ABSTRACT
Balancing the rate of differentiation and proliferation in developing tissues is essential to produce organs of robust size and composition. Although many molecular regulators have been established, how these connect to physical and geometrical aspects of tissue architecture is poorly understood. Here, using high-resolution timelapse imaging, we find that changes to cell geometry associated with dense tissue packing play a significant role in regulating differentiation rate in the zebrafish neural tube. Specifically, progenitors that are displaced away from the apical surface due to crowding, tend to differentiate in a Notch-dependent manner. Using simulations we show that interplay between progenitor density, cell shape and changes in differentiation rate could naturally result in negative-feedback control on progenitor cell number. Given these results, we suggest a model whereby differentiation rate is regulated by density dependent effects on cell geometry to: (1) correct variability in cell number; and (2) balance the rates of proliferation and differentiation over development to ‘fill’ the available space.
KEY WORDS: Neurogenesis, Tissue packing, Feedback control, Differentiation
Highlighted Article: Live imaging demonstrates that neural tube progenitors differentiate when displaced from the apical surface, allowing negative feedback control on cell number.
INTRODUCTION
Growth is a central process in developmental programs, and its control is critical to generate tissues of a particular size. In many tissues, this growth is substantial. For example, cell number in the rat retina increases ∼400-fold during its development (Alexiades and Cepko, 1996), but occurs with a highly stereotyped rate and duration to ensure that the final number of cells in the tissue is tightly controlled.
Tissue growth rate, here defined as the rate of increase in cell number within the tissue, affects two interlinked and essential processes of development: (1) proliferation of a pool of dividing progenitors, which increases progenitor number; and (2) differentiation of progenitors into post-mitotic differentiated cells, which reduces progenitor number (with progenitor apoptosis typically negligible). In homeostatic tissues, proliferation and differentiation must be perfectly balanced to maintain a constant pool of cycling cells. However, in developing tissues, proliferation and differentiation must instead be coordinated (Hardwick and Philpott, 2014; Hindley and Philpott, 2012; Kicheva et al., 2014), so that, early on, proliferation dominates and the tissue grows, whereas later, more cells differentiate and exit the cell cycle, and the growth rate finally approaches zero (Míguez, 2015). It is crucial to know how these two processes are tuned as development progresses in order to understand how a tissue reaches a specified final size. Furthermore, stereotyped tissue growth must occur despite large variability in proliferation rates (i.e. cell cycle times), and inherently probabilistic modes of differentiation (He et al., 2012). Determining how differentiation and proliferation are controlled – in the engineering sense of correcting errors – is thus fundamental to understanding how tissues reach a robust final size, despite the stochastic and noisy mechanisms underpinning their development.
Here, we focus on the neural tube as a model system of growth control that shows stereotypic growth dynamics – specifically an initial phase of rapid proliferation, followed by a gradual shift to differentiation (Saade et al., 2013) Much is known about the molecular regulators of neural tube growth. For example, the Hes/Her transcription factors promote and maintain proliferation of the progenitor pool, whereas expression of genes such as neurogenin or p27 induces cell cycle exit and differentiation (Hardwick et al., 2015). Differentiation is also affected by the inheritance of specific proteins following division (Alexandre et al., 2010; Dong et al., 2012; Huttner and Kosodo, 2005; Noctor et al., 2004; Paolini et al., 2015). Expression and genetic analyses also implicate a number of extracellular regulators of cell fate. Some of these are local signals, such as the Delta-Notch pathway, whereas others, such as Wnt, TGFβ, Shh and BMP, can act over a longer range (Dessaud et al., 2007; Garcia-Campmany and Marti, 2007; Le Dréau et al., 2014; Saade et al., 2013; Zechner et al., 2003). These then define molecular gradients that give rise to differential differentiation rates across the dorsal-ventral axis of the neural tube (Megason and McMahon, 2002; Kicheva et al., 2014).
We wondered whether there are also mechanical, or physical, regulators of proliferation and/or differentiation during neural tube growth. Experiments on cell stretching in vitro reveal that proliferation can respond significantly to externally applied mechanical strain (Aragona et al., 2013; Benham-Pyle et al., 2015; Streichan et al., 2014). Furthermore, differentiation of various stem cells in culture has been shown to be highly dependent on the mechanical properties of their microenvironment (Arulmoli et al., 2015; Engler et al., 2006; Gilbert et al., 2010; Leipzig and Shoichet, 2009; Pan et al., 2016; Seidlits et al., 2010). However, the extent to which these observations apply to neural tube development is unclear, particularly as it has a much more complex tissue architecture than the 2D cell monolayers that have been studied previously. This tissue architecture is: (1) epithelial, and therefore has a distinct apical-basal polarity; (2) pseudostratified, in which multiple nuclei are densely packed at different depths within a single epithelial layer; and (3) highly dynamic, with apical mitoses driving extensive rearrangement of nuclei, termed ‘interkinetic nuclear migration’ (Leung et al., 2011; Norden et al., 2009). To what extent these properties impact mechanical influences on proliferation or differentiation in this tissue is unknown.
Here, we investigate the role of tissue packing and its physical/geometric nature, during neural tube development. Using high resolution in toto timelapse imaging (Megason, 2009), we show that crowding at the apical surface is correlated with an increased rate of differentiation within the tissue. At the single-cell level, this manifests itself as a correlation between cells whose nuclei have been displaced basally (due to apical crowding) and those that differentiate. Experimentally arresting a subset of cells in mitosis in apical but not basal positions causes a locally increased rate of differentiation. Notch is downregulated in cells that are displaced from the apical surface, and Notch inhibition causes an increase in differentiation rate. Using simulations, we show that such density-dependent feedback on differentiation rate could naturally provide control to guide robust developmental trajectories in the face of probabilistic differentiation processes and highly variable cell cycle progression. Given the prevalence of similar pseudostratified tissue architectures, both in developmental contexts [e.g. cortex (Kosodo et al., 2011), retina (Leung et al., 2011), pancreas (Bort et al., 2006)], as well as in homeostatic adult tissues [e.g. the intestine (Grosse et al., 2011; Jinguji and Ishikawa, 1992)], we speculate that tissue packing and apical crowding may be a widely used regulator of differentiation and growth across a range of different organisms and tissues.
RESULTS
The neural tube is densely packed and crowded at the apical surface
To investigate neurogenesis in the zebrafish neural tube, we collected high-resolution confocal stacks of embryos doubly transgenic for a ubiquitous membrane label, Tg(actb2:memCherry2) (Xiong et al., 2014), and a pan-neuronal marker, Tg(neurod:eGFP) (Obholzer et al., 2008), one of the earliest markers of neural differentiation (Lee, 1997) (Fig. 1A, Fig. S1C). For measurement, we define differentiation based on expression of neurod rather than cell cycle exit. Our tracking data suggests these are tightly correlated as we did not observe neurod in dividing cells (0/91). We further confirmed that Tg(neurod:eGFP) faithfully marked postmitotic neurons by showing that neurod expression overlapped significantly with expression of elavl3/HuC, an alternative and more terminal neuronal marker gene (see Fig. S1D).
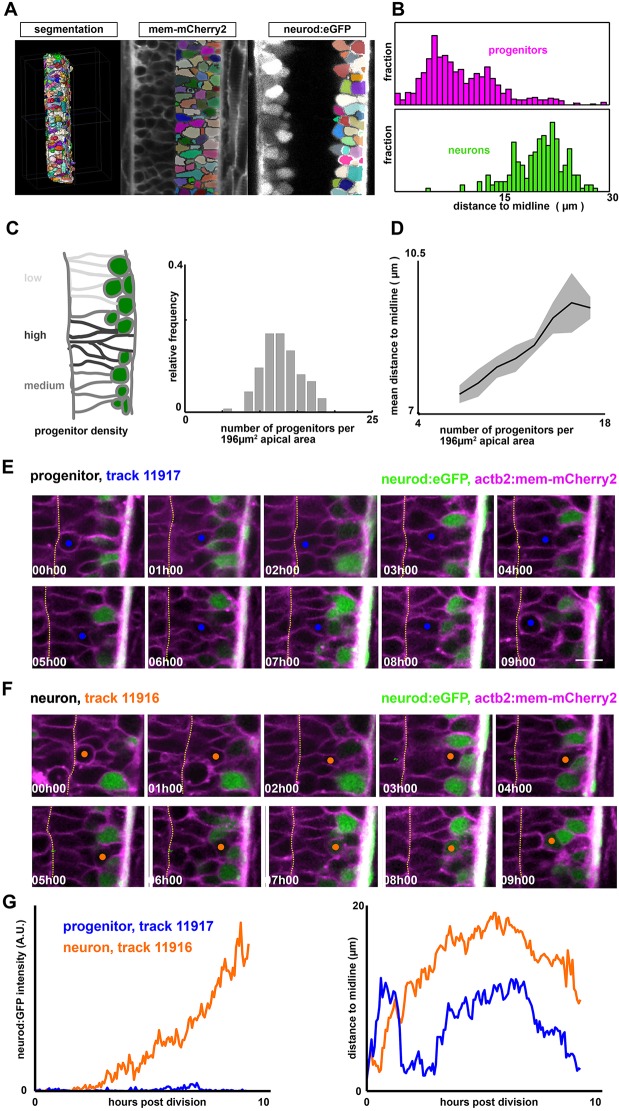
Fig. 1.
The neural tube is a densely packed and dynamic pseudostratified epithelium. (A) Analysis pipeline: membrane-labeled images are segmented and cropped using custom scripts to generate 3D cell meshes for the entire neural tube. Each mesh is then classified as a neuron or a progenitor, according to the expression level of the neural marker (neurod:eGFP) (see also Fig. S1C). (B) Distance of cell centroid position to the midline, for both progenitors and neurons. (C) Progenitor density (per unit apical area) varies significantly across the neural tube, even at the same dorsal-ventral position. Shown are measurements of progenitor number collected in a 14×14 µm section of apical area collected from different embryos across a range of anterior-posterior positions. (D) Regions of high progenitor density correlate with regions where progenitors are located far from the midline (data are mean±s.e.m.). (E,F) Representative cell tracks from GoFigure2, following two sister cells. (E) A cycling progenitor. Scale bar: 10 µm. (F) A nascent neuron. Orange and blue circles indicate one of the daughter cells from the mitotic cell. Dotted line indicates the midline (apical surface) (see also Movie 1). (G) neurod:eGFP intensity (left) and nuclear position (right) as a function of time for the two cells shown in E,F.
3D cell segmentations were generated using ACME (Mosaliganti et al., 2012) and revealed a densely packed, pseudostratified epithelial tissue architecture (Fig. 1A). Consistent with other neuroepithelia, neurons are located basally, whereas progenitors are predominantly apical (Fig. 1B), and divide with their nuclei at the midline (58/58 of tracked cells), although remaining attached to both the apical and basal surface (Fig. 2B). Progenitors in the neural tube show a large variability in cell shape. A simple measure of cell shape that can be accurately measured in our timelapse movies is the distance between the apical surface and the cell centroid, which is highly correlated with the nuclear distance to the apical surface (Fig. S1B). This is typical of pseudostratified epithelia in which there are multiple nuclei at different distances from the midline within a densely packed single-cell layer.
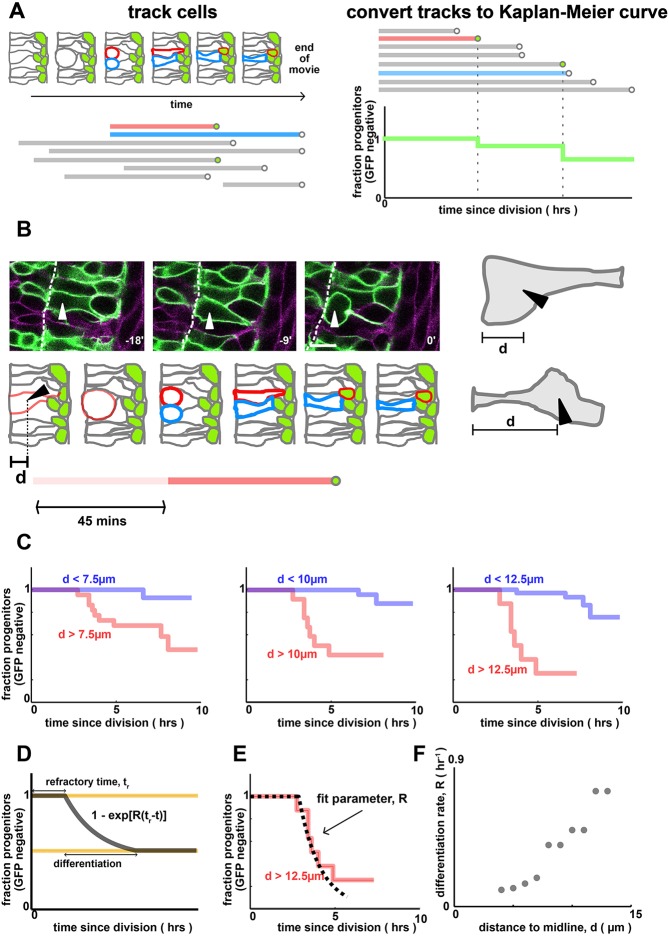
Fig. 2.
Progenitors that are far from the apical surface differentiate more frequently. (A) Quantifying cell-tracking data using Kaplan–Meier curves. Cells are manually tracked over time (schematic, upper left). Tracks begin at mitosis and end when (i) the cell turns on GFP (i.e. differentiates, see the red cell track), (ii) divides or (iii) becomes untrackable/moves out of frame (see the blue cell track). This generates an ensemble of tracks (lower left). To calculate the Kaplan–Meier curves, we align all tracks to begin at the same time, order them by length and, for each timepoint, compute the probability that progenitors remain GFP negative (right). (B) Quantification of pre-mitotic cell shape by distance to midline, d. Scale bar: 10 µm. Arrowhead shows a cell entering mitosis. Dashed line indicates the apical surface. (C) Single-cell tracking reveals that cells that are far from the apical surface pre-division turn on neurod more rapidly than those that are close. The dependence of differentiation rate on cell shape is independent of the threshold value that defines which cells are ‘far’ and which cells are ‘close’. P=0.01 (left), P=1e-6 (middle) and P=1e-7 (right). Data are from n=46 cells and n=3 embryos. The number of cells closer/further than each distance threshold is: 24/22 (7.5 µm), 34/12 (10 µm) and 38/8 (12.5 µm). (D) A simple model for differentiation, where for some time windows each cell differentiates at a constant rate per unit time, R (the differentiation rate). (E) Numerical fit of the model in D to the data in C to infer the differentiation rate parameter R. The dotted line refers to the best fit model. (F) Differentiation rate parameter, R, as a function of the distance to midline, d.
Next, we collected in toto timelapse imaging datasets that allowed single-cell tracking of neural progenitors over ∼12 h of development starting from 24 hpf (Xiong et al., 2013). These data revealed the highly dynamic aspect of tissue architecture, as evidenced by the significant movement seen in tracking single nuclei over time (Fig. 1E,F). By following individual progenitors, we see substantial, but largely undirected movement between divisions. As progenitors differentiate, they move basally and, as they divide, they move to the apical surface, but their movements during interphase appear largely undirected (Fig. 1G). This pattern of nuclear movements and apical mitoses (known as interkinetic nuclear migration, IKNM) closely resembles that seen in other neuroepithelia, such as the zebrafish retina (Norden et al., 2009; Leung et al., 2011) and is consistent with previous work on the zebrafish neural tube (Alexandre et al., 2010). Previous work (Norden et al., 2009) has argued that nuclear movements during IKNM in the retina are largely stochastic except at mitosis, at which time nuclei move to the apical surface to divide. They propose an analogy in which IKNM is like the movement of people at a party in a room with a bar at one end. People move to the bar to get a drink but are then pushed away by others getting drinks.
Progenitor cells in the neural tube, as in other pseudostratified epithelia, form a single epithelial layer that spans all the way from apical to basal. However, the nuclei, which we expect to be more round and tend to be more stiff (Lammerding, 2011) than the cytoplasm, must be at different depths due to packing considerations. We thus hypothesized that progenitor density (per unit apical surface area) would be correlated with average nuclear distance from the apical surface. To extend the analogy of Norden and colleagues (2009), the more crowded a party is, the further from the bar people are pushed, on average, in between drinks. We found that the density of progenitors (per unit apical surface) varies considerably across the tissue (Fig. 1C), and indeed we see a clear correlation between progenitor density and nuclear position. Specifically, in regions where there are many progenitors per unit apical surface area, their mean distance to the midline is higher (Fig. 1D). This follows from a purely geometric argument: more progenitors produce crowding at the apical surface, thereby forcing some progenitor nuclei to be displaced basally. In this way, there is a direct geometrical connection between epithelial cell density and the distribution of nuclear depths due to cell packing.
Progenitors far from the apical surface differentiate
Next, we used timelapse microscopy as a sensitive, single-cell assay to measure differentiation rates, by directly tracking progenitors and assigning them to a neural identity based on neurod:eGFP expression. To avoid biases caused by variations along the DV axis, we restricted analysis to cells located within the central third of the neural tube. By collecting many such tracks, we could generate Kaplan–Meier plots, as shown in Fig. 2A, which characterize the rate at which progenitors differentiate (Rich et al., 2010).
Kaplan–Meier curves are commonly used to analyze survival times in the medical community. For example, when studying recovery from a certain illness, they are used to compare recovery time between placebo- and drug-treated subjects. They are particularly useful when not all subjects complete the entire study (‘censoring’) as the calculation of the survival probabilities corrects for these censored subjects. Kaplan–Meier curves are therefore well suited for analyzing in toto image tracks, as they provide a measure of ‘survival’ (i.e. maintenance of the proliferative state) that is insensitive to incomplete cell tracks. This naturally corrects for effects of cells moving out of frame, or the timelapse ending before a cell has definitively divided or differentiated, which are commonly encountered in tracking data (Fig. 2A).
From these Kaplan–Meier curves, we saw a correlation between differentiation of progenitors and their apical-basal positioning. To quantify this, we analyzed the shapes of progenitors prior to their division (Fig. 2B). Restricting our shape measurements to the pre-mitotic mother cell was key in order to examine causation, as it is known that progenitors undergo stereotyped cell shape changes as they divide and differentiate, which would result in a trivial correlation between cell shape and differentiation. As a simple measure of cell shape, we measured the maximum distance of the cell nucleus to the apical surface observed in a time window 45-60 min prior to mitosis. This is not the only measure of cell shape – e.g. another possibility would be the size of the apical endfoot (Clark et al., 2012; Fig. S5) – but nuclear position is the most reliable measure using our timelapse data. We explicitly ignored any transient basal movement induced by neighboring cells as they divided, as we hypothesized that the long-term cell shape would be more informative (in Fig. S2, we confirm that the transient displacements have minimal effects on differentiation).
We then asked whether this pre-mitotic nuclear position correlated with the probability of a cell differentiating. Strikingly, we found a strong association between cells whose nuclei were far from the apical surface, and cells that rapidly turned on neurod after dividing (Fig. 2C). By fitting a simple parametric form to the Kaplan–Meier differentiation curves (Fig. 2D,E), we could quantify how the differentiation rate, R, depended on distance of nucleus to the midline, d, and found a significant positive correlation between the two (Fig. 2F). Interestingly, a potentially related observation has been made in the zebrafish retina (Baye and Link, 2007; Del Bene et al., 2008), in which the maximal basal position of the progenitor nucleus prior to division correlated with its chances of exiting the cell cycle to differentiate.
Together with the observations of tissue packing in Fig. 1, this suggests a model whereby apical crowding induces differentiation. More specifically, a high density of cells at the apical surface causes progenitors to be displaced away from the apical surface, which in turn leads to an increase in differentiation rate. Conversely, in regions of low apical pressure (i.e. few cells), we would expect a lower rate of differentiation.
Apical displacement promotes differentiation
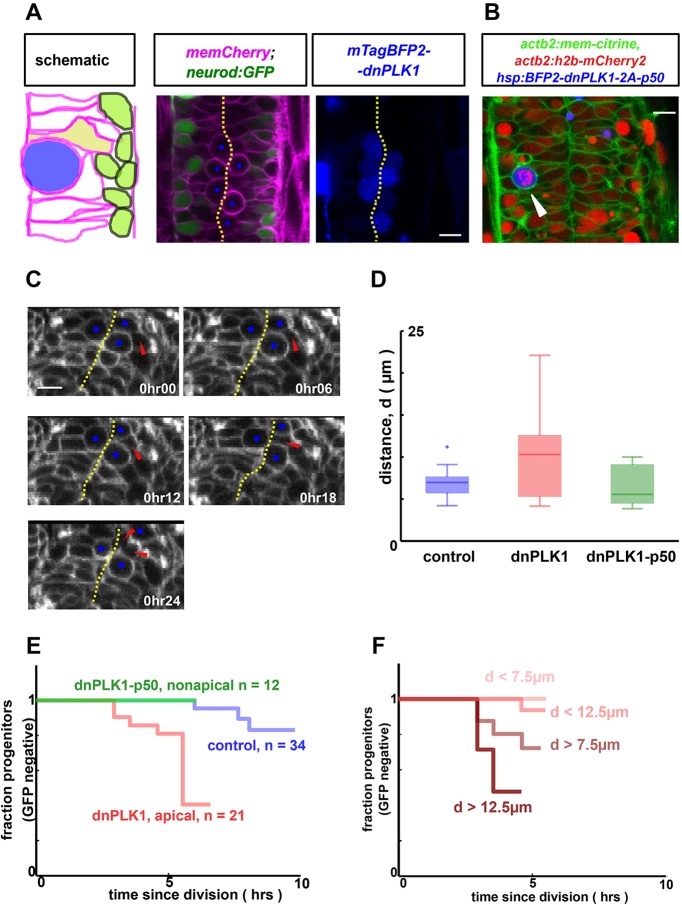
To test this hypothesis, we aimed to locally increase crowding at the apical surface and thereby push progenitors away from the midline. To do this, we exploited the fact that mitotic cells significantly deform their neighbors, a result of their large size and rounded morphology at the apical surface upon division (Fig. S2). Therefore, we could mimic an increase in crowding at the apical surface by simply using an M-phase cell that is prevented from dividing, and thus persistently occupies a significant volume of apical space. To achieve this, we arrested a small fraction of cells in mitosis, by inducing expression of a dominant-negative version of PLK1, a kinase that is necessary for mitotic exit (Strzyz et al., 2015). Following heat shock-induced mosaic dnPLK1 expression, the small fraction of cells that were expressing the construct (BFP positive) failed to exit mitosis and remained rounded and apical for extended periods of time (Fig. 3A). Furthermore, these arrested mitotic cells substantially deformed the shapes of neighboring progenitors and, as hoped, caused a significant increase in the distance of neighboring wild-type cell nuclei to the apical surface (Fig. 3C,D). We then measured whether such a perturbation to apical crowding and nuclear position impacted the proliferation and/or differentiation of the neighboring wild-type cells.
Fig. 3.
Pushing progenitors away from the apical surface by an arrested mitotic cell promotes their differentiation. (A) Cell shapes are perturbed by inducibly and mosaically arresting neighboring cells in mitosis by using a heat shock-inducible dnPLK1 construct that prevents mitotic exit. Scale bar: 10 µm. (B) Arrested mitotic cells can be nonapical (arrowhead) when p50 is co-expressed with dnPLK1. h2b-mCherry demonstrates condensed chromosomes, hence mitotic entry. Scale bar: 10 µm. (C) Arrested cells (blue dots) crowd the apical surface, pushing neighboring unperturbed cells away (red arrowhead). These cells still approach the midline to divide (last frame) and are then tracked to monitor differentiation status. Note that banding artifacts and pixellation visible in these panels result from data acquisition and processing methods (see Materials and Methods). Scale bar: 10 µm. (D) Cells adjacent to the arrested mitotic cell are shifted basally (P<0.01, n=10 for dnPLK1, n=26 for control). Here, d is the distance to the apical surface prior to division, as measured in Fig. 2. In contrast, progenitors remain apical when adjacent to dnPLK1-p50 arrested non-apical mitotic cells (P=0.6). (E) Tracking of single cells adjacent to an arrested mitotic cell reveals a significant increase in differentiation (P<0.01) compared with control (data from the same experiment as Fig. 2). In contrast, non-apical mitotic cells do not induce differentiation of their neighbors (P=0.8, n=12 versus control). (F) Single-cell tracking reveals the same correlation between cell shape and neurod dynamics as in Fig. 2 for cells adjacent to arrested mitotic cells (using the n=21 tracked dnPLK1 cells).
We generated in toto timelapse datasets of these perturbed embryos and tracked cells that were in close proximity to the mitotically arrested cells (but were themselves not arrested). Tracking data revealed that wild-type progenitors adjacent to the arrested mitotic cell more rapidly and extensively turned on neurod expression than in control embryos (Fig. 3E). Furthermore, within this dataset, we saw the same correlation between the pre-mitotic nuclear position of the cell and the neurod dynamics of its daughter, as above [i.e. those progenitors that were pushed far from the apical surface by the arrested cell were much more likely to rapidly turn on neurod following division (Fig. 3F)].
As a second assay for differentiation rates, we collected high-resolution confocal stacks to count neuron and progenitor numbers following prolonged deformation by arrested mitotic cells. We found that there was a significant increase in the number of neurons in close proximity to an arrested cell, compared with unperturbed control regions from the same embryo, indicating an increase in the differentiation rate (Fig. S3). We then measured proliferation rates by counting the total cell number at two time points, subtracting to get the number of division events, and then dividing by the number of progenitors to get an estimate of the proliferation rate per progenitor cell. No significant difference was found in proliferation rate between regions deformed by an arrested cell and unperturbed regions. Together, this suggests that crowding progenitor nuclei away from the apical surface leads to an increase in differentiation, but minimal changes to proliferation, consistent with our previous results.
The effect of the apical arrested mitotic cell is primarily physical
The strong correlation between nuclear position and differentiation rate in response to neighboring mitotic cells suggests that the effect of the arrested cell on its neighbors depends on its ability to physically deform them. However, it is conceivable that a non-physical mechanism such as expression of some secreted molecule or cell-surface protein by mitotic cells could also affect differentiation rate in neighbors. To test this possibility, we sought to arrest mitotic cells in such a way that they did not increase pressure at the apical surface and therefore do not deform their neighbors to the same extent. Inspired by previous studies on neuroepithelial nuclear migration, we expressed p50, which is known to inhibit the dynactin complex and thus impair apical movement of nuclei (Burkhardt et al., 1997; Tsuda et al., 2010; Tsujikawa et al., 2007), in the dnPLK1-arrested cells. Indeed, we found that a small number of the arrested cells (assayed by condensed chromosomes and rounded shape, Fig. 3B) were non-apical and therefore neighboring cell nuclei remained apical (Fig. 3D). By tracking cells adjacent to these basal mitotically arrested cells, we no longer observed a local increase in differentiation, suggesting that the apical location of the mitotic cell is necessary for its neurogenic effect (Fig. 3E). These results suggest that the extent of crowding at the apical surface, as measured by nuclear position, strongly influences the differentiation rate of neural progenitors.
Notch as a candidate molecular transducer of apical crowding
Next, we aimed to understand how apical crowding and cell shape differences could be sensed molecularly, and therefore how the physical effect of tissue packing connects to the molecular circuitry upstream of neural differentiation. We started by considering the Notch pathway (Bray, 2016), the activity of which is necessary for progenitor maintenance in the zebrafish neural tube. This pathway is particularly appealing since previous studies in the retina have suggested that Notch activity can depend on nuclear position potentially via an apical-to-basal gradient of the Notch intracellular domain (Aggarwal et al., 2016; Clark et al., 2012; Del Bene et al., 2008; Hatakeyama et al., 2014; Latasa et al., 2009).
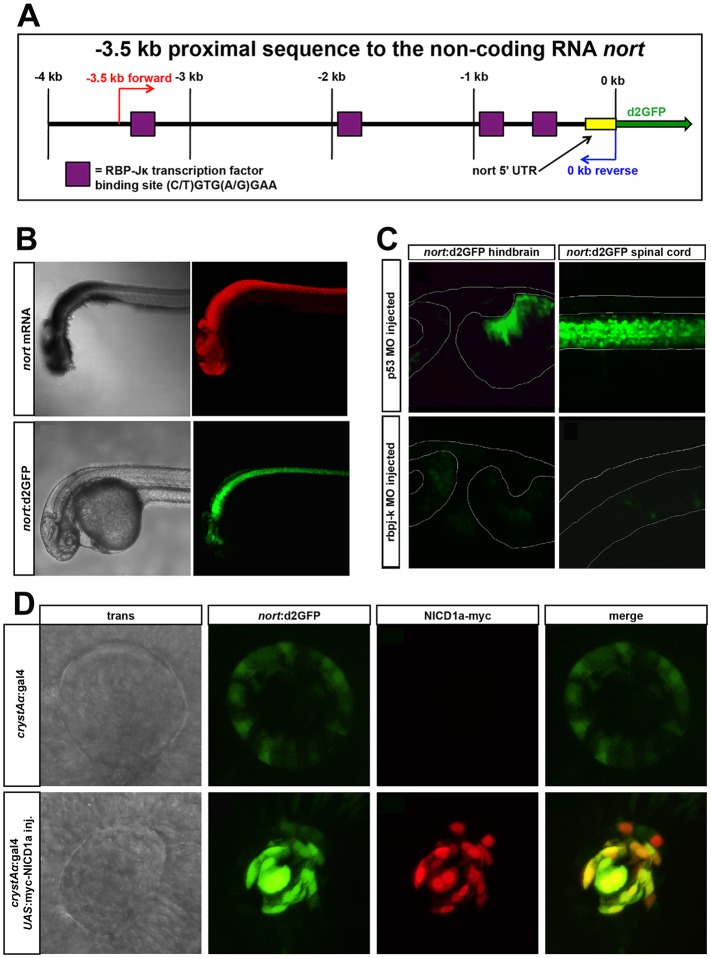
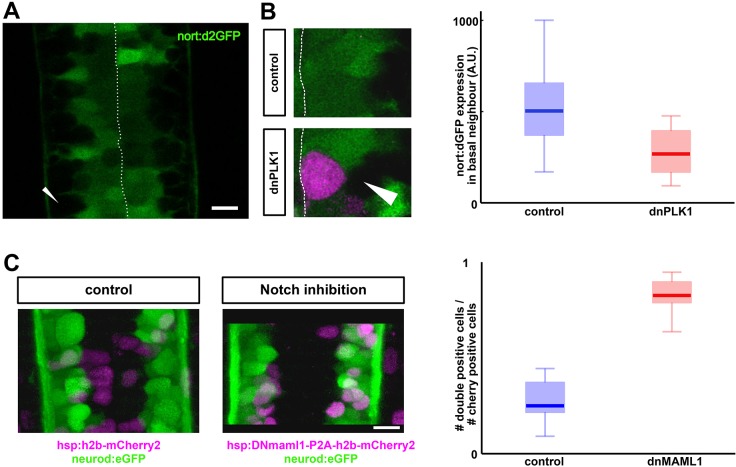
To test whether Notch was involved in cell shape sensing, we measured Notch activity in cells that were significantly and persistently deformed by an adjacent hsp:dnPLK1-arrested mitotic cell. We created a novel transgenic reporter to mark Notch activity, which drives destabilized GFP expression downstream of the nort promoter (Fig. 4A), a known direct target of Notch (Tsutsumi and Itoh, 2007). The transgene expressed fluorescence in a manner nearly identical to expression of the endogenous transcript, including robust expression in spinal cord neural progenitors (Fig. 4B). Importantly, abatement of Notch signaling by knockdown of Rbpj (Fig. 4C) or expression of dominant-negative Maml (not shown), which are essential co-factors for transcriptional activity of all Notch subtypes, resulted in nearly complete loss of reporter fluorescence. In addition, overexpression of the Notch1 intracellular domain (NICD1) strongly enhanced reporter activity in either endogenous sites of nort expression or within ectopic locations that normally do not express nort (Fig. 4D).
Fig. 4.
A nort transgenic reporter for Notch activity. (A) Schematic of the 3.5 kb nort proximal promoter. This region contains four canonical rbpj-K-binding sites. (B) nort mRNA (red) and nort:d2GFP (green) transgene expressed in the same tissues at 30 hpf. (C) nort:d2GFP expression was reduced in a rbpj-k MO-injected 48 hpf hindbrain and spinal cord compared with the p53 MO-injected controls. The morphology of the hindbrain was similar to the controls, whereas the rbpj-k MO-injected spinal cord was curved. (D) Normal expression of nort:d2GFP is found in the lens epithelium. Overexpression of myc-NICD1a in the lens using crystAα:gal4 causes enhanced ectopic nort:d2GFP expression. Myc immunofluorescence colocalizes with upregulated d2GFP at 28 hpf.
We hypothesized that the arrested mitotic cells would locally inhibit Notch activity to drive differentiation. Consistent with this hypothesis, we saw a significant downregulation of Notch activity in progenitors adjacent and basal to an arrested mitotic cell (Fig. 5A,B). Given that Notch is required for progenitor maintenance in the neural tube (Appel et al., 2001; Huang et al., 2012; Schier et al., 1996; Yeo and Chitnis, 2007), this supports a model whereby apical crowding inhibits Notch signaling, thus causing cells to differentiate. To test this idea further, we inducibly and mosaically inhibited Notch activity by overexpressing a dominant-negative version of mastermind-like 1 (MAML1), a necessary Notch co-factor. We saw that when cells had suppressed Notch signaling (hsp:dnMAML1-P2A-h2b-mCherry2), they had a much higher fraction of neurod+ neurons than those that expressed a control construct (hsp:h2b-mCherry2), supporting our hypothesis (Fig. 5C). Taken together, these data and previous work (Del Bene et al., 2008; Clark et al., 2012; Aggarwal et al., 2016) support a model in which Notch links the effects of cell packing on differentiation rate.
Fig. 5.
Notch activity as a potential readout of nuclear position. (A) nort:d2GFP expression reports Notch activity in the neural tube. Arrowhead indicates GFP-negative basally localized neurons. Scale bar: 10 µm. (B) Notch activity (green) is significantly inhibited in progenitors that are adjacent (and basal) to an arrested mitotic cell (magenta) (P<0.01, n=15 for control, n=12 for dnPLK1). (C) Inhibition of Notch signaling (via microinjection of hsp:dnMAML1-P2A-h2bCherry2) promotes differentiation compared with a control injection (hsp:h2bCherry2) (P<0.001 for n=11). Scale bar: 10 µm.
There are multiple models by which Notch could transduce differences in cell shape. In the simplest model, cell packing leads to nuclei being displaced further from the apical surface, which in concert with a proposed apical to basal gradient of Notch intracellular domain (Del Bene et al., 2008; Aggarwal et al., 2016), would cause these cells to differentiate. An alternative hypothesis to explain the connection between Notch and progenitor geometry involves oriented cell divisions at the apical surface (Das and Storey, 2012). In this model, nuclear position could play an important role in defining the orientation of dividing cells, and that division orientation in turn influences differentiation (Siller and Doe, 2009), e.g. by controlling the segregation of Notch pathway components at division (Kressmann et al., 2015). However, we saw that division orientation could predict differentiation rate much less clearly than distance to midline alone (Fig. S4B), suggesting that the division orientation is not the primary regulator here (see also Alexandre et al., 2010).
Another model for linking Notch to cell shape is that packing decreases the size of the apical endfoot, which was previously shown to relate to the level of active Notch signaling (Clark et al., 2012). We measured the apical contact area for each neural progenitor (by mosaic labeling) and found a strong negative correlation between the area of the apical endfoot and the distance of the nucleus to the apical surface (Fig. S4A). Akin to Fig. 1C, this makes sense geometrically – in regions of high progenitor density, not only are nuclei squeezed away from the midline, but the amount of apical surface occupied per progenitor must be less. Therefore, both nuclear position and apical area are readouts of the degree of crowding at the apical surface, and both could be contributing to differential Notch activation and altered rates of differentiation. Unfortunately, we cannot simultaneously track cells and accurately measure apical area in the same embryo, so further work is needed to disentangle these two shape parameters.
Finally, a related model linking Notch and cell packing is that cells are physically detached from the apical surface due to crowding (Hatakeyama et al., 2014), leading to a loss of Notch signaling and their differentiation. Indeed, we see that the majority of neurod:GFP+ cells are not connected to the apical domain (Fig. S4C). However, we do not favor this hypothesis as our observations from Fig. 2 suggest that cell shapes prior to division – when the cells are still attached to the apical surface – correlate strongly with differentiation.
Apical crowding provides a negative feedback on progenitor number
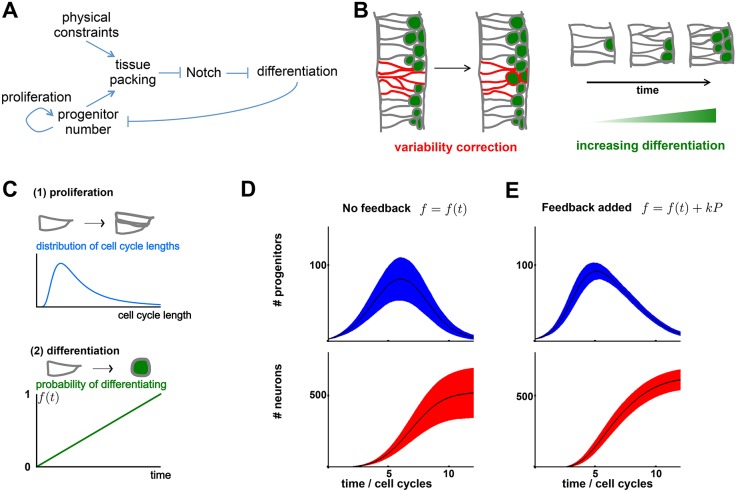
Regardless of how it is transduced molecularly, the effect of tissue packing and apical crowding on differentiation rate would naturally provide a negative-feedback loop between growth rate and cell number in this tissue. Specifically, as the number of progenitors increases within the tissue, we expect an increase in the pressure and/or crowding of cells at the apical surface. This will then change the distribution of cell shapes within the tissue, giving rise to a higher number of basally located nuclei with smaller apical area, and thus a higher rate of differentiation. Therefore, as the number of progenitors within a region increases, their rate of differentiation also increases. This in turn leads to a depletion of the progenitor pool, giving rise to negative feedback on progenitor cell number (Fig. 6A).
Fig. 6.
Apical crowding as a feedback mechanism to control progenitor number. (A) Proposed regulation of differentiation by cell shape to form a negative-feedback loop. This feedback could perform several functions, schematized in B. (B) Negative feedback naturally reduces variability in progenitor number. Left: a region of high progenitor density (red) corrects itself by differentiating. Right: if cells divide within a confined space, negative feedback predicts an increase in differentiation over time. (C) A phenomenological mathematical model of neural tube development with two main ingredients. Top: progenitors proliferate with a given cell cycle distribution; here, we assume a generalized extreme value distribution. Bottom: progenitors differentiate shortly after dividing, with probability f(t). (D) Progenitor and neuron numbers are variable without feedback. The black line is the mean trajectory; red and blue regions indicate the s.d. for 3000 independent simulations. (E) When we add feedback [by allowing f(t)=f0(t)+kP], the s.d. in neuron and progenitor number is significantly reduced.
One possible role for this negative feedback would be to suppress fluctuations. Neurogenesis dynamics are far from deterministic, and must operate despite highly variable cell cycle lengths and multiple stochastic influences on the differentiation machinery. This generates significant variability in neuron and progenitor numbers across the tissue, and between different embryos. This variability has been studied in most detail in the developing retina (He et al., 2012), although we expect similar effects in other neuroepithelia. A crucial role for negative feedback would be to reduce this variability: intuitively, regions with too many progenitors would compensate by differentiating more; and regions with too few progenitors by differentiating less (see Fig. 6B).
To formally test this intuition, we constructed a mathematical model of neural tube development. The goal here is not to fit the model to data, nor to construct a comprehensive model of neural tube development. This would require measurement of many more parameters in the system, which current data do not allow (e.g. the full distribution of cell cycle lengths). Plus, even if we could build such a model, it is not straightforward to interpret it. Instead, we built a simple model of neural tube development, hoping to reveal qualitative insights into feedback regulation.
We first assume that progenitors proliferate with a certain distribution of cell cycle times (Fig. 6C). Then, after dividing, there is a certain probability a given progenitor will either self-renew (fPP), asymmetrically divide (fNP) or generate two post-mitotic daughters (fNN). Motivated by work in the chick neural tube (Saade et al., 2013) and for simplicity, we assume that each daughter cell differentiates independently with probability f [i.e. division mode probabilities fPP=f 2, fNP=2f(1−f), fNN=(1−f)2 form a binomial distribution]. Over time, we assume that this differentiation probability increases [i.e. f(t) is an increasing function of time, t], so that initially the tissue grows, and later on differentiation dominates and the pool of progenitors is depleted. We find that, without any feedback mechanism, the numbers of progenitors and neurons are highly variable in the in silico neural tube (Fig. 6D), a consequence of the probabilistic differentiation and variable cell cycle lengths. Strikingly, the percentage variability (CV) is much higher than the simple Poissonian limit, both for progenitors and neurons, suggesting that fluctuations are particularly significant in a growing tissue. Furthermore, we saw high variability in cell number even when cell cycle lengths had low variability (Fig. S5). However, if we incorporate the feedback from apical crowding – whereby the differentiation probability, f(t, P), increases not only as a function of time, but also as a function of progenitor number, P – then we find that this variability is significantly reduced (Fig. 6E), consistent with our intuition.
DISCUSSION
In this study, using a combination of in toto timelapse imaging and physical perturbations, we have identified a role for apical crowding in neurogenesis. In particular, we found that when neural progenitor nuclei are displaced away from the midline (and/or their apical surface is compressed), they were more likely to differentiate. This suggests that neurogenesis dynamics within the neural tube are not entirely deterministic, nor cell-autonomously programmed, and instead can be regulated by the mechanical properties of the tissue, its environment and how these interact to regulate tissue packing. Using modeling, we argued that this phenomenon could result in negative-feedback control between progenitor number and differentiation rate, and that this can significantly reduce variability in developmental trajectories. To what extent this feedback mechanism is necessary to limit variability in vivo remains an open question.
This proposed negative-feedback module also provides a mechanism to coordinate changes in tissue size and growth rate over developmental time. In particular, during early neural tube development, there are few progenitors and the tissue is relatively loosely packed, and thus in our model differentiation is low. However, as the neural tube continues to grow, we expect that it becomes compressed by the tissues surrounding it (likely the skin, somites and notochord, each compressing the neural tube from different directions), causing cells to be densely packed and therefore more likely to differentiate (see Fig. 6B). In this way, the exit from the early proliferative phase of neural tube growth could be governed by this mechanical feedback, in addition to known molecular regulators (Hudish et al., 2016), and therefore growth continues until all the available space is filled. This hypothesis may provide an explanation for the hyperproliferation phenotypes in human open neural tube defects (NTDs), such as spina bifida, in which the spinal cord is ‘open’ or exposed at birth (Copp et al., 2013). We speculate that the increased growth is a result of the reduction in physical constraints acting on the neural tube. This has been directly observed in surgical models of NTDs, in which surgically removing the skin overlying the spinal cord results in increased proliferation in chick embryos (You et al., 1994). However, more experiments are required to determine to what extent such a space-filling mechanism is actually operating in the zebrafish neural tube, and its significance during unperturbed development. In particular, there are likely other control mechanisms at play in the tissue that could operate not just on differentiation rates but also on cell cycle progression. For example, it has been proposed that proliferation rates are inherently limited by the physical space available for apical mitoses (Smart, 1972, 1973), a limitation that has been overcome in larger brains by basal divisions (Fish et al., 2008). The relative importance of cell cycle control, and its coordination with differentiation control, should be the focus of future study.
In this work, we have largely focused on explaining our observations at the level of cells and tissues. Preliminary work has implicated the Delta-Notch signaling pathway as a potential mechanism by which cells measure their shape, although the precise details are unclear. One hypothesis is that, given that the Notch ligand and receptor are both apically enriched, one might expect the level of active nuclear Notch (NICD) to depend on the distance of the nucleus to the apical surface, provided NICD is rapidly degraded (or bound by an inhibitor, e.g. numb) en route to the nucleus (Del Bene et al., 2008; Aggarwal et al., 2016). In this case, having the nucleus in close proximity to the apical Notch receptors increases the chances that a given NICD molecule reaches the nucleus and activates transcription. If nuclei are displaced from the apical surface due to crowding, they would receive less NICD, leading to differentiation.
However, there are other possibilities. In particular, although we have focused on nuclear position as a readout of cell shape, there could also be a role for apical contact area. Correspondingly, another possible mechanism is that, as proposed elsewhere (Clark et al., 2012; Shaya et al., 2017), the amount of Notch signaling received by a cell is dependent on the size of its cell-cell contacts with neighboring cells (which is directly related to its apical area), as this is where the bulk of the Notch receptor is located. Therefore, a cell with smaller apical area will have a smaller contact with neighboring Delta-positive cells and consequently will receive lower active Notch signaling. A further possibility is that it is not just geometry but also force that is at play. Notch signaling has been shown to depend, at the single molecule level, on forces; therefore, the forces associated with apical compression could be modulating Notch activity directly, rather than indirectly via its effect on cell geometry (Gordon et al., 2015). Finally, there could be a role for the cell cycle in the regulation of Notch activity, as has been proposed elsewhere (Murciano et al., 2002). In this scenario, nuclear position and tissue packing would affect progression through the cell cycle, and this would subsequently result in changes to Notch activity. These possible mechanisms are not mutually exclusive and aspects of each may be coordinated to regulate neurogenesis. Testing these hypotheses will require higher resolution tools to measure and perturb Notch signal transduction, ideally approaches for measuring Notch activity in real time and with subcellular resolution rather than the currently available reporters, which integrate Notch-induced transcriptional activity over time to make a cell-wide reporter.
Although in this work we have focused on Notch activity as a readout of cell shape, other signaling pathways may also be involved. The Wnt pathway is a promising candidate, as it is known to be responsive to mechanical cues (Brunet et al., 2013; Fernández-Sánchez et al., 2015; Nowell et al., 2016) and has significant effects on neurogenesis (Zechner et al., 2003). Other mechanotransduction pathways such as the Hippo pathway (Dupont et al., 2011) or the piezo proteins (Coste et al., 2012) could also be determining the response to increased pressure at the apical surface. Finally, it is possible that it is not just apical crowding, but also signals from the basal compartment (e.g. TGFβ secreted by basally positioned neurons) that are important. Elucidating the molecular details of the shape-based feedback mechanism, and the interactions between Notch and other signaling pathways with the apical surface should be the subject of further work.
Finally, our work may yield important insights into understanding how differentiation and proliferation are balanced more generally. Many tissues have a similar architecture (i.e. densely packed, pseudostratified epithelia, with a large degree of nuclear movement), most notably other neuroepithelia, but also a range of other developmental and adult tissues (Spear and Erickson, 2012). We suggest that the transfer function that relates cell shape to differentiation rate can be adjusted between different tissues to result in different epithelial thicknesses. For example, in the zebrafish retina, there is also a correlation between nuclear position and differentiation rate (Del Bene et al., 2008), but the gradient is quantitatively shallower, which could allow the epithelium to grow thicker. It will be interesting to determine whether the feedback control between tissue packing and differentiation rate described in this work is a common feature in these tissues, how the relationship can be tuned to achieve different morphologies such as the thicker retina or folded primate brain (Otani et al., 2016; Tallinen et al., 2014), and how it can be broken resulting in aberrant growth during tumorigenesis or open neural tube defects (Fernández-Sánchez et al., 2015; Ou and Weaver, 2015; Copp et al., 2013).
MATERIALS AND METHODS
Zebrafish strains and maintenance
Tg(neurod:eGFP) (Obholzer et al., 2008), Tg(elavl3:kaede) (Sato et al., 2006), Tg(actb2:mem-mCherry2) (Xiong et al., 2014), Tg(crystAα:Gal4) (Hayes et al., 2012) and Tg(actb2:mem-citrine-citrine) (Xiong et al., 2013) (referred to as ‘mem-citrine’) have been described previously. Tg(actb2:h2b-mCherry2) was generated using a plasmid that encodes the h2b sequence fused to mCherry2, in a pMTB backbone as described previously (Xiong et al., 2014). Tg(−3.5 kb nort:d2GFP) was constructed using Gateway recombination and the Tol2 Kit (Kwan et al., 2007) to place the 3.5 kb nort promoter upstream of d2GFP (destabilized GFP; Clontech). Natural spawning was used and embryos were incubated at 28°C throughout their development (including during imaging), but excluding small amounts of time during experimental manipulation (such as microinjection and mounting), which occurred at room temperature.
Zebrafish work was approved by the Harvard Medical Area Standing Committee on Animals.
Confocal imaging
Embryos were anesthetized in two different ways depending on the type of experiment. First, for continuous timelapse imaging, α bungarotoxin protein was delivered via microinjection into the heart 1 h before imaging (4.6 nl, 0.5 mg/ml) or via mRNA microinjection at the single-cell stage (2.3 nl, 15 ng/µl). This method of anesthetizing produces fewer developmental delays and defects than the conventional method, tricaine (Swinburne et al., 2015). For endpoint images, in which embryo health was less crucial, we used tricaine (Sigma) at 0.2 mg/ml.
Prior to imaging, healthy embryos were selected and dechorionated on a glass dish then transferred to a 1.5% agarose 0.4 µm canyon mount (Megason, 2009). Using a stereoscope, embryos were carefully positioned within the canyon using a hair loop, with the dorsal neural tube oriented upwards. For the majority of experiments, embryos were mounted in egg water, except some of the embryos for the results in Fig. 2, where they were mounted in 1% low melt agarose (A9414, Sigma) for increased stability and longer-term imaging. For the control data in Fig. 3, we excluded these agarose-embedded embryos.
A Corning #1 coverslip was placed on top of the agarose mount, taking care not to disturb the embryo positioning.
Imaging was performed using a Zeiss 710 confocal microscope, C-Apochromat 40×1.2 NA objective, with a custom-made heating chamber to keep the embryos at 28°C. The following laser lines were used: 405 nm (eBFP2), 488 nm (eGFP), 514 nm (citrine) and 594 nm (mCherry2). Other parameters were optimized for each experiment (e.g. low laser powers were used for all timelapse imaging to prevent bleaching), but were consistent between experimental conditions. Timelapse movies were started at 24 hpf (±1 h). Endpoint measurements (Fig. S3 and Fig. 5) were taken at 32 hpf.
Elavl3/HuC expression was monitored using the Tg(huc:kaede) transgenic. Kaede was photoconverted into the red state using a 405 nm laser (3 mW for 120 s), and then imaged at the same time as neurod:eGFP. We confirmed that photoconversion was complete by repeating the experiment with longer photoconversion periods.
Figures are composed of single XY slices, dorsal view, of single timepoints from the timelapse data. Some images are flipped left to right for consistency of data presentation, and the brightness/contrast adjusted to show clear signal. The imaging from Fig. 4 was performed on a Nikon Eclipse E800 confocal microscope with the embryos anesthetized in tricaine (Sigma) and embedded in low melt agarose (1%) within glass-bottomed petri dishes.
Analysis of timelapse data
Raw Zeiss .lsm files were converted to formats compatible with GoFigure2, an open-source software package to manually analyze in toto timelapse imaging data. First, three or four cells were manually tracked for the entire length of the movie. These tracks were then used to register the data between timepoints, thus removing global translation and rotation of the embryo. Then, using this registered dataset, we assembled a set of tracks. We started each track at its division (evident by its spherical morphology), and tracked both forwards and backwards in time. We restricted our tracks to cells roughly within the central one-third of the neural tube along DV, and rejected cells that could not be tracked for long periods (e.g. those that moved out of the field of view too quickly or had poor membrane signal). GFP intensity (from neurod:eGFP) was used to identify neurons. To positively identify a neuron, we required that the entire cell was GFP positive in each of the xy, xz and yz image planes, to avoid the potential of GFP scatter from neighboring cells giving false positives. The first time at which a cell was identified as a neuron was recorded. In some cases, GFP was excited intermittently throughout the timelapse to reduce bleaching (e.g. every hour, instead of every 3 min). In this case, the time recorded was chosen to be midway between the time intervals (e.g. if a cell was negative at 3 h, but positive at 4 h, we record 3.5 h). Cells that did not turn on GFP were tracked either until they divided or were no longer trackable; the total track time was recorded. GFP-on times (‘events’) were combined with the total track time to generate Kaplan–Meier plots (MATLAB). For lists of cell tracks, see Tables S2-S4.
Manual image analysis
Distances are measured within GoFigure2 using a 3D distance tool. Cell (or nuclei) centroid positions are manually identified and recorded by the placement of a cell mesh, and its distance to the apical surface is measured again using the 3D distance tool. Apical area was measured as the product of the major and minor axes of the apical endfoot projected on the DV-AP plane. Division orientation was measured using an angle tool on GoFigure2.
Notch activity was measured by GFP intensity from the nort:dGFP reporter – GFP intensity was measured within a 3 µm radius spherical mesh, the center of which was placed 12 µm away from the apical surface in line with the arrested mitotic cell (BFP positive). For controls, two random numbers were chosen (MATLAB) to generate positions along the DV and AP axes, and a 3 µm mesh was placed 12 µm away from this point.
Automated image analysis: high-quality single-timepoint images
Raw Zeiss .lsm files were first converted to .mha files. Segmentation was then performed on the membrane channel, using the ACME algorithm (Mosaliganti et al., 2012). A mask was created in GoFigure2 to correctly identify meshes that fell within the neural tube, and excluded skin, notochord and somite cells. Cell position, volume, shape and median GFP intensity were extracted from the cell meshes and analyzed in MATLAB. Progenitor density was calculated by binning cells along the DV and AP axes into 14 µm bins and counting cell number within each bin. Segmentation and neuron classifications were visually inspected on ITKsnap and, where necessary, manually corrected. All image analysis was performed using custom C++ scripts.
DNA constructs
The hsp:mTagBFP2-dnPLK1 construct was generated by fusing the coding sequence for mTagBFP2 to a dominant-negative human polo-kinase 1 [a gift from Caren Norden (Strzyz et al., 2015), MPI-CBG, Dresden, Germany], using a flexible GA linker and inserting into a vector containing the hsp70 promoter (Xiong et al., 2015). The hsp:mTagBFP2-dnPLK1-2A-p50 was similarly made, but with two extra pieces: the P2A sequence (Addgene, #52421) and the p50 sequence (amplified from zebrafish cDNA). The hsp:h2b-mCherry2 construct was assembled by inserting h2b-mCherry2 cDNA into the hsp70 vector. The hsp:dnMAML1-P2A-h2b-mCherry2 had two extra pieces: a truncated dnMAML1 (as per Wu et al., 2000) plus the P2A sequence as above. All the above constructs were cloned by PCR amplifying pieces with 20-30 bp overlap regions, and then combined using Gibson isothermal assembly.
RT-PCR was performed to generate TOPO (Life Technologies) plasmids of the full-length cDNA sequence for zebrafish nort. The TOPO (Life Technologies) nort plasmid was used to generate an in situ primer. A list of primers is provided in Table S1.
Fluorescent in situ hybridization
Dechorionated embryos were fixed in fresh, ice-cold 4% paraformaldehyde/PBS overnight at 4°C. After fixation, embryos were washed twice in ice-cold PBS and then four times in ice-cold 100% methanol and stored in methanol (15-20 embryos per tube) at −20°C. After methanol fixation, embryos were re-hydrated in a dilution series of methanol:1×PBS/1%Tween-20 (3:1,1:1,1:3) and then standard in situ methodology (Thisse et al., 2004) was followed and Fast Red tablets (F4648 Sigma) were used to visualize nort mRNA.
Whole-mount zebrafish larvae immunofluorescence
Embryos were fixed in 4% paraformaldehyde in 1×PBS (pH 7.4) overnight at 4°C and after fixation rinsed three times for 5 min in 1×PBS. Prior to immunostaining, embryos were blocked for 60 min at room temperature in 2% normal goat serum/1%Triton X-100/1% Tween-20/1×PBS (pH 7.4) (blocking buffer). After block, embryos were incubated in diluted anti-Myc primary antibody (clone9E10, Thermo Fisher) at 1:200 dilution in blocking buffer overnight at room temperature. Embryos were then rinsed in 1% Tween-20/1×PBS (pH 7.4) and then washed three times for 60 min in 1% Tween-20/1×PBS (pH 7.4). Then embryos were incubated in Alexa-567 secondary antibodies (Invitrogen) at 1:800 in blocking buffer overnight at 4°C. Following secondary treatment, embryos were washed four times for 30 min in 1% Tween-20/1×PBS.
Microinjections of DNA and mRNA
Plasmid DNA (hsp:mTagBFP2-dnPLK1, hsp:mTagBFP2-dnPLK1-P2A-p50) was injected at the single-cell stage, delivering 2.3 nl (Nanoject) at a concentration of 10 ng/µl combined with 25 ng/µl transposase mRNA. mRNA (mem-citrine-citrine and centrin-tdTomato, a gift from Caren Norden) was synthesized using the mMESSAGE mMACHINE kits (Ambion), and injected either at the one-cell stage, at the 16- to 128-cell stage for mosaic labeling, at a concentration of 20 ng/µl. Prior to each experiment, embryos were screened for health. 10 ng/µl 5xUAS-E1b:6xMYC-notch1a (4.6 nl) (Scheer and Campos-Ortega, 1999) plasmid DNA was injected into one- to four-cell stage embryos.
Morpholinos (MO)
Morpholinos used were: tp53 MO, 5′-GCGCCATTGCTTTGCAAGAATTG-3′ (Robu et al., 2007, 9.2 nl of a 50 μM solution injected); rbpj ATG MO, 5′-CAAACTTCCCTGTCACAACAGGCGC-3′ (Ohata et al., 2011, 9.2 nl of a 50 μM MO solution injected).
Heat-shock treatment
Embryos were placed in a 1.5 ml Eppendorf tube containing (pre-warmed) egg water, at 37°C, for 45 min, between 19 and 20 hpf. Embryos were then removed and placed in fresh (22-28°C) water, and returned to the incubator.
Statistical tests
Statistical analysis of pairwise comparisons was mainly performed using a two-tailed t-test (ttest2 in MATLAB). Several variables had a highly skewed distribution [(1) distance of cell to apical surface; and (2) nort:dGFP expression] so in these cases we used a Mann–Whitney test (ranksum in MATLAB). Kaplan–Meier curves were analyzed using the log rank test (logrank in MATLAB) (Rich et al., 2010). For Fig. 2E,F, we fit the Kaplan–Meier curves to a parametric form ρ(t)=1−exp[R(tr−t)] for the first 6 h after division. R can be related to the division probability in Fig. 6, provided one knows when cells stop differentiating (e.g. when they enter S phase). The fitting was implemented as a linear fit of ln(1 -ρ(t)) in time. For the plot of R as a function of d, R(d) corresponds to the differentiation rate for all cells whose nuclear distance exceeds the value d.
Mathematical model
A stochastic simulation was implemented in MATLAB. Each progenitor is modeled independently and after birth is assumed to divide again with a cell cycle time taken from the distribution in Fig. 6C [a generalized extreme value distribution (Bogdan et al., 2014)]. Upon dividing, each daughter cell differentiates independently with probability f(t). In our simulations, f(t) increases linearly from zero to one over the course of 12 cell cycles. For Fig. 6D,E, we repeat each simulation 3000 times and plot the mean±s.d. as shown. For feedback, we modify f(t)→f(t)+kP−δ, where k is a constant controlling the strength of feedback, and δ is a constant that is manually tuned such that the mean dynamics are similar to the case without feedback.
Acknowledgements
We thank members of the Megason Lab, Connie Cepko, Cliff Tabin and Allon Klein for helpful discussions and feedback.
Footnotes
Competing interests
The authors declare no competing or financial interests.
Author contributions
Conceptualization: T.W.H., S.G.M.; Software: T.W.H., K.R.M.; Formal analysis: T.W.H.; Investigation: T.W.H.; Resources: J.B.M., B.A.L.; Writing - original draft: T.W.H.; Writing - review & editing: T.W.H., B.A.L., S.G.M.; Supervision: S.G.M.; Project administration: B.A.L., S.G.M.; Funding acquisition: B.A.L., S.G.M.
Funding
This work was supported by the National Institutes of Health [R01GM107733 to S.G.M.]. Deposited in PMC for release after 12 months.
Data availability
The original data for the images in Fig. 3C are saved as a 10 GB 4-dimensional (xyzt) image set in the Zeiss LSM format and can be downloaded from Dryad (doi:10.5061/dryad.212777q).
Supplementary information
Supplementary information available online at http://dev.biologists.org/lookup/doi/10.1242/dev.157040.supplemental
References
- Aggarwal V., Dickinson R. B. and Lele T. P. (2016). Concentration sensing by the moving nucleus in cell fate determination: a computational analysis. PLoS ONE 11, e0149213 10.1371/journal.pone.0149213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexandre P., Reugels A. M., Barker D., Blanc E. and Clarke J. D. W. (2010). Neurons derive from the more apical daughter in asymmetric divisions in the zebrafish neural tube. Nat. Neurosci. 13, 673-679. 10.1038/nn.2547 [DOI] [PubMed] [Google Scholar]
- Alexiades M. R. and Cepko C. (1996). Quantitative analysis of proliferation and cell cycle length during development of the rat retina. Dev. Dyn. 205, 293-307. [DOI] [PubMed] [Google Scholar]
- Appel B., Givan L. A. and Eisen J. S. (2001). Delta-Notch signaling and lateral inhibition in zebrafish spinal cord development. BMC Dev. Biol. 1, 13 10.1186/1471-213X-1-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aragona M., Panciera T., Manfrin A., Giulitti S., Michielin F., Elvassore N., Dupont S. and Piccolo S. (2013). A mechanical checkpoint controls multicellular growth through YAP/TAZ regulation by actin-processing factors. Cell 154, 1047-1059. 10.1016/j.cell.2013.07.042 [DOI] [PubMed] [Google Scholar]
- Arulmoli J., Pathak M. M., McDonnell L. P., Nourse J. L., Tombola F., Earthman J. C. and Flanagan L. A. (2015). Static stretch affects neural stem cell differentiation in an extracellular matrix-dependent manner. Sci. Rep. 5, 8499 10.1038/srep08499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baye L. M. and Link B. A. (2007). Interkinetic nuclear migration and the selection of neurogenic cell divisions during vertebrate retinogenesis. J. Neurosci. 27, 10143-10152. 10.1523/JNEUROSCI.2754-07.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benham-Pyle B. W., Pruitt B. L. and Nelson W. J. (2015). Cell adhesion. Mechanical strain induces E-cadherin-dependent Yap1 and beta-catenin activation to drive cell cycle entry. Science 348, 1024-1027. 10.1126/science.aaa4559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bogdan P., Deasy B. M., Gharaibeh B., Roehrs T. and Marculescu R. (2014). Heterogeneous structure of stem cells dynamics: statistical models and quantitative predictions. Sci. Rep. 4, 4826 10.1038/srep04826 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bort R., Signore M., Tremblay K., Barbera J. P. M. and Zaret K. S. (2006). Hex homeobox gene controls the transition of the endoderm to a pseudostratified, cell emergent epithelium for liver bud development. Dev. Biol. 290, 44-56. 10.1016/j.ydbio.2005.11.006 [DOI] [PubMed] [Google Scholar]
- Bray S. J. (2016). Notch signalling in context. Nat. Rev. Mol. Cell Biol. 17, 722-735. 10.1038/nrm.2016.94 [DOI] [PubMed] [Google Scholar]
- Brunet T., Bouclet A., Ahmadi P., Mitrossilis D., Driquez B., Brunet A.-C., Henry L., Serman F., Béalle G., Ménager C. et al. (2013). Evolutionary conservation of early mesoderm specification by mechanotransduction in Bilateria. Nat. Commun. 4, 2821 10.1038/ncomms3821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burkhardt J. K., Echeverri C. J., Nilsson T. and Vallee R. B. (1997). Overexpression of the dynamitin (p50) subunit of the dynactin complex disrupts dynein-dependent maintenance of membrane organelle distribution. J. Cell Biol. 139, 469-484. 10.1083/jcb.139.2.469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark B. S., Cui S., Miesfeld J. B., Klezovitch O., Vasioukhin V. and Link B. A. (2012). Loss of Llgl1 in retinal neuroepithelia reveals links between apical domain size, Notch activity and neurogenesis. Development 139, 1599-1610. 10.1242/dev.078097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Copp A. J., Stanier P. and Greene N. D. E. (2013). Neural tube defects: recent advances, unsolved questions, and controversies. Lancet Neurol. 12, 799-810. 10.1016/S1474-4422(13)70110-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coste B., Xiao B., Santos J. S., Syeda R., Grandl J., Spencer K. S., Kim S. E., Schmidt M., Mathur J., Dubin A. E. et al. (2012). Piezo proteins are pore-forming subunits of mechanically activated channels. Nature 483, 176-181. 10.1038/nature10812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das R. M. and Storey K. G. (2012). Mitotic spindle orientation can direct cell fate and bias Notch activity in chick neural tube. EMBO Rep. 13, 448-454. 10.1038/embor.2012.42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Bene F., Wehman A. M., Link B. A. and Baier H. (2008). Regulation of neurogenesis by interkinetic nuclear migration through an apical-basal notch gradient. Cell 134, 1055-1065. 10.1016/j.cell.2008.07.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dessaud E., Yang L. L., Hill K., Cox B., Ulloa F., Ribeiro A., Mynett A., Novitch B. G. and Briscoe J. (2007). Interpretation of the sonic hedgehog morphogen gradient by a temporal adaptation mechanism. Nature 450, 717-720. 10.1038/nature06347 [DOI] [PubMed] [Google Scholar]
- Dong Z., Yang N., Yeo S.-Y., Chitnis A. and Guo S. (2012). Intralineage directional Notch signaling regulates self-renewal and differentiation of asymmetrically dividing radial glia. Neuron 74, 65-78. 10.1016/j.neuron.2012.01.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dupont S., Morsut L., Aragona M., Enzo E., Giulitti S., Cordenonsi M., Zanconato F., Le Digabel J., Forcato M., Bicciato S. et al. (2011). Role of YAP/TAZ in mechanotransduction. Nature 474, 179-183. 10.1038/nature10137 [DOI] [PubMed] [Google Scholar]
- Engler A. J., Sen S., Sweeney H. L. and Discher D. E. (2006). Matrix elasticity directs stem cell lineage specification. Cell 126, 677-689. 10.1016/j.cell.2006.06.044 [DOI] [PubMed] [Google Scholar]
- Fernández-Sánchez M. E., Barbier S., Whitehead J., Béalle G., Michel A., Latorre-Ossa H., Rey C., Fouassier L., Claperon A., Brullé L. et al. (2015). Mechanical induction of the tumorigenic beta-catenin pathway by tumour growth pressure. Nature 523, 92-95. 10.1038/nature14329 [DOI] [PubMed] [Google Scholar]
- Fish J. L., Dehay C., Kennedy H. and Huttner W. B. (2008). Making bigger brains-the evolution of neural-progenitor-cell division. J. Cell Sci. 121, 2783-2793. 10.1242/jcs.023465 [DOI] [PubMed] [Google Scholar]
- Garcia-Campmany L. and Marti E. (2007). The TGFbeta intracellular effector Smad3 regulates neuronal differentiation and cell fate specification in the developing spinal cord. Development 134, 65-75. 10.1242/dev.02702 [DOI] [PubMed] [Google Scholar]
- Gilbert P. M., Havenstrite K. L., Magnusson K. E. G., Sacco A., Leonardi N. A., Kraft P., Nguyen N. K., Thrun S., Lutolf M. P. and Blau H. M. (2010). Substrate elasticity regulates skeletal muscle stem cell self-renewal in culture. Science 329, 1078-1081. 10.1126/science.1191035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon W. R., Zimmerman B., He L., Miles L. J., Huang J., Tiyanont K., McArthur D. G., Aster J. C., Perrimon N., Loparo J. J. et al. (2015). Mechanical allostery: evidence for a force requirement in the proteolytic activation of Notch. Dev. Cell 33, 729-736. 10.1016/j.devcel.2015.05.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grosse A. S., Pressprich M. F., Curley L. B., Hamilton K. L., Margolis B., Hildebrand J. D. and Gumucio D. L. (2011). Cell dynamics in fetal intestinal epithelium: implications for intestinal growth and morphogenesis. Development 138, 4423-4432. 10.1242/dev.065789 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardwick L. J. A. and Philpott A. (2014). Nervous decision-making: to divide or differentiate. Trends Genet. 30, 254-261. 10.1016/j.tig.2014.04.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardwick L. J. A., Ali F. R., Azzarelli R. and Philpott A. (2015). Cell cycle regulation of proliferation versus differentiation in the central nervous system. Cell Tissue Res. 359, 187-200. 10.1007/s00441-014-1895-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatakeyama J., Wakamatsu Y., Nagafuchi A., Kageyama R., Shigemoto R. and Shimamura K. (2014). Cadherin-based adhesions in the apical endfoot are required for active Notch signaling to control neurogenesis in vertebrates. Development 141, 1671-1682. 10.1242/dev.102988 [DOI] [PubMed] [Google Scholar]
- Hayes J. M., Hartsock A., Clark B. S., Napier H. R. L., Link B. A. and Gross J. M. (2012). Integrin alpha5/fibronectin1 and focal adhesion kinase are required for lens fiber morphogenesis in zebrafish. Mol. Biol. Cell 23, 4725-4738. 10.1091/mbc.E12-09-0672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- He J., Zhang G., Almeida A. D., Cayouette M., Simons B. D. and Harris W. A. (2012). How variable clones build an invariant retina. Neuron 75, 786-798. 10.1016/j.neuron.2012.06.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hindley C. and Philpott A. (2012). Co-ordination of cell cycle and differentiation in the developing nervous system. Biochem. J. 444, 375-382. 10.1042/BJ20112040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang P., Xiong F., Megason S. G. and Schier A. F. (2012). Attenuation of Notch and Hedgehog signaling is required for fate specification in the spinal cord. PLoS Genet. 8, e1002762 10.1371/journal.pgen.1002762 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hudish L. I., Galati D. F., Ravanelli A. M., Pearson C. G., Huang P. and Appel B. (2016). miR-219 regulates neural progenitors by dampening apical Par protein-dependent Hedgehog signaling. Development 143, 2292-2304. 10.1242/dev.137844 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huttner W. B. and Kosodo Y. (2005). Symmetric versus asymmetric cell division during neurogenesis in the developing vertebrate central nervous system. Curr. Opin. Cell Biol. 17, 648-657. 10.1016/j.ceb.2005.10.005 [DOI] [PubMed] [Google Scholar]
- Jinguji Y. and Ishikawa H. (1992). Electron microscopic observations on the maintenance of the tight junction during cell division in the epithelium of the mouse small intestine. Cell Struct. Funct. 17, 27-37. 10.1247/csf.17.27 [DOI] [PubMed] [Google Scholar]
- Kicheva A., Bollenbach T., Ribeiro A., Valle H. P., Lovell-Badge R., Episkopou V. and Briscoe J. (2014). Coordination of progenitor specification and growth in mouse and chick spinal cord. Science 345, 1254927 10.1126/science.1254927 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosodo Y., Suetsugu T., Suda M., Mimori-Kiyosue Y., Toida K., Baba S. A., Kimura A. and Matsuzaki F. (2011). Regulation of interkinetic nuclear migration by cell cycle-coupled active and passive mechanisms in the developing brain. EMBO J. 30, 1690-1704. 10.1038/emboj.2011.81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kressmann S., Campos C., Castanon I., Fürthauer M. and González-Gaitán M. (2015). Directional Notch trafficking in Sara endosomes during asymmetric cell division in the spinal cord. Nat. Cell Biol. 17, 333-339. 10.1038/ncb3119 [DOI] [PubMed] [Google Scholar]
- Kwan K. M., Fujimoto E., Grabher C., Mangum B. D., Hardy M. E., Campbell D. S., Parant J. M., Yost H. J., Kanki J. P. and Chien C.-B. (2007). The Tol2kit: a multisite gateway-based construction kit for Tol2 transposon transgenesis constructs. Dev. Dyn. 236, 3088-3099. 10.1002/dvdy.21343 [DOI] [PubMed] [Google Scholar]
- Lammerding J. (2011). Mechanics of the nucleus. Compr. Physiol. 1, 783-807. 10.1002/cphy.c100038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Latasa M. J., Cisneros E. and Frade J. M. (2009). Cell cycle control of Notch signaling and the functional regionalization of the neuroepithelium during vertebrate neurogenesis. Int. J. Dev. Biol. 53, 895-908. 10.1387/ijdb.082721ml [DOI] [PubMed] [Google Scholar]
- Le Dréau G., Saade M., Gutiérrez-Vallejo I. and Martí E. (2014). The strength of SMAD1/5 activity determines the mode of stem cell division in the developing spinal cord. J. Cell Biol. 204, 591-605. 10.1083/jcb.201307031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J. E. (1997). Basic helix-loop-helix genes in neural development. Curr. Opin. Neurobiol. 7, 13-20. 10.1016/S0959-4388(97)80115-8 [DOI] [PubMed] [Google Scholar]
- Leipzig N. D. and Shoichet M. S. (2009). The effect of substrate stiffness on adult neural stem cell behavior. Biomaterials 30, 6867-6878. 10.1016/j.biomaterials.2009.09.002 [DOI] [PubMed] [Google Scholar]
- Leung L., Klopper A. V., Grill S. W., Harris W. A. and Norden C. (2011). Apical migration of nuclei during G2 is a prerequisite for all nuclear motion in zebrafish neuroepithelia. Development 138, 5003-5013. 10.1242/dev.071522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Megason S. G. (2009). In toto imaging of embryogenesis with confocal time-lapse microscopy. Methods Mol. Biol. 546, 317-332. 10.1007/978-1-60327-977-2_19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Megason S. G. and McMahon A. P. (2002). A mitogen gradient of dorsal midline Wnts organizes growth in the CNS. Development 129, 2087-2098. [DOI] [PubMed] [Google Scholar]
- Míguez D. G. (2015). A branching process to characterize the dynamics of stem cell differentiation. Sci. Rep. 5, 13265 10.1038/srep13265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosaliganti K. R., Noche R. R., Xiong F., Swinburne I. A. and Megason S. G. (2012). ACME: automated cell morphology extractor for comprehensive reconstruction of cell membranes. PLoS Comput. Biol. 8, e1002780 10.1371/journal.pcbi.1002780 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murciano A., Zamora J., Lopez-Sanchez J. and Frade J. M. (2002). Interkinetic nuclear movement may provide spatial clues to the regulation of neurogenesis. Mol. Cell. Neurosci. 21, 285-300. 10.1006/mcne.2002.1174 [DOI] [PubMed] [Google Scholar]
- Noctor S. C., Martínez-Cerdeño V., Ivic L. and Kriegstein A. R. (2004). Cortical neurons arise in symmetric and asymmetric division zones and migrate through specific phases. Nat. Neurosci. 7, 136-144. 10.1038/nn1172 [DOI] [PubMed] [Google Scholar]
- Norden C., Young S., Link B. A. and Harris W. A. (2009). Actomyosin is the main driver of interkinetic nuclear migration in the retina. Cell 138, 1195-1208. 10.1016/j.cell.2009.06.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nowell C. S., Odermatt P. D., Azzolin L., Hohnel S., Wagner E. F., Fantner G. E., Lutolf M. P., Barrandon Y., Piccolo S. and Radtke F. (2016). Chronic inflammation imposes aberrant cell fate in regenerating epithelia through mechanotransduction. Nat. Cell Biol. 18, 168-180. 10.1038/ncb3290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obholzer N., Wolfson S., Trapani J. G., Mo W., Nechiporuk A., Busch-Nentwich E., Seiler C., Sidi S., Sollner C., Duncan R. N. et al. (2008). Vesicular glutamate transporter 3 is required for synaptic transmission in zebrafish hair cells. J. Neurosci. 28, 2110-2118. 10.1523/JNEUROSCI.5230-07.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohata S., Aoki R., Kinoshita S., Yamaguchi M., Tsuruoka-Kinoshita S., Tanaka H., Wada H., Watabe S., Tsuboi T., Masai I. et al. (2011). Dual roles of Notch in regulation of apically restricted mitosis and apicobasal polarity of neuroepithelial cells. Neuron 69, 215-230. 10.1016/j.neuron.2010.12.026 [DOI] [PubMed] [Google Scholar]
- Otani T., Marchetto M. C., Gage F. H., Simons B. D. and Livesey F. J. (2016). 2D and 3D stem cell models of primate cortical development identify species-specific differences in progenitor behavior contributing to brain size. Cell Stem Cell 18, 467-480. 10.1016/j.stem.2016.03.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ou G. and Weaver V. M. (2015). Tumor-induced solid stress activates beta-catenin signaling to drive malignant behavior in normal, tumor-adjacent cells. BioEssays 37, 1293-1297. 10.1002/bies.201500090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan Y., Heemskerk I., Ibar C., Shraiman B. I. and Irvine K. D. (2016). Differential growth triggers mechanical feedback that elevates Hippo signaling. Proc. Natl. Acad. Sci. USA 113, E6974-E6983. 10.1073/pnas.1615012113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paolini A., Duchemin A.-L., Albadri S., Patzel E., Bornhorst D., Gonzalez Avalos P., Lemke S., Machate A., Brand M., Sel S. et al. (2015). Asymmetric inheritance of the apical domain and self-renewal of retinal ganglion cell progenitors depend on Anillin function. Development 142, 832-839. 10.1242/dev.118612 [DOI] [PubMed] [Google Scholar]
- Rich J. T., Neely J. G., Paniello R. C., Voelker C. C. J., Nussenbaum B. and Wang E. W. (2010). A practical guide to understanding Kaplan–Meier curves. Otolaryngol. Head Neck Surg. 143, 331-336. 10.1016/j.otohns.2010.05.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robu M. E., Larson J. D., Nasevicius A., Beiraghi S., Brenner C., Farber S. A. and Ekker S. C. (2007). p53 activation by knockdown technologies. PLoS Genet. 3, e78 10.1371/journal.pgen.0030078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saade M., Gutierrez-Vallejo I., Le Dréau G., Rabadán M. A., Miguez D. G., Buceta J. and Martí E. (2013). Sonic hedgehog signaling switches the mode of division in the developing nervous system. Cell Rep. 4, 492-503. 10.1016/j.celrep.2013.06.038 [DOI] [PubMed] [Google Scholar]
- Sato T., Takahoko M. and Okamoto H. (2006). HuC:Kaede, a useful tool to label neural morphologies in networks in vivo. Genesis 44, 136-142. 10.1002/gene.20196 [DOI] [PubMed] [Google Scholar]
- Scheer N. and Campos-Ortega J. A. (1999). Use of the Gal4-UAS technique for targeted gene expression in the zebrafish. Mech. Dev. 80, 153-158. 10.1016/S0925-4773(98)00209-3 [DOI] [PubMed] [Google Scholar]
- Schier A. F., Neuhauss S. C., Harvey M., Malicki J., Solnica-Krezel L., Stainier D. Y., Zwartkruis F., Abdelilah S., Stemple D. L., Rangini Z. et al. (1996). Mutations affecting the development of the embryonic zebrafish brain. Development 123, 165-178. [DOI] [PubMed] [Google Scholar]
- Seidlits S. K., Khaing Z. Z., Petersen R. R., Nickels J. D., Vanscoy J. E., Shear J. B. and Schmidt C. E. (2010). The effects of hyaluronic acid hydrogels with tunable mechanical properties on neural progenitor cell differentiation. Biomaterials 31, 3930-3940. 10.1016/j.biomaterials.2010.01.125 [DOI] [PubMed] [Google Scholar]
- Shaya O., Binshtok U., Hersch M., Rivkin D., Weinreb S., Amir-Zilberstein L., Khamaisi B., Oppenheim O., Desai R. A., Goodyear R. J. et al. (2017). Cell-cell contact area affects notch signaling and Notch-dependent patterning. Dev. Cell 40, 505-511.e506. 10.1016/j.devcel.2017.02.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siller K. H. and Doe C. Q. (2009). Spindle orientation during asymmetric cell division. Nat. Cell Biol. 11, 365-374. 10.1038/ncb0409-365 [DOI] [PubMed] [Google Scholar]
- Smart I. H. (1972). Proliferative characteristics of the ependymal layer during the early development of the mouse diencephalon, as revealed by recording the number, location, and plane of cleavage of mitotic figures. J. Anat. 113, 109-129. [PMC free article] [PubMed] [Google Scholar]
- Smart I. H. (1973). Proliferative characteristics of the ependymal layer during the early development of the mouse neocortex: a pilot study based on recording the number, location and plane of cleavage of mitotic figures. J. Anat. 116, 67-91. [PMC free article] [PubMed] [Google Scholar]
- Spear P. C. and Erickson C. A. (2012). Interkinetic nuclear migration: a mysterious process in search of a function. Dev. Growth Differ. 54, 306-316. 10.1111/j.1440-169X.2012.01342.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Streichan S. J., Hoerner C. R., Schneidt T., Holzer D. and Hufnagel L. (2014). Spatial constraints control cell proliferation in tissues. Proc. Natl. Acad. Sci. USA 111, 5586-5591. 10.1073/pnas.1323016111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strzyz P. J., Lee H. O., Sidhaye J., Weber I. P., Leung L. C. and Norden C. (2015). Interkinetic nuclear migration is centrosome independent and ensures apical cell division to maintain tissue integrity. Dev. Cell 32, 203-219. 10.1016/j.devcel.2014.12.001 [DOI] [PubMed] [Google Scholar]
- Swinburne I. A., Mosaliganti K. R., Green A. A. and Megason S. G. (2015). Improved long-term imaging of embryos with genetically encoded alpha-bungarotoxin. PLoS ONE 10, e0134005 10.1371/journal.pone.0134005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tallinen T., Chung J. Y., Biggins J. S. and Mahadevan L. (2014). Gyrification from constrained cortical expansion. Proc. Natl. Acad. Sci. USA 111, 12667-12672. 10.1073/pnas.1406015111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thisse B., Heyer V., Lux A., Alunni V., Degrave A., Seiliez I., Kirchner J., Parkhill J. P. and Thisse C. (2004). Spatial and temporal expression of the zebrafish genome by large-scale in situ hybridization screening. Methods Cell Biol. 77, 505-519. 10.1016/S0091-679X(04)77027-2 [DOI] [PubMed] [Google Scholar]
- Tsuda S., Kitagawa T., Takashima S., Asakawa S., Shimizu N., Mitani H., Shima A., Tsutsumi M., Hori H., Naruse K. et al. (2010). FAK-mediated extracellular signals are essential for interkinetic nuclear migration and planar divisions in the neuroepithelium. J. Cell Sci. 123, 484-496. 10.1242/jcs.057851 [DOI] [PubMed] [Google Scholar]
- Tsujikawa M., Omori Y., Biyanwila J. and Malicki J. (2007). Mechanism of positioning the cell nucleus in vertebrate photoreceptors. Proc. Natl. Acad. Sci. USA 104, 14819-14824. 10.1073/pnas.0700178104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsutsumi M. and Itoh M. (2007). Novel transcript nort is a downstream target gene of the Notch signaling pathway in zebrafish. Gene Expr. Patterns 7, 227-232. 10.1016/j.modgep.2006.10.002 [DOI] [PubMed] [Google Scholar]
- Wu L., Aster J. C., Blacklow S. C., Lake R., Artavanis-Tsakonas S. and Griffin J. D. (2000). MAML1, a human homologue of Drosophila mastermind, is a transcriptional co-activator for NOTCH receptors. Nat. Genet. 26, 484-489. 10.1038/82644 [DOI] [PubMed] [Google Scholar]
- Xiong F., Tentner A. R., Huang P., Gelas A., Mosaliganti K. R., Souhait L., Rannou N., Swinburne I. A., Obholzer N. D., Cowgill P. D. et al. (2013). Specified neural progenitors sort to form sharp domains after noisy Shh signaling. Cell 153, 550-561. 10.1016/j.cell.2013.03.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong F., Ma W., Hiscock T. W., Mosaliganti K. R., Tentner A. R., Brakke K. A., Rannou N., Gelas A., Souhait L., Swinburne I. A. et al. (2014). Interplay of cell shape and division orientation promotes robust morphogenesis of developing epithelia. Cell 159, 415-427. 10.1016/j.cell.2014.09.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong F., Obholzer N. D., Noche R. R. and Megason S. G. (2015). Multibow: digital spectral barcodes for cell tracing. PLoS ONE 10, e0127822 10.1371/journal.pone.0127822 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeo S.-Y. and Chitnis A. B. (2007). Jagged-mediated Notch signaling maintains proliferating neural progenitors and regulates cell diversity in the ventral spinal cord. Proc. Natl. Acad. Sci. USA 104, 5913-5918. 10.1073/pnas.0607062104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- You H., Sim K. B., Wang K. C., Kim D. G. and Kim H. J. (1994). Morphological study of surgically induced open neural tube defects in chick embryos--postoperative 24 hours. J. Korean Med. Sci. 9, 116-122. 10.3346/jkms.1994.9.2.116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zechner D., Fujita Y., Hülsken J., Müller T., Walther I., Taketo M. M., Crenshaw E. B. III, Birchmeier W. and Birchmeier C. (2003). beta-Catenin signals regulate cell growth and the balance between progenitor cell expansion and differentiation in the nervous system. Dev. Biol. 258, 406-418. 10.1016/S0012-1606(03)00123-4 [DOI] [PubMed] [Google Scholar]