Abstract
Steroid-induced osteonecrosis of the femoral head (ONFH) is a common orthopedic disease. The lack of specific manifestations and effective diagnostic methods make it difficult for this disease to be diagnosed at early stages. Recent studies have shown that microRNAs (miRNA) participate in the development of steroid-induced ONFH, but there is limited research into the diagnostic use of circulating miRNAs. Blood samples from 23 human subjects (7 systemic lupus erythematosus (SLE) patients with steroid-induced ONFH; 7 SLE controls without ONFH; and 9 healthy controls) and 71 rats (19 with steroid-induced ONFH; 28 receiving steroids without ONFH; and 24 untreated controls) were collected to verify the abundance of changes of 6 previously identified ONFH-associated plasma miRNAs (miR-423-5p, miR-99a-5p, miR-10a-5p, miR-21-5p, miR-130a-3p and miR-6787-5p) by quantitative RT-PCR (Reverse Transcription-Polymerase Chain Reaction). In humans, the circulating levels of miR-10a-5p, miR-99a-5p and miR-21-5p were increased in SLE patients treated with cortico steroid regardless of ONFH status when compared with healthy controls. However, miR-423-5p, miR-6787-5p and miR-130a-3p showed no significant differences between the three groups. In the rat model, the success rate of steroid-induced ONFH was 40.4% (19/47) based on pathological examination and confirmation by micro-CT scan. Similar to human plasma, the circulating levels of miR-10a-5p, miR-99a-5p and miR-21-5p were increased in steroid-treated rats independent of ONFH development. The serum levels of miR-10a-5p, miR-99a-5p and miR-21-5p were increased by steroid treatment regardless of ONFH development in both humans and rats. These data suggested that miR-10a-5p, miR-99a-5p and miR-21-5p are steroid-responsive circulating miRNAs, but they are not specific for diagnosing steroid-induced ONFH.
Keywords: Osteonecrosis of the femoral head, systemic lupus erythematosus, plasma micrornas
Introduction
Steroid-induced osteonecrosis of femoral head (ONFH) is a common disease that mainly occurs in young adults [1,2]. The exact pathogenesis of steroid-induced ONFH is still unknown. Early diagnosis and intervention may help prevent the progression of ONFH and avoid the collapse of the femoral head and joint dysfunction. However, the lack of specific manifestations and sensitive diagnostic methods makes it difficult to diagnose steroid-induced ONFH at early stages. As a result, many patients have no choice but to undergo hip replacement surgery at a young age [3]. Recent studies have shown that microRNAs (miRNA) could regulate different cellular processes, such as apoptosis, angiogenesis, cell differentiation and extracellular matrix deposition in different orthopedic conditions, including ONFH [4,5]. These findings suggest that miRNAs may participate in the development of ONFH.
Previous studies have demonstrated that miRNAs are involved in the development of ONFH, but none of these studies focused on circulating miRNAs, which are stable, detectable, and can be purified and quantified from serum samples [6]. As it was unrealistic to perform biopsies for diagnosing ONFH, detection of circulating miRNAs could be a convenient and effective, non-invasive method for early diagnosis. It therefore would be useful to identify circulating miRNAs as plasma markers for early clinical screening of steroid-induced ONFH.
We previously published our results screening circulating miRNAs in steroid-induced ONFH by Illumina-based high-throughput sequencing technology, which was the only study of circulating miRNAs under these conditions [6]. Our previous results demonstrated that 27 circulating miRNAs were differentially expressed in the serum of patients with steroid-induced ONFH. Among them, levels of 15 and 12 miRNAs were increased and decreased, respectively, when compared to both patients with systemic lupus erythematosus (SLE) on steroid therapy and to healthy subjects. To establish plasma miRNAs as early diagnostic markers, the current study sought to validate differences in selected plasma miRNA in steroid-induced ONFH when compared with SLE patients.
Materials and methods
Patients and grouping
All subjects provided written informed consent, and this study was approved by the Ethics Committee of Peking Union Medical College Hospital (Beijing, China). Patients were divided into three groups: 7 patients with ONFH secondary to hormone therapy for the treatment of systemic lupus erythematosus (SLE), 7 matched controls with SLE and similar hormone treatment history but without the development of ONFH, and 9 healthy controls. Subjects in each group were strictly matched with age, gender, and history of hormone exposure. After blood was derived, serum was separated from the sample within 1 h. All serum samples were then stored at -80°C.
Animal experiments and grouping
Seventy-two Sprague-Dawley (SD) adult male rats (12 weeks old; body weight between 400 g and 450 g; from Charles River Company, Beijing, China) were used in this study. All rats were housed in standard laboratory conditions (12/12-h light/dark cycle, 24-25°C, with humidity 50-55%) and allowed free access to food and water during the study. Diet, hair, and activity state of the rats was recorded every day, and the weight of the rats was measured weekly. On the third day of the experiment, one of the rats died from an abdominal infection. All the other rats were healthy without death or infection. After a week of adjusting to the environment, the rats were randomized into 2 groups: a normal saline (NS) group and a lipopolysaccharide/methylprednisolone (LPS/MPS) group. Forty-seven rats from the LPS/MPS group were given two doses of intraperitoneal injection of 20 μg/kg of LPS (from Escherichia coli 055: B5, Sigma, St. Louis, MO, USA) on days 0 and 1. Twenty-four hours after the last LPS injection, the rats received three intramuscular injections of 40 mg/kg of methylprednisolone sodium succinate (Pfizer Pharmaceutical, China) on days 3, 4 and 5 at a 24 h time interval. Twenty-four rats from the NS group only received normal saline. At 4 weeks, blood from the 24 rats in NS group (control group) and the 47 rats in LPS/MPS group was collected, and the animals were sacrificed. The femoral heads were harvested for micro-CT and histological examination. The animal use and care protocols were reviewed and approved by the Committee for Animal Experiments of the Peking Union Medical College Hospital. The rats were sacrificed by CO2 asphyxiation.
Micro-CT scanning
The specimens were scanned by skyscan 1076 in vivo X-ray microtomograph (SKYSCAN Micro-CT 1076) at a voltage of 70 kV and a current of 139 μA. An entire scan length of 20 mm and a spatial resolution of 18 μm were used for animal experimental studies and reconstructed using NRecon. An area 1 mm below the center of the epiphyseal line with a total of 50 slices was chosen as the region of interest (ROI) for analysis and comparison of trabecula parameters. The bone volume/total volume (BV/TV) in the ROI was computed by the workstation.
Pathological examination
The femur specimens were fixed in a 10% buffered neutral formalin solution for 72 h and followed by decalcification with 10% ethylenediaminetetraacetic acid (EDTA)-0.1 M of phosphate buffer (pH 7.4). Then, the tissues were dehydrated in graded ethanol, embedded in paraffin, cut into 4-μm thick sections in the coronal plane, and processed for routine hematoxylin and eosin staining for the evaluation of osteonecrosis. All sections were blindly and independently assessed by two examiners using a microscope (Leica, Wetzlar, Germany). The diagnosis of osteonecrosis was established based on the presence of empty lacunae or pyknotic nuclei of osteocytes in the bone trabeculae, accompanied by surrounding bone marrow cell necrosis [7]. If the diagnosis differed between the two examiners, a consensus was reached by a third person.
RNA extraction and quantitative RT-PCR
Total RNA was extracted from plasma using a Total RNA Kit (OMEGA, Norocross, GA, USA), according to the manufacturer’s instructions. cDNA was synthesized using a High-Capacity cDNA Reverse Transcription Kits (cat.no.4368814). Quantitative reverse transcription (qRT)-PCR was carried out using EvaGreen with an ABI 7500 thermal cycler. The sequences of primers for the selected miRNAs are shown in Table 1. miRNA levels were normalized to GAPDH. All the experiments were performed in triplicate.
Table 1.
The primers for the micro-RNAs
| Name | Sequence | RT-primer | For primer |
|---|---|---|---|
| hsa-miR-16-5p (internal control) | UAGCAGCACGUAAAUAUUGGCG | GTCGTATCCAGTGCAGGGTCCGAGGTATTCGCACTGGATACGACTTGGCG | AGGGCCCTGTTCTCCATTACT |
| hsa-miR-29c-3p | UAGCACCAUUUGAAAUCGGUUA | GTCGTATCCAGTGCACGCTCCGAGGTATTCGCACTGGATACGACTAACCG | TCGCGCTAGCACCATTT |
| hsa-miR-423-5p | UGAGGGGCAGAGAGCGAGACUUU | GTCGTATCCAGTGCACGCTCCGAGGTATTCGCACTGGATACGACAAAGTCTC | TCGCGCTGAGGGGCAGA |
| hsa-miR-6787-5p | UGGCGGGGGUAGAGCUGGCUGC | GTCGTATCCAGTGCACGCTCCGAGGTATTCGCACTGGATACGACGCAGCCAG | TCGCGCTGGCGGGGGTA |
| hsa-miR-21 | UAGCUUAUCAGACUGAUGUUGA | GTCGTATCCAGTGCACGCTCCGAGGTATTCGCACTGGATACGACTCAACATC | TCGCGCTAGCTTATCAG |
| hsa-miR-130a | CAGUGCAAUGUUAAAAGGGCAU | GTCGTATCCAGTGCACGCTCCGAGGTATTCGCACTGGATACGACATGCCCTT | TCGCGCCAGTGCAATGT |
| hsa-miR-10a | UACCCUGUAGAUCCGAAUUUGUG | GTCGTATCCAGTGCACGCTCCGAGGTATTCGCACTGGATACGACCACAAATT | TCGCGCTACCCTGTAGA |
| hsa-miR-99a | AACCCGUAGAUCCGAUCUUGUG | GTCGTATCCAGTGCACGCTCCGAGGTATTCGCACTGGATACGACCACAAGAT | TCGCGCAACCCGTAGAT |
Statistical analysis
Data are presented as the mean ± standard deviation (SD). One-way ANOVA and pairwise comparison among groups was performed using multiple comparisons tests with GraphPad Prism 6.02 (USA, GraphPad Software). Differences with P<0.05 were considered significant.
Results
Serum levels of miR-10a-5p, miR-99a-5p and miR-21-5p were increased in steroid-treated SLE patients with or without ONFH
The levels of miR-10a-5p, miR-99a-5p, miR-423-5p, miR-21-5p, miR-6787-5p and miR-130a-3p were measured in the 23 human blood plasma samples (9 healthy controls, 7 non-ONFH SLE controls, and 7 ONFH) using qRT-PCR. The results showed that the levels of miR-10a-5p (Figure 1A), miR-99a-5p (Figure 1B) and miR-423-5p (Figure 1D) were all increased in both the steroid-treated ONFH group and non-ONFH SLE control group when compared to the healthy controls. However, the levels of miR-423-5p (Figure 1C), miR-6787-5p (Figure 1E) and miR-130a-3p (Figure 1F) were not significantly different among the three groups.
Figure 1.
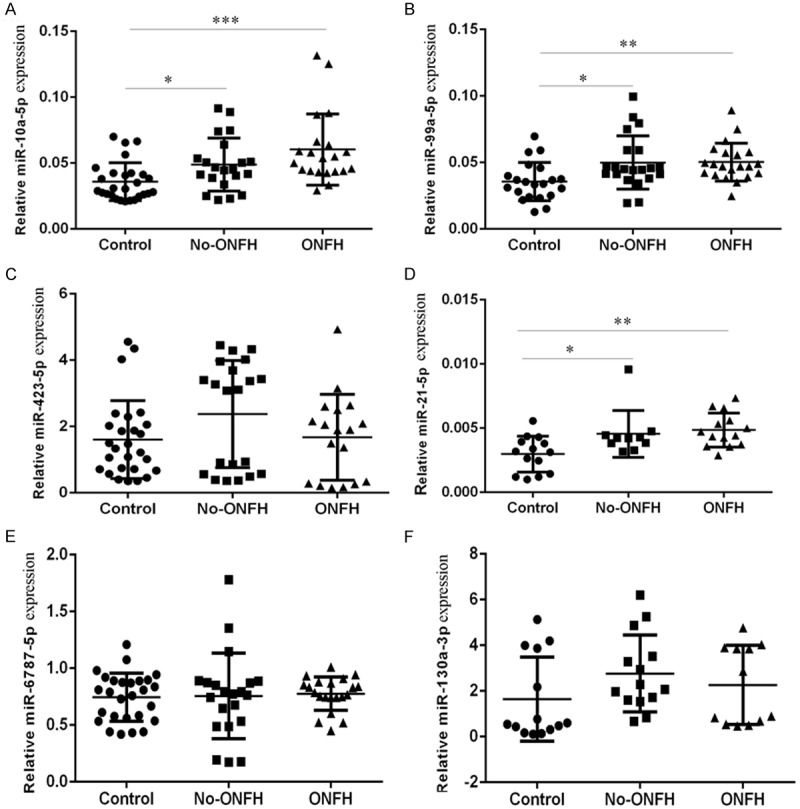
The expression of miR-10a-5p, miR-99a-5p, miR-423-5p, miR-21-5p, miR-6787-5p and miR-130a-3p in the 23 human blood plasma samples (9 healthy controls, 7 non-ONFH SLE controls, and 7 ONFH) was determined by using qRT-PCR. U6 was used as the internal control. *P<0.05, **P<0.01 and ***P<0.001.
Steroid-induced ONFH was established in rats
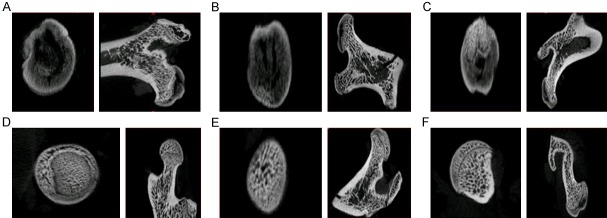
The histopathological appearance of the femoral heads of SD rats in both the NS and LPS/MPS groups was assessed by routine hematoxylin-eosin staining. Nineteen out of 47 (40.4%) rats in the LPS/MPS group developed ONFH, which presented empty bone lacuna or pyknotic nuclei of osteocytes in the bone trabeculae, accompanied by bone marrow necrosis. None of the mice in the NS control group demonstrated these features (Figure 4). The histological diagnosis was confirmed with micro-CT scanning, which was performed on the animals prior to sacrifice. The 3D reconstruction showed that mice with histologically confirmed ONFH exhibited collapsed femoral heads and lost the shape of the smooth hemisphere when compared with the NS control group (Figure 2). The horizontal and coronal sections of micro-CT from the ONFH group also showed broken trabecula and cystic degeneration when compared with the NS control group (Figure 3). Quantitative analysis showed that BV/TV of the ONFH group (Mean ± SD: 33.64±1.223%) was significantly lower than that of the non-ONFH group (Mean ± SD: 41.404±1.580%) and the NS control group (Mean ± SD: 51.23±2.162%) (ONFH vs non-ONFH: P=0.0002; ONFH vs NS control: P<0.0001).
5.1.5×; 5.2.10×; 5.3.20× (LEICA DM3000.
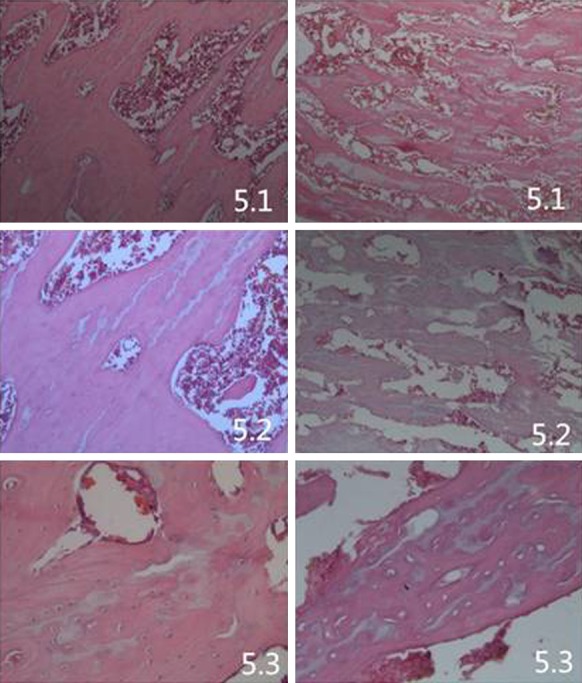
) The histopathological appearance of the femoral head, processed for routine hematoxylin and stained by eosin, in the ONFH group, which showed trabecular bone fracture and structure disorder, increased trabecular spacing, significantly increased empty bone lacuna, bleeding point of bone marrow necrosis and hyperplasia of fibrous structure. While the normal group and non-ONFH group showed better arranged and more condensed trabeculae compared with the ONFH group. By HE stained histological examination, the incidence of osteonecrosis was 40.4% (19/47).
Figure 2.
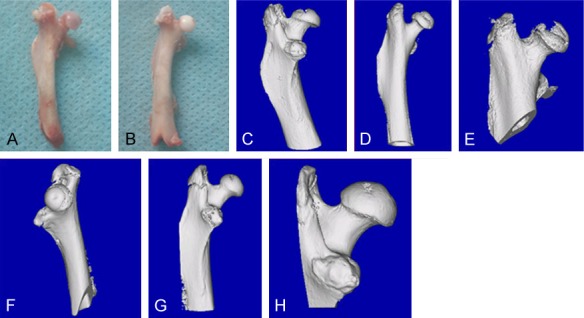
3D micro-CT reconstruction. A, C-E: ONFH group; B, F-H: non-ONFH group. The 3D reconstruction showed that mice with histologically confirmed ONFH exhibited collapsed femoral heads and lost the shape of the smooth hemisphere when compared with the NS control group.
Figure 3.
The horizontal and coronal section of micro-CT. A-C: ONFH group: Obvious necrosis and cyst degeneration; D-F: Control group. The horizontal and coronal sections of micro-CT from the ONFH group also showed broken trabecula and cystic degeneration when compared with the NS control group.
Serum levels of miR-10a-5p, miR-99a-5p and miR-21-5p were increased, while miR-6787-5p was decreased in LPS/MPS-treated rats regardless of ONFH development
Similar to the human study, the serum levels of miR-10a-5p, miR-99a-5p, miR-423-5p, miR-21-5p, miR-6787-5p and miR-130a-3p were determined by qRT-PCR in the 71 rat blood plasma samples (19 LPS/MPS-treated rats with ONFH, 28 LPS/MPS-treated rats without ONFH and 24 NS controls). Consistent with the levels of abundance of miRNAs in the human serum, the levels of miR-10a-5p (Figure 5A), miR-99a-5p (Figure 5B) and miR-423-5p (Figure 5D) were increased in the LPS/MPS-treated rats regardless of ONFH development when compared to the NS control group. Moreover, the levels of miR-6787-5p were found to be reduced in the in the LPS/MPS-treated rats (Figure 5E). However, the circulating levels of miR-423-5p (Figure 5C) and miR-130a-3p (Figure 5F) were not significantly different among the three groups.
Figure 5.
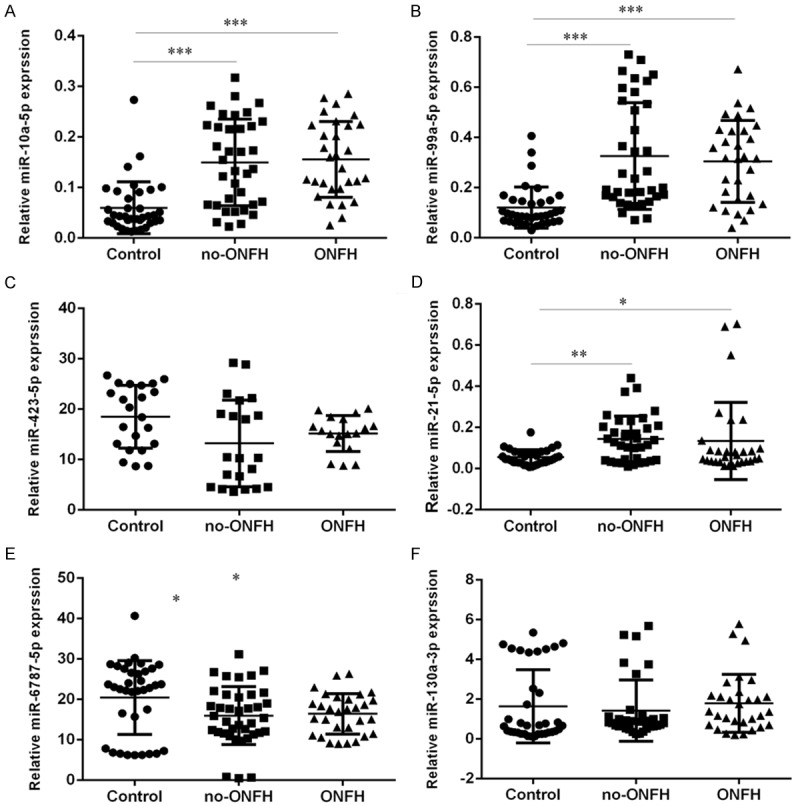
The expression of miR-10a-5p, miR-99a-5p, miR-423-5p, miR-21-5p, miR-6787-5p and miR-130a-3p in the 71 rat blood plasma samples (19 LPS/MPS-treated rats with ONFH, 28 LPS/MPS-treated rats without ONFH and 24 NS controls) was determined using qRT-PCR. U6 was used the internal control. *P<0.05, **P<0.01 and ***P<0.001.
Discussion
Several studies [8-20] on the association between steroid-induced ONFH and miRNA deregulation have been published, but none have focused on circulating miRNAs. As it is clinically infeasible to perform biopsies for diagnosing steroid-induced ONFH, biomarkers for early diagnosis are urgently needed. With this background, we conducted a pilot study for screening circulating microRNAs associated with steroid-induced ONFH [6]. This current endeavor sought to confirm the associations using animal models and a larger cohort of patients with steroid-induced ONFH. After analyzing the relative abundance of miRNA levels in combination with the results of a previous high-throughput sequencing study [16], six miRNAs with relatively high specificity and sensitivity were chosen for further validation by qRT-PCR. Notably, 3 miRNAs (miR-10a-5p, miR-423-5p, and miR-99a-5p) were selected from our previous study [6], in which 27 differentially abundant (15 increased and 12 reduced) miRNAs from ONFH serum were identified when compared with the SLE and healthy controls. Unfortunately, none of the 6 tested miRNAs showed specificity to steroid-induced ONFH.
In this study, we demonstrated that the serum levels of miR-10a-5p, miR-99a-5p and miR-21-5p were increased in steroid-exposed SLE patients and MPS-treated rats regardless of ONFH development, suggesting that these 3 miRNAs are steroid-responsive. In this connection, aberrant down-regulation of miR-10a-5p in synoviocytes has been shown to contribute to TBX5-controlled joint inflammation [21]. miR-10a could also alleviate vascular inflammation by down-regulating the pro-inflammatory GATA6/VCAM-1 signaling in endothelial cells [22]. miR-99a has also been shown to inhibit the LPS-induced vascular inflammation via inhibition of the mTOR/NF-κB signal [23]. Increased expression of miR-99a could also reduce the activity of STAT3 [24], which is a key mediator of pro-inflammatory signaling [25]. Moreover, the anti-inflammatory effects of miR-21 in the macrophage response to peritonitis has been demonstrated [26]. Our findings, together with the reported anti-inflammatory functions of miR-10a-5p, miR-99a-5p and miR-21-5p, suggested that these 3 miRNAs might be responsible for, at least in part, the systemic anti-inflammatory effect of corticosteroid. Nevertheless, further studies assessing the anti-inflammatory effects of corticosteroids in specific miRNA-knockout mice would be required to test this postulation.
To conclude, we have demonstrated that the circulating levels of miR-10a-5p, miR-99a-5p and miR-21-5p were all increased in both steroid-treated SLE patients and LPS/MPS-treated rats regardless of ONFH development. These miRNAs might serve as biomarkers of steroid exposure. However, their functional roles in the anti-inflammatory actions of cortico steroids remain unclear. Our data also suggest that other miRNAs should be sought for the specific diagnosis of steroid-induced ONFH.
Acknowledgements
This study was supported by the National Natural Science Foundation of China (Nos. 81572110 and 81171775).
Disclosure of conflict of interest
None.
References
- 1.Kang JS, Park S, Song JH, Jung YY, Cho MR, Rhyu KH. Prevalence of osteonecrosis of the femoral head: a nationwide epidemiologic analysis in Korea. J Arthroplasty. 2009;24:1178–1183. doi: 10.1016/j.arth.2009.05.022. [DOI] [PubMed] [Google Scholar]
- 2.Fukushima W, Fujioka M, Kubo T, Tamakoshi A, Nagai M, Hirota Y. Nationwide epidemiologic survey of idiopathic osteonecrosis of the femoral head. Clin Orthop Relat Res. 2010;468:2715–2724. doi: 10.1007/s11999-010-1292-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hong YC, Zhong HM, Lin T, Shi JB. Comparison of core decompression and conservative treatment for avascular necrosis of femoral head at early stage: a meta-analysis. Int J Clin Exp Med. 2015;8:5207–5216. [PMC free article] [PubMed] [Google Scholar]
- 4.Lu J, Getz G, Miska EA, Alvarez-Saavedra E, Lamb J, Peck D, Sweet-Cordero A, Ebert BL, Mak RH, Ferrando AA, Downing JR, Jacks T, Horvitz HR, Golub TR. MicroRNA expression profiles classify human cancers. Nature. 2005;435:834–838. doi: 10.1038/nature03702. [DOI] [PubMed] [Google Scholar]
- 5.Miska EA, Alvarez-Saavedra E, Townsend M, Yoshii A, Sestan N, Rakic P, Constantine-Paton M, Horvitz HR. Microarray analysis of microRNA expression in the developing mammalian brain. Genome Biol. 2004;5:R68. doi: 10.1186/gb-2004-5-9-r68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang X, Qian W, Wu Z, Bian Y, Weng X. Preliminary screening of differentially expressed circulating microRNAs in patients with steroidinduced osteonecrosis of the femoral head. Mol Med Rep. 2014;10:3118–3124. doi: 10.3892/mmr.2014.2660. [DOI] [PubMed] [Google Scholar]
- 7.Irisa T, Yamamoto T, Miyanishi K, Yamashita A, Iwamoto Y, Sugioka Y, Sueishi K. Osteonecrosis induced by a single administration of low-dose lipopolysaccharide in rabbits. Bone. 2001;28:641–649. doi: 10.1016/s8756-3282(01)00460-4. [DOI] [PubMed] [Google Scholar]
- 8.Yamasaki K, Nakasa T, Miyaki S, Yamasaki T, Yasunaga Y, Ochi M. Angiogenic microRNA-210 is present in cells surrounding osteonecrosis. J Orthop Res. 2012;30:1263–1270. doi: 10.1002/jor.22079. [DOI] [PubMed] [Google Scholar]
- 9.Sun J, Wang Y, Li Y, Zhao G. Downregulation of PPARgamma by miR-548d-5p suppresses the adipogenic differentiation of human bone marrow mesenchymal stem cells and enhances their osteogenic potential. J Transl Med. 2014;12:168. doi: 10.1186/1479-5876-12-168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jia J, Feng X, Xu W, Yang S, Zhang Q, Liu X, Feng Y, Dai Z. MiR-17-5p modulates osteoblastic differentiation and cell proliferation by targeting SMAD7 in non-traumatic osteonecrosis. Exp Mol Med. 2014;46:e107. doi: 10.1038/emm.2014.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yuan HF, Von Roemeling C, Gao HD, Zhang J, Guo CA, Yan ZQ. Analysis of altered microRNA expression profile in the reparative interface of the femoral head with osteonecrosis. Exp Mol Pathol. 2015;98:158–163. doi: 10.1016/j.yexmp.2015.01.002. [DOI] [PubMed] [Google Scholar]
- 12.Wu X, Zhang Y, Guo X, Xu H, Xu Z, Duan D, Wang K. Identification of differentially expressed microRNAs involved in non-traumatic osteonecrosis through microRNA expression profiling. Gene. 2015;565:22–29. doi: 10.1016/j.gene.2015.03.072. [DOI] [PubMed] [Google Scholar]
- 13.Bian Y, Qian W, Li H, Zhao RC, Shan WX, Weng X. Pathogenesis of glucocorticoid-induced avascular necrosis: a microarray analysis of gene expression in vitro. Int J Mol Med. 2015;36:678–684. doi: 10.3892/ijmm.2015.2273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wei B, Wei W. Identification of aberrantly expressed of serum microRNAs in patients with hormone-induced non-traumatic osteonecrosis of the femoral head. Biomed Pharmacother. 2015;75:191–195. doi: 10.1016/j.biopha.2015.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yuan HF, Christina VR, Guo CA, Chu YW, Liu RH, Yan ZQ. Involvement of microRNA-210 demethylation in steroid-associated osteonecrosis of the femoral head. Sci Rep. 2016;6:20046. doi: 10.1038/srep20046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang B, Yu P, Li T, Bian Y, Weng X. MicroRNA expression in bone marrow mesenchymal stem cells from mice with steroid-induced osteonecrosis of the femoral head. Mol Med Rep. 2015;12:7447–7454. doi: 10.3892/mmr.2015.4386. [DOI] [PubMed] [Google Scholar]
- 17.Gu C, Xu Y, Zhang S, Guan H, Song S, Wang X, Wang Y, Li Y, Zhao G. miR-27a attenuates adipogenesis and promotes osteogenesis in steroid-induced rat BMSCs by targeting PPARgamma and GREM1. Sci Rep. 2016;6:38491. doi: 10.1038/srep38491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liu G, Luo G, Bo Z, Liang X, Huang J, Li D. Impaired osteogenic differentiation associated with connexin43/microRNA-206 in steroid-induced avascular necrosis of the femoral head. Exp Mol Pathol. 2016;101:89–99. doi: 10.1016/j.yexmp.2016.07.009. [DOI] [PubMed] [Google Scholar]
- 19.Li T, Li H, Li T, Fan J, Zhao RC, Weng X. MicroRNA expression profile of dexamethasone-induced human bone marrow-derived mesenchymal stem cells during osteogenic differentiation. J Cell Biochem. 2014;115:1683–1691. doi: 10.1002/jcb.24831. [DOI] [PubMed] [Google Scholar]
- 20.Li T, Li H, Wang Y, Li T, Fan J, Xiao K, Zhao RC, Weng X. microRNA-23a inhibits osteogenic differentiation of human bone marrow-derived mesenchymal stem cells by targeting LRP5. Int J Biochem Cell Biol. 2016;72:55–62. doi: 10.1016/j.biocel.2016.01.004. [DOI] [PubMed] [Google Scholar]
- 21.Hussain N, Zhu W, Jiang C, Xu J, Wu X, Geng M, Hussain S, Cai Y, Xu K, Xu P, Han Y, Sun J, Meng L, Lu S. Down-regulation of miR-10a-5p in synoviocytes contributes to TBX5-controlled joint inflammation. J Cell Mol Med. 2018;22:241–250. doi: 10.1111/jcmm.13312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lee DY, Lin TE, Lee CI, Zhou J, Huang YH, Lee PL, Shih YT, Chien S, Chiu JJ. MicroRNA-10a is crucial for endothelial response to different flow patterns via interaction of retinoid acid receptors and histone deacetylases. Proc Natl Acad Sci U S A. 2017;114:2072–2077. doi: 10.1073/pnas.1621425114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bao MH, Li JM, Luo HQ, Tang L, Lv QL, Li GY, Zhou HH. NF-κB-Regulated miR-99a modulates endothelial cell inflammation. Mediators Inflamm. 2016;2016:5308170. doi: 10.1155/2016/5308170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lin K, Ye H, Han BW, Wang WT, Wei PP, He B, Li XJ, Chen YQ. Genome-wide screen identified let-7c/miR-99a/miR-125b regulating tumor progression and stem-like properties in cholangiocarcinoma. Oncogene. 2016;35:3376–3386. doi: 10.1038/onc.2015.396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hodge DR, Hurt EM, Farrar WL. The role of IL-6 and STAT3 in inflammation and cancer. Eur J Cancer. 2005;41:2502–2512. doi: 10.1016/j.ejca.2005.08.016. [DOI] [PubMed] [Google Scholar]
- 26.Barnett RE, Conklin DJ, Ryan L, Keskey RC, Ramjee V, Sepulveda EA, Srivastava S, Bhatnagar A, Cheadle WG. Anti-inflammatory effects of miR-21 in the macrophage response to peritonitis. J Leukoc Biol. 2016;99:361–371. doi: 10.1189/jlb.4A1014-489R. [DOI] [PMC free article] [PubMed] [Google Scholar]