Abstract
Objective: This study aimed to investigate the therapeutic effect of mesencephalic astrocyte-derived neurotrophic factor (MANF) on the MPTP/MPP+-induced model of Parkinson’s disease (PD) and the potential mechanism. Methods: Male C57BL/6 mice PD model with MPTP-induced were randomly injected bilaterally with MANF or PBS into the striatum. Two weeks later, Rotarod test, immunohistochemistry, and detection of dopamine (DA) and its metabolites, superoxide dismutase (SOD), glutathione (GSH), and malondialdehyde (MDA) were performed. A cell model of PD was established by incubating SH-SY5Y cells with MPP+, cells were pretreated for 2 h with different concentrations of MANF before 24 h incubation with MPP+. Cell viability, expression of Bax, and Bcl-2, gene expression levels of Heme oxygenase 1 (HMOX1) and Superoxide dismutase 2 (SOD2), and mitochondrial transmembrane potential were detected. Results: The latency reduction in PD mice was partially restored after MANF treatment (P<0.05); MANF significantly reduced the loss of tyrosine hydroxylase (TH)-positive dopaminergic neurons in the substantia nigra pars compacta (SNpc) (P<0.01); MANF significantly increased the striatal DA level in PD mice (P<0.05) and markedly increased the SOD activity (P<0.01) and GSH production (P<0.01). MANF pre-treatment significantly decreased the MPP+-induced reduction of cell viability (P<0.01), inhibited the ratio of Bax/Bcl-2 expression (P<0.01), activated gene expression levels of HMOX1 (P<0.01) and SOD2 (P<0.05), and reversed MPP+-induced loss of mitochondrial membrane potential (P<0.01). Conclusion: MANF can attenuate the neuronal lesion in MPTP/MPP+-induced PD mice, which may be related to the improvement of mitochondrial function and inhibition of oxidative stress.
Keywords: MANF, MPTP/MPP+, neurorestorative effect, Parkinson’s disease, mitochondrial function
Introduction
Parkinson’s disease (PD) is a disabling neurodegenerative disorder characterized clinically by tremor, rigidity, bradykinesia and postural instability [1] and pathologically by the extensive loss of dopaminergic neurons in the substantia nigra pars compacta (SNpc) [2]. Currently, the most widely used treatment for PD is dopamine replacement therapy, but long-term treatment can produce debilitating adverse effects and may not affect the progression of PD.
At the cellular level, PD is involved in the alteration of mitochondrial electron transport chain function, the change in dopamine metabolism, and the excess production of reactive oxygen species (ROS) due to oxidative stress. Thus, it has been proposed that to improve the mitochondrial function is effective to delay the progression of dopaminergic neurons loss.
Neurotoxin 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) and its metabolite 1-methyl-4-phenylpyridinium ion (MPP+) are widely used to establish model of PD since it shares tremendous similarities in neural biochemical, pathology and behaviors with human PD [3].
Mesencephalic astrocyte-derived neurotrophic factor (MANF) is a novel neurotrophic factor and can selectively protect nigral dopaminergic neurons. It has been proven that MANF has neuroprotective and neurorestorative effects on PD in both in vitro and in vivo models [4,5]. To date, however, the specific mechanisms underlying the neuroprotective effects of MANF are still unclear.
This study was designed to investigate the potential role of mitochondrial pathway in the neuroprotection of MANF in a mouse model of PD.
Materials and methods
Animals and treatment
All animal experiments were performed in accordance with the Guideline for the Use and Care of Animals in Biomedical Research of Tongji University. Male C57BL/6 mice aged 7 weeks and weighing 20-25 g used in this experiment were housed in a humidified environment (humidity: 55±5%) at 22±2°C with 12 h/12 h-light/dark cycle and given ad libitum access to food and water. After one week’s acclimation and behavioral training, then mice were intraperitoneally treated with MPTP (20 mg/kg; Sigma) or saline of equal volume once daily for consecutive 5 days [6]. One week after last MPTP injection, MPTP-treated mice were randomly divided into two groups (12-13 mice per group). Mice were intraperitoneally anesthetized with chloral hydrate (400 mg/kg) and placed in a stereotaxic apparatus. According to the atlas of Paxinos and Watson [7], mice were injected bilaterally with MANF (10 μg in 2 μl) or phosphate buffered saline (PBS) (2 μl/hemisphere) into the striatum (AP 0.5, ML 1.7, DV -3.5) at 0.5 μl/min with a Hamilton syringe. The needle was left in place for 5 minutes after each injection to minimize cell diffusion up the needle track. Animals were killed 2 weeks after the surgery.
Rotarod test
Rotarod test was conducted using a rotor with a 5-cm diameter [8]. In brief, mice received training session for 7 consecutive days before MPTP injection. At the first week after the last MPTP injection, and the first and second week after MANF treatment, mice were placed on the rotating rod that rotated at a constant accelerating rate, from 4 to 40 rpm in 300 s. The latency to fall was automatically recorded by magnetic trip plates or at 300 s. Each mouse was tested three times and the mean was calculated.
Tyrosine hydroxylase immunohistochemistry
Two weeks after MANF treatment, 6-7 mice from each group were intraperitoneally anesthetized with 10% chloral hydrate and the brain was perfusion-fixed with 4% paraformaldehyde following a normal saline flush, and then transferred to a 30% sucrose solution. Serial coronal sections through the substantia nigra were cut frozen at a thickness of 20 μm. Immunohistochemistry was performed for tyrosine hydroxylase (TH) as described [9]. After inhibition of endogenous peroxidase activity, sections were incubated in 5% bovine serum albumin (BSA) in PBS for 30 min at room temperature. Sections were then incubated with rabbit anti-TH primary antibody (1:1000, Millipore) overnight at 4°C. After rinsing with PBS, sections were incubated in a biotinylated secondary antibody (1:50) with horseradish peroxidase (HRP) for 2 h and then visualized with diaminobenzidine (DAB). At last, the sections were dehydrated through an alcohol series and mounted.
The brain sections were photographed at a magnification of 200× (Nikon) and TH positive dopaminergic neurons were manually counted in the SNpc of right hemisphere by an investigator blind to the experiment. At least three sections were used for each animal and a mean was calculated.
Brain tissue preparation and DA detection
Mice were sacrificed by cervical dislocation 14 days after MANF treatment and the dopamine and its metabolites 3,4-dihydroxyphenylaceticacid (DOPAC) and homovanillic acid (HVA) were detected by HPLC. The brains were rapidly removed fresh and the striatum was separated on an ice-cold plate. For each animal, the striatum of both hemispheres was transferred into a 1.5-ml flask, weighed and homogenized in ice-cold solution A (0.2 mM HClO4), and then the homogenate was centrifuged at 10,000 rpm for 15 min at 4°C. The supernatant was filtered through a nylon syringe filter (0.2 μm in pore size) and 20 μL of the filtrate was injected for HPLC. The mobile phase, containing 80 mM sodium dihydrogenphosphate monohydrate, 2.0 mM 1-octanesulfonic acid sodium salt, 100 μl/l triethylamine, 5 nM EDTA, and 10% acetonitrile, pH 3.0, was perfused at 0.25 ml/min. Data were calibrated with an external standard. The contents of dopamine and its metabolites were calculated and expressed as ng/mg tissue weight.
Detection of anti-oxidative activity
Mice were euthanized and the substantia nigra was separated, homogenized in PBS and centrifuged at 10,000 g for 10 min at 4°C. The protein concentration was quantified with BCA protein assay kit. Superoxide dismutase (SOD) activity, glutathione (GSH) content, and malondialdehyde (MDA) content were determined with commercially available kits according to the manufacturer’s instructions.
Cell culture and treatments
Human neuroblastoma SH-SY5Y cells were cultured in DMEM supplemented with 10% (v/v) FBS, and 100 U/mL penicillin/streptomycin at 37°C in an environment with 5% CO2 and 95% humidified air. SH-SY5Y cells were pretreated for 2 h with different concentrations of MANF (50, 200, 400 ng/mL) before 24-h incubation with 2 mM MPP+.
MTT assay of cell viability
MTT assay was used to evaluate the viability of SH-SY5Y cells. Cells were seeded in 96-well plates with five wells in each group. After 24-h treatment, 20 μL of MTT solution (5 mg/ml) was added to each well, followed by incubation at 37°C for 4 h. Then, the supernatant was removed and 150 μL of DMSO was added into each well. The absorbance of each well was measured at 570 nm with an automated microplate reader. The cell viability was averaged and normalized to that of control cells.
Western blot analysis
Cells were homogenized in RIPA lysis buffer and the protein concentration in the supernatant was quantified by BCA kit, proteins (30 μg) was loaded onto 10% SDS-PAGE. After electrophoresis, proteins were transferred to PVDF membranes. Membranes were blocked with 5% BSA in TBST for 1 h at room temperature and then were incubated with Bax (1:1000), Bcl-1 (1:1000) overnight at 4°C followed by incubation for 1 h with HRP secondary antibodies (1:5000). Bands of proteins were visualized using an ECL detection system and digitized data were quantified using Image J software and the relative intensities were normalized to that of GAPDH.
Quantitative real-time PCR analysis
Total RNA from SH-SY5Y cells was isolated using TRIzol reagent. mRNA was enriched from the total RNA using oligo dT magnetic beads. Then, the mRNA was fragmented into short fragments using an RNA fragmentation kit. Double stranded cDNA was purified using a QIAquick PCR extraction kit and used for end repair and base A addition. Then, the following RT-PCR primer pairs were used: TBP, 5’-AGCCAAGAGTGAAGAACAGTCC-3’ (forward) and 5’-CACAGCTCCCCACCATATTC-3’ (reverse); Heme oxygenase 1 (HMOX1), 5’-AACTTTCAGAAGGGCCAGGT-3’ (forward) and 5’-GTAGACAGGGGCGAAGACTG-3’ (reverse); Superoxide dismutase 2 (SOD2), 5’-TCAATAAGGAACGGGGACAC-3’ (forward) and 5’-ACACATCAATCCCCAGCAGT-3’ (reverse); Primers for TBP mRNA were used to normalize mRNA expression.
Measurement of mitochondrial membrane potential
JC-1, a lipophilic cationic dye, was used to detect mitochondrial transmembrane potential change. SH-SY5Y cells were seeded in 6-well plates and JC-1 kit was used according to manufacturer’s instructions. After the indicated treatments, cells were incubated with JC-1 solution for 20 min at 37°C, then rinsed twice with JC-1 buffer. Fluorescent signal was measured by flow cytometry. The mitochondrial membrane potential was calculated as the ratio of JC-1 red to green fluorescence intensity.
Statistical analysis
Data are expressed as mean ± standard error (S.E.M). Statistical analysis was performed using SPSS version 20.0. One-way analysis of variance (ANOVA) followed by Post-hoc LSD test was used to compare data among groups. A value of P<0.05 was considered statistically significant.
Results
MANF improves motor behavior in MPTP-treated mice
One week after the last MPTP administration, the latency of Rotarod test (206.7±7.2, n=26) reduced significantly as compared to control group (274.1±14.1, n=9) (P<0.01), which indicates that the MPTP-induced mouse PD model is established successfully (Figure 1A). Then, one week after MANF or PBS treatment, no significant difference was observed between PBS-treatment group (180.7±16.1, n=13) and MANF- treatment group (174.9±13.6, n=13) (P>0.05); however, after 2-week MANF treatment, the latency reduction after MPTP injection was significantly attenuated in MANF-treatment group (203.4±13.9, n=13) as compared to PBS-treatment group (182.5±15.9, n=13) (P<0.05) (Figure 1B).
Figure 1.
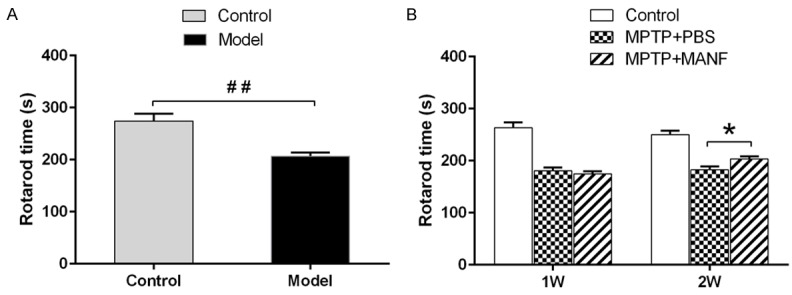
Behavioral test of MPTP-treated mice. A. Rotarod test revealed motor coordination ability of mice, which was impaired in MPTP-treated mice as compared to control mice, suggesting PD mouse model was established successfully. B. MANF improved motor deficits as shown by Rotarod test as compared to PBS-treated mice at 2 weeks after MANF treatment. Data are presented as mean ± S.E.M. *P<0.05, **P<0.01 vs PBS-treatment group. ##P<0.01 vs control group.
Loss of TH-positive neurons in the SNpc
TH is a rate-limiting enzyme in the dopamine biosynthesis and a marker of dopaminergic neuron. MPTP mainly damages the dopaminergic neurons which could cause a prominent TH-positive cell loss in the SNpc. [10]. In addition, results showed MANF significantly inhibited the loss of TH-positive dopaminergic neurons (37.1±4.4, n=6) as compared to PBS-treatment group (20.9±3.5, n=6) (P<0.01) (Figure 2). These demonstrate that MANF can rescue dopaminergic neurons from MPTP neurotoxicity in mice.
Figure 2.
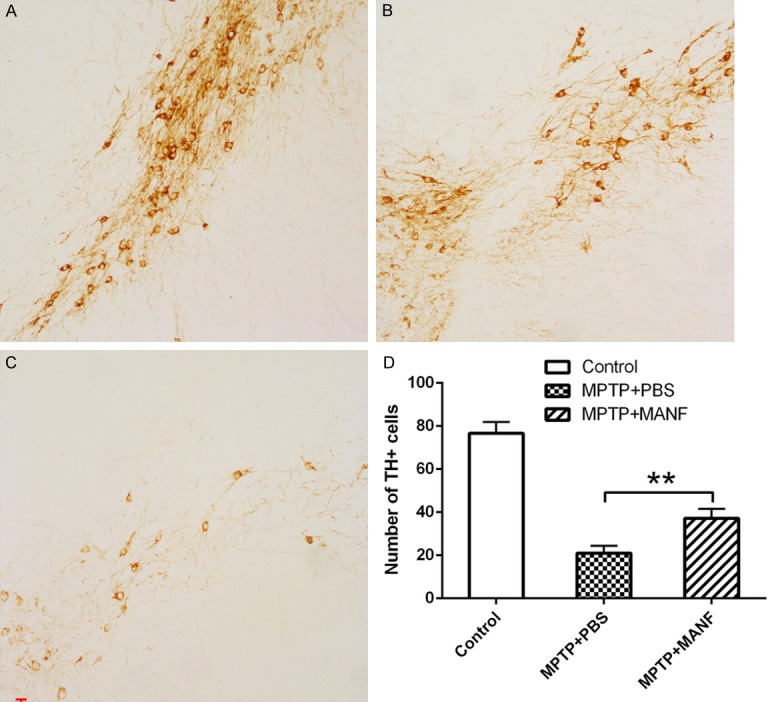
Effect of MANF on the loss of TH-positive neurons in the SNpc. Photographs were captured at a magnification of 100×. A. Control group. B. MANF+MPTP-treated group. C. PBS+MPTP-treated group. D. Quantitative analysis of TH-positive neurons in the substantia nigra pars compacta. MANF significantly inhibited the loss of TH-positive neurons as compared to PBS-treatment group. Data are presented as mean ± S.D. *P<0.05, **P<0.01 vs PBS-treatment group.
Levels of dopamine and its metabolites
MPTP markedly reduced in the levels of dopamine (DA) and its metabolites (DOPAC and HVA) in the striatum. However, after MANF treatment, the striatal DA, DOPAC and HVA levels increased significantly as compared to PBS treated mice (DA: 13.86±0.9 ng/mg, n=7, vs 11.0±2.0 ng/mg, n=7, P<0.05; DOPAC: 5.5±0.8 ng/mg, n=7, vs 3.8±1.1 ng/mg, n=7, P<0.05; HVA: 1.15±0.08 ng/mg, n=7, vs 0.84±0.13 ng/mg, n=7, P<0.01, Figure 3).
Figure 3.
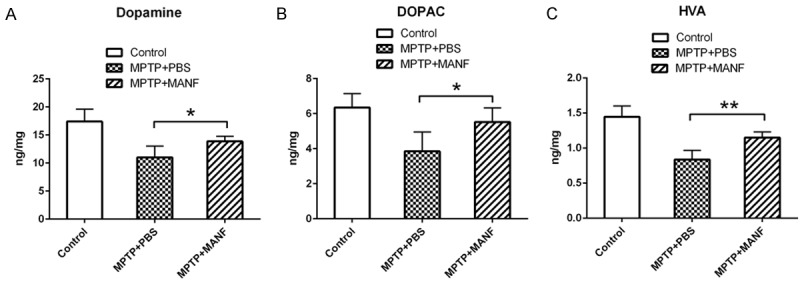
Effects of MANF treatment on the levels of striatal dopamine (DA) and its metabolites 3,4-dihydroxyphenylaceticacid (DOPAC) and homovanillic acid (HVA) in the brain. MANF significantly increased the levels of striatal DA, DOPAC and HVA as compared to PBS-treated mice. Data are presented as mean ± S.D. *P<0.05, **P<0.01 vs PBS-treatment group.
Anti-oxidative indexes
SOD is an important anti-oxidase in nearly all living cells. GSH is one of the most important non-enzymatic antioxidants in cells and possesses the capabilities of free radical scavenging, detoxification and cellular immunity. GSH can scavenge the O2- and H2O2, and thus the amount of GSH is an important factor related to anti-oxidative capability. MDA was a stable product of lipid peroxidation and may indirectly reflect the extent of cell damage. As mentioned previously, MPTP may induce the free radical production and lipid peroxidation in dopaminergic neurons. These results indicated that, compared with PBS-treatment group, MANF significantly increased the SOD activity (SOD: 375.0±15.0, n=7, vs 276.4±32.0, n=7, P<0.01) and GSH production (GSH: 290.2±34.6, n=7, 219.7±11.92, n=7, P<0.01; Figure 4A, 4B), and decreased the MDA production (0.88±0.067, n=7, vs 0.89±0.044, n=7, P>0.05; Figure 4C). These indicate that MANF may improve the anti-oxidative capacity in MTPT-treated mice.
Figure 4.
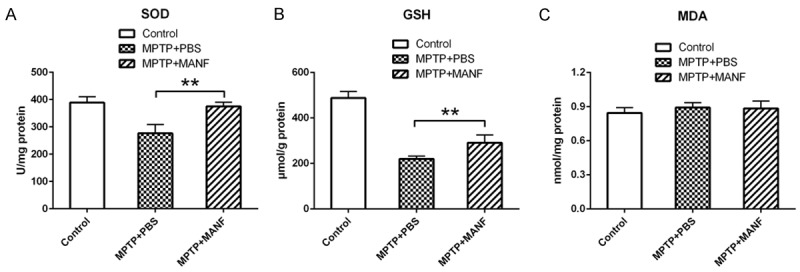
Effects of MANF on the anti-oxidative activity of PD mice. SOD, GSH, and MDA were measured in the brain. MANF increased the SOD activity and the GSH production, and decreased the MDA production. Data are presented as mean ± S.D. *P<0.05, **P<0.01 vs PBS-treatment group.
Effects of MANF on cell viability
The dose-cytotoxicity relationship of MPP+ was determined in SH-SY5Y cells which were treated with various concentrations of MPP+ for 24 h. As illustrated in Figure 5A, MTT assay showed MPP+ (2 mM for 24 h) decreased cell viability to 50.49%, however, pre-treatment with 50, 200 or 400 ng/mL MANF for 2 h before 2 mM MPP+ incubation for 24 h prevented SH-SY5Y cells from MPP+ induced injury, and could restore the cell viability to 52.33%, 66.93% and 75.58%, respectively (P>0.05, P<0.01, and P<0.01). Moreover, various concentrations of MANF (50, 200 and 400 ng/mL) alone failed to significantly affect the viability of SH-SY5Y cells after 24-h treatment (Figure 5B). It suggests that MANF significantly decreases MPP+-induced reduction of cell viability in a dose dependent manner.
Figure 5.
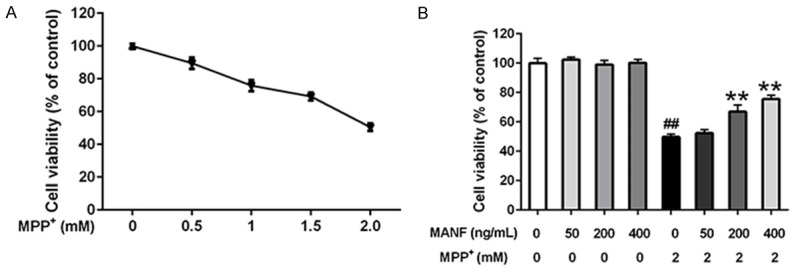
Effects of MANF on the viability of MPP+-treated SH-SY5Y cells. A. SH-SY5Y cells were seeded in 96-well plates and exposed to different concentrations of MPP+ for 24 h, and cell viability was assessed by MTT assay. ▲: control group, ▼: treatment with different concentrations of MPP+ for 24 h, SH-SY5Y cells treated with 2 mM MPP+ were used to establish the cytotoxic model of PD. B. Cells were pretreated with different concentrations of MANF (50, 200 and 400 ng/mL) for 2 h before incubation with 2 mM MPP+ for 24 h, and cell viability was assessed by MTT assay or cells were incubated with various concentrations of MANF for 24 h. Data are presented as mean ± S.E.M. *P<0.05, **P<0.01 vs MPP+-treated group. #P<0.05, ##P<0.01 vs control group.
Expression of Bax and Bcl-2 in SH-SY5Y cells
The Bcl-2 family proteins play a pivotal role in the cellular apoptotic machinery caused by MPP+ [11,12]. In this study, Bax protein expression significantly increased in the MPP+-treated group compared with control group, while pretreatment with MANF inhibited Bax expression. In contrast, the levels of Bcl-2 decreased in the MPP+ group and increased after MANF treatment. There was a significant increase in the ratio of Bax/Bcl-2 expression in MPP+-treated group compared with the control group, while the ratio decreased in cells pre-treated with MANF (Figure 6), suggesting that MPP+-induced alteration of Bcl-2 and Bax expression are inhibited by MANF treatment.
Figure 6.
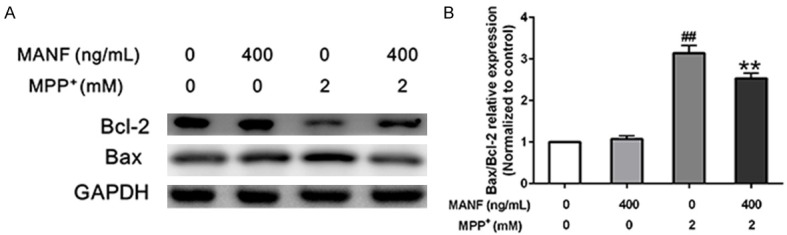
Expression of Bax and Bcl-2 in SH-SY5Y cells. A. Cells were pretreated with MANF (400 ng/ml) for 2 h, then incubated with 2 mM MPP+ for 24 h. Immunoblot was applied to measure the proteins expression of Bax and Bcl-2. Values of densitometric analysis were calculated from three independent experiments. GAPDH was used as internal control. B. MPP+-induced increase of Bax/Bcl-2 was attenuated by MANF pretreatment. Data are presented as mean ± S.E.M. *P<0.05, **P<0.01 vs MPP+-treated group. #P<0.05, ##P<0.01 vs control group.
Expression of HMOX1 and SOD2 in SH-SY5Y cells
Heme oxygenase 1 (HMOX1) and Superoxide dismutase 2 (SOD2) are two of oxidative stress responsive genes, which are involved in anti-oxidative pathway. In this study, MPP+ up-regulated the expression level of HMOX1, and MANF further strengthened the reaction (P<0.01). Meanwhile, MANF significantly increased the expression level of SOD2 (P<0.05) which was decreased by MPP+ treatment (Figure 7). It indicated that MANF might be involved in anti-oxidative pathway.
Figure 7.
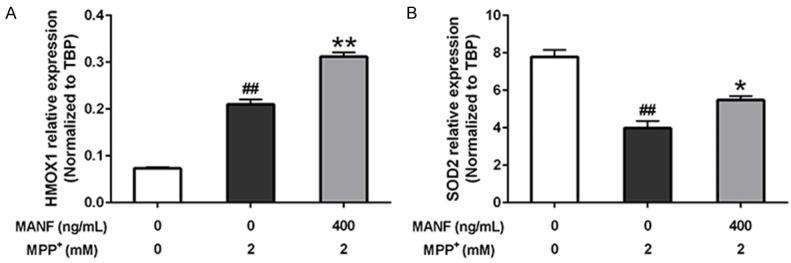
Expression of HMOX1 and SOD2 in SH-SY5Y cells. A. MPP+ up-regulated the expression level of HMOX1, and MANF further strengthened the reaction. B. MANF significantly increased the expression level of SOD2 which was decreased by MPP+ treatment. Data are presented as mean ± S.E.M. *P<0.05, **P<0.01 vs MPP+-treated group. #P<0.05, ##P<0.01 vs control group.
Mitochondrial membrane potential in SH-SY5Y cells
Results showed 2 mM MPP+ significantly decreased the ratio of red/green fluorescence to 38.4% (P<0.01), indicating the depolarization of mitochondrial membrane. In contrast, pretreatment with MANF (400 ng/mL) reversed the MPP+-induced reduction of mitochondrial membrane potential (P<0.01) (Figure 8). In brief, MANF decreases the MPP+-induced mitochondrial membrane potential decline.
Figure 8.
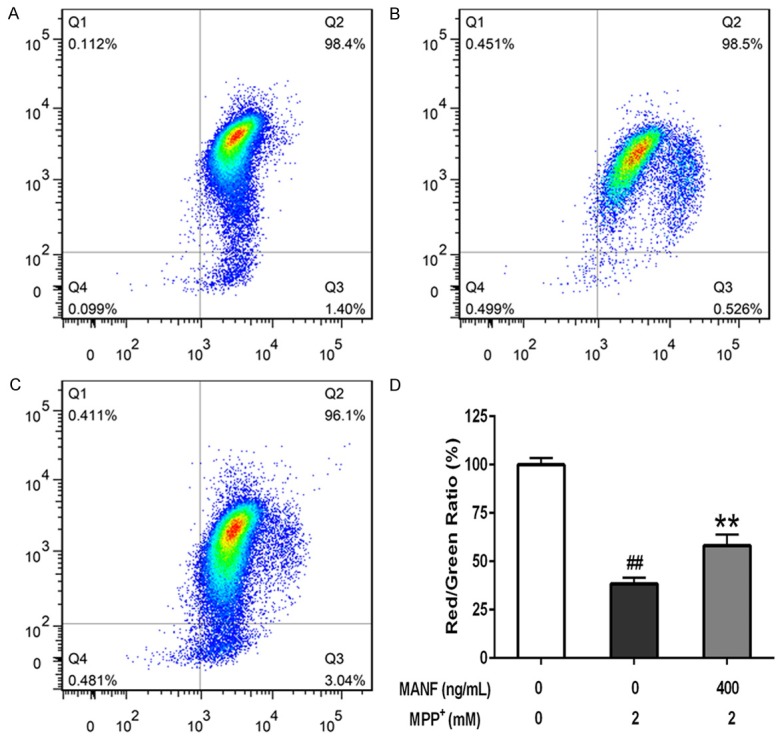
Effects of MANF on the mitochondrial membrane potential of MPP+-treated SH-SY5Y cells. SH-SY5Y cells were seeded in 6-well plates and pretreated MANF (400 ng/mL) for 2 h before incubation with 2 mM MPP+ for 24 h, and the mitochondrial membrane potential was detected with the JC-1 assay kit by flow cytometry. A. Control group; B. 2 mM MPP+ treatment alone; C. 400 ng/mL MANF+2 mM MPP+; D. Ratio of red/green fluorescence intensity. Data are presented as mean ± S.D. *P<0.05, **P<0.01 vs 2 mM MPP+-treated group. #P<0.05, ##P<0.01 vs control group.
Discussion
MANF is a novel evolutionarily conserved neurotrophic factor secreted by the endoplasmic reticulum and Golgi apparatus [5]. In vitro and in vivo, MANF has been found to exert prominent neuroprotective effects on nigral dopaminergic neurons [13]. In this study, we further investigated the effects of MANF on the MPTP/MPP+-induced PD model and the potential mechanism. These results showed MANF was able to improve the mitochondrial function and inhibit the oxidative stress in both in vivo and in vitro PD model.
Mitochondrial dysfunction and cellular oxidative stress are the most prominent features in PD [14-17]. It has been confirmed that mitochondrial dysfunction may cause oxidative stress and neurodegeneration [3,18], leading to neuronal loss. These findings indicate MANF can enhance the SOD activity and GSH production, scavenge lipid peroxidation product MDA, inhibit oxidative stress, and alleviate the ROS induced damage to dopaminergic neurons.
MPP+ accumulates in the mitochondria after being transported into the dopaminergic neurons, resulting in mitochondrial membrane potential decline [19-21]. In this study, MTT assay confirmed that MPP+ treatment reduced the viability of SH-SY5Y cells in a dose-dependent manner, which was consistent with previous findings [22]. However, pretreatment with MANF partially reversed the reduction of cell viability caused by MPP+ treatment, which indicates that MANF protects SH-SY5Y cells against MPP+-induced cytotoxicity in a dose-dependent pattern. The mitochondrial depolarization is thought to mediate the neuronal apoptosis induced by MPP+ [23]. There is evidence showing that excess production of mitochondrial ROS is a main cause of mitochondrial depolarization [24]. These results revealed that MANF could partially reverse the decrease of MPP+-induced mitochondrial membrane potential, suggesting that MANF protected mitochondria from oxidative damage, and it might involve in the inhibition of MPP+-induced mitochondrial apoptosis via up-regulated HMOX1 and SOD2 expression which encode antioxidant.
Meanwhile, MPTP may also cause limbs incoordination and motor deficits in mouse PD model. In present study, MPTP significantly impaired the neurological function, but MANF treatment increased the latency in Rotarod test, suggesting that MANF can ameliorate MPTP-induced behavioral deficits.
Immunohistoc hemistry for TH demonstrated that the TH-positive cells in MANF-treated group remarkably increased as compared to PBS-treated group, suggesting that MANF alleviates the loss of dopaminergic neurons in the substantia nigra after MPTP injection, which is consistent with findings from rat PD model and cell model [5,13]. These findings indicated the effect of MPTP on the dopaminergic neurons was more prominent than that on behavioral changes. Consistent with the features in human PD, the pathological changes often occur before the onset of clinical symptoms, which maybe related to the compensatory mechanism.
It has been reported that MPTP markedly reduces striatal dopamine, DOPAC and HVA [10]. These results further indicate that the depletion of dopamine, DOPAC and HVA in the striatum was partially inhibited by MANF-treatment, which was consistent with findings from immunohistochemistry for TH. This may be explained as that, in MPTP-induced PD model, MANF preserves more dopaminergic neurons and thus maintains dopamine at a relatively high level. Moreover, the amplitude of variation in dopamine level was not consistent with those in its metabolites, which was also reported in several previous studies [25,26].
MANF belongs to secretory protein family, but the precise mechanisms and signaling pathway related to its protection on dopaminergic neurons are still poorly understood. There is evidence showing that the neuroprotective effects of MANF may be related to the inhibition of ER stress-induced cell death [27-29]. Studies on MANF structure suggests that the neurotrophic activity may associate to its N-terminal domains and ER stress response to its C-terminal domains [30]. These several previous researches had found the neuroprotective effect of MANF involved in ER stress and autophagy pathways [31-33], the present study demonstrated the neuroprotective effect of MANF may rely on promoting mitochondrial function, inhibiting oxidative stress, rectifying imbalance between oxidative stress and antioxidant system. MANF may exert its neurotrophic role in dopaminergic neurons through multiple methods besides ER stress and autophagy pathways.
However, there were some limitations in this study. The receptor for MANF has not been identified until now, more further studies are needed to elucidate the exact downstream signaling pathways that triggering the specific biological effects of MANF.
In summary, this study reveals MANF may be a promising agent for PD, or other neurodegenerative disorders, and its neuroprotective effects may be ascribed to the improvement of impaired mitochondrial function and inhibition of oxidative stress.
Acknowledgements
We thank some members in the Department of Neurology, Shanghai Tongji Hospital and Tongji University School of Medicine. This study was supported by the National Major Scientific and Technological Special Project for Significant New Drugs Development (2014ZX09102043-003), National Natural Science Foundation of China (81371403, 81572234), and Shanghai Science and Technology Commission (13JC1401102).
Disclosure of conflict of interest
None.
References
- 1.Fahn S. Parkinsonism. In: Wyngaarden JB, Smith LH, editors. Cecil’s Textbook of Medicine. 18th edition. Philadelphia: Saunders; 1988. pp. 2143–2147. [Google Scholar]
- 2.Alexi T, Borlongan CV, Faull RL, Williams CE, Clark RG, Gluckman PD, Hughes PE. Neuroprotective strategies for basal ganglia degeneration: Parkinson’s and Huntington’s diseases. Prog Neurobiol. 2000;60:409–470. doi: 10.1016/s0301-0082(99)00032-5. [DOI] [PubMed] [Google Scholar]
- 3.Schmidt N, Ferger B. Neurochemical findings in the MPTP model of Parkinson’s disease. J Neural Transm (Vienna) 2001;108:1263–1282. doi: 10.1007/s007020100004. [DOI] [PubMed] [Google Scholar]
- 4.Barker RA. Continuing trials of GDNF in Parkinson’s disease. Lancet Neurol. 2006;5:285–286. doi: 10.1016/S1474-4422(06)70386-6. [DOI] [PubMed] [Google Scholar]
- 5.Petrova P, Raibekas A, Pevsner J, Vigo N, Anafi M, Moore MK, Peaire AE, Shridhar V, Smith DI, Kelly J, Durocher Y, Commissiong JW. MANF: a new mesencephalic, astrocyte-derived neurotrophic factor with selectivity for dopaminergic neurons. J Mol Neurosci. 2003;20:173–188. doi: 10.1385/jmn:20:2:173. [DOI] [PubMed] [Google Scholar]
- 6.Shiozaki S, Ichikawa S, Nakamura J, Kitamura S, Yamada K, Kuwana Y. Actions of adenosine A2A receptor antagonist KW-6002 on druginduced catalepsy and hypokinesia caused by reserpine or MPTP. Psychopharmacology (Berl) 1999;147:90–95. doi: 10.1007/s002130051146. [DOI] [PubMed] [Google Scholar]
- 7.Paxinos G, Franklin KBJ. The mouse brain in stereotaxic coordinates. Gulf Professional Publishing. 2004 [Google Scholar]
- 8.Chagniel L, Robitaille C, Lacharite-Mueller C, Bureau G, Cyr M. Partial dopamine depletion in MPTP-treated mice differentially altered motor skill learning and action control. Behav Brain Res. 2012;228:9–15. doi: 10.1016/j.bbr.2011.11.019. [DOI] [PubMed] [Google Scholar]
- 9.Fujita K, Seike T, Yutsudo N, Ohno M, Yamada H, Yamaguchi H, Sakumi K, Yamakawa Y, Kido MA, Takaki A, Katafuchi T, Tanaka Y, Nakabeppu Y, Noda M. Hydrogen in drinking water reduces dopaminergic neuronal loss in the 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine mouse model of Parkinson’s disease. PLoS One. 2009;4:e7247. doi: 10.1371/journal.pone.0007247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jackson-Lewis V, Przedborski S. Protocol for the MPTP mouse model of Parkinson’s disease. Nat Protoc. 2007;2:141–151. doi: 10.1038/nprot.2006.342. [DOI] [PubMed] [Google Scholar]
- 11.Cory S, Adams JM. The Bcl2 family: regulators of the cellular life-or-death switch. Nat Rev Cancer. 2002;2:647–656. doi: 10.1038/nrc883. [DOI] [PubMed] [Google Scholar]
- 12.O’Malley KL, Liu J, Lotharius J, Holtz W. Targeted expression of BCL-2 attenuates MPP+ but not 6-OHDA induced cell death in dopaminergic neurons. Neurobiol Dis. 2003;14:43–51. doi: 10.1016/s0969-9961(03)00013-5. [DOI] [PubMed] [Google Scholar]
- 13.Voutilainen MH, Back S, Porsti E, Toppinen L, Lindgren L, Lindholm P, Peranen J, Saarma M, Tuominen RK. Mesencephalic astrocytederived neurotrophic factor is neurorestorative in rat model of Parkinson’s disease. J Neurosci. 2009;29:9651–9659. doi: 10.1523/JNEUROSCI.0833-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Davidzon G, Greene P, Mancuso M, Klos KJ, Ahlskog JE, Hirano M, DiMauro S. Earlyonset familial parkinsonism due to POLG mutations. Ann Neurol. 2006;59:859–862. doi: 10.1002/ana.20831. [DOI] [PubMed] [Google Scholar]
- 15.Hudson G, Schaefer AM, Taylor RW, Tiangyou W, Gibson A, Venables G, Griffiths P, Burn DJ, Turnbull DM, Chinnery PF. Mutation of the linker region of the polymerase gamma-1 (POLG1) gene associated with progressive external ophthalmoplegia and Parkinsonism. Arch Neurol. 2007;64:553–557. doi: 10.1001/archneur.64.4.553. [DOI] [PubMed] [Google Scholar]
- 16.Kraytsberg Y, Kudryavtseva E, McKee AC, Geula C, Kowall NW, Khrapko K. Mitochondrial DNA deletions are abundant and cause functional impairment in aged human substantia nigra neurons. Nat Genet. 2006;38:518–520. doi: 10.1038/ng1778. [DOI] [PubMed] [Google Scholar]
- 17.Luoma P, Melberg A, Rinne JO, Kaukonen JA, Nupponen NN, Chalmers RM, Oldfors A, Rautakorpi I, Peltonen L, Majamaa K, Somer H, Suomalainen A. Parkinsonism, premature menopause, and mitochondrial DNA polymerase gamma mutations: clinical and molecular genetic study. Lancet. 2004;364:875–882. doi: 10.1016/S0140-6736(04)16983-3. [DOI] [PubMed] [Google Scholar]
- 18.Jakowec MW, Petzinger GM. 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-lesioned model of parkinson’s disease, with emphasis on mice and nonhuman primates. Comp Med. 2004;54:497–513. [PubMed] [Google Scholar]
- 19.Brieger K, Schiavone S, Miller FJ Jr, Krause KH. Reactive oxygen species: from health to disease. Swiss Med Wkly. 2012;142:w13659. doi: 10.4414/smw.2012.13659. [DOI] [PubMed] [Google Scholar]
- 20.Ghosh A, Chandran K, Kalivendi SV, Joseph J, Antholine WE, Hillard CJ, Kanthasamy A, Kanthasamy A, Kalyanaraman B. Neuroprotection by a mitochondria-targeted drug in a Parkinson’s disease model. Free Radic Biol Med. 2010;49:1674–1684. doi: 10.1016/j.freeradbiomed.2010.08.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mizuno Y, Sone N, Saitoh T. Effects of 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine and 1-methyl-4-phenylpyridinium ion on activities of the enzymes in the electron transport system in mouse brain. J Neurochem. 1987;48:1787–1793. doi: 10.1111/j.1471-4159.1987.tb05737.x. [DOI] [PubMed] [Google Scholar]
- 22.Lee DY, Lee KS, Lee HJ, Noh YH, Kim DH, Lee JY, Cho SH, Yoon OJ, Lee WB, Kim KY, Chung YH, Kim SS. Kynurenic acid attenuates MPP(+)-induced dopaminergic neuronal cell death via a Bax-mediated mitochondrial pathway. Eur J Cell Biol. 2008;87:389–397. doi: 10.1016/j.ejcb.2008.03.003. [DOI] [PubMed] [Google Scholar]
- 23.Liu WB, Zhou J, Qu Y, Li X, Lu CT, Xie KL, Sun XL, Fei Z. Neuroprotective effect of osthole on MPP+-induced cytotoxicity in PC12 cells via inhibition of mitochondrial dysfunction and ROS production. Neurochem Int. 2010;57:206–215. doi: 10.1016/j.neuint.2010.05.011. [DOI] [PubMed] [Google Scholar]
- 24.Vercesi AE, Kowaltowski AJ, Grijalba MT, Meinicke AR, Castilho RF. The role of reactive oxygen species in mitochondrial permeability transition. Biosci Rep. 1997;17:43–52. doi: 10.1023/a:1027335217774. [DOI] [PubMed] [Google Scholar]
- 25.Bender A, Krishnan KJ, Morris CM, Taylor GA, Reeve AK, Perry RH, Jaros E, Hersheson JS, Betts J, Klopstock T, Taylor RW, Turnbull DM. High levels of mitochondrial DNA deletions in substantia nigra neurons in aging and Parkinson disease. Nat Genet. 2006;38:515–517. doi: 10.1038/ng1769. [DOI] [PubMed] [Google Scholar]
- 26.Pyle A, Foltynie T, Tiangyou W, Lambert C, Keers SM, Allcock LM, Davison J, Lewis SJ, Perry RH, Barker R, Burn DJ, Chinnery PF. Mitochondrial DNA haplogroup cluster UKJT reduces the risk of PD. Ann Neurol. 2005;57:564–567. doi: 10.1002/ana.20417. [DOI] [PubMed] [Google Scholar]
- 27.Apostolou A, Shen Y, Liang Y, Luo J, Fang S. Armet, a UPR-upregulated protein, inhibits cell proliferation and ER stress-induced cell death. Exp Cell Res. 2008;314:2454–2467. doi: 10.1016/j.yexcr.2008.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mizobuchi N, Hoseki J, Kubota H, Toyokuni S, Nozaki J, Naitoh M, Koizumi A, Nagata K. ARMET is a soluble ER protein induced by the unfolded protein response via ERSE-II element. Cell Struct Funct. 2007;32:41–50. doi: 10.1247/csf.07001. [DOI] [PubMed] [Google Scholar]
- 29.Tadimalla A, Belmont PJ, Thuerauf DJ, Glassy MS, Martindale JJ, Gude N, Sussman MA, Glembotski CC. Mesencephalic astrocyte-derived neurotrophic factor is an ischemia-inducible secreted endoplasmic reticulum stress response protein in the heart. Circ Res. 2008;103:1249–1258. doi: 10.1161/CIRCRESAHA.108.180679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hellman M, Arumae U, Yu LY, Lindholm P, Peranen J, Saarma M, Permi P. Mesencephalic astrocyte-derived neurotrophic factor (MANF) has a unique mechanism to rescue apoptotic neurons. J Biol Chem. 2011;286:2675–2680. doi: 10.1074/jbc.M110.146738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Huang J, Chen C, Gu H, Li C, Fu X, Jiang M, Sun H, Xu J, Fang J, Jin L. Mesencephalic astrocytederived neurotrophic factor reduces cell apoptosis via upregulating GRP78 in SH-SY5Y cells. Cell Biol Int. 2016;40:803–811. doi: 10.1002/cbin.10621. [DOI] [PubMed] [Google Scholar]
- 32.Zhang J, Cai Q, Jiang M, Liu Y, Gu H, Guo J, Sun H, Fang J, Jin L. Mesencephalic astrocyte-derived neurotrophic factor alleviated 6-OHDAinduced cell damage via ROS-AMPK/mTOR mediated autophagic inhibition. Exp Gerontol. 2017;89:45–56. doi: 10.1016/j.exger.2017.01.010. [DOI] [PubMed] [Google Scholar]
- 33.Zhang J, Tong W, Sun H, Jiang M, Shen Y, Liu Y, Gu H, Guo J, Fang J, Jin L. Nrf2-mediated neuroprotection by MANF against 6-OHDA-induced cell damage via PI3K/AKT/GSK3β pathway. Exp Gerontol. 2017;100:77–86. doi: 10.1016/j.exger.2017.10.021. [DOI] [PubMed] [Google Scholar]


