Abstract
The dysregulation of transcription factors plays a vital role in tumor initiation and progression. Sex determining region Y-box 5 (SOX5) encodes a member of the SRY-related HMG-box family of transcription factors involved in the determination of the cell fate and the regulation of embryonic development. However, its functional roles in non-small cell lung cancer (NSCLC) remain unclear. Herein, we report that SOX5 sustains stem-like traits and enhances the malignant phenotype of NSCLC cells. We determine that SOX5 is preferentially expressed by cancer stem-like cells (CSLCs) of human NSCLC. In vitro gain- and loss-of-function studies demonstrate that SOX5 promotes self-renewal, invasion and migration in NSCLC cells. Importantly, knockdown of SOX5 potently inhibits tumor growth in a xenograft mouse model. Mechanistically, YAP1 can act as an interacting protein of SOX5 to drive the malignant potential of NSCLC cells. Silencing of YAP1 attenuates the malignant processes in NSCLC cells, which is consistent with the function of SOX5 loss. SOX5 overexpression reverses the attenuated malignant progression in YAP1 knockdown cancer cells. Taken together, these findings identify that SOX5 acts as an oncogenic factor by interacting with YAP1 in NSCLC cells and may be a potential therapeutic target for NSCLC patients.
Keywords: SOX5, YAP1, self-renewal, invasion, migration, non-small cell lung cancer
Introduction
Lung cancer is the most prevalent cancer and the leading cause of cancer death worldwide [1], and an estimated 1.4 million patients would die from lung cancer each year [2]. Non-small cell lung cancer (NSCLC) accounts for more than 85% of all lung cancer, which can be subdivided into squamous cell carcinoma, adenocarcinoma, and large cell carcinoma. Although much effort has been devoted to improve the treatment strategy of NSCLC, the therapeutic outcome of patients remains poor. Treatment failures can even occur in lung cancer patients who are diagnosed at an early stage, which might be due to the high rates of local recurrence and distant metastasis [2-4].
It has been demonstrated that a small subpopulation of cancer stem-like cells (CSLCs) exist in different types of solid tumors and are responsible for driving tumor initiation and progression, and these CSLCs possess a variety of potentially biological properties including self-renewal, propagation and production of differentiated progeny, cancer invasion, tumor angiogenesis and therapy resistance [5-9]. Accumulating evidence suggests that targeting molecular regulators of CSLCs may significantly improve NSCLC treatment strategy. Thus, there is an urgent need to identify the key molecular regulators that control CSLCs self-renewal and maintain the malignant potential of NSCLC.
Transcription factors control the rates of transcription of genetic information from DNA to messenger RNA, by binding to a specific DNA sequence, which regulates gene expression in the right cell, at the right time, and in the right amount throughout the life of the cell and the organism [10,11]. Transcription factors that are involved in the regulation of CSLCs include OCT4, NANOG and SOX2, all of which play vital roles in maintaining stemness of CSLCs, and promoting cancer progression [5,12-14]. SOX5 gene is ubiquitously expressed and located on chromosome 12p12.1, which encodes a member of the SRY-related HMG-box family of transcription factors involved in the maintenance of normal physiological functions [15-19]. Recent studies reveal that aberrant expression of SOX5 plays critical roles in tumor progression of colorectal cancer, melanoma, lymphoma and hepatocellular carcinoma [20-23]. It remains elusive whether SOX5 drives the malignant potential in NSCLC, and its regulatory mechanism for SOX5 needs to be elucidated.
In the present study, we investigate the malignant phenotype and regulatory mechanisms of SOX5 in NSCLC cells, and determine whether SOX5 may play oncogenic roles by interacting with YAP1 to promote cancer progression, and targeting SOX5 may be a promising therapeutic strategy for NSCLC.
Materials and methods
Cell culture
Human NSCLC cell lines A549 and H1975 were purchased from the American Type Culture Collection (ATCC) (Rockville, MD, USA). Cells were cultured in Dulbecco’s Modified Eagle Medium (DMEM) (Hyclone, Logan, UT, USA) supplemented with 10% fetal bovine serum (FBS) (Hyclone, Logan, UT, USA) and 100 U/ml penicillin-streptomycin (Hyclone, Logan, UT, USA) and maintained at humidified 37°C incubator with 5% CO2.
Lentiviral cell infection
For stable silencing and overexpression of SOX5, as well as silencing of YAP1 in A549 and H1975 cells, the SOX5 shRNA and overexpression lentivirus were purchased from Genechem (Genechem, Shanghai, China) and Hanbio (Hanbio, Shanghai, China), YAP1-shRNA lentivirus were purchased from Genechem (Genechem, Shanghai, China). The detailed procedures were performed according to the manufacturer’s instructions. The infected efficiency was confirmed by qRT-PCR and Western blot.
Quantitative real-time PCR (qRT-PCR)
Total RNA was extracted from NSCLC cells using the RNA fast200 kit (Fastagen, Shanghai, China) and reverse transcribed to cDNA using Prime ScriptTM RT Master Mix (TaKaRa, Dalian, China). The PCR reaction was performed using SYBR® Premix ExTaqTM (TaKaRa, Dalian, China). Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was amplified as an internal control. The data was calculated using the 2-ΔΔCt method, and each cDNA sample was tested at least in triplicate.
Western blot and CO-IP
Western blot analysis was performed as previously described [6]. For CO-IP, NSCLC cells were infected with SOX5 overexpression lentivirus, which contains a 3 × flag sequence. Anti-FLAG M2 Magnetic Beads (Sigma-Aldrich, St.Louis, MO, USA) were used to detect and capture fusion proteins. The detailed procedures were performed according to the manufacturer’s instructions.
Tumorsphere formation assay
Cancer cells were seeded into 96-well plates (1 × 102/well). Cells were cultured in DMEM/F12 stem cell medium containing basic fibroblast growth factor (bFGF), epidermal growth factor (EGF) and B-27 for one week. The tumorspheres were photographed by a light microscope and counted to calculate the tumorsphere efficiency.
Cell invasion assay
A 24 well transwell chamber with 8 µm pore size (Millipore, Billerica, MA, USA) was coated with Matrigel (BD Bioscience, Franklin lakes, NJ, USA). Then, 1 × 104 cells were added to the upper chamber, 500 µl of DMEM containing 10% FBS was added to lower chambers. After incubation for 24 h, the chambers were wiped by using cotton swabs, fixed with 4% paraformaldehyde, and stained with crystal violet. The lower chamber was photographed by a light microscope.
Wound healing assay
Single cell suspension (2 × 105) was placed into a 24-well plate and cultured. When cells reached at 90%-100% confluency, cells were gently and vertically scratched using a 10 µl pipette tip to form wound, and detached cells were washed out with PBS. Medium was then discarded and replaced with 1 ml serum-free medium. The 24-well plate was cultured at Live Cell Imaging System (Leica, Brunswick, Saxony, Germany), the wound were photographed at 0 h and 24 h. The distances of wound were measured and rate of wound healing were assessed.
Immunofluorescence (IF) and immunohistochemistry (IHC)
For IF and IHC, the procedure was performed as previously described [5]. The primary antibodies were as follows: SOX5 (1:50, Abcam, Cambridge, UK) and YAP1 (1:100, Cell Signaling Technology, Boston, MA, USA). For tetramethyl rhodamine isothio-cyanate (TRITC) labeled Rhodamine Phalloidin to detected F-actin (Solarbio, Beijing, China), the detailed procedure was performed according the manufacturer’s instruction. We defined more than four invadopodia as a positive cell, and the positive cells were counted and analyzed.
In vivo xenograft tumor models
Four-week-old BALB/c athymic female nude mice were reared in the specific pathogen free (SPF) room. A total of 5 × 106 cells were injected into axillary subcutaneous, and xenograft tumor sizes were measured every four days by using a vernier caliper. The xenograft tumors were allowed to grow for one month. Then, all nude mice were sacrificed, and xenograft tumors were removed, photographed and weighed. Tumor volume (TV) was measured according to following formula: TV (mm3) = length × width2 × 0.5, the length and width represent the longest and shortest diameters. Then, the tumors were fixed in paraffin, and IHC were performed. All animal experimental procedures were performed in accordance with National Institutes of Health Sciences and approved by the Animal Care and Use Committee of the First Affiliated Hospital of The Third Military Medical University.
Statistical analysis
All data were analyzed by using SPSS 16.0 software (Chicago, IL, USA) and Graphpad Prism 6.0 (San Diego, CA, USA) and were expressed as mean ± SD. Unless otherwise noted, data were analyzed by using student’s t-test or one-way ANOVA, and P<0.05 (two-sided) was considered as statistical significance.
Results
SOX5 sustains the self-renewal of NSCLC cells
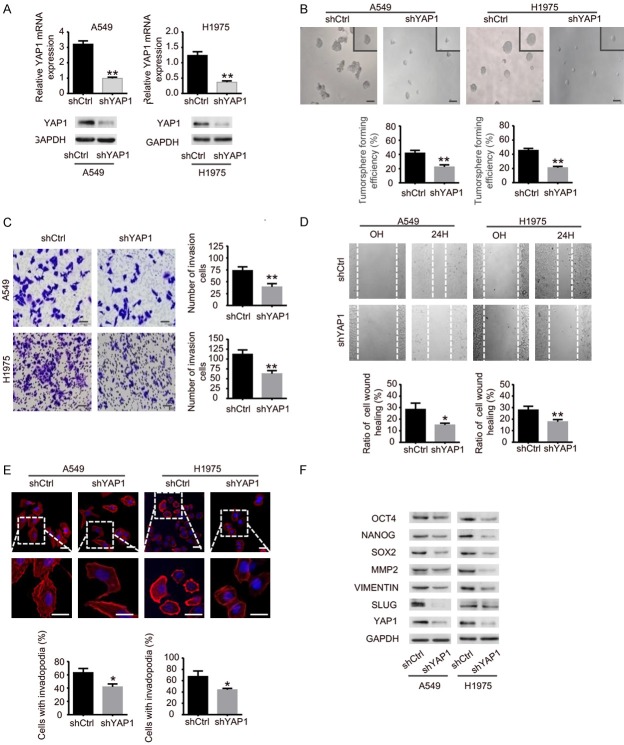
To investigate whether SOX5 can affect the self-renewal capability of NSCLC cells, we established a stable tumorsphere culture system for isolating and enriching CSLCs from A549 and H1975 cells. CSLCs were identified with a significantly higher expression of stemness markers, such as OCT4, NANOG and SOX2. The mRNA and protein levels of SOX5 were significantly up-regulated in CSLCs detected by qRT-PCR and Western blot (Figure 1A). To further identify the influence of SOX5 on tumorsphere formation capacity of NSCLC cells, stable knockdown and overexpression of SOX5 in A549 and H1975 cells were established (Figure 1B). We found that knockdown of SOX5 significantly reduced the size and the number of tumorspheres compared to the control group. In contrast, overexpression of SOX5 could reverse this phenomenon (Figure 1C and 1D). In addition, Western blot analysis showed that both knockdown and overexpression of SOX5 altered the expression of CSLCs makers, such as OCT4, NANOG and SOX2 (Figure 1E and 1F).
Figure 1.
SOX5 sustains the self-renewal of NSCLC cells. A. Relative mRNA levels and proteins abundances of SOX5, OCT4, NANOG, SOX2 in monolayer cells and tumorspheres of A549 and H1975 cells were determined by qRT-PCR and Western blot, respectively. B. Validation of stable knockdown and overexpression of SOX5 in A549 and H1975 cells by qRT-PCR and Western blot. C, D. Tumorsphere formation assay showed that knockdown of SOX5 reduced the size (left panel) (scale bar represents 100 µm) and the number (right panel) of tumorspheres, while overexpression of SOX5 increased the size and the number of tumorspheres in A549 and H1975 cells. E, F. Western blot showing that knockdown of SOX5 decreased the expression of OCT4, NANOG, SOX2, while overexpression of SOX5 increased the expression of those proteins. Data are shown as the mean ± standard deviation (SD). All experiments were repeated three times; *indicates P<0.05, **indicates P<0.01.
SOX5 promotes invasion and migration of NSCLC cells
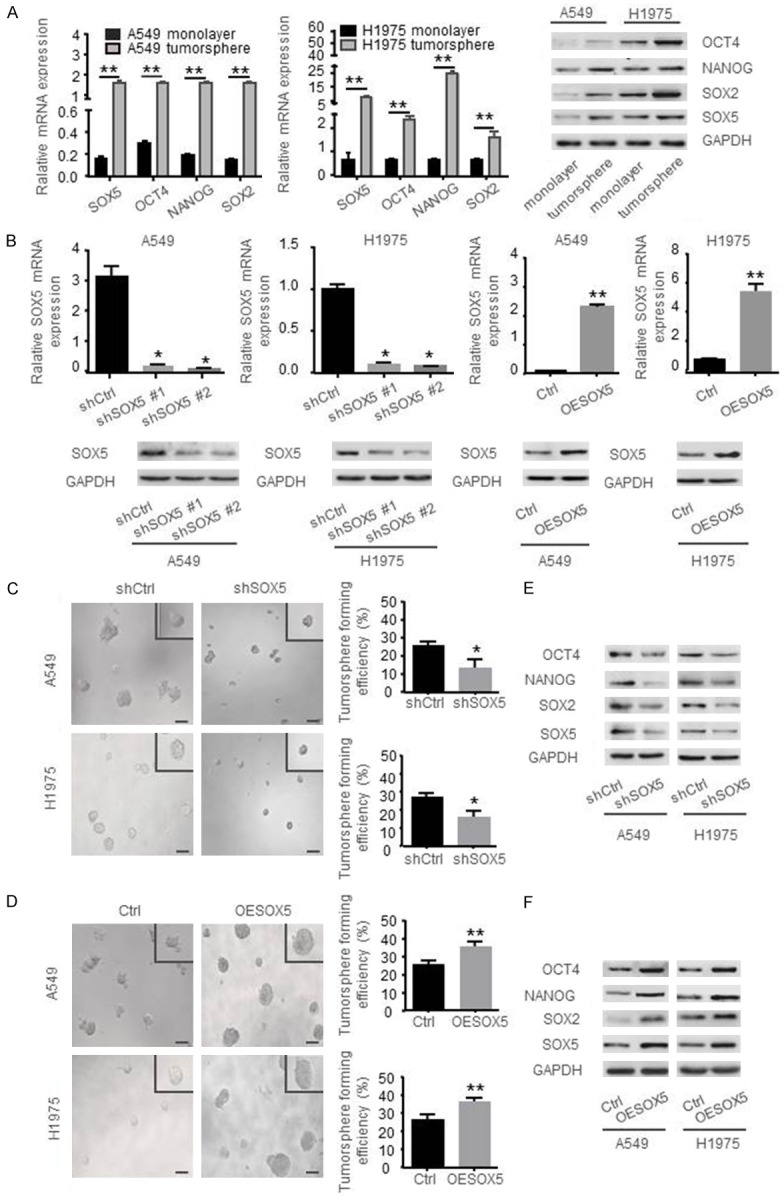
To evaluate the effects of SOX5 expression on cancer cell invasion and migration, transwell invasion assay, wound healing assay, and immunofluorescence invadopodia staining were performed. It was shown that the high invasive ability of cells on the lower chamber of shSOX5 groups were less than that of the control groups (Figure 2A), while overexpression of SOX5 increased the number of invasive cells (Figure 2B). The wound healing assay showed that closing of the wound was slowed down in the shSOX5 groups, while overexpression of SOX5 expression significantly increased the wound healing rate (Figure 2C and 2D). Moreover, invadopodia, as actin-based membrane protrusions in cancer cells, have the ability to degrade extracellular matrix (ECM) for tumor invasion and metastasis [24,25]. To investigate whether SOX5 can influence the forming of invadopodia, we used tetramethyl rhodamine isothiocyanate (TRITC) labeled Phalloidin to combine F-actin to assess the number of invadopodia. As shown in Figure 2E and 2F, knockdown of SOX5 reduced the formation of invadopodia in A549 and H1975 cells, while overexpression of SOX5 promoted its formation. Western blot analysis showed that protein expression of MMP2, VIMENTIN and SLUG was reduced after knockdown of SOX5, while overexpression of SOX5 increased the expression level of those proteins (Figure 2G and 2H). These results indicate that SOX5 promotes the invasion and migration capabilities in NSCLC cells.
Figure 2.
SOX5 promotes invasion and migration of NSCLC cells. A, B. The transwell invasion assay was used to evaluate the invasion ability of shSOX5 or OESOX5 groups compared with control groups (scale barrepresents 50 µm). C, D. The wound healing assay was performed to evaluate the migration ability of shSOX5 and OESOX5 groups as compared with control groups, cells were photographed at 0 H and 24 H after wounding (magnifcation × 100). E, F. Immunofluorescence showing that knockdown and overexpression of SOX5 affect invadopodia formation. The nuclei were stained with DAPI (blue) (scale bar represents 20 µm). G, H. Western blot showing that knockdown of SOX5 decreased protein expression of MMP2, VIMENTIN, SLUG, while overexpression of SOX5 increased the expression of those proteins. Data are shown as the mean ± SD. All experiments were repeated three times; *indicates P<0.05, **indicates P<0.01.
SOX5 interacts with YAP1 in NSCLC cells
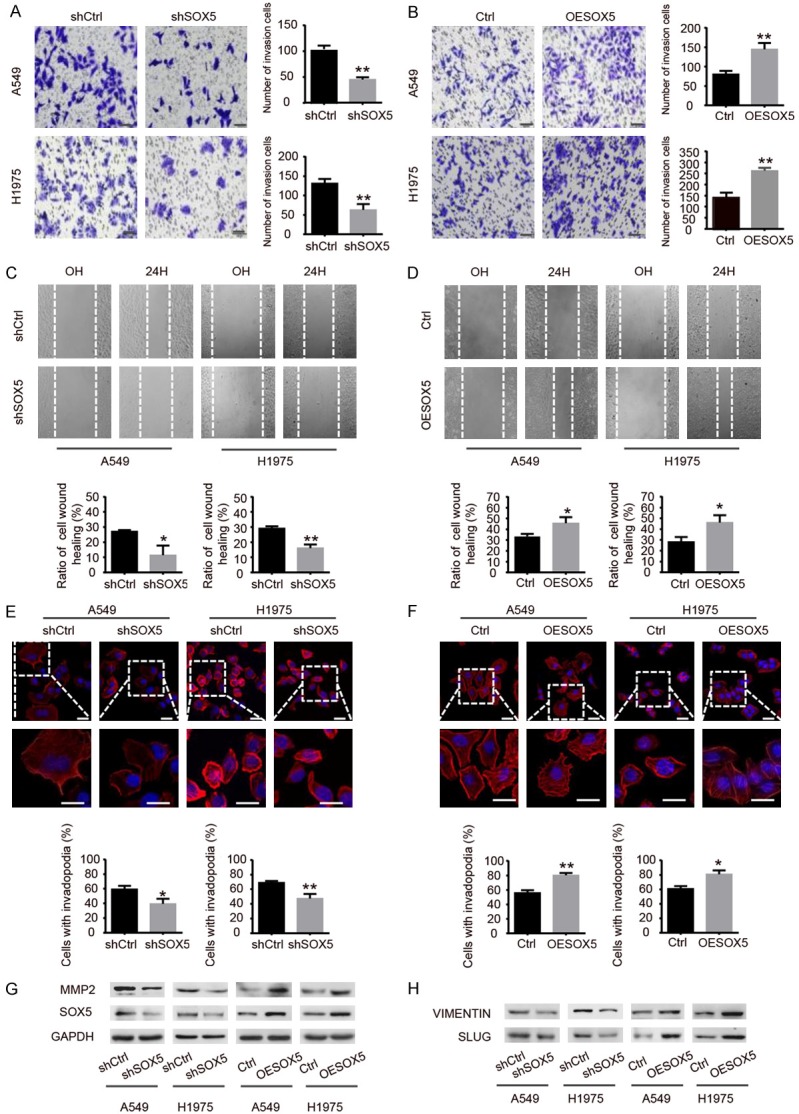
YAP1 is a key effector of the Hippo pathway. Since we found that both SOX5 and YAP1 were significantly up-regulated in CSLCs (Figure 3A and 3B), it would be of interest to investigate the relationship between these two molecules as they may influence the capabilities of cell self-renewal, invasion and migration. As shown in Figure 3C, Western blot showed that YAP1 synchronously changed along with either down- or up-regulated SOX5. Importantly, knockdown of YAP1 also decreased the expression of SOX5. Furthermore, SOX5 interacted with YAP1 to form a complex in both A549 and H1975 cells (Figure 3D). We also demonstrated that SOX5 and YAP1 preferentially localized in the nuclei of NSCLC cells as observed by the laser confocal scanning microscopy (LCSM) (Figure 3E). Cell fractionation analysis showed that overexpression of SOX5 led to translocation of YAP1 into the nuclei (Figure 3F). Taken together, these results indicate that SOX5 interacts with YAP1 to promote the progression of NSCLC cells.
Figure 3.
SOX5 interacts with YAP1 in NSCLC cells. A, B. qRT-PCR and Western blot analysis of SOX5 and YAP1 in tumorspheres and monolayer cells. C. Western blot showing that YAP1 level was decreased after knockdown of SOX5, and knockdown of YAP1 also down-regulated SOX5. D. CO-IP assays showed that SOX5 interacted with YAP1 in both A549 and H1975 cells. E. Immunofluorescence showing that SOX5 (green) and YAP1 (red) co-localized at nucleus, the nuclei were stained with DAPI (blue), scale bar represents 20 µm. F. Total cell lysates and nuclear and cytoplasmic extracts were prepared, overexpression of SOX5 increased the expression level of YAP1 in cytoplasm and nucleus. All experiments were repeated three times, **indicates P<0.01.
Knockdown of YAP1 decreases self-renewal, invasion and migration capacity in NSCLC cells
To confirm the effect of YAP1 dysregulation on NSCLC cell self-renewal, cell invasion and migration, we constructed stable YAP1 knockdown in A549 and H1975 cells (Figure 4A). As shown in Figure 4B, knockdown of YAP1 significantly inhibited the self-renewal capacity of cancer cells. Transwell invasion assay and wound healing assay also showed that down-regulated expression of YAP1 significantly decreased the invasion cell numbers and slowed the rate of wound healing (Figure 4C and 4D). In addition, we also found that knockdown of YAP1 decreased invadopodia formation (Figure 4E). In Figure 4F, Western blot analysis showed that knockdown of YAP1 decreased the expression of stemness transcription factors (OCT4, NANOG, SOX2) and invasion- and migration-associated proteins (MMP2, VIMENTIN, SLUG). Taken together, those results suggest that down-regulation of YAP1 decrease self-renewal, invasion and migration capacity of NSCLC cells.
Figure 4.
Knockdown of YAP1 decreases self-renewal, invasion and migration capacity in NSCLC cells. A. Knockdown of YAP1 in A549 and H1975 cells were validated by qRT-PCR and Western blot. B. Tumorsphere formation assays showing that down-regulated YAP1 decreased the size and the number of tumorspheres (scale bar represents 100 µm). C. Transwell invasion assays showed that knockdown of YAP1 efficiently reduced the invasion cells. Scale bar represents 50 µm. D. The wound healing assay showing that knockdown of YAP1 decreased the migration rate of A549 and H1975 cells (magnification × 100). E. Immunofluorescence showing that stable silencing YAP1 decreased the formation of invadopodia, the nuclei were stained with DAPI (blue), scale bar represents 20 µm. F. Western blot showing that knockdown of YAP1 attenuated the protein expression of OCT4, NANOG, SOX2, MMP2, VIMENTIN and SLUG. Data are shown as the mean ± standard deviation (SD). All experiments were repeated three times. *indicates P<0.05, **indicates P<0.01.
Overexpressed SOX5 reverses the attenuated malignant potential in YAP1 knockdown NSCLC cells
To further explore the association between YAP1 and SOX5 in regulating NSCLC cells progression, we stably overexpressed SOX5 in shYAP1 A549 cells (shYAP1&OESOX5). It was shown that down-regulated YAP1 attenuated the expression of SOX5, up-regulated SOX5 in shYAP1 cancer cells increased the expression of SOX5, NANOG and VIMENTIN (Figure 5A and 5B). Furthermore, we found that SOX5 overexpression could reverse the decreased tumorsphere formation in YAP1 knockdown A549 cells (Figure 5C). Meanwhile, SOX5 overexpression in shYAP1 cells resulted in higher invasion ability (Figure 5D) and faster wound healing (Figure 5E) compared to shYAP1 cells. Collectively, SOX5 may act as a target of YAP1, and overexpression of SOX5 could reverse the attenuated self-renewal, invasion and migration capabilities resulting from the silencing of YAP1 in NSCLC cells.
Figure 5.
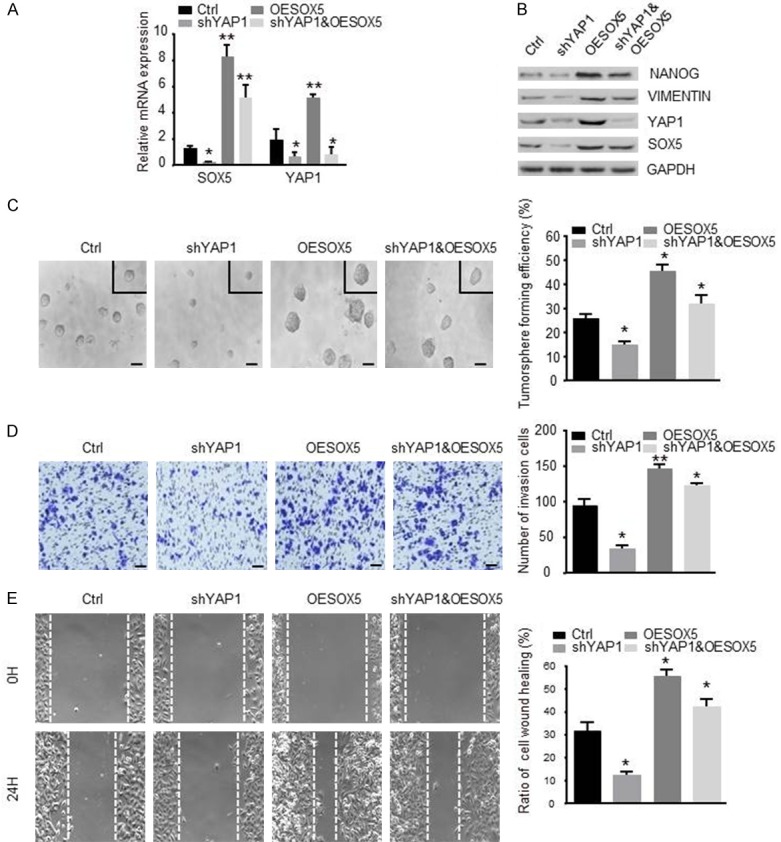
Overexpression of SOX5 rescues loss of self-renewal and motile ability of silencing YAP1 cells. A. qRT-PCR analysis of YAP1 knockdown A549 cells with stably overexpressed SOX5 (shYAP1&OESOX5). B. Western blot showing the protein expressions of NANOG, VIMENTIN, YAP1 and SOX5 after overexpression of SOX5 in shYAP1 A549 cells. C. Tumorsphere formation assay showed that up-regulated SOX5 in shYAP1 A549 cells increased the volume (left panel) (scale bar represents 100 µm) and the number (right panel) of tumorspheres compared to shYAP1 group. D. The transwell invasion assay was performed to evaluate the invasion ability of shYAP1&OESOX5 group compared to control group (scale bar represents 50 µm). E. The wound healing assay was performed to evaluate the migration ability of shYAP1&OESOX5 group compared to control group, cells were photographed at 0 H and 24 H after wounding (magnifcation × 100). Data are shown as the mean ± SD. *indicates P<0.05, **indicates P<0.01.
Stable knockdown of SOX5 inhibits xenograft tumor growth in vivo
To investigate whether SOX5 can influence tumor growth in vivo, stable silencing of SOX5 cancer cells were subcutaneously injected into the right flanks of nude mice. We found that knockdown of SOX5 significantly reduced the volume and weight of xenograft tumors (Figure 6A-C). Consistent with our previous data, IHC showed that silencing of SOX5 reduced, YAP1, SOX2 and MMP2 protein expressions in xenograft tumors (Figure 6D). These results suggest that silencing SOX5 inhibits the growth of xenograft tumors in vivo.
Figure 6.
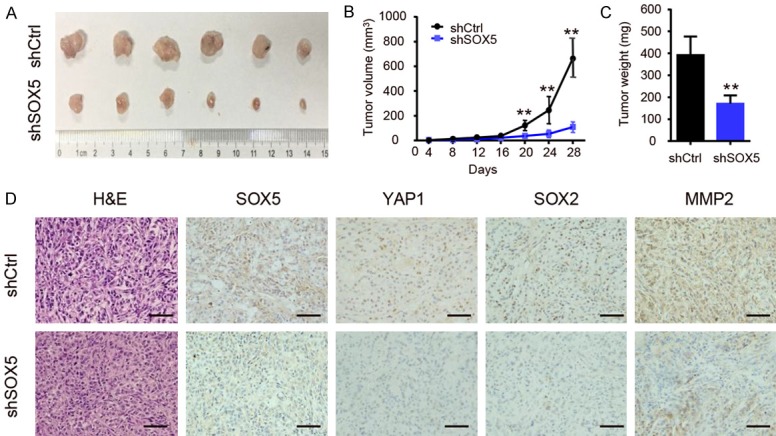
Stable knockdown of SOX5 inhibits xenograft tumor growth in vivo. A. Control-shRNA- or SOX5-shRNA-infected A549 cells were injected subcutaneously into the flank of BALB/c-nude mice for tumorigenicity analysis. The xenografts were harvested and photographed on day 28. B. SOX5-knockdown cancer cells produced significantly smaller xenografts tumor volume than that of control cells. C. The harvested tumor weight of shSOX5 group was lower than that of control group. D. IHC showing that tumor with low expression level of SOX5 displayed decreased protein level of YAP1, SOX2 and MMP2 (scale bar represents 100 µm). *indicates P<0.05, **indicates P<0.01.
Discussion
As a nuclear transcription factor, high expression of SOX5 is detected in many malignant tumors, such as hepatocellular carcinoma, melanoma, nasopharyngeal carcinoma, B lymphomas, prostate cancer and glioma. Aberrant expression of SOX5 can promote epithelial-mesenchymal transition (EMT), proliferation, invasion and migration of cancers by targeting different down-stream genes such as Twist1, Snail, secreted protein acidic and rich in cysteine (SPARC), and microphthalmia-associated transcription factor (MITF), as well as up-regulating the protein abundances of p27 and β-catenin, etc. [20,22,26-29]. However, whether SOX5 can influence the tumor initiation and progression of NSCLC has not been elucidated. Cancer stem cells (CSLCs) are a small subpopulation of cancer cells that are responsible for cancer initiation, invasion, metastasis and therapy resistance [30]. Understanding and targeting CSLCs would find out new treatment strategies for NSCLC. Thus, it is necessary to explore the relationship between SOX5 and CSLCs in NSCLC.
Recently, it has been shown that SOX5 is responsible for TGF-β-induced EMT and that Smad3-Sox5-Twist1 signaling, acting as an axis to promote EMT and contribute to prostate cancer metastasis [31]. Here, our findings demonstrate that SOX5 sustains the self-renewal capability of NSCLC cells, and promotes the ability of cancer cell invasion and migration. In vivo mouse tumor models, knockdown of SOX5 in A549 cells significantly suppresses the tumor growth. Collectively, we determine that SOX5 functions as an oncogene in NSCLC, which is consistent with previous report.
YAP1, as a key effector of the Hippo pathway, has been reported in varieties of cancers to promote cancer cell tumorsphere formation, proliferation, chemoresistance, and metastasis [32-34]. Our study confirmed that knockdown of YAP1 decreased the tumorsphere formation, invasion and migration, and also decreased the formation of invadopodia in NSCLC cells, and regulated stemness maker (OCT4, NANOG, SOX2) and invasion and migration associated protein (MMP2, VIMENTIN, SLUG) expression in NSCLC cells. We demonstrate that YAP1 plays an important role in the progression of NSCLC cells.
Previous studies showed that SOX2 can interact with YAP1 to maintain stemness and vascular mimicry to promote tumorigenesis [35-37]. Considering both SOX5 and SOX2 are the homologs of SOX family and as a whole the SOX family controls cell fate and differentiation in a multitude of processes, we deduce whether SOX5 is similar with SOX2 and can interact with YAP1 to promote NSCLC progression. Our data showed that YAP1 together with SOX5 was expressed at higher level in tumorspheres than monolayer cells derived from NSCLC, suggesting that both SOX5 and YAP1 may be involved in self-renewal of NSCLC cells. We also found that knockdown or overexpression of SOX5 synchronously changed the protein expression level of YAP1, and knockdown of YAP1 decreased the expression level of SOX5. We demonstrated for the first time that SOX5 interacted with YAP1 and colocalized in nucleus of cancer cells. Moreover, SOX5 was associated with increased translocation of YAP1 to the nucleus, SOX5 overexpression reversed the malignant progression in YAP1 knockdown cancer cells. Collectively, we found that SOX5 can interact with YAP1 and may be a potential target of YAP1.
In summary, our findings identify that SOX5 acts as an oncogenic factor to enhance the malignant potential of NSCLC, and reveal a new mechanism underlying the physical interaction of SOX5 with YAP1 to promote YAP1 nucleus translocation, indicating that SOX5 may be a potential therapeutic target against CSLCs hidden in human NSCLC.
Acknowledgements
We would like to thank all the lab members for their critical reading of the manuscript. This study was supported by the National Natural Science Foundation of China (No. 81572892; No. 81773040) and Sichuan Provincial Science Fund for Distinguished Young Scholars of China (No. 2015JQO055).
Disclosure of conflict of interest
None.
References
- 1.Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 2.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin. 2013;63:11–30. doi: 10.3322/caac.21166. [DOI] [PubMed] [Google Scholar]
- 3.Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM. Cancer incidence and mortality worldwide: IARC cancerbase No. International Journal of Cancer Journal International Du Cancer. 2010;136:E359–E386. doi: 10.1002/ijc.29210. [DOI] [PubMed] [Google Scholar]
- 4.Fang L, Cai J, Chen B, Wu S, Li R, Xu X, Yang Y, Guan H, Zhu X, Zhang L, Yuan J, Wu J, Li M. Aberrantly expressed miR-582-3p maintains lung cancer stem cell-like traits by activating Wnt/beta-catenin signalling. Nat Commun. 2015;6:8640. doi: 10.1038/ncomms9640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Xu C, Xie D, Yu SC, Yang XJ, He LR, Yang J, Ping YF, Wang B, Yang L, Xu SL, Cui W, Wang QL, Fu WJ, Liu Q, Qian C, Cui YH, Rich JN, Kung HF, Zhang X, Bian XW. beta-Catenin/POU5F1/SOX2 transcription factor complex mediates IGF-I receptor signaling and predicts poor prognosis in lung adenocarcinoma. Cancer Res. 2013;73:3181–3189. doi: 10.1158/0008-5472.CAN-12-4403. [DOI] [PubMed] [Google Scholar]
- 6.Xin YH, Bian BS, Yang XJ, Cui W, Cui HJ, Cui YH, Zhang X, Xu C, Bian XW. POU5F1 enhances the invasiveness of cancer stem-like cells in lung adenocarcinoma by upregulation of MMP-2 expression. PLoS One. 2013;8:e83373. doi: 10.1371/journal.pone.0083373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhou W, Chen C, Shi Y, Wu Q, Gimple RC, Fang X, Huang Z, Zhai K, Ke SQ, Ping YF, Feng H, Rich JN, Yu JS, Bao S, Bian XW. Targeting glioma stem cell-derived pericytes disrupts the blood-tumor barrier and improves chemotherapeutic efficacy. Cell Stem Cell. 2017;21:591–603. e594. doi: 10.1016/j.stem.2017.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tammela T, Sanchez-Rivera FJ, Cetinbas NM, Wu K, Joshi NS, Helenius K, Park Y, Azimi R, Kerper NR, Wesselhoeft RA, Gu X, Schmidt L, Cornwall-Brady M, Yilmaz OH, Xue W, Katajisto P, Bhutkar A, Jacks T. A Wnt-producing niche drives proliferative potential and progression in lung adenocarcinoma. Nature. 2017;545:355–359. doi: 10.1038/nature22334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhang WC, Shyh-Chang N, Yang H, Rai A, Umashankar S, Ma S, Soh BS, Sun LL, Tai BC, Nga ME, Bhakoo KK, Jayapal SR, Nichane M, Yu Q, Ahmed DA, Tan C, Sing WP, Tam J, Thirugananam A, Noghabi MS, Pang YH, Ang HS, Mitchell W, Robson P, Kaldis P, Soo RA, Swarup S, Lim EH, Lim B. Glycine decarboxylase activity drives non-small cell lung cancer tumor-initiating cells and tumorigenesis. Cell. 2012;148:259–272. doi: 10.1016/j.cell.2011.11.050. [DOI] [PubMed] [Google Scholar]
- 10.Takemoto T, Uchikawa M, Yoshida M, Bell DM, Lovell-Badge R, Papaioannou VE, Kondoh H. Tbx6-dependent Sox2 regulation determines neural or mesodermal fate in axial stem cells. Nature. 2011;470:394–398. doi: 10.1038/nature09729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wong ES, Schmitt BM, Kazachenka A, Thybert D, Redmond A, Connor F, Rayner TF, Feig C, Ferguson-Smith AC, Marioni JC, Odom DT. Interplay of cis and trans mechanisms driving transcription factor binding and gene expression evolution. Nat Commun. 2017;8:1092. doi: 10.1038/s41467-017-01037-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jen J, Tang YA, Lu YH, Lin CC, Lai WW, Wang YC. Oct4 transcriptionally regulates the expression of long non-coding RNAs NEAT1 and MALAT1 to promote lung cancer progression. Mol Cancer. 2017;16:104. doi: 10.1186/s12943-017-0674-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Guo T, Kong J, Liu Y, Li Z, Xia J, Zhang Y, Zhao S, Li F, Li J, Gu C. Transcriptional activation of NANOG by YBX1 promotes lung cancer stem-like properties and metastasis. Biochem Biophys Res Commun. 2017;487:153–159. doi: 10.1016/j.bbrc.2017.04.033. [DOI] [PubMed] [Google Scholar]
- 14.Justilien V, Walsh MP, Ali SA, Thompson EA, Murray NR, Fields AP. The PRKCI and SOX2 oncogenes are coamplified and cooperate to activate Hedgehog signaling in lung squamous cell carcinoma. Cancer Cell. 2014;25:139–151. doi: 10.1016/j.ccr.2014.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wunderle VM, Critcher R, Ashworth A, Goodfellow PN. Cloning and characterization of SOX5, a new member of the human SOX gene family. Genomics. 1996;36:354–358. doi: 10.1006/geno.1996.0474. [DOI] [PubMed] [Google Scholar]
- 16.Ikeda T, Zhang J, Chano T, Mabuchi A, Fukuda A, Kawaguchi H, Nakamura K, Ikegawa S. Identification and characterization of the human long form of Sox5 (L-SOX5) gene. Gene. 2002;298:59–68. doi: 10.1016/s0378-1119(02)00927-7. [DOI] [PubMed] [Google Scholar]
- 17.Lai T, Jabaudon D, Molyneaux BJ, Azim E, Arlotta P, Menezes JR, Macklis JD. SOX5 controls the sequential generation of distinct corticofugal neuron subtypes. Neuron. 2008;57:232–247. doi: 10.1016/j.neuron.2007.12.023. [DOI] [PubMed] [Google Scholar]
- 18.Stolt CC, Schlierf A, Lommes P, Hillgartner S, Werner T, Kosian T, Sock E, Kessaris N, Richardson WD, Lefebvre V, Wegner M. SoxD proteins influence multiple stages of oligodendrocyte development and modulate SoxE protein function. Dev Cell. 2006;11:697–709. doi: 10.1016/j.devcel.2006.08.011. [DOI] [PubMed] [Google Scholar]
- 19.Hersh CP, Silverman EK, Gascon J, Bhattacharya S, Klanderman BJ, Litonjua AA, Lefebvre V, Sparrow D, Reilly JJ, Anderson WH, Lomas DA, Mariani TJ. SOX5 is a candidate gene for chronic obstructive pulmonary disease susceptibility and is necessary for lung development. Am J Respir Crit Care Med. 2011;183:1482–1489. doi: 10.1164/rccm.201010-1751OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang D, Han S, Wang X, Peng R, Li X. SOX5 promotes epithelial-mesenchymal transition and cell invasion via regulation of Twist1 in hepatocellular carcinoma. Med Oncol. 2015;32:461. doi: 10.1007/s12032-014-0461-2. [DOI] [PubMed] [Google Scholar]
- 21.Shiseki M, Masuda A, Yoshinaga K, Mori N, Okada M, Motoji T, Tanaka J. Identification of the SOX5 gene as a novel IGH-involved translocation partner in BCL2-negative follicular lymphoma with t(12;14)(p12.2;q32) Int J Hematol. 2015;102:633–638. doi: 10.1007/s12185-015-1823-z. [DOI] [PubMed] [Google Scholar]
- 22.Kordass T, Weber CE, Oswald M, Ast V, Bernhardt M, Novak D, Utikal J, Eichmuller SB, Konig R. SOX5 is involved in balanced MITF regulation in human melanoma cells. BMC Med Genomics. 2016;9:10. doi: 10.1186/s12920-016-0170-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wu K, Zhao Z, Liu K, Zhang J, Li G, Wang L. Long noncoding RNA lnc-sox5 modulates CRC tumorigenesis by unbalancing tumor microenvironment. Cell Cycle. 2017;16:1295–1301. doi: 10.1080/15384101.2017.1317416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Eckert MA, Lwin TM, Chang AT, Kim J, Danis E, Ohno-Machado L, Yang J. Twist1-induced invadopodia formation promotes tumor metastasis. Cancer Cell. 2011;19:372–386. doi: 10.1016/j.ccr.2011.01.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ngan E, Stoletov K, Smith HW, Common J, Muller WJ, Lewis JD. LPP is a Src substrate required for invadopodia formation and efficient breast cancer lung metastasis. Nat Commun. 2017;8:15059. doi: 10.1038/ncomms15059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Edwards SK, Desai A, Liu Y, Moore CR, Xie P. Expression and function of a novel isoform of Sox5 in malignant B cells. Leuk Res. 2014;38:393–401. doi: 10.1016/j.leukres.2013.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Renjie W, Haiqian L. MiR-132, miR-15a and miR-16 synergistically inhibit pituitary tumor cell proliferation, invasion and migration by targeting Sox5. Cancer Lett. 2015;356:568–578. doi: 10.1016/j.canlet.2014.10.003. [DOI] [PubMed] [Google Scholar]
- 28.Zhang D, Liu S. SOX5 promotes epithelialmesenchymal transition in osteosarcoma via regulation of Snail. J BUON. 2017;22:258–264. [PubMed] [Google Scholar]
- 29.Huang DY, Lin YT, Jan PS, Hwang YC, Liang ST, Peng Y, Huang CY, Wu HC, Lin CT. Transcription factor SOX-5 enhances nasopharyngeal carcinoma progression by down-regulating SPARC gene expression. J Pathol. 2008;214:445–455. doi: 10.1002/path.2299. [DOI] [PubMed] [Google Scholar]
- 30.Liu CC, Lin SP, Hsu HS, Yang SH, Lin CH, Yang MH, Hung MC, Hung SC. Suspension survival mediated by PP2A-STAT3-Col XVII determines tumour initiation and metastasis in cancer stem cells. Nat Commun. 2016;7:11798. doi: 10.1038/ncomms11798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hu J, Tian J, Zhu S, Sun L, Yu J, Tian H, Dong Q, Luo Q, Jiang N, Niu Y, Shang Z. Sox5 contributes to prostate cancer metastasis and is a master regulator of TGF-beta-induced epithelial mesenchymal transition through controlling Twist1 expression. Br J Cancer. 2018;118:88–97. doi: 10.1038/bjc.2017.372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dai Y, Liu S, Zhang WQ, Yang YL, Hang P, Wang H, Cheng L, Hsu PC, Wang YC, Xu Z, Jablons DM, You L. YAP1 regulates ABCG2 and cancer cell side population in human lung cancer cells. Oncotarget. 2017;8:4096–4109. doi: 10.18632/oncotarget.13686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zanconato F, Cordenonsi M, Piccolo S. YAP/TAZ at the roots of cancer. Cancer Cell. 2016;29:783–803. doi: 10.1016/j.ccell.2016.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lin L, Sabnis AJ, Chan E, Olivas V, Cade L, Pazarentzos E. The Hippo effector YAP promotes resistance to RAF- and MEK-targeted cancer therapies. Nat Genet. 2015;47:250–256. doi: 10.1038/ng.3218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Basu-Roy U, Bayin NS, Rattanakorn K, Han E, Placantonakis DG, Mansukhani A, Basilico C. Sox2 antagonizes the Hippo pathway to maintain stemness in cancer cells. Nat Commun. 2015;6:6411. doi: 10.1038/ncomms7411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bora-Singhal N, Nguyen J, Schaal C, Perumal D, Singh S, Coppola D, Chellappan S. YAP1 regulates OCT4 activity and SOX2 expression to facilitate self-renewal and vascular mimicry of stem-like cells. Stem Cells. 2015;33:1705–1718. doi: 10.1002/stem.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Slemmons KK, Crose LES, Riedel S, Sushnitha M, Belyea BC, Linardic CM. A novel notchYAP circuit drives stemness and tumorigenesis in embryonal rhabdomyosarcoma. Mol Cancer Res. 2017;15:1777–1791. doi: 10.1158/1541-7786.MCR-17-0004. [DOI] [PMC free article] [PubMed] [Google Scholar]