Abstract
Introduction
Stroke registries are used in many settings to measure stroke treatment and outcomes, but rarely include data on health economic outcomes. We aimed to extend the Sentinel Stroke National Audit Programme registry of England, Wales and Northern Ireland to derive and report patient-level estimates of the cost of stroke care.
Methods
An individual patient simulation model was built to estimate health and social care costs at one and five years after stroke, and the cost-benefits of thrombolysis and early supported discharge. Costs were stratified according to age, sex, stroke type (ischaemic or primary intracerebral haemorrhage) and stroke severity. The results were illustrated using data on all patients with stroke included in Sentinel Stroke National Audit Programme from April 2015 to March 2016 (n = 84,184).
Results
The total cost of health and social care for patients with acute stroke each year in England, Wales and Northern Ireland was £3.60 billion in the first five years after admission (mean per patient cost: £46,039). There was fivefold variation in the magnitude of costs between patients, ranging from £19,101 to £107,336. Costs increased with older age, increasing stroke severity and intracerebral hemorrhage stroke. Increasing the proportion of eligible patients receiving thrombolysis or early supported discharge was estimated to save health and social care costs by five years after stroke.
Discussion
The cost of stroke care is large and varies widely between patients. Increasing the proportion of eligible patients receiving thrombolysis or early supported discharge could contribute to reducing the financial burden of stroke.
Conclusion
Extending stroke registers to report individualised data on costs may enhance their potential to support quality improvement and research.
Keywords: Cost, health economics, registers, stroke
Introduction
Stroke is one of the major global causes of mortality and disability. Each year, 10 million people are estimated to have a first ever stroke, and 6.5 million people die as a result of stroke.1 In the United Kingdom, stroke is the third leading cause of years of life lost and of disability-adjusted life years.2 This human burden is mirrored by the very large cost of providing healthcare to people with stroke, with stroke care accounting for approximately 3–5% of all healthcare expenditure.3,4
Many health systems are faced with the challenge of tackling the rising costs of healthcare services, and it is now increasingly recognised that cost is a fundamental dimension of healthcare quality. Cost reduction is one of the components of the Institute of Healthcare Improvement’s ‘Triple Aim’5 and some have argued that the concept of quality in healthcare should be reframed in terms of ‘value’: health outcomes achieved per unit of expenditure.6 Although registries have been established in many settings7–9 to measure stroke care and outcomes after stroke, they typically do not include information about healthcare costs and are not specifically designed to measure the value that healthcare services provide.
The Sentinel Stroke National Audit Programme (SSNAP) is the national stroke register of England, Wales and Northern Ireland, collecting data on an estimated 95% of all patients admitted to hospital with acute stroke.10 It provides individualised data and analytics about the quality of stroke care to hospitals, rehabilitation services, payers (‘Commissioners’ in the terminology of the National Health Service), stroke survivors and policy makers. The aims of this study were to estimate patient-level health and social care costs of stroke for all patients admitted to hospital with stroke in England, Wales and Northern Ireland and integrate this alongside near real-time reporting of SSNAP data on quality of care and patient outcomes.
Methods
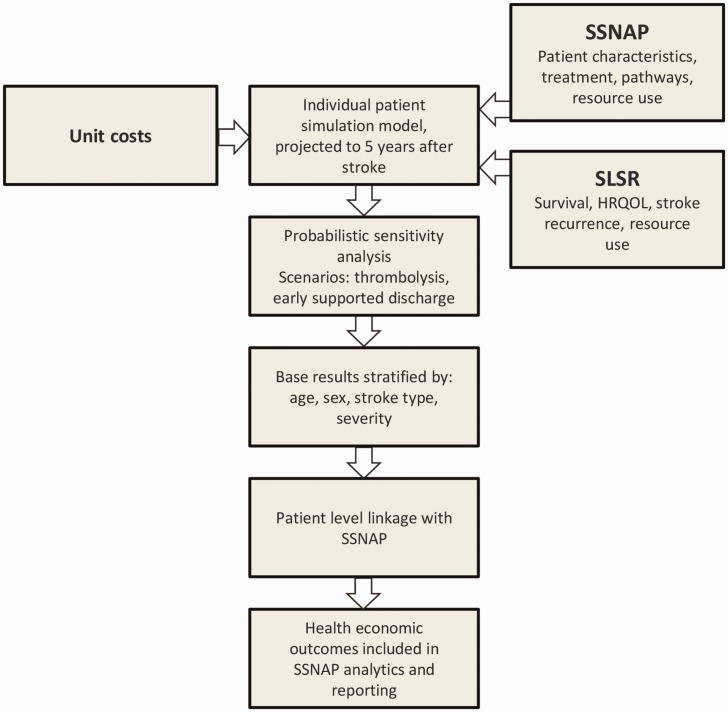
The study was carried out in two phases (Figure 1). Firstly, a health economic simulation was developed to produce patient-level estimates of the health and social care cost of acute stroke, taking account of patient’s age, stroke type, stroke severity and sex. Secondly, these estimates were integrated into SSNAP, and interactive tools were developed to model the health economic consequences of increasing access to intravenous thrombolysis and early supported discharge.
Figure 1.
Design of the study.
Health economic simulation
A detailed description of the health economic methods is provided in the online Appendix and is summarised below.
The model was a time-to-event individual patient simulation model.11 In this model, patients go through different treatment units for healthcare (Table 1 and Figure A1, online Appendix). Their health status, stroke-related resource use and costs and outcomes are modelled through the simulation, up to five years after stroke.
Table 1.
Summary of methods used in the model.
| Treatment unit | Health state change | Length of stay | Mortality | Next destinations |
|---|---|---|---|---|
| ASU and thrombolysis | Thrombolysed patients: mRS when leaving ASU was dependent on NIHSS 24 h after thrombolysis and age group Not thrombolysed patients – mRS when leaving ASU was dependent on NIHSS when admitted to hospital and age group | Generalised linear model with family of gamma and log link • Age • Sex • Thrombolysed patients – NIHSS after thrombolysis • Not thrombolysed patients – NIHSS when admitted to hospital • Discharge destination Bounded at 90th percentile of the SSNAP data: 36.0 days | Mortality probability in ASU was dependent on age group and mRS and was different in thrombolysed and not thrombolysed patients. All causes mortality was used for both groups. | ESD SU CRT Discharge with no need for rehabilitation |
| GMWs | No health state change involved in GMW due to the lack of data and relatively short period of time in GMW | Fixed average length of stay | No mortality in the model. | ASU |
| SU – inpatient rehabilitation | Health state change was dependent on age group and mRS when entering SU | Length of stay was sampled from a lognormal distribution with the parameters dependent on the next destination (including death), age group and mRS when entering SU Bounded at 90th percentile of the SSNAP data: 70.8 days | Dependent on age and mRS on arrival at SU | ESD CRT Discharge with no need for rehabilitation |
| ESD | Health state change is dependent on age group and mRS when entering ESD | Length of stay was sampled from an exponential distributions with the parameters dependent on age and mRS when entering ESD Bounded at 90th percentile of the SSNAP data: 63.1 days | Dependent on age and mRS when arrived to ESD | CRT Discharged to own home or nursing home |
| CRT | Health state change is dependent on age group and mRS when admitted by a CRT | Length of stay with CRT is determined by a discrete event algorithm | Survival curve was fitted dependent on age, sex, mRS at discharge and stroke type (Cox regression) | Discharged to own home or care home |
| Discharge to own home or care home | No health state change after discharge (unless they have a recurrence). Admission to care home only included if newly institutionalised after stroke | Length of stay at own home/care home after discharge is determined by a discrete event algorithm | Survival curve is dependent on age, sex, mRS at discharge and stroke type (Cox regression) | Patients might have stroke recurrence after discharge |
| Stroke recurrence | Recurrence severity is measured in NIHSS, dependent on recurrence type, independent from age and previous severity | Same pathway as first stroke (dependent on age and severity) | Same pathway as first stroke | Same pathway as first stroke |
ASU: Acute stroke unit; mRS: modified Rankin scale; ESD: early supported discharge; SU: stroke unit; CRT: community rehabilitation team; NIHSS: National Institutes of Health Stroke Scale, or NIH Stroke Scale; GMW: general medical ward.
The principal model parameters were: age, sex, stroke type (ischaemic stroke and primary intracerebral haemorrhage) and stroke severity. Health status as well as other parameters including mortality, next event and length of stay in each treatment unit were probabilistically updated at the end of each treatment unit, depending on their characteristics when entering the unit. Health states were measured by the National Institute of Health Stroke Score (NIHSS; a measure of stroke severity) at stroke onset and then subsequently by the modified Rankin Score (mRS; a measure of functional status). The change in mRS from before to after the stroke was used to estimate change in disability caused by the stroke and attribute changes in health and social care utilisation to the stroke itself rather than pre-existing or co-morbid illnesses. Health-Related Quality of Life (health utility on a 0–1 scale) was estimated by mapping from the mRS.12
Probabilities and distributions were largely estimated using SSNAP data, with additional data on long-term outcomes, stroke recurrence and resource use obtained from the South London Stroke Register (SLSR). SSNAP data included in the model included all patients aged 40–100 years admitted for acute stroke from April 2013 to 2015 (n = 111,846). The SLSR is a population-based register with prospective long-term follow-up of all adults with first ever stroke in South London,13 including data on 6000 patients. The SLSR was used to provide data to model survival after stroke, stroke recurrence and to estimate long-term health and social care utilisation after stroke. Resource use was estimated based on the parameters in Table 1, which were combined with unit costs (online Appendix) to estimate the costs generated by individuals in the model.
Cost estimates took a health and social care perspective (healthcare is free for users of the National Health Service in the United Kingdom but social care may incur charges). Unit costs for events and treatment units were derived from existing cost sources (online Appendix) and were not discounted due to the relatively short time-horizon used. Quality-adjusted life-years (QALYs), the primary health outcomes, were estimated by weighting survival with health utility. Health cost estimates included the costs of pre-hospital care, acute care, diagnostics, prescribing, inpatient rehabilitation, community rehabilitation, early supported discharge, primary care, secondary prevention and stroke recurrence. Social care included nursing home care, formal care at home, supported meals and day services. The population mean of these costs and QALY were then estimated according to 80 different combinations of baseline characteristics (age, sex, stroke type and stroke severity).
Baseline results were estimated to reflect the current situation of stroke care. Inputs estimated from SSNAP were used directly for this purpose. Scenario analyses were conducted to estimate the cost-effectiveness results of increasing the proportion of patients treated with intravenous thrombolysis or early supported discharge (online Appendix). The treatment effects of thrombolysis and early supported discharge were then calibrated to the results of Cochrane reviews of randomised controlled trials of these interventions,14,15 by comparing models using treatment effects from the Cochrane reviews to those obtained using the real-world treatment effects observed in SSNAP. Treatment effects reported by the Cochrane reviews were used to optimise the inputs of the model. Mean healthcare cost, mean social care cost and mean QALYs over all stroke patients at one and five years after primary stroke were plotted and the line of best fit calculated to estimate the cost savings and QALYs gained for each extra person thrombolysed or discharged to ESD (online Appendix). Probabilistic sensitivity analysis (PSA) was conducted using Monte Carlo simulation to assess uncertainty of our results and estimate 95% confidence intervals for the base case results.
Statistical analyses were carried out with R and Stata 13, and the health economic model was built in MS Excel VBA (2010).
Integration into SSNAP
All patients in SSNAP were assigned one of the model subgroups based on sex, age group, stroke severity and stroke type. The data were then linked with a table of cost estimates from the health economics model to generate individualised cost estimates for all patients in SSNAP. Total and average costs were integrated into reports alongside SSNAP outputs describing quality of care metrics and outcomes (survival, mRS at discharge, new institutionalisation). SSNAP outputs incorporating health economic data include executive summaries designed for hospital executive boards and dashboards designed for commissioners. The results of the model were illustrated for one year of SSNAP data (n = 84,184 patients admitted between April 2015 and March 2016), plotting five-year health and social care cost against age, stroke severity and stroke type.
Ethics
Data analyses were carried out using fully anonymised datasets from SSNAP and the SLSR. SSNAP has approval from the Clinical Advisory Group of the NHS Health Research Authority to collect patient-level data under section 251 of the NHS Act 2006. All patients or their relatives gave written informed consent to participate in the SLSR. The design of the SLSR was approved by the ethics committees of Guy's and St Thomas' National Health Service Foundation Trust, King's College Hospital Foundation Trust, St George's University Hospital, National Hospital for Nervous Diseases, and Westminster Hospital.
Results
The mean per-patient cost of healthcare attributable to stroke was £13,452 in the first year after stroke and £17,963 after five years (Table 2). Mean social care costs attributable to stroke were lower than healthcare costs in the first year (£8977) but by five years after stroke social care costs accounted for the larger burden of cost (£28,076). Total mean costs were £22,429 by one year and £46,039 at five years.
Table 2.
Baseline mean costs per patient at one and five years.
| 1 Year | 5 Year | |
|---|---|---|
| Mean healthcare costs per patienta | £13,452 | £17,963 |
| Mean social care costs per patientb | £8977 | £28,076 |
| Mean total health and social care costs per patient | £22,429 | £46,039 |
| Combined total cost for all patients included in SSNAP, April 2015–March 2016 (n = 84,184) | £1,736,338,300 | £3,604,672,200 |
| Mean health and social costs per patient with ischaemic stroke | £20,121 | £41,432 |
| Mean health and social costs per patient with ICH stroke | £24,297 | £52,726 |
ICH: intracerebral hemorrhage; SSNAP: Sentinel Stroke National Audit Programme.
Healthcare costs include: ambulance, MRI or CT scan, thrombolysis, acute stroke unit care, rehabilitation stroke unit care, general medical ward care, community rehabilitation, GP visits, secondary prevention, and ESD therapists.
Social care costs include: care home, home help, meals on wheels, and social service day centre visits.
In England, Wales and Northern Ireland, there were 84,184 patients admitted with stroke between April 2015 and March 2016 and entered onto SSNAP. Of these, 73,318 (87.1%) had ischaemic stroke, 10,267 (12.2%) had ICH, 599 (0.7%) had undetermined stroke type. Total health and social care costs attributable to stroke for this cohort of patients were £1.74 billion at one year and £3.60 billion at five years.
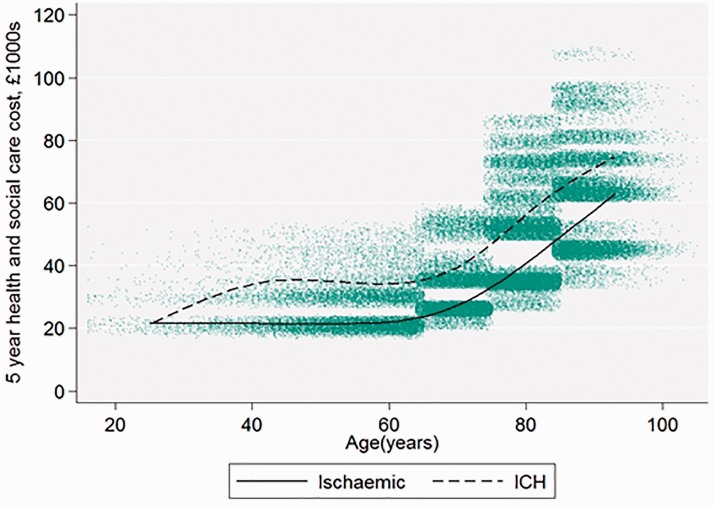
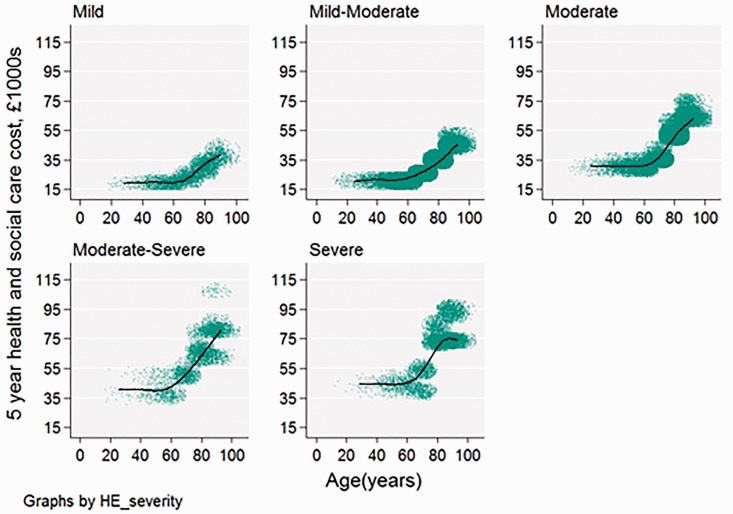
The estimated stroke cost varied widely according to patient characteristics, ranging from £7322 to £44,854 at one year and £19,101 to £107,336 at five years. Costs increased with age and with stroke severity (Figure 2). Mean costs were £4000 higher for ICH stroke than for ischaemic stroke at one year and £11,000 higher by five years (Figure 3)
Figure 2.
Five-year health and social costs by age and stroke severity, for all patients (n = 84,184) admitted between April 2015 and March 2016. Each dot on the scatter plot is one patient with stroke; the best fit line is a restricted cubic spline with four knots.
Figure 3.
Five-year health and social cost age and stroke type, for all patients (n = 84,184) admitted between April 2015 and March 2016. Each dot on the scatter plot is one patient with stroke; the best fit line is a restricted cubic spline with four knots.
Scenario analysis
We found that increasing the proportion of patients receiving thrombolysis or early supported discharge was cost saving, particularly for long-term social care costs. For thrombolysis, we estimated healthcare savings of £3200 and £4000, social care savings of £2900 and £5300 and 0.08 and 0.26 QALYs gained in total for each extra patient thrombolysed after one and five years, respectively (Figure A2, online Appendix). For ESD, the cost savings for health and social care were estimated to be £1600 and £2400 after one year, £1600 and £8100 after five years, with QALY gains of 0.04 and 0.14 per at one and five years, respectively, for each extra patient discharged with ESD (Figure A3, online Appendix). In the sensitivity analyses, 95% confidence intervals were estimated for three scenarios: base case, 95% of the patients who met the SSNAP minimum criteria were thrombolysed and 35% of patients who were not discharged to ESD redirected to ESD treatment (online Appendix).
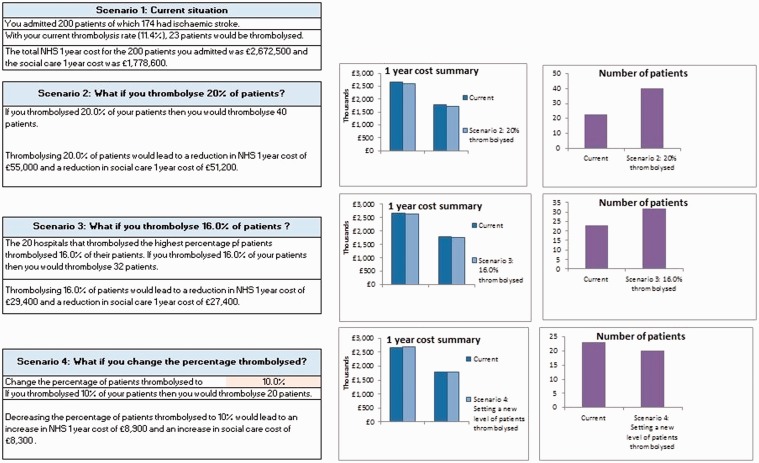
We used these results to support quality improvement and service development in stroke care services by building interactive Excel tools for SSNAP participants (illustration of the thrombolysis tool in Figure 4). These enable SSNAP participants to compare their own data to the results of the health economic model and estimate the potential cost effectiveness of increasing access to thrombolysis and ESD in their patient cohorts. The tool allows participants to model different scenarios (such as increasing the number of patients arriving at hospital within the thrombolysis time window), allowing them to identify opportunities for improvement.
Figure 4.
Screenshot from the Thrombolysis tool.
Discussion
The care of patients with stroke is expensive, both at the individual level and at the level of health systems and society as a whole. These costs vary considerably between patients, in the first five years after stroke ranging fivefold between patient subgroups with the lowest and highest care costs. Five-year costs were highest in patients with haemorrhagic strokes, older patients and those with more severe stroke. Costs were lowest in patients with ischaemic stroke and mild strokes. Costs over the first year after stroke largely arise from the costs of acute treatment, but over time the costs of providing social care account for a greater proportion of the total care costs of stroke. We found that both thrombolysis and early supported discharge services were both cost effective and cost saving, resulting in financial savings to both the healthcare and social care costs of stroke. Increasing the proportion of patients receiving these interventions therefore has the potential not only to improve patient outcomes after stroke but also help to control the high financial burden of stroke.
As far as we are aware, this is the first time that a detailed patient-level health economic model has been integrated into a large-scale stroke register. The development of a stratified model enabled patient-level cost estimates to take account of how costs vary according to patients’ age, stroke type, stroke severity and sex, rather than simply using an average across all patients. By integrating this model into an existing stroke register, we were able to extend SSNAP to report patient-level data on estimated costs alongside data about stroke care quality and outcomes. Although we have not yet evaluated the effectiveness and usefulness of feeding back cost data in this way, the methodology could be adopted by other stroke registries and has the potential to expand the role of registries in supporting quality improvement and research.
These cost estimates are consistent with previous studies of the costs of stroke. Recent estimates of the costs in high-income countries with health systems similar to the United Kingdom have reported costs in the first year after stroke of €29,484 in the Netherlands16 and €21,200 in Sweden.17 A previous national analysis of stroke data in England in 2009 estimated a mean cost of £28,568 (in 2017 prices).18 Since that time there have been major changes in the organisation and delivery of stroke care in the United Kingdom, resulting in reduced length of stay, increase in the provision and use of stroke units and greater use of interventions which reduce long-term disability (e.g. thrombolysis)10 and recurrence (e.g. antiplatelet therapy). These improvements in care may have contributed to the reduction in the estimated costs of stroke care since 2009.
Collecting data on large cohorts of patients, typically at the regional or national level, registries are one of the major sources of ‘real world data’ about human health and healthcare services.19,20 It is too early to evaluate the impact of including data on health costs within a large-scale clinical registry, but we anticipate that these data will have several uses. For example, bespoke and clearly sourced estimates of costs could be used by clinicians and managers to make business cases for quality improvement or service development. Similarly, these data make it simpler to estimate the financial return on investment in spending money on stroke prevention, through for example detection and management of atrial fibrillation20 and in predicting the future financial burden of stroke care. These data could also be useful in the planning, design and conduct of observational and interventional research studies. For example, embedding randomised controlled trials (RCTs) within registries that include data on health economic outcomes could help researchers carry out cheaper and more useful RCTs of new interventions in stroke care21 and aid in the health economic evaluation of new interventions.
Strengths and limitations
The strength of this study was that it estimated a fuller range of costs than are usually available in healthcare payment or reimbursement systems – in the UK, for example, previous sources of stroke cost data included only inpatient care but not the longer term costs of rehabilitation, nursing and medical care. By making use of the detailed real-world stroke data about stroke collected through registries, we were able to model stroke care with a level of detail that has typically not been possible in previous studies of the cost of stroke.22
There are, however, a number of limitations. Firstly, the model only takes a health and social care perspective and does not include the wider societal costs of stroke (such as costs to patients or family members through loss of income and informal care). Cost estimates were also only based on patients who had a full NIHSS score in SSNAP (76% out of the all stroke patients). In comparison with the entire patient population, these patients were slightly younger (median = 76 vs. 80) and slightly more independent (median mRS = 0 vs. 1), likely resulting in a bias towards lower cost estimates. Cost estimates were also based only on patients aged above 40 years due to insufficient data being available in the SLSR of patients aged less than 40 years to fit the models. In implementing these estimates in SSNAP, we therefore assumed that costs in younger patients are the same as for those aged 40–64 years. The model describes the typical care pathways experienced by the majority of patients admitted with acute stroke in the UK, but this is by necessity a simplification of the real world. For example, the model assumes that all patients are admitted to a stroke unit, and although this is true for 95% of stroke patients in England, Wales and Northern Ireland,10 the model does not take account of the 5% of patient which do not. It also does not take account of the impact of thrombectomy in patients with acute ischemic stroke. Although in the UK this is still not in widespread use and so is unlikely to have made a significant difference to current cost estimates, future estimates of the cost of stroke should take account of this emerging therapy, as it is likely to result in lower long-term costs.23 To gain more power from the data, we combined all SSNAP data collected from England, Wales, and Northern Ireland and applied the same unit costs to all patients. In the real world, costs might vary between these three countries, although the differences are likely to be small as their health systems are similar.
Conclusion
We developed and implemented a detailed health economic model within a national stroke register. Not only does this help to provide more up to date estimates of the large financial costs arising from stroke in the United Kingdom, but provides new data about how costs vary between patients and a new source of information to help plan and improve stroke care services. In particular, these results imply that improving access to thrombolysis and early supported discharge services can contribute to reducing the financial burden of stroke on health and social care services. Health economic models are inevitably a simplification of the real world, and it is important to understand the assumptions that support the estimates from models such as this. Nonetheless, integrating the measurement and reporting of health economic outcomes data into clinical registries could help them become increasingly useful resources for quality improvement and research.
Data sharing
Data from SSNAP are available at www.strokeaudit.org. This includes extensive and unrestricted access to publicly available datasets and contact details to request access to anonymised patient-level data.
Supplementary Material
Acknowledgements
This work uses data provided by patients and collected by the National Health Service as part of their care and support. We would like to thank the many hundreds of people and organisations participating in SSNAP, and on their behalf specifically thank the members of the SSNAP collaboration (www.strokeaudit.org/Research/SSNAP-Collaboration.aspx).
The SLSR and CDAW are funded/supported by the National Institute for Health Research (NIHR) Biomedical Research Centre based at Guy's and St Thomas' NHS Foundation Trust and King's College London, and the NIHR Collaboration for Leadership in Applied Health Research (award number NIHR CLAHRC-2013-10022) and Care South London at King's College Hospital NHS Foundation Trust.
Declaration of conflicting interests
The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: AGR is the National Clinical Director for Stroke, NHS England.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This project was funded by a grant from NHS England. SSNAP is funded by the Healthcare Quality Improvement Partnership on behalf of NHS England. The SLSR and CDAW are funded/supported by the National Institute for Health Research (NIHR) Biomedical Research Centre based at Guy's and St Thomas' NHS Foundation Trust and King's College London, and the NIHR Collaboration for Leadership in Applied Health Research (award number NIHR CLAHRC-2013-10022) and Care South London at King's College Hospital NHS Foundation Trust.
Informed consent
SSNAP has permission from the NHS Health Research Authority under section 251 of the Health and Social Care Act 2006 to collect patient data without prospective consent. Patients can opt out of data collection.
Ethical approval
SSNAP: SSNAP has been granted permission by the NHS Health Research Authority to collect patient data under Section 251 of the Health and Social Care Act 2006. Patients may opt out of data collection and record linkage. South London Stroke Register (SLSR): All patients and/or their relatives gave written informed consent to participate in the study, and over the study period very few patients have declined to be registered. The design of the study was approved by the ethics committees of Guy's and St Thomas' NHS Foundation Trust, King's College Hospital Foundation Trust, St George's University Hospital, National Hospital for Nervous Diseases, and Westminster Hospital.
Guarantor
BDB.
Contributions
XMX, EV, LP, BDB carried out the analyses. BDB, XMX, DW designed the study. DW supervised the health economic methods. AD prepared data extracts and analyses from the SLSR. AH, CDAW, AGR supervised the study and provided clinical input. BDB and XMX wrote the first draft of the paper. All authors contributed to the final version of the manuscript.
References
- 1.Feigin VL, Krishnamurthi RV, Parmar P, et al. GBD 2013 Writing Group.; GBD 2013 Stroke Panel Experts Group. Update on the global burden of ischemic and hemorrhagic stroke in 1990-2013: the GBD 2013 study. Neuroepidemiology 2015; 45: 161–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Newton JN, Briggs AD, Murray CJ, et al. Changes in health in England, with analysis by English regions and areas of deprivation, 1990-2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet 2015; 386: 2257–2274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Evers SM, Struijs JN, Ament AJ, et al. International comparison of stroke cost studies. Stroke 2004; 35: 1209–1215. [DOI] [PubMed] [Google Scholar]
- 4.Saka O, McGuire A, Wolfe C. Cost of stroke in the United Kingdom. Age Ageing 2009; 38: 27–32. [DOI] [PubMed] [Google Scholar]
- 5.Whittington JW, Nolan K, Lewis N, et al. Pursuing the triple aim: the first 7 years. Milbank Q 2015; 93: 263–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Porter ME. What is value in health care? N Engl J Med 2010; 363: 2477–2481. [DOI] [PubMed] [Google Scholar]
- 7.Johnsen SP, Ingeman A, Hundborg HH, et al. The Danish Stroke Registry. Clin Epidemiol 2016; 8: 697–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fonarow GC, Reeves MJ, Smith EE, et al. GWTG-Stroke Steering Committee and Investigators. Characteristics, performance measures, and in-hospital outcomes of the first one million stroke and transient ischemic attack admissions in get with the guidelines – stroke. Circ Cardiovasc Qual Outcomes 2010; 3: 291–230. [DOI] [PubMed] [Google Scholar]
- 9.Asplund K, Hulter Åsberg K, Appelros P, et al. The Riks-Stroke story: building a sustainable national register for quality assessment of stroke care. Int J Stroke 2011; 6: 99–108. [DOI] [PubMed] [Google Scholar]
- 10.Sentinel Stroke National Audit Programme, www.strokeaudit.org. (accessed 23 May 2017).
- 11.Brennan A, Chick SE, Davies R. A taxonomy of model structures for economic evaluation of health technologies. Health Econ 2006; 15: 1295–1310. [DOI] [PubMed] [Google Scholar]
- 12.Whynes DK, Sprigg N, Selby J, et al. Investigators ENOS. Testing for differential item functioning within the EQ-5D. Med Decis Making 2013; 33: 252–260. [DOI] [PubMed] [Google Scholar]
- 13.Wolfe CD, Crichton SL, Heuschmann PU, et al. Estimates of outcomes up to ten years after stroke: analysis from the prospective South London Stroke Register. PLoS Med 2011; 8: e1001033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wardlaw JM, Murray V, Berge E, et al. Thrombolysis for acute ischaemic stroke. Cochrane Database Syst Rev 2014; 7: CD000213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Early Supported Discharge Trialists. Services for reducing duration of hospital care for acute stroke patients. Cochrane Database Syst Rev 2005; 2: CD000443. [DOI] [PubMed] [Google Scholar]
- 16.van Eeden M, van Heugten C, van Mastrigt GA, et al. The burden of stroke in the Netherlands: estimating quality of life and costs for 1 year poststroke. BMJ Open 2025; 5: e008220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Persson J, Ferraz-Nunes J, Karlberg I. Economic burden of stroke in a large county in Sweden. BMC Health Serv Res 2012; 12: 341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.National Audit Office, Progress in improving stroke care. Report on the findings from our modelling of stroke care provision, www.nao.org.uk/report/department-of-health-progress-in-improving-stroke-care (2010, accessed 23 May 2017).
- 19.Nelson EC, Dixon-Woods M, Batalden PB, et al. Patient focused registries can improve health, care, and science. BMJ 2016; 354: i3319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sterne JA, Bodalia PN, Bryden PA, et al. Oral anticoagulants for primary prevention, treatment and secondary prevention of venous thromboembolic disease, and for prevention of stroke in atrial fibrillation: systematic review, network meta-analysis and cost-effectiveness analysis. Health Technol Assess 2017; 21: 1–386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lauer MS, D'Agostino RB., Sr The randomized registry trial – the next disruptive technology in clinical research? N Engl J Med 2013; 369: 1579–1581. [DOI] [PubMed] [Google Scholar]
- 22.Demaerschalk BM, Hwang HM, Leung G. US cost burden of ischemic stroke: a systematic literature review. Am J Manag Care 2010; 16: 525–533. [PubMed] [Google Scholar]
- 23.Aronsson M, Persson J, Blomstrand C, et al. Cost-effectiveness of endovascular thrombectomy in patients with acute ischemic stroke. Neurology 2016; 86: 1053–1059. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.