Abstract
Conjunctival microcirculation imaging provides a non-invasive means for detecting hemodynamic alterations due to systemic and ocular diseases. However, reliable longitudinal monitoring of hemodynamic changes due to disease progression requires establishment of measurement variability over time. The purpose of the current study was to determine inter-visit variability of conjunctival microvascular hemodynamic measurements in non-diabetic control (NC, N = 7) and diabetic retinopathy (DR, N = 10) subjects. Conjunctival microvascular imaging was performed during 2 visits, which were 17 ± 12 weeks apart. Images were analyzed to determine vessel diameter (D), axial blood velocity (V), blood flow (Q), wall shear rate (WSR) and wall shear stress (WSS). The inter-visit variability was determined based on mean inter-visit differences. In NC, inter-visit variability of D, V, Q, WSR and WSS were 0.2 ± 0.5 µm, −0.01 ± 0.16 mm/s, −8 ± 46 pl/s, −3 ± 46 s−1 and −0.01 ± 0.10 dyne/cm2, respectively. Inter-visit variability of D, V, Q, WSR and WSS were beyond the normal 95% confidence limits in 60%, 20%, 40%, 20% and 20% of DR subjects, respectively. The variability of hemodynamic measurements over time was established in non-diabetic subjects, suggestive of the potential of the method for detecting longitudinal changes due to progression of DR.
Keywords: Conjunctival microvasculature, Inter-visit variability, Microcirculation, Microvascular hemodynamics, Diabetic retinopathy
1. Introduction
The bulbar conjunctiva is a vascularized mucus membrane covering the outer layer of the eye. Conjunctiva has gained attention in the literature due to the ease of accessibility and visibility of blood flow within the microvascular network. Imaging modalities including orthogonal polarization spectral imaging (van Zijderveld et al., 2014), slit-lamp biomicroscopy (Jiang et al., 2014; Khansari et al., 2016b; Koutsiaris et al., 2007; Shahidi et al., 2010), and intravital microscopy (Cheung et al., 2002a; Cheung et al., 1999) have been developed for assessment of conjunctival microvascular hemodynamics. Furthermore, commercial devices such as retinal functional imager (Jiang et al., 2013) and Heidelberg retinal flowmeter (Duench et al., 2007) have been modified to measure conjunctival hemodynamics. Application of these imaging modalities has shown conjunctival microvasculopathy and hemodynamic alterations due to systemic diseases such as Alzheimer's disease (Smith et al., 2009), hypertension (To et al., 2013), hypotension (Gaynes et al., 2012), diabetes (Khansari et al., 2017; Cheung et al., 2009; Cheung et al., 2001; Owen et al., 2008; To et al., 2011), and sickle cell disease (Cheung et al., 2002a; Kord Valeshabad et al., 2015; Paton, 1962; Wanek et al., 2013). Furthermore, a recent study showed a significant decrease in conjunctival blood flow, vessel density and non-perfused areas in brain dead subjects as compared to normal controls (Tamosuitis et al., 2016). Moreover, abnormal conjunctival hemodynamics was reported during internal carotid artery surgery (Schaser et al., 2003).
The study of conjunctival microvasculature may help elucidate information relevant to the study of microcirculation in other human organs. Conjunctival microvascular complications due to diabetes have been reported (Cheung et al., 2009; Owen et al., 2008; To et al., 2011), similar to those reported in the retina (Ditzel, 1967; Tarr et al., 2013). Additionally, conjunctival blood flow has shown to be correlated with sublingual microcirculation in rats (Yin et al., 2016), and with cerebral blood flow in dogs (Ohtani, 1996).
Since systemic diseases can cause alterations in the conjunctival microvascular hemodynamics, studying inter-visit variability of the measurements is crucial to determine sensitivity for detection of changes due to diseases. Previous studies have reported inter-visit variability of blood flow in native arteriovenous fistula in chronic hemodialysis subjects and retinal vascular oxygen saturation in healthy subjects (O'Connell et al., 2014; Valek et al., 2008). In the conjunctiva, intra-visit variability of hemodynamics has been established within one or multiple sessions during a single day (Duench et al., 2007; Khansari et al., 2016b; Xu et al., 2015). Nevertheless, to the best of our knowledge, inter-visit variability of conjunctival microvascular hemodynamics was not reported previously. The purpose of the current study was to determine inter-visit variability of conjunctival microvascular hemodynamic measurements in non-diabetic subjects and report the incidence of longitudinal variations in diabetic retinopathy (DR) subjects.
2. Materials and methods
2.1. Subjects
This study was approved by an institutional review board of the University of Illinois at Chicago. The study was explained to subjects and informed consents were obtained according to the tents of Declaration of Helsinki. The study population included 17 subjects: 7 non-diabetic control (NC) (4 males and 3 females) and 10 DR (6 males and 4 females) subjects. Diagnosis was based on retinal examination performed by retinal specialists based on clinical examination. The exclusion criteria were stroke or myocardial infarction within 3 months of imaging, active angina, age-related macular degeneration, glaucoma, dry eye syndrome, retinal vascular occlusions, history of intraocular surgery, or cataract surgery within 4 months of imaging. Subjects' age were 36 ± 19 years (mean ± standard deviation (SD)) and 57 ± 12 years in NC and DR, respectively (P = 0.01). Before imaging, subjects were asked to sit for approximately 10 min to facilitate a cardiovascular and respiratory resting state. During imaging, subjects were seated in front of the slit lamp biomicroscope with chin and forehead support. An external fixation target was presented to the fellow eye to minimize eye movements. The same imaging protocol was performed at the follow-up visit. The follow-up durations were 11 ± 15 weeks and 22 ± 8 weeks in NC and DR subjects, respectively (P = 0.06). Data in one eye of each subject with repeated measurements in 3 or more vessel segments was included.
2.2. Image acquisition
Image acquisition was performed by our previously established non-invasive imaging system, EyeFlow (Khansari et al., 2016b). The system incorporated a slit lamp biomicroscope coupled with a digital camera. Imaging was performed on conjunctival regions temporal to the limbus. Several 1-second high magnification image sequences were recorded at 5.1× at a rate of 50 Hz (exposure of 20 ms). The high magnification images composed of 1360 × 550 pixels with a pixel resolution of 1.25 µm on the object plane. Contiguous low magnification images of conjunctival microvascular were acquired at 2× magnification. The low magnification images composed of 1024 × 1360 pixels with a pixel resolution of 3.12 µm on the object plane. The high and the low magnification images covered approximately a conjunctival region of 1.7 mm × 0.8 mm and 3.2 mm × 4.2 mm, respectively.
2.3. Image processing and analysis
High magnification image sequences were analyzed quantitatively using our previously developed automated method (Khansari et al., 2016b). In summary, on average 17 (range; 6–41) consecutive image frames were registered using an intensity based image registration technique to correct for eye movements. A mean image was generated by averaging the registered images. Different size conjunctival microvessels were then segmented using Frangi vesselness filter applied to the mean image. Vessel caliber (D) and axial blood velocity (V) measurements were obtained by full width at half maximum of intensity profiles and from the slope of prominent bands in the spatial-temporal images (STI), respectively. Average cross-sectional blood velocity (Vs) was computed from measurements of D and V. Blood flow (Q = VsπD2 /4) and wall shear rate (WSR = 8Vs/D) were computed from Vs and D. Finally, wall shear stress (WSS = ηWSR) was determined based on WSR and dynamic blood viscosity (η), where η was calculated from clinical hematocrit values as described previously (Koutsiaris et al., 2007; Shahidi et al., 2010).
2.4. Detection of repeated vessel segments
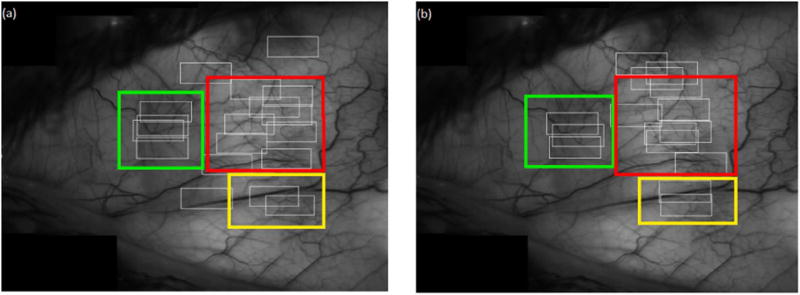
Conjunctival microvasculature regions imaged repeatedly were identified by generating a mosaic image and locating regions of imaging during each visit. The conjunctival mosaic image was generated per subject per visit as described previously (Khansari et al., 2016a). Briefly, contiguous low magnification images were processed semi-automatically using MosaicJ, a plug-in for ImageJ (ImageJ 1.48 V), to form a mosaic image. A human observer then used the best quality mosaic image from the 2 visits to locate conjunctival microvascular regions covered by each of the registered image sequences. Fig. 1 shows an example of the imaging regions (white boxes) in a NC subject at 2 visits. The mosaic image from the first visit was used to locate the repeated overlapping regions (color boxes).
Fig. 1.
Example of a conjunctival microvasculature mosaic image in an NC subject showing regions of image sequences (white boxes) for (a) the first and (b) the second visits. Overlapping regions between the 2 visits are shown by similarly colored boxes overlaid on the mosaic image. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
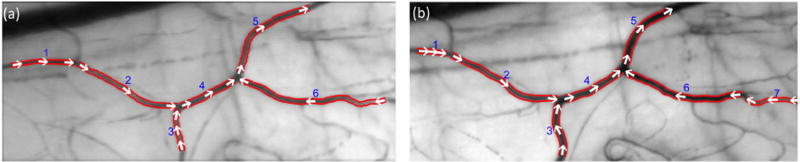
Image sequences acquired from the same conjunctival regions were inspected by a human observer to find repeated vessel segments with at least 50% overlap. Since the center of the image region may have been slightly shifted between visits, in some cases, repeated measurements in only part of vessel segments could be obtained. An example of conjunctival microvascular images showing the same microvascular region between the 2 visits in the same NC subject is displayed in Fig. 2. Vessel segments were numbered automatically, and the detected vessel boundaries were outlined by red lines, representing D. As shown in Fig. 2, the imaged vessel segment number 5 is longer in the first visit than in the second visit. Since there was more than 50% overlap, the data was included for analysis. Direction of RBC movement within microvasculature which was determined based on the sign of the slope of prominent bands in the STI is shown by white arrows. Conjunctival hemodynamic measurements (D, V, Q, WSR and WSS) were then compared between repeated vessel segment pairs to determine intervisit variability of the measurements.
Fig. 2.
Example of conjunctival microvascular images obtained by averaging registered image sequences for a NC subject in (a) the first and (b) the second visit. Vessel segments were numbered automatically, and the detected vessel boundaries were outlined by red lines. Direction of RBC movements is shown by white arrows. Vessel segments 1 to 5 were representing repeated microvasculature between the two visits. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
2.5. Data analysis
Compiled data from all subjects was analyzed using Excel software (Microsoft Corp., Redmond, WA, USA). Inter-visit variability of hemodynamic measurements was quantified in NC subjects by both the mean and SD of inter-visit differences. The 95% confidence interval (CI) of mean inter-visit differences was also calculated. Percentage of DR subjects with D, V, Q, WSR and WSS with mean inter-visit differences beyond CI of NC subjects was determined. Bland and Altman (Bland et al., 2007) plots were used to provide visualization for the mean intervisit differences and CI in NC subjects.
3. Results
In NC, a total of 67 repeated vessel segments were identified with D ranging from 11 µm to 38 µm and V ranging from 0.1 mm/s to 2 mm/s. In DR, a total of 48 repeated vessel segments were identified with D ranging from 10 µm to 48 µm and V ranging from 0.1 mm/s to 2.8 mm/s.
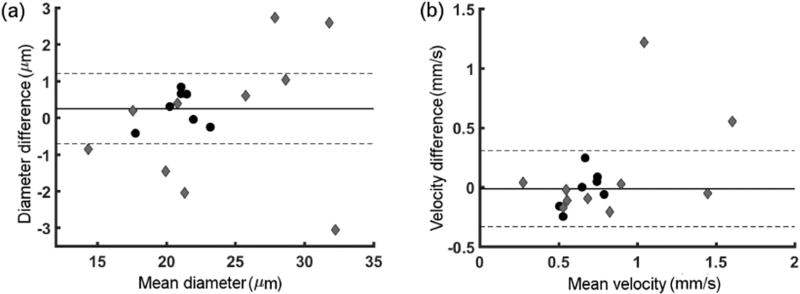
Inter-visit variability of conjunctival microvascular D in NC and DR subjects is shown in Fig. 3(a). In NC subjects, the mean difference was 0.2 ± 0.5 µm (CI: −0.7 µm to 1.2 µm). As can be seen from Fig. 3(a), 60% of DR had D difference beyond CI of NC subjects. However, directions of D changes were not consistent across DR subjects. Inter-visit variability of conjunctival microvascular V in NC and DR subjects is shown in Fig. 3(b). In NC subjects, the mean difference was −0.01 ± 0.16 mm/s (CI: −0.3 mm/s to 0.3 mm/s). As can be seen from Fig. 3(b), 20% of DR had V difference higher than CI of NC subjects.
Fig. 3.
Inter-visit variability of conjunctival microvascular D (a) and V (b) using Bland and Altman analysis. Difference against hemodynamic measurements per subject are shown for NC (black circles) and DR (gray diamond) subjects. Mean of differences (solid line) and 95% confidence interval (dashed lines) of NC subjects are also indicated.
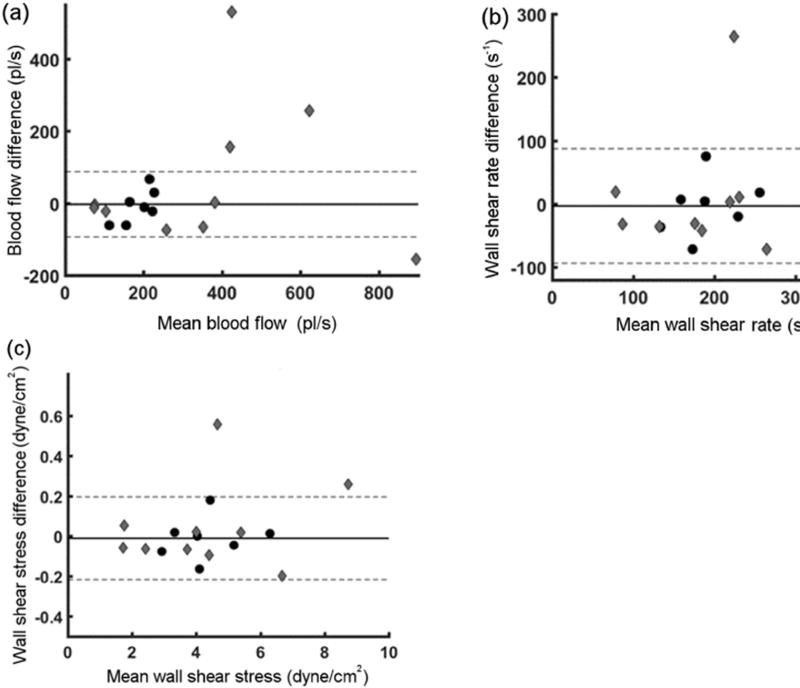
Inter-visit variability of conjunctival microvascular Q in NC and DR subjects is shown in Fig. 4(a), with mean difference of −8 ± 46 pl/s (CI: −99 pl/s to 83 pl/s) in NC subjects. As can be seen from Fig. 4(a), 40% of DR had Q difference beyond CI of NC subjects. Similar to D, directions of Q changes were not consistent across DR subjects. Intervisit variability of conjunctival microvascular WSR in NC and DR subjects is shown in Fig. 4(b), with mean difference of −3 ± 46 s−1 (CI: −93 s−1 to 87 s−1) in NC subjects. As can be seen from Fig. 4(b), 20% of DR had WSR difference higher than CI of NC subjects. Finally, intervisit variability of conjunctival microvascular WSS in NC and DR subjects is shown in Fig. 4(c), with mean difference of −0.01 ± 0.10 dyne/cm2 (CI: −0.2 dyne/cm2 to 0.2 dyne/cm2) in NC subjects. As can be seen from Fig. 4(c), 20% of DR had WSS difference higher than CI of NC subjects.
Fig. 4.
Inter-visit variability of conjunctival microvascular Q (a), WSR (b) and WSS (c) using Bland and Altman analysis. Difference against hemodynamic measurements per subject are shown for NC (black circles) and DR (gray diamond) subjects. Mean of differences (solid line) and 95% confidence interval (dashed lines) of NC subjects are also indicated.
In DR, inter-visit variability of D, V, Q, WSR and WSS were 0.01 ± 2 µm, 0.12 ± 0.44 mm/s, 60 ± 202 pl/s, 22 ± 102 s−1 and 0.04 ± 0.22 dyne/cm2, respectively.
4. Discussion
Alterations in the conjunctival microvascular hemodynamics can be readily quantified due to microcirculation accessibility for direct imaging (Cheung et al., 2002a; Cheung et al., 2009; Devaraj et al., 2007; Khansari et al., 2016b; Khansari et al., 2017; Kord Valeshabad et al., 2015; Koutsiaris et al., 2013; Smith et al., 2009). Furthermore, conjunctival microcirculation can provide insight into pathophysiology of conditions that can alter systemic circulation (Ohtani, 1996; Schaser et al., 2003; Tamosuitis et al., 2016; Yin et al., 2016). Establishing measurement variability is essential for discriminating between true hemodynamic alterations and random fluctuations. In the current study, inter-visit variability of conjunctival microvascular hemodynamics (D, V, Q, WSR and WSS) were reported in non-diabetic and DR subjects. The results showed some DR subjects had hemodynamic changes beyond the CI in NC subjects.
Previous studies have reported intra-visit variability of conjunctival microvascular hemodynamics in healthy subjects (Khansari et al., 2016b; Xu et al., 2015). However, to our knowledge, inter-visit variability of conjunctival microvascular hemodynamics has not been reported in NC subjects. In the current study, the 95% CI for detection of changes in D, V, Q, WSR and WSS was established. Several factors such as diet and stress could contribute to variability of hemodynamics measurements (Cohen et al., 2015; Davis et al., 2007). To enable comparison of current inter-visit variability with our previous report of intra-visit variability (Khansari et al., 2016b), mean SD of repeated measurements for the current data was calculated. In NC subjects, the intra-visit variability of D (Khansari et al., 2016b) was lower than the inter-visit variability (mean SD of repeated measurements between visits) reported in the current study (0.7 µm vs 1.08 µm), whereas intravisit and inter-visit variability of V were similar (0.17 mm/s vs 0.14 mm/s).
The primary conjunctival microvascular hemodynamic metrics are D and V which were shown to significantly change due to diabetes (Cheung et al., 2002a; Cheung et al., 2002b; Cheung et al., 2001; Kord Valeshabad et al., 2015). D variability was higher in more than half of DR than NC subjects, though the direction of change from the baseline to the follow-up visit was not consistent across subjects. V variability was higher in less than a quarter of DR and V increased in the follow-up visit. Q, WSR and WSS were determined based on D and V (Koutsiaris et al., 2007), and hence their variabilities depend on combined variations of D and V. The results of the current study showed between 20% to 40% of DR subjects had higher Q, WSR and WSS variability than NC subjects. It is important to note that apart from D and V, WSS is influenced by dynamic blood viscosity which increases with DR progression (Lowe et al., 1986). Dynamic blood viscosity is related to hematocrit (HCT). Nevertheless, the HCT values of DR subjects in the current study showed no significant change between visits (P = 0.4; t-test).
The current study had limitations. First, the number of subjects and repeated measurements in each group were limited. In fact, finding same vessels between visits was time-intensive due to a large number of vessels within the network and limited number of image sequences that could be acquired from each subject in each session. Nevertheless, 3 or more repeated measurements were obtained per subject to improve reliability of the results. Second, arterioles and venules were not discriminated in the current study since branching of the vessel segments could not be visualized in some of the image sequences, precluding reliable vessel type identification. Nevertheless, multiple measurements were averaged per each eye to minimize previously reported variation due to pulsation of blood flow in arterioles (Koutsiaris, 2016). Future studies can be helpful in determining inter-visit variability of conjunctival microvascular hemodynamics differentially in arterioles and venules. Third, reported inter-visit variability of conjunctival hemodynamics is specific to the imaging equipment and image processing algorithms employed in the current study. Some differences in measurement variability are likely with the use of different imaging techniques. Additionally, future studies with larger number of subjects and longer follow-ups can be useful for determining the effect of disease progression on conjunctival microvascular hemodynamics in DR subjects.
Overall, the current study established variability of conjunctival microvascular hemodynamics over time in NC subjects and reported feasibility of detecting variations in DR subjects over time. Longitudinal monitoring of conjunctival hemodynamic measurements shows promise for detecting changes due to disease progression.
Acknowledgments
Funding
This work was supported by the National Institutes of Health [DK104393 and EY001792]; Senior Scientific Investigator Award (MS) and departmental award from Research to Prevent Blindness.
Footnotes
Commercial relationship
None.
Financial disclosure
Mahnaz Shahidi holds a patent for the EyeFlow system used in the study and has the potential for financial benefits from its future commercialization. None of the other authors have any proprietary interest in any materials or methods.
References
- Bland J, et al. Agreement between methods of measurement with multiple observations per individual. J. Biopharm. Stat. 2007;17:571–582. doi: 10.1080/10543400701329422. [DOI] [PubMed] [Google Scholar]
- Cheung ATW, et al. Improvements in diabetic microangiopathy after successful simultaneous pancreas-kidney transplantation: a computer-assisted intravital microscopy study on the conjunctival microcirculation1, 2. Transplantation. 1999;68:927–932. doi: 10.1097/00007890-199910150-00005. [DOI] [PubMed] [Google Scholar]
- Cheung ATW, et al. Microvascular abnormalities in the bulbar conjunctiva of patients with type 2 diabetes mellitus. Endocr. Pract. 2001;7:358–363. doi: 10.4158/EP.7.5.358. [DOI] [PubMed] [Google Scholar]
- Cheung AT, et al. Microvascular abnormalities in sickle cell disease: a computer- assisted intravital microscopy study. Blood. 2002a;99:3999–4005. doi: 10.1182/blood.v99.11.3999. [DOI] [PubMed] [Google Scholar]
- Cheung AT, et al. Microvascular abnormalities in pediatric diabetic patients. Microvasc. Res. 2002b;63:252–258. doi: 10.1006/mvre.2001.2386. [DOI] [PubMed] [Google Scholar]
- Cheung ATW, et al. Correlation of microvascular abnormalities and endothelial dysfunction in Type-1 Diabetes Mellitus (T1DM): a real-time intravital microscopy study. Clin. Hemorheol. Microcirc. 2009;42:285–295. doi: 10.3233/CH-2009-1199. [DOI] [PubMed] [Google Scholar]
- Cohen BE, et al. State of the art review: depression, stress, anxiety, and cardiovascular disease. Am. J. Hypertens. 2015;28:1295–1302. doi: 10.1093/ajh/hpv047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis N, et al. The effect of diet on endothelial function. Cardiol. Rev. 2007;15:62–66. doi: 10.1097/01.crd.0000218824.79018.cd. [DOI] [PubMed] [Google Scholar]
- Devaraj S, et al. Evidence of increased inflammation and microcirculatory abnormalities in patients with type 1 diabetes and their role in microvascular complications. Diabetes. 2007;56:2790–2796. doi: 10.2337/db07-0784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ditzel J. Haemorheological factors in the development of diabetic microangiopathy. Br. J. Ophthalmol. 1967;51:793–803. doi: 10.1136/bjo.51.12.793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duench S, et al. Assessment of variation in bulbar conjunctival redness, temperature, and blood flow. Optom. Vis. Sci. 2007;84:511–516. doi: 10.1097/OPX.0b013e318073c304. [DOI] [PubMed] [Google Scholar]
- Gaynes B, et al. Feasibility of conjunctival hemodynamic measurements in rabbits: reproducibility, validity, and response to acute hypotension. Microcirculation. 2012;19:521–529. doi: 10.1111/j.1549-8719.2012.00182.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang H, et al. Human conjunctival microvasculature assessed with a retinal function imager (RFI) Microvasc. Res. 2013;85:134–137. doi: 10.1016/j.mvr.2012.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang H, et al. Functional slit lamp biomicroscopy for imaging bulbar conjunctival microvasculature in contact lens wearers. Microvasc. Res. 2014;92:62–71. doi: 10.1016/j.mvr.2014.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khansari MM, et al. Automated fine structure image analysis method for discrimination of diabetic retinopathy stage using conjunctival microvasculature images. Biomed. Opt. Express. 2016a;7:2597–2606. doi: 10.1364/BOE.7.002597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khansari MM, et al. Automated assessment of hemodynamics in the conjunctival microvasculature network. IEEE Trans. Med. Imaging. 2016b;35:605–611. doi: 10.1109/TMI.2015.2486619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khansari MM, et al. Assessment of conjunctival microvascular hemodynamics in stages of diabetic microvasculopathy. Sci. Rep. 2017;7 doi: 10.1038/srep45916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kord Valeshabad A, et al. Conjunctival microvascular haemodynamics in sickle cell retinopathy. Acta Ophthalmol. 2015;93:e275–e280. doi: 10.1111/aos.12593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koutsiaris AG. Pulsatility Index quantification in the human precapillary arterioles of the eye. Microvasc. Res. 2016;106:36–38. doi: 10.1016/j.mvr.2016.03.008. [DOI] [PubMed] [Google Scholar]
- Koutsiaris AG, et al. Volume flow and wall shear stress quantification in the human conjunctival capillaries and post-capillary venules in vivo. Biorheology. 2007;44:375–386. [PubMed] [Google Scholar]
- Koutsiaris AG, et al. Wall shear stress quantification in the human conjunctival pre-capillary arterioles in vivo. Microvasc. Res. 2013;85:34–39. doi: 10.1016/j.mvr.2012.11.003. [DOI] [PubMed] [Google Scholar]
- Lowe G, et al. Increased blood viscosity in diabetic proliferative retinopathy. Diabetes Res. (Edinburgh, Scotland) 1986;3:67–70. [PubMed] [Google Scholar]
- O'Connell RA, et al. Test-retest reliability of retinal oxygen saturation measurement. Optom. Vis. Sci. 2014;91:608–614. doi: 10.1097/OPX.0000000000000257. [DOI] [PubMed] [Google Scholar]
- Ohtani N. Laser Doppler flowmetry of the bulbar conjunctiva as a monitor of the cerebral blood flow. [Zasshi] [Journal] Nihon Kyobu Geka Gakkai. 1996;44:1721–1728. [PubMed] [Google Scholar]
- Owen CG, et al. Diabetes and the tortuosity of vessels of the bulbar conjunctiva. Ophthalmology. 2008;115:e27–e32. doi: 10.1016/j.ophtha.2008.02.009. [DOI] [PubMed] [Google Scholar]
- Paton D. The conjunctival sign of sickle-cell disease: further observations. Arch. Ophthalmol. 1962;68:627–632. doi: 10.1001/archopht.1962.00960030631010. [DOI] [PubMed] [Google Scholar]
- Schaser KD, et al. Noninvasive analysis of conjunctival microcirculation during carotid artery surgery reveals microvascular evidence of collateral compensation and stenosis-dependent adaptation. J. Vasc. Surg. 2003;37:789–797. doi: 10.1067/mva.2003.139. [DOI] [PubMed] [Google Scholar]
- Shahidi M, et al. Quantitative assessment of conjunctival microvascular circulation of the human eye. Microvasc. Res. 2010;79:109–113. doi: 10.1016/j.mvr.2009.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith MM, et al. Whole blood viscosity and microvascular abnormalities in Alzheimer's Disease. Clin. Hemorheol. Microcirc. 2009;41:229–239. doi: 10.3233/CH-2009-1174. [DOI] [PubMed] [Google Scholar]
- Tamosuitis T, et al. Conjunctival microcirculatory blood flow is altered but not abolished in brain dead patients: a prospective observational study. BMC Neurol. 2016;16(1) doi: 10.1186/s12883-016-0618-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarr JM, et al. Pathophysiology of diabetic retinopathy. ISRN Ophthalmol. 2013;2013 doi: 10.1155/2013/343560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- To WJ, et al. Correlation of conjunctival microangiopathy with retinopathy in type-2 diabetes mellitus (T2DM) patients. Clin. Hemorheol. Microcirc. 2011;47:131–141. doi: 10.3233/CH-2010-1374. [DOI] [PubMed] [Google Scholar]
- To WJ, et al. Real-time studies of hypertension using non-mydriatic fundus photography and computer-assisted intravital microscopy. Clin. Hemorheol. Microcirc. 2013;53:267–279. doi: 10.3233/CH-2012-1567. [DOI] [PubMed] [Google Scholar]
- Valek M, et al. Physiologic variability of vascular access blood flow for hemodialysis. Blood Purif. 2008;26:468–472. doi: 10.1159/000157324. [DOI] [PubMed] [Google Scholar]
- Wanek J, et al. Human bulbar conjunctival hemodynamics in hemoglobin SS and SC disease. Am. J. Hematol. 2013;88:661–664. doi: 10.1002/ajh.23475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Z, et al. Measurement variability of the bulbar conjunctival microvasculature in healthy subjects using functional slit lamp biomicroscopy (FSLB) Microvasc. Res. 2015;101:15–19. doi: 10.1016/j.mvr.2015.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin L, et al. Changes in sublingual microcirculation is closely related with that of bulbar conjunctival microcirculation in a rat model of cardiac arrest. Shock (Augusta, Ga.) 2016;45:428–433. doi: 10.1097/SHK.0000000000000508. [DOI] [PubMed] [Google Scholar]
- van Zijderveld R, et al. Orthogonal polarization spectral imaging of conjunctival microcirculation. Graefes Arch. Clin. Exp. Ophthalmol. 2014;252:773–779. doi: 10.1007/s00417-014-2603-9. [DOI] [PubMed] [Google Scholar]