1136/jech-2015-206556. [DOI] [PubMed] [Google Scholar]
- 25.Kitahara CM, González AB, Freedman ND, Huxley R, Mok Y, Jee SH, Samet JM. Total cholesterol and cancer risk in a large prospective study in Korea. J. Clin. Oncol. 2011;29:1592–1598. doi: 10.1200/JCO.2010.31.5200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rodriguez-Broadbent H, Law PJ, Sud A, Palin K, Tuupanen S, Gylfe A, Hänninen UA, Cajuso T, Tanskanen T, Kondelin J, Kaasinen E, Sarin AP, Ripatti S, Eriksson JG, Rissanen H, Knekt P, Pukkala E, Jousilahti P, Salomaa V, Palotie A, Renkonen-Sinisalo L, Lepistö A, Böhm J, Mecklin JP, Al-Tassan NA, Palles C, Martin L, Barclay E, Farrington SM, Timofeeva MN, Meyer BF, Wakil SM, Campbell H, Smith CG, Idziaszczyk S, Maughan TS, Kaplan R, Kerr R, Kerr D, Passarelli MN, Figueiredo JC, Buchanan DD, Win AK, Hopper JL, Jenkins MA, Lindor NM, Newcomb PA, Gallinger S, Conti D, Schumacher F, Casey G, Aaltonen LA, Cheadle JP, Tomlinson IP, Dunlop MG, Houlston RS. Mendelian randomization implicates hyperlipidaemia as a risk factor for colorectal cancer. Int J Cancer. 2017;140:2701–2708. doi: 10.1002/ijc.30709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liao F, He W, Jiang C, Yin C, Guo G, Chen X, Qiu H, Rong Y, Zhang B, Xu D, Xia L. A high LDL-C to HDL-C ratio predicts poor prognosis for initially metastatic colorectal cancer patients with elevations in LDL-C. Onco Targets Ther. 2015;8:3135–3142. doi: 10.2147/OTT.S90479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhou T, Zhan JH, Fang WF, Zhao Y, Yang Y, Hou X, Zhang Z, He X, Zhang Y, Huang Y, Zhang L. Serum low-density lipoprotein and low-density lipoprotein expression level at diagnosis are favorable prognostic factors in patients with small-cell lung cancer (SCLC) BMC Cancer. 2017;17:269. doi: 10.1186/s12885-017-3239-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Morel S, Leahy J, Fournier M, Lamarche B, Garofalo C, Grimard G, Poulain F, Delvin E, Laverdière C, Krajinovic M, Drouin S, Sinnett D, Marcil V, Levy E. Lipid and lipoprotein abnormalities in acute lymphoblastic leukemia survivors. J Lipid Res. 2017;58:982–993. doi: 10.1194/jlr.M072207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liu Z, Gao Y, Hao F, Lou X, Zhang X, Li Y, Wu D, Xiao T, Yang L, Li Q, Qiu X, Wang E. Secretomes are a potential source of molecular targets for cancer therapies and indicate that APOE is a candidate biomarker for lung adenocarcinoma metastasis. Mol Biol Rep. 2014;41:7507–7523. doi: 10.1007/s11033-014-3641-4. [DOI] [PubMed] [Google Scholar]
- 31.Sakashita K, Tanaka F, Zhang X, Mimori K, Kamohara Y, Inoue H, Sawada T, Hirakawa K, Mori M. Clinical significance of ApoE expression in human gastric cancer. Oncol Rep. 2008;20:1313–1319. [PubMed] [Google Scholar]
- 32.Cho HJ, Kwon GT, Park H, Song H, Lee KW, Kim JI, Park JH. A high-fat diet containing lard accelerates prostate cancer progression and reduces survival rate in mice: possible contribution of adipose tissue-derived cytokines. Nutrients. 2015;7:2539–2561. doi: 10.3390/nu7042539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Huang M, Narita S, Numakura K, Tsuruta H, Saito M, Inoue T, Horikawa Y, Tsuchiya N, Habuchi T. A high-fat diet enhances proliferation of prostate cancer cells and activates MCP-1/CCR2 signaling. Prostate. 2012;72:1779–1788. doi: 10.1002/pros.22531. [DOI] [PubMed] [Google Scholar]
- 34.Xu H, Hu MB, Bai PD, Zhu WH, Ding Q, Jiang HW. Will metformin postpone high-fat diet promotion of TRAMP mouse prostate cancer development and progression? Int Urol Nephrol. 2014;46:2327–2334. doi: 10.1007/s11255-014-0823-x. [DOI] [PubMed] [Google Scholar]
- 35.Nara T, Narita S, Mingguo H, Yoshioka T, Koizumi A, Numakura K, Tsuruta H, Maeno A, Saito M, Inoue T, Tsuchiya N, Satoh S, Habuchi T. Altered miRNA expression in highfat diet-induced prostate cancer progression. Carcinogenesis. 2016;37:1129–1137. doi: 10.1093/carcin/bgw108. [DOI] [PubMed] [Google Scholar]
- 36.Xu H, Hu MB, Bai PD, Zhu WH, Liu SH, Hou JY, Xiong ZQ, Ding Q, Jiang HW. Proinflammatory cytokines in prostate cancer development and progression promoted by high-fat diet. Biomed Res Int. 2015;2015:249741. doi: 10.1155/2015/249741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Day SD, Enos RT, McClellan JL, Steiner JL, Velázquez KT, Murphy EA. Linking inflammation to tumorigenesis in a mouse model of high-fat-diet-enhanced colon cancer. Cytokine. 2013;64:454–462. doi: 10.1016/j.cyto.2013.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhang L, Li Z, Ding G, La X, Yang P, Li Z. GRP78 plays an integral role in tumor cell inflammationrelated migration induced by M2 macrophages. Cell Signal. 2017;37:136–148. doi: 10.1016/j.cellsig.2017.06.008. [DOI] [PubMed] [Google Scholar]
- 39.Pascual G, Avgustinova A, Mejetta S, Martín M, Castellanos A, Attolini CS, Berenguer A, Prats N, Toll A, Hueto JA, Bescós C, Di Croce L, Benitah SA. Targeting metastasis-initiating cells through the fatty acid receptor CD36. Nature. 2017;541:41–45. doi: 10.1038/nature20791. [DOI] [PubMed] [Google Scholar]
- 40.Tsutsumi T, Inoue M, Okamoto Y, Ishihara A, Tokumura A. Daily intake of high-fat diet with lysophosphatidic acid-rich soybean phospholipids augments colon tumorigenesis in Kyoto apc delta rats. Dig Dis Sci. 2017;62:669–677. doi: 10.1007/s10620-016-4434-5. [DOI] [PubMed] [Google Scholar]
- 41.Wang W, Yang J, Nimiya Y, Lee KSS, Sanidad K, Qi W, Sukamtoh E, Park Y, Liu Z, Zhang G. ω-3 polyunsaturated fatty acids and their cytochrome P450-derived metabolites suppress colorectal tumor development in mice. J Nutr Biochem. 2017;48:29–35. doi: 10.1016/j.jnutbio.2017.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jones JE, Esler WP, Patel R, Lanba A, Vera NB, Pfefferkorn JA, Vernochet C. Inhibition of acetyl-CoA carboxylase 1 (ACC1) and 2 (ACC2) reduces proliferation and de novo lipogenesis of egfrviii human glioblastoma cells. PLoS One. 2017;12:e0169566. doi: 10.1371/journal.pone.0169566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hilvo M, Denkert C, Lehtinen L, Müller B, Brockmöller S, Seppänen-Laakso T, Budczies J, Bucher E, Yetukuri L, Castillo S, Berg E, Nygren H, Sysi-Aho M, Griffin JL, Fiehn O, Loibl S, Richter-Ehrenstein C, Radke C, Hyötyläinen T, Kallioniemi O, Iljin K, Oresic M. Novel theranostic opportunities offered by characterization of altered membrane lipid metabolism in breast cancer progression. Cancer Res. 2011;71:3236–3245. doi: 10.1158/0008-5472.CAN-10-3894. [DOI] [PubMed] [Google Scholar]
- 44.Weille JD, Fabre C, Bakalara N. Oxysterols in cancer cell proliferation and death. Biochem Pharmacol. 2013;86:154–160. doi: 10.1016/j.bcp.2013.02.029. [DOI] [PubMed] [Google Scholar]
- 45.Tekade RK, Sun SK. The Warburg effect and glucose-derived cancer theranostics. Drug Discov Today. 2017;22:1637–1653. doi: 10.1016/j.drudis.2017.08.003. [DOI] [PubMed] [Google Scholar]
- 46.Cheng C, Ru P, Geng F, Liu J, Yoo JY, Wu X, Cheng X, Euthine V, Hu P, Guo JY, Lefai E, Kaur B, Nohturfft A, Ma J, Chakravarti A, Guo D. Glucose-mediated n-glycosylation of SCAP is essential for SREBP-1 activation and tumor growth. Cancer Cell. 2015;28:569–581. doi: 10.1016/j.ccell.2015.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bao J, Zhu L, Zhu Q, Su J, Liu M, Huang W. SREBP-1 is an independent prognostic marker and promotes invasion and migration in breast cancer. Oncol Lett. 2016;12:2409–2016. doi: 10.3892/ol.2016.4988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ru P, Hu P, Geng F, Mo X, Cheng C, Yoo JY, Cheng X, Wu X, Guo JY, Nakano I, Lefai E, Kaur B, Chakravarti A, Guo D. Feedback loop regulation of SCAP/SREBP-1 by miR-29 modulates EGFR signaling-driven glioblastoma growth. Cell Rep. 2016;16:1527–1535. doi: 10.1016/j.celrep.2016.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pizer ES, Chrest FJ, Digiuseppe JA, Han WF. Pharmacological inhibitors of mammalian fatty acid synthase suppress DNA replication and induce apoptosis in tumor cell lines. Cancer Res. 1998;58:4611–4615. [PubMed] [Google Scholar]
- 50.Jiang L, Wang H, Li J, Fang X, Pan H, Yuan X, Zhang P. Up-regulated FASN expression promotes transcoelomic metastasis of ovarian cancer cell through epithelial-mesenchymal transition. Int J Mol Sci. 2014;15:11539–11554. doi: 10.3390/ijms150711539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Li JQ, Dong LH, Wei DP, Wang X, Zhang S, Li H. Fatty acid synthase mediates the epithelialmesenchymal transition of breast cancer cells. Int J Biol Sci. 2014;10:171–180. doi: 10.7150/ijbs.7357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wu X, Daniels G, Lee P. Lipid metabolism in prostate cancer. Am J Clin Exp Urol. 2014;2:111–120. [PMC free article] [PubMed] [Google Scholar]
- 53.Svensson RU, Parker SJ, Eichner LJ, Kolar MJ, Wallace M, Brun SN, Lombardo PS, Van Nostrand JL, Hutchins A, Vera L, Gerken L, Greenwood J, Bhat S, Harriman G, Westlin WF, Harwood HJ Jr, Saghatelian A, Kapeller R, Metallo CM, Shaw RJ. Inhibition of acetyl-CoA carboxylase suppresses fatty acid synthesis and tumor growth of non-small-cell lung cancer in preclinical models. Nat Med. 2016;22:1108–1109. doi: 10.1038/nm.4181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Yang W, Bai YB, Xiong Y, Zhang J, Chen S, Zheng X, Meng X, Li L, Wang J, Xu C, Yan C, Wang L, Chang CC, Chang TY, Zhang T, Zhou P, Song BL, Liu W, Sun SC, Liu X, Li BL, Xu C. Potentiating the antitumor response of CD8 (+) T cells by modulating cholesterol metabolism. Nature. 2016;531:651–655. doi: 10.1038/nature17412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Luo DX, Cao DL, Xiong Y, Peng XH, Liao DF. A novel model of cholesterol efflux from lipidloaded cells. Acta Pharmacol Sin. 2010;31:1243–1257. doi: 10.1038/aps.2010.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Smith B, Land H. Anticancer activity of the cholesterol exporter ABCA1 gene. Cell Rep. 2012;2:5805–5890. doi: 10.1016/j.celrep.2012.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bi DP, Yin CH, Zhang XY, Yang NN, Xu JY. MiR-183 functions as an oncogene by targeting ABCA1 in colon cancer. Oncol Rep. 2016;35:2873–2879. doi: 10.3892/or.2016.4631. [DOI] [PubMed] [Google Scholar]
- 58.Chou JL, Huang RL, Shay J, Chen LY, Lin SJ, Yan PS, Chao WT, Lai YH, Lai YL, Chao TK, Lee CI, Tai CK, Wu SF, Nephew KP, Huang TH, Lai HC, Chan MW. Hypermethylation of the TGF-β target, ABCA1 is associated with poor prognosis in ovarian. Clin Epigenetics. 2015;7:1. doi: 10.1186/s13148-014-0036-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Schimanski S, Wild PJ, Treeck O, Horn F, Sigruener A, Rudolph C, Blaszyk H, Klinkhammer-Schalke M, Ortmann O, Hartmann A, Schmitz G. Expression of the lipid transporters ABCA3 and ABCA1 is diminished in human breast cancer tissue. Horm Metab Res. 2010;42:102–109. doi: 10.1055/s-0029-1241859. [DOI] [PubMed] [Google Scholar]
- 60.Lee BH, Taylor MG, Robinet P, Smith JD, Schweitzer J, Sehayek E, Falzarano SM, MagiGalluzzi C, Klein EA, Ting AH. Dysregulation of cholesterol homeostasis in human prostate cancer through loss of ABCA1. Cancer Res. 2013;73:1211–1218. doi: 10.1158/0008-5472.CAN-12-3128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Villa GR, Hulce JJ, Zanca C, Bi J, Ikegami S, Cahill GL, Gu Y, Lum KM, Masui K, Yang H, Rong X, Hong C, Turner KM, Liu F, Hon GC, Jenkins D, Martini M, Armando AM, Quehenberger O, Cloughesy TF, Furnari FB, Cavenee WK, Tontonoz P, Gahman TC, Shiau AK, Cravatt BF, Mischel PS. An LXR-cholesterol axis creates a metabolic co-dependency for brain cancers. Cancer Cell. 2016;30:683–693. doi: 10.1016/j.ccell.2016.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Guo D, Reinitz F, Youssef M, Hong C, Nathanson D, Akhavan D, Kuga D, Amzajerdi AN, Soto H, Zhu S, Babic I, Tanaka K, Dang J, Iwanami A, Gini B, Dejesus J, Lisiero DD, Huang TT, Prins RM, Wen PY, Robins HI, Prados MD, Deangelis LM, Mellinghoff IK, Mehta MP, James CD, Chakravarti A, Cloughesy TF, Tontonoz P, Mischel PS. An LXR agonist promotes glioblastoma cell death through inhibition of an EGFR/AKT/SREBP-1/LDLR-dependent pathway. Cancer Discov. 2011;1:442–456. doi: 10.1158/2159-8290.CD-11-0102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Choi Y, Oh ST, Won MA, Choi KM, Ko MJ, Seo D, Jeon TW, Baik IH, Ye SK, Park KU, Park IC, Jang BC, Seo JY, Lee YH. Targeting ODC1 inhibits tumor growth through reduction of lipid metabolism in human hepatocellular carcinoma. Biochem Biophys Res Commun. 2016;478:1674–1681. doi: 10.1016/j.bbrc.2016.09.002. [DOI] [PubMed] [Google Scholar]
- 64.Rajora MA, Zheng G. Targeting SR-BI for cancer diagnostics, imaging and therapy. Front Pharmacol. 2016;7:326. doi: 10.3389/fphar.2016.00326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Nwosu ZC, Ebert MP, Dooley S, Meyer C. Caveolin-1 in the regulation of cell metabolism: a cancer perspective. Mol Cancer. 2016;15:71. doi: 10.1186/s12943-016-0558-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Deb M, Sengupta D, Kar S, Rath SK, Roy S, Das G, Patra SK. Epigenetic drift towards histone modifications regulates CAV1 gene expression in colon cancer. Gene. 2016;581:75–84. doi: 10.1016/j.gene.2016.01.029. [DOI] [PubMed] [Google Scholar]
- 67.Cannon CP, Blazing MA, Giugliano RP, McCagg A, White JA, Theroux P, Darius H, Lewis BS, Ophuis TO, Jukema JW, De Ferrari GM, Ruzyllo W, De Lucca P, Im K, Bohula EA, Reist C, Wiviott SD, Tershakovec AM, Musliner TA, Braunwald E, Califf RM. Ezetimibe added to statin therapy after acute coronary syndromes. N Engl J Med. 2015;372:2387–2397. doi: 10.1056/NEJMoa1410489. [DOI] [PubMed] [Google Scholar]
- 68.Apostolova SN, Toshkova RA, Momchilova AB, Tzoneva RD. Statins and alkylphospholipids as new anticancer agents targeting lipid metabolism. Anticancer Agents Med Chem. 2016;16:1512–1522. doi: 10.2174/1871520616666160624093955. [DOI] [PubMed] [Google Scholar]
- 69.Huang BZ, Chang JI, Li E, Xiang AH, Wu BU. Influence of statins and cholesterol on mortality among patients with pancreatic cancer. J Natl Cancer Inst. 2016:109. doi: 10.1093/jnci/djw275. [DOI] [PubMed] [Google Scholar]
- 70.Gbelcová H, Svéda M, Laubertová L, Varga I, Vítek L, Kolář M, Strnad H, Zelenka J, Böhmer D, Ruml T. The effect of simvastatin on lipid droplets accumulation in human embryonic kidney cells and pancreatic cancer cells. Lipids Health Dis. 2013;12:126. doi: 10.1186/1476-511X-12-126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Gong J, Sachdev E, Robbins LA, Lin E, Hendifar AE, Mita MM. Statins and pancreatic cancer. Oncol Lett. 2017;13:1035–1040. doi: 10.3892/ol.2017.5572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Liu Y, Chen L, Gong Z, Shen L, Kao C, Hock JM, Sun L, Li X. Lovastatin enhances adenovirusmediated TRAIL induced apoptosis by depleting cholesterol of lipid rafts and affecting CAR and death receptor expression of prostate cancer cells. Oncotarget. 2015;6:3055–3070. doi: 10.18632/oncotarget.3073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Notarnicola M, Messa C, Refolo MG, Tutino V, Miccolis A, Caruso MG. Synergic effect of eicosapentaenoic acid and lovastatin on gene expression of HMG-CoA reductase and LDL receptor in cultured HepG2 cells. Lipids Health Dis. 2010;9:135. doi: 10.1186/1476-511X-9-135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Suárez Y, Fernández C, Gómez-Coronado D, Ferruelo AJ, Dávalos A, Martínez-Botas J, Lasunción MA. Synergistic upregulation of lowdensity lipoprotein receptor activity by tamoxifen and lovastatin. Cardiovasc Res. 2004;64:346–355. doi: 10.1016/j.cardiores.2004.06.024. [DOI] [PubMed] [Google Scholar]
- 75.Barrera G, Gentile F, Pizzimenti S, Canuto RA, Daga M, Arcaro A, Cetrangolo GP, Lepore A, Ferretti C, Dianzani C, Muzio G. Mitochondrial dysfunction in cancer and neurodegenerative diseases: spotlight on fatty acid oxidation and lipoperoxidation products. Antioxidants (Basel) 2016;5:16–20. doi: 10.3390/antiox5010007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Roberts MJ, Yaxley JW, Coughlin GD, Gianduzzo TR, Esler RC, Dunglison NT, Chambers SK, Medcraft RJ, Chow CW, Schirra HJ, Richards RS, Kienzle N, Lu M, Brereton I, Samaratunga H, Perry-Keene J, Payton D, Oyama C, Doi SA, Lavin MF, Gardiner RA. Can atorvastatin with metformin change the natural history of prostate cancer as characterized by molecular, metabolomic, imaging and pathological variables? A randomized controlled trial protocol. Contemp Clin Trials. 2016;50:16–20. doi: 10.1016/j.cct.2016.06.014. [DOI] [PubMed] [Google Scholar]
- 77.Wang SQ, Wang N, Zheng YF, Zhang J, Zhang F, Wang Z. Caveolin-1: an oxidative stressrelated target for cancer prevention. Oxid Med Cell Longev. 2017;2017:7454031. doi: 10.1155/2017/7454031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Favari E, Zanotti I, Zimetti F, Ronda N, Bernini F, Rothblat GH. Probucol inhibits ABCA1mediated cellular lipid efflux. Arterioscler Thromb Vasc Biol. 2004;24:2345–2350. doi: 10.1161/01.ATV.0000148706.15947.8a. [DOI] [PubMed] [Google Scholar]
- 79.Nishimura G, Yanoma S, Mizuno H, Kawakami K, Tsukuda M. An antioxidant, probucol, induces anti-angiogenesis and apoptosis in athymic nude mouse xenografted human head and neck squamous carcinoma cells. Jpn J Cancer Res. 1999;90:1224–1230. doi: 10.1111/j.1349-7006.1999.tb00700.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Pfeuffer MA, Richard BM. Probucol increases the selective uptake of HDL cholesterol esters by HepG2 human hepatoma cells. Arterioscler Thromb. 1992;12:870–878. doi: 10.1161/01.atv.12.7.870. [DOI] [PubMed] [Google Scholar]
- 81.Zhang ZW, Cao HQ, Jiang SJ, Liu Z, He X, Yu H, Li Y. Nanoassembly of probucol enables novel therapeutic efficacy in the suppression of lung metastasis of breast cancer. Small. 2014;10:4735. doi: 10.1002/smll.201400799. [DOI] [PubMed] [Google Scholar]
- 82.Wang Z, Zhai Z, Du X. Celastrol inhibits migration and invasion through blocking the NFκB pathway in ovarian cancer cells. Exp Ther Med. 2017;14:819–824. doi: 10.3892/etm.2017.4568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Zhang Y, Si Y, Zhai L, Guo S, Zhao J, Sang H, Pang X, Zhang X, Chen A, Qin S. Celastrus orbiculatus thunb. Reduces lipid accumulation by promoting reverse cholesterol transport in hyperlipidemic mice. Lipids. 2016;51:677–692. doi: 10.1007/s11745-016-4145-x. [DOI] [PubMed] [Google Scholar]
- 84.Wang C, Shi C, Yang X, Yang M, Sun H, Wang C. Celastrol suppresses obesity process via increasing antioxidant capacity and improving lipid metabolism. Eur J Pharmacol. 2014;744:52–58. doi: 10.1016/j.ejphar.2014.09.043. [DOI] [PubMed] [Google Scholar]
- 85.Dai CS, Lei L, Li B, Lin Y, Xiao X, Tang S. Involvement of the activation of Nrf2/HO-1, p38 MAPK signaling pathways and endoplasmic reticulum stress in furazolidone induced cytotoxicity and S phase arrest in human hepatocyte L02 cells: modulation of curcumin. Toxicol Mech Methods. 2017;27:165–172. doi: 10.1080/15376516.2016.1273424. [DOI] [PubMed] [Google Scholar]
- 86.Zhang I, Cui Y, Amiri A, Ding Y, Campbell RE, Maysinger D. Pharmacological inhibition of lipid droplet formation enhances the effectiveness of curcumin in glioblastoma. Eur J Pharm Biopharm. 2016;100:66–76. doi: 10.1016/j.ejpb.2015.12.008. [DOI] [PubMed] [Google Scholar]
- 87.Dong SZ, Zhao SP, Wu ZH, Yang J, Xie XZ, Yu BL, Nie S. Curcumin promotes cholesterol efflux from adipocytes related to PPARgamma-LXRalpha-ABCA1 pathway. Mol Cell Biochem. 2011;358:281–285. doi: 10.1007/s11010-011-0978-z. [DOI] [PubMed] [Google Scholar]
- 88.Daker M, Bhuvanendran S, Ahmad M, Takada K, Khoo AS. Deregulation of lipid metabolism pathway genes in nasopharyngeal carcinoma cells. Mol Med Rep. 2013;7:731–741. doi: 10.3892/mmr.2012.1253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Tabaczar S, Pieniążek A, Czepas J, Piasecka-Zelga J, Gwoździński K, Koceva-Chyła A. Quercetin attenuates oxidative stress in the blood plasma of rats bearing DMBA-induced mammary cancer and treated with a combination of doxorubicin and docetaxel. Gen Physiol Biophys. 2013;32:535–543. doi: 10.4149/gpb_2013048. [DOI] [PubMed] [Google Scholar]
- 90.Ali H, Dixit S. Quercetin attenuates the development of 7, 12-dimethyl benz (a) anthracene (DMBA) and croton oil-induced skin cancer in mice. J Biomed Res. 2015;29:139–144. doi: 10.7555/JBR.29.20130025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Sharmila G, Athirai T, Kiruthiga B, Senthilkumar K, Elumalai P, Arunkumar R, Arunakaran J. Chemopreventive effect of quercetin in MNU and testosterone induced prostate cancer of Sprague-Dawley rats. Nutr Cancer. 2014;66:38–46. doi: 10.1080/01635581.2014.847967. [DOI] [PubMed] [Google Scholar]
- 92.Zhou Y, Cao S, Wang Y, Xu P, Yan J, Bin W, Qiu F, Kang N. Berberine metabolites could induce low density lipoprotein receptor up-regulation to exert lipid-lowering effects in human hepatoma cells. Fitoterapia. 2014;92:230–237. doi: 10.1016/j.fitote.2013.11.010. [DOI] [PubMed] [Google Scholar]
- 93.Tan W, Zhong Z, Wang S, Suo Z, Yang X, Hu X, Wang Y. Berberine regulated lipid metabolism in the presence of C75, compound C, and TOFA in breast cancer cell line MCF-7. Evid Based Complement Alternat Med. 2015;5:396035. doi: 10.1155/2015/396035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Thirupurasundari CJ, Padmini R, Devaraj SN. Effect of berberine on the antioxidant status, ultrastructural modifications and protein bound carbohydrates in azoxymethane-induced colon cancer in rats. Chem Biol Interact. 2009;177:190–195. doi: 10.1016/j.cbi.2008.09.027. [DOI] [PubMed] [Google Scholar]


