Abstract
Rationale
Prescription opioid abuse and transition to heroin use are growing problems in the United States. However, the long-term consequences of adolescent prescription opioid abuse on subsequent drug use and affective-like behavior are unknown.
Objectives
This study aims to determine if adolescent exposure to oxycodone alters the rewarding effects of morphine, anxiety-like behavior, and reward-related gene expression later in adulthood.
Methods
Adolescent male C57Bl/6 mice were exposed to oxycodone (3 mg/kg/day) via osmotic minipumps for 28-days. Following a 28-day withdrawal period, mice were tested in morphine conditioned place preference paradigm (CPP), morphine sensitization, open field, marble burying, and forced swim (FST) tests. To determine if effects were specific to adolescent exposure, adult mice were exposed to oxycodone for 28-days and underwent 28-days of withdrawal prior to the same behavioral testing schedule. Expression of reward related genes including dopamine receptor 1 (D1) and dopamine transporter (DAT) in the nucleus accumbens (NAc) and ventral tegmental area (VTA) was examined. Results: Adolescent oxycodone exposure significantly increased (300%) response to morphine CPP during adulthood and significantly reduced D1 expression (30%) in the NAc and DAT expression (75%) in the VTA. Adult oxycodone exposure did not affect subsequent responses to morphine CPP. Oxycodone exposure did not affect the development of morphine sensitization or affective-like behaviors. Corticosterone response to a stressor (FST) was significantly reduced (65%) in mice exposed to oxycodone during adolescence but not adulthood.
Conclusions
Adolescent oxycodone exposure enhances rewarding effects of morphine in adulthood with no effect on other affective-like behaviors.
Keywords: oxycodone, morphine, opioid, dopamine, reward, conditioned place preference, anxiety
Introduction
Prescription opioid abuse is a growing health concern in the United States and worldwide. Current estimates indicate 4.3 million people in the United States abuse prescription opioids (Center for Behavioral Health Statistics and Quality (CBHSQ) 2015) and they are the second most abused illicit drugs by youth between the ages of 12 and 17 (CBHSQ 2015). Use of prescription opioids, such as oxycodone, increases propensity to initiate heroin use (Unick et al. 2013; Jones 2013; Mars et al. 2014; Cerdá et al. 2015), which has steadily increased over the last decade (CBHSQ 2015). In addition, there is an association between adolescent opioid abuse and other affective psychiatric disorders (Morley et al. 2015; Edlund et al. 2015).
Adolescence, in humans and animals, is a developmental period associated with increased sensitivity to drugs of abuse (Clark et al. 1998; Spear 2000; Doremus-Fitzwater et al. 2010). Oxycodone, like other μ-opioid receptor agonists, indirectly stimulates dopamine neurons within the ventral tegmental area (VTA) resulting in an increase in dopamine release in projection regions including the nucleus accumbens (NAc; Di Chiara and Imperato 1988; Spanagel et al. 1990; Johnson and North 1992). This dopamine release is believed to underlie the rewarding effects of opioid drugs. During adolescence, the reward pathway undergoes significant modifications (for review see, Spear 2000; Burke and Miczek 2013). Specifically, within the nucleus accumbens, there is a progressive increase in the density of dopaminergic innervation (Tarazi et al. 1998a) and expression of dopamine receptors through mid-adolescence when adult levels are reached (Tarazi et al. 1998b; Tarazi et al. 1999). Basal levels of dopamine, measured by microdialysis, are lower in adolescents compared to adults (Laviola et al. 2001). However, oxycodone elicits a larger dopamine response in the nucleus accumbens of adolescents compared to adults (Zhang et al. 2009). This may be due in part to the higher, rates of dopamine turnover (Stamford 1989; Naneix et al. 2012) and/or larger pools of releasable dopamine (Stamford 1989) that are evident in adolescents. Thus, ongoing neurodevelopment within the reward pathway can significantly alter adolescent response to drugs of abuse.
The long-term effects of adolescent morphine exposure in animals can impact adult response to morphine. For example, locomotor activating effects of morphine (White and Holtzman 2005) and reinstatement of morphine conditioned place preference (Schwarz and Bilbo 2013) are enhanced in animals that were exposed to morphine during adolescence. In addition, adolescent morphine exposure results in depression-like behavior in adulthood (Lutz et al. 2013). In contrast, the long-term effects of oxycodone exposure during adolescence on subsequent adult behavior have not been examined.
The purpose of the current study was to examine the impact of adolescent oxycodone exposure on subsequent response to reward-related behaviors. To reflect the shift of prescription opioid abuse to heroin abuse in humans, we modeled our drug paradigm to expose mice to oxycodone and test sensitivity to the heroin metabolite morphine. To this end, adolescent mice were exposed to oxycodone and tested as adults in a morphine conditioned place preference (CPP) paradigm to test sensitivity to the rewarding value of opioids following adolescent exposure to oxycodone. In addition, acute locomotor and sensitized response to morphine following adolescent exposure to oxycodone was evaluated. Furthermore, due to the strong association between adolescent drug use and affective disorders in human adolescents, the same model was used to assess if this exposure differentially affects vulnerability to affective-like and anxiety-like behaviors later in adulthood. To determine if these effects were specific to adolescence, the same behaviors were examined in mice exposed to oxycodone during adulthood. The potential underlying neurobiological adaptations that may contribute to altered behavior were also investigated by measuring gene expression in key drug reward regions in the brain.
Materials and Methods
General Methods
Animals
C57Bl/6NTac mice (Taconic, Hudson, New York) were bred at the University of Pennsylvania for 2 generations to eliminate potential effects of stress due to shipping. The male offspring of the second generation were used for these experiments. Mice were group housed (3-5/cage) with ad libitum access to food and water under a 12-hour light/dark cycle (lights on at 0700). All procedures were approved by the University of Pennsylvania Animal Care and Use Committee and were in compliance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals.
Surgery
All surgical procedures were conducted using aseptic techniques and under 1-3% isoflurane/oxygen vapor mixture. Osmotic minipumps (model 1004; Alzet, Cupertino, CA) were inserted subcutaneously. Minipumps delivered oxycodone (oxycodone HCl, Spectrum Chemical, New Brunswick, NJ) dissolved in 0.9% saline at a dosage of 3 mg kg−1 day−1 for 28 days. To adjust for animal growth during exposure, the concentration of oxycodone solution added to the minipumps was determined based on average C57Bl/6 mouse weight at 6 weeks of age (i.e. approximate weight 2 weeks following minipump implant, Fig 1A). Thus while adolescents receive a significantly higher dose for the first week of exposure, they received similar doses as adults did for the last 3 weeks of exposure (Fig 1B). The dose was selected based on previous studies indicating that this dose is rewarding in a conditioned place preference paradigm and leads to a significant increase in locomotor activity in adolescent and adult C57Bl/6 mice (Niikura et al. 2013). Furthermore, our preliminary studies indicated that this dose was sufficient to produce spontaneous withdrawal in adolescent and adult mice measured as total occurrences of somatic signs (wet-dog shakes, jumping, backing-up, tremors, diarrhea, and genital licking) during 20-minute observations 1, 2, and 3 days following minipump or pellet removal (Fig 1C). Importantly, there was no association between withdrawal scores and dose of oxycodone in either age group. After 28 days of exposure, minipumps were surgically removed. Sham surgeries were performed on a subset of mice, during which a small cellulose pellet (NIDA Drug Supply, Research Triangle Park, NC) was implanted subcutaneously, to control for the effects of surgery and implantation. Pilot studies in our laboratory indicate that there are no differences between mice implanted with cellulose pellets or saline minipumps in behavioral responses to tests performed in this study. Minimpumps were selected for these experiments to maximize drug exposure without the stress of multiple injections and to control the onset of withdrawal.
Fig 1.
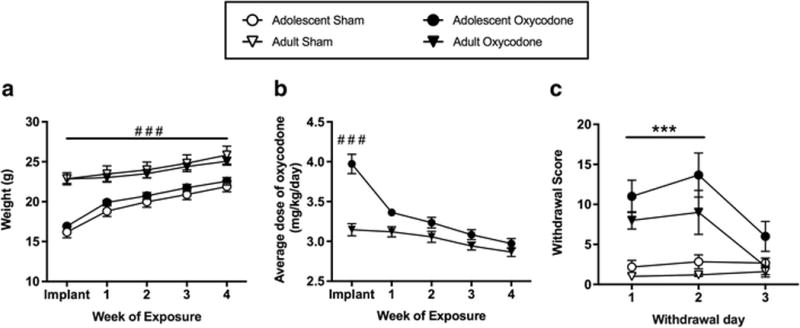
Animal weights (A) are represented on the day of surgery (implant) and at the end of each week for sham (open symbols) and oxycodone (filled symbols) adolescent (circles) and adult (triangles) exposure groups. The average (mean ± SEM) dose of oxycodone (B) is represented for the adolescent and adult exposure groups on the day of surgery and at the end of each week of exposure. Spontaneous somatic withdrawal score (C) 1, 2, and 3 days following sham and oxycodone exposure in adolescent and adult groups. Number sign indicates significant difference between age groups (###, p < 0.001) and asterisk indicates significant difference between drug exposure groups (***, p < 0.001)
Marble Burying
The marble burying test was performed as previously described (Turner et al. 2011). Briefly, 20 marbles were arranged with equal distribution atop bedding (5-cm deep) in a standard mouse cage (26×20×14cm). Mice were acclimated to the testing room for 1-hour then individually placed into a test cage for 15-minutes. After the test, mice were removed and an experimenter blind to the drug condition counted the number of marbles buried ¾ or more in the bedding.
Open Field
Activity in an open field was measured in Plexiglas open field boxes (43×43 cm2) with 32 infrared photobeams in a 16×16 arrangement using Photobeam Activity System software (San Diego Instruments, San Diego, CA). The open field was divided into 2 areas, a center area (the innermost 6×6 photobeams) and the outer area that surrounded the center and was closest to the walls. Mice were placed in the open field close to a wall and allowed to explore for 5 minutes while the program recorded total distance traveled and time spent in the center area.
Forced Swim Test and Plasma Corticosterone Radioimmunoassay
The forced swim test was performed as previously described (Turner et al. 2010). Mice were placed in a Plexiglass cylinder filled with water at room temperature for 6 minutes. Video tape recordings were scored by an experimenter blinded to the experimental conditions for time spent immobile (i.e. movement necessary for floating only) during the full 6 minute test. Trunk blood was collected from each mouse 20 minutes after the start of the forced swim test. Plasma was separated by centrifugation and stored at −20°C until assayed for coriticosterone using a commercially available radioimmunoassay kit (ICN Biomedicals Inc., Cleveland, OH). Intra-assay coefficient variation was < 20%.
Conditioned Place Preference
The rewarding properties of morphine were assessed using an unbiased conditioned place preference paradigm as described previously (Walters et al. 2005). During the pre-conditioning session, mice were allowed to explore both sides of a two-chambered CPP apparatus (20 × 20 × 20 cm/chamber) for 900 s, and time spent in each side was recorded. Beginning on day 2, animals were conditioned once daily for 6 days, with each group receiving morphine (5 mg/kg; morphine sulfate, NIDA Drug Supply, Research Triangle Park, NC) on one side and saline on the other side. Drug-paired sides were randomized among groups. One day after the last conditioning session, animals were allowed to explore freely between the two sides for 900 s, and time spent on each side was recorded. The preference score (time spent in drug-paired side minus time in saline-paired side on the post-conditioning day minus the pre-conditioning day) was calculated for each mouse.
Morphine Behavioral Sensitization
Locomotor activity was assessed as described previously (Walters et al. 2005) in a “home cage” activity monitoring system (Med Associates, St. Albans, VT). The home cage was placed in a photobeam frame with sensors arranged in 8 beam array strips. To determine baseline activity, mice were given intraperitoneal (i.p.) injections of saline and activity was recorded for 1-hour for three consecutive days. Mice were then given 10 mg/kg morphine i.p. injections every other day for a total of 4 days. Mice were left undisturbed for 1-week and then injected with 10 mg/kg morphine (challenge). Locomotor activity was recorded for 2-hours following all morphine injections. Beam break data were read into Med Associates personal computer-designed software and activity during the last 1-hour baseline session and the 2-hour test session are reported.
Quantitative real-time PCR
Following exposure and 28-days of withdrawal, brains from a subset of mice were rapidly removed and flash frozen in isopentane (−80°C). Coronal brain sections (300 μm) between bregma 1.10 and 1.94 (Paxinos and Franklin 2008) were used to collect 1.2 mm punches of the Nucleus Accumbens (NAc) bilaterally. Sections between bregma −2.92 and −3.10 were used to collect 1.5 mm punches of the Ventral Tegmental Area (VTA). RNA was extracted from NAc and VTA punches by homogenizing in 800μl of TRizol and 160μl of chloroform. RNA was purified using the RNeasy Mini Kit. RNA concentration and integrity were determined using a Nanodrop spectrophotometer (Nanodrop Technologies, Wilmington, DE) and the RNA NanoChip on the Bioanalyzer (Agilent), respectively. cDNA was synthesized from RNA (100ng) using the High-Capacity cDNA Reverse Transcription Kit (Applied Biosystems). Quantitative real-time PCR (qRT-PCR) was performed using primers (Table 1) for the dopamine 1 and 2 receptors (D1 and D2, respectively) and μ-opioid receptor (MOR) with cDNA prepared from NAc region and tyrosine hydroxylase (TH; rate-limiting enzyme of dopamine synthesis), the dopamine transporter (DAT), and MOR with cDNA prepared from the VTA region. Reactions were assembled according to manufacturers instructions using SYBR-Green master mix (Applied Biosystems) and 300ng (final concentration) primer, for a total reaction volume of 25 μl. Quantitative real-time PCR (qRT-PCR) was performed on the Stratagene Mx3000 and Mxpro Software. Cycling parameters were 95°C for 10 min and then 40 cycles of 95°C (30 s) and 60°C (1 min), followed by a melting curve analysis. All reactions were run in triplicate and median cycles to threshold (Ct) values were used for analysis. Housekeeping genes (B-actin, GAPDH, and HPRT) were used to normalize against experimental genes and relative gene expression was determined using the 2−ΔΔCT method (Livak and Schmittgen 2001).
Table 1.
List of primer sequences (5′-to-3′) for dopamine 1 (D1) and 2 (D2) receptors (D1 and D2, respectively), tyrosine hydroxylase (TH), the dopamine transporter (DAT), the μ-opioid receptor (MOR) and housekeep genes (GAPDH, HPRT, and B-actin).
| Gene | Forward | Reverse |
|---|---|---|
|
| ||
| D1 | ATGGCTCCTAACACTTCTACCA | GGGTATTCCCAAGAGAGTGGAC |
| GAPDH | ATTCAACGGCACAGTCAAGG | TGGATGCAGGGATGATGTTTC |
| D2 | GCCCTTCATCGTCACCCTGCT | TGGGCATGGTCTGGATCTCAA |
| TH | GTCTCAGAGCAGGATACCAAGC | CTCTCCCTCGAATACCACAGCC |
| MOR | TGGTGGCAGTCTTCATTTT | CACCATCATGGCCCTCTATT |
| HPRT | CAAAGCCTAAGATGAGCGCAAG | TTACTAGGCAGATGGCCACAGG |
| DAT | GCATCCTGTTCACATATTACAC | TTGTCTCCCAACCTGAATTC |
| B-actin | AGCCATGTACGTAGCCATCC | CTCTCAGCTTGTGGTGGTGAA |
Statistical Analysis
All data are presented as the mean ± SEM. Repeated measures ANOVAs were used to compare weights, oxycodone dose, and withdrawal scores between age groups over time. Pearson product-moment correlation was computed to determine association between oxycodone dose over time and withdrawal scores. Student’s t-test was used to compare the number of marbles buried, time spent in the center and total distance traveled in the open field test, the CPP preference score, time spent immobile in the forced swim test and relative gene expression data between oxycodone and placebo exposure in adolescents and adults. A two-way ANOVA was used to compare corticosterone levels from oxycodone and placebo exposure in adolescents and adults with stress or no stress exposure. A repeated measures ANOVA was used to compare locomotor activity during the last saline baseline day, the first morphine injection, and the challenge morphine injection test days between oxycodone and placebo exposure in adolescents and adults. Statistical analyses were performed using Graphpad Prism 6 (Graphpad Software, La Jolla, CA) with alpha set at 0.05 (p < 0.05) for all tests.
Experiment 1: Adolescent oxycodone exposure
The purpose of this experiment was to determine if adolescent exposure to oxycodone would alter subsequent response to the rewarding and locomotor activating effects of morphine as well as anxiety-like behaviors in adulthood. Adolescent mice (postnatal day 28) were implanted with cellulose pellets (N = 25) or were implanted with minipumps containing oxycodone (N = 26) for a 28-day exposure. Minipumps and pellets were removed and mice underwent a 28-day withdrawal period prior to behavioral testing. Thus, mice exposed during adolescence were not tested until adulthood on postnatal day 84. Three separate groups of mice were used for behavioral and molecular experiments. The first subset of mice (sham, n = 11; oxycodone, n = 12) was tested in the marble burying task, open field test, and CPP with 2-3 days between each test. Two weeks after the CPP test, trunk blood was collected from the first subset of mice either after a forced swim test stress (sham, n = 5; oxycodone, n = 5) or no stress (sham, n = 6; oxycodone, n = 6). The second group of mice (sham, n = 6; oxycodone, n = 7) was tested in the marble burying task and the morphine behavioral sensitization. No differences were observed between the first and second cohort in the marble burying task and data were therefore combined for analysis. The final group of mice (sham, n = 8; oxycodone, n = 7) did not undergo behavioral testing and instead were killed 28 days after the drug or placebo exposure, on postnatal day 84, and their brains used for gene expression analysis.
Experiment 2: Adult oxycodone exposure
The purpose of this experiment was to determine if adult exposure to oxycodone would alter subsequent vulnerability to the rewarding and locomotor activating effects of morphine as well as anxiety-like behaviors. Adult mice (postnatal day 56) underwent sham surgery (N = 22) or were implanted with oxycodone minipumps (N=20) for a 28-day exposure. Minipumps were removed and mice underwent a 28-day withdrawal period prior to behavioral testing. Mice exposed during adulthood were therefore not tested until postnatal day 112. Three separate groups of mice were used for the following tests. The first subset of mice (sham, n = 9; oxycodone, n = 8) was tested in the marble burying task, open field test, and CPP with 2-3 days between each test. Two weeks after the CPP test, trunk blood was collected from the first subset of mice either after a forced swim test stress (sham, n = 4; oxycodone, n = 4) or no stress (sham, n = 3; oxycodone, n = 4). The second group of mice (sham; n = 6; oxycodone, n = 5) was tested in the marble burying task and morphine behavioral sensitization. No differences were observed in the marble burying task in the first and second cohorts and therefore data were combined for this analysis. The third group of mice (sham, n = 7; oxycodone; n = 7) did not undergo behavioral testing and instead were killed 28 days after the drug or placebo exposure, on postnatal day 112, and their brains were used for gene expression analysis.
Results
Experiment 1
Adult mice exposed to oxycodone during adolescence displayed similar behavior to the sham controls in anxiety-like behaviors tested in adulthood. Specifically, in the marble burying test, no significant differences in the number of marbles buried were observed between mice exposed to oxycodone (n=19) or sham surgery (n=17) during adolescence (Table 2). In the open field test, mice exposed to oxycodone (n = 11) during adolescence spent a similar amount of time exploring the center area of the arena as compared to sham controls (n = 12; Table 2). In addition, no difference was observed in the total distance traveled in the open field arena between sham and oxycodone treated mice (Table 2). Similarly, there was no difference in the time spent immobile in the forced swim test between mice pre-treated with oxycodone and sham surgery during adolescence (Table 2). The exposure to the forced swim test during adulthood did increase plasma corticosterone as compared to no stress controls in both sham and oxycodone pre-treated groups (Fig 2; effect of stress, F1,18 = 461.1, p < 0.0001). Interestingly, a significant effect of drug pre-treatment (F1,18 = 23.24, p < 0.001) and drug pre-treatment by stress interaction (F1,18 = 24.21, p < 0.0001) was observed. Adolescent oxycodone pre-treatment significantly attenuated plasma corticosterone levels following forced swim test (t18 = 6.636, p < 0.0001). No difference in plasma corticosterone levels was found at baseline in the no stress condition between drug treatment groups.
Table 2.
Results (mean ± SEM) from affective-like behavioral tests in adult mice that were exposed to sham surgery or oxycodone during adolescence are presented.
| Behavioral Test | Adolescent Sham Exposure | Adolescent Oxycodone Exposure |
|---|---|---|
|
| ||
| Marble Burying | ||
| Number of marbles buried | 7.6 ± 1.0 | 7.4 ± 1.1 |
| Open Field | ||
| Time spent in center (sec) | 13.5 ± 4.2 | 13.1 ± 4.2 |
| Total distance traveled (m) | 11.8 ± 0.9 | 13.6 ± 1.0 |
| Forced Swim | ||
| Time spent immobile (sec) | 181.8 ± 25.3 | 198 ± 24.3 |
Fig 2.
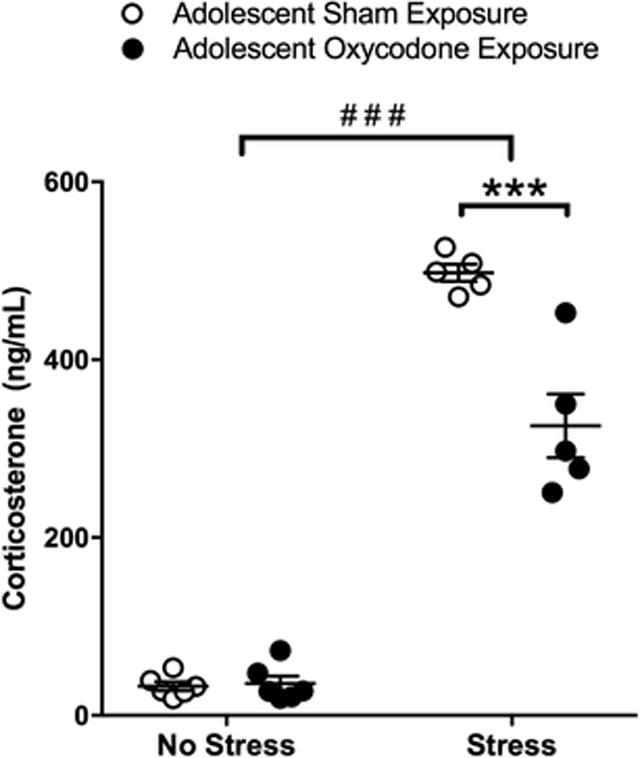
Plasma corticosterone in mice exposed to sham (open circles) or oxycodone (filled circles) during adolescence under control conditions (no stress) or following forced swim test (stress). Number sign indicates significant difference between stress exposure groups (###, p < 0.001) and asterisk indicates significant difference between drug exposure groups (***, p < 0.001)
The CPP paradigm was used to examine if adolescent oxycodone exposure affected sensitivity to the rewarding effects of morphine later in adulthood. A low dose of morphine (5 mg/kg) resulted in a low overall preference score in the sham control group (Fig 3). However, a significant increase in the preference score was observed in the adult mice that had been exposed to oxycodone during adolescence as compared to the sham controls (t16 = 2.169, p < 0.05; Fig 3).
Fig 3.
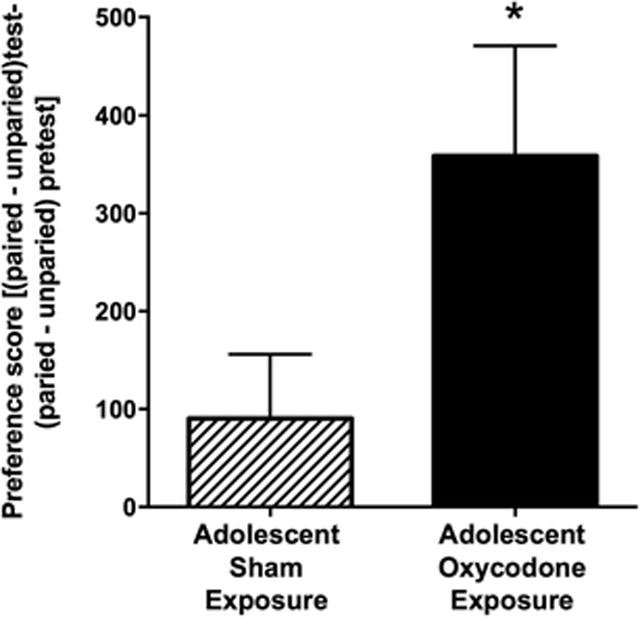
Oxycodone exposure in adolescence increases sensitivity to the rewarding effects of morphine in adulthood. The preference scores (mean ± SEM) are displayed for adult mice exposed to oxycodone (solid bar; n = 8) during adolescence and those exposed to sham (striped bar; n = 10) during adolescence. Asterisk indicates a significant difference between drug exposure groups (*, p < 0.05)
Sensitization to the locomotor activating effects of a moderate dose of morphine (10 mg/kg) was used to determine if adolescent exposure affected sensitivity to morphine induced plasticity later in adulthood. Regardless of adolescent treatment, both groups developed behavioral sensitization to the locomotor activating effects of repeated morphine exposure in adulthood. A repeated measure ANOVA revealed a significant effect of time (F2,22 = 49.16, p < 0.01) but no significant effect of adolescent treatment group or interaction (Fig 4).
Fig 4.
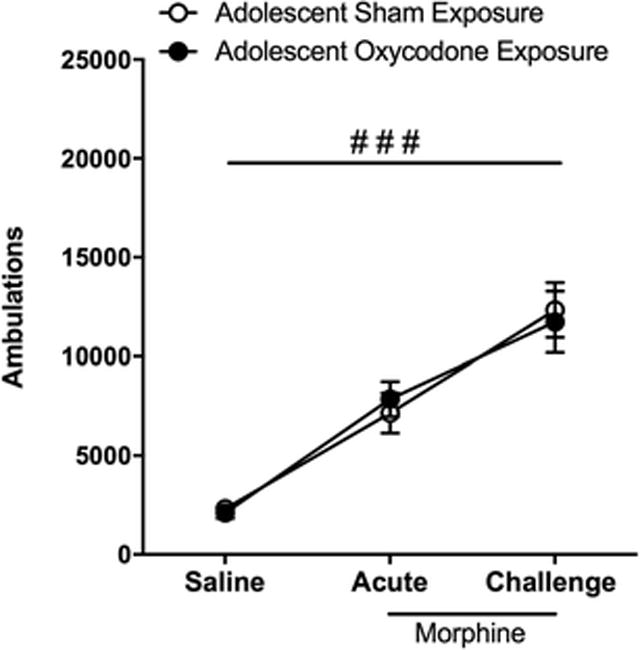
Locomotor sensitization to morphine is not affected by adolescent exposure to oxycodone. Locomotor activity (ambulations) recorded following the last saline baseline day (saline), the first injection 10 mg/kg morphine (acute), and following 4 daily injections of morphine and 1-week abstinence (challenge) are represented for adult mice exposed to sham (open circles; n = 6) and oxycodone (filled circles; n = 7) during adolescence. Both groups display a significant increase in ambulations over time (###, p < 0.01)
To examine underlying molecular changes associated with adolescent oxycodone exposure, we measured alterations in the expression of dopamine related genes within the reward pathway. We found a significant reduction in dopamine D1 receptor expression in NAc in adult mice previously exposed to oxycodone as adolescents (t11 = 2.612, p < 0.05; Fig 5a). No difference was observed in the expression of the D2 receptor within the NAc (Fig 5b). In the VTA, adolescent exposure to oxycodone significantly reduced DAT expression as compared to sham controls (t10 = 2.129, p < 0.05; Fig 5d). However, there was no effect of oxycodone exposure on the expression of TH (Fig 5e). Further, we found no difference in gene expression for MOR between groups in neither the NAc nor the VTA (Fig 5c,f).
Fig 5.
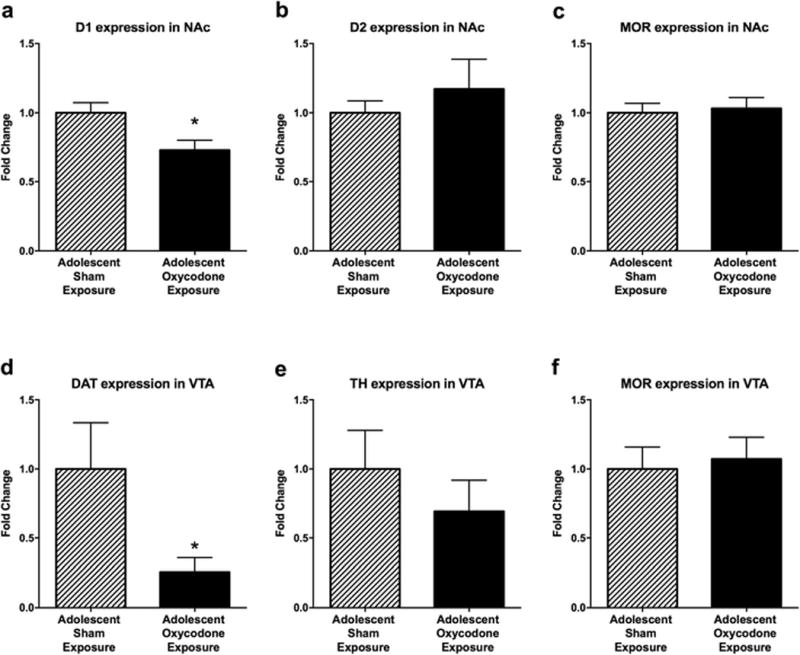
Adolescent exposure to oxycodone modulates dopamine-related gene expression in adulthood. The average (mean ± SEM) relative expression of (a) D1 receptor in the NAc, (b) D2 receptor in the NAc, (c) MOR in the NAc, (d) DAT in the VTA, (e) TH in the VTA, and (f) MOR in the VTA for adult mice that were exposed to sham surgery (striped bars; n = 6 - 8) and oxycodone (solid bars; n = 6 -7) during adolescence. Asterisk indicates a significant difference between drug exposure groups (*, p < 0.05)
Experiment 2
The purpose of this experiment was to determine if the effects of oxycodone exposure were contingent on a developmental period (adolescence) or if any drug exposure would also have long-lasting effects on behavior and gene expression in adulthood. We found that adult oxycodone exposure did not have long-lasting effects on anxiety-like behaviors. Specifically, no effect of previous oxycodone exposure was observed in the number of marbles buried (Table 3). Further, there was no difference between groups in time spent exploring the center area of the open field arena, total distance traveled within the open field arena, or time spent immobile in the forced swim test (Table 3). In addition, although forced swim test stress significantly increased plasma corticosterone levels as compared to no stress controls (Fig 6; F1,11 = 57.77, p < 0.0001), there was no difference in the plasma corticosterone levels between drug exposure groups following stress or no stress.
Table 3.
Results (mean ± SEM) from affective-like behavioral tests in adult mice that were exposed to sham surgery or oxycodone earlier in adulthood are presented.
| Behavioral Test | Adult Sham Exposure | Adult Oxycodone Exposure |
|---|---|---|
|
| ||
| Marble Burying | ||
| Number of Marbles Buried | 10.9 ± 1.1 | 12.9 ± 1.0 |
| Open Field | ||
| Time spent in center (sec) | 2.9 ± 1.0 | 2.8 ± 0.6 |
| Total Distance Traveled (m) | 13.3 ± 0.8 | 12.7 ± 1.6 |
| Forced Swim | ||
| Time spent immobile (sec) | 186.5 ± 27.4 | 217.5 ± 19.4 |
Fig 6.
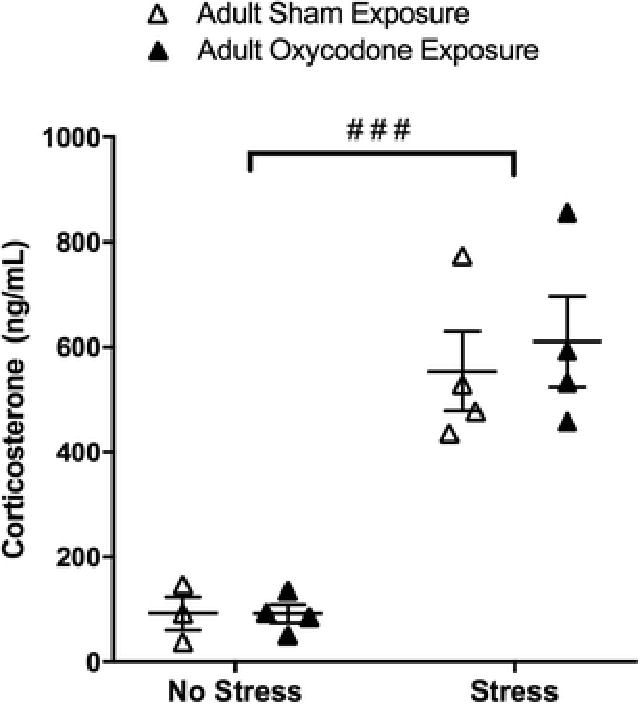
Plasma corticosterone in mice exposed to sham (open circles) or oxycodone (filled circles) during adulthood under control conditions (no stress) or following forced swim test (stress).
In contrast to findings in adolescent exposure, adult exposure to oxycodone did not alter the rewarding value of morphine. Specifically, similar preference scores were observed in adult mice regardless of oxycodone exposure following conditioning with a low dose (5mg/kg) of morphine (Fig 7). In addition, adult exposure did not affect acute or sensitized response to morphine (10mg/kg). Specifically, an overall repeated measures ANOVA of locomotor activity revealed a significant effect of time (F2,16 = 62.47, p < 0.01) with both groups displaying increased locomotor activity in response to a single and repeated morphine injections (Fig 8). However, no effects of adult treatment group or time by treatment group interaction were observed.
Fig 7.
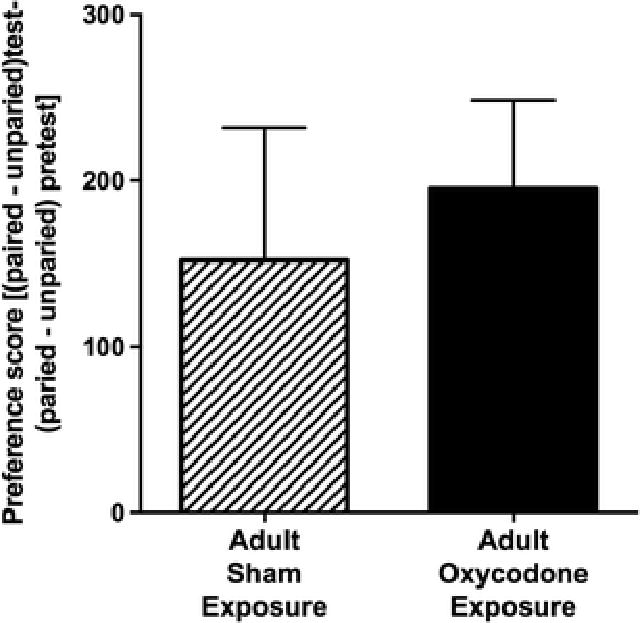
Adult exposure to oxycodone does not affect subsequent sensitivity to the rewarding effect of morphine later in adulthood. The preference scores (mean ± SEM) are displayed for adult mice exposed to oxycodone (solid bar; n = 9) during adulthood and those exposed to placebo (striped bar; n = 8) during adulthood.
Fig 8.
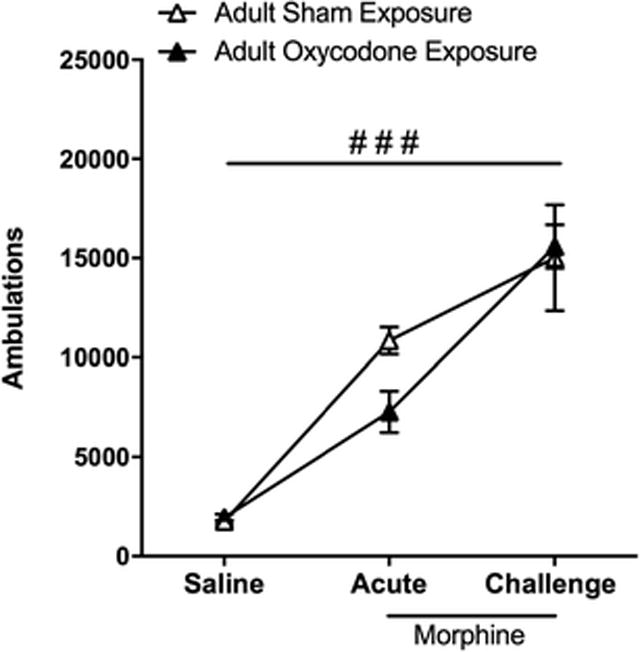
Locomotor sensitization to morphine is not affected by adult exposure to oxycodone Locomotor activity (ambulations) recorded following the last saline baseline day (saline), the first injection of 10 mg/kg morphine (acute), and following 4 daily injections of morphine and 1-week abstinence (challenge) are represented for adult mice exposed to placebo (open triangles; n = 6) and oxycodone (filled triangles; n = 5) during adulthood. Both groups display a significant increase in ambulations on acute and challenge days as compared saline day (###, p < 0.001)
Adult exposure to oxycodone did affect the expression of dopamine related genes within the reward pathway. In the NAc, D1 and D2 receptor expression were not significantly different between mice that had or had not been exposed to oxycodone in adulthood (Fig 9a and b). However, adult oxycodone exposure significantly increased DAT (t9 = 3.214, p < 0.01; Fig 9d) and TH (t11 = 2.485, p < 0.05; Fig 9e) expression in the VTA. Oxycodone exposure during adulthood did not alter expression of MOR in the NAc or VTA (Fig 8c,f).
Fig 9.
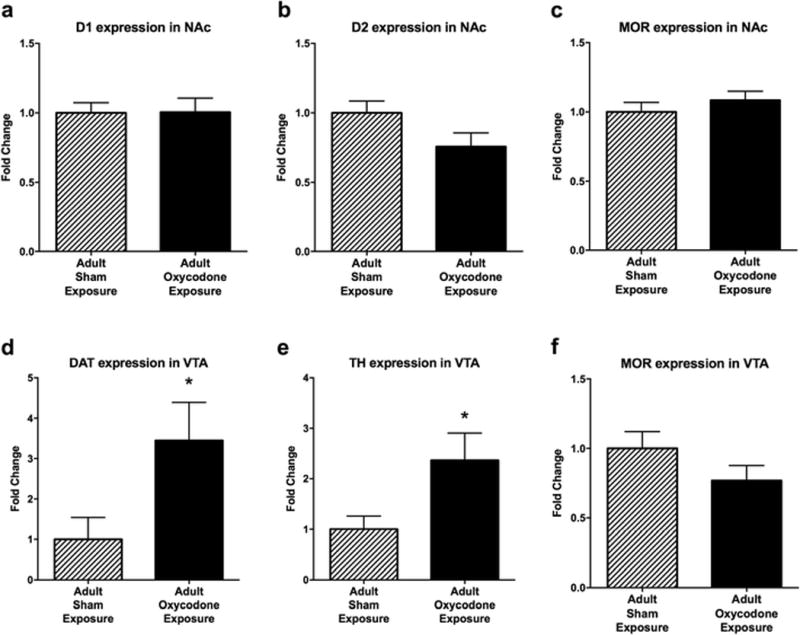
Adult exposure to oxycodone modulates dopamine-related gene expression later in adulthood. The average (mean ± SEM) relative expression of (a) D1 receptor in the NAc, (b) D2 receptor in the NAc, (c) MOR in the NAc, (d) DAT in the VTA, (e) TH in the VTA, and (f) MOR in the VTA for adult mice that were exposed to sham surgery (striped bars; n = 5 - 7) and oxycodone (solid bars; n = 6 - 7) earlier in adulthood. Asterisk indicates a significant difference between drug exposure groups (*, p < 0.05)
Discussion
Given the rise of prescription opioid abuse, particularly in youth, we determined if adolescent exposure to oxycodone had long-lasting effects on reward-related and anxiety-like behaviors later in adulthood. We observed that after adolescent oxycodone exposure, mice displayed increased response to the rewarding effects of morphine. Interestingly, adolescent oxycodone exposure did not affect morphine-induced locomotor sensitization or anxiety-like behaviors in adulthood, suggesting that long-lasting alterations may be specific to drug reward. To determine if long-lasting effects of oxycodone were specific to exposure during adolescence, a separate experiment was performed in which oxycodone exposure occurred during adulthood and behaviors were tested later in adulthood. Of importance, the period of drug withdrawal from oxycodone prior to behavioral testing was the same in both adolescent and adult exposure (28 days), to control for any effects of withdrawal on morphine reward. Indeed, both groups exhibit similar somatic signs of withdrawal. In contrast to mice exposed during adolescence, mice exposed to oxycodone during adulthood did not show enhanced response to morphine reward. Together, these findings indicate that adolescent oxycodone exposure may induce long-lasting alterations in the reward pathway, specifically in response to opioids.
Adolescence is a period of ongoing neurodevelopment particularly in the mesolimbic dopamine circuitry (Spear 2000; Burke and Miczek 2013). Therefore, it is possible that administration of drugs of abuse during this period could alter normal development of the reward pathway and lead to long-term changes in behavior and gene expression. Along these lines, previous work has demonstrated that prior exposure to other drugs of abuse during adolescence affects sensitivity to opioids. For example, a brief 3-day exposure to morphine during adolescence increases sensitivity to the locomotor activating effects of morphine later in adulthood (White and Holtzman 2005). Similarly other drugs of abuse that are known to act on the dopamine reward pathway have also been found to alter drug response later in life. Adolescent Δ(9)-tetrahydrocannabinol (THC) exposure increases heroin self-administration in adults (Ellgren et al. 2006; Tomasiewicz et al. 2012) and adolescent nicotine exposure increases fentanyl consumption in adulthood (Klein 2001). The work presented here adds oxycodone to the growing body of literature indicating adolescent exposure to drugs of abuse has long-lasting effects on vulnerability to subsequent drug abuse.
We found that adolescent exposure to oxycodone alters gene expression within the mesolimbic reward pathway later in adulthood. Specifically, we found a significant decrease in D1 receptor mRNA expression in the NAc and a reduction of DAT mRNA expression in the VTA. Developing adolescent brains show increasing expression of D1 and D2 receptor in the striatum that declines with age (Tarazi et al. 1998b; Tarazi et al. 1999; Naneix et al. 2012). Pruning and fine-tuning of the mesolimbic dopamine system is thought to be essential for adult development (Teicher et al. 1995), and maladaptive alterations may increase vulnerability to drug use later in life (Wise 1996). It is possible that the alterations we observed in D1 and DAT underlie the increased sensitivity to the rewarding effects of morphine observed in adulthood. The rewarding effects of morphine are mediated by both D1 and D2 receptors (Leone and Di Chiara 1987; Shippenberg and Herz 1988; Maldonado et al. 1997). Although reduced expression of D1 receptor mRNA was observed, suggesting decreased production of D1 receptors perhaps due to an over-pruning of neuronal pathways, the expression of D2 receptor mRNA was unchanged which could facilitate the rewarding properties of morphine.
However, dopamine is not the only mechanism mediating the rewarding effects of opioid drugs. Some evidence suggests that direct stimulation of mu receptors within the nucleus accumbens may contribute to morphine reward (Nutt et al., 2015). Regardless, adolescent exposure to oxycodone did not alter expression of MOR in the NAc or VTA. We did detect lower levels of DAT within the VTA, which may result in a stronger dopamine signal in response to morphine due to a reduced availability of transporters to remove dopamine from the synaptic cleft in the NAc. Indeed, knockout of the DAT increases morphine conditioned place preference in mice (Spielewoy et al. 2000). Taken together, oxycodone exposure during adolescence changes expression of key reward pathway genes, which may reflect an altered developmental trajectory leading to the observed behavioral differences in adulthood. However, future studies are necessary to determine if the alterations in gene expression in fact are the cause of the enhanced morphine reward in mice exposed to oxycodone during adolescence.
Adult exposure to oxycodone did not affect subsequent response to morphine in the CPP paradigm. There was a trend for a decrease in locomotor activity in response to an acute morphine exposure in adults pre-exposed to oxycodone during adulthood (Fig 8). Although this effect did not reach statistical significance, future studies with larger doses of morphine may provide evidence of attenuated acute locomotor response to morphine in adults pre-exposed to oxycodone. However, when tested on challenge day (1 week following chronic morphine exposure) locomotor activity was enhanced similarly to controls indicating the development of behavioral sensitization was not impaired.
Chronic exposure to oxycodone during adulthood did not significantly alter any expression of reward related genes in the NAc, but a significant increase in DAT gene expression in the VTA of adults pre-exposed to oxycodone was observed. This increase may reflect greater capacity for dopamine reuptake from the synaptic cleft in the striatum. It is important to note that in these studies gene expression analysis was conducted in tissue from mice that were never exposed to morphine. Therefore the increased DAT expression might result in lower levels of basal dopamine and this could account for the attenuated acute locomotor response to morphine found in adults pre-exposed to oxycodone. However, a significant increase in TH gene expression was also observed in the VTA. TH is the rate-limiting enzyme in dopamine synthesis. Thus, increased dopamine synthesis may compensate for increased DAT. While speculative, the lack of behavioral consequences associated with adult oxycodone exposure may reflect a plasticity in adults to regulate genes in the reward pathway to compensate for any adverse response. Future studies that examine gene expression as well as protein levels of DA and DAT in the VTA following an acute morphine exposure may help to clarify long-lasting neurochemical effects of adult oxycodone exposure.
Anxiety-like behavior, as assessed by the marble burying and open field tests, was not affected by oxycodone exposure during adolescence or adulthood. These findings were surprising given the strong association between affective disorders and opioid addiction in adolescents and adult humans (Morley et al. 2015; Edlund et al. 2015). Previous work in adolescent and adult mice has shown a significant effect of prior opioid exposure on affective-like behaviors following brief and extended withdrawal periods (Molina et al. 1994; Grasing and Ghosh 1998; Anraku et al. 2001; Hodgson et al. 2009). However, these findings are specific to depression-like behaviors rather then anxiety-like behaviors.
The forced swim test is a rodent behavioral test most often used for evaluation of antidepressant drugs but it has also been used to evaluate experimental manipulations that are aimed at rendering depressive-like states. We did not see differences in depression-like behavior in the forced swim test in adults, regardless of when oxycodone exposure occurred. This was also surprising because opioid receptor agonists produce antidepressant-like effects in the FST (Lutz and Kieffer 2013). Interestingly, we did observe a blunted corticosterone response in mice that were exposed to oxycodone during adolescence but not during adulthood.
While some types of stress can promote drug reward, others can depress drug intake presumable due to a loss of reward/pleasure (for review see, Miczek et al. 2008). Corticosterone is involved in a variety of behavioral and neurochemical effects of exposure to stress, however the relationship between blunted corticosterone following a stress and drug sensitivity is not known. In this case, both a blunted corticosterone response and an enhanced drug response were observed in adults that were exposed to oxycodone during adolescence. It is important to note that the altered stress response is not directly related to the enhanced morphine CPP behavior as these outcomes were assayed in separate cohorts of mice, but it is tempting to speculate that such an altered stress response in general might impact drug reward.
In conclusion, these data demonstrate that adolescent oxycodone exposure alters certain aspects of development of the mesolimbic reward pathway, which may underlie subsequent sensitivity to the rewarding effects of morphine. Furthermore, these data indicate that adolescents may be particularly sensitive to the long-lasting effects of oxycodone as the same effects were not observed when adults were exposed to oxycodone. Of interest, the increase in both TH and DAT in adults previously exposed to oxycodone could reflect no net change in dopamine signaling and may explain the lack of reward enhancing effects of morphine in these mice.
Future research examining molecular response to a drug challenge within the reward pathway of adults exposed to oxycodone during adolescence may yield insight into mechanism underlying subsequent enhanced vulnerability to morphine. In addition, females are known to be more sensitive to various drugs of abuse (Lynch et al. 2002; Carroll and Anker 2010), thus future studies to examine potential sex differences in the long-lasting effects of oxycodone exposure are critical.
Acknowledgments
This work was supported by grants from the National Institute on Drug Abuse [R01DA033646 (J.A.B.) and T32DA28874 (N.L.Y)] and the Postdoctoral Fellowship for Academic Diversity through the Vice Provost’s Office at the University of Pennsylvania (V.S.).
Footnotes
The authors have no conflicts of interest to disclose.
References
- Anraku T, Ikegaya Y, Matsuki N, Nishiyama N. Withdrawal from chronic morphine administration causes prolonged enhancement of immobility in rat forced swimming test. Psychopharmacology. 2001;157:217–220. doi: 10.1007/s002130100793. [DOI] [PubMed] [Google Scholar]
- Burke AR, Miczek KA. Stress in adolescence and drugs of abuse in rodent models: Role of dopamine, CRF, and HPA axis. Psychopharmacology. 2013;231:1557–1580. doi: 10.1007/s00213-013-3369-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll ME, Anker JJ. Sex differences and ovarian hormones in animal models of drug dependence. Horm Behav. 2010;58:44–56. doi: 10.1016/j.yhbeh.2009.10.001. [DOI] [PubMed] [Google Scholar]
- Cerdá M, Santaella J, Marshall BDL, et al. Nonmedical Prescription Opioid Use in Childhood and Early Adolescence Predicts Transitions to Heroin Use in Young Adulthood: A National Study. J Pediatr. 2015;167:605–12. e1–2. doi: 10.1016/j.jpeds.2015.04.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark DB, Kirisci L, Tarter RE. Adolescent versus adult onset and the development of substance use disorders in males. Drug and Alcohol Dependence. 1998;49:115–121. doi: 10.1016/s0376-8716(97)00154-3. [DOI] [PubMed] [Google Scholar]
- Di Chiara G, Imperato A. Drugs abused by humans preferentially increase synaptic dopamine concentrations in the mesolimbic system of freely moving rats. Proc Natl Acad Sci USA. 1988;85:5274–5278. doi: 10.1073/pnas.85.14.5274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doremus-Fitzwater TL, Varlinskaya EI, Spear LP. Motivational systems in adolescence: Possible implications for age differences in substance abuse and other risk-taking behaviors. Brain and Cognition. 2010;72:114–123. doi: 10.1016/j.bandc.2009.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edlund MJ, Forman-Hoffman VL, Winder CR, et al. Opioid abuse and depression in adolescents: Results from the National Survey on Drug Use and Health. Drug and Alcohol Dependence. 2015;152:131–138. doi: 10.1016/j.drugalcdep.2015.04.010. [DOI] [PubMed] [Google Scholar]
- Ellgren M, Spano SM, Hurd YL. Adolescent Cannabis Exposure Alters Opiate Intake and Opioid Limbic Neuronal Populations in Adult Rats. Neuropsychopharmacology. 2006;32:607–615. doi: 10.1038/sj.npp.1301127. [DOI] [PubMed] [Google Scholar]
- Grasing K, Ghosh S. Selegiline prevents long-term changes in dopamine efflux and stress immobility during the second and third weeks of abstinence following opiate withdrawal. NP. 1998;37:1007–1017. doi: 10.1016/s0028-3908(98)00093-8. [DOI] [PubMed] [Google Scholar]
- Hodgson SR, Hofford RS, Wellman PJ, Eitan S. Different affective response to opioid withdrawal in adolescent and adult mice. Life Sciences. 2009;84:52–60. doi: 10.1016/j.lfs.2008.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson SW, North RA. Opioids excite dopamine neurons by hyperpolarization of local interneurons. J Neurosci. 1992;12:483–488. doi: 10.1523/JNEUROSCI.12-02-00483.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones CM. Heroin use and heroin use risk behaviors among nonmedical users of prescription opioid pain relievers - United States, 2002–2004 and 2008–2010. Drug and Alcohol Dependence. 2013;132:95–100. doi: 10.1016/j.drugalcdep.2013.01.007. [DOI] [PubMed] [Google Scholar]
- Klein LC. Effects of adolescent nicotine exposure on opioid consumption and neuroendocrine response in adult male and female rats. Experimental and Clinical Psychopharmacology. 2001;9:251–261. doi: 10.1037//1064-1297.9.3.251. [DOI] [PubMed] [Google Scholar]
- Laviola G, Pascucci T, Pieretti S. Striatal dopamine sensitization to D-amphetamine in periadolescent but not in adult rats. Pharmacology Biochemistry and Behavior. 2001;68:115–124. doi: 10.1016/s0091-3057(00)00430-5. [DOI] [PubMed] [Google Scholar]
- Leone P, Di Chiara G. Blockade of D-1 receptors by SCH 23390 antagonizes morphine- and amphetamine-induced place preference conditioning. European Journal of Pharmacology. 1987;135:251–254. doi: 10.1016/0014-2999(87)90621-2. [DOI] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Lutz PE, Kieffer BL. Opioid receptors: distinct roles in mood disorders. Trends Neurosci. 2013;36:195–206. doi: 10.1016/j.tins.2012.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lutz PE, Reiss D, Ouagazzal AM, Kieffer BL. A history of chronic morphine exposure during adolescence increases despair-like behaviour and strain-dependently promotes sociability in abstinent adult mice. Behav Brain Res. 2013;243:44–52. doi: 10.1016/j.bbr.2012.12.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch WJ, Roth ME, Carroll ME. Biological basis of sex differences in drug abuse: preclinical and clinical studies. Psychopharmacology. 2002;164:121–137. doi: 10.1007/s00213-002-1183-2. [DOI] [PubMed] [Google Scholar]
- Maldonado R, Saiardi A, Valverde O, et al. Absence of opiate rewarding effects in mice lacking dopamine D2 receptors. Nature. 1997;388:586–589. doi: 10.1038/41567. [DOI] [PubMed] [Google Scholar]
- Mars SG, Bourgois P, Karandinos G, et al. “Every ‘never’ I ever said came true”: transitions from opioid pills to heroin injecting. Int J Drug Policy. 2014;25:257–266. doi: 10.1016/j.drugpo.2013.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miczek KA, Yap JJ, Covington HE. Social stress, therapeutics and drug abuse: Preclinical models of escalated and depressed intake. Pharmacol Ther. 2008;120:102–128. doi: 10.1016/j.pharmthera.2008.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molina VA, Heyser CJ, Spear LP. Chronic variable stress or chronic morphine facilitates immobility in a forced swim test: reversal by naloxone. Psychopharmacology. 1994;114:433–440. doi: 10.1007/BF02249333. [DOI] [PubMed] [Google Scholar]
- Morley KI, Lynskey MT, Moran P, et al. Polysubstance use, mental health and high-risk behaviours: Results from the 2012 Global Drug Survey. Drug Alcohol Rev. 2015;34:427–437. doi: 10.1111/dar.12263. [DOI] [PubMed] [Google Scholar]
- Naneix F, Marchand AR, Di Scala G, et al. Parallel Maturation of Goal-Directed Behavior and Dopaminergic Systems during Adolescence. J Neurosci. 2012;32:16223–16232. doi: 10.1523/JNEUROSCI.3080-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niikura K, Ho A, Kreek MJ, Zhang Y. Oxycodone-induced conditioned place preference and sensitization of locomotor activity in adolescent and adult mice. Pharmacology Biochemistry and Behavior. 2013;110:112–116. doi: 10.1016/j.pbb.2013.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G, Franklin KBJ. The Mouse Brain in Stereotaxic Coordinates. Academic Press; 2008. [Google Scholar]
- Schwarz JM, Bilbo SD. Adolescent Morphine Exposure Affects Long-Term Microglial Function and Later-Life Relapse Liability in a Model of Addiction. J Neurosci. 2013;33:961–971. doi: 10.1523/JNEUROSCI.2516-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shippenberg TS, Herz A. Motivational effects of opioids: influence of D-1 versus D-2 receptor antagonists. European Journal of Pharmacology. 1988;151:233–242. doi: 10.1016/0014-2999(88)90803-5. [DOI] [PubMed] [Google Scholar]
- Spanagel R, Herz A, Shippenberg TS. The effects of opioid peptides on dopamine release in the nucleus accumbens: an in vivo microdialysis study. Journal of Neurochemistry. 1990;55:1734–1740. doi: 10.1111/j.1471-4159.1990.tb04963.x. [DOI] [PubMed] [Google Scholar]
- Spear LP. The adolescent brain and age-related behavioral manifestations. Neuroscience and Biobehavioral Reviews. 2000;24:417–463. doi: 10.1016/s0149-7634(00)00014-2. [DOI] [PubMed] [Google Scholar]
- Spielewoy C, Gonon F, Roubert C, et al. Increased rewarding properties of morphine in dopamine-transporter knockout mice. Eur J Neurosci. 2000;12:1827–1837. doi: 10.1046/j.1460-9568.2000.00063.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stamford JA. Development and ageing of the rat nigrostriatal dopamine system studied with fast cyclic voltammetry. Journal of Neurochemistry. 1989;52:1582–1589. doi: 10.1111/j.1471-4159.1989.tb09212.x. [DOI] [PubMed] [Google Scholar]
- Tarazi FI, Tomasini EC, Baldessarini RJ. Postnatal development of dopamine and serotonin transporters in rat caudate-putamen and nucleus accumbens septi. Neuroscience Letters. 1998a;254:21–24. doi: 10.1016/s0304-3940(98)00644-2. [DOI] [PubMed] [Google Scholar]
- Tarazi FI, Tomasini EC, Baldessarini RJ. Postnatal development of dopamine D1-like receptors in rat cortical and striatolimbic brain regions: An autoradiographic study. Dev Neurosci. 1999;21:43–49. doi: 10.1159/000017365. [DOI] [PubMed] [Google Scholar]
- Tarazi FI, Tomasini EC, Baldessarini RJ. Postnatal development of dopamine D4-like receptors in rat forebrain regions: comparison with D2-like receptors. Brain Res Dev Brain Res. 1998b;110:227–233. doi: 10.1016/s0165-3806(98)00111-4. [DOI] [PubMed] [Google Scholar]
- Teicher MH, Andersen SL, Hostetter JC. Evidence for dopamine receptor pruning between adolescence and adulthood in striatum but not nucleus accumbens. Brain Res Dev Brain Res. 1995;89:167–172. doi: 10.1016/0165-3806(95)00109-q. [DOI] [PubMed] [Google Scholar]
- Tomasiewicz HC, Jacobs MM, Wilkinson MB, et al. Proenkephalin Mediates the Enduring Effects of Adolescent Cannabis Exposure Associated with Adult Opiate Vulnerability. BPS. 2012;72:803–810. doi: 10.1016/j.biopsych.2012.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner JR, Castellano LM, Blendy JA. Parallel anxiolytic-like effects and upregulation of neuronal nicotinic acetylcholine receptors following chronic nicotine and varenicline. Nicotine Tob Res. 2011;13:41–46. doi: 10.1093/ntr/ntq206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unick GJ, Rosenblum D, Mars S, Ciccarone D. Intertwined epidemics: national demographic trends in hospitalizations for heroin- and opioid-related overdoses, 1993–2009. PLoS ONE. 2013;8:e54496. doi: 10.1371/journal.pone.0054496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walters CL, Godfrey M, Li X, Blendy JA. Alterations in morphine-induced reward, locomotor activity, and thermoregulation in CREB-deficient mice. Brain Research. 2005;1032:193–199. doi: 10.1016/j.brainres.2004.11.013. [DOI] [PubMed] [Google Scholar]
- White DA, Holtzman SG. Periadolescent morphine exposure alters subsequent behavioral sensitivity to morphine in adult rats. European Journal of Pharmacology. 2005;528:119–123. doi: 10.1016/j.ejphar.2005.10.026. [DOI] [PubMed] [Google Scholar]
- Wise RA. Neurobiology of addiction. Curr Opin Neurobiol. 1996;6:243–251. doi: 10.1016/s0959-4388(96)80079-1. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Picetti R, Butelman ER, et al. Behavioral and neurochemical changes induced by oxycodone differ between adolescent and adult mice. Neuropsychopharmacology. 2009;34:912–922. doi: 10.1038/npp.2008.134. [DOI] [PMC free article] [PubMed] [Google Scholar]


