Abstract
The goal of this study was to assess the ability of quercetin (Qu) to protect against myocardial ischemia-reperfusion injury. Cardiac injury was assessed in the context of global ischemia of isolated hearts, coronary artery ligated rats, and H9C2 cells. Qu was shown to significantly inhibit inflammatory cytokine production in coronary artery occlusion-induced rats, isolated hearts, and H9C2 cells. Electrocardiographic analysis revealed a restoration of the ST segment to normal levels following treatment of Qu. Triphenyltetrazolium chloride (TTC) staining and pathological analysis showed that Qu could significantly alleviate myocardial injury in vivo. Furthermore, ex vivo analyses showed improved recovery of heart function in response to Qu, characterized by enhanced myocardial contractility and coronary flow in isolated hearts. From a mechanistic standpoint, these effects appeared to be mediated through the HMGB1-related pathway, with expression of downstream targets significantly downregulated in rats, isolated hearts, and H9C2 cells following Qu treatment. Taken together, these data demonstrate the protective effects of Qu against myocardial injury via inhibition of the HMGB1 pathway in a myocardial ischemia-reperfusion injury (I/R) model.
Keywords: Quercetin, myocardial ischemia-reperfusion, inflammation
Introduction
Cardiovascular diseases (CVDs) remain the leading cause of morbidity and mortality worldwide. Theoretically, restoring blood supply to the ischemic myocardium should limit the degree of injury caused by a myocardial event; however, reperfusion of the affected area has been shown to increase myocardial damage resulting in an ischemia/reperfusion (I/R) injury [1]. Such an injury can have serious negative consequences for affected patients, making prevention of I/R injuries an important consideration when treating ischemic heart disease. The myocardial I/R injury typically manifests as a combination of functional impairment, arrhythmia, and accelerated progression of apoptosis [2], with evidence of inflammation also evident in I/R lesions in isolated hearts [3]. Several studies found anti-apoptotic and anti-inflammatory treatments to be protective against I/R injury, with evidence suggesting that both mechanisms are factors in this injury [4].
High mobility group box-1 (HMGB1) is generally regarded as a non-histone DNA binding protein involved in the stabilization of DNA and promotion of transcription. However, HMGB1 has recently been found to be passively released by necrotic cardiomyocytes in response to ischemia, serving as an early mediator of inflammation following I/R injury [5]. Furthermore, HMGB1 is actively secreted by immune cells including monocytes, macrophages, and dendritic cells, which further enhances the inflammatory reaction. More importantly, clinical data indicate that increased circulating HMGB1 levels are associated with poor cardiac and neurological outcomes, including increased mortality in cardiac disease patients [6,7].
Quercetin (3,5,7,3’,4’-pentahydroxyflavone) is a polyphenolic compound present in many vegetables and fruits such as onions and apples. It has been shown to exhibit a variety of biological functions including anti-inflammatory, anti-coagulation, and oxygen radical-scavenging activities [8,9]; however, the effects of Qu on myocardial ischemia/reperfusion are unknown. Therefore, we sought to evaluate the effects of Qu on I/R-induced heart injury and investigated its potential mechanism of action.
Materials and methods
Reagents
Quercetin was obtained from Sigma Aldrich (St Louis, MO, USA). All antibodies were provided by Cell Signaling Technology (Danvers, USA).
Animals
Adult Sprague-Dawley rats weighing 250-300 g were obtained from Xinuosai Biological Technology Ltd. (Suzhou, China) and housed in an air-conditioned room at 23 ± 2°C with a 12-h light/dark cycle. Water and food were provided ad libitum. All experimental procedures were performed according to the National Institute of Health Guidelines for the Care and Use of Laboratory Animals.
Surgical ligation of the left coronary artery and experimental protocol
The surgical protocol was carried out as follows: First, rats were anesthetized and restrained. The trachea was then incised, and wrapped in catgut for later use. An incision was then made in the skin at the position of the heart, and the underlying ribs exposed by blunt-dissection to provide convenient access during the heart operation. After exposing the heart, the left anterior descending coronary artery was blocked with a special suture. Meanwhile, a positive pressure respirator was applied to provide respiration of experimental rats during the procedure. Elevation of the ST segment was used to confirm successful implementation of the surgical model. Recovery of a strong, regular heart rhythm indicated that the thoracic cavity could be successfully sutured. The suture of rats in the sham group was not tied. Taking accidental deaths caused by failed surgery into consideration, the number of rat in each group was as follows: sham group, I/R group (model group), I/R + Diltiazem (Dil, 10 mg/kg) group, and I/R + Qu (50 mg/kg) group. Qu and Dil treatments were administered for 5 days after surgery. Rats were anesthetized again 24 h after the operation. Blood samples were collected from carotid artery and centrifuged at 3500 g for 15 min, and the supernatant stored at -80°C for biochemical indicator analysis. Subsequently, rats were sacrificed, and the hearts collected for TTC staining, western blot, and pathology analysis.
Langendorff-perfusion protocol
Sprague Dawley rats (250-300 g) were anesthetized with an intraperitoneal injection of chloral hydrate (350 mg/kg) and restrained. To prevent coagulation of the blood, 250 U/kg of heparin was administered intraperitoneally. The heart was then quickly excised by thoracic surgery and immediately mounted on a Langendorff’s apparatus. The hearts were retrogradely perfused under a constant pressure of 70 cm H2O with complex Krebs-Henseleit (K-H) buffer (118 mM NaCl, 1.2 mM KH2PO4, 4.7 mM KCl, 1.7 mM CaCl2, 1.2 mM MgSO4, 20 mM sodium acetate, and 10 mM glucose), equilibrated with 95% O2 plus 5% CO2 at 37°C (pH 7.4). Heart and perfusate temperature were maintained at 37°C using a water jacket. Thereafter, the isolated hearts were perfused with K-H solution at a constant pressure (80-90 mmHg).
The isolated rat hearts were randomly assigned into four groups: control group, I/R group, I/R + Dil group, and I/R + Qu group. Hearts in the control group were perfused for the 80min stabilization period. Hearts in the I/R group were allowed to equilibrate for 20 min followed by global ischemia (no flow) for 30 min. Subsequent reperfusion was conducted for 30 min using K-H buffer. Hearts in the I/R + Dil or I/R + Qu groups were treated as described in the I/R group, with the exception of reperfusion, for which either Dil (0.2 mg/kg) or Qu (1 mg/kg) was added to the K-H buffer.
Detection of coronary flow and myocardial contractility
The coronary flow (CF) rate was recorded at 5, 10, 20 and 30 min after reperfusion by measuring the K-H effluent, which drained from the right atrium into a graduated beaker. The heart with a flow rate of > 8 mL/min during the stabilization period was selected for further manipulation. Coronary effluent (CE) was harvested at the last time point for use in downstream analyses. A portable heart clip was applied to monitor and record the myocardial contractility during the entire experimental period.
Electrocardiogram (ECG) measurement
ECG measurements were recorded using the BL-420S Biologic Function Experiment system (Chengdu, China). Ischemia was verified by ST segment elevation recorded by ECG. The results were expressed relative to sham controls.
Measurements of cardiac output
For cardiac output detection, rats were anaesthetized and placed on a heating pad supplied with ventilation. The thymus lobes were pulled apart to expose the aorta. The ascending aorta was dissected and a transonic perivascular MA2.5 PSL flow probe (Transonic Systems, USA) was positioned around the aorta. An appropriate amount of ultrasound transmission gel was then injected into the space between the probe and the aorta, allowing for detection of flow signals, which were acquired using a TS420 flowmeter and Power Lab recording unit.
Determination of myocardial infarct size
The cardiac infarct area was evaluated by 2,3,5-triphenyltetrazolium chloride (TTC) (Sigma, St. Louis, MO, USA) staining. Briefly, the heart was placed at -20°C for 15 min and cut into five pieces parallel to the coronary sulcus and below the ligation of the heart. All slices were incubated with phosphate-buffered saline (PBS) containing 2% TTC at 37°C for 15 min in the dark for pathological analysis. TTC stained area (red, non-infarct area) and non-TTC stained area (white or pale, infarct area) were analyzed using Image-Pro Plus image analysis software (Version 4.1, Media Cybernetics, LP, USA). The ratio of infarcted myocardium to risk region was calculated as entire myocardial tissues (infarct area/whole heart area) × percentage.
Cell culture
H9C2 rat cardiomyocyte cells were obtained from the American Type Culture Collection and cultured with Dulbecco’s modified Eagle medium (DMEM, NanJing KeyGen Biotech Co., Ltd) containing 10% fetal bovine serum (FBS, Gibco), 100 IU/mL penicillin, and 100 IU/mL streptomycin in a humidified incubator at 37°C under 5% CO2 atmosphere, and passage at preconfluent densities by use of 0.25% trypsin solution (Gibco) every 2 to 3 days.
Hypoxia/reoxygenation (H/R) injury model in vitro
H9C2 cells were incubated in a hypoxic/ischemic chamber (Anaerocult® A mini, Merck) in serum-free, low-glucose DMEM at 37°C for 10 h in a humidified atmosphere of 5% CO2 and 95% nitrogen, after which cells were switched to fresh medium and restored to 95% nitrogen/5% CO2 for 2 h reoxygenation. At the onset of hypoxia, cardiomyocytes were randomly treated with either 40 uM Qu or 10 uM Dil dissolved in Tyrode buffer. H9C2 cells cultured under normal conditions in a CO2 incubator served as negative controls.
Assessment of inflammatory cytokines in serum and cell supernatant
IL-6, IL-1β and TNF-α protein levels in serum and cell supernatant were measured using ELISA kits according to the manufacturer’s instructions. Cytokine concentrations were quantified relative to a standard curve. The optical density (OD) of each well was read at 450 nm.
Detection of cardiac marker enzymes
Myocardial cellular damage was detected by measuring serum LDH and CK. Serum CK and LDH activities were assayed spectrophotometrically using commercially available kits, according to the manufacturer’s instructions.
Histological examination of the myocardium
Rat hearts were excised immediately, fixed in 10% (V/V) neutral buffered formalin solution, paraffin-embedded, cut into 4 μm thicknesses, stained with Hematoxylin and Eosin (H&E), and observed by light microscopy (Nikon, Tokyo, Japan) at 200× magnification.
Knockdown of HMGB1 in H9C2 cells
H9C2 cells were transfected with HMGB1 siRNA (Shanghai GenePharma, China) or negative control siRNA (Shanghai GenePharma, China) according to the manufacturer’s instructions. Briefly, a complex of 0.5 mL Opti-MEM medium and 6 μL of Lipofecramine 2000 containing 50 nM HMGB1 siRNA or 50 nM negative control siRNA per well was premixed and added to a 6-well plate, and incubated for 48 h. Silencing efficiency was determined by western blot analysis.
Immunohistochemistry
Expression of HMGB1, TLR4 and p-NF-κB in heart tissue was evaluated by immunohistochemistry. Briefly, tissues were fixed with 4% paraformaldehyde (PFA), embedded in paraffin, and sectioned. The paraffin sections were then dewaxed in xylene and dehydrated ethanol, microwaved in sodium citrate buffer, and washed with PBS. Endogenous peroxidase activity was blocked by 3% hydrogen peroxide for 20 min. Each sample were blocked with 5% goat serum for 20 min and then treated with primary antibodies at 4°C overnight. Samples were then washed three times with PBS, treated with goat anti-rabbit IgG secondary antibody for 20 min, incubated in a horseradish peroxidase substrate for 20 min, and washed three times with PBS. Samples were then stained with 3-3’diaminobenzidine (DAB), and re-stained with hematoxylin. After dehydrating and drying, the sections were mounted with neutral gum and observed under a microscope.
Western blot
Heart tissues were homogenized, washed with PBS, and lysed in RIPA buffer (Beyotime, Nanjing, China). Samples were then centrifuged at 12,000 rpm for 20 min, and the dissolved proteins collected from the supernatant. Protein concentrations were determined using a BCA protein assay (Beyotime, Nanjing, China). After quantification, samples were resolved on a 10% sodium dodecyl sulfate polyacrylamide gel (SDS-PAGE), and electro-transferred to nitrocellulose membranes. The membrane was then blocked with 5% skim milk in Tris buffer, and incubated with the appropriate specific antibodies. After washing, the blots were incubated with horseradish peroxidase-conjugated second antibodies. The antibody-reactive bands were visualized using enhanced chemiluminescence detection reagents and a gel imaging system (Tanon Science & Technology Co., Ltd., China). Quantification of protein expression was normalized to GAPDH using a densitometer (Imaging System).
Statistical analysis
Data are expressed as means ± the standard deviation (SD) of at least three separate experiments. Statistical comparisons between experimental groups were performed by ANOVA with Tukey multiple comparison test. P values ≤ 0.05 were considered statistically significant.
Results
Effect of Qu on myocardial infarct size
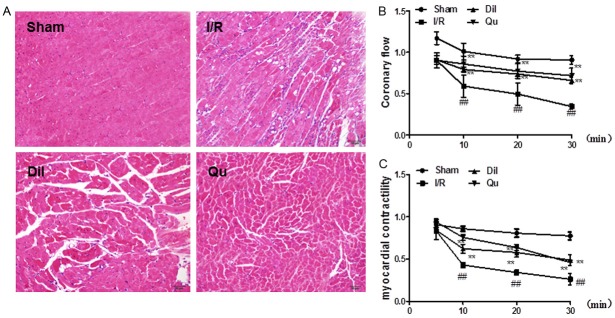
TTC staining of the myocardium following I/R injury was used to assess the degree of damage incurred in response to ischemic injury. Significantly higher infarct damage was observed in the I/R group compared to sham-treated controls (Figure 1). This damage was significantly attenuated in rats treated with Dil and Qu, with substantial reduction in infarct size compared to the I/R group.
Figure 1.
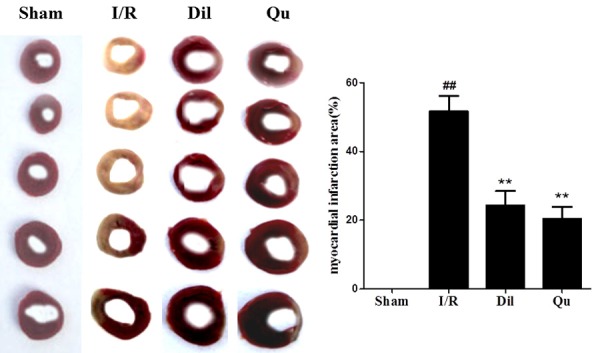
Effect of Qu on myocardial infarct size in I/R-induced isolated hearts. The data are expressed as mean values ± SDs. #P < 0.05, ##P < 0.01 relative to controls; *P < 0.05, **P < 0.01 compared with the I/R group.
Effect of Qu on myocardial histology
Sections of myocardial tissue taken from the sham treatment group exhibited intact myocardial membranes, a normal myofibrillar structure with striations, branched appearance, and continuity with adjacent myofibrils under light microscopy (Figure 2A). Hearts subjected to the ligation operation exhibited large numbers of infiltrating inflammatory cells, myocardial cell swelling, degeneration, cardiac necrosis, and loss of transverse striations. Qu and Dil significantly attenuated these pathological changes (Figure 2A).
Figure 2.
Effect of Qu on myocardial histology (A); coronary flow (B) and myocardial contractility (C) in I/R-induced isolated heart. The data are expressed as mean values ± SDs. #P < 0.05, ##P < 0.01 relative to controls; *P < 0.05, **P < 0.01 compared with the I/R group.
Effect of Qu on coronary flow in I/R-induced isolated heart
Myocardial ischemia following reperfusion in isolated rat heart caused significant decrease in coronary flow rate, noted in a time-dependent manner. The I/R group exhibited dramatic decreases in coronary flow, relative to controls; these effects were significantly attenuated following administration of Dil and Qu (Figure 2B).
Effects of Qu on myocardial contractility in I/R-induced isolated heart
Assessment of myocardial contractility of isolated hearts was assessed using a physiological recorder. The blockade of K-H flow markedly reduced myocardial contractility of isolated hearts in the I/R group, relative to controls. This phenotype was partially reversed following treatment with Qu and Dil (Figure 2C).
Effects of Qu on ST-segment elevation
Electrocardiographic patterns of normal and experimental rats were used to assess cardiac function in treated rats. The ST-segment was dramatically elevated, and R-amplitude was decreased in the I/R group, relative to the control group (Figure 3). This outcome provides definitive evidence that the I/R damage model was well established. Treatment with Qu ameliorated these effects, consistent with other protective effects seen for this compound (Figure 3).
Figure 3.
Effects of Qu on ST-segment elevation.
Effects of Qu on inflammatory cytokines in serum and cell supernatants
To determine whether Qu could inhibit inflammatory responses during myocardial I/R injury, the serum and cell supernatants were assessed for TNF-α, IL-6 and IL-1β. Significant increases in both serum and cell supernatant concentrations of TNF-α, IL-6 and IL-1β were observed in the I/R group. In contrast, cytokine levels were dramatically decreased in the Qu-treated group compared with those of the I/R group (Figure 4). These data indicate that Qu reduced the inflammatory cytokine content in serum of I/R injury rats.
Figure 4.
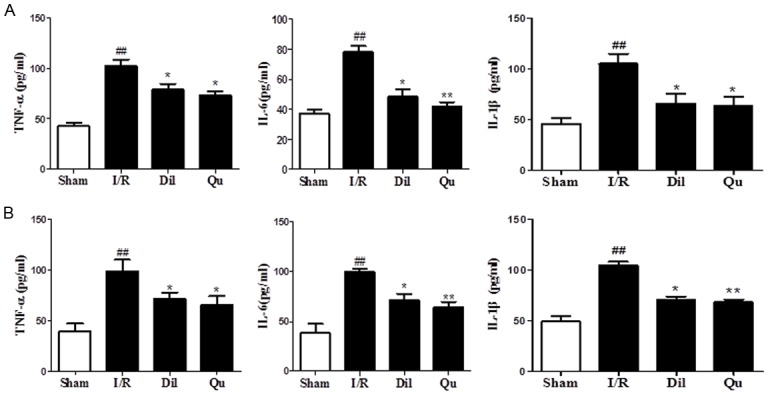
Effects of Qu on inflammatory cytokine levels in serum (A) and cell supernatant (B). The data are expressed as mean values ± SDs. #P < 0.05, ##P < 0.01 relative to controls; *P < 0.05, **P < 0.01 compared with the I/R group.
Effects of Qu on cardiac marker enzymes
To assess the expression of myocardial injury markers in response to I/R injury, CK and LDH levels were assessed in rat serum. Increased expression of CK and LDH was evident in I/R animals, relative to controls (Figure 5). These levels were restored to near-WT levels in both the Qu and Dil groups (Figure 5), further supporting the protective effects of these compounds in vivo.
Figure 5.
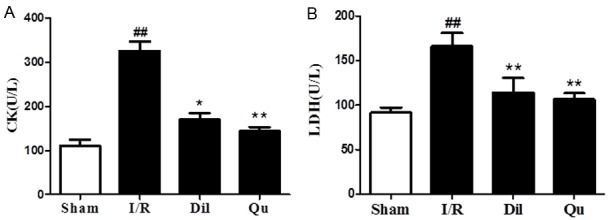
Effects of Qu on cardiac marker enzymes, CK (A) and LDH (B). The data are expressed as mean values ± SDs. #P < 0.05, ##P < 0.01 compared with control group; *P < 0.05, **P < 0.01 compared with the I/R group.
Effects of Qu on HMGB1, TLR4 and p-NF-κB by immunohistochemistry
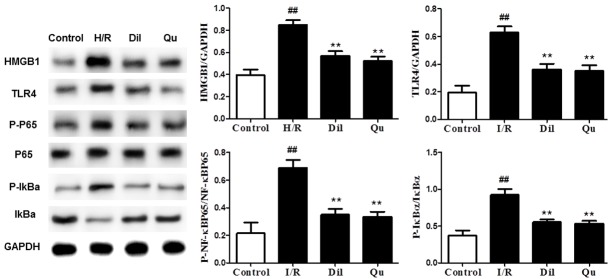
To understand more fully the mechanism by which Qu attenuated I/R injury, we next assessed expression of HMGB1, TLR4 and p-NF-κB in rat hearts by immunohistochemistry. Protein expression levels were significantly upregulated in heart tissues after I/R stimulation in coronary artery ligated rats (Figure 6). Treatment with either Dil or Qu significantly inhibited expression of these proteins (Figure 6), suggesting that these genes may be involved in the Qu-related suppression of I/R injury phenotype.
Figure 6.
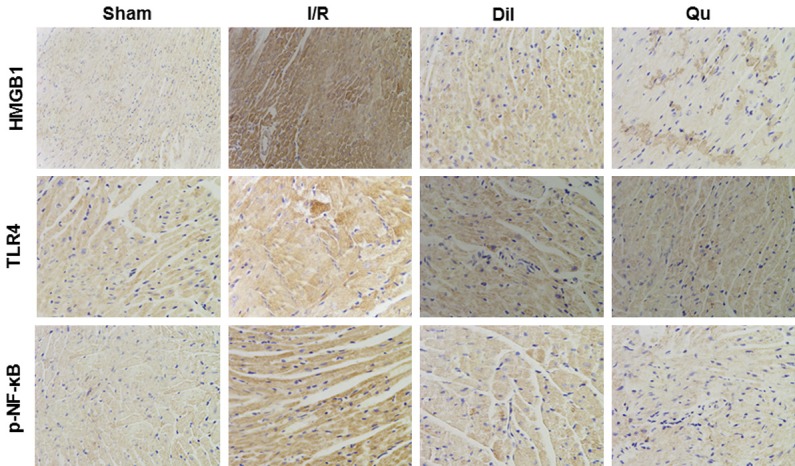
Effects of Qu on HMGB1, TLR4 and p-NF-κB protein expression by immunohistochemistry (× 200).
Effects of Qu on HMGB1/TLR/NF-κB pathway in I/R-induced heart
Further evaluation of the HMGB1/TLR/NF-κB pathway in I/R-induced hearts was performed by Western blot using heart tissues after I/R stimulation in global ischemia of isolated hearts and coronary artery ligated rats. Western blot analyses revealed strong activation of the HMGB1/TLR/NF-κB pathway in affected animals, which was reduced in response to Dil and Qu (Figure 7), further supporting the role of this pathway as a target of Qu treatment.
Figure 7.
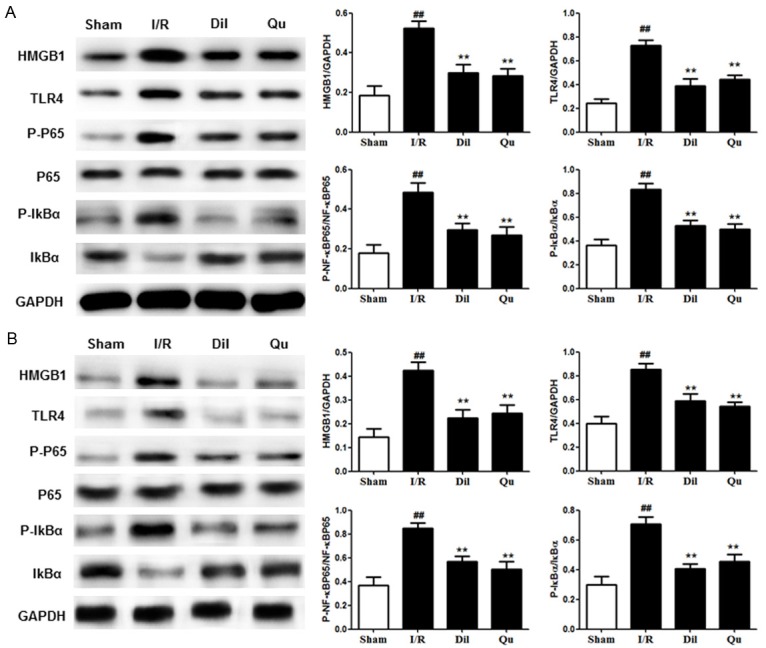
Effects of Qu on the HMGB1/TLR/NF-κB pathway in coronary artery ligated rats (A) and global ischemia of (B) isolated hearts. The data are expressed as mean values ± SDs. #P < 0.05, ##P < 0.01 relative to controls; *P < 0.05, **P < 0.01 compared with the I/R group.
Effects of Qu on HMGB1/TLR/NF-κB in H9C2 cells
Expression of the HMGB1/TLR/NF-κB pathway was significantly upregulated in H9C2 cells after I/R stimulation. As before, treatment with Dil and Qu effectively suppressed these effects, reducing the expression of this pathway to near-WT levels (Figure 8). Taken together, these results demonstrate that Qu successfully attenuates activation of the HMGB1/TLR/NF-κB pathway after I/R stimulation.
Figure 8.
Effects of Qu on the HMGB1/TLR/NF-κB pathway in H9C2 cells. The data are expressed as mean values ± SDs. #P < 0.05, ##P < 0.01 compared with control group; *P < 0.05, **P < 0.01 compared with H/R group.
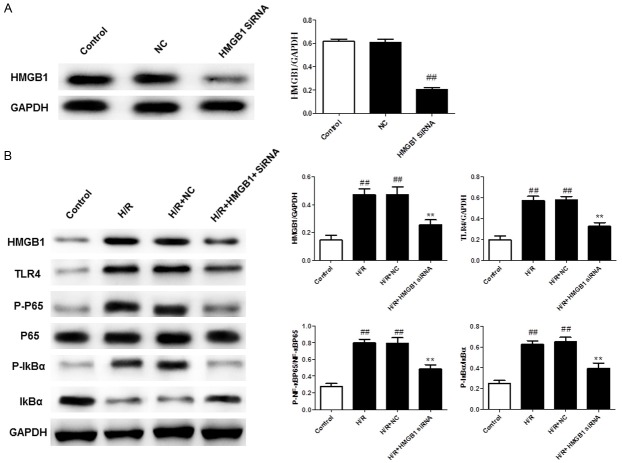
HMGB1 siRNA suppressed H/R injury in H9C2 cells
To confirm the role of HMGB1 in H/R injury, HMGB1 siRNA was used to knockdown HMGB1 gene expression in H9C2 cells. Following transfection for 48 h, cells exhibited a substantial decrease in HMGB1 protein abundance relative to controls (Figure 9A). As shown in Figure 9B, suppression of HMGB1 expression reduced the expression of HMGB1, TLR4 and p-NF-κB during H/R injury in H9C2 cells. These results are consistent with those seen for Qu during H/R injury in H9C2 cells, indicating that HMGB1 may participate in the anti-inflammatory effects of Qu in H/R injury in H9C2 cells.
Figure 9.
HMGB1 siRNA knockdown reduced HMGB1 gene expression (A) and the levels of HMGB1/TLR/NF-κB (B) in H9C2 cells. The data are expressed as mean values ± SDs. #P < 0.05, ##P < 0.01 relative to controls; *P < 0.05, **P < 0.01 compared with the H/R group.
Discussion
Current interventions for cardiovascular diseases include treatments such as thrombolytic therapy and coronary artery bypass grafting. Despite an overall decrease in mortality associated with cardiac events, the I/R injuries that arise in response to these treatments remain a significant obstacle. Interventions capable of both preventing and treating I/R injuries are therefore needed as an adjuvant to help salvage the ischemic-reperfused heart tissue in these patients.
In this study, Qu was shown to substantially reduce the myocardial infarct size in affected tissues, as well as preserve tissue integrity in rats subjected to heart ligation. Other benefits of Qu included improvement of heart function recovery through increasing coronary flow, elevation of myocardial contractility, and amelioration of ST-segment elevation. From a mechanistic standpoint, Qu was shown to decrease levels of TNF-α, IL-6 and IL-1β in serum and cell supernatants, indicating that Qu could inhibit inflammatory responses during myocardial I/R injury. Meanwhile, Qu significantly decreased CK and LDH levels compared with the I/R group. Taken together, these results show that Qu treatment confers a strong protective effect against myocardial ischemia-reperfusion injury.
HMGB1, a chromosomal protein with high electrophoretic mobility, plays a variety of roles, depending on the cellular location. In the nucleus, it promotes nuclear transcription, replication, recombination and DNR repair [10], while in the cytoplasm or extracellular phase it functions as a pro-inflammatory cytokine [11]. Various lines of evidence have shown HMGB1 mediates an inflammatory reaction through direct interactions with TLR2, TLR4 and RAGE [12,13]. Interaction of HMGB1 with TLR2/TLR4, in turn, triggers ERK1/2 and NF-κB signaling, which further enhances the inflammatory reaction [14]. NF-κB plays an important role in regulating inflammatory cytokine production, oxidative stress, and apoptosis [15], with significant implications for endothelial dysfunction and various heart diseases [16]. Our results confirmed that Qu successfully inhibited the expression of the HMGB1/TLR pathway, which may, in turn, lead to the suppression of NF-κB activation.
The goal of this study was to evaluate the effects of Qu on I/R-induced heart injury and investigate its potential mechanism of action. In vitro analyses showed that Qu successfully attenuated activation of the HMGB1/TLR/NFκB pathway in rats and isolated hearts. siRNA knockdown of HMGB1 in H9C2 cells inhibited the expression of HMGB1, TLR4 and p-NF-κB following H/R injury. Based on these results, HMGB1 may play an important role in the anti-inflammatory effects of Qu during H/R injury in H9C2 cells.
Taken together, the data presented here clearly demonstrate the cardioprotective effects of Qu on I/R injury-induced isolated heart tissues. These effects appear to be mediated through the HMGB1/TLR/NF-κB pathway, in part, through the induction of apoptotic pathogenesis. Further research will be necessary to both validate and expand upon these findings.
Disclosure of conflict of interest
None.
References
- 1.Hu Q, Wei B, Wei L, Hua K, Yu X, Li H, Ji H. Sodium tanshinone IIA sulfonate ameliorates ischemia-induced myocardial inflammation and lipid accumulation in beagle dogs through NLRP3 inflammasome. Int J Cardiol. 2015;196:183–92. doi: 10.1016/j.ijcard.2015.05.152. [DOI] [PubMed] [Google Scholar]
- 2.Zhu L, Wei T, Gao J, Chang X, He H, Luo F, Zhou R, Ma C, Liu Y, Yan T. The cardioprotective effect of salidroside against myocardial ischemia reperfusion injury in rats by inhibiting apoptosis and inflammation. Apoptosis. 2015;20:1433–1443. doi: 10.1007/s10495-015-1174-5. [DOI] [PubMed] [Google Scholar]
- 3.Weinreuter M, Kreusser MM, Beckendorf J, Schreiter FC, Leuschner F, Lehmann LH, Hofmann KP, Rostosky JS, Diemert N, Xu C. CaM kinase II mediates maladaptive post-infarct remodeling and pro-inflammatory chemoattractant signaling but not acute myocardial ischemia/reperfusion injury. EMBO Mol Med. 2014;6:1231–45. doi: 10.15252/emmm.201403848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hua K, Sheng X, Li T, Wang L, Zhang Y, Huang Z, Ji H. The edaravone and 3-n-butylphthalide ring-opening derivative 10b effectively attenuates cerebral ischemia injury in rats. Acta Pharmacol Sin. 2015;36:917–927. doi: 10.1038/aps.2015.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Andrassy M, Volz HC, Igwe JC, Funke B, Eichberger SN, Kaya Z, Buss S, Autschbach F, Pleger ST, Lukic IK. High-mobility group box-1 in ischemia-reperfusion injury of the heart. Circulation. 2008;117:3216–26. doi: 10.1161/CIRCULATIONAHA.108.769331. [DOI] [PubMed] [Google Scholar]
- 6.Klune JR, Dhupar R, Cardinal J, Billiar TR, Tsung A. HMGB1: endogenous danger signaling. Mol Med. 2008;14:476–84. doi: 10.2119/2008-00034.Klune. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Volz HC, Laohachewin D, Schellberg D, Wienbrandt AR, Nelles M, Zugck C, Kaya Z, Katus HA, Andrassy M. HMGB1 is an independent predictor of death and heart transplantation in heart failure. Clin Res Cardiol. 2012;101:427–35. doi: 10.1007/s00392-011-0409-x. [DOI] [PubMed] [Google Scholar]
- 8.Erden IM, Kahraman A. The protective effect of flavonol quercetin against ultraviolet a induced oxidative stress in rats. Toxicology. 2000;154:21–29. doi: 10.1016/s0300-483x(00)00268-7. [DOI] [PubMed] [Google Scholar]
- 9.Lee M, Son M, Ryu E, Yu SS, Kim JG, Kang BW, Sung GH, Cho H, Kang H. Quercetin-induced apoptosis prevents EBV infection. Oncotarget. 2015;6:12603–12624. doi: 10.18632/oncotarget.3687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Qin YH, Dai SM, Tang GS, Zhang J, Ren D, Wang ZW, Shen Q. HMGB1 enhances the proinflammatory activity of lipopolysaccharide by promoting the phosphorylation of MAPK p38 through receptor for advanced glycation end products. J Immunol. 2009;183:6244–50. doi: 10.4049/jimmunol.0900390. [DOI] [PubMed] [Google Scholar]
- 11.Karuppagounder V, Arumugam S, Thandavarayan RA, Pitchaimani V, Sreedhar R, Afrin R, Harima M, Suzuki H, Nomoto M, Miyashita S. Modulation of HMGB1 translocation and RAGE/NFκB cascade by quercetin treatment mitigates atopic dermatitis in NC/Nga transgenic mice. Exp Dermatol. 2015;24:418–23. doi: 10.1111/exd.12685. [DOI] [PubMed] [Google Scholar]
- 12.van Beijnum JR, Buurman WA, Griffioen AW. Convergence and amplification of toll-like receptor (TLR) and receptor for advanced glycation end products (RAGE) signaling pathways via high mobility group B1 (HMGB1) Angiogenesis. 2008;11:91–99. doi: 10.1007/s10456-008-9093-5. [DOI] [PubMed] [Google Scholar]
- 13.de Haan JJ, Smeets MB, Pasterkamp G, Arslan F. Danger signals in the initiation of the inflammatory response after myocardial infarction. Mediators Inflamm. 2013;2013:206039. doi: 10.1155/2013/206039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Favre J, Musette P, Douinechinard V, Laude K, Henry JP, Arnal JF, Thuillez C, Richard V. Toll-like receptors 2-deficient mice are protected against postischemic coronary endothelial dysfunction. Arterioscler Thromb Vasc Biol. 2007;27:1064–71. doi: 10.1161/ATVBAHA.107.140723. [DOI] [PubMed] [Google Scholar]
- 15.Zhang K, Liu J, You X, Kong P, Song Y, Cao L, Yang S, Wang W, Fu Q, Ma Z. P2X7 as a new target for chrysophanol to treat lipopolysaccharide-induced depression in mice. Neurosci Lett. 2015;613:60–65. doi: 10.1016/j.neulet.2015.12.043. [DOI] [PubMed] [Google Scholar]
- 16.Chorianopoulos E, Heger T, Lutz M, Frank D, Bea F, Katus HA, Frey N. FGF-inducible 14kDa protein (Fn14) is regulated via the RhoA/ROCK kinase pathway in cardiomyocytes and mediates nuclear factor-kappaB activation by TWEAK. Basic Res Cardiol. 2010;105:301–13. doi: 10.1007/s00395-009-0046-y. [DOI] [PubMed] [Google Scholar]