Abstract
This study aimed to examine whether exogenous NaHS can protect myocardial mitochondrial injury from sepsis by enhancing the peroxisome proliferator-activated receptor gamma co-activator 1α (PGC-1α)/ nuclear factor erythroid-2-related factor 2 (NRF2) pathway and mitochondrial biosynthesis in mice. Animals were divided into sham-operated, sepsis, sepsis + 25 μmol/L NaHS, sepsis + 50 μmol/L NaHS, sepsis + 100 μmol/L NaHS, and sepsis + 200 μmol/L NaHS groups. The myocardial damage was evaluated by hematoxylin and eosin staining for myocardial microstructure and serum cardiac troponin I (cTnI) detection. The myocardial mitochondrial damage was evaluated through transmission electron microscopic observation of mitochondrial microstructure and detection of the degree of myocardial mitochondrial swelling. The adenosine triphosphate (ATP) level was used to appraise the mitochondrial function. The mRNA expression levels of Nrf2, PGC-1α, and Tfam were analyzed to explore the molecular mechanism. Results: In the sepsis group, the structure of myocardial tissue and mitochondria were significantly damaged, the serum cTnI level increased (P < 0.05), the ATP level reduced, the degree of myocardial mitochondrial swelling aggravated, and the mRNA expression levels of Nrf2, PGC-1α, and Tfam increased (P < 0.05). After NaHS treatment, the structure of myocardial tissue and mitochondria improved, the cTnI level reduced, the ATP level increased, the degree of myocardial mitochondrial swelling alleviated, and the mRNA expression level of Nrf2, PGC-1α, and Tfam increased continuously in a dose-dependent manner (P < 0.05). Conclusions: Exogenous NaHS had a protective effect against myocardial mitochondrial injury in sepsis. The mechanism might lie in enhancing the PGC-1α/NRF2 pathway and mitochondrial biosynthesis.
Keywords: NaHS, mitochondria biosynthesis, Nrf2, PGC-1α, sepsis, Tfam
Introduction
Sepsis may result from microbic pathogen infections, such as bacterial, fungal, viral, and parasitic, which trigger the release of inflammatory mediators and induce cellular dysfunction inthe affected host. Patients suffering from this syndrome may present with a dysregulated host response to infection and life-threatening organ dysfunction [1,2]. Multiple-organ dysfunctions are the major cause of death in patients with severe sepsis. Approximately 44% of these patients have cardiac dysfunction [3]. Therefore, early effective treatment of myocardial lesions can reduce the morbidity due to complications and can raise the cure rate of the disease.
Previous studies have demonstrated that impaired endogenous hydrogen sulfide (H2S) production is involved in the pathophysiological processes in sepsis-associated multiple-organ dysfunction [4,5]. Mitochondrial damage had been recognized as an important molecular pathology in sepsis. It is linked with the severity of organ dysfunction and, possibly, outcome of sepsis [6]. Endogenous H2S can protect cardiomyocytes via varied mechanisms, such as protection against oxidative stress and reduction of mitochondrial damage [7]. A previous study has revealed the role of H2S in cardioprotective signaling pathways that converge toward the mitochondria [8]. NaHS is an important exogenous donator of H2S. The mechanism underlying the improvement in mitochondrial function by NaHS is worth exploring. Many regulatory factors regulate mitochondrial biogenesis, such as peroxisome proliferator-activated receptor gamma co-activator 1α (PGC-1α) and Nrf2. PGC-1α is closely related to mitochondrial respiratory regulation and ATP synthesis, and can effectively promote mitochondrial biogenesis and reduce mitochondrial damage. Nrf2 is an important molecular family member [9,10] that can regulate mitochondrial function. PGC-1α can strongly induce the expression of Nrf2 gene [11] and further synergistically regulate the expression of mitochondrial genes encoded by the nucleus, such as mitochondrial transcription factor A (Tfam), cytochrome C, and mitochondrial complexes. These factors co-regulate mitochondrial biogenesis [12]. The next question is whether H2S improves mitochondrial function by adjusting PGC-1α and Nrf2 levels in sepsis. Therefore, this study investigated the protective role of NaHS against myocardial injury and its molecular mechanism using the Cecal ligation puncture (CLP) models of sepsis. The findings would provide new insights into clinical treatments of septic myocardial injury.
Materials and methods
Animals
C57BL/6 male specific pathogen free (SPF)-grade mice, aged 6-8 weeks and weighing 20-30 g were obtained from Beijing HFK Bioscience Co. Ltd. They were fed pellet feed in the cages ad libitum. They were acclimatized for 1 week before the start of the experiment. They were randomly divided into the following groups (10 mice per group): sham-operated (underwent a similar operation as the sepsis group without cecal ligation and puncture), sepsis (underwent a CLP procedure), and sepsis + NaHS (underwent a CLP procedure + an NaHS intervention, with four subgroups). NaHS concentrations were 25, 50, 100, and 200 μmol/L).
Ethics statement
All procedures and housing were in accordance with the United Kingdom Animal (Scientific Procedures) Act (1986) and the National Institutes of Health Guide for the Care and Use of Laboratory Animals (NIH Publications No. 8023, revised 1978).
Establishment of CLP models
The CLP models of sepsis were established in accordance with the original protocol developed by Tomasz Skirecki’s lab [13]. The mice were anesthetized with 7% chloral hydrate solution (5 mL/kg, i.p.). After anesthesia, the abdominal skin was prepared, and 75% alcohol disinfection was performed. The midline abdominal incision was made, and the cecum was ligated (1 cm from the apex) after externalization. The cecum was punctured twice (through and through) with a needle. The fecal mass from the punctured cecum was squeezed out to ensure patency of punctures. The cecum was relocated, and the peritoneum and skin were closed [13]. The mice underwent only incision and cecum exteriorization in the sham-operated group. After the operation, a hypodermic i.p. injection of normal saline was administered in the sham-operated and sepsis groups. In the sepsis + NaHS group, intraperitoneal an injection of NaHS (Sigma) at concentrations of 25, 50, 100, and 200 μmol/L (20 mL/kg) was administered. The mice were placed in the cage until postanesthetic recovery. After 12-h induction of CLP, the mice were anesthetized with 7% chloral hydrate solution (5 mL/kg). The serum and myocardial tissues were collected for subsequent analysis.
Isolation of mitochondria
A Mitochondria Fractionation Kit (Beyotime Biotech, China) was used to isolate rat heart mitochondria according to the Wang’s method described elsewhere [14]. The heart was quickly removed and placed in a buffer containing 250 mM sucrose, 10 mM Tris, and 1 mM EGTA, pH 7.4, at 4°C. The myocardium samples were minced into homogenates. The debris was removed by centrifugation at 700 rpm for 10 min. Then, the supernatant was centrifuged at 1000 rpm for 10 min. The myocardial mitochondrial pellets were obtained.
Detection of plasma endotoxin and serum cTnI
The serum level of the plasma endotoxin was detected using an endotoxin Limulus kit by the dynamic turbidity method. The serum level of cardiac troponin I (cTnI) was detected using an enzyme-linked immunosorbent assay kit by the double-antibody sandwich method.
Hematoxylin and eosin staining
The myocardial tissue was fixed using 4% paraformaldehyde for 30-50 min. Hematoxylin and eosin (HE) staining was conducted after the section was dehydrated using gradient alcohol, cleared using xylene, embedded using paraffin, and cut into sections. The sections were stained with hematoxylin and 0.5% eosin and sealed using neutral balsam.
Transmission electron microscopy
The myocardial tissue was cut into a square (about 1 mm3). After fixing with glutaraldehyde + osmium chloride and dehydrating using gradient acetone solution, the square was embedded and cut into semi-thin sections. The sections were stained using a compound dye (0.25% sodium borate: 0.25% basic fuchsin = 1:1). After cell image observation using the microscope, the sections were cut into ultrathin sections. The ultrathin sections were obtained using a copper wire mesh attached to a film prepared using 0.45% Fonnvar solution. After staining at room temperature using uranyl acetate and lead staining fluids, the sample was dried using a filter paper and observed using a transmission electron microscope (TEM).
Evaluation of myocardial mitochondrial damage
The swelling degree of myocardial mitochondria was evaluated using the optical density values, which were measured using a tube luminometer. The ATP level of myocardial mitochondria was detected using an ATP kit (BioVision U.S.A).
Detection of Nrf2, PGC-1α, and Tfam mRNA expression levels
TRIzol reagent (1 mL; Qiagen) was added to 50-100 mg tissue, and the homogenate was obtained. The homogenate was centrifuged at 4°C and 12,000 g for 10 min. After the removal of insoluble substances, the subsequent RNA extraction steps were conducted. After total RNA was extracted, random primer reverse transcription reaction and SYBR green I fluorescent quantitative polymerase chain reaction (PCR) (Eppendorf) were carried out. The mRNA expression levels of Nrf2, PGC-1α, and Tfam were detected. The primer sequences are shown in Table 1. The PCR condition was 94°C for 4 min, 94°C for 20 s, 60°C for 30 s, and 72°C for 30 s, a total of 35 cycles. Three repeated wells were set for each sample.
Table 1.
Primers of fluorescent quantitative PCR
| Primer name | Sequence (5’-3’) | Amplification length (bp) |
|---|---|---|
| Nrf2 F | CTTCCATTTACGGAGACCCAC | 175 |
| Nrf2 R | GATTCACGCATAGGAGCACTG | |
| PGC-1α F | TGGCACGCAGCCCTATTC | 210 |
| PGC-1α R | GAGGATCTACTGCCTGGGGAC | |
| Tfam F | GCAAAGGATGATTCGGCTCAGGGAA | 200 |
| Tfam R | CCGGATCGTTTCACACTTCGACGG |
Statistical analyses
Data were analyzed using the Statistical Product for Social Sciences (SPSS; version 20.0). The results of plasma endotoxin levels between two groups were analyzed using the Student t test. All data sets were presented as mean ± standard deviation (SD). For the degree of swelling, serum cTnI and ATP levels, and q-PCR experiments, the results were analyzed using one-way analysis of variance. The Pearson’s correlation coefficient was used to describe the correlation between indicators. The scatter plot was applied to describe the relevant trends of indicators. A P value < 0.05 indicated statistical significance.
Results
Plasma endotoxin levels
Plasma endotoxin levels in the sepsis group significantly increased compared with those in sham-operated group, indicating that the sepsis model was established successfully (F = 59.22; P = 0.00) (Figure 1).
Figure 1.

Comparison of plasma plasma endotoxin levels of mice in different groups. After 12 h of operation, the plasma endotoxin level was measured in the sham-operated and CLP groups (n = 10 for each group). Data were presented as the mean ± SD. Statistically significant differences are indicated by *P < 0.01 versus sham-operated group.
Detection of myocardial tissue structure using HE staining
In the sham-operated group, the cardiomyocytes were intact with no evidence of necrosis or inflammatory cell infiltration. In the sepsis group, the cardiac muscle showed cell degeneration and necrosis, inflammatory changes, nuclear fragmentation and dissolution, myocardial fiber fracture and dissolution, fuzzy or disappearing cross striations, interstitial edema, widened red blood cells, fat deposition or overflow, and so forth. Exogenous NaHS could attenuate the sepsis-induced injury in a concentration-dependent manner (Figure 2).
Figure 2.
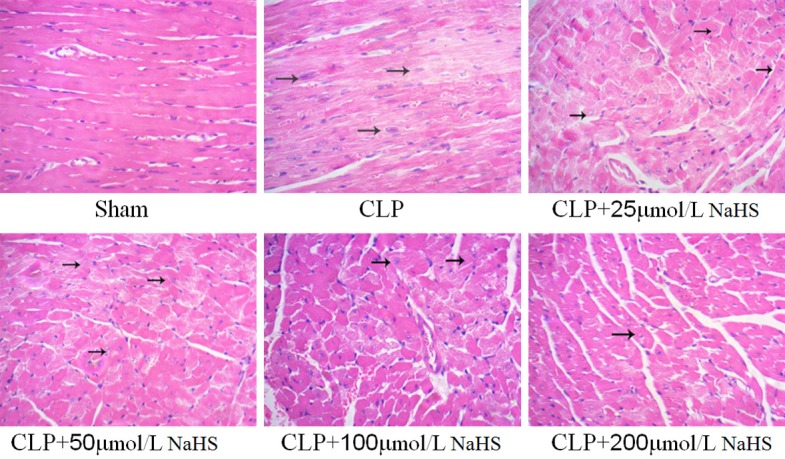
NaHS could protect the structure of myocardium. Representative images of cardiomyocyte HE staining (400×) in mice. In the CLP group, cardiac muscle showed cell degeneration and necrosis, inflammatory changes, and nuclear fragmentation and dissolution. In the presence of different concentrations of NaHS (25, 50, 100, and 200 µmol/L), the cardiac muscle improved.
Serum cTnI levels
The cTnI level in the sepsis group significantly increased compared with the sham-operated group, whereas the NaHS-treated groups showed a significant decrease in cTnI level. The cTnI level in the 200 μmol/L NaHS group significantly decreased compared with the sepsis group (Figure 3).
Figure 3.
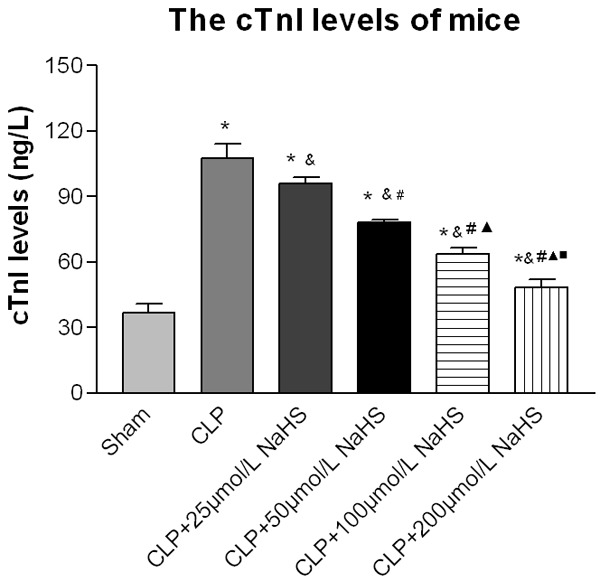
cTnI levels of mice. After 12 h of operation, the serum level of the plasma endotoxin was measured. After the interference of NaHS, the cTnI levels decreased. Data were presented as the mean ± SD. Statistically significant differences are indicated by *P < 0.01 versus the sham-operated group, &P < 0.01 versus the CLP group, # versus 25 µmol/L group, ▲ versus the 50 µmol/L group, and ■ versus the 100 µmol/L group.
Detection of myocardial mitochondrial structure using a TEM
The study then assessed ultrastructural changes in the myocardial mitochondria using an electron microscopic analysis. In the sham-operated group, the myocardial mitochondria showed regular shape, clear boundary, even matrix, and dense mitochondrial crest, with no evident swelling and vacuolar degeneration. The myocytes had a complete cell membrane and a clear intercellular boundary, the filaments were regularly arranged, and the fibrous structure in the interstitium did not increase significantly. In the sepsis group, the number of myocardial mitochondria reduced, the mitochondria showed irregular shape, obvious swelling and vacuolar degeneration were observed, and evident mitochondrial crest rupture was noted in the swelling mitochondria. The filaments were irregularly arranged, and the fibrous structure increased significantly in the interstitium. Also, fiber fracture and lysis were obvious in some parts of the myocardium. In the sepsis + 25 μmol/L NaHS group, the myocardial mitochondrial morphology was similar to that in the sepsis group. In other NaHS groups, the myocardial mitochondrial morphology improved compared with the sepsis group, Also, the number of mitochondria increased in an NaHS concentration-dependent manner, the mitochondrial shape became regular, mitochondrial swelling and vacuolar degeneration were attenuated, and the fibrous structure in the interstitium reduced compared with the sepsis group (Figure 4).
Figure 4.
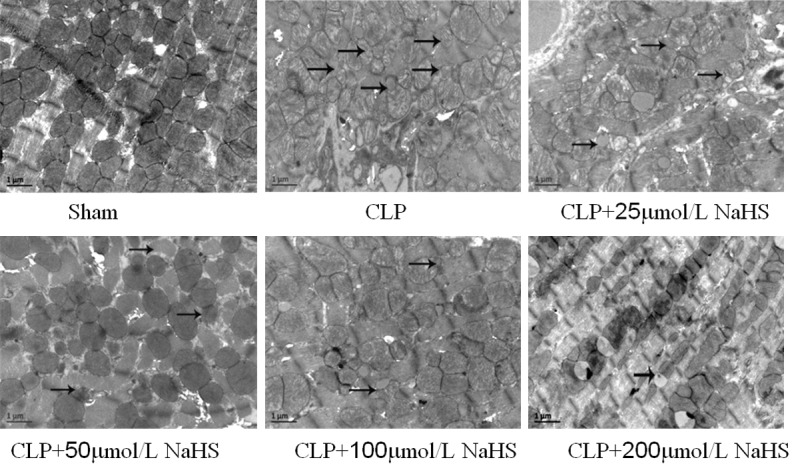
Observation of myocardial mitochondrial morphology of mice using a transmission electron microscope (×20000). In the CLP group, the number of myocardial mitochondria reduced, and in other NaHS groups, the myocardial mitochondrial morphology improved.
Swelling and functional changes of myocardial mitochondria
The result indicated the difference in the degree of myocardial mitochondrial swelling. In the sepsis group, the degree of myocardial mitochondrial swelling was aggravated and the ATP level decreased significantly. After NaHS treatment, the degree of myocardial mitochondrial swelling was alleviated and the ATP level increased significantly in a concentration-dependent manner (Swelling: F = 3524.07, P = 0.00; ATP: F = 1834.25, P = 0.00) (Figure 5).
Figure 5.
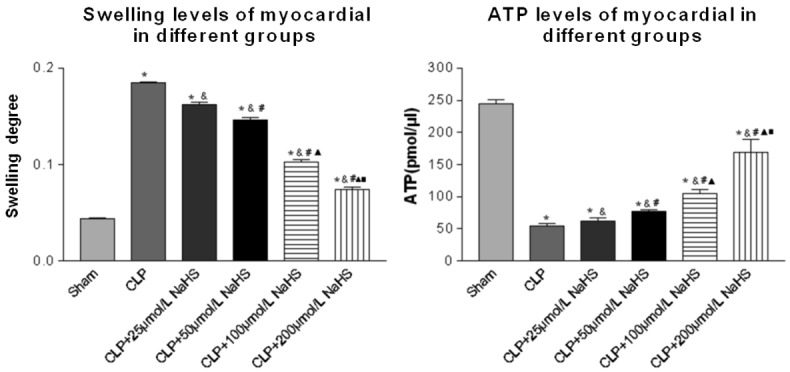
Comparison of swelling and ATP levels of myocardial cells of mice. The degree of swelling and ATP level changes showed mitochondrial morphological and functional changes. Data were presented as the mean ± SD. Statistically significant differences are indicated by *P < 0.01 versus the sham-operated group, &P < 0.01 versus the CLP group, # versus the 25 µmol/L group, ▲ versus the 50 µmol/L group, ■ versus the 100 µmol/L group.
Nrf2, PGC-1α, and Tfam mRNA expression levels
The result indicated no significant difference in the mRNA expression of PGC-1α, Nrf2, and Tfam between the normal and sham-operated groups. The mRNA expression of PGC-1α, Nrf2, and Tfam in the sepsis group increased significantly compared with that in the normal control and sham-operated groups. After NaHS intervention, the mRNA expression of PGC-1α, Nrf2, and Tfam increased markedly in a dose-dependent manner (PGC-1: F = 665.15, P = 0.00; αNrf: F = 2644.64, P = 0.00; Tfam: F = 145.76, P = 0.00) (Figure 6).
Figure 6.
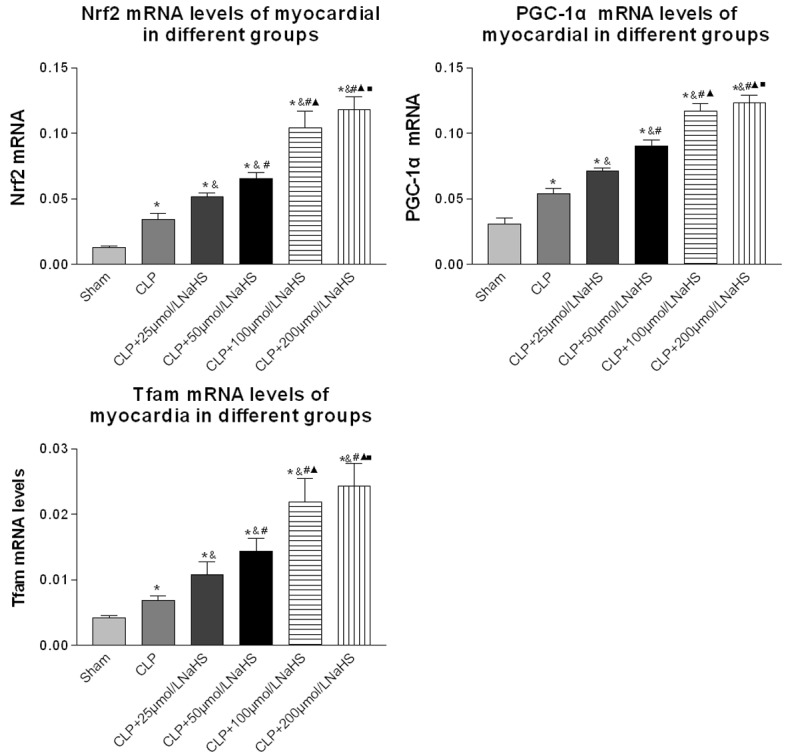
Comparison of Nrf2, PGC-1α, and Tfam mRNA expression levels of myocardial cells of mice. RNA was isolated using an RNA Isolation Kit. The expression levels of Nrf2, PGC-1α, and Tfam genes were measured using qPCR (n = 10 for each group). Data were presented as the mean ± SD. Statistically significant differences are indicated by *P < 0.01 versus the sham-operated group, &P < 0.01 versus the CLP group, # versus the 25 µmol/L group, ▲ versus the 50 µmol/L group, ■ versus the 100 µmol/L group.
Correlation analysis
In the NaHS intervention group, the degree of myocardial mitochondrial swelling and serum cTnI level were negatively correlated with PGC-1α and Nrf2 mRNA expression levels (mitochondrial swelling R = -0.955, -0.962; cTnI R = -0.963, -0.950, all P < 0.05). ATP content was positively correlated with Nrf2, PGC-1α, and Tfam mRNA expression levels (R = 0.863, 0.821, and 0.950, all P < 0.05). The Tfam mRNA expression level was also positively correlated with Nrf2 and PGC-1α mRNA expression levels (R = 0.925, 0.937, all P < 0.05). The result showed that exogenous NaHS could protect the myocardium in sepsis through improving myocardial mitochondrial biosynthesis by upregulating the mRNA expression levels of Nrf2 and PGC-1α.
Discussion
Sepsis manifests microcirculatory abnormalities and tissue cell damage caused by excessive systemic inflammatory response and host autonomic dysregulation. Without timely and effective therapeutic intervention, this scenario evolves to multiple-organ dysfunction syndrome and ultimately death. Myocardial depression is a well-recognized manifestation of organ dysfunction in sepsis. The degree of myocardial structural derangement and functional impairment is related to the severity and mortality of sepsis [17]. The present study showed that the myocardium of mice in the sepsis group had cell degeneration and necrosis, inflammatory changes, nuclear fragmentation and dissolution, myocardial fiber fracture and dissolution, fuzzy or disappearing cross striations, and interstitial edema, indicating that CLP-induced sepsis caused myocardial structural damage. In addition, the cTnI level in the sepsis group significantly increased compared with that in the sham-operated group. The quantitative indicator was consistent with the qualitative indicator, confirming that CLP-induced sepsis resulted in myocardium injury. Therefore, while treating sepsis, the myocardium should be protected to reduce the incidence of cardiac dysfunction and improve the survival rate of sepsis.
The precise mechanisms by which sepsis leads to cardiac dysfunction are not clear. However, awareness exists about a central role of mitochondrial dysfunction, which has become the hot spot for many researchers in recent years [18]. The mitochondrion is the energy center of cells. It provides energy to cells through the synthesis of ATP by oxidative phosphorylation in the inner mitochondrial membrane. Mitochondria are main sites for cellular respiration and ATP generation [19,20]. In the study, the TEM observation and analysis of the swelling degree of mitochondria showed that the myocardial mitochondria had obvious swelling, and the mitochondria ridge was disordered, partly disrupted, reduced, and even disappeared into a vacuolated structure. All these results indicated that the myocardial mitochondrial structure was damaged by CLP-induced sepsis. Compared with the sham-operated group, ATP levels in the sepsis group decreased significantly, indicating that the myocardial mitochondria function was injured. Thus, strategies aimed at improving the myocardial mitochondrial structure and function may be potentially beneficial to the treatment of sepsis.
H2S is an important endogenous biological signaling molecule. It has been shown to be crucial in the regulation of a number of vital biological functions in mammals, including antioxidant, anti-apoptotic, and anti-inflammatory activities [21]. It plays an important role in the physiological and pathophysiological processes of the cardiovascular system, inflammatory system, nervous system, and so on. Also, the protective effects have been observed after treatment with exogenous H2S in a number of disease states. Therefore, H2S has been widely explored by researchers [22,23]. H2S is a reducing agent easily consumed by various oxides. Hence, exogenous donors are needed to supply H2S, which has a therapeutic effect. Exogenous sodium hydrosulfide has been increasingly used as an exogenous H2S donor in various studies, such as ischemia-reperfusion injury [24], myocardial infarction [25], and hemorrhagic shock [26]. The experimental results showed that ATP was significantly unregulated in NaHS intervention groups compared with the sepsis group. The increase in ATP was positively correlated with the concentration of NaHS. The results revealed that NaHS might prevent against sepsis-induced cardiac muscle dysfunction by preserving mitochondrial function and promoting the production of ATP. This was correlated with the concentration of NaHS.
Mitochondrial biogenesis is an important pathway to renew the mitochondria, maintain the number and quality of mitochondria, improve mitochondrial function, and promote ATP synthesis. It is orchestrated by specific nuclear transcription factors that regulate the expression of genes encoding mitochondrial proteins. Mitochondrial transcription factor A is an important transcription factor implicated in the regulation of mitochondrial biogenesis [27]. The results showed that the T-fam mRNA expression level in myocardial cells of mice in the NaHS intervention groups was statistically significantly different from that in the sepsis group. With a gradual increase in the Tfam mRNA expression level, the level of ATP also rose, indicating a distinct direct relation between them. The result showed that the mitochondrial damage in myocardial cells of mice in the NaHS intervention groups was less than that in the sepsis group, and the number of mitochondria increased. Hence, NaHS improved mitochondrial function, promoted ATP production, and increased mitochondrial number by activating mitochondrial biogenesis in mice with sepsis. PGC-1α and Nrf2 were two of the important modulating factors in mitochondrial biogenesis, both of which are closely related and contribute to Tfam transcriptional activation. This study also found that the ATP and Tfam mRNA expression levels were distinctly positively correlated with PGC-1α and Nrf2 mRNA expression levels in the NaHS intervention groups. Exogenous NaHS could upregulate PGC-1α and Nrf2 expression and Tfam transcriptional activation, and promote mitochondrial biogenesis, which was to ensure the quality and quality of mitochondria, improve the function of mitochondria, and increase the synthesis of ATP.
The administration of exogenous NaHS could upregulate the expression levels of PGC-1α and Nrf2 in myocardial cells of mice with sepsis and promote mitochondrial biogenesis by transcription of Tfam so as to improve the function of mitochondria.
Acknowledgements
This work was supported by Wenzhou Public Welfare Science and Technology Projects (Y20150020). This work is supported by the Department of Pediatric Emergency, The Second Affiliated Hospital & Yuying Children’s Hospital, Wenzhou Medical University.
Disclosure of conflict of interest
None.
References
- 1.Sagy M, Al-Qaqaa Y, Kim P. Definitions and pathophysiology of sepsis. Curr Probl Pediatr Adolesc Health Care. 2013;43:260–263. doi: 10.1016/j.cppeds.2013.10.001. [DOI] [PubMed] [Google Scholar]
- 2.Mira JC, Gentile LF, Mathias BJ, Efron PA, Brakenridge SC, Mohr AM, Moore FA, Moldawer LL. Sepsis pathophysiology, chronic critical illness, and persistent inflammation-immunosuppression and catabolism syndrome. Crit Care Med. 2017;45:253–262. doi: 10.1097/CCM.0000000000002074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kong W, Kang K, Gao Y, Liu H, Meng X, Yang S, Yu K, Zhao M. Dexmedetomidine alleviates LPS-induced septic cardiomyopathy via the cholinergic anti-inflammatory pathway in mice. Am J Transl Res. 2017;9:5040–5047. [PMC free article] [PubMed] [Google Scholar]
- 4.Ahmad A, Druzhyna N, Szabo C. Delayed treatment with sodium hydrosulfide improves regional blood flow and alleviates cecal ligation and puncture (CLP)-induced septic shock. Shock. 2016;46:183–193. doi: 10.1097/SHK.0000000000000589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhang H, Zhi L, Moore PK, Bhatia M. Role of hydrogen sulfide in cecal ligation and punctureinduced sepsis in the mouse. Am J Physiol Lung Cell Mol Physiol. 2006;290:L1193–L1201. doi: 10.1152/ajplung.00489.2005. [DOI] [PubMed] [Google Scholar]
- 6.Gong Y, Zou L, Feng Y, Li D, Cai J, Chen D, Chao W. Importance of Toll-like receptor 2 in mitochondrial dysfunction during polymicrobial sepsis. Anesthesiology. 2014;121:1236–1247. doi: 10.1097/ALN.0000000000000470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fu M, Zhang W, Wu L, Yang G, Li H, Wang R. Hydrogen sulfide (H2S) metabolism in mitochondria and its regulatory role in energy production. Proc Natl Acad Sci U S A. 2012;109:2943–2948. doi: 10.1073/pnas.1115634109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Banu SA, Ravindran S, Kurian GA. Hydrogen sulfide post-conditioning preserves interfibrillar mitochondria of rat heart during ischemia reperfusion injury. Cell Stress Chaperones. 2016;21:571–582. doi: 10.1007/s12192-016-0682-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Williams LM, Timme-Laragy AR, Goldstone JV, McArthur AG, Stegeman JJ, Smolowitz RM, Hahn ME. Developmental expression of the Nfe2-related factor (Nrf) transcription factor family in the zebrafish, Danio rerio. PLoS One. 2013;8:e79574. doi: 10.1371/journal.pone.0079574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gleyzer N, Vercauteren K, Scarpulla RC. Control of mitochondrial transcription specificity factors (TFB1M and TFB2M) by nuclear respiratory factors (NRF-1 and NRF-2) and PGC-1 family coactivators. Mol Cell Biol. 2005;25:1354–1366. doi: 10.1128/MCB.25.4.1354-1366.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wenz T. Regulation of mitochondrial biogenesis and PGC-1alpha under cellular stress. Mitochondrion. 2013;13:134–142. doi: 10.1016/j.mito.2013.01.006. [DOI] [PubMed] [Google Scholar]
- 12.Johri A, Chandra A, Beal MF. PGC-1alpha, mitochondrial dysfunction, and Huntington’s disease. Free Radic Biol Med. 2013;62:37–46. doi: 10.1016/j.freeradbiomed.2013.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Skirecki T, Kawiak J, Machaj E, Pojda Z, Wasilewska D, Czubak J, Hoser G. Early severe impairment of hematopoietic stem and progenitor cells from the bone marrow caused by CLP sepsis and endotoxemia in a humanized mice model. Stem Cell Res Ther. 2015;6:142. doi: 10.1186/s13287-015-0135-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang CN, Liu YJ, Duan GL, Zhao W, Li XH, Zhu XY, Ni X. CBS and CSE are critical for maintenance of mitochondrial function and glucocorticoid production in adrenal cortex. Antioxid Redox Signal. 2014;21:2192–2207. doi: 10.1089/ars.2013.5682. [DOI] [PubMed] [Google Scholar]
- 15.Fink MP. Animal models of sepsis. Virulence. 2014;5:143–53. doi: 10.4161/viru.26083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kingsley SM, Bhat BV. Differential paradigms in animal models of sepsis. Curr Infect Dis Rep. 2016;18:26. doi: 10.1007/s11908-016-0535-8. [DOI] [PubMed] [Google Scholar]
- 17.Rudiger A, Singer M. Mechanisms of sepsisinduced cardiac dysfunction. Crit Care Med. 2007;35:1599–1608. doi: 10.1097/01.CCM.0000266683.64081.02. [DOI] [PubMed] [Google Scholar]
- 18.Alvarez S, Vico T, Vanasco V. Cardiac dysfunction, mitochondrial architecture, energy production, and inflammatory pathways: interrelated aspects in endotoxemia and sepsis. Int J Biochem Cell Biol. 2016;81:307–314. doi: 10.1016/j.biocel.2016.07.032. [DOI] [PubMed] [Google Scholar]
- 19.Vanasco V, Magnani ND, Cimolai MC, Valdez LB, Evelson P, Boveris A, Alvarez S. Endotoxemia impairs heart mitochondrial function by decreasing electron transfer, ATP synthesis and ATP content without affecting membrane potential. J Bioenerg Biomembr. 2012;44:243–252. doi: 10.1007/s10863-012-9426-3. [DOI] [PubMed] [Google Scholar]
- 20.Bertoncheli CM, Zimmermann CE, Jaques JA, Leal CA, Ruchel JB, Rocha BC, Pinheiro KV, Souza VC, Stainki DR, Luz SC, Schetinger MR, Leal DB. Increased NTPDase activity in lymphocytes during experimental sepsis. ScientificWorldJournal. 2012;2012:941906. doi: 10.1100/2012/941906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.D’Araio E, Shaw N, Millward A, Demaine A, Whiteman M, Hodgkinson A. Hydrogen sulfide induces heme oxygenase-1 in human kidney cells. Acta Diabetol. 2014;51:155–157. doi: 10.1007/s00592-013-0501-y. [DOI] [PubMed] [Google Scholar]
- 22.Tokuda K, Kida K, Marutani E, Crimi E, Bougaki M, Khatri A, Kimura H, Ichinose F. Inhaled hydrogen sulfide prevents endotoxin-induced systemic inflammation and improves survival by altering sulfide metabolism in mice. Antioxid Redox Signal. 2012;17:11–21. doi: 10.1089/ars.2011.4363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Greabu M, Totan A, Miricescu D, Radulescu R, Virlan J, Calenic B. Hydrogen sulfide, oxidative stress and periodontal diseases: a concise review. Antioxidants (Basel) 2016:5. doi: 10.3390/antiox5010003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mard SA, Neisi N, Solgi G, Hassanpour M, Darbor M, Maleki M. Gastroprotective effect of NaHS against mucosal lesions induced by ischemia-reperfusion injury in rat. Dig Dis Sci. 2012;57:1496–1503. doi: 10.1007/s10620-012-2051-5. [DOI] [PubMed] [Google Scholar]
- 25.Li N, Wang MJ, Jin S, Bai YD, Hou CL, Ma FF, Li XH, Zhu YC. The H2S donor NaHS changes the expression pattern of H2S-Producing enzymes after myocardial infarction. Oxid Med Cell Longev. 2016;2016:6492469. doi: 10.1155/2016/6492469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gao C, Xu DQ, Gao CJ, Ding Q, Yao LN, Li ZC, Chai W. An exogenous hydrogen sulphide donor, NaHS, inhibits the nuclear factor kappaB inhibitor kinase/nuclear factor kappab inhibitor/nuclear factor-kappaB signaling pathway and exerts cardioprotective effects in a rat hemorrhagic shock model. Biol Pharm Bull. 2012;35:1029–1034. doi: 10.1248/bpb.b110679. [DOI] [PubMed] [Google Scholar]
- 27.Gleyzer N, Vercauteren K, Scarpulla RC. Control of mitochondrial transcription specificity factors (TFB1M and TFB2M) by nuclear respiratory factors (NRF-1 and NRF-2) and PGC-1 family coactivators. Mol Cell Biol. 2005;25:1354–1366. doi: 10.1128/MCB.25.4.1354-1366.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]


