Abstract
Objective
To systematically review and analyze the efficacy and tolerability of different anti-depressant pharmacologic treatments for depressive symptoms in Parkinson’s disease (PD)
Methods
We searched PubMed, EMBASE, Cochrane database (CENTRAL), clinicaltrials.gov, and bibliographies for randomized controlled trials investigating the efficacy of antidepressant medications versus a non-treatment, placebo, or active treatment groups for depressive symptoms in PD. Twenty of 3191 retrieved studies (1893 patients) were included, but not all could be meta-analyzed. We used a random-effects model meta-analysis to compare depression scores between an active drug and placebo or control group then used a network meta-analysis to compare the effectiveness of different antidepressant classes. The primary outcome was the efficacy of different classes of antidepressant medications in PD patients with depressive symptoms, measured by standardized mean difference (SMD) in depression score from baseline compared with control.
Results
Pairwise meta-analysis suggested that type B-selective monoamine oxidase inhibitors (SMD = −1.28, CI = −1.68, −0.88), selective serotonin reuptake inhibitors (SMD = −0.49, CI = −0.93, −0.05), and tricyclics (SMD = −0.83, CI = −1.53, −0.13) are effective antidepressants in PD. Network meta-analysis showed that monoamine oxidase inhibitors had the largest effect on depression in PD (SMD (vs selective serotonin reuptake inhibitors) = −0.78, CI = −1.55, −0.01), but these might not be considered traditional antidepressants given their type B selectivity.
Conclusions
Although limited by few data, this review suggests that multiple antidepressant classes are potentially efficacious in the treatment of depression in PD, but that further comparative efficacy and tolerability research is needed.
Keywords: antidepressant, depression, meta-analysis, Parkinson’s disease
1 | INTRODUCTION
Depression is a common neuropsychiatric disturbance in Parkinson’s disease (PD), occurring in up to 35% of patients.1 Although PD is diagnosed and staged based on its motor symptoms, non-motor symptoms like depression have been shown to have a greater adverse impact on health-related quality of life.2–4 Depression in PD has been associated with greater disability, more rapid cognitive decline, higher rates of anxiety, increased mortality, and an increased burden on families and caregivers.5–8
Although the exact mechanisms are incompletely understood, both autopsy9,10 and functional connectivity11 studies in PD patients show abnormalities in the limbic system and other areas of the brain associated with depression and the production of monoamines. Derangements in the dopaminergic system may be even more relevant in PD depression than in depression in non-PD patients.12,13 Because this additional influence of dopaminergic denervation may represent a potential difference in pathophysiological mechanisms underlying depression in PD versus non-PD patients, treatment recommendations for depression in the non-PD population may not be generalizable to PD patients with depressive symptoms.
Treatments for depressive symptoms in PD have limited evidence for efficacy and tolerability, and there are no evidence-based guidelines to inform a “best” strategy for their use in clinical practice. Previous systematic reviews have focused on specific classes of pharmacologic treatments for depression (eg, selective serotonin reuptake inhibitors [SSRIs]).14 The objective of this review is to conduct a systematic review and network meta-analysis comparing the efficacy and tolerability of all classes of antidepressant medications that have been tested in the PD population.
2 | METHODS
2.1 | Study and patient inclusion/exclusion criteria
This systematic review included all randomized controlled trials investigating any antidepressant medications for treatment of depressive symptoms in a study population with PD. We included studies in which antidepressant therapies were compared with other antidepressants (active control), placebo, or no therapy. Observational studies were excluded. We included studies, written in English, of adult (age 20 and older) patients with idiopathic PD (as defined by study original authors) and depressive symptoms. We did not include trials comparing antidepressants to cognitive/behavioral therapy because that was beyond the purview of our research question regarding the efficacy of antidepressant pharmacotherapies in PD. We excluded patients with juvenile PD, atypical parkinsonism, and secondary parkinsonism due to the likely differential response to pharmacotherapy. We included patients with dementia, which commonly co-occurs with mood disorders in PD, although several trials excluded these patients. We excluded studies that explicitly excluded depressed PD patients. For the purposes of the network meta-analysis, medications were clustered by antidepressant class in each “node” to compare class effects, which are generally viewed as fairly homogenous within a particular class.15 To avoid publication bias, peer-reviewed journal publications, conference abstracts, and trials with outcomes reported in clinicaltrials.gov were all reviewed for inclusion.
2.2 | Search, selection, and extraction
We searched the Cochrane Central Register of Controlled Trials—CENTRAL in The Cochrane Library, Pubmed, EMBASE, and clinicaltrials.gov without any date or language restrictions. We used controlled vocabulary (MeSH and EMTREE) in addition to plain text searching (see Supplementary Material). We searched the reference lists of all included studies and relevant review articles using Web of Science. Two independent reviewers screened titles and abstracts then full text with discordance resolved through group consensus.
We reported the effect of each medication (and drug class) on depressive symptoms as a standardized mean difference (SMD) between groups to allow for comparison between various depression symptom severity scales. The primary time point for follow-up was 12 weeks, although we accepted measures between 4 and 24 weeks post-randomization.
To assess tolerability, the proportion of subjects experiencing discontinuation of intervention prior to trial completion in each study arm was compared using odds ratios with 95% confidence intervals. Change in motor symptoms, as measured using the Unified Parkinson’s Disease Rating Scale Part III (UPDRS III), was measured using a mean difference (95% CI). We assessed all included studies for any evidence that within-study missing data were not random. If missing data were not missing at random, differential between study arms, or common (eg, >20%), the study was labeled as high risk for attrition bias.
2.3 | Analytic methods
We analyzed classes of pharmacologic treatments separately because various mechanisms of action provide different degrees of efficacy in the non-PD literature.16 Within antidepressant class, clinical, methodological, and statistical heterogeneity was investigated by qualitative observation and later quantified using Q, τ2, and I2 statistics.17,18 Studies were compared with respect to sampling, inclusion and exclusion criteria, intervention, and outcome definition heterogeneity. Methodological heterogeneity was assessed by qualitative comparisons of study design and risk of bias between studies. Forest plots were used to visually inspect variability in estimates of intervention effect between studies.19
The results of all eligible studies were included in a qualitative review discussing the effects of each treatment modality on depressive symptoms. We explored differences in study design, depression measures, and population characteristics and discussed how these factors may contribute to reported outcomes. We planned to conduct a meta-analysis using Stata (Stata Statistical software: Release 14. College Station, TX, USA) with study level data if our qualitative review suggested sufficient clinical and methodological homogeneity between studies and limited statistical heterogeneity (ie, I2 is less than 50%). As the true treatment effect on depression likely depends on the severity of PD, other pharmacologic therapy for PD and other variables that were expected to vary between studies, we used a random-effects model for all analyses. The traditional meta-analysis compared the mean depression score between an active drug and a placebo or control group. We subsequently conducted a network meta-analysis that allowed us to investigate the comparative effectiveness of different antidepressant classes included in the reviewed studies.
2.4 | Assessment of consistency and bias across studies
If we found considerable statistical, clinical (such as dementia), or methodological heterogeneity among included studies, we planned to explore possible subgroup analyses focusing on likely contributors to heterogeneity. Because depressive symptom severity was measured using different survey instruments, we explored this source of methodological heterogeneity. We assessed reporting bias by examining funnel plots. Study characteristics that may affect symmetry of the funnel plot such as difference in methodological quality, selective outcome reporting, true heterogeneity, and other factors were considered.
3 | RESULTS
3.1 | Qualitative analysis (systematic review)
3.1.1 | Included studies
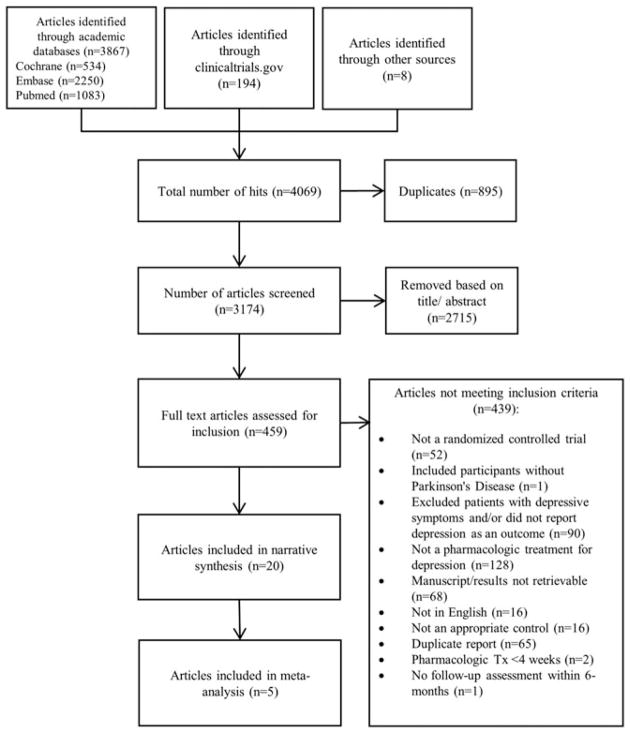
Electronic searches identified 3867 results from academic databases (CENTRAL: n = 534, Embase: n = 2250; Pubmed: n = 1083), 194 trial records through clinicaltrials.gov, and 8 articles through cross-referencing and hand searches. After de-duplication (n = 895), 3174 records were reviewed, and 459 of these were retained for full text review after title/abstract screening. The most common reasons for exclusion at the full text screening phase were not evaluating a pharmacologic treatment for depression (n = 128) and not reporting depression or excluding patients with depression (n = 90; Figure 1). Twenty randomized controlled trials published between 1988 and 2017 were included in this systematic review, 5 of which were abstracts (Table 1).
FIGURE 1.
Flow chart for systematic review study selection and screening
TABLE 1.
Studies included in the systematic review on the effect of antidepressant medications in PD.
| Author, Year | Setting | Arms: Drug (Class), Comparison |
Number of Patients Randomized |
Patient Age (Years)a |
Cognitive Impairment Eligibility Criteria |
Depression Eligibility Criteria |
Outcome Timing (Weeks) |
Depression: Primary or Secondary Outcome |
Depression Scales Used |
Tolerability Outcomes |
|---|---|---|---|---|---|---|---|---|---|---|
| Anderson 198035 | Denmark | Nortriptyline (tricyclic), placebo | 22 | NR | NR | Depressive symptoms ≥13 | 16 | Primary | 31-item rating scale | NR |
| Indaco 198836 | NR | Amytriptyline (tricyclic), placebo | 31 | Range: 41–70 | Excluded MMSE <24 | NR | 12 | Secondary | Zung self-rating scale | NC |
| Wermuth 199832 | Denmark | Citalopram (SSRI), placebo | 37 | 64 range: 44–79 | Excluded dementia | HAM-D ≥ 13 | 6 | Primary | HAM-D, MES | UPDRS III, NC, side effects |
| Trivedi 200220, (abstract) | NR | Sertraline (SSRI), bupropion (aminoketone) | 46 | NR | NR | NR | 6 | Primary | CGI, HAM-D | NR |
| Leentjens 200330 | Netherlands | Sertraline (SSRI), placebo | 12 | 67, SD = 7.8 | NR | DSM-IV depression | 10 | Primary | MADRS | UPDRS III |
| Avila 200333 | Spain | Nefazodone (SARI), fluoxetine | 16 | Range: 59–78 | Excluded “clinically relevant” | DSM-IV depression or dysthymic disorder | 12 | Primary | BDI | UPDRS III, NC |
| Antonini 200628 | Italy | Amytriptyline (tricyclic), sertraline (SSRI) | 31 | Range: 68.5–71.8 | Excluded dementia and MMSE < 24 | DSM-IV depression | 12 | Primary | HAM-D | UPDRS III, NC |
| Barone 200629 | Italy | Pramipexole, sertraline (SSRI) | 67 | Pramipexole: 64.8, SD = 8.3; SSRI: 68.1, SD = 6.5 | NR | HAM-D ≥ 16 | 14 | Primary | HAM-D, Zung Self-Rating Scale | UPDRS III, AEs, NC |
| Devos 200821 | France | Citalopram (SSRI), Desipramine (tricyclic), placebo | 48 | Range: 57–65 | Excluded MMSE <27, Mattis Dementia Rating Scale <130 | DSM-IV ongoing MDE and MADRS ≥20 | 4 | Primary | MADRS | UPDRS III, NC |
| Menza 200924 | United States | Paroxetine (SSRI), nortriptyline (tricyclic), placebo | 52 | 62.2, SD = 8.7 | Excluded MMSE < 26 | DSM-IV major depression or dysthymia | 8 | Primary | HAM-D | UPDRS III, SAEs, NC, side effects |
| Werneck 200939 | Brazil | Trazadone (SARI), control | 20 | 62.3 range: 45–75 | Excluded organic mental syndrome | NR | 20 | Primary | HAM-D | UPDRS II and III, NC |
| Djokic & Pavicevic 201022 (abstract) | NR | Sertraline (SSRI), mirtazapine, control | 141 | Range: 36–90 | NR | ICD-10/DSM IV depression | 12 | Primary | HAM-D | UPDRS37 I and II, AEs |
| Djokic & Zivkovix 201027 (abstract) | NR | Clomipramine (tricyclic), fluoxetine (SSRI), sertraline (SSRI), escitalopram (SSRI), mirtazapine (tetracyclic), Tianeptine (tricyclic), control | 339 | Range: 36–90 | NR | ICD-10/DSM IV depression | 12 | Primary | HAM-D | NR |
| Vasile 201026 (abstract) | NR | Escitalopram (SSRI), duloxetine (SNRI), trazadone (SSRI) | 23 | 72.1 | Included dementia | Admitted for MDD | 24 | Primary | CGI, HAM-D, MADRS | NR |
| Weintraub 201034 | United States | Atomoxetine (SNRI), placebo | 55 | 64.3, SD = 10.5 | Excluded if MMSE <15 | IDS-C ≥ 22 | 8 | Primary | CGI, IDS-C | UPDRS III, SAEs, NC |
| Richard 201225 | Canada, Puerto Rico, United States | Paroxetine (SSRI), venlafaxine (SNRI), placebo | 115 | 63.5, SD = 10.7 | Excluded dementia | DSM-IV depression | 12 | Primary | BDI-II, HAM-D | UPDRS III, AEs, NC |
| Lou 201323 (abstract) | NR | Atomoxetine (SNRI), Rivastigmine (cholinergic agent), placebo | 16 | NR | Included if MoCA >21 | NR | 6 | Secondary | CES-D | NR |
| Borgohain 201420 | Italy | Safinamide 100 mg (MAOI), safinamide 50 mg (MAOI), placebo | 669 | MAOI 100 mg: 60.1, SD = 9.2; MAOI 50 mg: 60.1, SD = 9.7; placebo: 59.4, SD = 9.4 | Excluded dementia | NR | 24 | Secondary | GRID HAM-D | UPDRS III, SAEs, AEs, NC, |
| Barone 201537 | India, Italy, Romania | Rasagiline (MAOI), placebo | 123 | MAOI: 66, SD = 8.7; placebo: 66.1, SD = 8.4 | Excluded if MMSE <26 | BDI ≥15, DSM-IV MDE within 1 year | 12 | Primary | BDI-IA | UPDRS III, SAEs, NC |
| Lim 201540 | NR | Rasagiline (MAOI), placebo | 30 | MAOI: 68.7, SD = 7.4; placebo:65.4, SD = 6.42 | Included MMSE Score > = 25 | NR | 12 | Secondary | BDI | AEs, side effects |
Summary statistic reported is the mean unless otherwise specified.
Abbreviations: AE: Adverse events; BDI: Beck Depression Inventory; CES-D: Center for Epidemiologic Studies Depression Scale; CGI: Clinical Global Impression; DSM: Diagnostic and Statistical Manual of Mental Disorders; HAM-D: Hamilton Rating Scale for Depression; ICD: International Classification of Diseases; IDS-C: Inventory of Depressive Symptomatology-Clinician; MADRS: Montgomery Asberg Depression Rating Scale; MAOI: Monoamine oxidase inhibitor; MDD: Major depressive disorder; MDE: Major depressive episode; Med: median; MES: Melancholia Scale; MMSE: Mini–mental state examination; MoCA: Montreal Cognitive Assessment; NC: Non-compliance/discontinued intervention; NR: not reported; SAE: Serious adverse events; SD: standard deviation; SNRI: Serotonin-norepinephrine reuptake inhibitor; SSRI: Serotonin-specific reuptake inhibitor; UPDRS: Unified Parkinson’s Disease Rating Scale
3.1.2 | Participants
Sample sizes ranged from 12 to 669 participants and all included persons with PD, most of whom were 60 years and older recruited from neurological departments and outpatient PD clinics in Denmark, Italy, India, Romania, France, Canada, Puerto Rico, Brazil, the Netherlands, Spain, and the United States. The majority of trials reported an average duration of PD ranging from 3 to 8 years. Inclusion criteria for all studies included a diagnosis of PD and 14 studies required that participants meet criteria for depressive disorder.
3.1.3 | Interventions and comparisons
Twelve studies were 2-arm randomized trials; however, there were seven 3-arm trials21–27 and one 7-arm trial.28 The trials had a combination of no-treatment (n = 3), placebo (n = 12), and active treatment only comparison groups (n = 5). The included studies contained arms evaluating the efficacy of multiple antidepressant classes: SSRIs (n = 12),22,23,25–34 selective norepinephrine reuptake inhibitors (SNRIs, n = 4),24,26,27,35 tricyclics (n = 6),22,25,28,29,36,37 monoamine oxidase inhibitors (MAOIs, n = 3),21,38,39 serotonin antagonist and reuptake inhibitors (SARIs),34,40 and other drugs including mirtazapine,23 combined atomoxetine and rivastigmine,24 bupropion,32 and trazodone.27
3.1.4 | Outcomes
All studies included a follow-up assessment between 4 and 24 weeks post-randomization. Studies mainly used the Hamilton Depression Rating Scale (HAM-D; n = 10) followed by the Beck Depression Inventory (BDI; n = 4) and the Montgomery Asberg Depression Rating Scale (MADRS; n = 3). Five studies classified participants as remitters22,25,29,30,35 or responders.22,25,29,31,35
Fourteen of the included studies provided information on our secondary outcome, tolerability measured as adverse events, changes in motor symptoms, and treatment non-compliance or dropout. Reporting of adverse events and side effects was inconsistent, so we analyzed treatment discontinuation as a measure of tolerability. Changes in motor symptoms were consistently measured using the UPDRS.
3.1.5 | Risk of bias
The risk of bias summary and judgments by risk of bias domain is depicted in Figure 2. Unclear risk of bias was common due to insufficient reporting of methods. Nine studies used random number sequence generation, and only 5 studies described specific methods of allocation. In 3 studies, participants were not blinded to intervention,29,30,40 and assessors were un-masked in 2 studies.30,34
FIGURE 2.
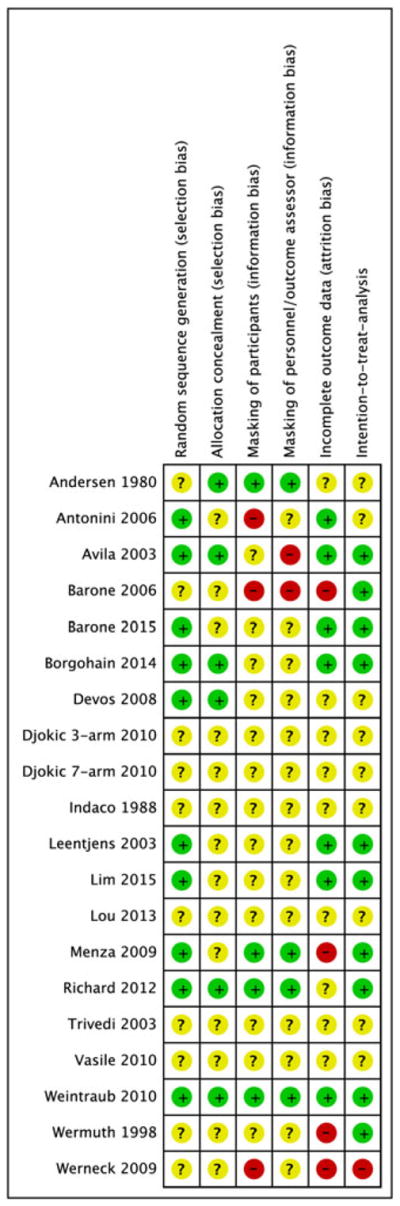
Green/+ = low likelihood of bias. Yellow/? = unclear bias (not enough information to assess). Red/− = high likelihood of bias. MAOI = monoamine oxidase inhibitor, SNRI = serotonin-norepinephrine reuptake inhibitor, SSRI = serotonin-specific reuptake inhibitor [Colour figure can be viewed at wileyonlinelibrary.com]
Ten studies described an intention-to-treat analysis such that all participants were analyzed as randomized.21,25,26,30,31,33–35,38,39 Two studies had differential losses to follow-up,30,40 and 2 had high rates of non-differential attrition bias25,33 and were rated as having a high risk of attrition bias. Seven of our included studies were funded by industry.21,29,31,33,36,38,41 Two studies were government funded, but with industry-supplied medications.25,26 Two studies were exclusively government funded,22 and 8 studies did not report a source of funding.20,23,24,27,28,30,37,40
3.2 | Summary of systematic review
We found mixed efficacy of antidepressants. Eight studies21–23,26,28,36,40,41 concluded that antidepressants were more efficacious than placebo or control, 531,33,35,37,38 found no benefit of antidepressant therapy, 1 concluded that a tricyclic was efficacious but an SSRI was not,25 and 1 abstract did not have interpretable results.24
The instruments used to assess depressive symptoms and differences in data reporting were important sources of methodological heterogeneity. PD and depression eligibility criteria were similar across studies that reported this information. Four studies included participants with dementia,24,27,34,39 in whom antidepressants have been shown to be less effective, thereby potentially modifying treatment effects.42,43
Differences in study design and outcome measurement contributed to methodological heterogeneity. The majority of studies were individual randomized-controlled trials; however, one of the included studies was a randomized crossover design.36 Each depression scale has its own sensitivity, specificity, and sensitivity to longitudinal change.43 While the majority of studies included a placebo comparison group (see Table 1), 3 included a no-treatment control group, and 5 only included active study arms. Research on the placebo effect has found some therapeutic benefit of placebos in placebo-control groups that are blind to study assignment in randomized trials of antidepres-sants.44 Thus, we might expect the effect size for trials employing a placebo group blind to study assignment to be smaller relative to trials employing a no-treatment control group.
3.3 | Meta-analyses and network meta-analysis
3.3.1 | Effect of treatment on depression
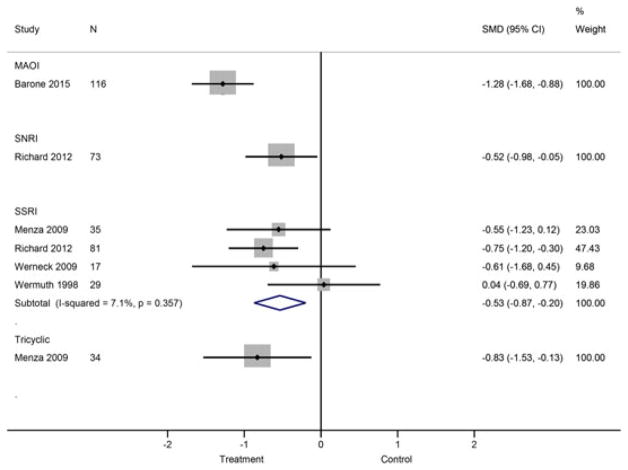
Standardized mean differences between active and control groups were calculated in 5 studies25,26,33,38,40 stratified by drug class (Figure 3). At the individual study level 3 studies reported significant intervention effects favoring MAOIs, SNRIs, SSRIs, and tricyclics over placebo.25,26,38 The 2 remaining SSRI versus placebo conditions25,33 and 1 SARI (trazodone) versus placebo condition40 found no significant differences.
FIGURE 3.
Meta-analysis of depression response in PD patients receiving antidepressants vs control or placebo. MAOI = monoamine oxidase inhibitor, SNRI = serotonin-norepinephrine reuptake inhibitor, SSRI = serotonin-specific reuptake inhibitor [Colour figure can be viewed at wileyonlinelibrary.com]
In a subgroup analysis comparing the SMD between SSRI and placebo groups, participants randomized to receive SSRI had lower depression scores relative to the comparison group (SMD = −0.534, 95% CI: −0.871, −0.198) (Figure 3). Statistical heterogeneity was fairly low across these studies: Q = 3.23 (df = 3, P = 0.357), I2 = 7.1%, τ2 = 0.0093. The planned sensitivity analyses could not be performed given the low number of studies in each of the drug classes.
To assess the relative efficacy of these drug classes and the comparison groups, we conducted a network meta-analysis to allow for direct and indirect comparisons to contribute to the estimation of all pairwise comparisons of treatment effects. As shown in the network map (Figure 4), the most common comparisons were between SSRIs and placebo. The League Table (Table 2) provides quantitative estimates of the pairwise comparisons by study condition. Participants randomized to SSRIs (SMD = 0.49, 95% CI: 0.02, 0.99), tricyclics (SMD = 0.77, 95% CI: 0.02, 1.53), and MAOIs (SMD = −1.27, 95% CI: −1.89, −0.66) experienced significantly lower depressive symptoms relative to participants randomized to placebo at approximately 12 weeks post-randomization (Table 2). There were no significant differences between the SNRI (SMD = −0.34, 95% CI: −1.57, 0.88) or SARI (SMD = 0.34, 95% CI: −0.88, 1.57) and placebo groups. Furthermore, participants in the MAOI condition had significantly better depression outcomes relative to the SSRI (SMD = −0.78, 95% CI: −1.55, −0.01) and SNRI (SMD = −0.90, 95% CI: −1.77, −0.03) groups.
FIGURE 4.
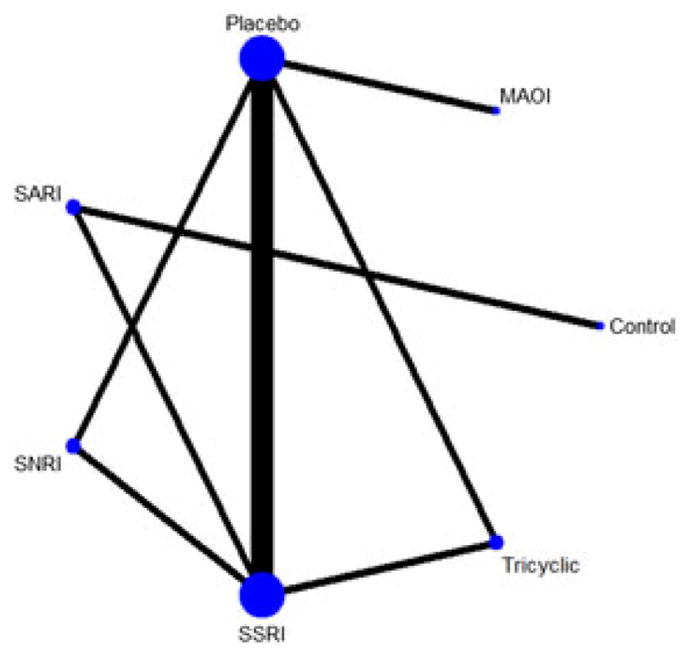
Network map for analysis comparing efficacy of antidepressant classes in PD. Nodes represents antidepressant drug classes. Node size represents size of study (N). Width of connecting lines represents number of studies assessed in each comparison. MAOI = monoamine oxidase inhibitor, SNRI = serotonin-norepinephrine reuptake inhibitor, SSRI = serotonin-specific reuptake inhibitor [Colour figure can be viewed at wileyonlinelibrary.com]
TABLE 2.
League table for network meta-analysis on antidepressant medication effect, by class, in PD
| CONTROL | 0.15 (−1.65, 1.94) | 0.43 (−1.19, 2.06) | 0.55 (−1.19, 2.28) | 0.58 (−0.58, 1.74) | 0.92 (−0.77, 2.61) | −0.35 (−2.15, 1.45) |
| TRICYCLIC | 0.28 (−0.46, 1.03) | 0.40 (−0.52, 1.31) | 0.43 (−0.93, 1.79) | 0.77 (0.02, 1.53) | −0.50 (−1.47, 0.47) | |
| SSRI | 0.11 (−0.50, 0.73) | 0.15 (−0.99, 1.28) | 0.49 (0.03, 0.95) | −0.78 (−1.55, −0.01) | ||
| SNRI | 0.03 (−1.26, 1.32) | 0.37 (−0.24, 0.99) | −0.90 (−1.77, −0.03) | |||
| SARI | 0.34 (−0.88, 1.57) | −0.93 (−2.30, 0.44) | ||||
| PLACEBO | −1.27 (−1.89, −0.66) | |||||
| MAOI |
Negative favors the column, positive favors the row; bold indicates significant difference from the null (zero).
Abbreviations: MAOI, monoamine oxidase inhibitor; SARI, serotonin antagonist and reuptake inhibitor; SNRI, serotonin-norepinephrine reuptake inhibitor; SSRI, serotonin-specific reuptake inhibitor.
3.3.2 | Effect of treatment on discontinuation and motor symptoms
Data on non-compliance and treatment discontinuation due to adverse events was available for all drug classes (Supplementary Material Figure 1). No significant differences in odds of discontinuation were found comparing MAOIs, SARIs, SSRIs, tricyclics, and SNRIs to their respective comparison groups. However, only 1 study including a SARI (trazodone)40 compared with placebo/control was analyzed, and a large confidence interval indicates an unreliable estimate (OR for discontinuation = 15.9, 95% CI: 0.70, 363.3).
Sufficient data were available to analyze mean difference in motor symptoms for SSRI and SNRI versus placebo, but no trials using MAOIs to target depression reported motor scores. Results suggest a significantly greater reduction in motor symptoms in participants randomized to SSRI as compared with placebo (MD = −3.29, 95% CI: −6.40, −0.18) with very little statistical heterogeneity (Q = 0.27 (df = 2, P = 0.875), I2 = 0.0%, τ2 = 0.000). The difference in motor symptoms was not significantly different between SNRI and placebo groups (MD = −0.10, 95% CI: −2.31, 2.11) with very little statistical heterogeneity Q = 0.37 (df = 1, P = 0.546), I2 = 0.0%, τ2 = 0.000.
4 | DISCUSSION
This systematic review and meta-analysis of the efficacy and tolerability of antidepressants for the treatment of depression in PD found support for the efficacy of SSRIs, MAOIs, and tricyclics in reducing depressive symptoms in patients with PD and depression when compared with placebo. None of the treatments appear to significantly worsen motor symptoms or increase the likelihood of treatment discontinuation. Our network meta-analysis comparing between-class efficacy of antidepressants suggests that the MAOIs such as safinamide or rasagaline had a larger effect size on depression outcomes compared with SSRIs, SNRIs, or placebo. Notably, the difference in SMD in depression severity was not statistically different between SSRIs and tricyclics or between SSRIs and SNRIs, suggesting the possible usefulness of multiple antidepressant drug classes. Within the SSRI studies, a similar SMD between SSRI and placebo was seen except for 1 study of citalopram,33 but this study did not describe clinical characteristics of the participants so the heterogeneity in study populations across SSRI studies could not be explored. These data may be particularly useful to neurologists and psychiatrists providing care for depressed PD patients given that PD-related depression may be more refractory to SSRIs and may require use of other antidepressant classes.
While our systematic review identified 20 included studies, many of these did not report continuous follow-up measurements for depression, allowing a meta-analysis of only 5 of the 20 included studies. Authors were contacted via email but did not respond within 3 months. Data on the secondary outcome, tolerability and adverse events, could not be synthesized due to differences in reporting across studies. Tolerability is important for depression treatments because it influences compliance and quality of life, and compliance was reasonably good in the 11 trials that reported discontinuation rates. Also problematic is the definition of “depression” or “depressive symptoms” in PD and the overlap with cognitive impairment, apathy, and behavioral disturbances. We acknowledge that considerable heterogeneity in the definition of depression exists among these studies and the relationship between treatment-associated changes in depression rating scales and related symptoms such as cognitive dysfunction and apathy could not be assessed given the available data in these trials. Use of a mixed effects rather than fixed effects meta-analysis accounts for some of this variability in patient populations between studies.
Risk of bias risk was assessed as “unclear” for many of the domains we examined in most studies (Figure 2). In the 5 studies included in the meta-analysis, only 2 studies were categorized as “high” risk of bias in any domain.33 Across studies, we found a slight asymmetry in the distribution of effects (Supplementary Material Figure 2), suggesting that publication bias may be present. This may be due to the inability to obtain outcomes data from several abstracts despite attempted contact with the authors.
Our findings are consistent with previous reviews evaluating the effect of antidepressants in PD, although our study includes more anti-depressant drug classes.14,45 One of these reviews focused on SSRIs,14 while the other included non-pharmacologic and non-antidepressant pharmacologic treatments for depression.45 A previous network meta-analysis found that TCAs had a larger effect than SSRIs on depression,46 and while our analysis trended in that direction, it was not significant (TCA vs SSRI 0.28, 95%CI: −0.46, 1.03). That study also did not include other antidepressant classes that were included in our network meta-analysis, such as SARIs (trazodone and nefazodne) or MAOIs (safinamide, rasagaline), but did compare SSRIs, SNRIs, and TCAs dopamine agonists including pramipexole and pergolide.46 Overall, our analysis was restricted to classes of medications considered to be antidepressants so as to potentially inform clinicians on the efficacy of antidepressant-class medications that patients may not already be taking for their motor symptoms of PD. Generally, our findings are in line with those of Liu et al with regard to comparative efficacy within antidepressant class medications. Notably, the largest effect on depressive symptoms in our network meta-analysis was found with MAOIs, including rasagaline,38,41 and safinamide,21 which is concordant with a recent finding from the ADAGIO trial that the combination of rasagaline and another antidepressant improved depression scores beyond what was found with placebo (instead of rasagaline) combined with another antidepressant.47
5 | CONCLUSION
Most of the research on pharmacologic treatments for depression in PD focuses on SSRIs, the first line of treatment in non-PD depression; however, our review incorporated other pharmacologic depression therapies, such as SARIs and MAOIs, which are less commonly used due to their known side effects. Based on the limited evidence evaluating the effect of MAOIs on depressive symptoms in PD, our network meta-analysis suggests that MAOIs might also be considered as therapeutic alternatives for depressive symptoms in PD, and they appear to be reasonably tolerated based on discontinuation rates. However, the included studies evaluating MAOIs have important caveats that limit generalizability. Nevertheless, MAOIs are also used to manage motor symptoms in PD, and although motor scores were not reported in the MAOI trials for depression in this review, they could help reduce polypharmacy if also effective for depressive symptoms. Given the difficulty that is sometimes seen in the treatment of PD-related depressive symptoms, broadening comparative effectiveness research to include other classes of antidepressants (beyond SSRIs and SNRIs) is warranted.
Supplementary Material
Key points.
SSRIs, TCAs, and MAOB-Is are effective in treating depressive symptoms in Parkinson’s disease
Multiple antidepressant classes can be considered efficacious in the treatment of Parkinson’s disease depression.
Acknowledgments
Funding information
National Center for Advancing Translational Sciences, Grant/Award Number: KL2TR001077; NIH/NIA, Grant/Award Number: K23AG044441; NIH/NHLBI, Grant/Award Numbers: F32HL129815 and T32HL007534; NIH/NIDA, Grant/Award Number: T32DA007292; NIH/NCATS, Grant/Award Number: UL1TR001079; KL2, Grant/Award Number: KL2TR001077
The authors would like to thank Drs. Kay Dickerson and Tianjing Li for their advice and oversight. None of the authors report any conflicts of interest with this research. Ethics approval was not required for this research. Kelly Mills is funded by KL2 scholar fund (KL2TR001077), a National Center for Advancing Translational Sciences grant to Johns Hopkins (UL1TR001079). Claire Greene is funded by a T32 award through NIH/NIDA (T32DA007292). Rebecca Dezube is funded by NIH/NHLBI (F32HL129815). Carrie Goodson is funded by NIH/NHLBI (T32HL007534). Gregory Pontone is funded by NIH/NIA (K23AG044441).
Footnotes
Additional Supporting Information may be found online in the supporting information tab for this article.
References
- 1.Reijnders JS, Ehrt U, Weber WE, Aarsland D, Leentjens AF. A systematic review of prevalence studies of depression in Parkinson’s disease. Mov Disord. 2008;23(2):183–189. doi: 10.1002/mds.21803. quiz 313. [DOI] [PubMed] [Google Scholar]
- 2.Barone P, Antonini A, Colosimo C, et al. The PRIAMO study: a multicenter assessment of nonmotor symptoms and their impact on quality of life in Parkinson’s disease. Mov Disord. 2009;24(11):1641–1649. doi: 10.1002/mds.22643. [DOI] [PubMed] [Google Scholar]
- 3.Carod-Artal FJ, Ziomkowski S, Mourao Mesquita H, Martinez-Martin P. Anxiety and depression: main determinants of health-related quality of life in Brazilian patients with Parkinson’s disease. Parkinsonism Relat Disord. 2008;14(2):102–108. doi: 10.1016/j.parkreldis.2007.06.011. [DOI] [PubMed] [Google Scholar]
- 4.Quelhas R, Costa M. Anxiety, depression, and quality of life in Parkinson’s disease. J Neuropsychiatry Clin Neurosci. 2009;21(4):413–419. doi: 10.1176/jnp.2009.21.4.413. [DOI] [PubMed] [Google Scholar]
- 5.Hughes TA, Ross HF, Mindham RH, Spokes EG. Mortality in Parkinson’s disease and its association with dementia and depression. Acta Neurol Scand. 2004;110(2):118–123. doi: 10.1111/j.1600-0404.2004.00292.x. [DOI] [PubMed] [Google Scholar]
- 6.Muller B, Assmus J, Herlofson K, Larsen JP, Tysnes OB. Importance of motor vs. non-motor symptoms for health-related quality of life in early Parkinson’s disease. Parkinsonism Relat Disord. 2013;19(11):1027–1032. doi: 10.1016/j.parkreldis.2013.07.010. [DOI] [PubMed] [Google Scholar]
- 7.Starkstein SE, Mayberg HS, Leiguarda R, Preziosi TJ, Robinson RG. A prospective longitudinal study of depression, cognitive decline, and physical impairments in patients with Parkinson’s disease. J Neurol Neurosurg Psychiatry. 1992;55(5):377–382. doi: 10.1136/jnnp.55.5.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pontone GM, Bakker CC, Chen S, et al. The longitudinal impact of depression on disability in Parkinson disease. Int J Geriatr Psychiatry. 2016;31(5):458–465. doi: 10.1002/gps.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Braak H, Ghebremedhin E, Rub U, Bratzke H, Del Tredici K. Stages in the development of Parkinson’s disease-related pathology. Cell Tissue Res. 2004;318(1):121–134. doi: 10.1007/s00441-004-0956-9. [DOI] [PubMed] [Google Scholar]
- 10.Politis M, Niccolini F. Serotonin in Parkinson’s disease. Behav Brain Res. 2015;277:136–145. doi: 10.1016/j.bbr.2014.07.037. [DOI] [PubMed] [Google Scholar]
- 11.Hu X, Song X, Yuan Y, et al. Abnormal functional connectivity of the amygdala is associated with depression in Parkinson’s disease. Mov Disord. 2015;30(2):238–244. doi: 10.1002/mds.26087. [DOI] [PubMed] [Google Scholar]
- 12.Thobois S, Ardouin C, Lhommee E, et al. Non-motor dopamine withdrawal syndrome after surgery for Parkinson’s disease: predictors and underlying mesolimbic denervation. Brain. 2010;133(Pt 4):1111–1127. doi: 10.1093/brain/awq032. [DOI] [PubMed] [Google Scholar]
- 13.Weintraub D, Newberg AB, Cary MS, et al. Striatal dopamine transporter imaging correlates with anxiety and depression symptoms in Parkinson’s disease. J Nucl Med. 2005;46(2):227–232. [PubMed] [Google Scholar]
- 14.Skapinakis P, Bakola E, Salanti G, Lewis G, Kyritsis AP, Mavreas V. Efficacy and acceptability of selective serotonin reuptake inhibitors for the treatment of depression in Parkinson’s disease: a systematic review and meta-analysis of randomized controlled trials. BMC Neurol. 2010;10:49. doi: 10.1186/1471-2377-10-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Trivedi MH, Fava M, Wisniewski SR, et al. Medication augmentation after the failure of SSRIs for depression. N Engl J Med. 2006;354(12):1243–1252. doi: 10.1056/NEJMoa052964. [DOI] [PubMed] [Google Scholar]
- 16.Furukawa TA, Salanti G, Atkinson LZ, et al. Comparative efficacy and acceptability of first-generation and second-generation antidepressants in the acute treatment of major depression: protocol for a network meta-analysis. BMJ Open. 2016;6(7):e010919. doi: 10.1136/bmjopen-2015-010919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Borenstein M, Hedges LV, Higgins JPT, Rothstein HR. Identifying and Quantifying Heterogeneity. Introduction to Meta-Analysis. West Sussex, UK: John Wiley & Sons, Ltd; 2009. [Google Scholar]
- 18.Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327(7414):557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sutton AJ, Abrams KR, Jones DR, Sheldon TA, Song F. Assessing Between Study Heterogeneity. Methods for Meta-analysis in Medical Research. West Sussex, UK: John Wiley & Sons, Ltd; 2000. [Google Scholar]
- 20.Trivedi A, Srivastava T, Batra D. Comperative Study of Efficacy for Bupropion vs Sertraline (SSRI) in Depression with Parkinson’s Disease. Yokohama, Japan. XII World Congress of Psychiatry, Aug 24–9; 2002; 2002. [Google Scholar]
- 21.Borgohain R, Szasz J, Stanzione P, et al. Randomized trial of safinamide add-on to levodopa in Parkinson’s disease with motor fluctuations. Mov Disord. 2014;29(2):229–237. doi: 10.1002/mds.25751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Devos D, Dujardin K, Poirot I, et al. Comparison of desipramine and citalopram treatments for depression in Parkinson’s disease: a double-blind, randomized, placebo-controlled study. Mov Disord. 2008;23(6):850–857. doi: 10.1002/mds.21966. [DOI] [PubMed] [Google Scholar]
- 23.Djokic G, Pavicevic D, Zivkovic N, Nenadovic M, Nenadovic N, Duisin D. Sertraline versus mirtazapine in treatment of depression in Parkinson’s disease. Eur Neuropsychopharmacol. 2010;20:S376–S377. [Google Scholar]
- 24.Lou JS, Dimitrova D, Chung KA, Andrea SB, Nutt J. The effects rivastigmine and atomoxetine on attention in nondemented Parkinson’s disease (PD) Mov Disord. 2013;28:S191–S192. [Google Scholar]
- 25.Menza M, Dobkin RD, Marin H, et al. A controlled trial of antidepressants in patients with Parkinson disease and depression. Neurology. 2009;72(10):886–892. doi: 10.1212/01.wnl.0000336340.89821.b3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Richard IH, McDermott MP, Kurlan R, et al. A randomized, double-blind, placebo-controlled trial of antidepressants in Parkinson disease. Neurology. 2012;78(16):1229–1236. doi: 10.1212/WNL.0b013e3182516244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vasile D, Vasiliu O, Vasile ML, et al. The efficacy of psychopharmacologic treatment in depressive disorder associated with Parkinson’s disease dementia. Eur Psychiatry. 2010;25 [Google Scholar]
- 28.Djokic G, Zivkovic N, Pavicevic D, Lazic D. Depression in Parkinson’s disease—searching for the most potent antidepressant. Eur Psychiatry. 2010;25 [Google Scholar]
- 29.Antonini A, Tesei S, Zecchinelli A, et al. Randomized study of sertraline and low-dose amitriptyline in patients with Parkinson’s disease and depression: effect on quality of life. Mov Disord. 2006;21(8):1119–1122. doi: 10.1002/mds.20895. [DOI] [PubMed] [Google Scholar]
- 30.Barone P, Scarzella L, Marconi R, et al. Pramipexole versus sertraline in the treatment of depression in Parkinson’s disease: a national multicenter parallel-group randomized study. J Neurol. 2006;253(5):601–607. doi: 10.1007/s00415-006-0067-5. [DOI] [PubMed] [Google Scholar]
- 31.Leentjens AF, Vreeling FW, Luijckx GJ, Verhey FR. SSRIs in the treatment of depression in Parkinson’s disease. Int J Geriatr Psychiatry. 2003;18(6):552–554. doi: 10.1002/gps.865. [DOI] [PubMed] [Google Scholar]
- 32.Trivedi A. Comparative study of efficacy for bupropion vs. sertraline (SSRI) in depression with Parkinsons disease [conference presentation]. International Psychogeriatrics [abstracts from The International Psychogeriatric Association Eleventh International Congress; Chicago, United States. 17–22 August 2003]; 2003. [Google Scholar]
- 33.Wermuth L, Sorensen PS, Timm S, et al. Depression in idiopathic Parkinson’s disease treated with citalopram. A placebo-controlled trial. Nordic Journal of Psychiatry—Nordisk Psykiatrisk Tidsskrift. 1998;52(2):163–169. [Google Scholar]
- 34.Avila A, Cardona X, Martin-Baranera M, Maho P, Sastre F, Bello J. Does nefazodone improve both depression and Parkinson disease? A pilot randomized trial. J Clin Psychopharmacol. 2003;23(5):509–513. doi: 10.1097/01.jcp.0000088908.24613.db. [DOI] [PubMed] [Google Scholar]
- 35.Weintraub D, Mavandadi S, Mamikonyan E, et al. Atomoxetine for depression and other neuropsychiatric symptoms in Parkinson disease. Neurology. 2010;75(5):448–455. doi: 10.1212/WNL.0b013e3181ebdd79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Andersen J, Aabro E, Gulmann N. Anti-depressive treatment in Parkinson’s disease. A controlled trial of the effect of nortriptyline in patients with Parkinson’s disease treated with L-dopa. Acta Neurol Scand. 1980;62(4):210–219. doi: 10.1111/j.1600-0404.1980.tb03028.x. [DOI] [PubMed] [Google Scholar]
- 37.Indaco A, Carrieri PB. Amitriptyline in the treatment of headache in patients with Parkinson’s disease: a double-blind placebo-controlled study. Neurology. 1988;38(11):1720–1722. doi: 10.1212/wnl.38.11.1720. [DOI] [PubMed] [Google Scholar]
- 38.Barone P, Santangelo G, Morgante L, et al. A randomized clinical trial to evaluate the effects of rasagiline on depressive symptoms in non-demented Parkinson’s disease patients. Eur J Neurol. 2015;22(8):1184–1191. doi: 10.1111/ene.12724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Malaty I. Rasagiline for the symptomatic treatment of fatigue in Parkinson’s disease. 2012. [DOI] [PubMed] [Google Scholar]
- 40.Werneck AL, Rosso AL, Vincent MB. The use of an antagonist 5-HT2a/c for depression and motor function in Parkinson’ disease. Arq Neuropsiquiatr. 2009;67(2b):407–412. doi: 10.1590/s0004-282x2009000300007. [DOI] [PubMed] [Google Scholar]
- 41.Lim TT, Kluger BM, Rodriguez RL, et al. Rasagiline for the symptomatic treatment of fatigue in Parkinson’s disease. Mov Disord. 2015;30(13):1825–1830. doi: 10.1002/mds.26429. [DOI] [PubMed] [Google Scholar]
- 42.Banerjee S, Hellier J, Dewey M, et al. Sertraline or mirtazapine for depression in dementia (HTA-SADD): a randomised, multicentre, double-blind, placebo-controlled trial. Lancet (London, England) 2011;378(9789):403–411. doi: 10.1016/S0140-6736(11)60830-1. [DOI] [PubMed] [Google Scholar]
- 43.Schrag A, Barone P, Brown RG, et al. Depression rating scales in Parkinson’s disease: critique and recommendations. Mov Disord. 2007;22(8):1077–1092. doi: 10.1002/mds.21333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kirsch I. Antidepressants and the placebo effect. Z Psychol. 2014;222(3):128–134. doi: 10.1027/2151-2604/a000176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bomasang-Layno E, Fadlon I, Murray AN, Himelhoch S. Antidepressive treatments for Parkinson’s disease: a systematic review and meta-analysis. Parkinsonism Relat Disord. 2015;21(8):833–842. doi: 10.1016/j.parkreldis.2015.04.018. discussion 833. [DOI] [PubMed] [Google Scholar]
- 46.Liu J, Dong J, Wang L, Su Y, Yan P, Sun S. Comparative efficacy and acceptability of antidepressants in Parkinson’s disease: a network meta-analysis. PLoS ONE. 2013;8(10):e76651. doi: 10.1371/journal.pone.0076651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Smith KM, Eyal E, Weintraub D. Combined rasagiline and antidepressant use in Parkinson disease in the ADAGIO study: effects on nonmotor symptoms and tolerability. JAMA Neurol. 2015;72(1):88–95. doi: 10.1001/jamaneurol.2014.2472. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.