Abstract
4-Aminopyridine is a clinically approved drug to improve motor symptoms in multiple sclerosis. A fluorine-18-labeled derivative of this drug, 3-[18F]fluoro-4-aminopyridine, is currently under investigation for positron emission tomography (PET) imaging of demyelination. Herein, the Yamada-Curtius reaction has been successfully applied for the preparation of this PET radioligand with a better radiochemical yield and improved specific activity. The overall radiochemical yield was 5 to 15% (n = 12, uncorrected) with a specific activity of 37 to 148 GBq/µmol (end of synthesis) in a 90 minute synthesis time. It is expected that this 1 pot Yamada-Curtius reaction can be used to prepare similar fluorine-18-labeled amino substituted heterocycles.
Keywords: aminopyridine, F-18, multiple sclerosis, PET, Yamada-Curtius rearrangement
1 | INTRODUCTION
Multiple sclerosis is a common immune-mediated disease of the central nervous system in which myelin, the insulating membrane that wraps around axons, is damaged. This damage—which is often repaired through the process of remyelination—disrupts the ability of parts of the nervous system to communicate, resulting in neurological deficits that may include physical, mental, and sometimes psychiatric problems. 4-Aminopyridine is a clinically approved drug to improve walking ability in multiple sclerosis patients.1 Its fluorinated derivative 3-[18F]fluoro-4-aminopyridine is currently being studied as a potential tracer for demyelination.2–4
Pyridine derivatives are common in pharmaceuticals and radiopharmaceuticals for a variety of biological activities.5–8 Compared with other heterocycles, these derivatives are the most widely studied for fluorine-18 labeling due to the availability of the precursor and the ease of incorporation of fluorine-18 at the ortho and/or para position(s). Unlike in benzene, nucleophilic aromatic substitution (SNAr) at the ortho or para position in a pyridine ring does not require additional electron-withdrawing groups.9,10 The nitrogen atom of the pyridine lowers the aromatic stability and stabilizes the intermediate Meisenheimer complex by both inductive and mesomeric effects.11,12 However, substitution at meta position remains challenging, often requiring an electron-withdrawing group at ortho and/or para to the leaving group for successful fluorination.13,14
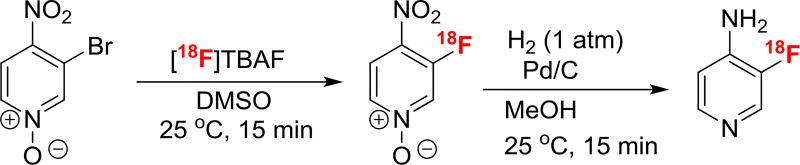
Chun and Pike reported a radiosynthesis of meta- [18F] fluoropyridine from its iodonium salt precursor.15 Abrahim et al reported meta fluorination of pyridine via displacement of a Br or Cl substituent with an activating group (cyano, nitro, or carboxamide) at the ortho position to the pyridine nitrogen.16 In this substitution, the 4-bromo-2-nitro derivative does not yield the desired fluorinated nitro pyridine, as the nitro group is preferentially displaced by fluorine. Brugarolas et al reported a novel approach to direct the fluorination at the position ortho to the nitro group by using pyridine N-oxide derivative. They prepared the fluorine-18 analog of 3-fluoro-4-aminopyridine (Scheme 1) by halogen (Br or F) exchange followed by hydrogenation. Nevertheless, this method suffered from low overall radiochemical yield or low specific activity.17 Therefore, new methods to prepare fluorine-18 labeled 3-fluoro-4-aminopyridine in better radiochemical yield and higher specific activity are warranted.
SCHEME 1.
Literature method17 for the preparation of 3-[18F] fluoro-4-aminopyridine
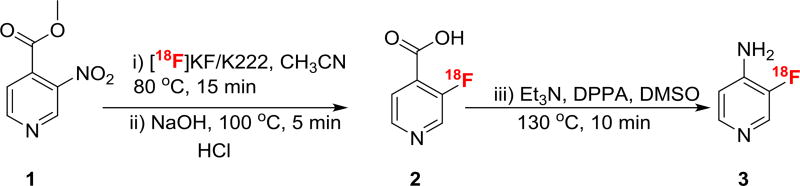
Here, we explore the possibility of labeling methyl-3-nitroisonicotinate followed by hydrolysis of the ester and conversion of the acid to the amine via the Yamada-Curtius rearrangement (Scheme 2). Nonradioactive fluorination of the methyl-3-nitroisonicotinate has been reported using CsF with 38% yield.18 To our knowledge, the Yamada-Curtius rearrangement has never been used in the synthesis of PET radiopharmaceuticals. This method may be a superior alternative to other literature methods for preparing fluorine-18-labeled aminoheterocycles.17,19
SCHEME 2.
Preparation of 3-[18F] fluoro-4-aminopyridine via Yamada- Curtius rearrangement
2 | MATERIALS AND METHODS
Precursors and nonradioactive cold standards were obtained from Combi-Blocks (San Diego, CA, USA). All other chemicals and solvents were received from Sigma- Aldrich (St Louis, MO, USA) and used without further purification. Fluorine-18 was obtained from National Institutes of Health cyclotron facility (Bethesda, MD, USA). Chromafix 30-PS-HCO3 anion-exchange cartridges were purchased from Macherey-Nagel (Düren, Germany). A semipreparative column for the purification of the final labeled product was obtained from Phenomenex (Torrance, CA, USA). Columns, iTLC-SG plates, and all other Sep-Pak cartridges used in this synthesis were obtained from Agilent Technologies (Santa Clara, CA, USA) and Waters (Milford, MA, USA), respectively. Alumina N cartridge was conditioned with 5 mL of water. High-performance liquid chromatography (HPLC) purification and analytical HPLC analyses for radiochemical work were performed on an Agilent 1200 Series instrument equipped with multiwavelength detectors. iTLC-SG papers were developed by using acetonitrile. The papers were read in an Eckert & Ziegler TLC scanner (B-AR2000-1).
2.1 | Experimental procedure for manual synthesis
2.1.1 | Synthesis of 3-[18F]fluoroisonicotinic acid (2)
Methyl 3-nitroisonicotinate (10 mg) in acetonitrile (0.3 mL) was added to the azeotropically dried mixture of [18F]KF/K222 (2 mg K2CO3, 10 mg K222) and heated at 80°C for 15 minutes. The reaction mixture was cooled to 40°C and treated with 100 µL sodium hydroxide (0.25 N) followed by heating at 100°C for 10 minutes. The solution was cooled to 40°C and acidified with hydrochloric acid (150 µL, 0.25 N). About 200 µL of triethylamine in 0.5 mL acetonitrile was added to the solution and evaporated to dryness. The residue was further dried by coevaporation with 1 mL acetonitrile.
2.1.2 | Synthesis of 3-[18F]fluoro-4-aminopyridine (3)
A solution of 20 µL diphenylphosphoryl azide (DPPA) in dimethyl sulfoxide (300 µL) was added to the dried residue of intermediate (2). The mixture was heated at 130°C for 10 minutes, then diluted with 2 mL HPLC solvent and passed through an activated (5 mL water) alumina Sep-Pak plus and washed with 2 mL HPLC solvent. The combined crude product was purified by HPLC using a semipreparative column. HPLC conditions: Phenomenex Luna column, 5 µm, 9.4 × 250 mm; 5% ethanol in water (0.1% triethylamine); flow rate, 4 mL/min, tR = ~16 minutes.
2.2 | Experimental procedure for automated synthesis
2.2.1 | Azeotropic drying of [18F]fluoride
Typically, 11.1 GBq [18F]fluoride in 2.5 mL of target water was passed through the PS-HCO3 cartridge, and the cartridge was rinsed with 1 mL of acetonitrile. [18F]Fluoride was eluted from the cartridge into Reactor 1 with the eluent (2 mg K2CO3, 10 mg K222 in 1 mL MeOH, and 200 µL water) in Vial 1 and dried under N2/vacuum at 75°C for 4 minutes. Reactor 1 was cooled to 50°C, acetonitrile in Vial 2 was added, and the activity was azeotropically dried at 55°C for 3 minutes, then at 95°C for 3 minutes under N2/vacuum. The activity was further dried by using a vacuum for 3 minutes. The [18F]fluoride drying cycle took about 20 minutes.
2.2.2 | Synthesis of [18F]fluoroisonicotinic acid (2)
Methyl 3-nitroisonicotinate (10 mg) in acetonitrile (0.5 mL) in Vial 3 was added to the azeotropically dried mixture of [18F]KF/K222 in Reactor 1. The mixture was heated at 80°C for 15 minutes. The reaction mixture was cooled to 40°C, and 150 µL sodium hydroxide (0.25 N) in Vial 4 was added, followed by heating at 100°C for 10 minutes. The solution was cooled to 40°C and acidified with hydrochloric acid (200 µL, 0.25 N) in Vial 5. The content in Reactor 1 was transferred to Reactor 2. Reactor 1 was rinsed by adding triethylamine (200 µL) in 0.5 mL acetonitrile in Vial 6; the mixture was also transferred to Reactor 2. The combined mixture in Reactor 2 was evaporated to dryness under N2 and vacuum. The residue was further dried with 1 mL acetonitrile in Vial 7.
2.2.3 | Synthesis of 3-[18F]fluoro-4-aminopyridine (3)
A solution of DPPA (20 µL) in dimethyl sulfoxide (300 µL) in Vial 8 was added to the dried residue of intermediate (2). The mixture was heated at 130°C for 10 minutes, diluted with 2 mL ammonium hydroxide (5%) in water in Vial 9, and passed through an activated (5 mL water) Alumina Sep-Pak plus. HPLC solvent in Vial 10 (2 mL) was added to wash Reactor 2, which was also passed through the Alumina Sep-Pak plus. The combined crude product was purified by HPLC by using a semipreparative column. HPLC conditions: Phenomenex Luna C182 column, 5 µm, 9.4 × 250 mm; 5% ethanol in water (0.1% ammonium hydroxide); flow rate, 4 mL/min, tR = 15 to 17 minutes. The fraction containing the product was collected in a product vial through a 0.22 µm sterile filter. The pH value of the final dose was adjusted to ~7 by using 0.1 MH3PO4 solution in water. The identity of the product was checked by analytical HPLC. HPLC conditions: Agilent XDB-C18 column, 5 µm, 4.6 × 150 mm; 5% acetonitrile in water (0.1% triethylamine), 1 mL/min. The total synthesis time is approximately 90 to 95 minutes.
3 | RESULTS AND DISCUSSION
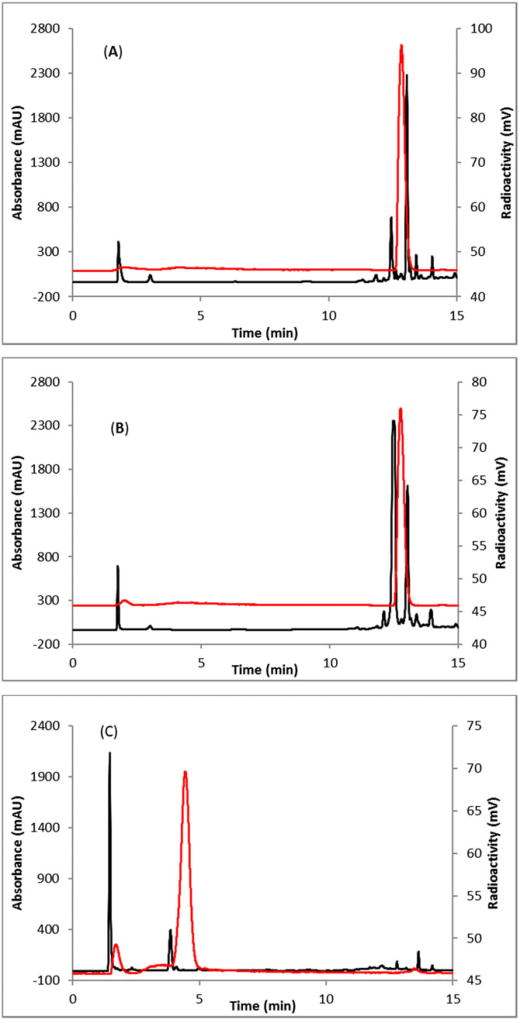
Fluorine 18 labeling was achieved in 3 steps (Scheme 2). Labeling efficiency was first standardized manually by using low amounts of starting activity (0.11-0.37 GBq). Briefly, nitro-precursor 1 (10 mg) was heated with [18F] KF/K222 at 80°C for 15 minutes. Fluorine-18 incorporation was monitored by analytical HPLC analysis (Figure 1A). The identity of the fluorinated ester was confirmed by comparing its HPLC retention time with authentic nonradioactive methyl 3-fluoroisonicotinate coinjected in analytical HPLC (Figure 1B). In the HPLC system, radiodetector is connected after UV detector. The slight difference (0.2 min) of retention time between UV and radiation trace is due to the dead volume between these 2 detectors.
FIGURE 1.
HPLC analysis of crude A, methyl 3-[18F] fluoroisonicotinate, B, methyl 3-[18F]fluoroisonicotinate coinjected with nonradioactive cold standard, and C, [18F]fluoroisonicotinic acid (2). HPLC conditions: Agilent Eclipse XDB C18 column (5 µm, 4.6 × 150 mm), mobile phase: 1 to 5% B in 8 minutes, 5 to 90% B in 15 minutes. A = water (0.1% TFA), B = acetonitrile, with a flow rate of 1 mL/min. Red line, in-line radiodetection; black line, UV detection at 254 nm
Deprotection of the methyl ester group was achieved with sodium hydroxide at 100°C for 5 minutes in the same pot with no need for purification of the methyl ester. Progress of the reaction was monitored by analytical HPLC (Figure 1C).
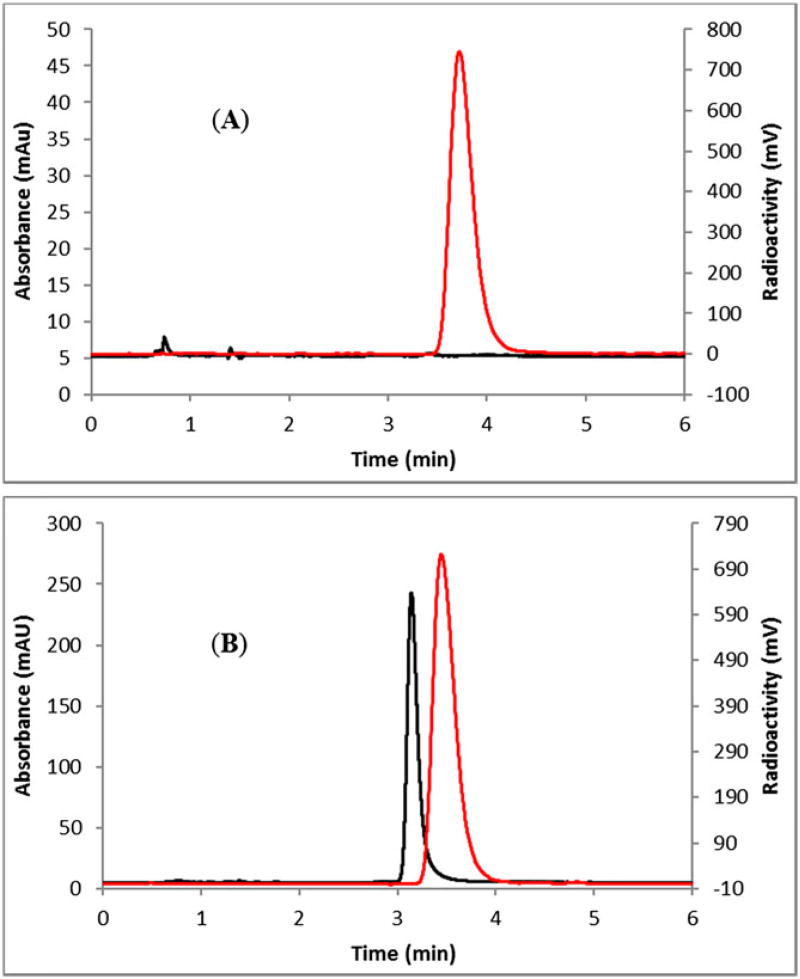
Reaction of the intermediate 3-[18F]fluoroisonicotinic acid 2 with DPPA at 130°C in the presence of triethylamine produced the final product 3-[18F]fluoro-4-aminopyridine 3 via Yamada-Curtius rearrangement. This reaction was also carried out on the same flask without the need for purification of the intermediate. The final product (3) was purified by HPLC using a semipreparative column. The overall radiochemical yield was 5 to 15% (uncorrected, n = 12) in 90 minutes, with >98% radiochemical purity by analytical HPLC (Figure 2A). The identity of the final product (3) was confirmed by comparing its HPLC retention time with authentic nonradioactive 3-fluoro-4-aminopyridine coinjected in analytical HPLC (Figure 2B). The final product was also checked by radio TLC and found to be free of unreacted fluoride- 18 (Figure S1).
FIGURE 2.
HPLC analysis of A, 3-[18F]fluoro-4-aminopyridine (3) and B, 3-[18F]fluoro-4-aminopyridine (3) coinjected with the nonradioactive standard. HPLC conditions: Phenomenex Luna C18 column (5 µm, 4.6 × 100 mm), mobile phase: 5% acetonitrile in water (0.1% triethylamine), with a flow rate of 1 mL/min. Red line, in-line radiodetection; black line, UV detection at 254 nm
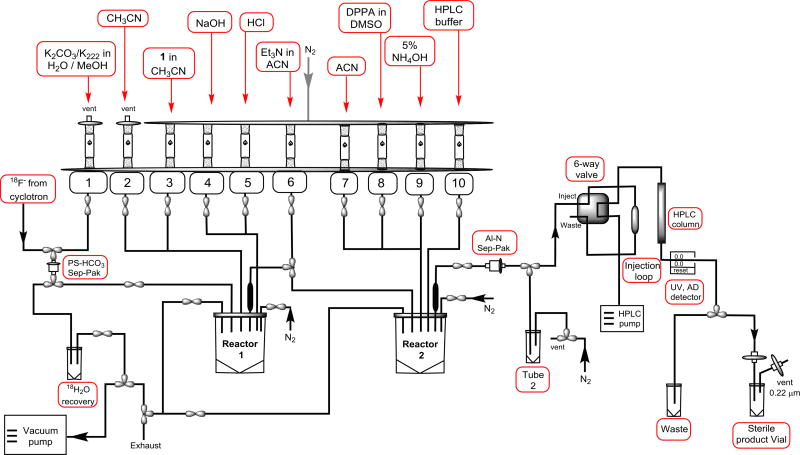
After successful manual standardization of the procedure, the fully automated synthesis was performed in a GE Tracerlab FX-N Pro module (Figure 3). Azeotropic drying, fluorination, and hydrolysis were performed in Reactor 1. After hydrolysis, compound 2 was transferred to Reactor 2 and converted to the final product, 3-[18F] fluoro-4-aminopyridine (3). The overall radiochemical yield was comparable with the manual synthesis with a specific activity of 37 to 148 GBq/µmol (n = 12). As reported in the recent literature,17 this tracer was synthesized with 2.5% (noncorrected) of radiochemical yield and with a specific activity of 0.37 to 3.7 GBq/µmol.
FIGURE 3.
Schematic diagram of the automated synthesis on GE FX-N Pro Module
4 | CONCLUSIONS
3-[18F]Fluoro-4-aminopyridine (3) is a PET radioligand under investigation for imaging demyelination and remyelination. Despite the simple nature of this compound, its labeling is challenging due to the meta position of the fluorine substituent. Previous syntheses solved this problem by starting from pyridine N -oxide, but this required hydrogen gas and suffered from low radiochemical yield or low specific activity. We improved the synthesis by employing the Yamada-Curtius rearrangement and here describes a fully automated method to prepare 3 with moderate radiochemical yield and high specific activity in 90 minutes of synthesis time. Successful radiolabeling performed at multiple institutions indicates that this radiolabeling method is robust. Finally, the automated synthesis allows facile translation to clinical production of tracer, repeatedly, in a safe and sterile manner for patient applications.
Supplementary Material
Acknowledgments
This project has been funded in whole or in part with federal funds from the National Institutes of Health, under contract no HHSN261200800001E, as well as the National Institute of Neurological Disorders and Stroke. It was also supported by grant NIH/NIBIB 1K99EB020075 to Pedro Brugarolas. The content of this publication does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the US Government.
Footnotes
Additional Supporting Information may be found online in the supporting information tab for this article.
References
- 1.Jensen HB, Ravnborg M, Dalgas U, Stenager E. 4-Aminopyridine for symptomatic treatment of multiple sclerosis: a systematic review. Ther Adv Neurol Disord. 2014;7:97–113. doi: 10.1177/1756285613512712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brugarolas P, Sanchez-Rodriguez J, Caprariello AV, et al. Fluorinated 4-aminopyridines as PET tracers for multiple sclerosis. Abstr Pap Am Chem S. 2014;248 [Google Scholar]
- 3.Brugarolas P, Sanchez-Rodriguez J, Lacroix J, et al. Development of a PET tracer for MS. J Nucl Med. 2014;55:1124. [Google Scholar]
- 4.Brugarolas P, Sanchez-Rodriguez J, Lacroix J, Chen C-T, Bezanilla F, Popko B. Fluorinated 4-aminopyrdines as PET tracers for MS. J Nucl Med. 2015;56:493–493. [Google Scholar]
- 5.Altaf AA, Shahzad A, Gul Z, Badshah NRA, Lal B, Khan E. A review on the medicinal importance of pyridine derivatives. Journal of Drug Design and Medicinal Chemistry. 2015;1:1–11. [Google Scholar]
- 6.Prachayasittikul S, Pingaew R, Worachartcheewan A, et al. Roles of pyridine and pyrimidine derivatives as privileged scaffolds in anticancer agents. Mini Rev Med Chem. 2016 doi: 10.2174/1389557516666160923125801. [DOI] [PubMed] [Google Scholar]
- 7.Dolle F. Fluorine-18-labelled fluoropyridines: advances in radiopharmaceutical design. Curr Pharm Des. 2005;11:3221–3235. doi: 10.2174/138161205774424645. [DOI] [PubMed] [Google Scholar]
- 8.Preshlock S, Tredwell M, Gouverneur V. 18F-Labeling of arenes and heteroarenes for applications in positron emission tomography. Chem Rev. 2016;116:719–766. doi: 10.1021/acs.chemrev.5b00493. [DOI] [PubMed] [Google Scholar]
- 9.Dolci L, Dolle F, Jubeau S, Vaufrey F, Crouzel C. 2-[18F] Fluoropyridines by no-carrier-added nucleophilic aromatic substitution with [18F]FK-K222—a comparative study. J Label Compd Radiopharm. 1999;42:975–985. [Google Scholar]
- 10.Malik N, Solbach C, Voelter W, Machulla H-J. Nucleophilic aromatic substitution by [18F]fluoride at substituted 2-nitropyridines. J Radioanal Nucl Chem. 2010;283:757–764. [Google Scholar]
- 11.Illuminati G, Stegel F. The formation of anionic σ-adducts from heteroaromatic compounds: structures, rates, and equilibria. Adv Heterocycl Chem. 1983;34:305–444. [Google Scholar]
- 12.Schlosser M, Ruzziconi R. Nucleophilic substitutions of nitroarenes and pyridines: new insight and new applications. Synthesis-Stuttgart. 2010:2111–2123. [Google Scholar]
- 13.Karramkam M, Hinnen F, Vaufrey F, Dolle F. 2-, 3- and 4-[F-18] fluoropyridine by no-carrier-added nucleophilic aromatic substitution with K[F-18]F-K-222—a comparative study. J Labelled Compd Rad. 2003;46:979–992. [Google Scholar]
- 14.Beer HF, Haeberli M, Ametamey S, Schubiger PA. Comparison of 2 synthetic methods to obtain [F-18] N-(2-aminoethyl)-5-fluoropyridine-2-carboxamide, a potential Mao-B imaging tracer for PET. J Labelled Compd Rad. 1995;36:933–945. [Google Scholar]
- 15.Chun JH, Pike VW. Selective syntheses of no-carrier-added 2-and 3-[F-18]fluorohalopyridines through the radiofluorination of halopyridinyl(4′-methoxyphenyl)iodonium tosylates. Chem Commun. 2012;48:9921–9923. doi: 10.1039/c2cc35005j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Abrahim A, Angelberger P, Kletter K, et al. Synthesis of fluorine-18-labelled 5-and 6-fluoro-2-pyridinamine. J Labelled Compd Rad. 2006;49:345–356. [Google Scholar]
- 17.Brugarolas P, Freifelder R, Cheng SH, DeJesus O. Synthesis of meta-substituted [F-18]3-fluoro-4-aminopyridine via direct radiofluorination of pyridine N-oxides. Chem Commun. 2016;52:7150–7152. doi: 10.1039/c6cc02362b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tjosaas F, Fiksdahl A. A simple synthetic route to methyl 3-fluoropyridine-4-carboxylate by nucleophilic aromatic substitution. Molecules. 2006;11:130–133. doi: 10.3390/11020130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liang SH, Collier TL, Rotstein BH, Lewis R, Steck M, Vasdev N. Rapid microfluidic flow hydrogenation for reduction or deprotection of F-18-labeled compounds. Chem Commun. 2013;49:8755–8757. doi: 10.1039/c3cc45166f. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.