Abstract
Ovarian carcinoma is a fatal malignancy in gynecological malignancies, and the prognosis still remains poor due to the lack of effective therapeutic targets. This study demonstrated that centromere protein U (CENPU) was up-regulated in ovarian cancer. The ectopic expression of CENPU in ovarian cancer cells expedited the proliferation, migration and invasion of ovarian cancer cells in vitro. Besides, the over-expression of CENPU markedly advanced the tumorigenicity of ovarian cancer cells in vivo whereas knocked-down CENPU resulted in opposite outcome. In addition, high mobility group box 2 (HMGB2) identified as a down-target of CENPU in ovarian cancer cells was positively correlated with the expression level of CENPU in ovarian cancer tissues. Finally, it was demonstrated that CENPU could enhance the aggressiveness ability of the ovarian cancer cells by regulating HMGB2. This research provided new insight for CENPU, promoted the progression of ovarian cancer and represented a novel target for anti-ovarian cancer therapy.
Keywords: CENPU, HMGB2, metastasis, ovarian carcinoma
Introduction
Ovarian carcinoma with the highest mortality in gynecological malignant tumors has high invasiveness and metastatic features [1,2]. Due to the advanced stages at diagnosis and the recurrence of chemotherapy-resistant ovarian cancer, it brings about poor prognosis [3-5]. Insufficiency investigations of the molecular mechanisms regulate that ovarian cancer progression remain to be challenge in improving clinical outcomes [6]. Thus, elucidating the functional-related molecular markers of cancer progression is critical in developing targeted treatment and improving the survival outcomes of ovarian cancer patients.
Aberrant expression of centromere protein U (CENPU), which also called MLF1IP, KLIP1 [7], is discovered in various types of cancers, including breast cancer, prostate cancer, colorectal carcinoma and lymphoma [8]. Substantial evidence demonstrates that CENPU is closely associated with tumorigenesis. Previous study verifies that CENPU is over-expression in glioblastoma (GBM) cell lines and plays a critical role in erythroleukemia [9]. Furthermore, the excessive expression of CENPU is an independent risk factor and possesses a positive correlation with the distant metastasis in luminal breast cancer [10]. Nevertheless, the basic biological functions of CENPU in ovarian cancer remain poorly investigated.
The family of human high mobility group box (HMGB) contains four-members (known as HMGB1, HMGB2, HMGB3 and HMGB4) [11]. HMGB which is ubiquitous in eukaryotic cells and non-specifically binds with DNA could enhance the flexibility of DNA, induce large-angle bends in DNA and facilitate various crucial biological interactions [12]. Despite the structure of HMGB2 analogue to HMGB1, the HMGB member exhibits different physiological functions in the tumor progression. The altered expression of HMGB1 is an innovative prognostic marker and a potential target for therapeutic pathway of multifarious human cancers [13,14]. Although the comprehensive characterization of the oncogene of HMGB1 in certain forms of tumors is much less known about HMGB2 in tumorigenesis and its prognostic significance in ovarian cancer, except the prognostic value of HMGB2 in hepatocellular carcinoma (HCC) breast cancer and skin squamous cell carcinoma (SSCC) [15,16].
In this study, it was demonstrated that CENPU was remarkably over-expressed in ovarian cancer and was strongly associated with clinical features of ovarian cancer. The over-expression of CENPU could enhance the proliferation, the mobility in vitro and the tumorigenic growth in vivo of ovarian cancer cells. It was identified that HMGB2 as a co-expression gene of CENPU could be assessed by TCGA analysis method. The comprehensive experiments in this study suggested that HMGB2 played crucial roles in the growth and metastasis of CENPU-regulated ovarian cancer cells. Altogether, it was suggested that CENPU/HMGB2 axis played a key role in the ovarian cancer development and highlighted its promising as a therapeutic target in ovarian cancer.
Materials and methods
Cell lines and ovarian cancer specimens
Ovarian cancer cell lines (SKOV3, OVCAR3 and Caov-3) and normal ovarian epithelial cells IOSE80 were purchased from Chinese Academy of Sciences Cell Bank of Type Culture Collection (CBTCCCAS, Shanghai, China). Cells were maintained in high-glucose Dulbecco’s modified Eagle’s medium (HyClone) or RPMI-1640 culture media supplemented with 10% fetal bovine serum, 100 U/mL penicillin and 100 U/mL streptomycin. A total of 46 ovarian cancer and matched adjacent tissues were obtained from ovarian cancer patients in Zhengzhou Central Hospital Affiliated of Zhengzhou University from January 2010 to December 2016. After surgery, tissues were frozen in liquid nitrogen. The clinical characteristics of patients with ovarian cancer were described in Supplementary Table 1. Informed consent of patients was obtained and the research procedures were approved by Medical Ethics Committee of Zhengzhou University.
Knock-down and over-expression treatment
CENPU or HMGB2 expression constructs were synthesized by sub-cloning PCR-amplied CENPU or HMGB2 cDNA into pMSCV retrovirus plasmid, respectively. CENPU short hairpin RNA (shRNA) was purchased from (Santa Cruz, sc-61057-SH, USA). siRNA against HMGB2 (siHMGB2) and the negative control (siNC) were generated by Genepharma (Shanghai, China). The sequence of siHMGB2 was: 5’-CUGAACAUCGCCCAAAGAU-3’. The sequence of siNC was: 5’-UUCUCCGAACGUGUCACGUTT-3’. Lipofectamine® 2000 (Invitrogen, Carlsbad, CA, USA) was applied for transfection.
MTT and colony formation assay
Cell viability was tested by MTT assay. 1000 cells were seeded in 96 well plates. Cell proliferation was determined at 12, 24, 48, or 72 h with Synergy™ HT Multi-Mode Micro-plate Reader (Bio-Tek, Winooski, VT, USA). In colonies formation assay, cells were seeded into 25 mm2 culture dish and colonies were counted after two weeks [17].
Wound closure analysis
Cells were seeded into six-well plate. After a confluent monolayer was formed, the monolayer was scratched. The medium was replaced with FBS free medium and the wound were photographed at different time points (0 and 24 h). The percentage of wound closure was measured based on the wound width at 0 h [18].
Invasion assay
24-well Transwell plate with 8 μm pore polycarbonate membrane insert (Corning-Costar, Cambridge, MA) was subjected to determine the invasion of ovarian cancer cells. Cells were plated into the upper chamber of Transwell and the medium containing 20% FBS were plated into the lower chamber. After 6 h, the invaded cells were stained with 0.1% crystal violet and then counted.
Quantitative real time PCR (qRT-PCR) analysis
Trizol reagent from Invitrogen, Carlsbad, CA, USA was used for total RNA extraction. cDNA was generated using RNA (1 μg) with a PrimeScript RT reagent kit (Tiangen, China). qRT-PCR was conducted with IQTM SYBR Green supermix and Applied Biosystems 7500 Detection system. The expression levels of target genes were quantified through the 2(-ΔΔCt) method. The primers of genes were as follows (sense and antisense, respectively): GAPDH, forward primer: 5’-CTCACCGGATGCACCAATGTT-3’ and reverse primer:5’-CGCGTTGCTCACAATGTTCAT-3’; CENPU, forward primer: 5’-ACCCACCTAGAGCATCAACAA-3’ and reverse primer: 5’-ACTTCAATCATACGCTGCCTTT-3’; HMGB2, forward primer: 5’-CGGGGCAAAATGTCCTCGTA-3’ and reverse primer: 5’-CGGAAGAGTCCGGGTGTTT-3’; Ecadherin, forward primer: 5’-AAAGGCCCATTTCCTAAAAACCT-3’ and reverse primer: 5’-TGCGTTCTCTATCCAGAGGCT-3’; N-cadherin, forward primer: 5’-TCAGGCTGTGGACATAGAAACC-3’ and reverse primer: 5’-GCTGTAAACGACTCTGGCACT-3’.
Western blotting analysis
Total protein was prepared with lysis in RIPA and the concentration was assessed by BCA protein assay Kit. The separated total proteins were transferred onto PVDF. PVDF was blocked with TBST containing 0.1% Tween and incubated with antibodies against CENPU (Santa Cruz, sc-514294, USA), HMGB2 (Abcam, ab133540, USA), N-Cadherin (Santa Cruz, sc-59987, USA), E-Cadherin (Santa Cruz, sc-71009, USA), or β-actin (Santa Cruz, sc-293335, USA) followed by the incubation with horseradish peroxidase-conjugated secondary antibodies. Blots were visualized with ECL detection kit (Pierce, IL).
In vivo tumor growth
Balb/c nude mice were purchased from Shanghai Slack laboratory animal co., LTD (Shanghai, China). The experimental protocol was approved by the Institutional Animal Care and Use Committee of Zhengzhou University. 0.1 ml SKOV3 cells (5 × 106) were implanted subcutaneously into nude mice (each group with three mice). Tumor volume was determined by the formula: Volume = 0.5 × length × width2. The mice were finally euthanized and the tumor mass were isolated [19]. In immunohistochemistry (IHC) assay, tumor was fixed with 4% paraformaldehyde and embedded in paraffin. Carry out the immunohistochemical staining of HMGB2 and Ki-67.
Experimental metastasis assay
Balb/c nude mice were purchased from Shanghai Slack laboratory animal co., LTD (Shanghai, China). The experimental protocol was approved by the Institutional Animal Care and Use Committee of Zhengzhou University. 5 × 105 ovarian cancer SKOV3 cells were injected into mice via the lateral tail vein (each group with three mice). After two weeks, the mice were sacrificed and all organs were examined [20]. Lung tissues fixed with 4% paraformaldehyde were subjected to H&E stain. Lung metastatic nodules were determined under a dissecting microscope.
Statistical analysis
Data were presented as mean ± SD of three independent experiments. One-way ANOVA or student t-test was applied to compare the results of two groups. P < 0.05 was considered statistically significant.
Results
CENPU is over-expression in ovarian cancer
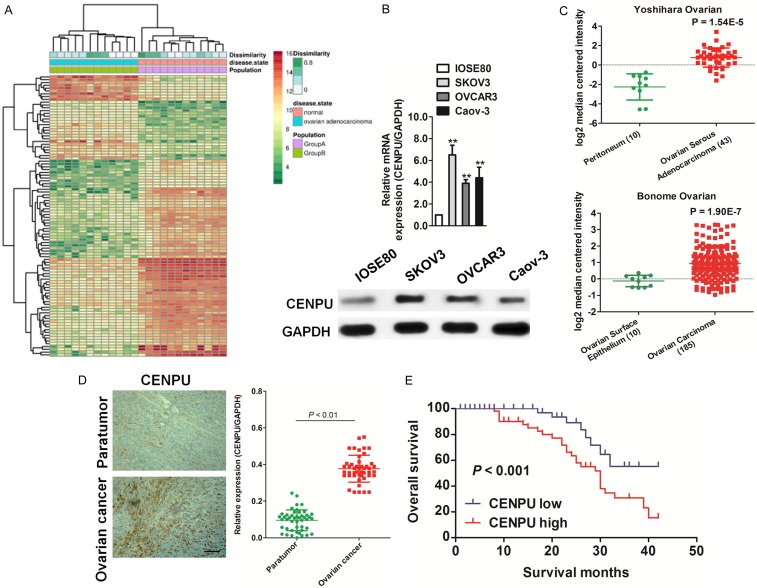
After analyzing the published expression profiles obtained from ovarian cancer and normal tissues (GDS3592) (https://www.ncbi.nlm.nih.gov/sites/GDSbrowser?acc=GDS3592), it was found that CENPU was remarkably up-regulated in ovarian cancer compared with the corresponding normal tissue (Figure 1A). Consistently, qRT-PCR and immunoblotting analysis demonstrated that CENPU was significantly over-expressed in three ovarian cancer cell lines (OVCAR3, SKOV3 and Caov-3) and normal ovarian epithelial cells IOSE80, compared with IOSE80 (Figure 1B). To investigate whether the expression of CENPU was involved in ovarian cancer progression, the expression patterns of CENPU in the Oncomine microarray database was determined. From Bonome Ovarian data-set (containing 10 cases of ovarian surface epithelium samples and 185 cases of ovarian carcinoma samples) and Yoshihara Ovarian data-set (containing 10 cases of peritoneum samples and 42 cases of ovarian serous adenocarcinoma samples) [21,22], it was found that the level of CENPU was increased in ovarian cancer in comparison with normal tissues (Figure 1C). CENPU was evaluated by qRT-PCR in clinical ovarian cancer tissues to furtherly confirm the function of CENPU in ovarian cancer. As shown in Figure 1D, CENPU was noteworthy over-expressing in ovarian cancer vs. normal tissues. Also, the ovarian cancer patients with higher CENPU level had poor OS and those with lower CENPU possessed longer survival (Figure 1E). The results suggested that CENPU functioned as an oncogene in ovarian cancer progression.
Figure 1.
The expression of CENPU in ovarian cancer. A. The expression profiling of mRNAs shown that CENPU was over-expressed in ovarian cancer as compared to normal (GDS3592). B. qRT-PCR and western blotting analyzed the expression of CENPU in IOSE80 cells and ovarian cancer cells. C. Box plots shown elevated levels of CENPU in ovarian cancer compared to normal from two microarray data-sets. **P < 0.01, compared with normal tissues. D. Representative staining result of CENPU in ovarian cancer tissues (left panel). Scale bar: 200 μm. qRT-PCR assay was conducted to analyze the expression of CENPU in ovarian cancer tissues. E. Kaplan-Meier survival curves of ovarian cancer patients with different expression level of CENPU.
Overexpression of CENPU promotes ovarian cancer cell proliferation and motility
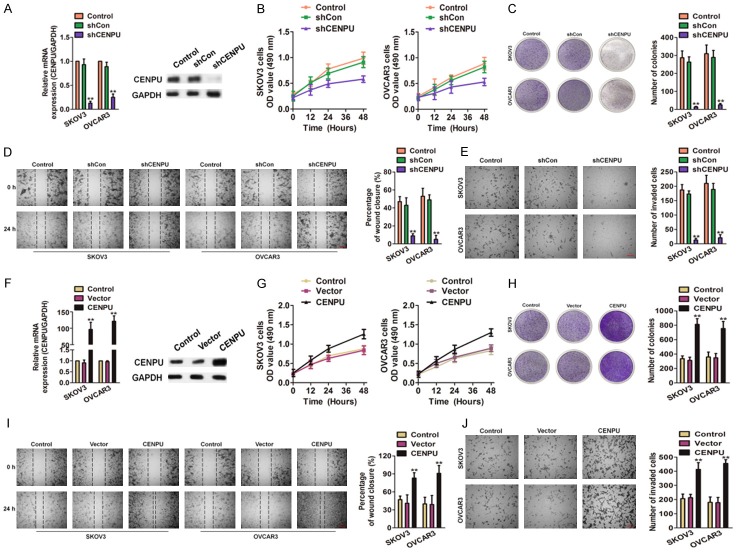
To investigate the functions of CENPU in ovarian cancer, the shRNA targeting CENPU (shCENPU) was applied to attenuate the CENPU expression in OVCAR3 and SKOV3 cells. The efficacy of transfection was confirmed by qRT-PCR and western blotting (Figure 2A), two cell lines were then subjected for MTT assay. As shown in Figure 2B, SKOV3 and OVCAR3 cell lines transfected with shCENPU exhibited remarkably the decreased growth compared with the control cells. In the colony formation assay, CENPU down-regulation SKOV3 and OVCAR3 cells exhibited significantly the decreased colonies formation as compared to cells transfected with shCon (Figure 2C). To explore the effects of CENPU on the mobility and invasion of ovarian cancer cells, both wound healing and Transwell analysis were conducted. The migratory (Figure 2D) and invasive (Figure 2E) abilities of down-regulation SKOV3 and OVCAR3 were remarkably inhibited compared with cells treated with shCon. To confirm the promoter role of CENPU in ovarian cancer, two cell lines were transfected with the CENPU to achieve CENPU overexpression (Figure 2F). Conversely, the over-expression of CENPU significantly accelerated the growth of SKOV3 and OVCAR3 cells compared with the control cells (Figure 2G). As consistent, the SKOV3 and OVCAR3 cells with CENPU over-expressed remarkably exhibited the elevated colonies growth compared with the control cells (Figure 2H). The mobility and invasion of SKOV3 and OVCAR3 cells with CENPU over-expressed markedly increased (Figure 2I, 2J). Altogether, these results suggested that CENPU could promote the proliferation, migration and invasion of ovarian cancer cells in vitro.
Figure 2.
The role of CENPU in ovarian cancer growth and mobility. A. Alteration of the CENPU expression in SKOV3 and OVCAR3 cells after transfection with shCENPU or shCon. B. MTT analysis showed that CENPU knocked-down significantly attenuate the growth of ovarian cancer cells in vitro. C. Effect of CENPU down-expression on the colonies formation of SKOV3 and OVCAR3 cells. D. Wound closure analysis was performed to reveal that the CENPU inhibition hampered the cells mobility. Scale bar: 200 μm. E. Representative pictures of Transwell assays of SKOV3 and OVCAR3 cells after transfected with shCENPU or shCon. Scale bar: 200 μm. F. SKOV3 and OVCAR3 cells were transfected with the CENPU or control vector and CENPU was determined by qRT-PCR and western blotting analysis. G. CENPU over-expression facilitated the growth of ovarian cancer cells as assessed by MTT. H. Effect of CENPU over-expression on the colonies formation of ovarian cancer cells in vitro. I. In SKOV3 and OVCAR3 cells, over-expression of CENPU increased cell motility in wound healing analysis. Scale bar: 200 μm. J. Representative pictures of Transwell assays of SKOV3 and OVCAR3 cells after transfected with CENPU or control vector. Scale bar: 200 μm. **P < 0.01 compared to control.
CENPU accelerates ovarian cancer cells growth and metastasis in vivo
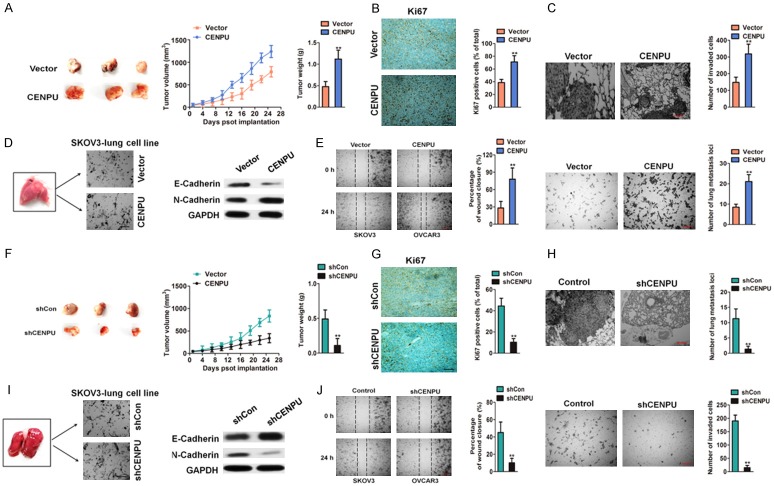
The xenograft model was utilized to identify the function of CENPU in ovarian cancer aggressiveness in vivo [23]. SKOV3 cells with CENPU over-expressed were subcutaneously inoculated into nude mice. Consistent with the results in vitro, the over-expression of CENPU in SKOV3 cells boosted tumor growth as compared to the control group (Figure 3A). Tumor mass were obtained and subjected to immumohistochemical staining. The tumors formed by SKOV3 cells with over-expressed CENPU exhibited higher staining intensity of Ki67 vs. the control group (Figure 3B). Then, it was investigated whether CENPU in cancer cells regulates metastatic implantation in vivo. The parental and CENPU over-expressed SKOV3 cells were injected into nude mice via the tail veins. The mice were dissected and the lungs were fixed and stained with H&E. Histology examination confirmed the higher frequency of lung metastases in CENPU over-expressed SKOV3 cells versus the control cells (Figure 3C). The epithelial-mesenchymal transition (EMT) was an essential process in cancer metastasis [24] and the overexpression of CENPU altered ovarian cancer cells metastasis, the expression of EMT-associated markers (E-Cadherin and N-Cadherin) in SKOV3 cells isolated from metastasis loci of lung were further examined. The expression of E-Cadherin significantly decreased whereas that of N-Cadherin was greatly enhanced in SKOV3-lung cell line compared with the corresponding observations in CENPU over-expressing SKOV3-lung cell line (Figure 3D). Meanwhile, the remarkably increased of the mobility and the invasiveness of the CENPU over-expressed SKOV3-lung cell line were observed compared with the control SKOV30 lung cell line (Figure 3E), which was similar to the increased motility according to the experiment in vitro. Conversely, the tumor mass formed in the SKOV3 cells with CENPU knocked-down were smaller and had significantly lower tumor weight than the control (Figure 3F). IHC analysis suggested that CENPU-silenced tumors displayed lower Ki67 proliferation index (Figure 3G). Meanwhile, the ability of SKOV3 cells metastasis in vivo was inhibited in the CENPU knocked-down cells (Figure 3H). Additionally, CENPU down-expressed SKOV3-lung cell line decreased the cell migration and invasive abilities (Figure 3J), accompany with the significantly decreased N-Cadherin whereas that of E-Cadherin was greatly increased (Figure 3I). Hence, all results indicated that CENPU promoted the growth and metastasis of ovarian cancer cells in vivo.
Figure 3.
Effect of CENPU on ovarian cancer growth and metastasis in vivo. A. Tumors were obtained four weeks after subcutaneous injection of CENPU over-expressing SKOV3 cells. Comparison of tumor volumes and weights between the CENPU over-expressing SKOV3 cells and control groups. B. The positive of Ki67 of tumor was determined in the experimental and control groups. Scale bar: 200 μm. C. Representative images of H&E-stained lungs with metastases. Scale bar: 200 μm. **P < 0.01 compared to vector. D. E-cadherin and N-cadherin were assessed by western blotting. E. Wound-closure analysis was performed to determine the effects of CENPU over-expressing SKOV3-lung cell line by calculating percentage of wound closure (left panel). CENPU over-expressing SKOV3-lung cell line was seeded onto Matrigel-coated Transwell chambers. After 24 h, the invaded cells were counted (right panel). Scale bar: 200 μm. **P < 0.01 compared to vector. F. Tumors were obtained after subcutaneous inoculation of shCENPU SKOV3 cells. Comparison of tumor volumes and weights between the shCENPU SKOV3 cells and control groups. G. The positive of Ki67 was evaluated in the experimental and control groups. H. Representative images of H&E-stained lungs with metastases. Scale bar: 200 μm. **P < 0.01 compared to shCon. I. E-cadherin and N-cadherin were determined by western blotting assay. J. Wound closure assay was performed to assess the effects of CENPU down-expressing SKOV3-lung cell line by calculating percentage of wound closure (left panel). CENPU down-expressing SKOV3-lung cell line was seeded onto Matrigel coated Transwell chambers. After 24 h, the invaded cells were counted (right panel). Scale bar: 200 μm. **P < 0.01 compared to shCon.
CENPU increases HMGB2 expression in ovarian cancer
The mechanisms underlying the potent effects of CENPU on ovarian cancer cells growth and metastasis were explored. Firstly, the CENPU expression in Oncomine database were analyzed and it was found that the co-expression of 114 genes was positively correlated with CENPU (Correlation > 0.5) (Supplementary Figure 1). qRT-PCR assay was applied to verify the top 30 co-expressed genes which were closely correlated with CENPU, and it was found that the expression of 6 gene among these markedly decreased in CENPU knocked-down cells compared with the parental cells (Fold change > 5). Notably, HMGB2 was highly co-expressed with CENPU and significantly decreased after cells transfected with shCENPU (Figure 4A). Considering the role of HMGB2 in the proliferation and metastasis of tumor cells, it was assumed that the growth and metastasis of CENPU which promoted the ovarian cancer cells might through the way of increasing HMGB2. Consistent with qRT-PCR results, CENPU knocked-down decreased HMGB2 levels in the SKOV3 and OVCAR3 cell lines (Figure 4B) and the overexpression of CENPU increased the expression of HMGB2 (Figure 4C). Moreover, it was discovered that the expression of HMGB2 was inhibited in the tumor tissues from xenografts of CENPU knocked-down SKOV3 cells (Figure 4D). In addition, HMGB2 levels were higher in ovarian cancer cells compared with IOSE80 cells (Figure 4E). The mRNA level of HMGB2 in the same set of 46 pairs of ovarian cancer tissues was also measured and it was found that HMGB2 was significantly higher in ovarian cancer than in the corresponding normal tissues (Figure 4F). Importantly, the HMGB2 level was correlated with the CENPU level in ovarian cancer tissues (Figure 4G). Kaplan-Meier survival analysis (http://kmplot.com/analysis) future indicated that the high level of HMGB2 was associated with poor overall survival (OS) (Figure 4H). These data suggested that CENPU could increase the expression of HMGB2 in ovarian cancer.
Figure 4.
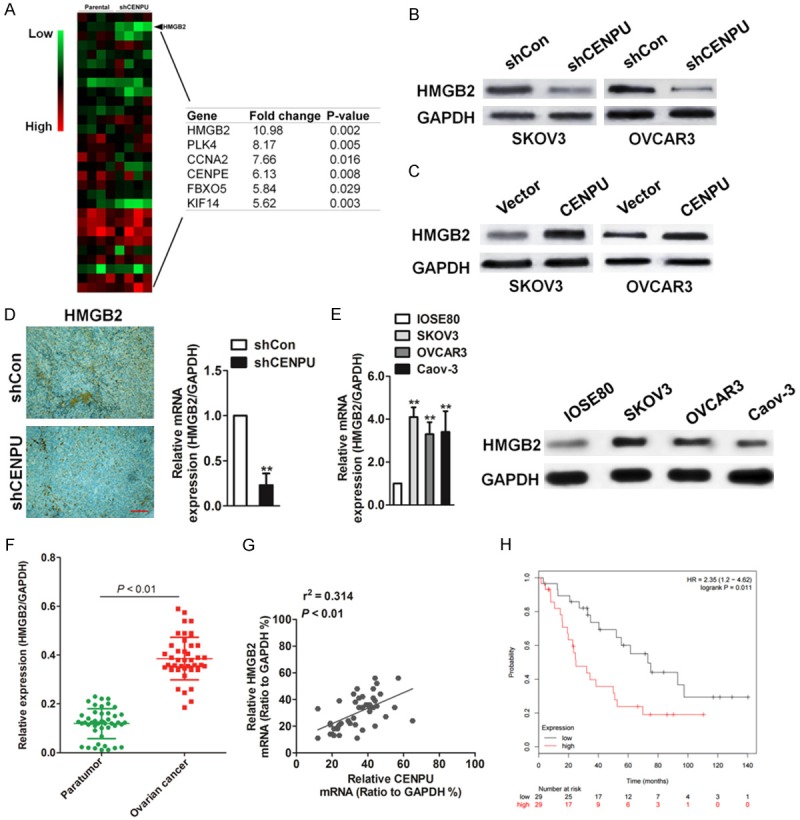
CENPU increases HMGB2 expression in ovarian cancer. A. qRT-PCR analysis of genes in parental SKOV3 cells and the CENPU knock-down SKOV3 cells. B. Western blot analysis of HMGB2 protein levels in CENPU knockdown SKOV3 and OVCAR3 cell lines. C. The expression of HMGB2 CENPU overexpression SKOV3 and OVCAR3 cells were determined by western blot analysis. D. Immunohistochemistry of HMGB2 in tumors from shCon cells or shCENPU cells. Scale bar: 200 μm. **P < 0.01 compared to shCon. E. The expression pattern of HMGB2 in ovarian cancer cell lines was assessed by qRT-PCR and western blot. **P < 0.01 compared to IOSE80. F. mRNA level of HMGB2 in 46 paired ovarian cancer and adjacent non-tumor tissues as analyzed by qRT-PCR. G. The correlation between the CENPU and HMGB2 level was measured in the same set of ovarian cancer tissues. H. Kaplan-Meier survival curves of overall survival (OS) in patients.
HMGB2 acts as a tumor promoter in ovarian cancer
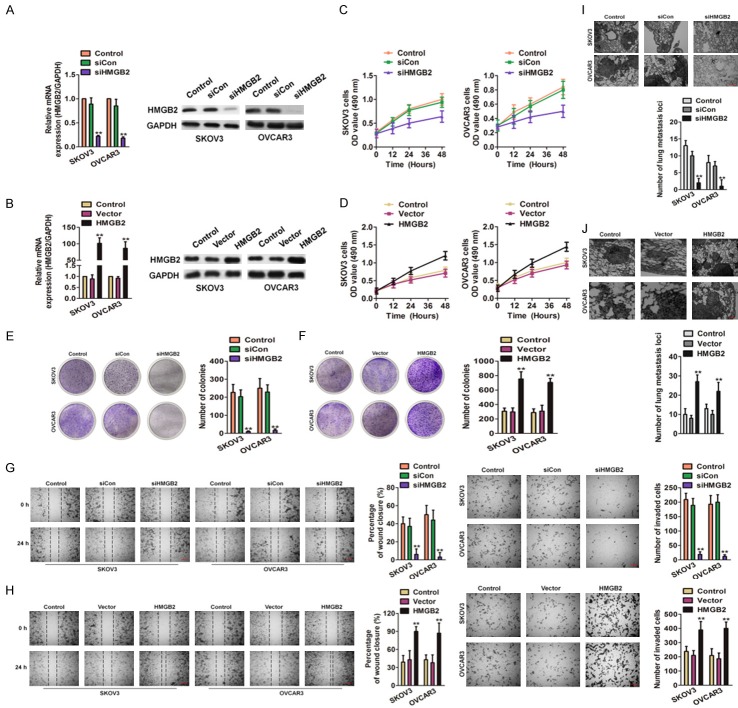
In order to future reveal the precise role of HMGB2 in ovarian cancer, the small interfering RNA (siRNA) to knocked-down HMGB2 (siHMGB2) and lentiviral particles to over-express HMGB2 in ovarian cancer cells was applied. qRT-PCR and immunoblotting assays were performed to assess the level of HMGB2 after siHMGB2 transfection and lentiviral infection (Figure 5A and 5B). After transfected with siHMGB2, the growth of SKOV3 and OVCAR3 decreased markedly while compared with cells transfected with siCon (Figure 5C). The ovarian cancer cells that infected with lentiviral-HMGB2 exhibited boosted proliferation compared with the control group (Figure 5D). In colonies formation assay, HMGB2 knocked-down SKOV3 and OVCAR3 cells had remarkably decreased colony growth compared with the control group (Figure 5E), and HMGB2 over-expressed cells obviously accelerated colonies formation (Figure 5F). In the wound closure and Transwell invasion analysis, SKOV3 and OVCAR3 cells bearing siHMGB2 showed obviously decreased mobility and invasion as compared to the control group (Figure 5G). Conversely, the ability of ovarian cancer cells mobility and invasive increased in cells transfected with the HMGB2-packed lentivirus (Figure 5H). Finally, the HMGB2 down-expressing or ectopic expressions of HMGB2 cells were injected into the nude mice via tail veins. Histology examination showed higher frequency of lung metastases in HMGB2 over-expressed cells versus the control group (Figure 5I) whereas siHMGB2 ovarian cancer cells exhibited few metastasis loci vs. the control group (Figure 5J). These finding suggested that down-regulation of HMGB2 impaired the growth and metastasis of ovarian cancer cells.
Figure 5.
Effects of HMGB2 on ovarian cancer cells growth and metastasis. A. Alteration of the HMGB2 expression levels of SKOV3 and OVCAR3 cells after transfection with the siHMGB2 or siCon. **P < 0.01 compared to control. B. SKOV3 and OVCAR3 cells were transfected with vector containing HMGB2 or control vector and the expression of HMGB2 was determined by qRT-PCR and immunoblotting. **P < 0.01 compared to control. C. MTT assay showed that transfection of the siHMGB2 attenuate the proliferation of ovarian cancer cells. D. The cells viability of HMGB2 over-expressing SKOV3 and OVCAR3 were assessed by MTT. E. Effect of HMGB2 down-expression on the colony growth of SKOV3 and OVCAR3 cells. **P < 0.01 compared to control. F. Effect of HMGB2 over-expression on the colony formation of ovarian cancer cells in vitro. **P < 0.01 compared to control. G. Wound closure assay was subjected to reveal that the CENPU inhibition hampered the cells mobility (left panel). Representative pictures of Transwell assays of SKOV3 and OVCAR3 cells after transfected with shCENPU or shCon (right panel). Scale bar: 200 μm. **P < 0.01 compared to control. H. In SKOV3 and OVCAR3 cells, over-expression of HMGB2 increased cell motility in wound healing analysis (left panel). Representative pictures of Transwell assays of SKOV3 and OVCAR3 cells after transfected with HMGB2 or control vector (right panel). Scale bar: 200 μm. **P < 0.01 compared to control. I. Representative images of H&E-stained lungs with metastases. Scale bar: 200 μm. **P < 0.01 compared to control. J. Representative images of H&E-stained lungs with metastases. Scale bar: 200 μm. **P < 0.01 compared to control.
CENPU promotes tumor growth and aggressive behavior dependent on HMGB2
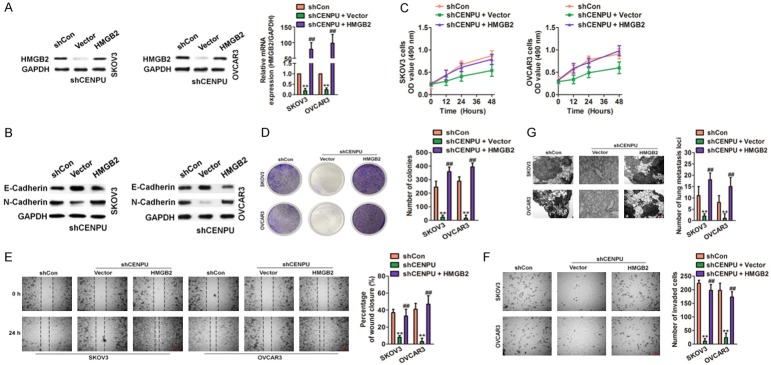
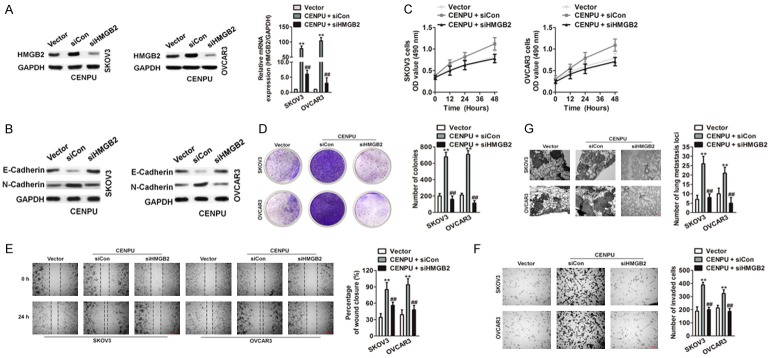
It was examined whether HMGB2 over-expression rescued the inhibition of CENPU on ovarian cancer cell growth and metastasis. Lentiviral HMGB2 and control vector were introduced to ovarian cancer cells that previously transfected with shCENPU and shCon. qRT-PCR and immunoblotting analysis confirmed that the HMGB2 overexpression markedly increased HMGB2 expression (Figure 6A). Moreover, the EMT-associated markers (E-Cadherin and N-Cadherin) were determined (Figure 6B). Cellular proliferation and colony formation were impaired in shCENPU ovarian cancer cells whereas the overexpression of HMGB2 rescued the inhibition effect of growth induced by shCENPU (Figure 6C and 6D). Mobility and metastasis abilities in vitro and in vivo were impaired in shCENPU SKOV3 and OVCAR3 cells whereas ectopic expression of HMGB2 rescued the inhibition effect of mobility induced by shCENPU (Figure 6E-G). In addition, co-transfecting HMGB2 siRNA and the vector containing CENPU into SKOV3 and OVCAR3 cells (Figure 7A) were subject to the growth and metastasis assays. Furthermore, the expression of E-Cadherin and N-Cadherin was evaluated (Figure 7B). The growth (Figure 7C and 7D) and metastasis (Figure 7E-G) of SKOV3 and OVCAR3 cells induced by the overexpressed CENPU were reversed by HMGB2 down-regulation. Taken together, these data clearly revealed that CENPU was in regulation with the proliferation of ovarian cancer cells and behaved aggressive behavior which was dependent on HMGB2.
Figure 6.
CENPU promotes the growth and metastasis of ovarian cancer dependent on HMGB2. A. SKOV3 and OVCAR3 cells were co-transfected with the shCENPU and the HMGB2 plasmid. The levels of HMGB2 in both cells were analyzed by qRT-PCR and immunoblotting. B. After co-transfection of cells with shCENPU and HMGB2, the levels of EMT-related proteins were measured by western blot. C. Cells were co-transfected with the shCENPU and the HMGB2 plasmid. Cells proliferation was determined by MTT assays. D. HMGB2 overexpression counteracted the inhibition effects of colony formation in CENPU down-expression ovarian cancer cells. E. Cells were co-transfected with lentiviral particles containing HMGB2 and shRNA targeting CENPU. Cell migration in SKOV3 and OVCAR3 cells were analyzed by wound healing assays. F. Over-expression of HMGB2 may reverse the effect of the CENPU down-regulation on invasion in ovarian cancer cells. G. Indicated cells were injected into nude mice via tail vein. H&E staining of lung nodules formed after injection of cells carrying shCENPU and vector containing HMGB2. Scale bar: 200 μm. **P < 0.01 compared to shCon, ##P < 0.01 compared to shCENPU + vector.
Figure 7.
Knock down of HMGB2 inhibits the growth and metastasis of ovarian cancer cells accelerated by HMGB2. A. CENPU over-expressing SKOV3 and OVCAR3 cells were transfected with siHMGB2. The mRNA levels of HMGB2 were analyzed by qRT-PCR and western blotting assays. B. After co-transfection of cells with siHMGB2 and CENPU, the levels of EMT-related proteins were measured by western blotting assay. C. HMGB2 down-expression counteracted the positive proliferative effects of CENPU over-expression in SKOV3 and OVCAR3 cells. D. HMGB2 inhibition hampered the colony formation of CENPU over-expression in ovarian cancer cells. E. CENPU over-expression SKOV3 or OVCAR3 cells were transfected with siHMGB2 and cell migration of ovarian cancer cells were analyzed by wound closure assay. F. Cell invasion in indicated SKOV3 or OVCAR3 cells were analyzed by Transwell assays. G. CENPU over-expressing cells were transfected with siHMGB2. Cells were injected into nude mice via tail vein. H&E staining of lung nodules. Scale bar: 200 μm. **P < 0.01 compared to vector, ##P < 0.01 compared to CENPU + siCon group.
Discussion
The issues of tumor relapse, chemotherapy resistance, and metastasis are still challenges in the clinical treatment of ovarian cancer [25,26]. Therefore, the identify molecular drivers of ovarian cancer metastasis is crucial for the development of novel therapeutic target ovarian cancer [27]. CENPU gene (also known as KLIP1 and MLF1IP) encodes a nuclear-localizing transcription suppressor protein, which is closely associated with malignancy [28,29]. In addition, CENPU is needed for proper chromosome segregation, stable kinetochore-microtubule attachment and recovery from spindle damage during mitosis. CENPU was firstly identified in 2004 by Hanissian et al, who implied the possible roles of CENPU in the pathogenesis of erythroleukemias [8]. Subsequently, the same team demonstrated that the up-regulation of CENPU is associated with glioblastoma tumor development in both rodents and humans [9]. More recently, CENPU up-regulation has been found in patients with familial colorectal cancer (Lynch syndrome) and breast cancer [30]. Nevertheless, there was no obvious alteration of CENPU observed in ovarian cancer. Our studies in vitro suggested a variable expression of CENPU in ovarian cancer cells compared with normal cells as determined by qPCR and western blot. Consistently, the evidence of over-expressed CENPU was found in ovarian cancer tissues and was associated with poor over survival of patients with ovarian cancer. Subsequently, it was found that the proliferation and colony formation of ovarian cancer cells were significantly reduced in the shCENPU-transfected cells. Additionally, cells transfected with shRNA targeting CENPU also exhibited the impaired capacities of migration and invasion in vitro. As expected, the tumor growth and metastasis significantly decreased in the CENPU-silenced ovarian cancer cells. The EMT is acknowledged to play critical roles in ovarian cancer metastasis. The current research revealed that the expression of E-Cadherin and N-Cadherin remarkably changed upon CENPU treatment. Taken together, CENPU knocked-down in ovarian cancer cells deregulated cell growth, colony formation, migration and metastasis whereas the up-expression of CENPU caused the increase of the growth and metastasis of ovarian cancer cells.
The CENPU co-expression genes were predicted to investigate the mechanism behind the function of CENPU in ovarian cancer. To explore the mechanism by which CENPU contributes to ovarian cancer proliferation and invasion, TCGA database was analyzed and it was found that the correlation coefficient of CENPU and HMGB2 co-expression was obvious (correlation = 0.614). This study confirmed a positive correlation between the levels of CENPU and HMGB2 in ovarian cancer (r2 = 0.314, P < 0.01). High mobility group box (HMGB) is an architectural factor which constitutes the critical hubs in chromatin network. HMGB family members (HMGB1, HMGB2, HMGB3, and HMGB4) play crucial roles in stem cell self-renewal, differentiation and proliferation [31]. In normal cells, HMGB is restricted to embryogenesis and is almost absent in normal adult cells [32]. Nevertheless, HMGB is at high level in transformed cells and represents a general feature of human malignancies. Several studies demonstrate that HMGB2 is elevated in a variety of cancers, including prostate cancer, thyroid carcinomas, breast cancer and colon cancer.
In the current studies, the over-expression of HMGB2 was closely associated with metastasis and the poor prognosis in ovarian cancer, however the down-regulation of HMGB1 inhibited the bioactivity of ovarian cancer cells. The down-expression of HMGB2 in ovarian cancer cells restrained cell growth and inhibited ovarian cancer cell mobility and invasion, similar to the phenotypic alterations observed upon CENPU knocked-down cells whereas the ectopic expression of HMGB2 resulted in completely opposite outcomes. Up-regulation of HMGB2 in ovarian cancer cells rescued the inhibition effects of knocked-down CENPU on the growth and metastasis of ovarian cancer cell. By contrast, down-regulation HMGB2 attenuated the promoted effects of over-expressed CENPU on ovarian cancer cells proliferation, migration and metastasis. In summary, it was demonstrated that CENPU was remarkably up-regulated in ovarian cancer cells and clinical ovarian cancer samples and the positive correlation between CENPU and the prognosis of patients with ovarian cancer. Over-expression of CENPU augmented ovarian cancer aggressiveness and this effect required HMGB2. Hence, the function of CENPU in ovarian cancer advances the knowledge of mechanisms underlying ovarian cancer metastasis, and establishes CENPU as a novel therapeutic target for the treatment of ovarian cancer.
Disclosure of conflict of interest
None.
Supporting Information
References
- 1.Segev Y, Segev L, Schmidt M, Auslender R, Lavie O. Palliative care in ovarian carcinoma patients-a personalized approach of a team work: a review. Arch Gynecol Obstet. 2017;296:691–700. doi: 10.1007/s00404-017-4484-8. [DOI] [PubMed] [Google Scholar]
- 2.Nasioudis D, Chapman-Davis E, Frey MK, Caputo TA, Witkin SS, Holcomb K. Should epithelial ovarian carcinoma metastatic to the inguinal lymph nodes be assigned stage IVB? Gynecol Oncol. 2017;147:81–84. doi: 10.1016/j.ygyno.2017.07.124. [DOI] [PubMed] [Google Scholar]
- 3.Tian J, Liu R, Qu Q. Role of endoplasmic reticulum stress on cisplatin resistance in ovarian carcinoma. Oncol Lett. 2017;13:1437–1443. doi: 10.3892/ol.2017.5580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ju B, Wang J, Yang B, Sun L, Guo Y, Hao Q, Wu J. Morphologic and immunohistochemical study of clear cell carcinoma of the uterine endometrium and cervix in comparison to ovarian clear cell carcinoma. Int J Gynecol Pathol. 2017 doi: 10.1097/PGP.0000000000000430. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 5.Li Y, Lu S, Xu Y, Qiu C, Jin C, Wang Y, Liu Z, Kong B. Overexpression of CD47 predicts poor prognosis and promotes cancer cell invasion in high-grade serous ovarian carcinoma. Am J Transl Res. 2017;9:2901–2910. [PMC free article] [PubMed] [Google Scholar]
- 6.Zhu Q, Qu Y, Zhang Q, Lu L, Weng W, Zhang H, Zhang L, Ning Y, Wang Y. IMP3 is upregulated in primary ovarian mucinous carcinoma and promotes tumor progression. Am J Transl Res. 2017;9:3387–3398. [PMC free article] [PubMed] [Google Scholar]
- 7.Feng G, Zhang T, Liu J, Ma X, Li B, Yang L, Zhang Y, Xu Z, Qin T, Zhou J, Huang G, Shi L, Xiao Z. MLF1IP promotes normal erythroid proliferation and is involved in the pathogenesis of polycythemia vera. FEBS Lett. 2017;591:760–773. doi: 10.1002/1873-3468.12587. [DOI] [PubMed] [Google Scholar]
- 8.Hanissian SH, Akbar U, Teng B, Janjetovic Z, Hoffmann A, Hitzler JK, Iscove N, Hamre K, Du X, Tong Y, Mukatira S, Robertson JH, Morris SW. cDNA cloning and characterization of a novel gene encoding the MLF1-interacting protein MLF1IP. Oncogene. 2004;23:3700–3707. doi: 10.1038/sj.onc.1207448. [DOI] [PubMed] [Google Scholar]
- 9.Hanissian SH, Teng B, Akbar U, Janjetovic Z, Zhou Q, Duntsch C, Robertson JH. Regulation of myeloid leukemia factor-1 interacting protein (MLF1IP) expression in glioblastoma. Brain Res. 2005;1047:56–64. doi: 10.1016/j.brainres.2005.04.017. [DOI] [PubMed] [Google Scholar]
- 10.Zhang L, Ji G, Shao Y, Qiao S, Jing Y, Qin R, Sun H, Shao C. MLF1 interacting protein: a potential gene therapy target for human prostate cancer? Med Oncol. 2015;32:454. doi: 10.1007/s12032-014-0454-1. [DOI] [PubMed] [Google Scholar]
- 11.Wang Y, Li XP, Yin JY, Zhang Y, He H, Qian CY, Chen J, Zheng Y, Smieszkol K, Fu YL, Chen ZY, Zhou HH, Liu ZQ. Association of HMGB1 and HMGB2 genetic polymorphisms with lung cancer chemotherapy response. Clin Exp Pharmacol Physiol. 2014;41:408–415. doi: 10.1111/1440-1681.12232. [DOI] [PubMed] [Google Scholar]
- 12.Shin YJ, Kim MS, Kim MS, Lee J, Kang M, Jeong JH. High-mobility group box 2 (HMGB2) modulates radioresponse and is downregulated by p53 in colorectal cancer cell. Cancer Biol Ther. 2013;14:213–221. doi: 10.4161/cbt.23292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Redmond AM, Byrne C, Bane FT, Brown GD, Tibbitts P, O’Brien K, Hill AD, Carroll JS, Young LS. Genomic interaction between ER and HMGB2 identifies DDX18 as a novel driver of endocrine resistance in breast cancer cells. Oncogene. 2015;34:3871–3880. doi: 10.1038/onc.2014.323. [DOI] [PubMed] [Google Scholar]
- 14.An Y, Zhang Z, Shang Y, Jiang X, Dong J, Yu P, Nie Y, Zhao Q. miR-23b-3p regulates the chemoresistance of gastric cancer cells by targeting ATG12 and HMGB2. Cell Death Dis. 2015;6:e1766. doi: 10.1038/cddis.2015.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li J, Gao J, Tian W, Li Y, Zhang J. Long noncoding RNA MALAT1 drives gastric cancer progression by regulating HMGB2 modulating the miR-1297. Cancer Cell Int. 2017;17:44. doi: 10.1186/s12935-017-0408-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cai X, Ding H, Liu Y, Pan G, Li Q, Yang Z, Liu W. Expression of HMGB2 indicates worse survival of patients and is required for the maintenance of Warburg effect in pancreatic cancer. Acta Biochim Biophys Sin (Shanghai) 2017;49:119–127. doi: 10.1093/abbs/gmw124. [DOI] [PubMed] [Google Scholar]
- 17.Pass HI, Lavilla C, Canino C, Goparaju C, Preiss J, Noreen S, Blandino G, Cioce M. Inhibition of the colony-stimulating-factor-1 receptor affects the resistance of lung cancer cells to cisplatin. Oncotarget. 2016;7:56408–56421. doi: 10.18632/oncotarget.10895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li Y, Sun Z, Liu B, Shan Y, Zhao L, Jia L. Tumorsuppressive miR-26a and miR-26b inhibit cell aggressiveness by regulating FUT4 in colorectal cancer. Cell Death Dis. 2017;8:e2892. doi: 10.1038/cddis.2017.281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tsuboki J, Fujiwara Y, Horlad H, Shiraishi D, Nohara T, Tayama S, Motohara T, Saito Y, Ikeda T, Takaishi K, Tashiro H, Yonemoto Y, Katabuchi H, Takeya M, Komohara Y. Onionin a inhibits ovarian cancer progression by suppressing cancer cell proliferation and the protumour function of macrophages. Sci Rep. 2016;6:29588. doi: 10.1038/srep29588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Matsumoto R, Tsuda M, Yoshida K, Tanino M, Kimura T, Nishihara H, Abe T, Shinohara N, Nonomura K, Tanaka S. Aldo-keto reductase 1C1 induced by interleukin-1beta mediates the invasive potential and drug resistance of metastatic bladder cancer cells. Sci Rep. 2016;6:34625. doi: 10.1038/srep34625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yoshihara K, Tajima A, Komata D, Yamamoto T, Kodama S, Fujiwara H, Suzuki M, Onishi Y, Hatae M, Sueyoshi K, Fujiwara H, Kudo Y, Inoue I, Tanaka K. Gene expression profiling of advanced-stage serous ovarian cancers distinguishes novel subclasses and implicates ZEB2 in tumor progression and prognosis. Cancer Sci. 2009;100:1421–1428. doi: 10.1111/j.1349-7006.2009.01204.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bonome T, Levine DA, Shih J, Randonovich M, Pise-Masison CA, Bogomolniy F, Ozbun L, Brady J, Barrett JC, Boyd J, Birrer MJ. A gene signature predicting for survival in suboptimally debulked patients with ovarian cancer. Cancer Res. 2008;68:5478–5486. doi: 10.1158/0008-5472.CAN-07-6595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kuang Y, Lu F, Guo J, Xu H, Wang Q, Xu C, Zeng L, Yi S. Histone demethylase KDM2B upregulates histone methyltransferase EZH2 expression and contributes to the progression of ovarian cancer in vitro and in vivo. Onco Targets Ther. 2017;10:3131–3144. doi: 10.2147/OTT.S134784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhu X, Wei L, Bai Y, Wu S, Han S. FoxC1 promotes epithelial-mesenchymal transition through PBX1 dependent transactivation of ZEB2 in esophageal cancer. Am J Cancer Res. 2017;7:1642–1653. [PMC free article] [PubMed] [Google Scholar]
- 25.Ge L, Li N, Liu M, Xu NZ, Wang MR, Wu LY. Copy number variations of neurotrophic tyrosine receptor kinase 3 (NTRK3) may predict prognosis of ovarian cancer. Medicine (Baltimore) 2017;96:e7621. doi: 10.1097/MD.0000000000007621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sakr S, Abdulfatah E, Thomas S, Al-Wahab Z, Beydoun R, Morris R, Ali-Fehmi R, Bandyopadhyay S. Granulosa cell tumors: novel predictors of recurrence in early-stage patients. Int J Gynecol Pathol. 2017;36:240–252. doi: 10.1097/PGP.0000000000000325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cheng WT, Rosario R, Muthukaruppan A, Wilson MK, Payne K, Fong PC, Shelling AN, Blenkiron C. MicroRNA profiling of ovarian granulosa cell tumours reveals novel diagnostic and prognostic markers. Clin Epigenetics. 2017;9:72. doi: 10.1186/s13148-017-0372-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hua S, Wang Z, Jiang K, Huang Y, Ward T, Zhao L, Dou Z, Yao X. CENP-U cooperates with Hec1 to orchestrate kinetochore-microtubule attachment. J Biol Chem. 2011;286:1627–1638. doi: 10.1074/jbc.M110.174946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Park CH, Park JE, Kim TS, Kang YH, Soung NK, Zhou M, Kim NH, Bang JK, Lee KS. Mammalian Polo-like kinase 1 (Plk1) promotes proper chromosome segregation by phosphorylating and delocalizing the PBIP1. CENP-Q complex from kinetochores. J Biol Chem. 2015;290:8569–8581. doi: 10.1074/jbc.M114.623546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Huang DP, Luo RC. MLF1IP is correlated with progression and prognosis in luminal breast cancer. Biochem Biophys Res Commun. 2016;477:923–926. doi: 10.1016/j.bbrc.2016.06.159. [DOI] [PubMed] [Google Scholar]
- 31.Zhang Z, Chang Y, Zhang J, Lu Y, Zheng L, Hu Y, Zhang F, Li X, Zhang W, Li X. HMGB3 promotes growth and migration in colorectal cancer by regulating WNT/beta-catenin pathway. PLoS One. 2017;12:e0179741. doi: 10.1371/journal.pone.0179741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pan C, Wang Y, Qiu MK, Wang SQ, Liu YB, Quan ZW, Ou JM. Knockdown of HMGB1 inhibits cell proliferation and induces apoptosis in hemangioma via downregulation of AKT pathway. J Biol Regul Homeost Agents. 2017;31:41–49. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.