Abstract
The present study aimed to investigate the gene expression changes in prostate cancer (PC) and screen the hub genes and associated pathways of PC progression. The authors employed integrated analysis of GSE46602 downloaded from the Gene Expression Omnibus and The Cancer Genome Atlas databases to identify 484 consensual differentially expressed genes (DEGs) in PC, when compared with adjacent normal tissue samples. Functional annotation and pathway analysis were performed. The protein-protein interaction (PPI) networks and module were constructed. RT-qPCR was used to validate the results in clinical PC samples. Survival analysis of hub genes was performed to explore their clinical value. GO analysis results revealed that DEGs were significantly enriched in negative regulation of nitrobenzene metabolic process, extracellular space and protein homodimerization activity. KEGG pathway analysis results revealed that DEGs were most significantly enriched in focal adhesion. The top 10 hub genes were identified to be hub genes from the PPI network, and the model revealed that these genes were enriched in various pathways, including neuroactive ligand-receptor interaction, p53 and glutathione metabolism signaling pathways. RT-qPCR results validated that expression levels of eight genes (PIK3R1, BIRC5, ITGB4, RRM2, TOP2A, ANXA1, LPAR1 and ITGB8) were consistent with the bioinformatics analysis. ITGB4 and RRM2 with genetic alterations exhibited association with a poorer survival rate, compared with those without alterations. These results revealed that PC-related genes and pathways have an important role in tumor expansion, metastasis and prognosis. In summary, these hub genes and related pathways may act as biomarkers or therapeutic targets for PC.
Keywords: Prostate cancer, TCGA, GEO, bioinformatic analysis, differentially expressed genes
Introduction
Among the diagnosed American male cancer patients, prostate cancer is the most common cancer except skin cancer [1]. In 2016, there were 181,000 newly diagnosed cases and 26,000 cases of mortality in the United States [2]. With the development of clinical and experimental research, progress has been made regarding the treatment and understanding of the fundamental biology underlying PC. In terms of detection, prostate-specific antigen (PSA) is still the commonly used marker to identify increased risk [3]. Various studies have shown that certain genes have an important role in the development and progression of PC, such as MXI1, BRCA1 and BRCA2. Regarding associated signaling pathways, it has been demonstrated that glioma and integrated breast cancer pathways, in addition to notch signaling and androgen receptor (AR) pathways, are associated with PC [4]. Furthermore, numerous novel biomarkers aid with profitable prognostic information, which may have vital therapeutic implications. This information may be used as selection criteria for patients eligible for active surveillance or candidates for radiotherapy/surgery [5].
However, identification of a valid biomarker to complement PSA for screening, molecular stratification methods and treatment of metastatic disease is of primary concern. The excavation of disease-related genes or biomarkers associated with the pathogenesis and molecular mechanism of PC is of great significance in the diagnosis and treatment of patients [6].
With the rapid development of molecular biology and bioinformatics, chip and sequencing technology is widely used, and research in this area is continuous. The Gene Expression Omnibus (GEO) and The Cancer Genome Atlas (TCGA) database have accumulated abundant genomic and gene expression profiles for different diseases during the past decade. Through the analysis of these data, various key genes and signaling pathways related to the disease may be identified, which will result in a better understanding of the occurrence and development mechanism of the disease.
In the present study, large-scale gene data sets regarding PC were downloaded from the GEO and TCGA databases. GEO2R and The R Programming Language (R) was utilized for preprocessing and analysis of these data to obtain the differentially expressed genes (DEGs). For these DEGs, the Database for Annotation Visualization and Integrated Discovery (DAVID) database was used to facilitate the functional annotation and pathway analysis, and the STRING database was used to construct the protein-protein interaction (PPI) network and modules selection. Then, RT-PCR was used to validate the hub genes in clinical PC samples. Finally, a survival analysis of the hub genes was conducted to explore their clinical value. The present study aimed to identify critical genes involved in PC, which may be helpful for the development of novel targets for therapeutic intervention.
Materials and methods
Microarray data
The gene expression profile GSE46602 was downloaded from the GEO database of the National Center for Biotechnology Information (http://www.ncbi.nlm.nih.gov/geo). Then, the probe-level information was converted into the corresponding gene symbol according to the explanation data downloaded from platform GPL570 (Affymetrix Human Genome U133 Plus 2.0 Array), and 54,675 probes were used to detect levels of gene transcription. The genome expression dataset consisted of 36 tumor sample specimens from patients with prostate cancer and 14 control samples from patients with benign prostate glands adjacent to cancer or benign prostate glands. In the present study, the dataset including the 14 control and 35 PC samples was selected. The genomic data and clinical data of PC from TCGA (https://cancergenome.nih.gov/) were also downloaded. These RNA sequencing (RNA-seq) data from Illumina HiSeq RNASeq platform included 498 tumor sample specimens from patients with prostate cancer and 52 control samples from patients with benign prostate glands adjacent to cancerous glands.
Data preprocessing and DEGs screening
GEO2R (https://www.ncbi.nlm.nih.gov/geo/geo2r/) is an interactive web tool which was applied to detect DEGs by comparing two or more groups of samples in a GEO series [7]. GEOquery and Limma R package in GEO2R was applied to identify the DEGs between PC samples and control samples. The Benjamini-Hochberg (BH) method [8] was introduced to adjust the raw P-values into a false discovery rate to avoid the multi-test problem, which might produce too many false positive results. The adjust P value <0.05 and |log2 fold change (FC)| ≥1 were set as the thresholds for identifying DEGs.
The RNA-Seq data of PC samples and control samples were downloaded from TCGA in September 2017. The edgeR package in R was subsequently used for the calculation of DEGs by comparing PC samples and control samples. The adjust P value <0.05 and I log2 fold change (FC) I ≥1 were set as the cut-off criteria. The genes that presented in both GEO and TCGA analysis results were selected as the final DEGs.
Functional and pathway enrichment analysis of DEGs
The DAVID (https://david.ncifcrf.gov/) database is a biological database regularly used to facilitate functional annotation and pathway analysis. In order to better understand the biological functions and characteristics, the present study performed Gene Ontology (GO) and Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway analyses in the DAVID database to identify DEGs. The human genome was selected as the background list parameter, and P value <0.05 and count ≥2 were chosen as the thresholds to indicate a statistically significant difference.
PPI network construction and modules selection
The present study used the STRING database (http://string-db.org/) to construct a PPI network. PPI analysis provides novel insights into protein function and may help to reveal the generic organization principles of functional cell systems and aid in the discovery of functional associations between proteins on a genome-wide scale. All the DEGs were imported into Cytoscape plugin to create network visualizations. Then, the resulting PPI network was subjected to module analysis with the Plugin Molecular Complex Detection (MCODE) with the default parameters (degree cutoff ≥2, node score cutoff ≥2, K-core ≥2, and maximum depth =100).
Validation based on clinical samples of PC
The aforementioned section described the creation of the PPI network using STRING and Cytoscape software. The hub genes were screened out according to the degree. To further verify the data of the hub genes, reverse transcription-quantitative polymerase chain reaction (RT-qPCR) was conducted to detect the expression levels of the hub genes within clinical PC samples (n=12) obtained from the First Affiliated Hospital of Guangzhou Medical University. All of the individuals participating in the project gave informed consent, and the study was approved by the human study ethics committee of the First Affiliated Hospital of Guangzhou Medical University. Total RNA was extracted from tissues using TRIzol® reagent (Invitrogen; Thermo Fisher Scientific, Inc., Waltham, MA, USA). A total of 1 µg total RNA was reverse transcribed to cDNA, which was amplified by PCR within a 10 μl reaction system using PrimeScript™ RT Reagent Kit (Takara Bio, Inc., Otsu, Japan). RT-qPCR procedures were performed using SYBR Premix Ex Taq™ GC (Takara Bio, Inc.) on a BIO-RAD system, according to the manufacturer’s instructions. Relative expression values were calculated using the 2-ΔCT method [9]. The primers were synthesized by Generay Biotech Co., Ltd. (Shanghai, China), and sequences are listed in Table 1. All values were normalized against GAPDH expression levels. A paired-sample t-test was performed to compare the hub genes between PC and para-cancerous prostate tissues, using SPSS software, version 22.0 (IBM SPSS, Armonk, NY, USA).
Table 1.
Primer sequences for RT-qPCR
| Gene | Forward primer (5’-3’) | Reverse primer (5’-3’) |
|---|---|---|
| PIK3R1 | ACCACTACCGGAATGAATCTCT | GGGATGTGCGGGTATATTCTTC |
| BIRC5 | AGGACCACCGCATCTCTACAT | AAGTCTGGCTCGTTCTCAGTG |
| ITGB4 | GCAGCTTCCAAATCACAGAGG | CCAGATCATCGGACATGGAGTT |
| RRM2 | GTGGAGCGATTTAGCCAAGAA | CACAAGGCATCGTTTCAATGG |
| TOP2A | ACCATTGCAGCCTGTAAATGA | GGGCGGAGCAAAATATGTTCC |
| ANXA1 | CTAAGCGAAACAATGCACAGC | CCTCCTCAAGGTGACCTGTAA |
| LPAR1 | CTTTGCTGGGTTGGCCTACTT | GCCATGTGCTAACAGTCAGTCT |
| ITGB8 | ACCAGGAGAAGTGTCTATCCAG | CCAAGACGAAAGTCACGGGA |
| GAPDH | GAGGTGAAGGTCGGAGT | GAAGATGGTGATGGGATTT |
Survival analysis
In order to reveal the genetic alterations and Kaplan-Meier curves, cBioportal (http://www.cbioportal.org/) [10,11] was applied to analyze the hub genes. The hub genes were imported to cBioPortal to investigate gene expression changes in PC with mRNA expression data (n=499) from the TCGA Prostate Project dataset, as compared with normal prostate samples. The aberrant mRNA expression threshold was defined as z-score ±2.0.
Results
Data preprocessing and DEG screening
The gene data were downloaded from GEO and TCGA. Based on the GEO2R and R analysis, a total of 3,714 DEGs were identified in PC compared with the control samples in GEO, and 1,415 DEGs were identified in PC compared with the control samples in TCGA. A total of 484 DEGs presented in both the GEO and TCGA analysis results. These genes included 168 upregulated and 316 downregulated genes. Two volcano plots of DEGs and one Venn diagram of the DEG screening are presented in Figure 1.
Figure 1.
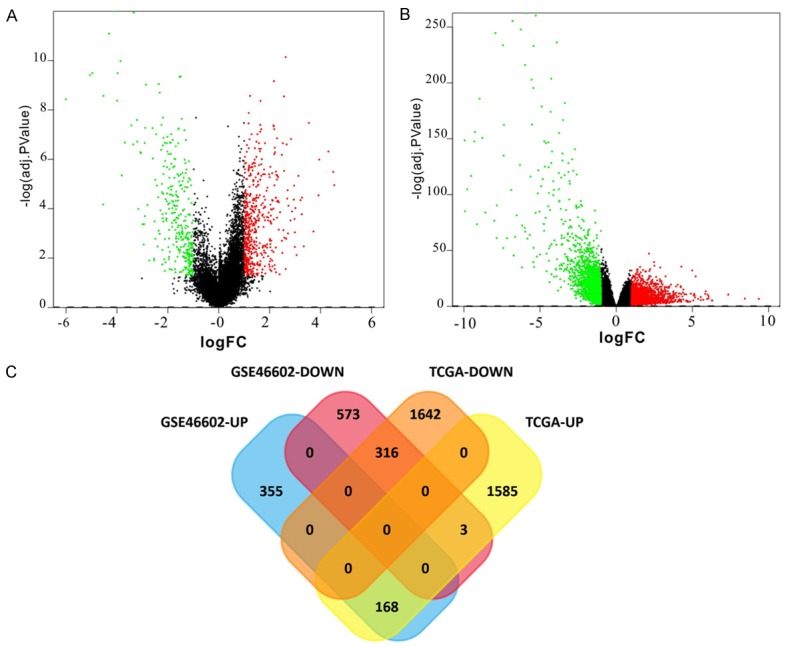
Two volcano plots of DEGs and one Venn diagram of the DEGs screening. For the volcano, the volcano plot on the left (A) is the result of the GEO database and the volcano plot on the right (B) represents the result of the TCGA database. The abscissa is logFC and the ordinate is -log10 (adj. P Value). The red and green spots represent DEGs. The black dots represent genes that are not differentially expressed between PC and control samples. Red: upregulated; green: downregulated. The Venn diagram (C) indicates the number of DEGs in four different datasets and the crossing area indicates the cross-DEGs in different datasets. 168 upregulated and 316 downregulated genes were identified from the data obtained from the TCGA and GEO databases. GEO, Gene Expression Omnibus; TCGA, The Cancer Genome Atlas; PC, prostate cancer; DEG, differentially expressed gene.
Functional and pathway enrichment analysis of DEGs
Three GO category results are presented, through the use of DAVID, including biological processes, cellular components and molecular functions. The biological process results revealed that DEGs were primarily enriched in nitrobenzene metabolic processes, glutathione derivative biosynthetic process and cochlea development. The cellular component results indicated that DEGs were mainly enriched in extracellular space, plasma membrane and proteinaceous extracellular matrix. The molecular function results showed that DEGs were mainly enriched in protein homodimerization activity, calcium ion binding and glutathione binding (Figure 2A-C; Table 2A). To investigate pathway enrichment, KEGG signaling pathway analysis was used to identify the top five pathways, which included ‘focal adhesion’, ‘glutathione metabolism’, and ‘chemical carcinogenesis’ (Figure 2D; Table 2B).
Figure 2.
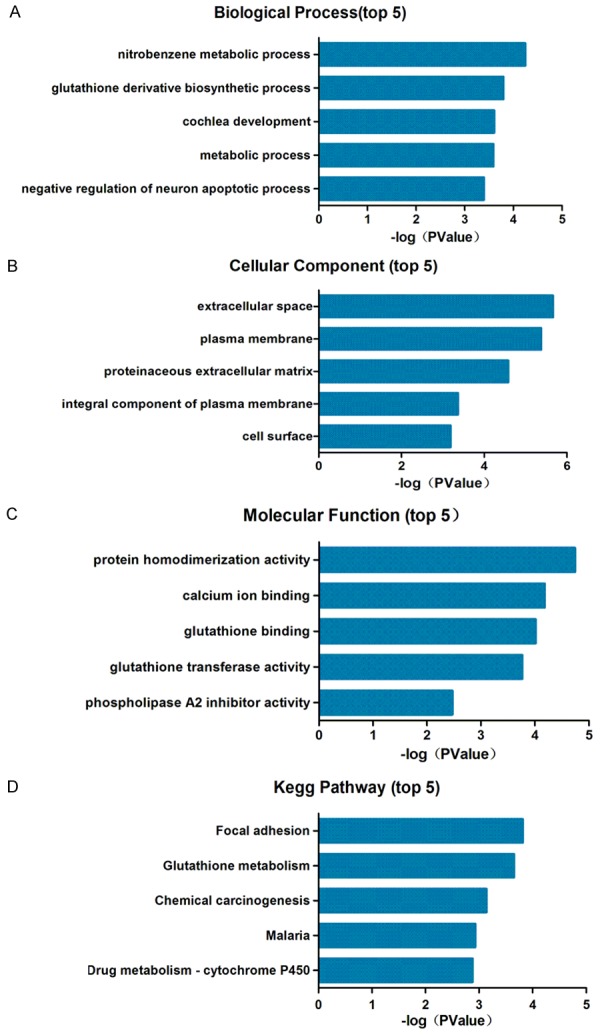
Top five Gene Ontology enrichment analysis and KEGG pathways. (A) Biological processes, (B) cellular components, (C) molecular functions and (D) KEGG pathway analysis. KEGG, Kyoto Encyclopedia of Genes and Genome.
Table 2A.
The top 15 enriched Gene Ontology terms of differentially expressed genes
| Category | Term | Count | P Value |
|---|---|---|---|
| BP | Nitrobenzene metabolic process | 4 | 5.72E-05 |
| BP | Glutathione derivative biosynthetic process | 6 | 1.60E-04 |
| BP | Cochlea development | 6 | 2.48E-04 |
| BP | Metabolic process | 14 | 2.57E-04 |
| BP | Negative regulation of neuron apoptotic process | 12 | 4.04E-04 |
| CC | Extracellular space | 61 | 2.16E-06 |
| CC | Plasma membrane | 140 | 4.21E-06 |
| CC | Proteinaceous extracellular matrix | 20 | 2.60E-05 |
| CC | Integral component of plasma membrane | 55 | 4.32E-04 |
| CC | Cell surface | 27 | 6.50E-04 |
| MF | Protein homodimerization activity | 38 | 1.81E-05 |
| MF | Calcium ion binding | 36 | 6.59E-05 |
| MF | Glutathione binding | 5 | 9.70E-05 |
| MF | Glutathione transferase activity | 7 | 1.72E-04 |
| MF | Phospholipase A2 inhibitor activity | 3 | 3.39E-03 |
BP: biological process; CC: cellular component; MF: molecular function.
Table 2B.
The top five enriched pathways of differentially expressed genes
| Category | Term | Count | P Value |
|---|---|---|---|
| KEGG_PATHWAY | Focal adhesion | 16 | 1.54E-04 |
| KEGG_PATHWAY | Glutathione metabolism | 8 | 2.24E-04 |
| KEGG_PATHWAY | Chemical carcinogenesis | 9 | 7.31E-04 |
| KEGG_PATHWAY | Malaria | 7 | 1.18E-03 |
| KEGG_PATHWAY | Drug metabolism-cytochrome P450 | 8 | 1.32E-03 |
PPI network construction and module selection
All DEGs were analyzed using the STRING online database and Cytoscape software. ‘Confidence score ≥0.7’ was set as the cut-off criterion. A total of 145 DEGs of the 484 commonly altered DEGs were filtered into the DEG PPI network complex, including 145 nodes and 288 edges (Figure 3A). Of the 145 DGEs, 14 hub genes were identified with the criteria of degree >10. The top 10 node degree genes were PIK3R1, BIRC5, ITGB4, AGTR1, RRM2, TOP2A, ANXA1, LPAR1, CCNB2 and ITGB8.
Figure 3.
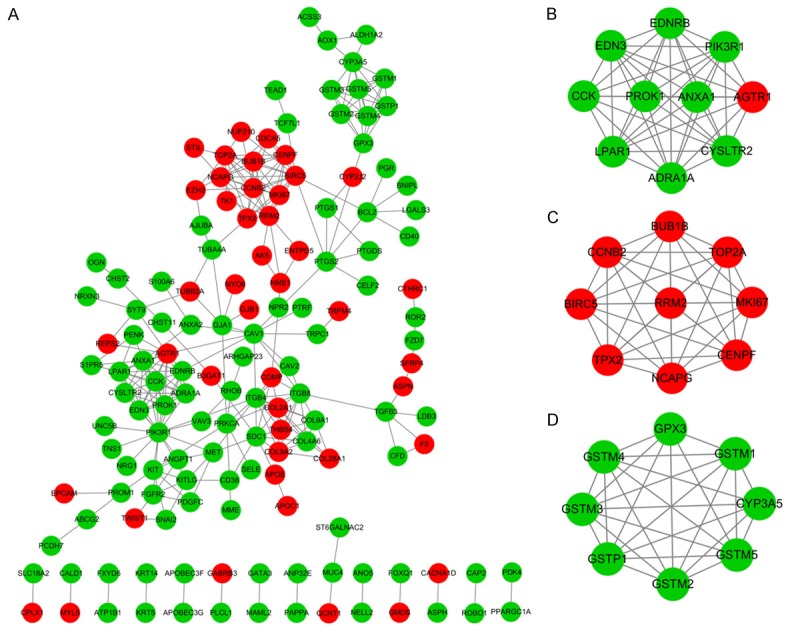
PPI network of the DEGs and modular analysis. (A) DEG PPI network complex, (B) module 1 of DEGs from PPI network, (C) module 2 of DEGs from PPI network and (D) module 3 of DEGs from PPI network. Red nodes represent the upregulated DEGs and green nodes represent the downregulated DEGs. Increased node interaction suggests a greater biological significance. PPI, protein-protein interaction; DEG, differentially expressed gene.
Furthermore, the top 3 significant modules from the PPI network were identified using MCODE plugin in Cytoscape (Figure 3B-D). The DEGs in the top 3 modules were selected to perform pathway enrichment analysis. The pathway enrichment analysis results revealed that the genes in module 1 and module 3 were predominantly associated with neuroactive ligand-receptor interaction, p53 signaling pathway and glutathione metabolism (Table 3).
Table 3.
Top three significant modules selected from the protein-protein interaction network
| Module | Name | Count | P value | Genes |
|---|---|---|---|---|
| Module 1 | Neuroactive ligand-receptor interaction | 5 | 1.22E-05 | EDNRB, AGTR1, CYSLTR2, ADRA1A, LPAR1 |
| cGMP-PKG signaling pathway | 4 | 1.31E-04 | EDNRB, AGTR1, ADRA1A, PIK3R1 | |
| Calcium signaling pathway | 4 | 1.65E-04 | EDNRB, AGTR1, CYSLTR2, ADRA1A | |
| Pathways in cancer | 4 | 1.68E-03 | EDNRB, AGTR1, LPAR1, PIK3R1 | |
| Adrenergic signaling in cardiomyocytes | 3 | 4.25E-03 | AGTR1, ADRA1A, PIK3R1 | |
| Module 2 | p53 signaling pathway | 2 | 2.8E-02 | CCNB2, RRM2 |
| Module 3 | Glutathione metabolism | 7 | 8.31E-13 | GSTM1, GSTM2, GSTM3, GSTM4, GPX3, GSTM5, GSTP1 |
| Drug metabolism-cytochrome P450 | 7 | 5.04E-12 | GSTM1, GSTM2, CYP3A5, GSTM3, GSTM4, GSTM5, GSTP1 | |
| Metabolism of xenobiotics by cytochrome P450 | 7 | 8.52E-12 | GSTM1, GSTM2, CYP3A5, GSTM3, GSTM4, GSTM5, GSTP1 | |
| Chemical carcinogenesis | 7 | 1.38E-11 | GSTM1, GSTM2, CYP3A5, GSTM3, GSTM4, GSTM5, GSTP1 |
Validation based on clinical samples of PC
To validate the findings in the integrated analysis, eight hub genes were selected for RT-qPCR in 12 tissues obtained from PC patients, compared with matched para-cancerous tissue. According to the experimental results, the expression pattern of selected genes in PC and matched para-cancerous tissue was similar to that observed in the integrated analysis (Figure 4). The expression levels of BIRC5, RRM2 and TOP2A were up-regulated in PC compared with matched para-cancerous tissue, whereas the expression levels of PIK3R1, ITGB4 and ANXA1, LPAR1 and ITGB8 were down-regulated.
Figure 4.
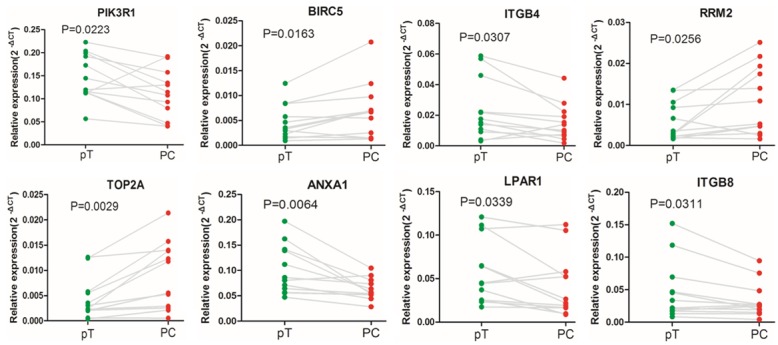
The expression levels of eight hub genes were detected in 12 tissues of PC patients and their matched para-cancerous tissue, using reverse transcription-quantitative polymerase chain reaction. GAPDH was used as an internal reference gene for normalization.
Survival analysis
Finally, two genes (ITGB4 and RRM2) were screened from cBioPortal. The Oncoprint from cBioPortal revealed that a total of 12% of cases with genetic alterations could be obtained. A total of two genes (ITGB4 and RRM2) had genetic alterations which included amplification, deep deletion, mRNA upregulation, truncating mutation (putative passenger) and missense mutation (putative passenger) (Figure 5A). It was demonstrated that the cases with genetic alterations in these genes (ITGB4, P=0.00158; RRM2, P=0.00771) exhibited a poorer survival rate compared with cases without alterations (Figure 5B).
Figure 5.
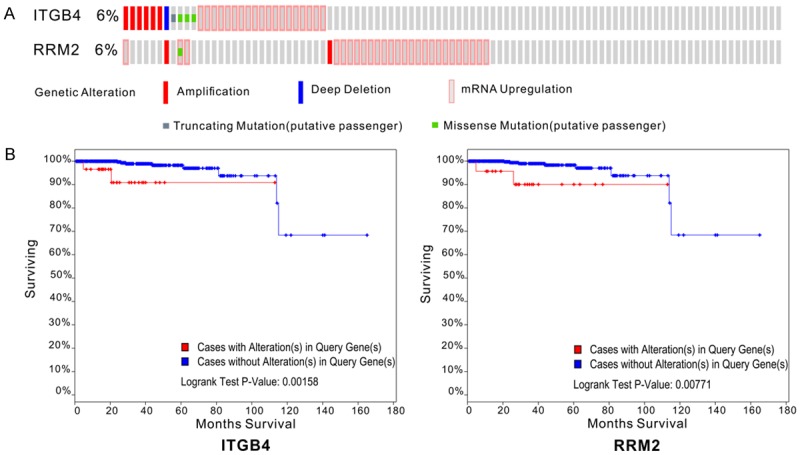
Genetic alterations and the prognostic value of differentially expressed genes in prostate cancer. A. Genetic alterations: Red represents amplification, blue represents deep deletion, pink represents mRNA upregulation, gray represents truncating mutation (putative passenger) and green represents missense mutation (putative passenger). B. Kaplan-Meier curves of two hub genes between group with alterations and group without alterations. The Kaplan-Meier survival curves showed the significant prognostic value of ITGB4 and RRM2 alteration regarding survival. Red line represents cases with alterations in query genes. Blue line represents cases without alterations in query genes. The x-axis indicates overall survival time (months) and the y-axis represents the survival rate. These curves were downloaded from cBioPortal.
Discussion
PC has become one of the most common non-skin malignancies among men with an incidence of approximately 0.01% worldwide [12]. Efficient progress has been made in genetics and molecular pathogenesis, however the detection of PC and treatment of the localized disease remains of primary concern, and requires further investigation [6].
In the present study, GEO2R and R was used to analyze the gene data downloaded from GEO and TCGA databases. A total of 484 DEGs in PC compared with control samples were identified, which included 168 upregulated and 316 downregulated genes. DEGs were mainly enriched in 15 GO terms, including negative regulation of nitrobenzene metabolic process, extracellular space and protein homodimerization activity. The KEGG pathway enrichment analysis result showed that the DEGs were related to focal adhesion, glutathione metabolism and chemical carcinogenesis. The focal adhesion pathway has great significance in the transfer and treatment of prostate cancer. In this pathway, talin1, a focal adhesion complex protein, enhances prostate cancer cell adhesion, migration and invasion [13]. Previous studies indicate that the overexpression of bone sialoprotein (BSP) in PC is correlated with tumor progression [14-16]. Gordon et al. demonstrated that BSP stimulates focal adhesion kinase and focal adhesion-related signaling pathways [17]. Therefore, monitoring of this signaling pathway may be beneficial to understanding the mechanism of carcinogenesis and researching treatment of prostate cancer.
Furthermore, the present study constructed PPI networks to investigate the critical DEGs, and 10 hub genes were identified. Furthermore, the differential expression of eight of these genes (PIK3R1, BIRC5, ITGB4, RRM2, TOP2A, ANXA1, LPAR1 and ITGB8) was verified in 12 tissues of PC patients, compared with matched paracancerous tissues, via RT-qPCR. The phosphoinositide 3-kinase (PI3K) signaling pathway and AR signaling may mediate prostate cancer survival signals and androgen inhibits PIK3R1 in prostate cancer cells [18]. Therefore, PIK3R1 may be a target for the treatment of PC, however this hypothesis requires further investigation. Survivin (encoded by the gene BIRC5) is an anti-apoptotic protein that is overexpressed in many cancer types, including gastric, lung, colon and breast cancer [19]. BIRC5 levels are correlated with Ras signaling signature expression, which is upregulated in PC [20]. Danilewicz et al. had confirmed that the immunoexpression of survivin is augmented in PC compared with benign prostatic hyperplasia, and positively correlated with parameters of tumor aggressiveness [21]. These results suggest that BIRC5 may be used as a therapeutic target in PC treatment. TOP2A encodes topoisomerase IIα, which is a ribozyme that controls DNA topology and cell cycle progression. This enzyme is a marker of cell proliferation in normal and tumor tissue [22]. Previous studies have reported that increased expression of TOP2A is linked to shortened survival in breast, ovary, brain, skin and small cell lung cancers [23-27]. Sullivan et al. suggested that increased TOP2A is a strong predictor of advancing stages and tumor grade in PC [28]. De Resende et al. suggested that TOP2A protein expression levels may act as a prognostic index for patients with PC [22]. Annexin A1 (ANXA1) is a Ca2+-binding protein in the invasive stages of PC. Bizzarro et al. indicated that ANXA1 may be a pivotal mediator of hypoxia-related metastasis-associated processes in PC [29]. D’Acunto et al. reported that the expression of ANXA1 is a contributing factor to the promotion of apoptosis in PC [30]. ANXA1 may increase the accuracy of prognostication as a biomarker of PC following radical prostatectomy [31]. Therefore, ANXA1 is important in PC and may be used as a prognostic indicator. Lysophosphatidic acid (LPA) is a growth factor in many cells, including prostate and ovarian cancer-derived cell lines [32]. In both in vivo and in vitro systems, LPA has been demonstrated to be involved in multiple aspects of cancer progression, including cell proliferation, growth, survival, migration, invasion and progression of angiogenesis [33-35]. LPA and LPA receptor 1 (LPAR1), mediated by activation of nuclear factor-κB, promotes proliferation, survival and migration of PC cells [36,37]. HärmäV et al. suggested that LPAR1 and Gα (12/13) signaling regulates cellular motility and invasion with epithelial maturation in PC [38]. These results suggest that LPAR1 has a potential therapeutic benefit in PC. Integrin (ITGB)8 is one of the members of the integrin family. ITGB8 has been shown to be upregulated in some cancers, including head and neck cancer, hepatocellular carcinoma, ovarian cancers and melanoma cell lines, in addition to primary non-small lung cancer samples and brain metastases from several epithelial cancers [39-41]. Furthermore, a six-gene expression signature biomarker, which includes ITGB8, may predict the occurrence of lung metastasis from breast cancer [42]. Rutkowski et al. reports that the overexpression of EPH Receptor (Eph) B4 leads to aggressive phenotypes in PC cells. The study additionally revealed that EphB4 regulates ITGB8 expression [43]. However, the role of ITGB8 in the motility of PC cells remains to be fully elucidated.
ITGB4 is also a member of the integrin family. Aberrant expression of integrin subunits has been implicated in the malignant phenotype of a variety of cancers [44]. Brendle et al. suggests that ITGB4 may influence tumor aggressiveness and survival, and it may have prognostic value in breast cancer [45]. In pancreatic ductal adenocarcinoma, ITGB4 overexpression promotes cell scattering and motility, downregulates E-cadherin and upregulates vimentin expression. Masugi et al. revealed that IGTB4 has a potential role in the regulation of cancer invasion and epithelial-mesenchymal transition [46]. Kettunen et al. demonstrated that the ITGB4 is upregulated in malignant pleural mesothelioma (MM) suggesting that the ITGB4 has link with the development of MM [47]. ITGB4 is important in PC migration and expansion of prostate tumor progenitors [48]. Kawakami et al. reports that ITGB and vinculin may be useful markers for the progression of PC associated with taxane resistance, and this result may provide a basis for the diagnosis of PC [49]. In the present study, it was demonstrated that the PC patients with ITGB4 alterations exhibited a poorer survival rate compared with those without the genetic alterations. This result suggests that the mutation in ITGB4 reduces the survival rate of patients with PC. Ribonucleotide reductase is required for DNA synthesis and repair [50], and is responsible for the de novo conversion of the ribonucleoside diphosphates to deoxyribonucleoside diphosphates. The ribonucleotide reductase M2 subunit (RRM2) determines malignant cellular behavior in a range of human cancers [51], such as colorectal cancer, oral squamous cell carcinoma, nasopharyngeal carcinoma, hepatocellular carcinoma, adrenocortical cancer, pancreatic adenocarcinoma and breast cancer. In the present study, the PC patients with an RRM2 alteration had a lower survival rate compared with patients without alteration. Notably, Huang et al. demonstrated that RRM2 is important in the proliferation and invasion of PC, which suggests that RRM2 may act as a novel biomarker for assessment of patients with low-risk PC [52]. The results of the present study are consistent with the results of previous studies. RRM2 may have an important role in the progression of PC, however this requires further study, in order to verify the specific molecular marker role of RRM2 in the diagnosis of patients with low-risk PC.
The module analysis result of the PPI network demonstrated that the development of PC was associated with neuroactive ligand-receptor interaction, the p53 signaling pathway and the glutathione metabolism pathway. The neuroactive ligand-receptor interaction signaling pathway is a collection of receptors and ligands on the plasma membrane that are associated with intracellular and extracellular signaling pathways [53]. Fang et al. and Liu et al. used bioinformatics to demonstrate that the neuroactive ligand-receptor interaction signaling pathway is associated with progression of bladder cancer and renal cell carcinoma [54,55]. Myers et al. revealed that five pathways were enriched in prostate tumors in members of the African-American population, including the neuroactive ligand-receptor interaction signaling pathway, via protein analysis [56]. In accordance with the results of previous studies, the present study demonstrated that neuroactive ligand-receptor interactions are involved in the progression of PC, however the specific molecular mechanisms in PC require further investigation. Furthermore, previous studies have demonstrated that glutathione metabolism is associated with PC. Glutathione peroxidase 1 polymorphism is involved in prostate carcinogenesis [57]. Glutathione exhibits an important role in survival mechanisms of PC cells [58] and this pathway is linked to the antineoplastic function and recrudescence in PC [59,60]. The p53 gene is the most common mutant gene in human tumors, and the primary function of the p53 protein is to prevent the cells into the DNA synthesis period and make it stagnation in the G1 phase to repair damaged DNA [61,62]. The mutation rate of the p53 gene in patients with primary PC is 10-20%, whereas the rate in the progression of PC is 42% and is closely related to malignant features such as bone metastasis and androgen dependence [63,64]. Kluth et al. analyzed tissue microarrays including 11,152 prostate cancer samples using immunohistochemistry and fluorescence in situ hybridization, and demonstrated that p53 may be a useful clinical molecular feature of PC [65]. These results indicate that p53 has an important role in the progression and prognosis of PC. Therefore, monitoring and blocking of neuroactive ligand-receptor interaction, p53 signaling pathway, and glutathione metabolism pathway are promising therapeutic strategies for future investigation and treatment of PC patients.
Conclusion
In conclusion, the present study identified various DEGs by using comprehensive bioinformatics analysis. Furthermore, various hub genes and pathways involved in the progression of prostate cancer, which may be predictors or therapeutic targets for PC were identified. However, lack of experimental verification is a limitation of the present study. Further experimental research is necessary to fully elucidate the mechanisms underlying PC tumorigenesis.
Acknowledgements
The present study was supported by Guangzhou Science and Technology Project of China (grant no. 201510010272), Science and Technology Planning Project of Guangdong Province (grant no.2017B030314108).
Disclosure of conflict of interest
None.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin. 2016;66:7–30. doi: 10.3322/caac.21332. [DOI] [PubMed] [Google Scholar]
- 2.Barry MJ, Simmons LH. Prevention of prostate cancer morbidity and mortality: primary prevention and early detection. Med Clin North Am. 2017;101:787–806. doi: 10.1016/j.mcna.2017.03.009. [DOI] [PubMed] [Google Scholar]
- 3.Roberts MJ, Chow CW, Schirra HJ, Richards R, Buck M, Selth LA, Doi SA, Samaratunga H, Perry-Keene J, Payton D, Yaxley J, Lavin MF, Gardiner RA. Diagnostic performance of expression of PCA3, Hepsin and miR biomarkers inejaculate in combination with serum PSA for the detection of prostate cancer. Prostate. 2015;75:539–549. doi: 10.1002/pros.22942. [DOI] [PubMed] [Google Scholar]
- 4.Maughan BL, Antonarakis ES. Androgen pathway resistance in prostate cancer and therapeutic implications. Expert Opin Pharmacother. 2015;16:1521–1537. doi: 10.1517/14656566.2015.1055249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ferro M, Buonerba C, Terracciano D, Lucarelli G, Cosimato V, Bottero D, Deliu VM, Ditonno P, Perdona S, Autorino R, Coman I, De Placido S, Di Lorenzo G, De Cobelli O. Biomarkers in localized prostate cancer. Future Oncol. 2016;12:399–411. doi: 10.2217/fon.15.318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bjurlin MA, Taneja SS. Prostate cancer. Urol Clin North Am. 2017;44:xv–xvi. doi: 10.1016/j.ucl.2017.08.001. [DOI] [PubMed] [Google Scholar]
- 7.Barrett T, Wilhite SE, Ledoux P, Evangelista C, Kim IF, Tomashevsky M, Marshall KA, Phillippy KH, Sherman PM, Holko M, Yefanov A, Lee H, Zhang N, Robertson CL, Serova N, Davis S, Soboleva A. NCBI GEO: archive for functional genomics data sets--update. Nucleic Acids Res. 2013;41:D991–995. doi: 10.1093/nar/gks1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hardcastle TJ. Generalized empirical Bayesian methods for discovery of differential data in high-throughput biology. Bioinformatics. 2016;32:195–202. doi: 10.1093/bioinformatics/btv569. [DOI] [PubMed] [Google Scholar]
- 9.Schmittgen TD, Livak KJ. Analyzing realtime PCR data by the comparative C(T) method. Nat Protoc. 2008;3:1101–1108. doi: 10.1038/nprot.2008.73. [DOI] [PubMed] [Google Scholar]
- 10.Gao J, Aksoy BA, Dogrusoz U, Dresdner G, Gross B, Sumer SO, Sun Y, Jacobsen A, Sinha R, Larsson E, Cerami E, Sander C, Schultz N. Integrative analysis of complex cancer genomics and clinical profiles using the cBioPortal. Sci Signal. 2013;6:pl1. doi: 10.1126/scisignal.2004088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cerami E, Gao J, Dogrusoz U, Gross BE, Sumer SO, Aksoy BA, Jacobsen A, Byrne CJ, Heuer ML, Larsson E, Antipin Y, Reva B, Goldberg AP, Sander C, Schultz N. The cBio cancer genomics portal: an open platform for exploring multidimensional cancer genomics data. Cancer Discov. 2012;2:401–404. doi: 10.1158/2159-8290.CD-12-0095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang L, Ma L, Wang X, Li B, Guo S, Qiao Q. Association of serum EPCA-2 level with prostate cancer in Chinese Han population. Int J Clin Exp Pathol. 2015;8:9397–9403. [PMC free article] [PubMed] [Google Scholar]
- 13.Sakamoto S, McCann RO, Dhir R, Kyprianou N. Talin1 promotes tumor invasion and metastasis via focal adhesion signaling and anoikis resistance. Cancer Res. 2010;70:1885–1895. doi: 10.1158/0008-5472.CAN-09-2833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Huang WC, Wu D, Xie Z, Zhau HE, Nomura T, Zayzafoon M, Pohl J, Hsieh CL, Weitzmann MN, Farach-Carson MC, Chung LW. beta2-microglobulin is a signaling and growth-promoting factor for human prostate cancer bone metastasis. Cancer Res. 2006;66:9108–9116. doi: 10.1158/0008-5472.CAN-06-1996. [DOI] [PubMed] [Google Scholar]
- 15.Chaplet M, Waltregny D, Detry C, Fisher LW, Castronovo V, Bellahcene A. Expression of dentin sialophosphoprotein in human prostate cancer and its correlation with tumor aggressiveness. Int J Cancer. 2006;118:850–856. doi: 10.1002/ijc.21442. [DOI] [PubMed] [Google Scholar]
- 16.Jung K, Lein M, Stephan C, Von Hosslin K, Semjonow A, Sinha P, Loening SA, Schnorr D. Comparison of 10 serum bone turnover markers in prostate carcinoma patients with bone metastatic spread: diagnostic and prognostic implications. Int J Cancer. 2004;111:783–791. doi: 10.1002/ijc.20314. [DOI] [PubMed] [Google Scholar]
- 17.Gordon JA, Sodek J, Hunter GK, Goldberg HA. Bone sialoprotein stimulates focal adhesion-related signaling pathways: role in migration and survival of breast and prostate cancer cells. J Cell Biochem. 2009;107:1118–1128. doi: 10.1002/jcb.22211. [DOI] [PubMed] [Google Scholar]
- 18.Munkley J, Livermore KE, McClurg UL, Kalna G, Knight B, McCullagh P, McGrath J, Crundwell M, Leung HY, Robson CN, Harries LW, Rajan P, Elliott DJ. The PI3K regulatory subunit gene PIK3R1 is under direct control of androgens and repressed in prostate cancer cells. Oncoscience. 2015;2:755–764. doi: 10.18632/oncoscience.243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Altieri DC. Survivin, versatile modulation of cell division and apoptosis in cancer. Oncogene. 2003;22:8581–8589. doi: 10.1038/sj.onc.1207113. [DOI] [PubMed] [Google Scholar]
- 20.Chen WY, Liu SY, Chang YS, Yin JJ, Yeh HL, Mouhieddine TH, Hadadeh O, Abou-Kheir W, Liu YN. MicroRNA-34a regulates WNT/TCF7 signaling and inhibits bone metastasis in Ras-activated prostate cancer. Oncotarget. 2015;6:441–457. doi: 10.18632/oncotarget.2690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Danilewicz M, Stasikowska-Kanicka O, Wagrowska-Danilewicz M. Augmented immunoexpression of survivin correlates with parameters of aggressiveness in prostate cancer. Pol J Pathol. 2015;66:44–48. doi: 10.5114/pjp.2015.51152. [DOI] [PubMed] [Google Scholar]
- 22.de Resende MF, Vieira S, Chinen LT, Chiappelli F, da Fonseca FP, Guimaraes GC, Soares FA, Neves I, Pagotty S, Pellionisz PA, Barkhordarian A, Brant X, Rocha RM. Prognostication of prostate cancer based on TOP2A protein and gene assessment: TOP2A in prostate cancer. J Transl Med. 2013;11:36. doi: 10.1186/1479-5876-11-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mu XC, Tran TA, Ross JS, Carlson JA. Topoisomerase II-alpha expression in melanocytic nevi and malignant melanoma. J Cutan Pathol. 2000;27:242–248. doi: 10.1034/j.1600-0560.2000.027005242.x. [DOI] [PubMed] [Google Scholar]
- 24.Holden JA, Townsend JJ. DNA topoisomerase II-alpha as a proliferation marker in astrocytic neoplasms of the central nervous system: correlation with MIB1 expression and patient survival. Mod Pathol. 1999;12:1094–1100. [PubMed] [Google Scholar]
- 25.Costa MJ, Hansen CL, Holden JA, Guinee D Jr. Topoisomerase II alpha: prognostic predictor and cell cycle marker in surface epithelial neoplasms of the ovary and peritoneum. Int J Gynecol Pathol. 2000;19:248–257. doi: 10.1097/00004347-200007000-00009. [DOI] [PubMed] [Google Scholar]
- 26.Dingemans AM, Witlox MA, Stallaert RA, van der Valk P, Postmus PE, Giaccone G. Expression of DNA topoisomerase IIalpha and topoisomerase IIbeta genes predicts survival and response to chemotherapy in patients with small cell lung cancer. Clin Cancer Res. 1999;5:2048–2058. [PubMed] [Google Scholar]
- 27.Depowski PL, Rosenthal SI, Brien TP, Stylos S, Johnson RL, Ross JS. Topoisomerase IIalpha expression in breast cancer: correlation with outcome variables. Mod Pathol. 2000;13:542–547. doi: 10.1038/modpathol.3880094. [DOI] [PubMed] [Google Scholar]
- 28.Sullivan GF, Amenta PS, Villanueva JD, Alvarez CJ, Yang JM, Hait WN. The expression of drug resistance gene products during the progression of human prostate cancer. Clin Cancer Res. 1998;4:1393–1403. [PubMed] [Google Scholar]
- 29.Bizzarro V, Belvedere R, Migliaro V, Romano E, Parente L, Petrella A. Hypoxia regulates ANXA1 expression to support prostate cancer cell invasion and aggressiveness. Cell Adh Migr. 2017;11:247–260. doi: 10.1080/19336918.2016.1259056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.D’Acunto CW, Fontanella B, Rodriquez M, Taddei M, Parente L, Petrella A. Histone deacetylase inhibitor FR235222 sensitizes human prostate adenocarcinoma cells to apoptosis through up-regulation of Annexin A1. Cancer Lett. 2010;295:85–91. doi: 10.1016/j.canlet.2010.02.016. [DOI] [PubMed] [Google Scholar]
- 31.Long Q, Johnson BA, Osunkoya AO, Lai YH, Zhou W, Abramovitz M, Xia M, Bouzyk MB, Nam RK, Sugar L, Stanimirovic A, Williams DJ, Leyland-Jones BR, Seth AK, Petros JA, Moreno CS. Protein-coding and microRNA biomarkers of recurrence of prostate cancer following radical prostatectomy. Am J Pathol. 2011;179:46–54. doi: 10.1016/j.ajpath.2011.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Klomsiri C, Rogers LC, Soito L, McCauley AK, King SB, Nelson KJ, Poole LB, Daniel LW. Endosomal H2O2 production leads to localized cysteine sulfenic acid formation on proteins during lysophosphatidic acid-mediated cell signaling. Free Radic Biol Med. 2014;71:49–60. doi: 10.1016/j.freeradbiomed.2014.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Anliker B, Chun J. Cell surface receptors in lysophospholipid signaling. Semin Cell Dev Biol. 2004;15:457–465. doi: 10.1016/j.semcdb.2004.05.005. [DOI] [PubMed] [Google Scholar]
- 34.Anliker B, Chun J. Lysophospholipid G protein-coupled receptors. J Biol Chem. 2004;279:20555–20558. doi: 10.1074/jbc.R400013200. [DOI] [PubMed] [Google Scholar]
- 35.Gardell SE, Dubin AE, Chun J. Emerging medicinal roles for lysophospholipid signaling. Trends Mol Med. 2006;12:65–75. doi: 10.1016/j.molmed.2005.12.001. [DOI] [PubMed] [Google Scholar]
- 36.Guo R, Kasbohm EA, Arora P, Sample CJ, Baban B, Sud N, Sivashanmugam P, Moniri NH, Daaka Y. Expression and function of lysophosphatidic acid LPA1 receptor in prostate cancer cells. Endocrinology. 2006;147:4883–4892. doi: 10.1210/en.2005-1635. [DOI] [PubMed] [Google Scholar]
- 37.Raj GV, Sekula JA, Guo R, Madden JF, Daaka Y. Lysophosphatidic acid promotes survival of androgen-insensitive prostate cancer PC3 cells via activation of NF-kappaB. Prostate. 2004;61:105–113. doi: 10.1002/pros.20083. [DOI] [PubMed] [Google Scholar]
- 38.Harma V, Knuuttila M, Virtanen J, Mirtti T, Kohonen P, Kovanen P, Happonen A, Kaewphan S, Ahonen I, Kallioniemi O, Grafstrom R, Lotjonen J, Nees M. Lysophosphatidic acid and sphingosine-1-phosphate promote morphogenesis and block invasion of prostate cancer cells in three-dimensional organotypic models. Oncogene. 2012;31:2075–2089. doi: 10.1038/onc.2011.396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Liu LX, Jiang HC, Liu ZH, Zhou J, Zhang WH, Zhu AL, Wang XQ, Wu M. Integrin gene expression profiles of human hepatocellular carcinoma. World J Gastroenterol. 2002;8:631–637. doi: 10.3748/wjg.v8.i4.631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Goodman SL, Grote HJ, Wilm C. Matched rabbit monoclonal antibodies against alphavseries integrins reveal a novel alphavbeta3-LIBS epitope, and permit routine staining of archival paraffin samples of human tumors. Biol Open. 2012;1:329–340. doi: 10.1242/bio.2012364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Vogetseder A, Thies S, Ingold B, Roth P, Weller M, Schraml P, Goodman SL, Moch H. alphav-Integrin isoform expression in primary human tumors and brain metastases. Int J Cancer. 2013;133:2362–2371. doi: 10.1002/ijc.28267. [DOI] [PubMed] [Google Scholar]
- 42.Landemaine T, Jackson A, Bellahcene A, Rucci N, Sin S, Abad BM, Sierra A, Boudinet A, Guinebretiere JM, Ricevuto E, Nogues C, Briffod M, Bieche I, Cherel P, Garcia T, Castronovo V, Teti A, Lidereau R, Driouch K. A six-gene signature predicting breast cancer lung metastasis. Cancer Res. 2008;68:6092–6099. doi: 10.1158/0008-5472.CAN-08-0436. [DOI] [PubMed] [Google Scholar]
- 43.Rutkowski R, Mertens-Walker I, Lisle JE, Herington AC, Stephenson SA. Evidence for a dual function of EphB4 as tumor promoter and suppressor regulated by the absence or presence of the ephrin-B2 ligand. Int J Cancer. 2012;131:E614–624. doi: 10.1002/ijc.27392. [DOI] [PubMed] [Google Scholar]
- 44.Desgrosellier JS, Cheresh DA. Integrins in cancer: biological implications and therapeutic opportunities. Nat Rev Cancer. 2010;10:9–22. doi: 10.1038/nrc2748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Brendle A, Lei H, Brandt A, Johansson R, Enquist K, Henriksson R, Hemminki K, Lenner P, Forsti A. Polymorphisms in predicted microRNA-binding sites in integrin genes and breast cancer: ITGB4 as prognostic marker. Carcinogenesis. 2008;29:1394–1399. doi: 10.1093/carcin/bgn126. [DOI] [PubMed] [Google Scholar]
- 46.Masugi Y, Yamazaki K, Emoto K, Effendi K, Tsujikawa H, Kitago M, Itano O, Kitagawa Y, Sakamoto M. Upregulation of integrin beta4 promotes epithelial-mesenchymal transition and is a novel prognostic marker in pancreatic ductal adenocarcinoma. Lab Invest. 2015;95:308–319. doi: 10.1038/labinvest.2014.166. [DOI] [PubMed] [Google Scholar]
- 47.Kettunen E, Nicholson AG, Nagy B, Wikman H, Seppanen JK, Stjernvall T, Ollikainen T, Kinnula V, Nordling S, Hollmen J, Anttila S, Knuutila S. L1CAM, INP10, P-cadherin, tPA and ITGB4 over-expression in malignant pleural mesotheliomas revealed by combined use of cDNA and tissue microarray. Carcinogenesis. 2005;26:17–25. doi: 10.1093/carcin/bgh276. [DOI] [PubMed] [Google Scholar]
- 48.Drake JM, Barnes JM, Madsen JM, Domann FE, Stipp CS, Henry MD. ZEB1 coordinately regulates laminin-332 and {beta}4 integrin expression altering the invasive phenotype of prostate cancer cells. J Biol Chem. 2010;285:33940–33948. doi: 10.1074/jbc.M110.136044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kawakami K, Fujita Y, Kato T, Mizutani K, Kameyama K, Tsumoto H, Miura Y, Deguchi T, Ito M. Integrin beta4 and vinculin contained in exosomes are potential markers for progression of prostate cancer associated with taxane-resistance. Int J Oncol. 2015;47:384–390. doi: 10.3892/ijo.2015.3011. [DOI] [PubMed] [Google Scholar]
- 50.Lee B, Ha SY, Song DH, Lee HW, Cho SY, Park CK. High expression of ribonucleotide reductase subunit M2 correlates with poor prognosis of hepatocellular carcinoma. Gut Liver. 2014;8:662–668. doi: 10.5009/gnl13392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Duxbury MS, Whang EE. RRM2 induces NF-kappaB-dependent MMP-9 activation and enhances cellular invasiveness. Biochem Biophys Res Commun. 2007;354:190–196. doi: 10.1016/j.bbrc.2006.12.177. [DOI] [PubMed] [Google Scholar]
- 52.Huang Y, Liu X, Wang YH, Yeh SD, Chen CL, Nelson RA, Chu P, Wilson T, Yen Y. The prognostic value of ribonucleotide reductase small subunit M2 in predicting recurrence for prostate cancers. Urol Oncol. 2014;32:51, e59–19. doi: 10.1016/j.urolonc.2013.08.002. [DOI] [PubMed] [Google Scholar]
- 53.Lauss M, Kriegner A, Vierlinger K, Noehammer C. Characterization of the drugged human genome. Pharmacogenomics. 2007;8:1063–1073. doi: 10.2217/14622416.8.8.1063. [DOI] [PubMed] [Google Scholar]
- 54.Fang ZQ, Zang WD, Chen R, Ye BW, Wang XW, Yi SH, Chen W, He F, Ye G. Gene expression profile and enrichment pathways in different stages of bladder cancer. Genet Mol Res. 2013;12:1479–1489. doi: 10.4238/2013.May.6.1. [DOI] [PubMed] [Google Scholar]
- 55.Liu X, Wang J, Sun G. Identification of key genes and pathways in renal cell carcinoma through expression profiling data. Kidney Blood Press Res. 2015;40:288–297. doi: 10.1159/000368504. [DOI] [PubMed] [Google Scholar]
- 56.Myers JS, von Lersner AK, Sang QX. Proteomic upregulation of fatty acid synthase and fatty acid binding protein 5 and identification of cancer- and race-specific pathway associations in human prostate cancer tissues. J Cancer. 2016;7:1452–1464. doi: 10.7150/jca.15860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Arsova-Sarafinovska Z, Matevska N, Eken A, Petrovski D, Banev S, Dzikova S, Georgiev V, Sikole A, Erdem O, Sayal A, Aydin A, Dimovski AJ. Glutathione peroxidase 1 (GPX1) genetic polymorphism, erythrocyte GPX activity, and prostate cancer risk. Int Urol Nephrol. 2009;41:63–70. doi: 10.1007/s11255-008-9407-y. [DOI] [PubMed] [Google Scholar]
- 58.Sainz RM, Reiter RJ, Tan DX, Roldan F, Natarajan M, Quiros I, Hevia D, Rodriguez C, Mayo JC. Critical role of glutathione in melatonin enhancement of tumor necrosis factor and ionizing radiation-induced apoptosis in prostate cancer cells in vitro. J Pineal Res. 2008;45:258–270. doi: 10.1111/j.1600-079X.2008.00585.x. [DOI] [PubMed] [Google Scholar]
- 59.Maldonado L, Brait M, Loyo M, Sullenberger L, Wang K, Peskoe SB, Rosenbaum E, Howard R, Toubaji A, Albadine R, Netto GJ, Hoque MO, Platz EA, Sidransky D. GSTP1 promoter methylation is associated with recurrence in early stage prostate cancer. J Urol. 2014;192:1542–1548. doi: 10.1016/j.juro.2014.04.082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Cao DL, Ye DW, Dai B, Zhang HL, Shen YJ, Zhu Y, Zhu YP, Shi GH, Ma CG, Xiao WJ, Qin XJ, Lin GW. Association of glutathione S-transferase T1 and M1 polymorphisms with prostate cancer susceptibility in populations of Asian descent: a meta-analysis. Oncotarget. 2015;6:35843–35850. doi: 10.18632/oncotarget.5346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Karan D, Lin MF, Johansson SL, Batra SK. Current status of the molecular genetics of human prostatic adenocarcinomas. Int J Cancer. 2003;103:285–293. doi: 10.1002/ijc.10813. [DOI] [PubMed] [Google Scholar]
- 62.Hughes C, Murphy A, Martin C, Sheils O, O’Leary J. Molecular pathology of prostate cancer. J Clin Pathol. 2005;58:673–684. doi: 10.1136/jcp.2002.003954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Bookstein R, MacGrogan D, Hilsenbeck SG, Sharkey F, Allred DC. p53 is mutated in a subset of advanced-stage prostate cancers. Cancer Res. 1993;53:3369–3373. [PubMed] [Google Scholar]
- 64.Navone NM, Labate ME, Troncoso P, Pisters LL, Conti CJ, von Eschenbach AC, Logothetis CJ. p53 mutations in prostate cancer bone metastases suggest that selected p53 mutants in the primary site define foci with metastatic potential. J Urol. 1999;161:304–308. [PubMed] [Google Scholar]
- 65.Kluth M, Harasimowicz S, Burkhardt L, Grupp K, Krohn A, Prien K, Gjoni J, Hass T, Galal R, Graefen M, Haese A, Simon R, Huhne-Simon J, Koop C, Korbel J, Weischenfeld J, Huland H, Sauter G, Quaas A, Wilczak W, Tsourlakis MC, Minner S, Schlomm T. Clinical significance of different types of p53 gene alteration in surgically treated prostate cancer. Int J Cancer. 2014;135:1369–1380. doi: 10.1002/ijc.28784. [DOI] [PubMed] [Google Scholar]


