Abstract
The tumor microenvironment (TME) is a key factor regulating tumor cell invasion and metastasis. The effects of biochemical factors such as stromal cells, immune cells, and cytokines have been previously investigated. Owing to restrictions by the natural barrier between physical and biochemical disciplines, the role of physical factors in tumorigenesis is unclear. However, with the emergence of interdisciplinary mechanobiology and continuous advancements therein in the past 30 years, studies on the effect of physical properties such as hardness or shear stress on tumorigenesis and tumor progression are constantly renewing our understanding of mechanotransduction mechanisms. Shear stress, induced by liquid flow, is known to actively participate in proliferation, apoptosis, invasion, and metastasis of tumor cells. The present review discusses the progress and achievements in studies on tumor fluid microenvironment in recent years, especially fluid shear stress, on tumor metastasis, and presents directions for future study.
Keywords: Fluid shear stress, metastatic cascade, tumor microenvironment, mechanotransduction, circulating tumor cells
Introduction
Metastasis is a complex dynamic cascade, accounting for approximately 90% of tumor-related mortalities [1]. Previous studies have focused on the effects of biochemical factors, such as stromal cells, immune cells, and cytokines, on tumor metastasis. However, during metastasis, tumor cells also interact with various biochemical and biophysical factors in the tumor microenvironment (TME). Therefore, it is essential to elucidate the dynamic response of tumor cells to different physical and chemical factors in the TME.
In 2015, a landmark study reported that long-term 1-kPa magnetic load in intestinal crypts can upregulate the oncogene c-Myc and lead to carcinogenesis, which indicates that a simple physical process can induce tumorigenesis [2]. Moreover, the biophysical characteristics of tumor cells have been gradually unveiled with developments in biomechanics for approximately 60 years [3]. Biomechanics refers to the study of the deformation and movement of living bodies and validates the laws of mechanics in life. In the 1990s, with the emerging mechanics tools such as atomic force microscopy and Förster resonance energy transfer (FRET), physicists shifted their focus on biomechanics from the tissue level to the cellular or gene level, and subsequently gradually shifted from biomechanics to mechanobiology. During this shift, a series of mechanosensitive molecules such as Cav-1, BMP, IGF-2, VEGF [4,5], and nuclear transcription factors YAP/YAZ [6], c-Myc [2], and Atoh8 [7] were identified, which play an important role in tumorigenesis or tumor progression [8,9]. This aspect triggered a widespread concern among oncologists, especially in the past 4 years, and numerous studies then focused on the mechanobiological mechanism of tumorigenesis and metastasis.
As a classic mechanical feature, matrix hardness has been considered a peculiar mechanical feature in predicting tumor metastasis and prognosis [10,11]. Wei et al. confirmed that matrix hardness can activate the TWIST1-G3BP2 pathway to promote tumor cell invasion and metastasis [12]. Nonetheless, the flow of biological fluids is a vital physical property of the TME; however, owing to continuous changes in the parameters including flow diameter and fluid velocity in vivo, in vitro modeling of the tumor fluid microenvironment has been faced with numerous technical challenges. In recent years, with the application of microfluidic technology and mechanical measurement methods in studies on cancer, developments in tumor fluid mechanics accelerated. Increasing evidence now indicates that fluid shear stress (FSS) is an essential factor affecting fluid mechanics, and its role in metastasis has received increasing attention.
FSS is defined as the internal frictional force between moving layers in laminar flow. Additionally, FSS, the product of fluid viscosity and shear rate, is an important parameter of cellular stress in flowing liquid, measured in Newtons per square meter (N/m2) or dynes per square centimeter (dyn/cm2) [13]. FSS is a key regulator of vascular endothelial phenotypes and to induce polarity in endothelial cell [14], cytoskeletal rearrangement [14], and post-translational modifications (e.g., phosphorylation, etc.) and gene expression [15]. Liquid laminar flow is prevalent in biological systems and is usually categorized as blood, lymphoid, and interstitial flow. Tumor cells primarily encounter interstitial shear stress and blood shear stress during metastasis to the target organs. The former plays a role in promoting tumor metastasis, lymphatic drainage, and anti-cancer drug delivery [16]. Current evidence suggests that on tumorigenesis, blood shear stress has dual effects. It could promote tumor invasion and metastasis, adhesion, and extravasation under certain circumstances while [17] conversely, mechanically eliminating circulating tumor cells (CTCs) [18], and they promote cell cycle arrest in tumor cells [19]. The development of related technology, four types of tumor-related fluid microenvironments and the mechanism of FSS in various stages of the tumor metastasis cascade are summarized herein to provide a reference for subsequent studies on tumor fluid mechanics.
Technological advancements in microfluidics
In the past few decades, the need to explore the biological significance of mechanical force has led to the development of several innovative approaches. Furthermore, the emergence of pN-level mechanical measurement and visualization tools such as biofilm probes, traction force microscopy, and atomic force microscopy have shifted the focus from traditional biomechanics to mechanotransduction at the cellular and subcellular level [20], and the use of microfluidic chips and 4-dimensional flow magnetic resonance imaging to model in vitro and in vivo mechanical microenvironments has received increasing attention [21,22]. The following sections focus on the advancements in fluid mechanic tools and their applications in studies on cancer (Table 1). These novel methods have enhanced the general understanding of the correlation between tumor metastasis and fluid shear stress.
Table 1.
Tools for the study of fluid mechanics of cancer
| Tool or technique | Application | Refs | |
|---|---|---|---|
| Mechanical measurement | Microfluidic traction force microscopy | Observe the traction and shear deformation of cells under fluid environment; | [23,24] |
| To study mechanotransduction in angiogenesis and the initial growth of tumors; | |||
| Intracellular tension sensors/FRET | Realize the visualization of intracellular forces; | [25] | |
| To detect the location and interactions of cellular structures (including intergrins and membrane proteins); | |||
| Confocal microscopy or optical coherence tomography | Investigate the effect of shear stress on cell-cell interactions and mechanotransduction mechanism; | [22,26,27] | |
| To measure biomechanical properties of developing, engineered, and natural tissues and to understand the role of mechanical stimuli such as shear stress; | |||
| 4-dimensional flow magnetic resonance imaging | Analyze the flow and wall shear stress; | [21,28] | |
| To monitor the chemoembolization of hepatocellular carcinoma; | |||
| Mechanical simulation | Parallel plate flow chamber | Mimic the fluid environment of cancer cell growth; | [29,30] |
| To investigate the effect of shear stress on cell-cell interactions and mechanotransduction mechanism; | |||
| Bionic chips or microfluidic platforms | Model and study the cell-cell interactions and mechanotransduction mechanism under shear stress; | [31-33] | |
| To detect cancer biomarkers and to isolate characteristic cancer cells; | |||
| Computational fluid dynamics modeling | Simulate drug distribution in a single tumor nodule or tumor-induced angiogenesis, etc.; | [34-36] | |
| To observe various cell behaviors on micro-rheology of cancer cells in 3D environments; | |||
Pioneering advancements have been made in fluid mechanics. However, it is important to investigate the biological mechanisms involved in fluid mechanics. At present, the process of integrating mechanical sensing and mechanical simulation, or in other words, during dynamic hydrodynamic sensing, simulating positive and negative feedback regulation mechanisms and adjusting the parameters of the microfluidic model in real time to restore the complexity of fluid mechanics may be the direction for future studies. In addition, the need to establish a reliable in vivo model of fluid dynamics is still urgent for the development of mechanical technology.
Tumor metastasis-related fluid microenvironment
Tumor growth and metastasis are influenced by changes in the fluid microenvironment, such as interstitial flow, lymph flow, blood flow, and other organ-specific components.
Interstitial flow
The gradual flow of fluid in tumor tissues is known as interstitial flow. In a physiological state, most of the fluid that leaks out of capillaries is directed back to the capillaries, and only a fraction of fluid that passes through tumor tissues is recycled by the lymphatic vessels. The aforementioned process completes the exchange of material between the capillaries and the surrounding tissues and prevents the accumulation of fluid in interstitial spaces. In tumor tissues, however, it was reported that owing to the increased flow rate and high vascular permeability [15], interstitial pressure increased and therefore interstitial shear stress approached approximately 0.1 dyn/cm2 [13,37] (Figure 1). Under continuous flow of interstitial fluid in an in vitro 3D culture, the migration rate of breast cancer cells tended to increase [38]. Munson et al. reported a similar result in glioma cells [39]. Apart from promoting tumor invasion and metastasis, higher interstitial flow rate is also an independent predictor of poor prognosis in cancer patients [40].
Figure 1.
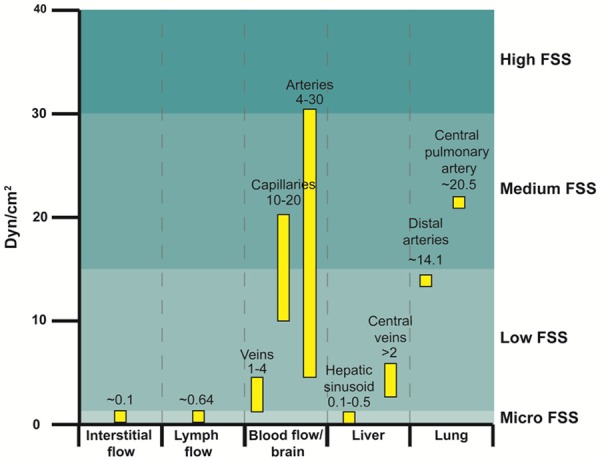
Shear stress levels are variable in tumor metastasis-related fluid microenvironment. Blood shear stress levels are higher than interstitial flow and lymph flow. Additionally, the FSS in hepatic sinusoid and central veins is 0.1-0.5 dyn/cm2 and over 2 dyn/cm2, respectively. The FSS in the central pulmonary artery and distal arteries are higher, approximately 20.5 ± 4.0 and 14.1 ± 0.7 dyn/cm2 in that order.
Blood flow
When primary tumor cells intravasate into blood vessels, they become CTCs and are then widely disseminated through circulation. Many clinical studies have suggested that CTCs are responsible for most postoperative recurrences and distant metastasis in patients with malignant tumors [41]. Moreover, metastatic colonization of CTCs is much less efficient. Although millions of tumor cells differentiate into CTCs per day, only 0.02% survive to successfully undergo metastasis [42]. Apart from anoikis and killing by natural killer cells, the mechanical damage from FSS is the main cause of death of CTCs. The mean FSS in veins, capillaries, and arteries is 1-4 dyn/cm2, 10-20 dyn/cm2, and 4-30 dyn/cm2 [43], respectively (Figure 1). In addition, FSS is significantly higher in close proximity to large vessels, heart turbulence, and blood vessel bifurcations, where the FSS of tumor cells encountering may approach 3000 dyn/cm2 [13,44]. FSS is omnipresent in circulation; hence, CTCs are exposed to varying levels of FSS. There is growing evidence that blood shear stress can bilaterally regulate tumor cell proliferation [19], induce apoptosis [45], promote CTC adhesion and extravasation [46], etc., thereby serving as a vital factor affecting tumor metastasis.
Lymphatic flow
In general, the lymphatic system participates in blood and body fluid circulation by assisting the re-entry of body fluid into the circulatory system. In addition, it can also transport immune cells and deliver antigens, shouldering imperative immune function. The average FSS of lymphatic vessels is 0.64 ± 0.14 dyn/cm2 and the peak is 4-12 dyn/cm2 [47] (Figure 1), which is far lower than blood shear stress. Recent studies have reported that the FSS generated at sentinel lymph nodes can significantly upregulate ICAM-1 in lymphoid endothelial cells, thereby facilitating lymph node metastasis [48]. Overall, lymphatic shear stress is considered to probably increase lymph node metastasis and affect immune regulation in cancer.
Target organ-specific blood microenvironment
When primary tumor cells infiltrate the circulatory system, they usually stagnate in the vasculature of the target organs minutes after being in rapid blood flow [18]. Clinically, the liver [49], lung [50], and brain [51] are highly metastatic organs; all of these have unique blood microvasculature, and their FSS is enlisted in Figure 1.
For instance, the hepatic sinusoid and alveolar walls both have a dual blood supply system. A key process of organ-specific metastasis is that CTCs are captured by the different vasculature. Weiss et al. have analyzed the relationship between the metastatic rate of eight target organs and their arterial blood flow in colorectal cancer and esophageal squamous cell carcinoma. They found that the frequency of organ metastasis is positively correlated with blood flow [52]. Recently, researchers have developed a series of organ-specific microfluidic chips, such as liver, lung, brain chips, etc. [53-55]. Using these chips as experimental systems, they confirmed that FSS affects the metabolism of and secretion from hepatocytes, the immune response of lung tissue, and the integrity and penetrability of the blood-brain barrier [53-55]. Clinically, the mechanism underlying organ-specific metastasis and whether fluid mechanics is a contributor warrant further investigation. Hence, it seems worthwhile to investigate the following two aspects using microfluidic chips: first, to set up various tissue/organ-specific microfluidic chips in series and observe tumor cell invasion and metastasis under different fluid dynamics; second, to seed different cell types (tumor cells, endothelial cells, immune cells, etc.), and investigate the interactions between tumor cells and other stromal cells, using the microfluidic chips.
Fluid shear stress plays significant roles in the tumor metastasis cascade
Dynamic response of tumor cells to FSS
FSS induces tumor cell death
The size, action time, and acting form of FSS changes with time for CTCs. In general, CTCs undergo an FSS that can vary from 0.1 dyn/cm2 to 1-40 dyn/cm2, sometimes approaching 3000 dyn/cm2 [13,44]. To better understand the effects of FSS of various strengths on tumor metastasis, we have defined four grades of FSS, based on the existing literature: micro, low, medium, and high; these correspond to FSS ranges of 0-0.5 dyn/cm2, 0.5-15 dyn/cm2, 15-30 dyn/cm2, and >30 dyn/cm2, respectively (Figure 1). Lien et al. reported that laminar shear stress (LSS) of 0.5-12 dyn/cm2 can induce apoptosis of Hep3B, MG63, SCC25, and A549 cells, unlike oscillation shear stress (OSS), which suggests that the different effects of FSS-induced apoptosis might depend on the model of FSS (Figure 2) [45]. Under certain circumstances, such as liver fibrosis or high interstitial pressure, blood flow tends to be reversible [15,56] and easily induces OSS, leading to decreased FSS-induced apoptosis in tumor cells. Accordingly, a high metastatic characteristic in a particular region is likely to attribute to a variant FSS model.
Figure 2.
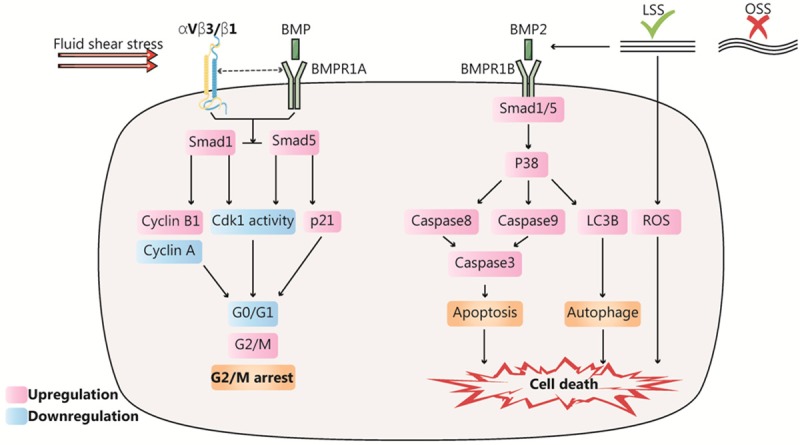
Dynamic response of tumor cells to fluid shear stress (FSS) related to cell survival. FSS targets bone morphogenetic protein (BMP) and integrin, and then accelerates the process of G2/M cell cycle arrest and cell death. On one hand, FSS upregulates BMPRIB, activating the Smad1/5/p38 MAPK signaling pathway, enhancing cleavage caspase-3 or LC3B-I and further promotes apoptosis or autophagy. On the other hand, FSS increases the levels of reactive oxygen species in tumor cells and directly induces tumor cell death. Moreover, FSS activated Smad1/5, causing cell cycle arrest at the G2/M phase and inhibiting cell differentiation.
Notably, FSS primarily induces tumor cell apoptosis or autophagy rather than necrosis. The human colorectal carcinoma cell line HCT116 has been reported to undergo almost no cell death within the first 2 min under continuous FSS of 8-60.5 dyn/cm2; however, tumor cell death rate increases to 60% after 20 h [57]. Similarly, Sagar reported that high FSS (60 dyn/cm2) eliminates more circulating tumor cells than low FSS (15 dyn/cm2), and high FSS can eliminate more than 90% of tumor cells within 4 h; apoptosis of tumor cells continued even after termination of FSS in 16-24 h [58]. These results indicate that FSS-specific cell death has a residual effect that is positively correlated with FSS size and action time. In terms of the molecular mechanism, Fu reported that high FSS increased reactive oxygen species (ROS) levels in tumor cells, which could cause oxidative stress and ultimately induce tumor cell death (Figure 2) [38]. Furthermore, FSS also upregulates BMPRIB, thereby activating the Smad1/5/p38 MAPK signaling pathway, enhancing protein expression of cleavage caspase-3 or LC3B-I, to promote apoptosis or autophagy (Figure 2) [45]. In addition, it has been reported that FSS-induced cell death may be attributed to cytoskeleton destruction, thereby preventing cell adhesion and inducing anoikis.
FSS regulates tumor proliferation
In addition to cell death, FSS was reported to influence cell proliferation. Studies have reported that tumor cell proliferation can be obviously reduced by increasing the FSS stimulation time [19]. Further experiments have indicated that FSS can lead to G1/S or G2/M cell cycle arrest in tumor cells. A previous study reported that 61% of colon cancer cells were arrested in the G1 phase with sustained FSS stimulation at 15 dyn/cm2, compared to 24% in the FSS-free group [59]. Similarly, Chang reported that low FSS stimulation of 2-20 dyn/cm2 activated Smad1/5, causing cell cycle arrest at the G2/M phase and downregulating cell differentiation in the adherent human osteosarcoma cell line (MG63) (Figure 2) [60]. Simultaneously, the study reported that p-Smad1/5 was upregulated with increasing FSS strength [60]. Fan reported that when FSS of different strengths were applied to circulating human colon cancer cells (HCT116) for a constant period, cancer cells in the high FSS group (60.5 dyn/cm2) had higher cell vitality and β-catenin expression than those in the low FSS group [19]. Recent studies have also reported that tumor cells have shear stress resistance, implying that tumor cells can adapt to shear stress stimulation up to 6000 dyn/cm2 and show increased survival after repeated exposure to FSS, relative to normal epithelial cells [61]. The findings of these two studies are not consistent with those of Chang, and we surmise the reasons may be related to the FSS range and cell state employed in the study. Although FSS has been reported to have various regulatory effects on tumor proliferation, FSS is consistently reported to play a vital role in tumor proliferation.
FSS promotes tumor invasion and metastasis
Increasing evidence indicates that low FSS stimulation upregulates or activates a series of cytokines or mechanosensitive molecules, such as IGF-2, VEGF, ROCK, and Cav-1, triggering downstream molecular pathways and promoting invasion and metastasis of tumor cells (Figure 3) [62,63]. For instance, Wang reported that application of low FSS (2 dyn/cm2) to chondrosarcoma cells promoted the synthesis of cAMP and IL-1β or activation of IGF-2 and VEGF-B/D, targeting the PI3-K, p38, or other signaling pathways, ultimately enhancing the invasion of chondrosarcoma cells in vitro [64,65]. Lee supported this conclusion by demonstrating that FSS (0.05 dyn/cm2) activated the ROCK-LIMK-cofilin signaling axis, inducing nuclear translocation of YAP1, and regulating transcription of metastasis-related genes in prostate cancer cells [17]. Yang’s team also verified that Cav-1 can activate the downstream PI3K Akt/mTOR pathway and promote metastasis of breast cancer cells under low FSS, using in vivo and in vitro experiments [66].
Figure 3.
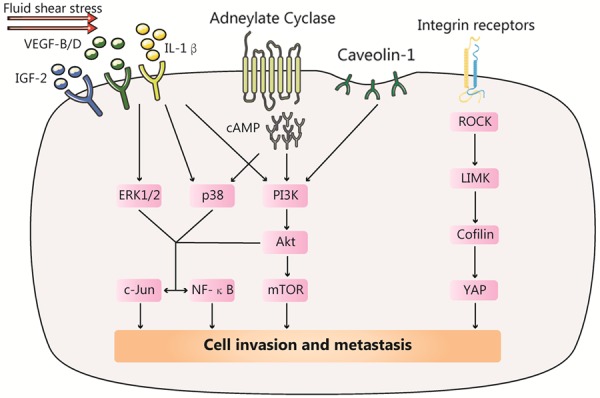
Various factors activated by fluid shear stress (FSS) can induce tumor cells metastasis. FSS stimulation upregulates a series of cytokines or mechanosensitive molecules, such as IGF-2, VEGF and Cav-1, activating PI3K/AKT, c-Jun and NF-kB pathway and promoting invasion and metastasis; FSS can also combine integrin receptors, activating the ROCK-LIMK-cofilin signaling axis, inducing nuclear translocation of YAP1 and promoting invasion and metastasis.
In conclusion, FSS has an important effect on proliferation, death, invasion, and metastasis of tumor cells, which is largely dependent on the type, size, and action time of the FSS.
FSS-dependent interaction among tumor cells and other blood components
Platelets
Clinically, thrombocytosis is often observed in metastatic cancer patients, suggesting that platelets may contribute to tumor metastasis [67]. Platelets can downregulate the NK cell-surface receptor NKG2D through paracrine TGF-β signaling [68]; meanwhile, platelet-derived VEGF can also repress antigen presentation in mature dendritic cells, thereby inhibiting their immune surveillance function. Besides mediating immunity escape of carcinoma cells, platelets can directly trigger EMT of CTCs [18] or mobilize neutrophils via the secretion of TGF-β/PDGF, and leading to tumor micrometastasis [69]. However, in most situations, platelets will directly bind to CTCs to form cell complexes, thus promoting the evasion of CTCs from the immune, inhibiting CTC apoptosis, and mediating CTC extravasation [70].
At low FSS of 1.84 dyn/cm2, thrombin-activated platelets can produce a 5-fold increase of endothelial adherence in cervical cancer cells (HeLa) [71]. At low FSS (5 dyn/cm2), tumor gangliosides can drastically enhance the dynamic adhesion of platelets in the bloodstream, facilitating the capture of platelet-CTC complexes by endothelial cells (Figure 4) [72]. However, at an FSS of 50 dyn/cm2, the adhesion efficiency was not further enhanced, thereby indicating that low FSS is sufficient to enhance the adhesion of platelet-CTC complexes to endothelial cells [72]. In addition, Egan et al. reported that low FSS would similarly reduce LDH levels in tumor cells; LDH can be a quantitative molecular marker of membrane damage induced by FSS, which indicates that platelets can protect cancer cells from FSS-induced mechanical damage (Figure 4) [73]. However, with the increase in FSS, although a similar number of platelets adhered to tumor cells, the protective effect from platelets in FSS-induced tumor cell death decreased [73]. Thus, low FSS can enhance platelet promotion of tumor metastasis; however, high FSS may reverse the effect.
Figure 4.
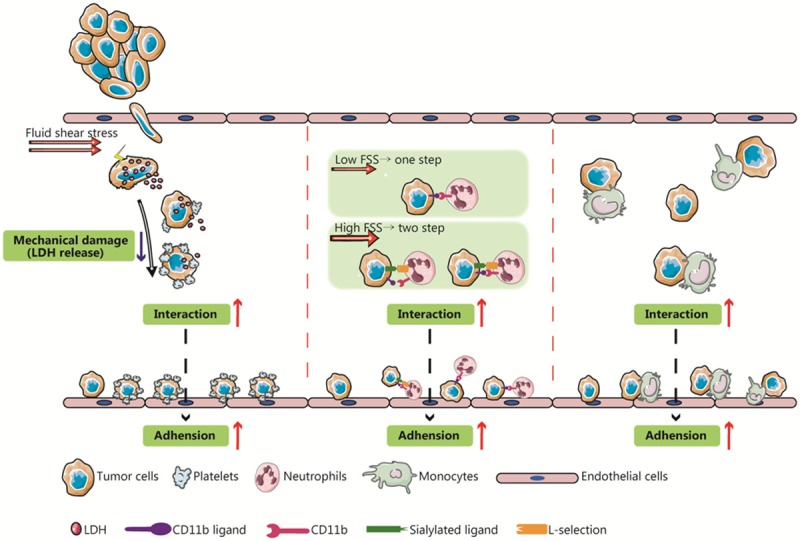
Fluid shear stress (FSS) regulates the interactions among tumor cells and other blood components. Blood shear stress can enhance the interactions between circulating tumor cells (CTCs) and platelets, neutrophils, monocytes, and other blood components, thereby protecting them from mechanical damage and promoting CTC adhesion to endothelial cell. Moreover, different magnitudes of shear stress can regulate the binding of CTCs to neutrophils.
Neutrophils
There is growing evidence that neutrophils may play a dual role in tumor metastasis. On one hand, when neutrophils have immediate contact with tumor cells, they can produce TNF-α, IL-1β, protease, membrane perforators, and other compounds to eliminate tumor cells [74]; on the other hand, gastrointestinal and other malignant tumors are characterized by neutrophil infiltration [75], and neutrophils can enhance tumorigenic potential [76].
The formation of neutrophil-tumor cell complexes is mediated by the sizes of the FSS; specifically, the number of neutrophils binding to tumor cells decreases with an increase in FSS. The mechanism underlying this phenomenon is that at low FSS, neutrophils can bind directly to tumor cells through the surface molecule CD11b, while at high FSS, binding is a two-step, sequential process. In detail, neutrophils first bind transiently to tumor cells via L-selectin, followed by conversion of the transient binding into stable adhesion via the synergistic effect of CD11a and CD11b (Figure 4) [77]. In 2008, another study confirmed that the accumulation of neutrophils and melanoma cells into a cellular mass is dependent on FSS size and shear rate, mainly via β2 integrin and selectin [78].
Monocytes and macrophages
Similar to neutrophils, macrophages are important immune cells, with phagocytosis, antigen presentation, and other immune functions, while various macrophage subtypes play different roles in tumor metastasis. Previously, tumor-associated macrophages (TAMs), which specifically infiltrate tumor tissue, were the focus of studies on cancer associated with macrophages. However, in recent years, in vivo studies have reported that circulating monocytes/macrophages are closely linked to the process of extravasation of breast cancer cells [79]. Based on an in vitro model loaded with a dynamic FSS, Evani et al. reported that breast cancer cells would not adhere to endothelial cells directly under low FSS (0.5-2 dyn/cm2), but instead formed a tumor cell/monocyte complex before binding to endothelial cells (Figure 4) [80]. Briefly, FSS determines the binding of tumor cells/monocyte complexes and endothelial cells.
These results suggest that FSS can mediate the interaction between CTCs and various blood components; moreover, the formation of tumor cell complexes contributes to CTC survival, adhesion, extravasation, etc. In the future, targeting the tumor cell complex-endothelium axis should be investigated as a promising therapy.
FSS is an essential regulator of tumor extravasation
Extravasation is a preliminary step in tumor metastasis. Similar to leukocyte exudation, tumor cell extravasation primarily involves three steps: adhesion, trans-endothelial migration (TEM), and crossing the vascular basement membrane (Figure 5) [81]. Previous studies indicate that FSS not only plays an important role in the neutrophil recruitment cascade (capture-scrolling-activating-adhesion), but also is closely related to the extravasation of CTCs [82].
Figure 5.
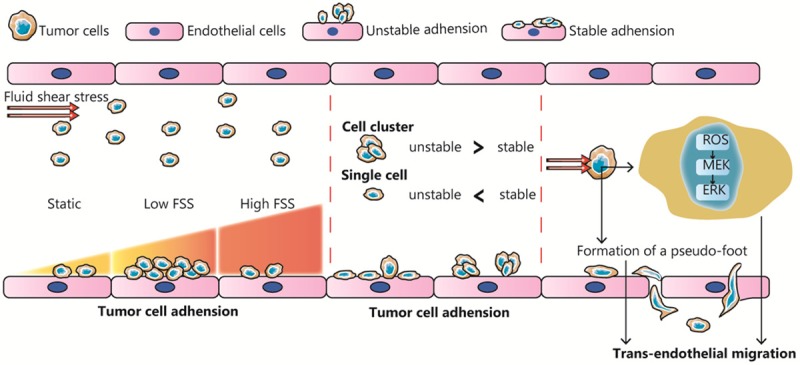
Fluid shear stress (FSS) is an essential regulator of tumor cell adhesion and extravasation. FSS plays a dual role in the adhesion of tumor cells to endothelial cells, and as the FSS increases, the adhesion efficiency first increases and then decreases. There may be different tendencies in the manner of adhesion of single tumor cells and tumor cell clusters in blood. FSS stimulation can promote intracellular generation of reactive oxygen species and pseudopodia formation, thereby triggering trans-endothelial migration.
FSS regulates tumor cell adhesion
The adhesion of CTCs to vasculature endothelial cells is a prerequisite for tumor extravasation, wherein selectin, cadherin, and integrin are key proteins. Essentially, cell adhesion involves binding between a specific receptor and its ligand, which can be subdivided into two stages: initial rolling adhesion and stable adhesion. Different adhesion molecules display diverse molecular dynamics of reaction rate or affinity; therefore FSS is likely to affect tumor cell adhesion via regulating adhesion/dissociation efficiency or expression levels of adhesion molecules.
Low FSS has been shown to affect the stable adhesion of tumor cells; furthermore, it has been reported that as the FSS increases, the adhesion efficiency first increases and then decreases. Fennewald reported that the quantity of cancer cells (HNSCCs) adhered to the matrix gel was significantly higher in the group with FSS (0-0.05 dyn/cm2). Similarly, another study reported that low FSS induced a 2-fold increase in the number of breast cancer cells adhered to endothelial cells, compared with the group without FSS [83]. However, Fennewald also reported that tumor cell adherence was gradually reduced to zero as the FSS increased (in the range of 0.05 to 1 dyn/cm2) [84]. Papadimitriou and Richter successively reported the same conclusion that FSS, within a particular range, can inhibit tumor cell adhesion [85,86]. It is noteworthy that although FSS is a two-way regulation of tumor adhesion, the response of different tumor cells to varying degrees of FSS may be positive or negative [87], which may be associated with the type or expression level of adhesion molecules at the tumor cell surface. Other than stable adhesion, FSS can negatively regulate rolling adhesion within a certain range (Figure 5). Aigner reported that the ratio of rolling adhesion in three cancer cell types (KS, HL-60, and SkW3) significantly declined with FSS stimulation (0.25-2.75 dyn/cm2) [88].
Since the effect of FSS varies with time and site of application, it is worth investigating how the dynamic FSS play roles in tumor cell adhesion. Through in silico modeling, Yan observed that the adhesion rate of tumor cells to curving vessels was 1.5-fold times greater than that to straight vessels, and nearly 45% of tumor cells preferentially adhered to medial curving vessels. Based on the hydrodynamic theory, the authors concluded that a positive shear stress gradient enhanced tumor cell adhesion while a negative gradient weakened it [89]. Thus, a constantly increasing FSS (within 50 dyn/cm2) is likely to promote tumor cell adhesion, and vice versa.
FSS has differing effects on cell adhesion in single cells and clustered cells, the two major forms of CTCs. Yano et al. compared adhesion rate between N17 single cells and NL17 cell clusters at low FSS. They found that the binding frequency increased in NL17 cell clusters, which was mostly short-term attachment, with less stable adhesion, but single cells are more likely to develop stable adhesion at the same FSS [90]. However, subsequent in vivo experiments reported that NL17 cell clusters result in greater tumor metastases than did N17 single cells (Figure 5) [90], which may attributed to the stronger killing effect of FSS on single tumor cells.
FSS regulates TEM of tumor cells
TEM refers to the process whereby tumor cells stably adhere to endothelial cells and then penetrate the endothelial tissue. There are two primary TEM pathways in tumors: 1) paracellular TEM, wherein tumor cells reduce the endothelial cell junction and cause endothelial cell retraction and separation via secretion of VEGF, TGF-β, and other cytokines, promoting TEM [91]; and 2) transcellular TEM, wherein tumor cells pass directly through vascular endothelial cells [46,92].
The potential for TEM increases as the retention time of tumor cells in endothelial cells increases, and that retention time largely depends on FSS [93]. As mentioned earlier, a stronger FSS can not only increase the adhesion between tumor cells and endothelial cells, but also reduce the duration and prevent TEM. However, using a zebrafish model, Ma reported that ROS levels in tumor cells are upregulated with FSS (10-15 dyn/cm2) stimulation, activating the MEK/ERK signaling pathway and promoting TEM (Figure 5) [94]. Another study reported that when the shear rate is greater than 400 s-1, that is, physiological shear conditions, FSS could induce the formation of a pseudo-foot in tumor cells, which is also conducive to TEM [95].
Future research directions for fluid mechanics in tumor metastasis
Establishment of guidelines for tumor research using fluid mechanics
Fluid mechanics of tumors, as an emerging interdisciplinary research field, faces many challenges. Unlike solid mechanics and structural mechanics, fluid mechanics involves the investigation of components that continually change their form and are in a constant state of motion. Therefore, it entails more complicated theoretical analysis and numerical calculations. However, in practical research, there are no uniform standards to determine FSS mode or microfluidic devices, leading to poor reproducibility and weak clinical translation of most findings. Hydrodynamics has a significant impact on vascular remodeling and tumor metastasis. Therefore, a multidisciplinary team of professionals including physicists, biologists, and clinicians should be constituted to promote interdisciplinary integration and establish guidelines for research in this field. The background and history of mechanobiology development, its scope, theoretical basis, research and experimental methodologies, existing achievements, scientific problems remain to be solved, and the future developmental directions urgently need to be comprehensively and systematically summarized.
Elucidation of the mechanism of mechanotransduction
Mechanotransduction, which constitutes the core of biomechanics, can be divided into five stages-stimulus, sensing, signaling, gene expression, cellular response, and cellular function [96]. Among these, molecular mechanosensing is the most widely studied process. The activity of biomolecules is closely related to their physical form or conformation, which is the basis for the mechanical sensitivity of mechanosensing molecules. For example, stress can induce a conformational change in p130Cas adhesion spot, exposing the phosphorylation site of Src protein and allowing it to phosphorylate and activate the downstream p38/MAPK signaling pathway [97]. Numerous studies have reported various mechanosensitive cell-surface molecules such as integrins, adherent proteins, and calcium channels [98], which can dynamically detect changes in mechanical forces and activate downstream signaling pathways, which regulate gene transcription and translation to effect phenotypic and functional changes. However, how these mechanosensitive molecules detect changes in the magnitude and direction of mechanical forces and subsequently mediate the activation of downstream pathways is unclear. Studies using intracellular tension sensors and other new technologies are needed to address these questions.
Furthermore, hydrodynamics influences cell fate through multiple routes, such as epigenetic modifications (DNA methylation) [99], modulation of mRNA and protein levels, and alteration of chromatin structure [100]. Fernandez-Sanchez et al. reported that long-term 1-kPa magnetic load can activate the Wnt pathway in intestinal crypt cells, thereby upregulating the oncogene cMyc and leading to carcinogenesis. These effects are not reversed by the withdrawal of the magnetic load, suggesting that mechanical forces can induce stable genetic effects and lead to tumorigenesis [2]. Therefore, the core molecules or pathways that mediate the biological effects of mechanical forces could be unique targets for antitumor therapy.
Strengthening of clinical applications
The tumor microenvironment is a network of biological, chemical, physical, and other signals that interact with each other. Thus, tumorigenesis and tumor progression cannot be explained without considering both mechanics and biology.
The use of microfluidic chips and computational fluid dynamics modeling tools can enhance our understanding of the biological mechanisms of tumor metastasis. Microfluidic technology, a popular tool, can simulate a wide array of complicated tumor microenvironments, allowing the study of various chemical and mechanical effects and facilitating the comprehensive study of the TME.
In addition, FSS not only directly promotes migration of T lymphocytes [101] and helps screen antigen-specific T cells [102], but also it induces M1 polarization of macrophages [103] and activates the immunoregulatory function of mesenchymal stem cells [104]. Therefore, detailed studies on molecular mechanisms through which FSS stimulates tumor cells, immune cell differentiation, and induces antigen presentation are needed. Furthermore, numerous studies have reported that PD-L1, CD47, and other immunosuppressive molecules expressed on the surface of CTCs are significantly increased compared with that on primary tumor cells [105-107]. Aside from the primary tumor, one of the biggest changes for CTCs in the tumor microenvironment is FSS. We speculate that FSS likely induces evasion from the immune system through upregulation of immunosuppressive molecules in CTCs. In the future, more basic studies are needed to determine whether and how FSS affects tumor immunity.
In recent years, researchers have developed numerous novel mechanosensing carriers such as liposomes and microaggregates for clinical application, especially for treating cardiovascular disease [108]. These materials can sense changes in shear force and respond by releasing their contents, diffusion, cohesion, or inducing polymerization to achieve mechanics-targeted drug delivery. Future studies on hydrodynamics-based antitumor strategies would focus on further clarifying how mechanistic and biological factors together impact tumor growth, designing fluid mechanics-based targeted drugs, and changing the tumor hydrodynamic microenvironment.
Outlook
Fluid dynamics is an important dynamic variable in physiological phenomena. Like Heraclitus famously said that no man ever steps in the same river twice. Despite infinite variations in mechanobiology, the goal of understanding the universal law and the vital signal transduction mechanisms can be attained with persistent effort. To determine the essence and verify the laws, a specific spatiotemporal event or a specific biological aspect for investigation (such as FSS) should be focused on to begin with, investigated progressively, and then the accumulated evidence should be interpreted and explained sequentially, thereby revealing the role of fluid mechanics in tumor development.
The tumor fluid microenvironment, especially the FSS, plays an indispensable role in tumor progression and metastasis. Rapid advancements in research tools and computer algorithms will certainly improve the general understanding of the mechanism underlying the induction of tumorigenesis and tumor progression by mechanotransduction, and fluid mechanics will provide novel strategies and new targets for antitumor therapy.
Acknowledgements
This study was supported by the National Natural Science Foundation of China (81672447), CSCO Merck Serono Oncology Research Fund (cc), and the Special Foundation of National Clinical Specialties of China (to The Department of Oncology, Nanfang Hospital).
Disclosure of conflict of interest
None.
References
- 1.Chaffer CL, Weinberg RA. A perspective on cancer cell metastasis. Science. 2011;331:1559–1564. doi: 10.1126/science.1203543. [DOI] [PubMed] [Google Scholar]
- 2.Fernandez-Sanchez ME, Barbier S, Whitehead J, Bealle G, Michel A, Latorre-Ossa H, Rey C, Fouassier L, Claperon A, Brulle L, Girard E, Servant N, Rio-Frio T, Marie H, Lesieur S, Housset C, Gennisson JL, Tanter M, Menager C, Fre S, Robine S, Farge E. Mechanical induction of the tumorigenic beta-catenin pathway by tumour growth pressure. Nature. 2015;523:92–95. doi: 10.1038/nature14329. [DOI] [PubMed] [Google Scholar]
- 3.Guilak F, Butler DL, Goldstein SA, Baaijens FP. Biomechanics and mechanobiology in functional tissue engineering. J Biomech. 2014;47:1933–1940. doi: 10.1016/j.jbiomech.2014.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Garcia-Cardena G, Slegtenhorst BR. Hemodynamic control of endothelial cell fates in development. Annu Rev Cell Dev Biol. 2016;32:633–648. doi: 10.1146/annurev-cellbio-100814-125610. [DOI] [PubMed] [Google Scholar]
- 5.Xiong N, Li S, Tang K, Bai H, Peng Y, Yang H, Wu C, Liu Y. Involvement of caveolin-1 in low shear stress-induced breast cancer cell motility and adhesion: roles of FAK/Src and ROCK/p-MLC pathways. Biochim Biophys Acta. 2017;1864:12–22. doi: 10.1016/j.bbamcr.2016.10.013. [DOI] [PubMed] [Google Scholar]
- 6.Elosegui-Artola A, Andreu I, Beedle AEM, Lezamiz A, Uroz M, Kosmalska AJ, Oria R, Kechagia JZ, Rico-Lastres P, Le Roux AL, Shanahan CM, Trepat X, Navajas D, Garcia-Manyes S, RocaCusachs P. Force triggers YAP nuclear entry by regulating transport across nuclear pores. Cell. 2017;171:1397–1410. e1314. doi: 10.1016/j.cell.2017.10.008. [DOI] [PubMed] [Google Scholar]
- 7.Fang F, Wasserman SM, Torres-Vazquez J, Weinstein B, Cao F, Li Z, Wilson KD, Yue W, Wu JC, Xie X, Pei X. The role of Hath6, a newly identified shear-stress-responsive transcription factor, in endothelial cell differentiation and function. J Cell Sci. 2014;127:1428–1440. doi: 10.1242/jcs.136358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Moroishi T, Hansen CG, Guan KL. The emerging roles of YAP and TAZ in cancer. Nat Rev Cancer. 2015;15:73–79. doi: 10.1038/nrc3876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Song Y, Pan G, Chen L, Ma S, Zeng T, Man Chan TH, Li L, Lian Q, Chow R, Cai X, Li Y, Li Y, Liu M, Li Y, Zhu Y, Wong N, Yuan YF, Pei D, Guan XY. Loss of ATOH8 increases stem cell features of hepatocellular carcinoma cells. Gastroenterology. 2015;149:1068–1081. e1065. doi: 10.1053/j.gastro.2015.06.010. [DOI] [PubMed] [Google Scholar]
- 10.Nagelkerke A, Bussink J, Rowan AE, Span PN. The mechanical microenvironment in cancer: how physics affects tumours. Semin Cancer Biol. 2015;35:62–70. doi: 10.1016/j.semcancer.2015.09.001. [DOI] [PubMed] [Google Scholar]
- 11.Liu C, Liu Y, Xie HG, Zhao S, Xu XX, Fan LX, Guo X, Lu T, Sun GW, Ma XJ. Role of three-dimensional matrix stiffness in regulating the chemoresistance of hepatocellular carcinoma cells. Biotechnol Appl Biochem. 2015;62:556–562. doi: 10.1002/bab.1302. [DOI] [PubMed] [Google Scholar]
- 12.Wei SC, Fattet L, Tsai JH, Guo Y, Pai VH, Majeski HE, Chen AC, Sah RL, Taylor SS, Engler AJ, Yang J. Matrix stiffness drives epithelialmesenchymal transition and tumour metastasis through a TWIST1-G3BP2 mechanotransduction pathway. Nat Cell Biol. 2015;17:678–688. doi: 10.1038/ncb3157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wirtz D, Konstantopoulos K, Searson PC. The physics of cancer: the role of physical interactions and mechanical forces in metastasis. Nat Rev Cancer. 2011;11:512–522. doi: 10.1038/nrc3080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Galbraith CG, Skalak R, Chien S. Shear stress induces spatial reorganization of the endothelial cell cytoskeleton. Cell Motil Cytoskeleton. 1998;40:317–330. doi: 10.1002/(SICI)1097-0169(1998)40:4<317::AID-CM1>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- 15.Haddad O, Chotard-Ghodsnia R, Verdier C, Duperray A. Tumor cell/endothelial cell tight contact upregulates endothelial adhesion molecule expression mediated by NFkappaB: differential role of the shear stress. Exp Cell Res. 2010;316:615–626. doi: 10.1016/j.yexcr.2009.11.015. [DOI] [PubMed] [Google Scholar]
- 16.Swartz MA, Lund AW. Lymphatic and interstitial flow in the tumour microenvironment: linking mechanobiology with immunity. Nat Rev Cancer. 2012;12:210–219. doi: 10.1038/nrc3186. [DOI] [PubMed] [Google Scholar]
- 17.Lee HJ, Diaz MF, Price KM, Ozuna JA, Zhang S, Sevick-Muraca EM, Hagan JP, Wenzel PL. Fluid shear stress activates YAP1 to promote cancer cell motility. Nat Commun. 2017;8:14122. doi: 10.1038/ncomms14122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Labelle M, Hynes RO. The initial hours of metastasis: the importance of cooperative host-tumor cell interactions during hematogenous dissemination. Cancer Discov. 2012;2:1091–1099. doi: 10.1158/2159-8290.CD-12-0329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fan R, Emery T, Zhang Y, Xia Y, Sun J, Wan J. Circulatory shear flow alters the viability and proliferation of circulating colon cancer cells. Sci Rep. 2016;6:27073. doi: 10.1038/srep27073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Huse M. Mechanical forces in the immune system. Nat Rev Immunol. 2017;17:679–690. doi: 10.1038/nri.2017.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang D, Jin B, Lewandowski RJ, Ryu RK, Sato KT, Mulcahy MF, Kulik LM, Miller FH, Salem R, Li D, Omary RA, Larson AC. Quantitative 4D transcatheter intraarterial perfusion MRI for monitoring chemoembolization of hepatocellular carcinoma. J Magn Reson Imaging. 2010;31:1106–1116. doi: 10.1002/jmri.22155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kang T, Park C, Lee BJ. Investigation of biomimetic shear stress on cellular uptake and mechanism of polystyrene nanoparticles in various cancer cell lines. Arch Pharm Res. 2016;39:1663–1670. doi: 10.1007/s12272-016-0847-0. [DOI] [PubMed] [Google Scholar]
- 23.Perrault CM, Brugues A, Bazellieres E, Ricco P, Lacroix D, Trepat X. Traction forces of endothelial cells under slow shear flow. Biophys J. 2015;109:1533–1536. doi: 10.1016/j.bpj.2015.08.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Boldock L, Wittkowske C, Perrault CM. Microfluidic traction force microscopy to study mechanotransduction in angiogenesis. Microcirculation. 2017:24. doi: 10.1111/micc.12361. [DOI] [PubMed] [Google Scholar]
- 25.Verma D, Bajpai VK, Ye N, Maneshi MM, Jetta D, Andreadis ST, Sachs F, Hua SZ. Flow induced adherens junction remodeling driven by cytoskeletal forces. Exp Cell Res. 2017;359:327–336. doi: 10.1016/j.yexcr.2017.08.009. [DOI] [PubMed] [Google Scholar]
- 26.Liang X, Oldenburg AL, Crecea V, Chaney EJ, Boppart SA. Optical micro-scale mapping of dynamic biomechanical tissue properties. Opt Express. 2008;16:11052–11065. doi: 10.1364/oe.16.011052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Perozziello G, La Rocca R, Cojoc G, Liberale C, Malara N, Simone G, Candeloro P, Anichini A, Tirinato L, Gentile F, Coluccio ML, Carbone E, Di Fabrizio E. Microfluidic devices modulate tumor cell line susceptibility to NK cell recognition. Small. 2012;8:2886–2894. doi: 10.1002/smll.201200160. [DOI] [PubMed] [Google Scholar]
- 28.Markl M, Wallis W, Harloff A. Reproducibility of flow and wall shear stress analysis using flow-sensitive four-dimensional MRI. J Magn Reson Imaging. 2011;33:988–994. doi: 10.1002/jmri.22519. [DOI] [PubMed] [Google Scholar]
- 29.Ma P, Shen X, Tan P, Yang L, Liu W. Effect of matrix rigidity on organ-specific capture of tumor cells by flow. Cell Mol Biol (Noisy-le-grand) 2017;63:10–15. doi: 10.14715/cmb/2017.63.4.2. [DOI] [PubMed] [Google Scholar]
- 30.Mohammadalipour A, Burdick MM, Tees DFJ. Deformability of breast cancer cells in correlation with surface markers and cell rolling. FASEB J. 2018;32:1806–1817. doi: 10.1096/fj.201700762R. [DOI] [PubMed] [Google Scholar]
- 31.Cui X, Guo W, Sun Y, Sun B, Hu S, Sun D, Lam RHW. A microfluidic device for isolation and characterization of transendothelial migrating cancer cells. Biomicrofluidics. 2017;11:014105. doi: 10.1063/1.4974012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sun B, Hu S, Sun D, Lam RHW, Biselli E, Agliari E, Barra A, Bertani FR, Gerardino A, De Ninno A, Mencattini A. Organs on chip approach: a tool to evaluate cancer-immune cells interactions. Biomicrofluidics. 2017;7:12737. doi: 10.1038/s41598-017-13070-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wu J, Dong M, Santos S, Rigatto C, Liu Y, Lin F. Lab-on-a-Chip platforms for detection of cardiovascular disease and cancer biomarkers. Sensors (Basel) 2017:17. doi: 10.3390/s17122934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mak M, Kamm RD, Zaman MH. Impact of dimensionality and network disruption on microrheology of cancer cells in 3D environments. PLoS Comput Biol. 2014;10:e1003959. doi: 10.1371/journal.pcbi.1003959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bonilla LL, Capasso V, Alvaro M, Carretero M. Hybrid modeling of tumor-induced angiogenesis. Phys Rev E Stat Nonlin Soft Matter Phys. 2014;90:062716. doi: 10.1103/PhysRevE.90.062716. [DOI] [PubMed] [Google Scholar]
- 36.Steuperaert M, Falvo D’Urso Labate G, Debbaut C, De Wever O, Vanhove C, Ceelen W, Segers P. Mathematical modeling of intraperitoneal drug delivery: simulation of drug distribution in a single tumor nodule. Drug Deliv. 2017;24:491–501. doi: 10.1080/10717544.2016.1269848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sabeh F, Shimizu-Hirota R, Weiss SJ. Protease-dependent versus-independent cancer cell invasion programs: three-dimensional amoeboid movement revisited. J Cell Biol. 2009;185:11–19. doi: 10.1083/jcb.200807195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fu A, Ma S, Wei N, Tan BX, Tan EY, Luo KQ. High expression of MnSOD promotes survival of circulating breast cancer cells and increases their resistance to doxorubicin. Oncotarget. 2016;7:50239–50257. doi: 10.18632/oncotarget.10360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Munson JM, Shieh AC. Interstitial fluid flow in cancer: implications for disease progression and treatment. Cancer Manag Res. 2014;6:317–328. doi: 10.2147/CMAR.S65444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hompland T, Lund KV, Ellingsen C, Kristensen GB, Rofstad EK. Peritumoral interstitial fluid flow velocity predicts survival in cervical carcinoma. Radiother Oncol. 2014;113:132–138. doi: 10.1016/j.radonc.2014.09.011. [DOI] [PubMed] [Google Scholar]
- 41.Rejniak KA. Circulating tumor cells: when a solid tumor meets a fluid microenvironment. Adv Exp Med Biol. 2016;936:93–106. doi: 10.1007/978-3-319-42023-3_5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Massague J, Obenauf AC. Metastatic colonization by circulating tumour cells. Nature. 2016;529:298–306. doi: 10.1038/nature17038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gray KM, Stroka KM. Vascular endothelial cell mechanosensing: new insights gained from biomimetic microfluidic models. Semin Cell Dev Biol. 2017;71:106–117. doi: 10.1016/j.semcdb.2017.06.002. [DOI] [PubMed] [Google Scholar]
- 44.Mitchell MJ, Denais C, Chan MF, Wang Z, Lammerding J, King MR. Lamin A/C deficiency reduces circulating tumor cell resistance to fluid shear stress. Am J Physiol Cell Physiol. 2015;309:C736–746. doi: 10.1152/ajpcell.00050.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lien SC, Chang SF, Lee PL, Wei SY, Chang MD, Chang JY, Chiu JJ. Mechanical regulation of cancer cell apoptosis and autophagy: roles of bone morphogenetic protein receptor, Smad1/5, and p38 MAPK. Biochim Biophys Acta. 2013;1833:3124–3133. doi: 10.1016/j.bbamcr.2013.08.023. [DOI] [PubMed] [Google Scholar]
- 46.Tremblay PL, Huot J, Auger FA. Mechanisms by which E-selectin regulates diapedesis of colon cancer cells under flow conditions. Cancer Res. 2008;68:5167–5176. doi: 10.1158/0008-5472.CAN-08-1229. [DOI] [PubMed] [Google Scholar]
- 47.Dixon JB, Greiner ST, Gashev AA, Cote GL, Moore JE, Zawieja DC. Lymph flow, shear stress, and lymphocyte velocity in rat mesenteric prenodal lymphatics. Microcirculation. 2006;13:597–610. doi: 10.1080/10739680600893909. [DOI] [PubMed] [Google Scholar]
- 48.Kawai Y, Kaidoh M, Yokoyama Y, Ohhashi T. Pivotal roles of shear stress in the microenvironmental changes that occur within sentinel lymph nodes. Cancer Sci. 2012;103:1245–1252. doi: 10.1111/j.1349-7006.2012.02289.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lalor PF, Adams DH. Adhesion of lymphocytes to hepatic endothelium. Mol Pathol. 1999;52:214–219. doi: 10.1136/mp.52.4.214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tang BT, Pickard SS, Chan FP, Tsao PS, Taylor CA, Feinstein JA. Wall shear stress is decreased in the pulmonary arteries of patients with pulmonary arterial hypertension: an image-based, computational fluid dynamics study. Pulm Circ. 2012;2:470–476. doi: 10.4103/2045-8932.105035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wong AD, Ye M, Levy AF, Rothstein JD, Bergles DE, Searson PC. The blood-brain barrier: an engineering perspective. Front Neuroeng. 2013;6:7. doi: 10.3389/fneng.2013.00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Weiss L, Bronk J, Pickren JW, Lane WW. Metastatic patterns and target organ arterial blood flow. Invasion Metastasis. 1981;1:126–135. [PubMed] [Google Scholar]
- 53.Du Y, Li N, Yang H, Luo C, Gong Y, Tong C, Gao Y, Lu S, Long M. Mimicking liver sinusoidal structures and functions using a 3D-configured microfluidic chip. Lab Chip. 2017;17:782–794. doi: 10.1039/c6lc01374k. [DOI] [PubMed] [Google Scholar]
- 54.Benam KH, Villenave R, Lucchesi C, Varone A, Hubeau C, Lee HH, Alves SE, Salmon M, Ferrante TC, Weaver JC, Bahinski A, Hamilton GA, Ingber DE. Small airway-on-a-chip enables analysis of human lung inflammation and drug responses in vitro. Nat Methods. 2016;13:151–157. doi: 10.1038/nmeth.3697. [DOI] [PubMed] [Google Scholar]
- 55.Adriani G, Ma D, Pavesi A, Kamm RD, Goh EL. A 3D neurovascular microfluidic model consisting of neurons, astrocytes and cerebral endothelial cells as a blood-brain barrier. Lab Chip. 2017;17:448–459. doi: 10.1039/c6lc00638h. [DOI] [PubMed] [Google Scholar]
- 56.Zardi EM, Uwechie V, Caccavo D, Pellegrino NM, Cacciapaglia F, Di Matteo F, Dobrina A, Laghi V, Afeltra A. Portosystemic shunts in a large cohort of patients with liver cirrhosis: detection rate and clinical relevance. J Gastroenterol. 2009;44:76–83. doi: 10.1007/s00535-008-2279-1. [DOI] [PubMed] [Google Scholar]
- 57.Rana K, Liesveld JL, King MR. Delivery of apoptotic signal to rolling cancer cells: a novel biomimetic technique using immobilized TRAIL and E-selectin. Biotechnol Bioeng. 2009;102:1692–1702. doi: 10.1002/bit.22204. [DOI] [PubMed] [Google Scholar]
- 58.Regmi S, Fu A, Luo KQ. High shear stresses under exercise condition destroy circulating tumor cells in a microfluidic system. Sci Rep. 2017;7:39975. doi: 10.1038/srep39975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Avvisato CL, Yang X, Shah S, Hoxter B, Li W, Gaynor R, Pestell R, Tozeren A, Byers SW. Mechanical force modulates global gene expression and beta-catenin signaling in colon cancer cells. J Cell Sci. 2007;120:2672–2682. doi: 10.1242/jcs.03476. [DOI] [PubMed] [Google Scholar]
- 60.Chang SF, Chang CA, Lee DY, Lee PL, Yeh YM, Yeh CR, Cheng CK, Chien S, Chiu JJ. Tumor cell cycle arrest induced by shear stress: roles of integrins and Smad. Proc Natl Acad Sci U S A. 2008;105:3927–3932. doi: 10.1073/pnas.0712353105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Chivukula VK, Krog BL, Nauseef JT, Henry MD, Vigmostad SC. Alterations in cancer cell mechanical properties after fluid shear stress exposure: a micropipette aspiration study. Cell Health Cytoskelet. 2015;7:25–35. doi: 10.2147/CHC.S71852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zhao F, Li L, Guan L, Yang H, Wu C, Liu Y. Roles for GP IIb/IIIa and alphavbeta3 integrins in MDA-MB-231 cell invasion and shear flow-induced cancer cell mechanotransduction. Cancer Lett. 2014;344:62–73. doi: 10.1016/j.canlet.2013.10.019. [DOI] [PubMed] [Google Scholar]
- 63.Qazi H, Palomino R, Shi ZD, Munn LL, Tarbell JM. Cancer cell glycocalyx mediates mechanotransduction and flow-regulated invasion. Integr Biol (Camb) 2013;5:1334–1343. doi: 10.1039/c3ib40057c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wang P, Chen SH, Hung WC, Paul C, Zhu F, Guan PP, Huso DL, Kontrogianni-Konstantopoulos A, Konstantopoulos K. Fluid shear promotes chondrosarcoma cell invasion by activating matrix metalloproteinase 12 via IGF-2 and VEGF signaling pathways. Oncogene. 2015;34:4558–4569. doi: 10.1038/onc.2014.397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Guan PP, Yu X, Guo JJ, Wang Y, Wang T, Li JY, Konstantopoulos K, Wang ZY, Wang P. By activating matrix metalloproteinase-7, shear stress promotes chondrosarcoma cell motility, invasion and lung colonization. Oncotarget. 2015;6:9140–9159. doi: 10.18632/oncotarget.3274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Yang H, Guan L, Li S, Jiang Y, Xiong N, Li L, Wu C, Zeng H, Liu Y. Mechanosensitive caveolin-1 activation-induced PI3K/Akt/mTOR signaling pathway promotes breast cancer motility, invadopodia formation and metastasis in vivo. Oncotarget. 2016;7:16227–16247. doi: 10.18632/oncotarget.7583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Sierko E, Wojtukiewicz MZ. Platelets and angiogenesis in malignancy. Semin Thromb Hemost. 2004;30:95–108. doi: 10.1055/s-2004-822974. [DOI] [PubMed] [Google Scholar]
- 68.Kopp HG, Placke T, Salih HR. Platelet-derived transforming growth factor-beta downregulates NKG2D thereby inhibiting natural killer cell antitumor reactivity. Cancer Res. 2009;69:7775–7783. doi: 10.1158/0008-5472.CAN-09-2123. [DOI] [PubMed] [Google Scholar]
- 69.Granot Z, Henke E, Comen EA, King TA, Norton L, Benezra R. Tumor entrained neutrophils inhibit seeding in the premetastatic lung. Cancer Cell. 2011;20:300–314. doi: 10.1016/j.ccr.2011.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Lou XL, Sun J, Gong SQ, Yu XF, Gong R, Deng H. Interaction between circulating cancer cells and platelets: clinical implication. Chin J Cancer Res. 2015;27:450–460. doi: 10.3978/j.issn.1000-9604.2015.04.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Liu Y, Zhao F, Gu W, Yang H, Meng Q, Zhang Y, Yang H, Duan Q. The roles of platelet GPIIb/IIIa and alphavbeta3 integrins during HeLa cells adhesion, migration, and invasion to monolayer endothelium under static and dynamic shear flow. J Biomed Biotechnol. 2009;2009:829243. doi: 10.1155/2009/829243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Jabbar AA, Kazarian T, Hakobyan N, Valentino LA. Gangliosides promote platelet adhesion and facilitate neuroblastoma cell adhesion under dynamic conditions simulating blood flow. Pediatr Blood Cancer. 2006;46:292–299. doi: 10.1002/pbc.20326. [DOI] [PubMed] [Google Scholar]
- 73.Egan K, Cooke N, Kenny D. Living in shear: platelets protect cancer cells from shear induced damage. Clin Exp Metastasis. 2014;31:697–704. doi: 10.1007/s10585-014-9660-7. [DOI] [PubMed] [Google Scholar]
- 74.Di Carlo E, Forni G, Lollini P, Colombo MP, Modesti A, Musiani P. The intriguing role of polymorphonuclear neutrophils in antitumor reactions. Blood. 2001;97:339–345. doi: 10.1182/blood.v97.2.339. [DOI] [PubMed] [Google Scholar]
- 75.Queen MM, Ryan RE, Holzer RG, Keller-Peck CR, Jorcyk CL. Breast cancer cells stimulate neutrophils to produce oncostatin M: potential implications for tumor progression. Cancer Res. 2005;65:8896–8904. doi: 10.1158/0008-5472.CAN-05-1734. [DOI] [PubMed] [Google Scholar]
- 76.Begandt D, Thome S, Sperandio M, Walzog B. How neutrophils resist shear stress at blood vessel walls: molecular mechanisms, subcellular structures, and cell-cell interactions. J Leukoc Biol. 2017;102:699–709. doi: 10.1189/jlb.3MR0117-026RR. [DOI] [PubMed] [Google Scholar]
- 77.Jadhav S, Konstantopoulos K. Fluid shearand time-dependent modulation of molecular interactions between PMNs and colon carcinomas. Am J Physiol Cell Physiol. 2002;283:C1133–1143. doi: 10.1152/ajpcell.00104.2002. [DOI] [PubMed] [Google Scholar]
- 78.Liang S, Fu C, Wagner D, Guo H, Zhan D, Dong C, Long M. Two-dimensional kinetics of beta 2-integrin and ICAM-1 bindings between neutrophils and melanoma cells in a shear flow. Am J Physiol Cell Physiol. 2008;294:C743–753. doi: 10.1152/ajpcell.00250.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Qian BZ, Li J, Zhang H, Kitamura T, Zhang J, Campion LR, Kaiser EA, Snyder LA, Pollard JW. CCL2 recruits inflammatory monocytes to facilitate breast-tumour metastasis. Nature. 2011;475:222–225. doi: 10.1038/nature10138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Evani SJ, Prabhu RG, Gnanaruban V, Finol EA, Ramasubramanian AK. Monocytes mediate metastatic breast tumor cell adhesion to endothelium under flow. FASEB J. 2013;27:3017–3029. doi: 10.1096/fj.12-224824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Strilic B, Offermanns S. Intravascular survival and extravasation of tumor cells. Cancer Cell. 2017;32:282–293. doi: 10.1016/j.ccell.2017.07.001. [DOI] [PubMed] [Google Scholar]
- 82.Heidemann F, Schildt A, Schmid K, Bruns OT, Riecken K, Jung C, Ittrich H, Wicklein D, Reimer R, Fehse B, Heeren J, Luers G, Schumacher U, Heine M. Selectins mediate small cell lung cancer systemic metastasis. PLoS One. 2014;9:e92327. doi: 10.1371/journal.pone.0092327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Gomes N, Berard M, Vassy J, Peyri N, Legrand C, Fauvel-Lafeve F. Shear stress modulates tumour cell adhesion to the endothelium. Biorheology. 2003;40:41–45. [PubMed] [Google Scholar]
- 84.Fennewald SM, Kantara C, Sastry SK, Resto VA. Laminin interactions with head and neck cancer cells under low fluid shear conditions lead to integrin activation and binding. J Biol Chem. 2012;287:21058–21066. doi: 10.1074/jbc.M112.360313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Papadimitriou MN, Menter DG, Konstantopoulos K, Nicolson GL, McIntire LV. Integrin alpha4beta1/VCAM-1 pathway mediates primary adhesion of RAW117 lymphoma cells to hepatic sinusoidal endothelial cells under flow. Clin Exp Metastasis. 1999;17:669–676. doi: 10.1023/a:1006747106885. [DOI] [PubMed] [Google Scholar]
- 86.Richter U, Schroder C, Wicklein D, Lange T, Geleff S, Dippel V, Schumacher U, Klutmann S. Adhesion of small cell lung cancer cells to E- and P-selectin under physiological flow conditions: implications for metastasis formation. Histochem Cell Biol. 2011;135:499–512. doi: 10.1007/s00418-011-0804-4. [DOI] [PubMed] [Google Scholar]
- 87.Richter U, Wicklein D, Geleff S, Schumacher U. The interaction between CD44 on tumour cells and hyaluronan under physiologic flow conditions: implications for metastasis formation. Histochem Cell Biol. 2012;137:687–695. doi: 10.1007/s00418-012-0916-5. [DOI] [PubMed] [Google Scholar]
- 88.Aigner S, Ramos CL, Hafezi-Moghadam A, Lawrence MB, Friederichs J, Altevogt P, Ley K. CD24 mediates rolling of breast carcinoma cells on P-selectin. FASEB J. 1998;12:1241–1251. doi: 10.1096/fasebj.12.12.1241. [DOI] [PubMed] [Google Scholar]
- 89.Yan WW, Cai B, Liu Y, Fu BM. Effects of wall shear stress and its gradient on tumor cell adhesion in curved microvessels. Biomech Model Mechanobiol. 2012;11:641–653. doi: 10.1007/s10237-011-0339-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Yano HJ, Hatano K, Tsuno N, Osada T, Watanabe T, Tsuruo T, Muto T, Nagawa H. Clustered cancer cells show a distinct adhesion behavior from single cell form under physiological shear conditions. J Exp Clin Cancer Res. 2001;20:407–412. [PubMed] [Google Scholar]
- 91.Miles FL, Pruitt FL, van Golen KL, Cooper CR. Stepping out of the flow: capillary extravasation in cancer metastasis. Clin Exp Metastasis. 2008;25:305–324. doi: 10.1007/s10585-007-9098-2. [DOI] [PubMed] [Google Scholar]
- 92.Khuon S, Liang L, Dettman RW, Sporn PH, Wysolmerski RB, Chew TL. Myosin light chain kinase mediates transcellular intravasation of breast cancer cells through the underlying endothelial cells: a three-dimensional FRET study. J Cell Sci. 2010;123:431–440. doi: 10.1242/jcs.053793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Schluter K, Gassmann P, Enns A, Korb T, Hemping-Bovenkerk A, Holzen J, Haier J. Organ-specific metastatic tumor cell adhesion and extravasation of colon carcinoma cells with different metastatic potential. Am J Pathol. 2006;169:1064–1073. doi: 10.2353/ajpath.2006.050566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Ma S, Fu A, Chiew GG, Luo KQ. Hemodynamic shear stress stimulates migration and extravasation of tumor cells by elevating cellular oxidative level. Cancer Lett. 2017;388:239–248. doi: 10.1016/j.canlet.2016.12.001. [DOI] [PubMed] [Google Scholar]
- 95.Lawler K, Meade G, O’Sullivan G, Kenny D. Shear stress modulates the interaction of platelet-secreted matrix proteins with tumor cells through the integrin alphavbeta3. Am J Physiol Cell Physiol. 2004;287:C1320–1327. doi: 10.1152/ajpcell.00159.2004. [DOI] [PubMed] [Google Scholar]
- 96.Iskratsch T, Wolfenson H, Sheetz MP. Appreciating force and shape-the rise of mechanotransduction in cell biology. Nat Rev Mol Cell Biol. 2014;15:825–833. doi: 10.1038/nrm3903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Sawada Y, Tamada M, Dubin-Thaler BJ, Cherniavskaya O, Sakai R, Tanaka S, Sheetz MP. Force sensing by mechanical extension of the Src family kinase substrate p130Cas. Cell. 2006;127:1015–1026. doi: 10.1016/j.cell.2006.09.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Chistiakov DA, Orekhov AN, Bobryshev YV. Effects of shear stress on endothelial cells: go with the flow. Acta Physiol (Oxf) 2017;219:382–408. doi: 10.1111/apha.12725. [DOI] [PubMed] [Google Scholar]
- 99.Dunn J, Simmons R, Thabet S, Jo H. The role of epigenetics in the endothelial cell shear stress response and atherosclerosis. Int J Biochem Cell Biol. 2015;67:167–176. doi: 10.1016/j.biocel.2015.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Shivashankar GV. Mechanosignaling to the cell nucleus and gene regulation. Annu Rev Biophys. 2011;40:361–378. doi: 10.1146/annurev-biophys-042910-155319. [DOI] [PubMed] [Google Scholar]
- 101.Luscinskas FW, Lim YC, Lichtman AH. Wall shear stress: the missing step for T cell transmigration? Nat Immunol. 2001;2:478–480. doi: 10.1038/88663. [DOI] [PubMed] [Google Scholar]
- 102.Moura Rosa P, Gopalakrishnan N, Ibrahim H, Haug M, Halaas O. The intercell dynamics of T cells and dendritic cells in a lymph nodeona-chip flow device. Lab Chip. 2016;16:3728–3740. doi: 10.1039/c6lc00702c. [DOI] [PubMed] [Google Scholar]
- 103.Seneviratne AN, Cole JE, Goddard ME, Park I, Mohri Z, Sansom S, Udalova I, Krams R, Monaco C. Low shear stress induces M1 macrophage polarization in murine thin-cap atherosclerotic plaques. J Mol Cell Cardiol. 2015;89:168–172. doi: 10.1016/j.yjmcc.2015.10.034. [DOI] [PubMed] [Google Scholar]
- 104.Diaz MF, Li N, Lee HJ, Adamo L, Evans SM, Willey HE, Arora N, Torisawa YS, Vickers DA, Morris SA, Naveiras O, Murthy SK, Ingber DE, Daley GQ, Garcia-Cardena G, Wenzel PL. Biomechanical forces promote blood development through prostaglandin E2 and the cAMP-PKA signaling axis. J Exp Med. 2015;212:665–680. doi: 10.1084/jem.20142235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Steinert G, Scholch S, Niemietz T, Iwata N, Garcia SA, Behrens B, Voigt A, Kloor M, Benner A, Bork U, Rahbari NN, Buchler MW, Stoecklein NH, Weitz J, Koch M. Immune escape and survival mechanisms in circulating tumor cells of colorectal cancer. Cancer Res. 2014;74:1694–1704. doi: 10.1158/0008-5472.CAN-13-1885. [DOI] [PubMed] [Google Scholar]
- 106.Kulasinghe A, Perry C, Kenny L, Warkiani ME, Nelson C, Punyadeera C. PD-L1 expressing circulating tumour cells in head and neck cancers. BMC Cancer. 2017;17:333. doi: 10.1186/s12885-017-3316-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Jaiswal S, Jamieson CH, Pang WW, Park CY, Chao MP, Majeti R, Traver D, van Rooijen N, Weissman IL. CD47 is upregulated on circulating hematopoietic stem cells and leukemia cells to avoid phagocytosis. Cell. 2009;138:271–285. doi: 10.1016/j.cell.2009.05.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Wang J, Kaplan JA, Colson YL, Grinstaff MW. Mechanoresponsive materials for drug delivery: harnessing forces for controlled release. Adv Drug Deliv Rev. 2017;108:68–82. doi: 10.1016/j.addr.2016.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]


