ABSTRACT
The enteric nervous system (ENS) arises from neural crest cells that migrate, proliferate, and differentiate into enteric neurons and glia within the intestinal wall. Many extracellular matrix (ECM) components are present in the embryonic gut, but their role in regulating ENS development is largely unknown. Here, we identify heparan sulfate proteoglycan proteins, including collagen XVIII (Col18) and agrin, as important regulators of enteric neural crest-derived cell (ENCDC) development. In developing avian hindgut, Col18 is expressed at the ENCDC wavefront, while agrin expression occurs later. Both proteins are normally present around enteric ganglia, but are absent in aganglionic gut. Using chick-mouse intestinal chimeras and enteric neurospheres, we show that vagal- and sacral-derived ENCDCs from both species secrete Col18 and agrin. Whereas glia express Col18 and agrin, enteric neurons only express the latter. Functional studies demonstrate that Col18 is permissive whereas agrin is strongly inhibitory to ENCDC migration, consistent with the timing of their expression during ENS development. We conclude that ENCDCs govern their own migration by actively remodeling their microenvironment through secretion of ECM proteins.
KEY WORDS: Enteric nervous system, Extracellular matrix, Neural crest cells, Collagen 18, Agrin, Hirschsprung disease, Chicken, Mouse
Summary: ECM heparan sulfate proteoglycans are cell-autonomous regulators of ENS development in mouse and chick, with Col18 expression at the wavefront being permissive and agrin expression post-colonization being inhibitory to ENCDC migration.
INTRODUCTION
The enteric nervous system (ENS) is a network of neurons and glia in the wall of the gastrointestinal tract that controls intestinal functions including motility, secretion, absorption, sensation and immunity (Furness, 2012). The ENS is frequently called the ‘second brain’ because of its autonomous function, extensive neurotransmitter diversity and complex cytoarchitecture. During embryogenesis, ENS formation begins with colonization of the foregut by a multipotent stem cell population that arises from the neural crest and migrates rostrocaudally through the gut mesenchyme (Nagy and Goldstein, 2017). This long journey requires the coordinated regulation of enteric neural crest-derived cell (ENCDC) migration and proliferation, differentiation into multiple subtypes of enteric neurons and glia, aggregation into ganglia, and circuit formation to form an interconnected functioning network. These processes rely on reciprocal interactions between ENCDCs and their microenvironment, including extracellular matrix (ECM) and soluble factors, that are essential for proper cell survival, migration, proliferation, differentiation and patterning. Perturbations of these processes can lead to neurointestinal diseases, including Hirschsprung disease (HSCR), a congenital neurocristopathy characterized by the absence of ganglia (aganglionosis) from variable lengths of distal bowel due to failure of ENCDCs to complete their migration (Goldstein et al., 2016).
Several mouse models of HSCR are associated with disruption of intestinal ECM expression. Mice deficient in endothelin 3 (Edn3−/−) or endothelin receptor B (Ednrb−/−) have increased expression of laminin, collagen IV (Col4), perlecan (also known as Hspg2), and other proteoglycans in the aganglionic segment (Payette et al., 1988; Tennyson et al., 1990). ENCDC migration relies on their expression of β1-integrin, an important receptor for ECM adhesion (Nagy et al., 2009). Homozygous deletion of β1-integrin from ENCDCs arrests their migration in the proximal hindgut, leading to distal aganglionosis (Breau et al., 2009; Gazquez et al., 2016). In addition, in both avian and mouse embryos, sonic hedgehog secretion from the gut epithelium promotes mesenchymal expression of chondroitin sulfate proteoglycans (CSPGs), including versican and Col9, which inhibit ENS migration and patterning (Nagy et al., 2016).
While the intestinal ECM regulates ENS development, ENCDCs in turn affect ECM composition. For example, ENCDCs produce tenascin-C (TNC), an ECM protein that promotes ENCDC migration during ENS development (Akbareian et al., 2013; Nagy and Goldstein, 2006a; Newgreen et al., 1980). A recent insertional mutagenesis study generated mice with ENCDC-specific upregulation of Col6α4, which inhibited the pro-migratory effect of fibronectin and significantly reduced the speed of ENCDC migration (Soret et al., 2015). ENCDCs also secrete matrix metalloproteases (MMPs) that remodel the gut ECM (Chevalier et al., 2016). These observations suggest that ENCDCs actively shape their local ECM environment in a cell-autonomous way and that these environmental changes in turn regulate ENCDC development.
In this study, we extend these observations by demonstrating an important role for members of the heparan sulfate proteoglycan (HSPG) family during ENS development. HSPGs are glycoproteins with attached polysaccharide heparan sulfate chains. HSPGs can be membrane-bound (syndecans and glypicans) or secreted into the ECM (agrin, Col18, perlecan) (Poulain and Yost, 2015). They are bound by many different ligands and regulate a wide variety of activities (Sarrazin et al., 2011). With respect to neural crest cell development, HSPGs are pro-migratory, both in vitro (Perris et al., 1989) and in vivo (Banerjee et al., 2013), but a role in ENS development has not been previously described. Abundant Col18 expression has been observed in the peripheral nervous system, where neural crest-derived Schwann cells are the principal source of this HSPG (Halfter et al., 1998). Interestingly, studies using cultured mouse embryonic hindgut have shown that heparan sulfate molecules are required for GDNF-Ret signaling (Davies et al., 2003), supporting a link between HSPGs and ENCDC migration. We find that Col18 and agrin are important cell-autonomous regulators of ENCDC development. Using intestinal chimeras, we show that both proteins are produced by ENCDCs. Whereas Col18 is expressed by ENCDCs at the migratory wavefront and is permissive to ENS migration, agrin is expressed later in ENS development and is inhibitory. This embryonic mechanism allows the ENCDC to remodel its niche and thereby establish local spatiotemporal control of its development. Understanding and recapitulating this developmental strategy will allow us to understand congenital neurointestinal diseases and to reprogram enteric neuronal stem cells for successful regenerative therapy.
RESULTS
HSPG proteins surround the enteric ganglia in embryonic and postnatal gut
ECM protein expression was characterized before (E5), during (E8) and after (E14) ENS colonization of the chicken hindgut (Nagy et al., 2012) to identify ECM patterns that might suggest a role in ENS development. As shown in Fig. S1, at E5, prior to ENCDC arrival in the hindgut, fibronectin and laminin are diffusely expressed throughout the mesenchyme, as are TNC and fibrillin. Col1, Col3 and Col4 are widely expressed, although Col1 is concentrated in the outer mesenchyme and Col3 is strongest in the inner mesenchyme. Col6, CS-56 signal (a pan-CSPG antibody), Col9 and versican are all concentrated in the inner mesenchyme, whereas HSPGs are expressed strongly in the outer gut wall. By E8 and E14, after ENS colonization of the hindgut is complete, ECM expression has changed markedly (Figs S2 and S3). TNC is now localized in the muscularis propria and around the enteric ganglia. Interestingly, HSPGs are strongly co-expressed around the submucosal and myenteric ganglia at E8 (Fig. S2L), and this is even more pronounced at E14 (Fig. S3L), which led us to hypothesize a potential role for HSPGs during ENS development.
To examine further the relationship of HSPGs and ENCDCs, sections of E14 chick hindgut were stained with antibodies specific for the enteric neuronal marker Hu (Fig. 1A) and for the ECM proteins Col18 (Fig. 1B), agrin (Fig. 1C) and perlecan (Fig. 1D). Col18 is specifically expressed around the ganglia. Agrin and perlecan are expressed more diffusely throughout the mesenchyme, but agrin also shows concentrated periganglionic immunoreactivity (Fig. 1C, inset). The ganglionic expression of Col18 (Fig. 1E) and agrin (Fig. 1F) persists postnatally in 2-day-old chick hindgut as well as in 4-week-old mouse colon (Fig. 1G-J). Colonic sections from Wnt1;tdT mice were stained with antibody specific for full-length Col18 (Fig. 1G,H), the cleavage product endostatin, which represents the C-terminus of Col18 (Fig. 1I), and agrin (Fig. 1J), demonstrating strong expression of these two HSPG proteins surrounding the mouse enteric ganglia.
Fig. 1.
HSPG proteins are expressed in developing and mature avian and mouse hindgut. Transverse sections of E14 chick colon stained for Hu (A), Col18 (B), agrin (C), and perlecan (D). Insets are magnified views of a submucosal ganglion. Col18 (E) and agrin (F) are similarly expressed around and within HNK1+ ganglia in 2-day-old postnatal chick colon. Immunofluorescence staining of 4-week-old Wnt1;tdT mouse colon shows Col18 (G,H), endostatin (I) and agrin (J) expressed around Wnt1+ ENCDCs. mp, myenteric plexus; NoR, nerve of Remak; smp, submucosal plexus. DAPI, blue.
Col18 is expressed at the migratory wavefront during ENS development, whereas agrin expression occurs later
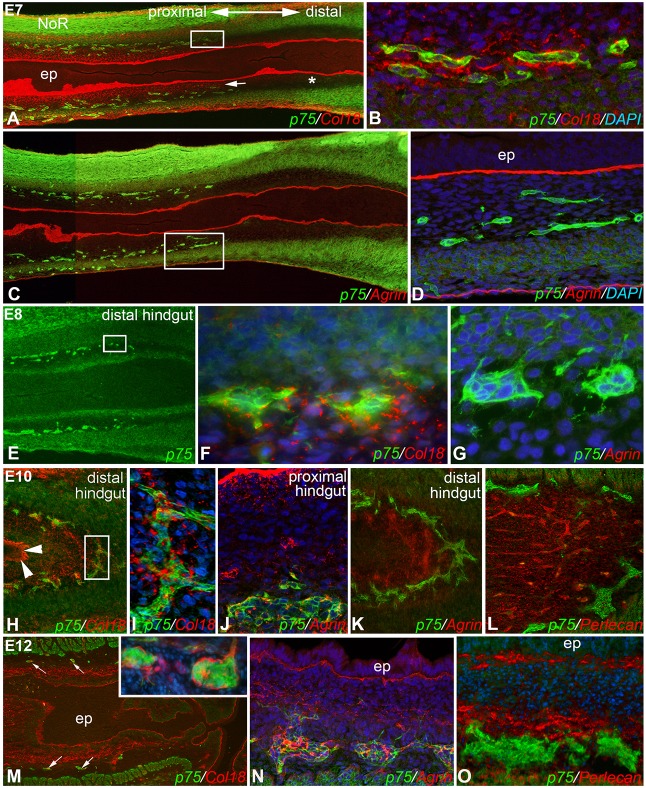
The spatiotemporal relationship between HSPG expression and ENS development was studied by immunofluorescence in longitudinal sections of E7-E12 chick hindgut. At E7, p75+ ENCDCs at the migratory wavefront are present in the mid-hindgut (Fig. 2A,C) and surrounded by Col18 (Fig. 2B). Interestingly, Col18 is also expressed in the subepithelial mesenchyme in the ganglionated portion of the gut (Fig. 2A, arrow), but is notably absent in the preganglionic distal segment (Fig. 2A, asterisk). Although agrin is expressed by the epithelial basement membrane in E7 hindgut, it is not expressed by the enteric ganglia (Fig. 2D). At E8, the wavefront has moved to the distal hindgut (Fig. 2E) and the leading edge of ENCDCs continue to express Col18 (Fig. 2F) and not agrin (Fig. 2G). At E10, ENCDCs in the distal hindgut still express Col18 (Fig. 2H,I). At this stage, agrin is now starting to be expressed by ENCDCs in the proximal (Fig. 2J), but not distal (Fig. 2K), hindgut. By E12, when ENS colonization is complete and gangliogenesis is underway in the distal hindgut (Fig. 2M, inset), both Col18 and agrin are expressed by the enteric ganglia throughout the hindgut (Fig. 2M,N). By contrast, perlecan remains notably absent from the ganglia at all embryonic stages examined (Fig. 2L,O).
Fig. 2.
Col18, but not agrin, surrounds ENCDCs at the migratory wavefront during ENS development. In E7 chick, the migratory ENS wavefront is in the mid-hindgut (composite images in A,C; boxed areas magnified in B,D, respectively). The p75+ ENCDCs at the wavefront express Col18 (B), but not agrin (D). This is also observed at E8, when the wavefront is in the distal hindgut (E, boxed area magnified in F and nearby area magnified in G.). Col18 is also expressed in the subepithelial mesenchyme in the ganglionated portion of the gut (A, arrow), but is absent in the preganglionic distal segment (A, asterisk). At E10, Col18 remains expressed around distal hindgut ENCDCs (H, arrowheads mark the Col18+ epithelial basement membrane; boxed area magnified in I). At E10, agrin is now expressed in the proximal hindgut ganglia (J), but not in the distal hindgut (K); perlecan is also absent here (L). At E12, enteric ganglia express both Col18 (M, arrows mark submucosal ganglia, magnified in inset) and agrin (N), but not perlecan (O). ep, epithelium; NoR, nerve of Remak.
ENCDCs are required for normal expression of Col18 and agrin by the gut mesenchyme
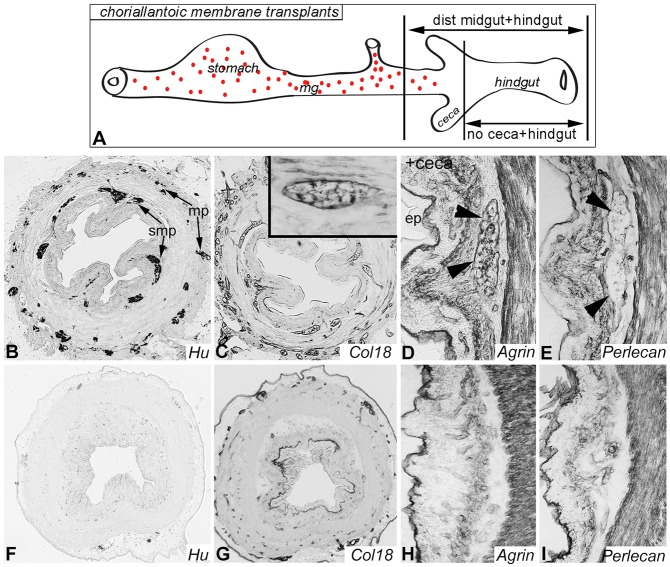
Given the proximity of Col18 and agrin to ENCDCs, we examined whether ENCDCs are required for their expression using experimentally and genetically aganglionic models. First, aganglionic chick hindgut was generated by explanting E5 hindgut without ceca or cloaca, and culturing it on the chorioallantoic membrane (CAM) of a host chick embryo for 9 days. The nerve of Remak was also excluded to prevent sacral neural crest-derived ENCDCs from entering the distal end of the gut. These hindguts remain aganglionic, whereas control explants that include the ENS-containing distal midgut and ceca become populated by a normal ENS (Fig. 3A). Normally ganglionated control explants reveal Hu+ enteric neurons (Fig. 3B) and normal HSPG expression (Fig. 3C-E). As expected, aganglionic explants have no Hu immunoreactivity (Fig. 3F). Importantly, expression of Col18 and agrin is markedly altered in these guts. The periganglionic expression of both HSPG proteins is now absent (Fig. 3G,H). Col18 continues to be expressed by the epithelial basement membrane and large blood vessels, and agrin is still expressed by the basement membranes of smooth muscle, epithelium and vessels. Perlecan expression, on the other hand, is unchanged (Fig. 3I).
Fig. 3.
ENCDCs are required for Col18 and agrin expression around the ENS. E5 chick hindgut was explanted with or without ceca onto the CAM of a host chick to generate ganglionic or aganglionic colon, respectively (A). After 9 days, Hu (enteric neurons; B), Col18 (C; inset shows a Col18-expressing ganglion), agrin (D) and perlecan (E) are expressed in the normally ganglionated colon. Arrowheads (D,E) indicate a myenteric ganglion. By contrast, aganglionic colon shows absence of enteric neurons (F), abnormal Col18 (G) and agrin (H), and preserved perlecan (I) expression. ep, epithelium; mg, midgut; mp, myenteric plexus; smp, submucosal plexus.
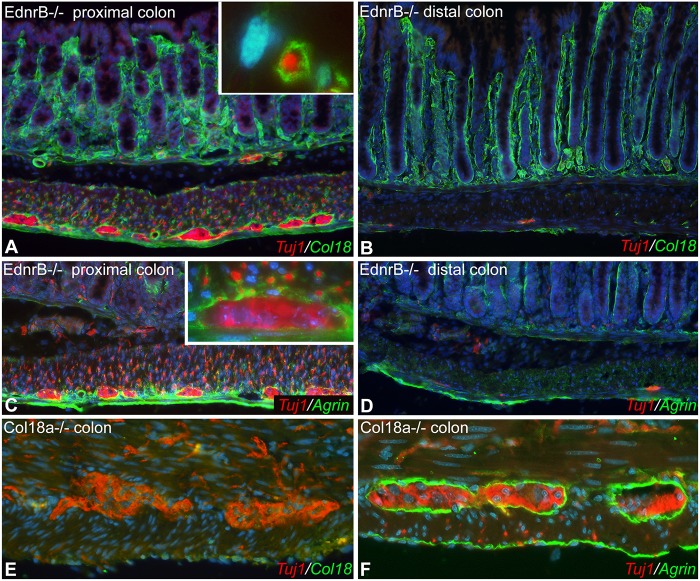
Genetically aganglionic colon was obtained from 3-week-old Ednrb−/− mice. The proximal, ganglionated colon of Ednrb−/− mice shows strong Col18 expression around enteric ganglia and the interconnecting fibers (Fig. 4A). By contrast, the aganglionic distal colon lacks Col18 expression in the mesenchyme (Fig. 4B). Periganglionic expression of agrin is also absent in the aganglionic segment (Fig. 4C,D). These results suggest that ENCDCs are indeed required for the periganglionic expression of Col18 and agrin. To test whether these HSPGs are, in turn, required for ENS development, we examined the colon of Col18a−/− mice. As expected, no Col18 expression is seen in mutant animals, although the myenteric ganglia appear morphologically normal (Fig. 4E). Agrin expression is normal in Col18a−/− mouse colon (Fig. 4F).
Fig. 4.
Ednrb−/− colon lacks expression of Col18 and agrin. Proximal, ganglionated colon of 3-week-old Ednrb−/− mouse colon shows Col18 expression surrounding Tuj1+ enteric ganglia and fibers (A, inset is a magnified view of a Tuj1+ fiber in the circular muscle layer). By contrast, the distal, aganglionic colon lacks Tuj1 and Col18 in the muscle layers (B). Agrin is also expressed around the ganglia in the proximal (normally ganglionated) colon, although not around the neuronal fibers (C, myenteric ganglion magnified in inset), while the aganglionic segment lacks agrin in the gut wall (D). Six-week-old Col18a-deficient mice have normal Tuj1 (E) and agrin (F) expression patterns.
Col18 and agrin are produced by ENCDCs
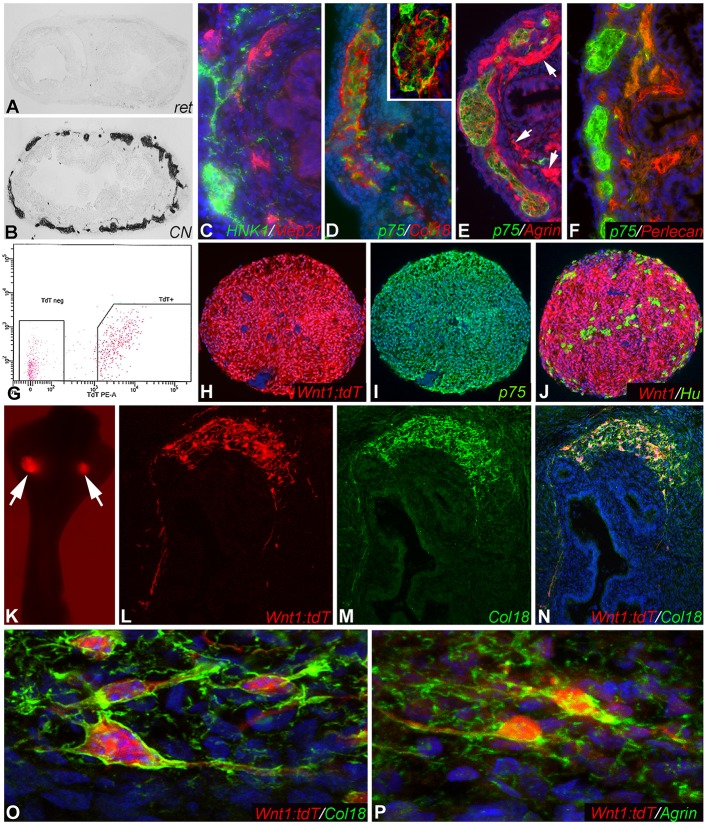
Since ENCDCs are required for Col18 and agrin expression, we examined whether these proteins are produced by the ENCDCs themselves. To answer this question, chick-mouse chimeras were generated. E11.5 mouse hindgut was isolated without the cecum or the cloaca. This preganglionated gut was transplanted into the coelomic cavity of an E3 chick host. As we previously described (Nagy and Goldstein, 2006b), the graft becomes colonized by host-derived neural crest cells and vascularized by host endothelial cells. The absence of mouse-specific Ret immunoreactivity (Fig. 5A), and the presence of immunoreactivity to the chick-specific neurite antibody CN (Fig. 5B), confirms the chick origin of the graft's ENS. Immunofluorescence imaging of serial sections of the grafted colon shows expression of HNK1+ and p75+ chick-derived neural crest cells (Fig. 5C-F) and MEP21+ chick-derived endothelial cells (Fig. 5C). Col18 (Fig. 5D), agrin (Fig. 5E) and perlecan (Fig. 5F) are all labeled by chick-specific antibodies, confirming that these ECM proteins are produced by chick-derived cells. Col18 (Halfter et al., 1998) is specifically expressed by chick-derived ENCDCs (Fig. 5D). Agrin (Tsen et al., 1995) is also expressed by the ENCDCs, and by host-derived blood vessels (Fig. 5E, arrows). Perlecan (Hummel et al., 2004) is strongly expressed by the chick-derived blood vessels, but not by the chick-derived ENCDCs (Fig. 5F). These results demonstrate that the ENCDCs themselves produce Col18 and agrin, but not perlecan. Of note, none of the ECM proteins is detected in the epithelial basement membrane of these mouse colon grafts because the antibodies used are all chick specific.
Fig. 5.
Col18 and agrin are produced by ENCDCs. E11.5 preganglionated wild-type mouse hindgut was transplanted into the coelomic cavity of an E3 chick embryo. After 9 days, the tissue does not label with a mouse-specific Ret antibody (A), but does stain for chick-specific neurons (B), confirming the chick origin of the graft's ENS. Serial sections reveal expression of chick-derived neural crest cells (C-F) and MEP21+ endothelial cells (C). In addition, chick-specific antibodies to Col18 (D), agrin (E) and perlecan (F) confirm the avian origin of these proteins in the mouse graft. Inset in D shows a magnified myenteric ganglion. Arrows (E) indicate agrin expression by host-derived blood vessels. To confirm this finding, neurospheres were generated from FACS-sorted 3-week-old Wnt1;tdT mouse intestine (G) and contain Wnt1+, p75+, and Hu+ cells (H-J). The neurospheres were implanted into E5 chick ceca (K, arrows). After 9 days, Wnt1+ cells migrate within the gut and produce mouse-derived Col18 (L-N). These Wnt1+ cells stain with mouse-specific anti-Col18 (O) and anti-agrin (P) antibodies, confirming the ENCDC origin of these ECM proteins.
Since the hindgut ENS is composed of both vagal neural crest-derived and sacral neural crest-derived cells, we asked whether one or both sources produces Col18 and agrin. The chick-mouse chimeras described above (Fig. 5) confirm that vagal crest cells can produce Col18 and agrin, since coelomic grafts are colonized by vagal crest-derived cells. To test the sacral crest-derived cells, we explanted preganglionated E5 chick hindgut without the ceca, but keeping the cloaca intact. This allows sacral crest cells to colonize the graft (Nagy et al., 2007). This E5 gut was grafted onto the CAM of a host chick embryo to generate hindguts containing an ENS composed exclusively of sacral crest-derived ENCDCs. After 9 days on the CAM, the grafts contain scattered Hu+ enteric ganglia that express both Col18 (Fig. S4A) and agrin (Fig. S4B). By contrast, and consistent with our prior report (Akbareian et al., 2013), TNC is not expressed by sacral neural crest-derived cells (Fig. S4C).
To confirm that ENCDCs are the source of Col18 and agrin, we generated neurospheres from 3-week-old Wnt1;tdT mouse intestine by dissociating the gut and sorting for tdT expression (Fig. 5G). Serial sections through the neurospheres demonstrate ubiquitous Wnt1 expression (Fig. 5H), extensive expression of p75 (Ngfr) (Fig. 5I), and scattered Hu+ enteric neurons (Fig. 5J). The neurospheres were transplanted into the cecal region of E5 chick embryos (Fig. 5K) and cultured on the CAM of host E9 embryos. After 9 days, the grafts were harvested and sections stained with antibodies that specifically recognize mouse-derived Col18 and agrin. Wnt1+ cells migrate within the gut wall (Fig. 5L) and co-express mouse-specific Col18 (Fig. 5M,N). High-magnification confocal images confirm that mouse-derived enteric neuronal stem cells (ENSCs) produce both Col18 (Fig. 5O) and agrin (Fig. 5P). Thus, as in the chick embryo, mouse-derived ENCDCs also produce Col18 and agrin.
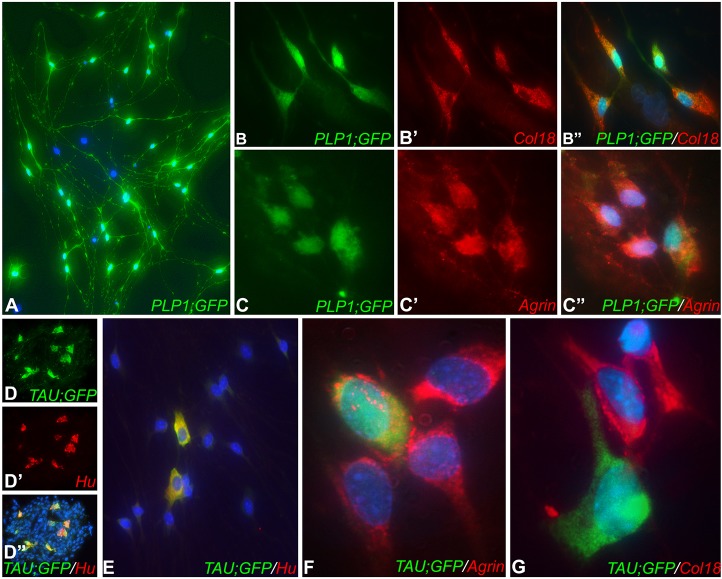
While the experiments above demonstrate that both embryonic and postnatal ENCDCs express Col18 and agrin, we asked which ENS cell type produces these proteins in the postnatal intestine. Neurospheres were spontaneously generated from Plp1GFP and TauGFP/+ intestine to generate ENCDC cultures containing fluorescently labeled glia and neurons, respectively. After 1 week, neurospheres were dissociated and cultured on a fibronectin-coated surface. PLP1+ enteric glial cells co-express both Col18 and agrin (Fig. 6A-C″). By contrast, Tau+ enteric neurons express agrin, but not Col18 (Fig. 6D-G).
Fig. 6.
Enteric glia express both Col18 and agrin. Enteric neurospheres were generated from postnatal Plp1GFP mice, dissociated, and cultured for 4 days (A). Co-expression of Col18 (B-B″) and agrin (C-C″) by enteric glia was seen. Neurospheres were also generated from TauGFP/+ mouse intestine and, after 1 week, Tau+/Hu+ enteric neurons were present (D-D″). Neurospheres were then dissociated and cultured (E). Tau+/Hu+ enteric neurons express agrin (F) but not Col18 (G).
Agrin strongly inhibits ENCDC migration, whereas Col18 is permissive to it
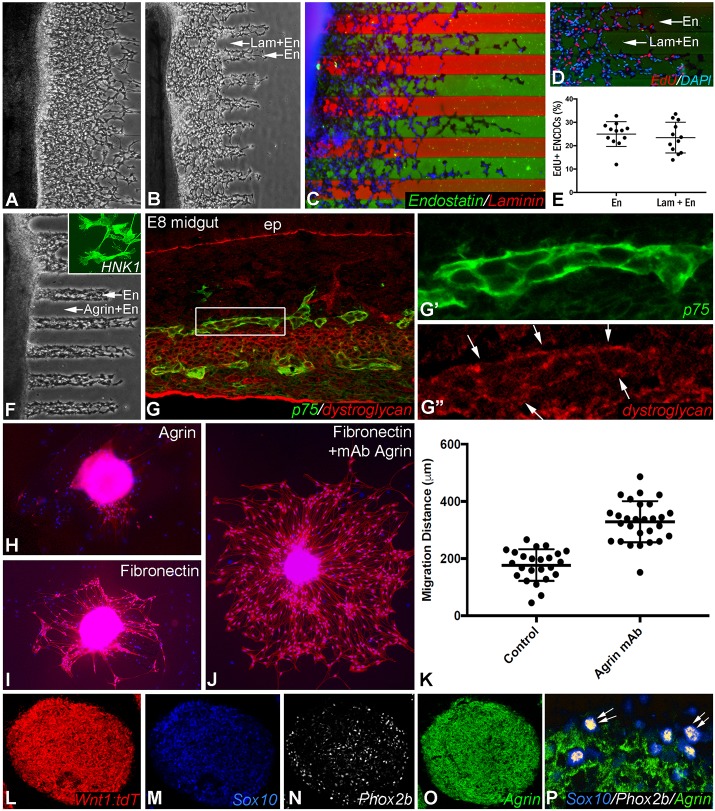
To determine the function of these ECM proteins, their effects on ENCDC migration and proliferation were examined. E7 chick midgut was cultured for 24 h in the presence of 10 ng/ml GDNF on a surface coated with alternating stripes of ECM proteins. For this assay, recombinant endostatin protein was utilized as a surrogate for Col18 since the latter protein is not available in purified form. Endostatin is a cleavage product of Col18 and a normal component of the basement membranes surrounding blood vessels (Rehn et al., 2001; Heljasvaara et al., 2017) and enteric ganglia (Fig. 1J). Similar to Col18, endostatin supports cell survival and stimulates cell migration in an integrin-dependent manner in both endothelial and non-endothelial (including neuronal) cells (Ackley et al., 2001; Kuo et al., 2001; Rehn et al., 2001; Su et al., 2012). We used recombinant endostatin for our in vitro studies as full-length Col18 in not commercially available. ENCDCs migrate equally well in lanes coated with endostatin (50 µg/ml) with or without added fibronectin (20 µg/ml), an ECM protein known to be permissive to ENCDC migration (Fig. 7A). Interestingly, ENCDCs migrate farther in lanes coated with endostatin alone as compared with lanes coated with endostatin and laminin (10 µg/ml) (Fig. 7B,C). Immunostaining with antibodies specific for laminin or endostatin confirms their relative positions in the stripes (Fig. 7C). Note that the green stripes contain endostatin alone, whereas the red stripes contain both endostatin and laminin. The stripe assay was repeated in the presence of EdU (Fig. 7D) to determine the effect of endostatin on ENCDC proliferation, and no significant difference was observed (Fig. 7E).
Fig. 7.
Agrin is inhibitory to ENCDC migration. E7 embryonic chick midgut was cultured for 24 h on a surface coated with alternating stripes of ECM proteins. ENCDCs migrate equally well along stripes containing fibronectin and endostatin as compared with stripes containing endostatin alone (A). ENCDCs migrate further in stripes coated with endostatin alone compared with those coated with both endostatin and laminin (B). Immunostaining with antibodies specific for laminin and endostatin confirms their relative positions in the stripes (C; green, endostatin; red, endostatin plus laminin). EdU incorporation was measured as an indicator of ENCDC proliferation and no difference was detected between the two stripes (D,E). Although ENCDCs migrate well in endostatin-coated lanes, they are unable to migrate in the presence of agrin (F; inset shows migrating HNK1+ ENCDCs). The inhibitory effect of agrin on ENCDCs may be mediated by their expression of dystroglycan (G, boxed area magnified in G′,G″, where arrows indicate expression in the enteric ganglia). The inhibitory role of agrin was confirmed by culturing Wnt1;tdT-derived neurospheres on an agrin-coated surface, which inhibits ENCDC migration (H). By contrast, cells migrate well on a fibronectin surface (I), with a marked increase in migration following addition of an agrin function-blocking antibody (J,K). In Wnt1;tdT neurospheres, agrin is not expressed by ENCDC progenitor cells, as shown by the absence of agrin expression by Sox10+/Phox2b+ double-immunoreactive cells (L-P, arrows). En, endostatin; ep, epithelium; Lam, laminin. Note that central bar and range bars in E and K represent mean and s.d., respectively.
Interestingly, whereas Col18 is permissive to ENCDC migration, agrin strongly inhibits migration. ENCDCs migrate normally in the presence of endostatin alone, but are completely inhibited by the addition of agrin (Fig. 7F). This inhibition suggests that agrin is acting directly on the ENCDCs. We therefore examined whether ENCDCs express the agrin receptors MuSK (Zong and Jin, 2013) and dystroglycan (Winder, 2001). MuSK is expressed in the intestinal smooth muscle, but not in the enteric ganglia (not shown), while dystroglycan is expressed by ENCDCs (Fig. 7G-G″). We confirmed the inhibitory role of agrin by culturing neurospheres derived from Wnt1;tdT mouse gut on an agrin-coated surface. No ENCDC migration was observed from those postnatal neurospheres (Fig. 7H), whereas robust migration occurred on a fibronectin-coated surface (Fig. 7I). The addition of agrin function-blocking antibody to the fibronectin-coated dish markedly increased the distance of ENCDC migration (Fig. 7J). Quantitative comparison of migratory distance in the absence (control) and presence of agrin blockade showed that, in control conditions, ENCDCs migrated 177±55 μm, whereas in the presence of agrin function-blocking antibody they migrated 329±72 μm, representing an 86% increase (Fig. 7K; P<0.0001, Student's t-test). This suggests that inhibition of agrin releases its inhibitory effect and allows ENCDCs in the neurosphere to become highly migratory. This observation, however, raises the question of what cells the agrin blockade is acting on, since we have shown that agrin is absent from the embryonic migratory wavefront (Fig. 2C,D), which contains undifferentiated progenitor cells. We performed immunofluorescence on Wnt1;tdT-derived enteric neurospheres and identified the undifferentiated progenitors based on their co-expression of Sox10 and Phox2b. As expected, these cells do not express agrin (Fig. 7L-P). Agrin inhibition is thus enhancing the migration of a population of agrin-expressing cells in the neurosphere that are not undifferentiated progenitors, but more likely committed precursors that retain migratory potential. The ongoing migratory capacity of committed precursors in the ENS has been described previously (Young et al., 2014).
DISCUSSION
Migration of ENCDCs within the gut relies on reciprocal interactions between the migrating cells and their surrounding ECM (Nagy and Goldstein, 2017). This matrix is a highly complex three-dimensional structure of glycoproteins, collagens and proteoglycans that interact with surface receptors on ENCDCs to regulate many aspects of ENS development, including ENCDC polarity, migration, proliferation, differentiation, ganglion formation, and patterning. The important role of ECM proteins during ENS development has been highlighted by several studies. Loss of β1-integrin, for example, a key ECM receptor expressed on the surface of ENCDCs (Nagy et al., 2009), leads to abnormal migration and aggregation of ENCDCs, resulting in distal intestinal aganglionosis (Breau et al., 2006, 2009). Several ECM proteins have been shown to regulate ENCDC migration, including laminin (Nagy et al., 2009), fibronectin (Akbareian et al., 2013), vitronectin (Breau et al., 2009), TNC (Akbareian et al., 2013; Breau et al., 2009), Col1 (Chevalier et al., 2016; Nagy and Goldstein, 2006a; Young et al., 2001) and Col6 (Soret et al., 2015). The role of each of these proteins in permitting, promoting or inhibiting migration regulates not only the rostrocaudal migration of these cells during gut colonization, but also the radial patterning of the ENS (Nagy et al., 2009; Nagy et al., 2016). Interestingly, recent evidence shows that ENCDCs not only respond to their local environment, but also actively modify it through the production of ECM proteins, such as TNC (Akbareian et al., 2013) and MMPs, including MMP2 (Anderson, 2010). These observations emphasize the need to elucidate how ENCDCs interact with and modify their niche, both to improve our understanding of neurointestinal diseases, such as HSCR, and to enhance the success of cell therapy approaches for the treatment of these diseases.
With the goal of identifying additional ECM-ENCDC interactions that regulate ENS development, we performed a comprehensive immunohistochemical analysis at multiple stages during gut development. We found strong expression of HSPG proteins around the submucosal and myenteric ganglia during ENS formation and consequently interrogated specific members of this ECM family. We show that Col18 is expressed by ENCDCs at the wavefront of migration and, in addition, that its expression in the submucosa is induced as the wavefront proceeds. By contrast, agrin is notably absent from the wavefront and is not expressed by undifferentiated progenitor cells of the ENS. Agrin is produced by ENCDCs only later during development, first appearing in the avian hindgut at E10, ∼2 days after ENS colonization has completed. To explore the functional significance of this expression pattern, we examined the effects of Col18 and agrin on ENCDC migration. We found that agrin strongly inhibits ENCDC migration, presumably through its interaction with its receptor, dystroglycan, which is expressed by enteric ganglia. Col18, on the other hand, is permissive to, although not essential for, migration. We conclude that agrin may prevent maturing ENCDCs from continuing to migrate once they reach their final destination and undergo commitment to a neuronal or glial lineage. These results are interesting in the context of our prior study, in which we showed that ENCDCs secrete TNC, which is expressed at the wavefront, specifically by vagal crest-derived cells, and actively promotes ENCDC migration (Akbareian et al., 2013). Together, these findings highlight the important functional role of the autonomous deposition of ECM proteins by ENCDCs during formation of the ENS and emphasize how the extracellular environment ‘educates’ developing ENCDCs and ensures proper spatiotemporal coordination of ENS development and patterning.
Agrin is a large HSPG that is abundantly expressed in the developing brain and in virtually all basal laminae of the developing organs. Agrin is also expressed by Schwann cell precursors, immature Schwann cells (Lasrado et al., 2017) and developing chicken sensory ganglia (Halfter et al., 1997). It has been shown to inhibit retinal neurite outgrowth in vitro (Halfter et al., 1997), consistent with its inhibitory role in our study. Interestingly, adherence of α5β1-expressing cells to agrin can be blocked by the addition of antibody to β1-integrin (Martin and Sanes, 1997), which is known to play an important role in ENS development (Breau et al., 2006, 2009; Nagy et al., 2009). Col18 was the first member of the collagen family to be found to have heparan sulfate side chains (Halfter et al., 1998). This HSPG protein is widely expressed in nearly all epithelial and endothelial basement membranes and has roles in neuronal and neural crest development. In the cerebellum, for example, Col18 and its matricryptin, endostatin, are produced by Purkinje cells and are required for the organization of climbing fiber terminals and the formation of brain synapses (Su et al., 2012). In studies performed in Caenorhabditis elegans (Ackley et al., 2001) and Drosophila melanogaster (Meyer and Moussian, 2009), these proteins play a role in neuronal cell migration and axon guidance. A role in neural crest development has also been documented, with morpholino-mediated knockdown of Col18 leading to trunk neural crest migration defects (Banerjee et al., 2013). In the ENS, a recent study using single-cell RNA sequencing of ENCDCs from early mouse embryos identified Col18 among the progenitor/gliogenic marker genes (Lasrado et al., 2017), consistent with our finding that Col18 protein is expressed at the wavefront of migration, where the undifferentiated ENCDC progenitors are present. Despite the widespread expression of Col18 and its many roles, the Col18 null mouse has only subtle phenotypes, including retinal detachment and degeneration, macular abnormalities, and neural tube closure defects (Heljasvaara et al., 2017). This might be due to functional redundancy, a phenomenon commonly observed among ECM proteins. We find that Col18 is permissive to ENCDC migration, and the absence of a permissive molecule, as in the Col18a−/− mouse, would not necessarily be expected to lead to a migratory phenotype, as long as the remaining ECM proteins continue to create a permissive environment. Further investigation of the functional role of Col18 protein during ENS development is warranted.
Col18 and agrin are both actively secreted by ENCDCs, similar to TNC (Akbareian et al., 2013). These results highlight an important cell-autonomous role for ENCDCs in remodeling their environment. Recent gene expression profiling identified several ECM-related genes specifically expressed by migrating hemocytes and muscle precursor cells in Drosophila embryos and shared by chick neural crest cells (Bae et al., 2017). Interestingly, similar to neural crest cells, the migration ability of mouse fetal muscle stem cells coincides with their Col6 and TNC production (Tierney et al., 2016), ECM proteins that have been shown to regulate cell migration via interactions with membrane-bound receptors or by altering tissue stiffness. Col18 and agrin may have additional roles in vivo during ENS development beyond cell migration, including regulation of neuronal or glial differentiation, formation of ganglionic aggregates, and extension of neurite processes. Their persistent expression in the postnatal intestine also suggests an ongoing role in maintenance of the mature ENS. Both proteins surround the ganglia and may play a role in creating a protective barrier to separate ganglia from smooth muscle or other nearby cells, to contain the cells that comprise the enteric ganglia, or to protect them from certain immune mediators. This would be consistent with the barrier function of agrin in the brain, where its accumulation in the basement membranes surrounding the capillaries coincides with development of microvascular impermeability and maturation of the blood-brain barrier (Barber and Lieth, 1997). Examining the in vivo significance of the microenvironmental remodeling caused by Col18 and agrin during ENS development and its physiological role in ENS maintenance will make important contributions to neurointestinal research.
Although we show that Col18 and agrin are produced by ENCDCs in both chick and mouse intestine, an interesting distinction was observed. In postnatal and embryonic chick, both proteins are expressed within and around the ganglia. This is in contrast to mouse, in which the protein is found only around the ganglia. Prior studies have reported a similar observation with other ECM molecules, including laminin, type IV collagen and HSPGs, which were found in the basement membrane that surrounds mammalian myenteric ganglia but not within the ganglia (Gabella, 1981; Bannerman et al., 1986), whereas laminin expression by avian enteric ganglia occurs both around and within the ganglia (Balaskas and Gabella, 1997). This fundamental difference might be of functional importance, although this is yet to be defined.
Recognition of the active role of ENCDCs in remodeling the intestinal matrisome has important clinical and therapeutic implications. In HSCR, for example, this implies that not only are ENCDCs absent from the aganglionic segment, but also that the ECM is perturbed, as confirmed by our observation that Col18 and agrin are absent from the periganglionic areas in the distal colon of Ednrb−/− mice. HSCR therefore needs to be considered in the context of the broader implications that it has on gut development and structure. From the perspective of regenerative therapy, stem cell strategies that aim to repopulate the aganglionic segment in HSCR must consider the abnormal matrisome into which the cells will be delivered and the potentially adverse effects this will have on enteric neuronal development in that host environment. Decreasing agrin activity, given its inhibitory effect on cell migration, or enhancing the activity of pro-migratory factors such as TNC, might offer novel approaches to improving ENSC migration following stem cell transplantation. Further elucidation of the functional role of the ECM during normal and abnormal ENS development is needed. Given the myriad of proteins that comprise the intestinal matrisome, an unbiased transcriptomic or proteomic approach would improve our understanding of normal ENS development, advance knowledge of the pathophysiology of enteric neuropathies, and provide new insights to enhance the success of cell therapy for these disorders.
MATERIALS AND METHODS
Animals
Fertilized White Leghorn chicken (Gallus gallus domesticus) eggs were obtained from commercial breeders and incubated at 38°C. Embryos were staged according to developmental tables of Hamburger and Hamilton (HH) (Hamburger and Hamilton, 1992) or the number of embryonic days (E). C57BL/6, Ednrb−/− (Ednrbtm1Ywa/J; #003295), TauGFP/+ [Tau (Mapt) KO, #004779], Wnt1-Cre (#003829), and control ROSA26R-tdTomato (R26R-tdT) reporter (#007914) mice were obtained from Jackson Laboratory. Since Wnt1 marks all neural crest-derived cells, Wnt1-Cre mice were crossed with R26R-tdT reporter mice to obtain Wnt1-Cre;tdTomato (Wnt1;tdT) mice in which all neural crest-derived cells are fluorescently labeled. Col18a1−/− mice were a kind gift from Dr Bjorn Olsen's laboratory at Harvard Medical School (Fukai et al., 2002). Plp1GFP (PLP-EGFP) transgenic mice were kindly provided by Dr Wendy Macklin, University of Colorado (Mallon et al., 2002). Avian and mouse experiments were approved by the Institutional Animal Care and Use Committees of Semmelweis University and Massachusetts General Hospital, respectively.
Immunohistochemistry
Chick gut was dissected between stages E5 and day 2 after hatching and at least five samples were used for each stage. Mice were sacrificed on postnatal weeks 2-6 and the gastrointestinal tract from duodenum to anus removed. Tissue samples were fixed in buffered 4% formaldehyde and infiltrated with 15% sucrose for 24 h. The medium was changed to 7.5% gelatin containing 15% sucrose at 37°C for 1 h, and the tissue rapidly frozen at −40°C in methylbutane (Sigma-Aldrich). Frozen sections were cut at 12 µm, or at 20 µm for confocal laser scanning microscopy, and collected on Probe On Plus slides (Fisher Scientific). Frozen sections were immunostained as described previously (Nagy and Goldstein, 2006a). Briefly, sections were incubated with primary antibody (Table S1), followed by biotinylated goat anti-mouse IgG (Vector Labs), and avidin-biotin-peroxidase complex (Vectastain Elite ABC Kit, Vector Labs). Endogenous peroxidase activity was blocked with 3% hydrogen peroxide (Sigma) for 10 min. The binding sites of the primary antibodies were visualized by 4-chloro-1-naphtol (Sigma).
For immunofluorescence, sections were incubated with primary antibody for 45 min, and secondary antibody for 1 h using Alexa Fluor 594-, 350- and 488-conjugated anti-mouse IgG, Alexa Fluor 594- and 488-conjugated anti-mouse IgG1, Alexa Fluor 488-conjugated anti-mouse IgM, Alexa Fluor 594- and 488-conjugated anti-goat, Alexa Fluor 350- and 647-conjugated anti-guinea pig, Alexa Fluor 594- and 488-conjugated anti-rabbit (all from Invitrogen).
For cell proliferation, 10 µM 5-ethynyl-20-deoxyuridine (EdU) was added to the medium 3 h prior to fixation. EdU incorporation was detected using the Click-iT EdU Imaging Kit (Invitrogen). Cell nuclei were counterstained with DAPI (4′,6-diamidino-2-phenylindole dihydrochloride; Vector Labs). Images were captured and photographed using a Nikon Eclipse 80i microscope or with a Nikon A1R laser-scanning confocal microscope and compiled using Adobe Photoshop.
Chorioallantoic membrane (CAM) transplants
CAM grafts (n=45) were performed as previously described (Akbareian et al., 2013). Briefly, for Fig. 3, E5 chick intestine was harvested and the cloaca removed. Ganglionic intestine, termed ‘+ceca’, included the hindgut, ceca, and distal midgut, which contains ENCDCs at this stage. Aganglionic intestine, termed ‘−ceca’, included only the post-cecal intestine and therefore has no ENCDCs. For Fig. 6, E5 hindgut (preganglionic) was harvested without the ceca, but with cloaca attached to serve as a source of sacral neural crest-derived cells. To generate a hindgut containing only sacral ENCDCs, these intestines were grafted onto the CAM of an E9 host embryo (n=12). After 9 days of incubation, the graft, together with the surrounding CAM, was excised and processed for immunohistochemistry.
Mouse-chick intestinal chimeras
Intestinal chimeras were constructed as previously described (Nagy and Goldstein, 2006b). Hindgut was dissected from E11.5 mouse embryos. Ceca were removed, and these preganglionic hindguts, labeled with charcoal, were transplanted into the coelomic cavity of E3 (HH stage 18) chick embryos using a blunt-ended glass needle. The eggs were closed with adhesive tape and allowed to develop at 37°C for 9 days. The survival rate for the chimeras (n=9) was 65-70%.
Isolation, propagation and in vitro differentiation of enteric neuronal stem cells (ENSCs)
Gastrointestinal tract from duodenum to anus was removed from postnatal day 14 (P14) mice, dissociated with dispase (250 µg/ml; StemCell Technologies) and collagenase XI (1 mg/ml; Sigma-Aldrich) at 37°C for 1 h with gentle pipetting. The cell suspension was passed through a 40 µm cell strainer and cultured at a density of 50,000 cells/ml in proliferation medium consisting of NeuroCult Basal Medium (StemCell Technologies) supplemented with 20 ng/ml epidermal growth factor (StemCell Technologies), 10 ng/ml basic fibroblast growth factor (FGF2; StemCell Technologies), 0.0002% heparin (StemCell Technologies), 100 U/ml penicillin and 100 μg/ml streptomycin (Sigma-Aldrich) for 7-10 days to form primary enteric neurospheres. Neurospheres were dissociated with Accutase (StemCell Technologies) at 37°C for 30 min and sorted for tdT expression by FACS (BD FACSAria, BD Biosciences). Cells were then plated onto round-bottom, low-attachment, sterile 96-well plates (Corning, Costar) at 10,000 cells/well (or 50,000 cells/ml) in 200 µl proliferation medium and cultured for 7 days. To induce differentiation, neurospheres were dissociated with Accutase (StemCell Technologies) at 37°C for 30 min and plated in an 8-well chamber slide (ibidi) coated with 20 µg/ml fibronectin (Sigma-Aldrich) at 10,000 cells/well. Cells were cultured for 7 days in Differentiation Medium (StemCell Technologies). Adherent cells were fixed in ice-cold methanol for 20 min and processed for immunofluorescence staining.
Neurosphere transplants to aneural chick hindgut
Chick hindgut was explanted on E5, prior to colonization by ENCDCs. One or two neurospheres were implanted into the proximal hindgut using fine forceps under microscope visualization. The hindgut was placed onto the CAM of E9 host chick embryo and, 7 days later, the gut was removed and processed for immunohistochemistry (n=25).
In vitro migration assay
ENCDC migration was assessed by stripe assay (n=12) as described previously (Vielmetter et al., 1990; Yamagishi et al., 2016). Briefly, 6 cm plastic culture dishes were coated for 2 h at 37°C in alternate stripes of control proteins (10 µg laminin or 20 µg/ml fibronectin; Sigma-Aldrich) or test proteins (2 µg/ml agrin or 50 µg/ml endostatin; R&D Systems) using silicon matrices available from Prof. Martin Bastmeyer (Karlsruhe Institute of Technology, Germany). For each assay, 8-10 E7 chick midguts were placed perpendicular to the stripe pattern and covered with a small volume of serum-free DMEM culture medium containing penicillin and streptomycin. After initial incubation for 2 h at 37°C to allow the guts to adhere to the substrate, DMEM containing 10 ng/ml GDNF was added to the culture. The distance of ENCDC migration along each stripe was assessed at 24 h.
ENCDC migration was also analyzed by plating Wnt1;tdT-derived neurospheres onto culture plates coated with 20 µg/ml fibronectin and culturing for 48 h in either serum-free DMEM or medium supplemented with 20 µg/ml function-blocking agrin antibody (MAB5204, Millipore) as described (Chakraborty et al., 2015). Cell migration was measured using ImageJ (National Institutes of Health) by dividing the field of view into octants and measuring the distance from the neurosphere edge to the farthest Wnt1;tdT cell in each octant. Three to four neurospheres were analyzed per condition.
Supplementary Material
Acknowledgements
Several antibodies in Table S1 were obtained from the Developmental Studies Hybridoma Bank developed under the auspices of the NICHD and maintained by the University of Iowa, Department of Biological Sciences, Iowa City, IA 52242, USA. We thank Dr Hideki Enomoto (Kobe University School of Medicine) for providing Phox2b antiserum, and Dr Mihaly Kalman (Semmelweis University) for several dystroglycan antibodies.
Footnotes
Competing interests
The authors declare no competing or financial interests.
Author contributions
Conceptualization: N.N., C.B., A.M.G.; Methodology: N.N., C.B., R.H., S.B., E.A., D.D.; Software: S.B., E.A.; Validation: N.N., S.B.; Formal analysis: S.B., D.D.; Investigation: N.N., C.B., R.H.; Data curation: E.A.; Writing - original draft: N.N., C.B., A.M.G.; Writing - review & editing: N.N., A.M.G.; Visualization: N.N., R.H., E.A., D.D., A.M.G.; Supervision: N.N., A.M.G.; Project administration: N.N.; Funding acquisition: N.N., A.M.G.
Funding
A.M.G. is supported by the National Institutes of Health (R01DK103785). N.N. is supported by a Bolyai Fellowship from the Hungarian Academy of Sciences (Magyar Tudományos Akadémia) and Hungarian Science Foundation (Nemzeti Fejlesztési Minisztérium) NKFI grant (124740). Deposited in PMC for release after 12 months.
Supplementary information
Supplementary information available online at http://dev.biologists.org/lookup/doi/10.1242/dev.160317.supplemental
References
- Ackley B. D., Crew J. R., Elamaa H., Pihlajaniemi T., Kuo C. J. and Kramer J. M. (2001). The NC1/endostatin domain of Caenorhabditis elegans type XVIII collagen affects cell migration and axon guidance. J. Cell Biol. 152, 1219-1232. 10.1083/jcb.152.6.1219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akbareian S. E., Nagy N., Steiger C. E., Mably J. D., Miller S. A., Hotta R., Molnar D. and Goldstein A. M. (2013). Enteric neural crest-derived cells promote their migration by modifying their microenvironment through tenascin-C production. Dev. Biol. 382, 446-456. 10.1016/j.ydbio.2013.08.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson R. B. (2010). Matrix metalloproteinase-2 is involved in the migration and network formation of enteric neural crest-derived cells. Int. J. Dev. Biol. 54, 63-69. 10.1387/ijdb.082667ra [DOI] [PubMed] [Google Scholar]
- Bae Y.-K., Macabenta F., Curtis H. L. and Stathopoulos A. (2017). Comparative analysis of gene expression profiles for several migrating cell types identifies cell migration regulators. Mech. Dev. 148, 40-55. 10.1016/j.mod.2017.04.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balaskas C. and Gabella G. (1997). Laminin immunoreactivity in enteric ganglia of the chick embryo. Cell Tissue Res. 289, 243-251. 10.1007/s004410050871 [DOI] [PubMed] [Google Scholar]
- Banerjee S., Isaacman-Beck J., Schneider V. A. and Granato M. (2013). A novel role for Lh3 dependent ECM modifications during neural crest cell migration in zebrafish. PLoS ONE 8, e54609 10.1371/journal.pone.0054609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bannerman P. G. C., Mirsky R., Jessen K. R., Timpl R. and Duance V. C. (1986). Light microscopic immunolocalization of laminin, type IV collagen, nidogen, heparan sulphate proteoglycan and fibronectin in the enteric nervous system of rat and guinea pig. J. Neurocytol. 15, 733-743. 10.1007/BF01625191 [DOI] [PubMed] [Google Scholar]
- Barber A. J. and Lieth E. (1997). Agrin accumulates in the brain microvascular basal lamina during development of the blood-brain barrier. Dev. Dyn. 208, 62-74. [DOI] [PubMed] [Google Scholar]
- Breau M. A., Pietri T., Eder O., Blanche M., Brakebusch C., Fassler R., Thiery J. P. and Dufour S. (2006). Lack of beta1 integrins in enteric neural crest cells leads to a Hirschsprung-like phenotype. Development 133, 1725-1734. 10.1242/dev.02346 [DOI] [PubMed] [Google Scholar]
- Breau M. A., Dahmani A., Broders-Bondon F., Thiery J.-P. and Dufour S. (2009). Beta1 integrins are required for the invasion of the caecum and proximal hindgut by enteric neural crest cells. Development 136, 2791-2801. 10.1242/dev.031419 [DOI] [PubMed] [Google Scholar]
- Chakraborty S., Lakshmanan M., Swa H. L. F., Chen J., Zhang X., Ong Y. S., Loo L. S., Akincilar S. C., Gunaratne J., Tergaonkar V. et al. (2015). An oncogenic role of Agrin in regulating focal adhesion integrity in hepatocellular carcinoma. Nat. Commun. 6, 6184 10.1038/ncomms7184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chevalier N. R., Gazguez E., Bidault L., Guilbert T., Vias C., Vian E., Watanabe Y., Muller L., Germain S., Bondurand N. et al. (2016). How tissue mechanical properties affect enteric neural crest cell migration. Sci. Rep. 6, 20927 10.1038/srep20927 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies J. A., Yates E. A. and Turnbull J. E. (2003). Structural determinants of heparan sulphate modulation of GDNF signalling. Growth Factors 21, 109-119. 10.1080/08977190310001621005 [DOI] [PubMed] [Google Scholar]
- Fukai N., Eklund L., Marneros A. G., Oh S. P., Keene D. R., Tamarkin L., Niemela M., Ilves M., Li E., Pihlajaniemi T. et al. (2002). Lack of collagen XVIII/endostatin results in eye abnormalities. EMBO J. 21, 1535-1544. 10.1093/emboj/21.7.1535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furness J. B. (2012). The enteric nervous system and neurogastroenterology. Nat. Rev. Gastroenterol. Hepatol. 9, 286-294. 10.1038/nrgastro.2012.32 [DOI] [PubMed] [Google Scholar]
- Gabella G. (1981). Ultrastructure of the nerve plexuses of the mammalian intestine: the enteric glial cells. Neuroscience 6, 425-436. 10.1016/0306-4522(81)90135-4 [DOI] [PubMed] [Google Scholar]
- Gazquez E., Watanabe Y., Broders-Bondon F., Paul-Gilloteaux P., Heysch J., Baral V., Bondurand N. and Dufour S. (2016). Endothelin-3 stimulates cell adhesion and cooperates with beta1-integrins during enteric nervous system ontogenesis. Sci. Rep. 6, 37877 10.1038/srep37877 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein A. M., Thapar N., Karunaratne T. B. and De Giorgio R. (2016). Clinical aspects of neurointestinal disease: pathophysiology, diagnosis, and treatment. Dev. Biol. 417, 217-228. 10.1016/j.ydbio.2016.03.032 [DOI] [PubMed] [Google Scholar]
- Halfter W., Schurer B., Yip J., Yip L., Tsen G., Lee J. A. and Cole G. J. (1997). Distribution and substrate properties of agrin, a heparan sulfate proteoglycan of developing axonal pathways. J. Comp. Neurol. 383, 1-17. [DOI] [PubMed] [Google Scholar]
- Halfter W., Dong S., Schurer B. and Cole G. J. (1998). Collagen XVIII is a basement membrane heparan sulfate proteoglycan. J. Biol. Chem. 273, 25404-25412. 10.1074/jbc.273.39.25404 [DOI] [PubMed] [Google Scholar]
- Hamburger V. and Hamilton H. L. (1992). A series of normal stages in the development of the chick embryo. 1951. Dev. Dyn. 195, 231-272. 10.1002/aja.1001950404 [DOI] [PubMed] [Google Scholar]
- Heljasvaara R., Aikio M., Ruotsalainen H. and Pihlajaniemi T. (2017). Collagen XVIII in tissue homeostasis and dysregulation-Lessons learned from model organisms and human patients. Matrix Biol. 57-58, 55-75. 10.1016/j.matbio.2016.10.002 [DOI] [PubMed] [Google Scholar]
- Hummel S., Osanger A., Bajari T. M., Balasubramani M., Halfter W., Nimpf J. and Schneider W. J. (2004). Extracellular matrices of the avian ovarian follicle. Molecular characterization of chicken perlecan. J. Biol. Chem. 279, 23486-23494. 10.1074/jbc.M312694200 [DOI] [PubMed] [Google Scholar]
- Kuo C. J., LaMontagne K. R. Jr, Garcia-Cardeña G., Ackley B. D., Kalman D., Park S., Christofferson R., Kamihara J., Ding Y.-H., Lo K.-M. et al. (2001). Oligomerization-dependent regulation of motility and morphogenesis by the collagen XVIII NC1/endostatin domain. J. Cell Biol. 152, 1233-1246. 10.1083/jcb.152.6.1233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lasrado R., Boesmans W., Kleinjung J., Pin C., Bell D., Bhaw L., McCallum S., Zong H., Luo L., Clevers H. et al. (2017). Lineage-dependent spatial and functional organization of the mammalian enteric nervous system. Science 356, 722-726. 10.1126/science.aam7511 [DOI] [PubMed] [Google Scholar]
- Mallon B. S., Shick H. E., Kidd G. J. and Macklin W. B. (2002). Proteolipid promoter activity distinguishes two populations of NG2-positive cells throughout neonatal cortical development. J. Neurosci. 22, 876-885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin P. T. and Sanes J. R. (1997). Integrins mediate adhesion to agrin and modulate agrin signaling. Development 124, 3909-3917. [DOI] [PubMed] [Google Scholar]
- Meyer F. and Moussian B. (2009). Drosophila multiplexin (Dmp) modulates motor axon pathfinding accuracy. Dev. Growth Differ. 51, 483-498. 10.1111/j.1440-169X.2009.01111.x [DOI] [PubMed] [Google Scholar]
- Nagy N. and Goldstein A. M. (2006a). Endothelin-3 regulates neural crest cell proliferation and differentiation in the hindgut enteric nervous system. Dev. Biol. 293, 203-217. 10.1016/j.ydbio.2006.01.032 [DOI] [PubMed] [Google Scholar]
- Nagy N. and Goldstein A. M. (2006b). Intestinal coelomic transplants: a novel method for studying enteric nervous system development. Cell Tissue Res. 326, 43-55. 10.1007/s00441-006-0207-3 [DOI] [PubMed] [Google Scholar]
- Nagy N. and Goldstein A. M. (2017). Enteric nervous system development: a crest cell's journey from neural tube to colon. Semin. Cell Dev. Biol. 66, 94-106. 10.1016/j.semcdb.2017.01.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagy N., Brewer K. C., Mwizerwa O. and Goldstein A. M. (2007). Pelvic plexus contributes ganglion cells to the hindgut enteric nervous system. Dev. Dyn. 236, 73-83. 10.1002/dvdy.20933 [DOI] [PubMed] [Google Scholar]
- Nagy N., Mwizerwa O., Yaniv K., Carmel L., Pieretti-Vanmarcke R., Weinstein B. M. and Goldstein A. M. (2009). Endothelial cells promote migration and proliferation of enteric neural crest cells via beta1 integrin signaling. Dev. Biol. 330, 263-272. 10.1016/j.ydbio.2009.03.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagy N., Burns A. J. and Goldstein A. M. (2012). Immunophenotypic characterization of enteric neural crest cells in the developing avian colorectum. Dev. Dyn. 241, 842-851. 10.1002/dvdy.23767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagy N., Barad C., Graham H. K., Hotta R., Cheng L. S., Fejszak N. and Goldstein A. M. (2016). Sonic hedgehog controls enteric nervous system development by patterning the extracellular matrix. Development 143, 264-275. 10.1242/dev.128132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newgreen D. F., Jahnke I., Allan I. J. and Gibbins I. L. (1980). Differentiation of sympathetic and enteric neurons of the fowl embryo in grafts to the chorio-allantoic membrane. Cell Tissue Res. 208, 1-19. 10.1007/BF00234168 [DOI] [PubMed] [Google Scholar]
- Payette R. F., Tennyson V. M., Pomeranz H. D., Pham T. D., Rothman T. P. and Gershon M. D. (1988). Accumulation of components of basal laminae: association with the failure of neural crest cells to colonize the presumptive aganglionic bowel of ls/ls mutant mice. Dev. Biol. 125, 341-360. 10.1016/0012-1606(88)90217-5 [DOI] [PubMed] [Google Scholar]
- Perris R., Paulsson M. and Bronner-Fraser M. (1989). Molecular mechanisms of avian neural crest cell migration on fibronectin and laminin. Dev. Biol. 136, 222-238. 10.1016/0012-1606(89)90144-9 [DOI] [PubMed] [Google Scholar]
- Poulain F. E. and Yost H. J. (2015). Heparan sulfate proteoglycans: a sugar code for vertebrate development? Development 142, 3456-3467. 10.1242/dev.098178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rehn M., Veikkola T., Kukk-Valdre E., Nakamura H., Ilmonen M., Lombardo C., Pihlajaniemi T., Alitalo K. and Vuori K. (2001). Interaction of endostatin with integrins implicated in angiogenesis. Proc. Natl. Acad. Sci. USA 98, 1024-1029. 10.1073/pnas.98.3.1024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarrazin S., Lamanna W. C. and Esko J. D. (2011). Heparan sulfate proteoglycans. Cold Spring Harb. Perspect. Biol. 3, a004952 10.1101/cshperspect.a004952 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soret R., Mennetrey M., Bergeron K. F., Dariel A., Neunlist M., Grunder F., Faure C., Silversides D. W. and Pilon N. (2015). A collagen VI-dependent pathogenic mechanism for Hirschsprung's disease. J. Clin. Invest. 125, 4483-4496. 10.1172/JCI83178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su J., Stenbjorn R. S., Gorse K., Su K., Hauser K. F., Ricard-Blum S., Pihlajaniemi T. and Fox M. A. (2012). Target-derived matricryptins organize cerebellar synapse formation through alpha3beta1 integrins. Cell Rep. 2, 223-230. 10.1016/j.celrep.2012.07.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tennyson V. M., Payette R. F., Rothman T. P. and Gershon M. D. (1990). Distribution of hyaluronic acid and chondroitin sulfate proteoglycans in the presumptive aganglionic terminal bowel of ls/ls fetal mice: an ultrastructural analysis. J. Comp. Neurol. 291, 345-362. 10.1002/cne.902910303 [DOI] [PubMed] [Google Scholar]
- Tierney M. T., Gromova A., Sesillo F. B., Sala D., Spenlé C., Orend G. and Sacco A. (2016). Autonomous extracellular matrix remodeling controls a progressive adaptation in muscle stem cell regenerative capacity during development. Cell Rep. 14, 1940-1952. 10.1016/j.celrep.2016.01.072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsen G., Halfter W., Kröger S. and Cole G. J. (1995). Agrin is a heparan sulfate proteoglycan. J. Biol. Chem. 270, 3392-3399. 10.1074/jbc.270.7.3392 [DOI] [PubMed] [Google Scholar]
- Vielmetter J., Stolze B., Bonhoeffer F. and Stuermer C. A. O. (1990). In vitro assay to test differential substrate affinities of growing axons and migratory cells. Exp. Brain Res. 81, 283-287. 10.1007/BF00228117 [DOI] [PubMed] [Google Scholar]
- Winder S. J. (2001). The complexities of dystroglycan. Trends Biochem. Sci. 26, 118-124. 10.1016/S0968-0004(00)01731-X [DOI] [PubMed] [Google Scholar]
- Yamagishi S., Kesavamoorthy G., Bastmeyer M. and Sato K. (2016). Stripe assay to study the attractive or repulsive activity of a protein substrate using dissociated hippocampal neurons. J. Vis. Exp. 112, e54096 10.3791/54096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young H. M., Hearn C. J., Farlie P. G., Canty A. J., Thomas P. Q. and Newgreen D. F. (2001). GDNF is a chemoattractant for enteric neural cells. Dev. Biol. 229, 503-516. 10.1006/dbio.2000.0100 [DOI] [PubMed] [Google Scholar]
- Young H. M., Bergner A. J., Simpson M. J., McKeown S. J., Hao M. M., Anderson C. R. and Enomoto H. (2014). Colonizing while migrating: how do individual enteric neural crest cells behave? BMC Biol. 12, 23 10.1186/1741-7007-12-23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zong Y. and Jin R. (2013). Structural mechanisms of the agrin-LRP4-MuSK signaling pathway in neuromuscular junction differentiation. Cell. Mol. Life Sci. 70, 3077-3088. 10.1007/s00018-012-1209-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.