Abstract
Introduction
Treatment restrictions in the first 2 days after intracerebral haemorrhage have been independently associated with an increased risk of early death. It is unknown whether these restrictions also affect mortality if these are installed several days after stroke onset.
Patients and methods
Sixty patients with severe functional dependence at day 4 after ischaemic stroke or intracerebral haemorrhage were included in this prospective two-centre cohort study. The presence of treatment restrictions was assessed at the day of inclusion. Information about mortality, functional outcome (modified Rankin scale) score and quality of life (visual analogue scale) was recorded 6 months after stroke onset. Poor outcome was defined as modified Rankin scale >3. Satisfactory quality of life was defined as visual analogue scale ≥ 60.
Results
At 6 months, 30 patients had died, 19 survivors had a poor functional outcome and 9 patients had a poor quality of life. Treatment restrictions were independently associated with mortality at 6 months (adjusted relative risk, 1.30; 95% confidence interval, 1.06–1.59; p = 0.01), but not with functional outcome.
Discussion
Our findings were observed in 60 selected patients with severe stroke.
Conclusion
The instalment of treatment restrictions by itself may increase the risk of death after stroke, even if the first 4 days have passed. In future stroke studies, this potential confounder should be taken into account. Quality of life was satisfactory in the majority of the survivors, despite considerable disability.
Keywords: Stroke, ethics, mortality, survival
Introduction
Most in-hospital deaths of patients with acute stroke occur after a decision to withhold or withdraw life-sustaining therapies.1,2 The process to make decisions about treatment restrictions in patients with acute stroke differs from that in patients with progressive disease such as cancer because stroke patients often cannot fully participate in this process and because continuation of treatment potentially allows patients to live for months or years at the cost of being left in a state of disability that might be against their wishes.3
Treatment restrictions in the first 2 days after intracerebral haemorrhage have been independently associated with an increased risk of early death,2,4,5 and avoidance of new do-not-resuscitate (DNR) orders during the first 5 days after intracerebral haemorrhage has been associated with a substantially lower 30-day mortality rate than predicted.6 Treatment restrictions are also frequently installed in a later stage,7 but it has not been investigated whether these are also associated with early mortality. Postponing the instalment of treatment restrictions increases the window of opportunity for patients to express their wishes regarding life-sustaining treatments.
In this prospective observational study, we assessed the relation between the placement of treatment restrictions and mortality in patients who had survived the first 4 days after severe ischaemic stroke or intracerebral haemorrhage. We also assessed functional outcome and quality of life in survivors.
Methods
This is a prospective two-centre cohort study. Consecutive patients admitted at the stroke unit with an acute severe ischaemic or haemorrhagic stroke with a very small chance of functional independency after 6 months (defined as Barthel Index (BI) ≤ 6 out of 20 at day 4)8 were included. Patients with a subarachnoid haemorrhage and incompetent patients without an available legal representative were excluded from the study. Patients were included between September 2012 and December 2013 in the University Medical Center Utrecht, and between January and December 2013 in the St. Elisabeth hospital in Tilburg, a large regional teaching hospital in the Netherlands.
We collected information on patient characteristics, type of stroke (ischaemic or haemorrhagic), stroke severity on admission (by means of National Institutes of Health Stroke Scale (NIHSS) and pre-stroke comorbidity (by means of the Charlson Comorbidity Index (CCI)).9 Treatment restrictions were assessed by a semi-structured questionnaire administered to the treating physician at the day of inclusion.
Treatment restrictions were coded for the following categories: (a) DNR order, (b) withhold admission to intensive care unit (ICU), (c) withhold curative treatment of complications and (d) withhold artificial nutrition and hydration. These are incremental steps: each treatment restriction is added up to the before-mentioned treatment restrictions. We assessed all in-hospital treatment restrictions that were installed at study inclusion.
One trained investigator (FASdK) visited each patient and his/her caregiver at 6
months (±6 weeks) after stroke to assess functional outcome and quality of life.
Functional outcome was assessed with the modified Rankin Scale (mRS); poor outcome
was defined as mRS > 3. Patients’ quality of life was measured with a visual
analogue scale (VAS).10 The VAS was a vertical line of 10 cm with a ‘ ’ at the top demarcating the
best possible quality of life and a ‘
’ at the top demarcating the
best possible quality of life and a ‘ ’ at the lower end for the worst
possible quality of life. Scores were calculated as the indicated level in
(cm/10) × 100. Quality of life was considered acceptable if VAS ≥ 60.
’ at the lower end for the worst
possible quality of life. Scores were calculated as the indicated level in
(cm/10) × 100. Quality of life was considered acceptable if VAS ≥ 60.
The primary outcome measure was mortality at 6 months. Secondary outcome measures were functional outcome (mRS) and quality of life (VAS) at 6 months. The association between treatment restrictions and these outcomes was calculated with Poisson regression analysis with a robust error after adjustment for age, sex, NIHSS on admission, BI at day 4, CCI and type of stroke (ischaemic or haemorrhagic). We expressed associations as adjusted relative risk (aRR) with 95% CI.
We performed post-hoc subgroup analyses in patients with acute ischaemic stroke and intracerebral haemorrhage separately. In this subgroup analyses, we adjusted for age, sex, NIHSS on admission, BI at day 4 and CCI.
The study was approved by the institutional review board of each centre, and written informed consent was obtained from each patient or a legal representative.
Results
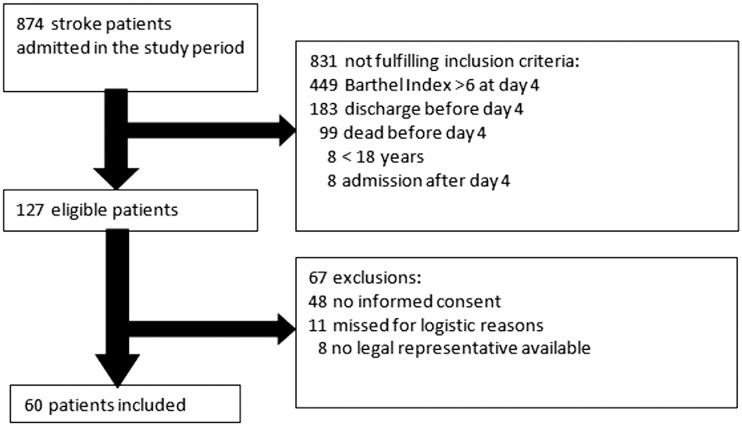
Of 874 stroke patients admitted during the course of the study, 127 fulfilled the inclusion criteria and 60 were included. Eight patients were excluded because they had no legal representative available, 48 patients declined participation and 11 were missed (Figure 1).
Figure 1.
Flow of patients through this study.
The median time between stroke onset and inclusion was 6 days (range, 4–10). The mean age of the patients was 72 years (SD 15); 30 (50%) were male; the median NIHSS on admission was 16 (3–28) and the median BI at day 4 was 2 (0–6). Additional patient characteristics are presented in Table 1.
Table 1.
Baseline characteristics.
| All patients n = 60 | Full care n = 18 | DNR-ordera n = 42 | Withhold admission at ICU n = 30 | No curative treatment of complications n = 12 | Withhold artificial nutrition and hydration n = 10 | |
|---|---|---|---|---|---|---|
| Age (years) | 72 (15) | 56 (11) | 79 (11) | 80 (12) | 78 (15) | 80 (8) |
| Men | 30 (50) | 13 (72) | 17 (41) | 11 (37) | 4 (33) | 3 (30) |
| Ischaemic stroke | 36 (60) | 10 (56) | 26 (62) | 19 (63) | 5 (42) | 4 (40) |
| NIHSS on admission | 16 (6) | 16 (6) | 16 (6) | 16 (7) | 19 (7) | 19 (7) |
| CCI | 1 (0–6) | 0 (0–4) | 1 (0–6) | 1 (0–6) | 1 (0–4) | 1 (0–4) |
| Barthel Index at day 4 | 0 (0–6) | 2 (0–3) | 0 (0–6) | 0 (0–6) | 0 (0–0) | 0 (0–0) |
Data are n (%), median (range) or mean (standard deviation (SD)) where appropriate.
DNR-order represents all treatment restrictions.
DNR: do not resuscitate; ICU: Intensive Care Unit; NIHSS: National Institutes of Health Stroke Scale; CCI: Charlson Comorbidity Index.
Forty-two patients (70%) had one or more treatment restrictions. Patients without treatment restrictions were younger than patients with treatment restrictions (56 vs 79 years, p < 0.001), and were more often men (72 vs 41%, p = 0.02) (Table 1).
At 6 months, 30 (50%) patients had died, of whom 12 during admission. The median time from stroke onset to in-hospital death was 9 days (range, 3–18). Twenty-eight of the patients who died (93%) had a treatment restriction.
The presence of any treatment restriction at study inclusion was independently associated with mortality at 6 months (aRR, 1.30; 95% confidence interval, 1.06–1.59; p = 0.01). Each individual type of treatment restriction was also associated with mortality at 6 months (Table 2).
Table 2.
Results on adjusted Poisson regression analysis on the relation between type of treatment restrictions and mortality.
| aRRa | 95%CI | P | |
|---|---|---|---|
| DNR-orderb | 1.30 | 1.06–1.59 | 0.01 |
| Withhold admission at ICU | 1.41 | 1.20–1.65 | <0.001 |
| No curative treatment of complications | 1.26 | 1.11–1.44 | 0.001 |
| Withhold artificial nutrition and hydration | 1.19 | 1.05–1.34 | 0.01 |
Adjusted for age, sex, National Institutes of Health Stroke Scale score on admission, Barthel Index at day 4, Charlson Comorbidity Index and type of stroke.
DNR-order represents all treatment restrictions.
aRR: adjusted relative risk; CI: confidence interval; DNR: do not resuscitate; ICU: Intensive Care Unit.
At 6 months, 19 of 30 survivors (63%) had a poor functional outcome (Table 3, Figure 2). Quality of life could be assessed in 26 survivors. Mean score on the VAS was 60 (SD 17). Quality of life was considered satisfactory in 11 of 16 (69%) survivors with a poor functional outcome, and in 6 of 10 (60%) patients with a good functional outcome (Table 3).
Table 3.
Outcome of survivors at 6 months.
| All patients n = 60 | Full care n = 18 | DNR-ordera n = 42 | Withhold admission at ICU n = 30 | No curative treatment of complications n = 12 | Withhold artificial nutrition and hydration n = 10 | |
|---|---|---|---|---|---|---|
| Alive at 6 months | 30 (50) | 16 (89) | 14 (33) | 5 (17) | 0 (0) | 0 (0) |
| Good functional outcomeb | 10 (34) | 7 (44) | 3 (23) | 1 (20) | 0 (0) | 0 (0) |
| Satisfactory quality of lifec | 17 (65) | 10 (63) | 7 (70) | 2 (66) | 0 (0) | 0 (0) |
Data are n (%), median (range) or mean (standard deviation (SD)) where appropriate.
DNR represents all treatment restrictions.
n = 29 survivors, 1 patient declined follow-up.
n = 26 survivors, 3 patients declined follow-up and one was aphasic.
DNR: do not resuscitate; ICU: Intensive Care Unit; NIHSS: National Institutes of Health Stroke Scale; CCI: Charlson Comorbidity Index.
Figure 2.
Functional outcome at 6 months.
Treatment restrictions were not associated with a poor functional outcome in survivors (Table 4), but patient numbers were small.
Table 4.
Results on adjusted Poisson regression analysis on the relation between treatment restrictions and poor functional outcome in survivors.
| aRRa | 95%CI | p | |
|---|---|---|---|
| DNR-orderb | 0.78 | 0.49–1.23 | 0.28 |
| Withhold admission at ICU | 0.87 | 0.64–1.17 | 0.34 |
| No curative treatment of complications | 0.96 | 0.81–1.15 | 0.68 |
| Withhold artificial nutrition and hydration | 1.02 | 0.87–1.18 | 0.84 |
Adjusted for age, sex, National Institutes of Health Stroke Scale score on admission, Barthel Index at day 4, Charlson Comorbidity Index and type of stroke.
DNR-order represents all treatment restrictions.
aRR: adjusted relative risk; CI: confidence interval; DNR: do not resuscitate; ICU: Intensive Care Unit.
Subgroup analysis
In a post-hoc subgroup analysis in the 36 patients with ischaemic stroke, results were essentially the same. The presence of any treatment restriction at study inclusion was independently associated with mortality at 6 months (aRR, 1.33; 95% confidence interval, 1.01–1.76; p = 0.04). Each individual type of treatment restriction was also associated with mortality at 6 months (Supplemental Table 1).
In 24 patients with intracerebral haemorrhage, results were comparable but did not reach statistical significance (aRR, 1.15; 95% confidence interval, 0.97–1.36; p = 0.11) (Supplemental Table 2).
Discussion
This study shows that in patients with severely disabling ischaemic stroke or intracerebral haemorrhage, treatment restrictions installed several days after stroke onset are associated with mortality at 6 months, independent of age, sex, stroke severity or pre-stroke comorbidity.
This association between treatment restrictions and mortality is probably at least partially causal, because the aim of these restrictions is to withhold potentially life-prolonging treatments when future quality of life expected to be insufficient, prioritising comfort care. The associations persist after adjustment for other factors that might affect survival such as age, pre-stroke comorbidity and stroke severity. Therefore, our findings suggest that treatment restrictions after the first 4 days increase the risk of death.
Previous studies have also shown that treatment restrictions are associated with mortality in patients with intracerebral haemorrhage2,4,11,12 and in study populations with both ischaemic and haemorrhagic stroke patients.13,14 Avoidance of early DNR orders has been associated with a substantially lower risk of death,6 supporting a causal relationship between treatment restrictions and early mortality. Whether this relation is causal indeed can only be tested in randomised trials of full medical support during a prespecified time period vs. ‘standard’ care, which includes the placement of treatment restrictions, but this design will likely be considered unethical.
Our findings have important consequences. In clinical practice, physicians should realise that treatment restrictions on their own may increase the risk of death, and that a poor functional outcome does not necessarily implicate an unsatisfactory quality of life. Therefore, physicians should be cautious to withhold their patients a chance on recovery by installing treatment restrictions too early. With respect to intervention trials and prognostic studies, confounding by treatment restrictions should also be avoided, and where this is not possible, the placement of treatment restrictions should be assessed. Confounding by treatment restrictions could be controlled by the adoption of a standard for withdrawal of life-sustaining treatment in the study protocol.
Treatment restrictions can be appropriate after severe stroke to prevent a patient for staying alive at the cost of being left in a state of disability that might be against his or her wishes. What constitutes a poor outcome is however difficult to adequately define. Although the majority of patients in our study had a poor functional outcome, the majority of the survivors had a satisfactory quality of life. While increasing disability is generally associated with a reduction in quality of life, this is not the first time that quality of life has been reported satisfactory in patients with a disabling stroke.15,16 Assessment of quality of life by these patients is probably influenced by a response shift, which includes a change in the internal standards and values in the self-assessment of quality of life,17 and by the capacity of patients with chronic illness or disability to adapt to their circumstances, a phenomenon often referred to as the disability paradox.18,19 Unfortunately, in the early phase after stroke, it is still unclear how to identify patients who will adapt well to their new situation and recapture a good quality of life.
We aimed to include patients with a very small chance on regaining functional independence, because treatment restrictions are probably most often installed in this patient group. The BI is an easy accessible and widely used scale to measure ADL dependency. A cut-off point of six on the BI at day 5 has previously been shown to be an accurate predictor of ADL independency at 6 months.8 We measured the BI on day 4 as part of routine clinical practice in both participating centres. According to the high rate of patients with poor outcome, the cut-off point of 6 on the BI at day 4 was appropriate.
This study has limitations. We could not include half of the eligible patients, because the majority of these patients declined participation. In addition, patients with more severe strokes or their relatives might have been more likely to decline consent, which may have led to selection bias. Our primary outcome was mortality at 6 months, and 60% of deaths occurred after discharge. We have no data on mortality at an earlier time point after discharge and not on treatment restrictions after discharge. We consider it likely that most restrictions already installed were not changed. Moreover, our findings were observed in 60 highly selected patients with severe stroke, and our findings do not apply to patients who are not severely disabled at day 4. We included both patients with severe ischaemic stroke or intracerebral haemorrhage, whereas patients who survive intracerebral haemorrhage to the point of rehabilitation have greater improvement in functional abilities than similarly affected patients with ischaemic stroke.12 However, our findings were independent of stroke type. We adjusted for pre-stroke comorbidities but did not collect data on the presence of complications that occurred after stroke, which may have had on impact on prognosis. Finally, quality of life data should be interpreted with caution because patients could have given desired answers during the home visit.
In conclusion, both clinicians and researchers should realise that placement of treatment restrictions by itself may increase the risk of death after stroke. ‘Our results need further confirmation. Randomised controlled trials on this topic will not be feasible for ethical reasons. Larger multi-centre cohort studies, prospectively assessing the relation between treatment limitations and mortality should further confirm our findings’. Future research should clarify the clinical practices in end-of-life decisions in stroke patients and focus on identifying patients who will recapture a good quality of life a severely disabling stroke.
Supplementary Material
Declaration of Conflicting Interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: M. Geurts and H.B. van der Worp are supported by the Dutch Heart Foundation (2010B239 and 2010T075, respectively).
Ethical approval
The study was approved by the institutional review board of the University Medical Center Utrecht and of the Elisabeth hospital.
Informed consent
Written informed consent was obtained from each patient or a legal representative.
Guarantor
HBW.
Contributorship
MG contributed to the study design, data collection, performed the data analysis, interpreted the data and prepared the first draft and subsequent versions. FASK helped to refine the study idea, contributed to the data collection and data analysis, helped interpreting the data and contributed to writing. JHT helped with data collection and contributed to writing. GJMWT, PLMK and LJK helped to refine the study idea, helped interpreting the data and contributed to writing. HBW conceived the study, helped to refine the study idea, helped interpreting the data and contributed to writing.
References
- 1.Kelly AG, Hoskins KD, Holloway RG. Early stroke mortality, patient preferences, and the withdrawal of care bias. Neurology 2012; 79: 941–944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Becker KJ, Baxter AB, Cohen WA, et al. Withdrawal of support in intracerebral hemorrhage may lead to self-fulfilling prophecies. Neurology 2001; 56: 766–772. [DOI] [PubMed] [Google Scholar]
- 3.Geurts M, Macleod MR, van Thiel GJ, et al. End-of-life decisions in patients with severe acute brain injury. Lancet Neurol 2014; 13: 515–524. [DOI] [PubMed] [Google Scholar]
- 4.Hemphill JC, 3rd, Newman J, Zhao S, et al. Hospital usage of early do-not-resuscitate orders and outcome after intracerebral hemorrhage. Stroke 2004; 35: 1130–1134. [DOI] [PubMed] [Google Scholar]
- 5.Silvennoinen K, Meretoja A, Strbian D, et al. Do-not-resuscitate (DNR) orders in patients with intracerebral hemorrhage. Int J Stroke 2014; 9: 53–58. [DOI] [PubMed] [Google Scholar]
- 6.Morgenstern LB, Zahuranec DB, Sanchez BN, et al. Full medical support for intracerebral hemorrhage. Neurology 2015; 84: 1739–1744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kelly AG, Zahuranec DB, Holloway RG, et al. Variation in do-not-resuscitate orders for patients with ischemic stroke: implications for national hospital comparisons. Stroke 2014; 45: 822–827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kwakkel G, Veerbeek JM, Harmeling-van der Wel BC, et al. Early Prediction of functional Outcome after Stroke (EPOS) Investigators. Diagnostic accuracy of the Barthel Index for measuring activities of daily living outcome after ischemic hemispheric stroke: does early poststroke timing of assessment matter? Stroke 2011; 42: 342–346. [DOI] [PubMed] [Google Scholar]
- 9.Charlson ME, Pompei P, Ales KL, et al. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis 1987; 40: 373–383. [DOI] [PubMed] [Google Scholar]
- 10.Indredavik B, Bakke F, Slordahl SA, et al. Stroke unit treatment improves long-term quality of life: a randomized controlled trial. Stroke 1998; 29: 895–899. [DOI] [PubMed] [Google Scholar]
- 11.Zurasky JA, Aiyagari V, Zazulia AR, et al. Early mortality following spontaneous intracerebral hemorrhage. Neurology 2005; 64: 725–727. [DOI] [PubMed] [Google Scholar]
- 12.Zahuranec DB, Brown DL, Lisabeth LD, et al. Early care limitations independently predict mortality after intracerebral hemorrhage. Neurology 2007; 68: 1651–1657. [DOI] [PubMed] [Google Scholar]
- 13.Alexandrov AV, Bladin CF, Meslin EM, et al. Do-not-resuscitate orders in acute stroke. Neurology 1995; 45: 634–640. [DOI] [PubMed] [Google Scholar]
- 14.Shepardson LB, Youngner SJ, Speroff T, et al. Increased risk of death in patients with do-not-resuscitate orders. Med Care 1999; 37: 727–737. [DOI] [PubMed] [Google Scholar]
- 15.Patel MD, Tilling K, Lawrence E, et al. Relationships between long-term stroke disability, handicap and health-related quality of life. Age Ageing 2006; 35: 273–279. [DOI] [PubMed] [Google Scholar]
- 16.Bruno MA, Bernheim JL, Ledoux D, et al. A survey on self-assessed well-being in a cohort of chronic locked-in syndrome patients: happy majority, miserable minority. BMJ Open 2011; 1: e000039–2010-000039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sprangers MA, Schwartz CE. Integrating response shift into health-related quality of life research: a theoretical model. Soc Sci Med 1999; 48: 1507–1515. [DOI] [PubMed] [Google Scholar]
- 18.Ubel PA, Loewenstein G, Schwarz N, et al. Misimagining the unimaginable: the disability paradox and health care decision making. Health Psychol 2005; 24: S57–S62. [DOI] [PubMed] [Google Scholar]
- 19.Albrecht GL, Devlieger PJ. The disability paradox: high quality of life against all odds. Soc Sci Med 1999; 48: 977–988. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.