Abstract
Little is known about the role of lincRNA-p21 in the development of diabetic nephropathy. The aim of the present study was to investigate the level of lincRNA-p21 in diabetic nephropathy, and explore its underlying mechanism. The current study revealed that down-regulation of lincRNA-p21 could alleviate pathological changes of diabetic nephropathy in mice. LincRNA-p21 expression was significantly up-regulated in MMCs under high glucose condition in vitro. Besides, lincRNA-p21 promoted the proliferation of MMCs, which was reversed by miR-18b targeted with 3’-UTR. Moreover, miR-18b suppressed the expression of connective tissue growth factor (CTGF) by binding with the 3’-UTR. Furthermore, down-regulation of lincRNA-p21 expression alleviated extracellular matrix under high glucose, which could be reversed by miR-18b inhibitor significantly. In short, our study suggests lincRNA-p21 plays as an important role in progression of diabetic nephropathy in an animal model through interaction with miR-18b, providing a novel insight for the pathogenesis and an underlying therapeutic target for diabetic nephropathy.
Keywords: Diabetic nephropathy, mouse mesangial cells, lincRNA-p21, miR-18b
Introduction
Diabetes mellitus, commonly referred to as diabetes, is a group of metabolic disorders characterized by hyperglycemia due to insulin-secreting or insulin-action disorders [1]. If left untreated, diabetes can increase the risk of long-term complications [2]. The primary complications of diabetes due to damage in small blood vessels can lead to systemic organs, especially in the eyes [3], kidneys [4], and nervous [5] system, and dysfunction and failure.
Diabetic nephropathy (DN) is one of the severe complications of diabetes and always leads to end-stage kidney diseases. The key pathologic features of DN include a thickening of the basement membrane, a widening of the slit membranes of the podocytes, an increase in the number of mesangial cells, and an accumulation in extracellular matrix [6]. Evidences suggest that proliferation of mesangial cells is critical in the initiation and progression of DN [7]. However, the molecular mechanisms underlying the development of DN have not been fully understood.
As a member of non-coding RNAs, long non-coding RNA (lncRNA) is defined as greater than 200 nucleotides in length and plays an important role in diverse cellular processes, such as regulation of gene expression, posttranslational processing and tumorigenesis [8,9]. Long intergenic non-coding RNA p21 (lincRNA-p21) was first reported as a direct transcriptional target of p53, it mainly regulates the cell cycle and apoptosis [10]. LincRNA-p21 has been previously found to play an important role in liver fibrosis and pulmonary fibrosis [11,12]. Nevertheless, the role of lincRNA-p21 in DN remains elusive.
MicroRNAs are a class of highly conservative non-coding small RNAs (about 19-23 nucleotides) that exhibit its biological functions by binding to the 3’ untranslated region (3’-UTR) of target gene [13]. Accumulating evidences suggest miRNAs are important regulators in pathology of diabetes and its relevant renal injure. For example, miR-192 could lead to renal fibrosis proteinuria by activating TGF-β signaling pathway and up-regulation of miR-21 induces the overactivation of Akt signaling pathway followed by renal fibrosis and hypertrophy [14,15]. Whether miR-18b acts on the development of DN is still not very clear.
Thus, the aim of the present study was to investigate the levels of lincRNA-p21 in the progression of DN in vitro and in a db/db mouse model of diabetes, and explore its underlying mechanism.
Materials and methods
Animals
The C57BL/6 db/db and control db/m mice at the age of 8 weeks old weighing 20-22 g were purchased from the laboratory animal center of Xinjiang Medical University. All mice were maintained in the same room under conventional conditions with a regular 12 h light/dark cycle with the temperature controlled at 20-22°C. Mice were given standard chow and autoclaved water ad libitum. Mouse models of diabetes were constructed by intraperitoneal injections of streptozotocin (STZ, 100 mg/kg) for three consecutive days [16]. Mice were considered DN when tail vein blood glucose levels ≥300 mg/dl and urine albumin creatine ratio (ACR) ≥300 µg/mg. Db/db and db/m mice were randomized into groups with 10 mice in each: (1) db/m group, (2) db/db group, (3) si-control-treated db/db group, (4) si-lincRNA-p21-treated db/db group. Renal cortical tissues were dissected from kidney as previously described [17]. The fresh kidney tissue samples were fixed by neutral formalin.
Biochemical determination
Tail vein blood glucose levels were measured by Glucose LiquiColor Test (Stanbio Laboratory, Boerne, TX, USA) every 4 weeks. Urinary samples (24 hours) were collected by metabolic cage every 4 weeks. BUN, creatinine and urine albumin were determined by competitive ELISA according to the manufacturer’s instruction.
Histological examination
Renal cortical tissues were fixed in 4% paraformaldehyde for 48 h, dehydrated through a graded series of ethanol, embedded in paraffin wax, and cut into 4 μm sections. The tissues were then stained with Hematoxylin-eosin (H&E) and Periodic acide Schiff (PAS) to observe glomerular morphological changes. Four random fields were chosen by light microscope.
Cell culture and transfection
293T cells were cultured in DMEM with 5 mmol/l glucose and 10% fetal bovine serum at 37°C in a humidified, 5% CO2 atmosphere. Mouse mesangial cells (MMCs) cultured in DMEM were treated with 25 and 5 mmol/l glucose respectively to mimic diabetic pathological and normal physiological environments as previously described [18]. For transfection, cells were seeded into plates and transfected with lincRNA-p21 mimic, lincRNA-p21 inhibitor, miR-18b mimic or miR-18b inhibitor (Gene Pharm, Shanghai, China) mixed with Lipofectamine 2000 reagent (Invitrogen, Carlsbad, MA, USA) according to the manufacturer’s protocols.
Real time quantitative PCR analysis
The total RNA was extracted from cells using Trizol reagent (Invitrogen, Shanghai, China) according to the manufacturer’s instructions. Complementary DNA (cDNA) was synthesized using a reverse transcription kit (Takara Biotechnology, Dalian, China). Relative levels of gene expression was expressed relative to GAPDH and calculated using the 2-ΔΔCt method.
Cell proliferation assay
Cells (3×103) were cultured in 96-well plates and incubated for 24 h and stained with 0.5 mg/ml MTT for 4 h. Supernatant was discarded and 200 μl of dimethylsulfoxide (DMSO) was added to dissolve precipitates. Samples were measured at 490 nm using an ELISA reader.
Luciferase activity assay
The 3’-UTR of lincRNA-p21 and CTGF containing miR-18b binding sites was amplified and cloned into the pGL3-basic luciferase vector (Promega, USA), respectively. Similarly, the mutant 3’-UTR of lincRNA-p21 and CTGF was cloned into the same vector. Transfected cells were detected using the Dual-Luciferase Reporter Assay System (Promega) 48 h later.
Western blotting analysis
The cultured cells were washed with ice-cold PBS and lysed in RIPA buffer supplemented with protease inhibitor mixture. After electrophoresis, the protein samples were incubated with the primary antibody (anti-CTGF, anti-Collagen I, anti-Collagen IV and anti-fibronectin, 1:1000 dilution). Samples were incubated with secondary antibodies conjugated by HRP. Bands were quantified using ImageJ software.
Statistical analysis
Results are expressed as the mean ± standard error. The Student’s t-test and ANOVA were performed among different groups. All calculations were performed using SPSS 17.0 software (IBM Software, Chicago, IL, USA) and GraphPad (vision 6.0, USA). A value of P<0.05 indicated a statistically significant difference.
Results
The role of lincRNA-p21 in renal function and pathological changes of DN in db/db mice
We first detected the change of blood glucose. The data of the present study demonstrated the level of blood glucose was up-regulated remarkably in db/db mice, which was significantly improved by si-lincRNA-p21 (Figure 1A, #P<0.05, *P<0.05). BUN, creatinine and urine albumin were considered as clinical indexes of renal function in DN [19,20]. Results showed that these indexes increased significantly with the progression of DN (Figure 1B-D; #P<0.05). When db/db mice were treated with si-lincRNA-p21, there was a significant decrease of the clinical indexes (Figure 1B-D; *P<0.05). To characterize the role of lincRNA-p21 in DN histologically, specimens obtained from renal cortex at 20 weeks showed increased mesangial matrix in db/db mice. HE and PAS staining also showed the degree of pathological changes was lightened when db/db mice received si-lincRNA-p21 treatment (Figure 1E).
Figure 1.
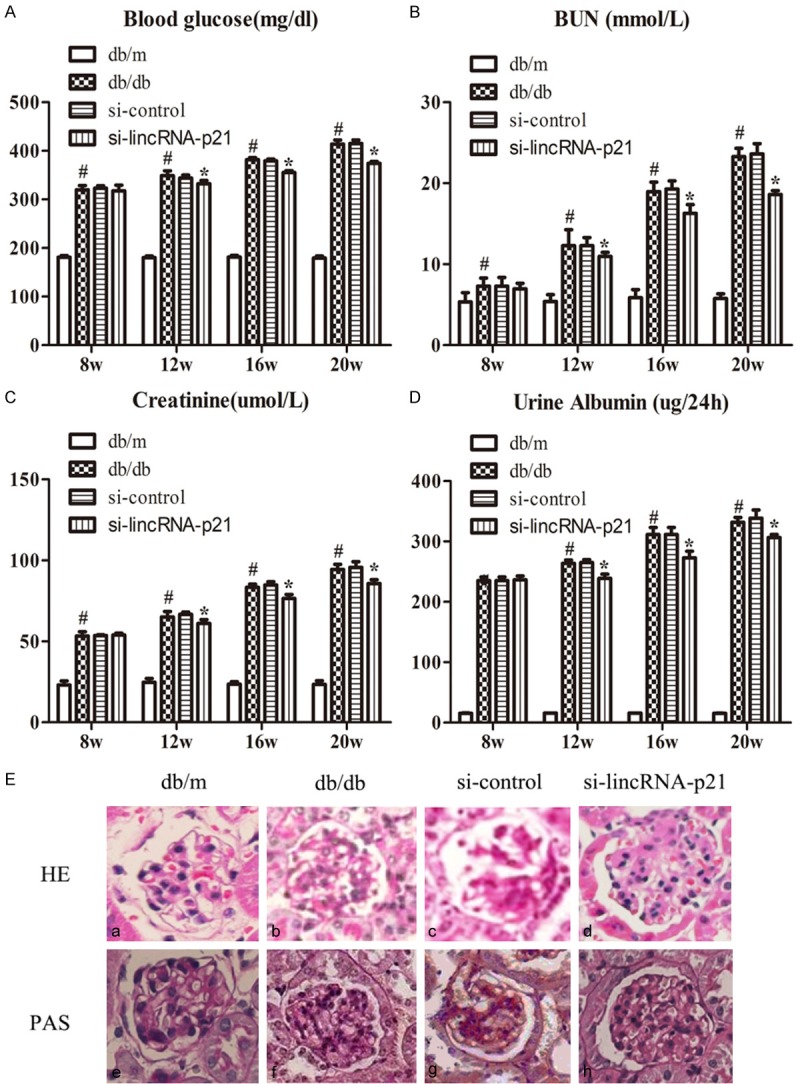
The role of lincRNA-p21 in renal function and pathological changes of DN in db/db mice. The level of clinical biochemical indexes were up-regulated remarkably in db/db mice, which was significantly improved by si-lincRNA-p21, including blood glucose, BUN, creatinine and urine albumin (A-D; #P<0.05, *P<0.05). HE and PAS staining showed the degree of pathological changes was lightened when db/db mice received si-lincRNA-p21 treatment (E).
LincRNA-p21 promoted the proliferation of MMCs in vitro, which was reversed by LincRNA-p21 silencing
To investigate the effect of lincRNA-p21 on the development of DN, the expression of lincRNA-p21 in MMCs was analyzed by RT-qPCR. The MMCs were induced by high glucose (25 mM) for 12, 24, 48, and 72 hours. Figure 2A showed that lincRNA-p21 mRNA expression was significantly up-regulated in MMCs under high glucose condition implying the elevated level of lincRNA-p21 mRNA induced by high glucose was time-dependent. Moreover, the lincRNA-p21-overexpression in MMCs was firstly established by retrovirus infection. Results of qRT-PCR manifested that lincRNA-p21 level was significantly elevated in MMCs transduced with lincRNA-p21 mimic compared with lincRNA-NC (Figure 2B, *P<0.05). To explore the role of lincRNA-p21 in cell proliferation, a MMT assay was performed to assess the viability of MMCs. Results revealed that the growth rate of MMCs in high glucose conditions was increased, which could be further elevated by lincRNA-p21 over-expression (Figure 2D, *P<0.05). To further verify the role of lincRNA-p21 in the development of DN, we stably established lincRNA-p21 silencing via siRNA transfection in cells in vitro (Figure 2C). MTT assay showed that lincRNA-p21 silencing inhibited the proliferation of MMCs in high glucose conditions significantly (Figure 2E).
Figure 2.
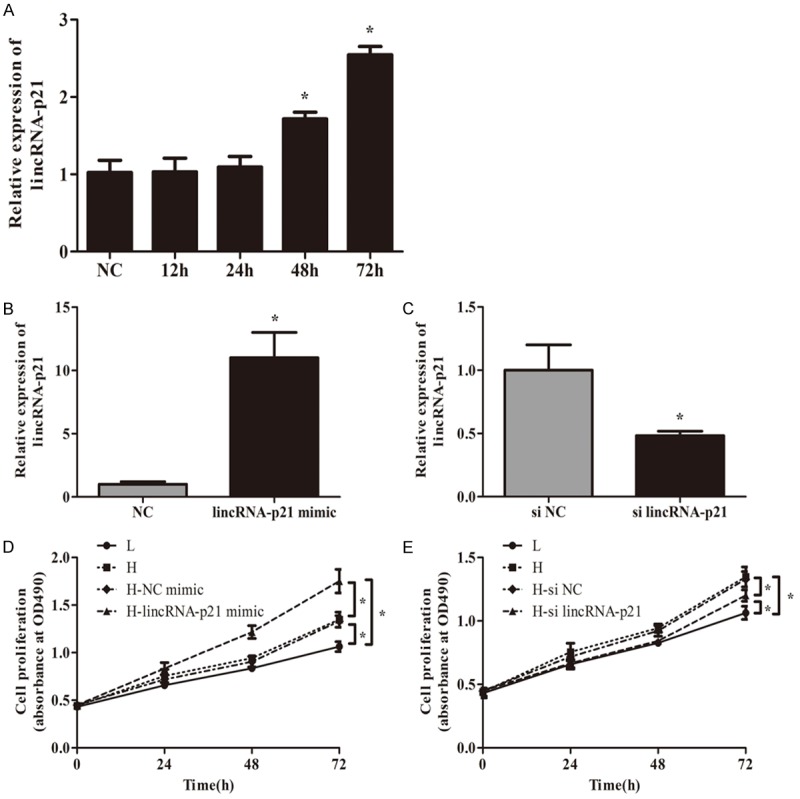
LincRNA-p21 promoted the proliferation of MMCs in vitro, which was reversed by LincRNA-p21 silencing. A showed that lincRNA-p21 mRNA expression was significantly up-regulated in MMCs under high glucose condition over time (A, *P<0.05). Results of qRT-PCR manifested that lincRNA-p21 level was significantly elevated in MMCs transduced with lincRNA-p21 mimic compared with lincRNA-NC (B, *P<0.05). A MMT assay revealed that the growth rate of MMCs in high glucose conditions was increased, which could be further elevated by lincRNA-p21 over-expression (D, *P<0.05). LincRNA-p21 silencing was established via siRNA transfection in cells in vitro (C, *P<0.05). MTT assay showed that lincRNA-p21 silencing inhibited the proliferation of MMCs in high glucose conditions significantly (E, *P<0.05).
LincRNA-p21 acts as a molecular sponge for miR-18b
Increasing evidence had illustrated that lincRNAs function as sponges to bind specific miRNAs. Bioinformatics analysis was used to predict the candidate targets of miRNAs binding with lincRNA-p21. Results found 3’UTR of LincRNA-p21 was highly conserved to bind with miR-18b. The 3’-UTR binding sites can be seen in Figure 3A. Luciferase reporter assay confirmed the binding with the decreasing fluorescence within miR-18b mimic and lincRNA-p21 wild type (Figure 3B). Moreover, after cells were transfected with si-lincRNA-p21, miR-18b expression levels were significantly up-regulated (Figure 3C). Figure 3D showed that miR-18b mRNA expression was significantly down-regulated in MMCs under high glucose condition over time. Furthermore, MTT assay revealed that miR-18b inhibitor could rescue the suppression by si-lincRNA-p21 in the proliferation of MMCs under high glucose for 72 h (Figure 3E).
Figure 3.
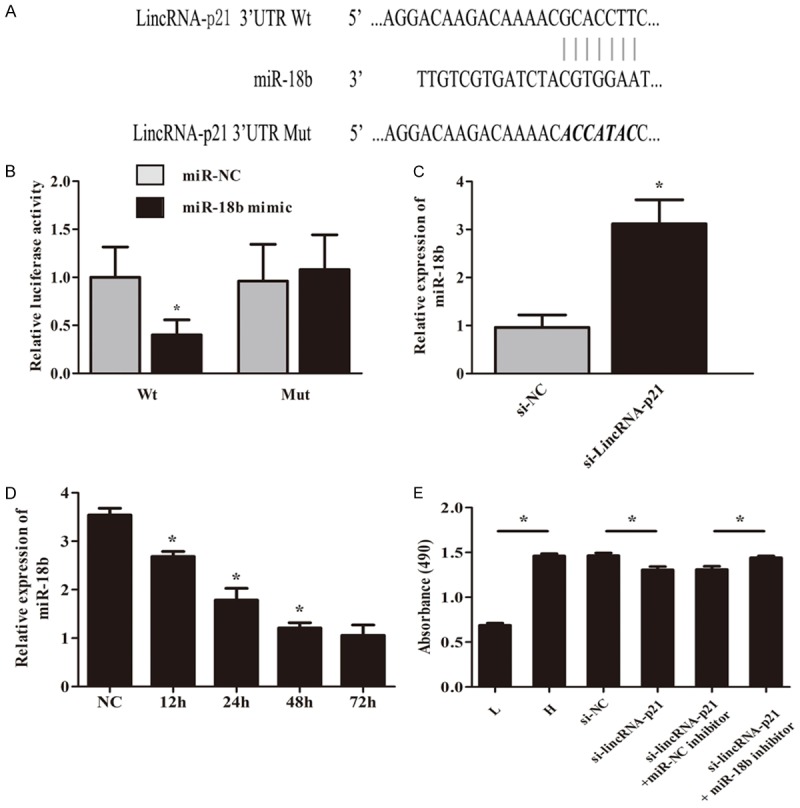
LincRNA-p21 acts as a molecular sponge for miR-18b. The 3’-UTR binding sites can be seen in (A). Luciferase reporter assay confirmed the binding with the decreasing fluorescence within miR-18b mimic and lincRNA-p21 wild type (B). MiR-18b expression levels were significantly up-regulated after cells were transfected with si-lincRNA-p21 (C, *P<0.05). (D) showed that miR-18b mRNA expression was significantly down-regulated in MMCs under high glucose condition over time (*P<0.05). MTT assay revealed that miR-18b inhibitor could rescue the suppression by si-lincRNA-p21 in the proliferation of MMCs under high glucose for 72 h (E, *P<0.05).
MiR-18b directly targets with 3’-UTR of CTGF
We examined computationally predicted targets of miR-18b using bioinformatics analysis. Results revealed 3’UTR of CTGF was highly conserved to bind with miR-18b. The 3’-UTR binding sites can be seen in Figure 4A. Luciferase reporter assay showed transfection of miR-18b could significantly restrict the relative luciferase activity in cells, suggesting miR-18b has inhibitory effects on CTGF expression via interaction with the 3’-UTR of CTGF (Figure 4B). Moreover, as shown in Figure 4C and 4D, overexpression of miR-18b obviously downregulated CTGF expression in cells at the mRNA and protein levels. Overall, our study discovered that miR-18b suppressed the expression of CTGF by binding with the 3’-UTR.
Figure 4.
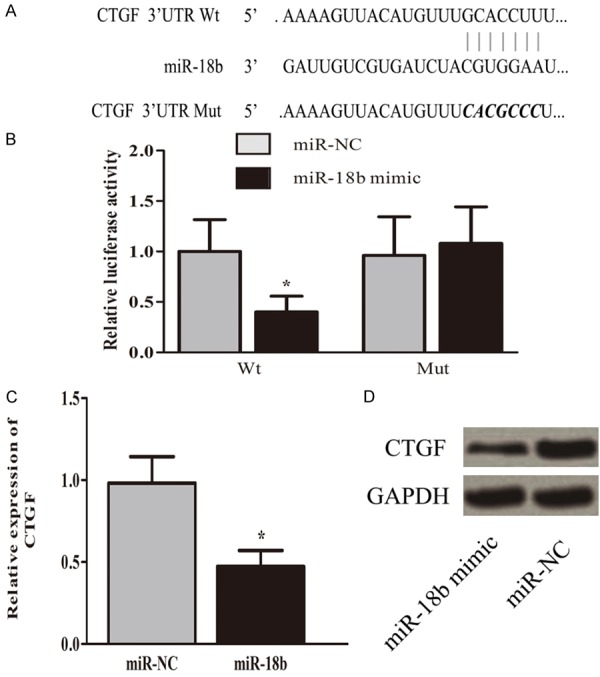
MiR-18b directly targets with 3’-UTR of CTGF. The 3’-UTR binding sites can be seen in (A). Luciferase reporter assay showed transfection of miR-18b could significantly restrict the relative luciferase activity in cells (B, *P<0.05). Overexpression of miR-18b obviously inhibited CTGF expression in cells at the mRNA and protein levels. (C, D; *P<0.05).
LincRNA-p21 silencing suppresses extracellular matrix accumulation in vitro
Excessive accumulation of extracellular matrix (ECM) leads to glomerulosclerosis and renal fibrosis [21]. As the major components of ECM in MMCs, the expression of Collagen I, Collagen IV and fibronectin were detected to predict the accumulation of ECM under high glucose condition by RT-PCR and western blot. When cells were transfected with si-lincRNA-p21, the expression of these indexes was down-regulated remarkably, which was reversed by miR-18b inhibitor significantly at mRNA and protein levels. Taken together, this suggests that lincRNA-p21 could promote extracellular matrix accumulation of MMCs in vitro. (Figure 5, *P<0.05).
Figure 5.
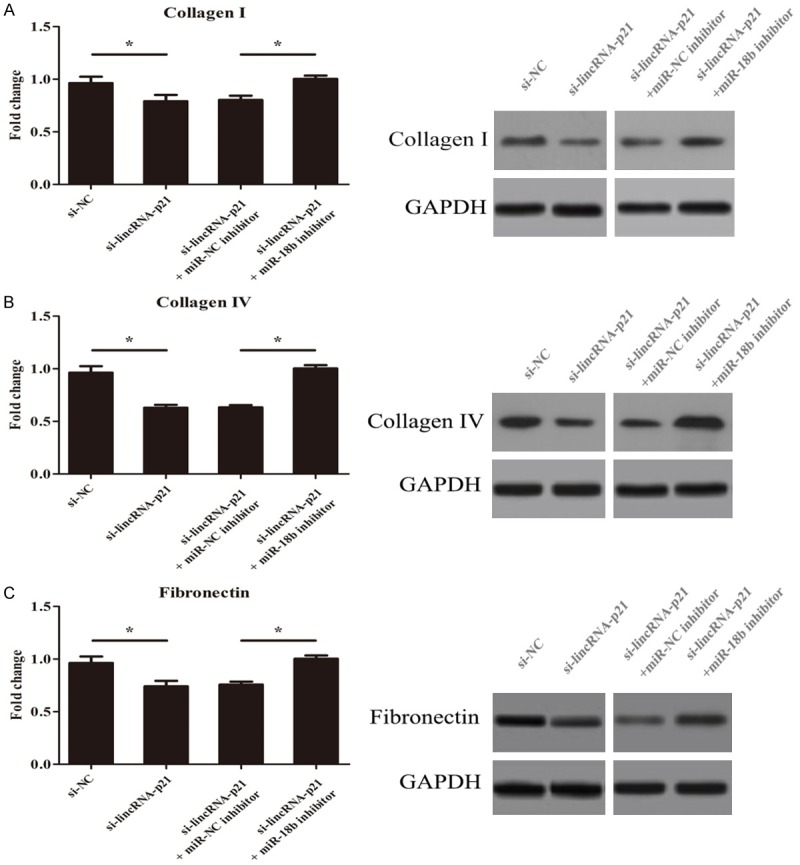
LincRNA-p21 silencing suppresses extracellular matrix accumulation in vitro. Cells were transfected with si-lincRNA-p21, the expression of Collagen I, Collagen IV and fibronectin was down-regulated remarkably, which was reversed by miR-18b inhibitor significantly. (*P<0.05).
Discussion
As one of the most important long-term complications of diabetes, DN is the major cause of end-stage renal disease [22]. Progression of diabetic nephropathy (DN) is characterized by the gradual scarring of the renal glomerulus and fibrosis of the tubulointerstitial region [23]. The function of lincRNA-p21 has been widely investigated in various diseases. Yu, F. et al. found lincRNA-p21 inhibits the progression of liver fibrosis via PTEN [24]. Conversely, Zhou, W. Q. et al. reported lincRNA-p21 could lead to pulmonary fibrosis in ARDS by inhibition of the expression of Thy-1 [12]. These studies suggested lincRNA-p21 serves different roles in different diseases.
To our knowledge, this is the first study investigating the impact of lincRNA-p21 on the development of DN. The main clinical features of DN are persistent albuminuria and progressively declined glomerular filtration rate. The results demonstrated the clinical indexes reflecting renal function was up-regulated remarkably in db/db mice, which was significantly improved by si-lincRNA-p21. These data suggested lincRNA-p21 could promote the progression of DN.
Given that mesangial expansion is the primary driver of glomerulosclerosis [23], we next observed the role of lincRNA-p21 in MMCs under high glucose condition. The result illustrated lincRNA-p21 mRNA expression was significantly up-regulated in MMCs under high glucose condition. Besides, lincRNA-p21 could promote the proliferation of MMCs in vitro, which was reversed by lincRNA-p21 silencing significantly. The study implied lincRNA-p21 act as a positive regulatory role in the growth of MMCs in vitro.
LncRNAs function as sponges for common miRNAs and abolished the endogenous suppressive effect of these miRNAs on their targeted transcripts [25]. In this study, we found that miR-18b was reduced by lincRNA-p21. Luciferase activity assays revealed that lincRNA-p21 is a target of miR-18b. Further study revealed that miR-18b inhibitor could rescue the suppression by si-lincRNA-p21 in the proliferation of MMCs under high glucose. These data showed lincRNA-p21 exerted the pro-fibrogenic role by targeting miR-18b.
CTGF is a 349-amino acid cysteine-rich polypeptide belonging to the CCN family [26]. Under the diabetic milieu, CTGF expression is found to be markedly upregulated in mesangial cells [27]. Bioinformatics analysis revealed 3’-UTR of CTGF was highly conserved to bind with miR-18b. Over-expression of miR-18b obviously inhibited CTGF expression in cells at the mRNA and the protein levels, suggesting miR-18b suppresses the progression of DN by targeting CTGF gene at 3’-UTR. When cells were transfected with si-lincRNA-p21, the expression of Collagen I, Collagen IV and fibronectin was down-regulated remarkably, which was reversed by miR-18b inhibitor significantly. These data suggests that lincRNA-p21 could promote extracellular matrix accumulation of MMCs in vitro.
In short, the present study characterizes the role of lincRNA-p21 in the pathogenesis of DN through interaction with miR-18b, and provides a novel therapeutic target for the treatment of DN.
Acknowledgements
This work was supported by Xinjiang Autonomous Region Regional Fund (2011211A058), Xinjiang Autonomous Region Natural Science Fundation (2016D01C319), National Natural Science Foundation of China (81160095) and Doctoral Scientific Fund of the Higher Education Institutions (2013651711003).
Disclosure of conflict of interest
None.
References
- 1.Rother KI. Diabetes treatment--bridging the divide. N Engl J Med. 2007;356:1499–1501. doi: 10.1056/NEJMp078030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sosale B, Chandrashekara S, Aravind SR, Renuka P, Anupama KR. Influence of cytokine status on insulin resistance and circulating endothelial progenitor cells in type 2 diabetes mellitus. Cytokine. 2017;99:179–185. doi: 10.1016/j.cyto.2017.09.012. [DOI] [PubMed] [Google Scholar]
- 3.Zhang X, Zhao L, Deng S, Sun X, Wang N. Dry eye syndrome in patients with diabetes mellitus: prevalence, etiology, and clinical characteristics. J Ophthalmol. 2016;2016:8201053. doi: 10.1155/2016/8201053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Blantz RC, Singh P. Glomerular and tubular function in the diabetic kidney. Adv Chronic Kidney Dis. 2014;21:297–303. doi: 10.1053/j.ackd.2014.03.006. [DOI] [PubMed] [Google Scholar]
- 5.Russell JW, Zilliox LA. Diabetic neuropathies. Continuum (Minneap Minn) 2014;20:1226–1240. doi: 10.1212/01.CON.0000455884.29545.d2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang M, Wang S, Yao D, Yan Q, Lu W. A novel long non-coding RNA CYP4B1-PS1-001 regulates proliferation and fibrosis in diabetic nephropathy. Mol Cell Endocrinol. 2016;426:136–145. doi: 10.1016/j.mce.2016.02.020. [DOI] [PubMed] [Google Scholar]
- 7.Wolf G, Schroeder R, Zahner G, Stahl RA, Shankland SJ. High glucose-induced hypertrophy of mesangial cells requires p27(Kip1), an inhibitor of cyclin-dependent kinases. Am J Pathol. 2001;158:1091–1100. doi: 10.1016/S0002-9440(10)64056-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Guttman M, Rinn JL. Modular regulatory principles of large non-coding RNAs. Nature. 2012;482:339–346. doi: 10.1038/nature10887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cheetham SW, Gruhl F, Mattick JS, Dinger ME. Long noncoding RNAs and the genetics of cancer. Br J Cancer. 2013;108:2419–2425. doi: 10.1038/bjc.2013.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Huarte M, Guttman M, Feldser D, Garber M, Koziol MJ, Kenzelmann-Broz D, Khalil AM, Zuk O, Amit I, Rabani M, Attardi LD, Regev A, Lander ES, Jacks T, Rinn JL. A large intergenic noncoding RNA induced by p53 mediates global gene repression in the p53 response. Cell. 2010;142:409–419. doi: 10.1016/j.cell.2010.06.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zheng J, Dong P, Mao Y, Chen S, Wu X, Li G, Lu Z, Yu F. lincRNA-p21 inhibits hepatic stellate cell activation and liver fibrogenesis via p21. FEBS J. 2015;282:4810–4821. doi: 10.1111/febs.13544. [DOI] [PubMed] [Google Scholar]
- 12.Zhou WQ, Wang P, Shao QP, Wang J. Lipopolysaccharide promotes pulmonary fibrosis in acute respiratory distress syndrome (ARDS) via lincRNA-p21 induced inhibition of Thy-1 expression. Mol Cell Biochem. 2016;419:19–28. doi: 10.1007/s11010-016-2745-7. [DOI] [PubMed] [Google Scholar]
- 13.Valencia-Sanchez MA, Liu J, Hannon GJ, Parker R. Control of translation and mRNA degradation by miRNAs and siRNAs. Genes Dev. 2006;20:515–524. doi: 10.1101/gad.1399806. [DOI] [PubMed] [Google Scholar]
- 14.Kato M, Arce L, Wang M, Putta S, Lanting L, Natarajan R. A microRNA circuit mediates transforming growth factor-beta1 autoregulation in renal glomerular mesangial cells. Kidney Int. 2011;80:358–368. doi: 10.1038/ki.2011.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dey N, Das F, Mariappan MM, Mandal CC, Ghosh-Choudhury N, Kasinath BS, Choudhury GG. MicroRNA-21 orchestrates high glucose-induced signals to TOR complex 1, resulting in renal cell pathology in diabetes. J Biol Chem. 2011;286:25586–25603. doi: 10.1074/jbc.M110.208066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yokoi H, Mukoyama M, Mori K, Kasahara M, Suganami T, Sawai K, Yoshioka T, Saito Y, Ogawa Y, Kuwabara T, Sugawara A, Nakao K. Overexpression of connective tissue growth factor in podocytes worsens diabetic nephropathy in mice. Kidney Int. 2008;73:446–455. doi: 10.1038/sj.ki.5002722. [DOI] [PubMed] [Google Scholar]
- 17.Putta S, Lanting L, Sun G, Lawson G, Kato M, Natarajan R. Inhibiting microRNA-192 ameliorates renal fibrosis in diabetic nephropathy. J Am Soc Nephrol. 2012;23:458–469. doi: 10.1681/ASN.2011050485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mahimainathan L, Das F, Venkatesan B, Choudhury GG. Mesangial cell hypertrophy by high glucose is mediated by downregulation of the tumor suppressor PTEN. Diabetes. 2006;55:2115–2125. doi: 10.2337/db05-1326. [DOI] [PubMed] [Google Scholar]
- 19.Wang J, Gao Y, Ma M, Li M, Zou D, Yang J, Zhu Z, Zhao X. Effect of miR-21 on renal fibrosis by regulating MMP-9 and TIMP1 in kk-ay diabetic nephropathy mice. Cell Biochem Biophys. 2013;67:537–546. doi: 10.1007/s12013-013-9539-2. [DOI] [PubMed] [Google Scholar]
- 20.Viberti G, Wheeldon NM. Microalbuminuria reduction with valsartan in patients with type 2 diabetes mellitus: a blood pressure-independent effect. Circulation. 2002;106:672–678. doi: 10.1161/01.cir.0000024416.33113.0a. [DOI] [PubMed] [Google Scholar]
- 21.Mason RM, Wahab NA. Extracellular matrix metabolism in diabetic nephropathy. J Am Soc Nephrol. 2003;14:1358–1373. doi: 10.1097/01.asn.0000065640.77499.d7. [DOI] [PubMed] [Google Scholar]
- 22.Dronavalli S, Duka I, Bakris GL. The pathogenesis of diabetic nephropathy. Nat Clin Pract Endocrinol Metab. 2008;4:444–452. doi: 10.1038/ncpendmet0894. [DOI] [PubMed] [Google Scholar]
- 23.McClelland AD, Herman-Edelstein M, Komers R, Jha JC, Winbanks CE, Hagiwara S, Gregorevic P, Kantharidis P, Cooper ME. miR-21 promotes renal fibrosis in diabetic nephropathy by targeting PTEN and SMAD7. Clin Sci (Lond) 2015;129:1237–1249. doi: 10.1042/CS20150427. [DOI] [PubMed] [Google Scholar]
- 24.Yu F, Lu Z, Chen B, Dong P, Zheng J. Identification of a novel lincRNA-p21-miR-181b-PTEN signaling cascade in liver fibrosis. Mediators Inflamm. 2016;2016:9856538. doi: 10.1155/2016/9856538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Deng L, Yang SB, Xu FF, Zhang JH. Long noncoding RNA CCAT1 promotes hepatocellular carcinoma progression by functioning as let-7 sponge. J Exp Clin Cancer Res. 2015;34:18. doi: 10.1186/s13046-015-0136-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bradham DM, Igarashi A, Potter RL, Grotendorst GR. Connective tissue growth factor: a cysteine-rich mitogen secreted by human vascular endothelial cells is related to the SRC-induced immediate early gene product CEF-10. J Cell Biol. 1991;114:1285–1294. doi: 10.1083/jcb.114.6.1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ito Y, Aten J, Bende RJ, Oemar BS, Rabelink TJ, Weening JJ, Goldschmeding R. Expression of connective tissue growth factor in human renal fibrosis. Kidney Int. 1998;53:853–861. doi: 10.1111/j.1523-1755.1998.00820.x. [DOI] [PubMed] [Google Scholar]


