Abstract
Systemic lupus erythematosus (SLE; lupus) is a prototypical autoimmune disease characterized by circulating autoantibodies to nuclear antigens and immune complex deposition, resulting in damage to target organs. To investigate the effects of tacrolimus (TAC) on effector T cells and B cells, we examined its involvement in the development of effector T cells, germinal center (GC) B cells, and plasma cells in an in vitro system using wild-type (WT) and lupus-prone mice. The population of T helper (Th) 1, Th2, and Th17 cells interleukin (IL)-17-producing T (Th17) cells and the production of interferon-γ and interleukin-17A IL-17A were suppressed by TAC. TAC also reduced the population of regulatory T (Treg) cells; however, a combination treatment with the signal transducer and activator of transcription 3 (STAT3) inhibitor STA-21 promoted the population of Treg cells. TAC also suppressed the populations of GC B cells and plasma cells synergistically with STA-21. These findings suggest that the application of TAC with a STAT3 signal inhibitor may provide benefits in SLE treatment.
Keywords: germinal center B cell, plasma cell, systemic lupus erythematosus, tacrolimus, Th17, Treg cell
Introduction
Systemic lupus erythematosus (SLE; lupus) is a systemic autoimmune disease characterized by defects in cellular apoptotic debris clearance and the breakdown of immunologic tolerance, resulting in the production of autoantibodies to nuclear antigens and cell surface and serum proteins, leading to damage to target organs including the skin, kidney, and brain.1
Until recently, SLE was thought to involve mainly dysregulated B cells. The breakdown of B-cell tolerance is believed to be a major mechanism promoting autoreactive B cell activation and differentiation into antibody-secreting plasma cells.2 However, accumulating evidence suggests that the adaptive and innate immune systems are involved in SLE pathogenesis. Aberrant T cells help autoreactive B cells differentiate, proliferate, and mature; secrete autoantibodies; and infiltrate and damage target organs.3
Tacrolimus (TAC), an antifungal natural product macrolide, is an immunosuppressive drug that blocks calcineurin activity, which inhibits interleukin (IL)-2 gene expression through nuclear factor of activated T cells (NFAT) in T lymphocytes. TAC is most widely used as a calcineurin inhibitor for prevention of graft-versus-host disease (GVHD) and for treatment of various autoimmune diseases, such as rheumatoid arthritis.4
TAC reduces proteinuria and prevents the progression of nephropathy in lupus nephritis, an inflammation of the kidney that is one of the most serious manifestations of SLE. TAC therapy in combination with other immunosuppressive drugs has shown additive effects in terms of reducing proteinuria and increasing serum C3 levels in patients with SLE during a maintenance phase.5 However, little is known about the regulatory effects of TAC on effector T cells and B cells.
STA-21 is a small molecule that inhibits constitutive signal transducer and activator of transcription 3 (STAT3) signaling. It targets the SH2 domain of STAT3 and inhibits STAT3 DNA-binding activity and STAT3–STAT3 dimerization. In a previous study, we demonstrated that STA-21 reciprocally regulates T helper (Th) 17 (IL-17-producing T (Th17)) and regulatory T (Treg) cells and ameliorates autoimmune inflammation in rheumatoid arthritis.6
The objective of this study was to assess the effect of TAC on effector T cells and B cells. We applied different TAC treatments to wild-type (WT) and lupus-prone mice to determine whether TAC is involved in the development of effector T cells, germinal center (GC) B cells, and plasma cells.
Materials and methods
Animals
We purchased 12- to 16-week-old C57BL/6 mice from Orient Bio Inc. (Seongnam, Korea). NZB/WF1 mice and Fas gene mutation (MRL/lpr) were obtained from the Jackson Laboratory (Bar Harbor, ME, USA). Mice harboring the M199R mutation in the Roquin protein (Roquinsan/san mice) were obtained from the Mutant Mouse Regional Resource Center (MMRRC) (Davis, CA). Animals were maintained under specific pathogen-free conditions at the Institute of Medical Science of the Catholic University of Korea and fed standard mouse chow and water. All experimental procedures were examined and approved by the Animal Research Ethics Committee of the Catholic University of Korea; the procedure conformed to all National Institutes of Health of the USA guidelines (Permit numbers: 2016-0105-03, 2016-0135-02). All surgery was performed under isoflurane anesthesia, and all efforts were made to minimize suffering. Mice were euthanized in a CO2 chamber at the end of the study for the purpose of sample collection and histologic examination. The experimental protocol was approved by, and all animals were treated and euthanized in accordance with the guidelines of, the Catholic University of Korea on Use and Care of Animals.
Isolation of splenocytes and stimulation
Mouse spleens were sieved through a mesh. Red blood cells were lysed with hypotonic ammonium-chloride-potassium buffer (0.15 mM NH4Cl, 1 mM KCO3, and 0.1 mM EDTA; pH 7.4). The remaining splenocytes were maintained in RPMI 1640 medium supplemented with 5% fetal bovine serum. For T-cell analysis, cells were cultured in the presence of plate-bound anti-CD3 (0.5 µg/mL) and soluble anti-CD28 (1 µg/mL) with or without TAC and STA-21 (Santa Cruz Biochemicals, Santa Cruz, CA, USA). For B-cell analysis, cells were cultured in the presence of lipopolysaccharide (LPS; 100 ng/mL) with or without TAC and STA-21. TAC was kindly provided by Astellas Pharma Korea, Inc. (Seoul, Korea). Cells were subjected to flow cytometry. Supernatants were used to determine cytokine levels.
Intracellular staining and flow cytometry
Splenocytes were immunostained with various combinations of the following fluorescence-conjugated antibodies: CD4, B220 (eBioscience, ThermoFisher Scientific, Waltham, MA, USA), CD138, GL-7 (BD Biosciences, San Jose, CA, USA), and CD25 (BioLegend, San Diego, CA, USA). These cells were also intracellularly stained with the following antibodies: interferon (IFN)-γ (BioLegend), IL-4, IL-17A, and Foxp3 (eBioscience). Before intracellular staining, cells were stimulated with phorbol myristate acetate (PMA; Sigma-Aldrich, St. Louis, MO, USA; 25 ng/mL) and ionomycin (250 ng/mL) in the presence of GolgiStop (BD Biosciences) for 4 h. Intracellular staining was conducted using an intracellular staining kit (eBioscience) according to the manufacturer’s protocol. Data were collected using a FACSCalibur instrument (BD Biosciences) and analyzed using the Flow Jo software (ver. 7.6; Treestar, Ashland, OR, USA).
Enzyme-linked immunosorbent assay
The amounts of IL-17A, IFN-γ, IL-10, and tumor necrosis factor (TNF)-α in culture supernatants were measured by sandwich enzyme-linked immunosorbent assay (ELISA; R&D Systems, Minneapolis, MN, USA). Horseradish peroxidase–avidin (R&D Systems) was used for color development. Absorbance was measured at 405 nM on an ELISA microplate reader (Molecular Devices, Sunnyvale, CA, USA).
Statistical analysis
Statistical analyses were performed using GraphPad Prism software for Windows (ver. 5; GraphPad Software Inc., San Diego, CA, USA). Experimental values are presented as mean ± standard deviation. P values were calculated by two-tailed t-test and two-way analysis of variance, using grouped data. Statistical significance (two-tailed) was determined at a level of P < 0.05.
Results
The population of effector T cells is suppressed by TAC
To evaluate the effect of TAC on T helper (Th)1, Th2, and Th17 cells, splenocytes from WT or lupus-prone NZB/WF1, Roquinsan/san, and MRL/lpr mice were stimulated with TAC in the presence of anti-CD3 and anti-CD28 for 3 days, and the population of effector T cells was determined by flow cytometry (Figure 1). The population of CD4+IFN-γ+ Th1 cells was dramatically decreased by TAC treatment compared with the untreated control (in WT and Roquinsan/san: P < 0.01; NZB/WF1 and MRL/lpr: P < 0.05; Figure 1(a)). Under this condition, TAC-treated cells were also less prone to differentiate toward CD4+IL-4+ Th2 (in WT, NZB/WF1, and MRL/lpr: P < 0.05) and CD4+IL-17A+ Th17 cells (in WT and Roquinsan/san: P < 0.05) than untreated cells (Figure 1(b) and (c)). However, TAC exerted a greater suppressive effect on Th1 cells than Th2 and Th17 cells.
Figure 1.
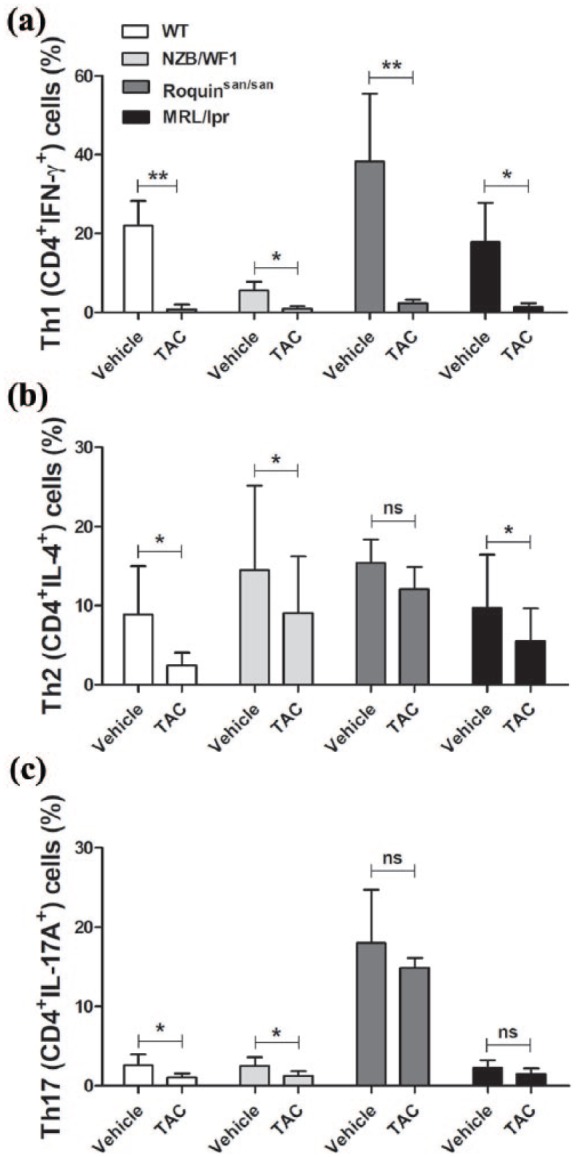
Suppression of effector T cells by tacrolimus (TAC) in mice. Splenocytes from the spleen of wild-type (WT) or lupus-prone NZB/WF1, Roquinsan/san, and MRL/lpr mice (n = 5) were stimulated with TAC (1 nM) in the presence of anti-CD3 and anti-CD28 for 3 days. Cells were stimulated with phorbol myristate acetate (PMA), ionomycin, and GolgiStop for 4 h and stained with antibodies against (a) CD4+IFN-γ+ Th1 cells, (b) CD4+IL-4+ Th2 cells, and (c) CD4+IL-17A+ Th17 cells for intracellular flow cytometric analysis. *P < 0.05, **P < 0.01 versus vehicle-treated condition. Data are mean ± standard deviation (SD).
Th1- and Th17-related cytokine production is suppressed by TAC
To investigate the effect of TAC on cytokine production in lupus-prone mice, splenocytes from spleens of WT or lupus-prone NZB/WF1, Roquinsan/san, and MRL/lpr mice were cultured with TAC in the presence of anti-CD3 and anti-CD28 for 3 days. IFN-γ and IL-17A levels in the supernatant from each mouse are shown in Figure 2. As expected, the supernatant from TAC-treated cells had a lower IFN-γ concentration than that from untreated cells (in WT, NZB/WF1, and Roquinsan/san: P < 0.001; MRL/lpr: P < 0.01; Figure 2(a)). TAC also significantly decreased the level of IL-17A in culture supernatant (in WT and Roquinsan/san: P < 0.001; NZB/WF1 and MRL/lpr: P < 0.05; Figure 2(b)).
Figure 2.
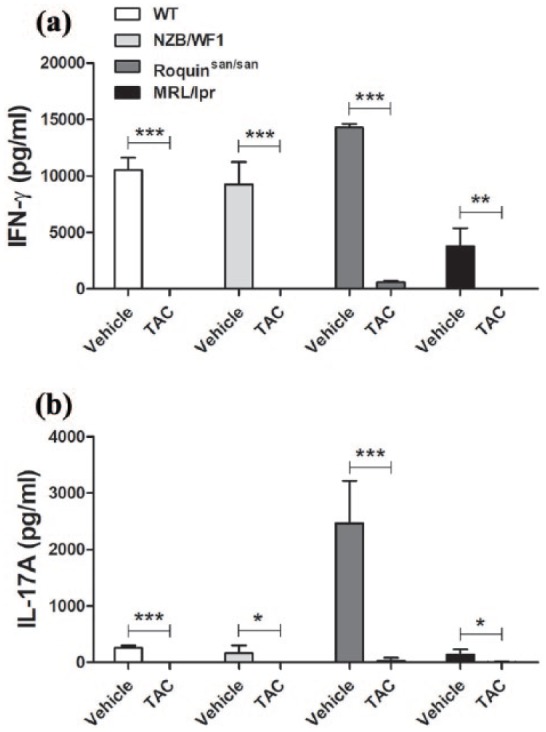
IFN-γ and IL-17A production was suppressed by TAC. Splenocytes from WT or lupus-prone NZB/WF1, Roquinsan/san, and MRL/lpr mice (n = 5) were stimulated with TAC (1 nM) in the presence of anti-CD3 and anti-CD28 for 3 days. (a) IFN-γ and (b) IL-17A concentrations in culture supernatants were determined by ELISA. *P < 0.05, **P < 0.01, ***P < 0.001 versus vehicle-treated condition. Data are mean ± SD.
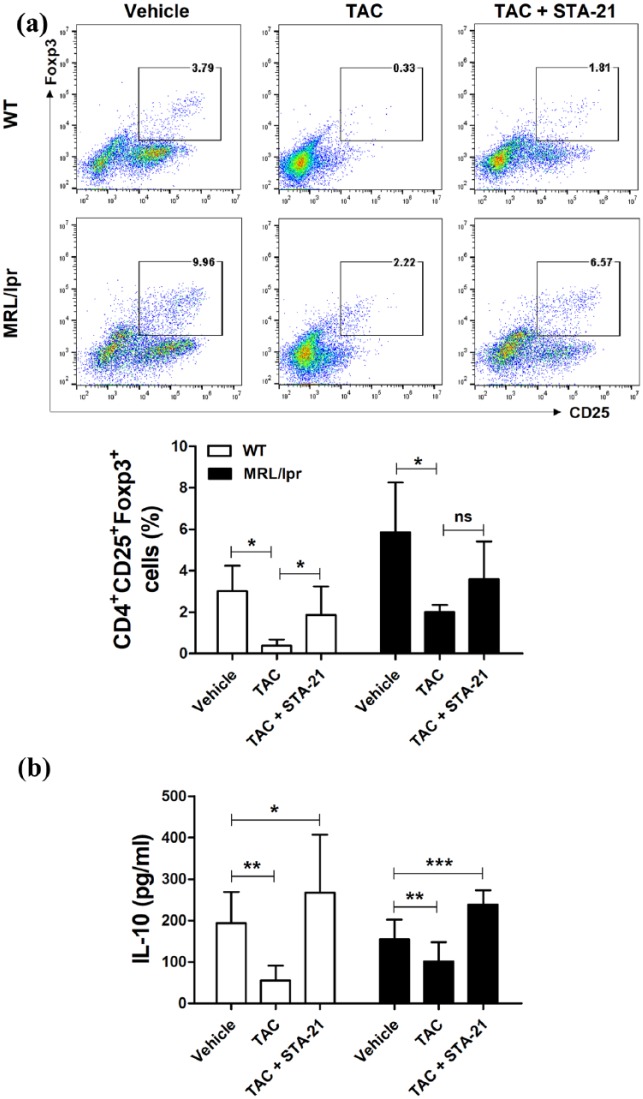
TAC and a STAT3 inhibitor, STA-21, induced Treg cells and IL-10 production
To assess the effects of TAC on Treg differentiation in vitro, isolated splenocytes of WT or lupus-prone MRL/lpr mice were cultured with TAC in the presence of anti-CD3 and anti-CD28 for 3 days. Flow cytometric analysis showed that in vitro treatment with TAC suppressed CD4+CD25+Foxp3+ Treg cells compared with untreated cells (Figure 3(a)). To investigate whether TAC in combination with STA-21 would affect the synergistic effects in the Treg cell population, we counted Treg cells among splenocytes treated with TAC and STA-21 by flow cytometry. As shown in Figure 3(a), the population of CD4+CD25+Foxp3+ Treg cells was dramatically decreased by TAC treatment compared with the untreated control (P < 0.05). Treatment with TAC and STA-21 exerted an additive effect, increasing the population of Treg cells in splenocytes from WT (P < 0.05) and lupus-prone MRL/lpr mice. The IL-10 level in culture supernatant from TAC-treated splenocytes of WT or lupus-prone MRL/lpr mice was also decreased (P < 0.01, respectively; Figure 3(b)). The addition of STA-21 to the TAC treatment increased the production of IL-10 in WT and lupus-prone MRL/lpr mice (P < 0.05 and P < 0.001, respectively).
Figure 3.
TAC and STA-21 induced Treg cells. Splenocytes of WT or lupus-prone MRL/lpr mice (n = 7) were cultured with TAC (1 nM) and STA-21 (10 μM) in the presence of anti-CD3 and anti-CD28 for 3 days. (a) After 3 days, cells were stained with antibodies against CD4+CD25+Foxp3+ Treg cells for intracellular flow cytometric analysis. (b) IL-10 concentrations in culture supernatants were determined by ELISA. *P < 0.05, **P < 0.01, ***P < 0.001. Data are mean ± SD.
TAC inhibited the production of TNF-α synergistically with STA-21
To test the negative regulation of TAC for the inflammatory mediator TNF-α, isolated splenocytes of WT or lupus-prone NZB/WF1, Roquinsan/san, and MRL/lpr mice were cultured with TAC in the presence of LPS for 3 days. Treatment with TAC did not suppress the production of TNF-α in splenocytes from WT or lupus-prone MRL/lpr mice (Figure 4). However, treatment with TAC and STA-21 significantly suppressed the production of TNF-α in a synergistic manner (in WT: P < 0.05; NZB/WF1, Roquinsan/san, and MRL/lpr: P < 0.001).
Figure 4.
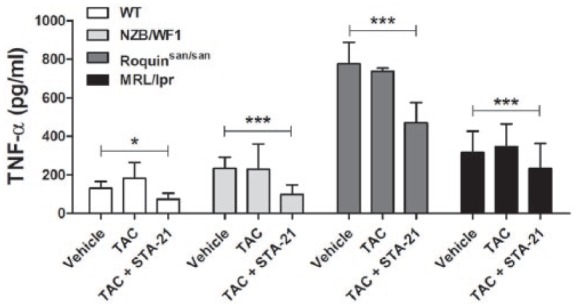
Inhibition of tumor necrosis factor (TNF)-α production by TAC and STA-21. Splenocytes of WT or lupus-prone NZB/WF1, Roquinsan/san, and MRL/lpr mice (n = 3) were cultured with TAC (1 nM) in the presence of LPS (100 ng/mL) for 3 days. TNF-α concentration in culture supernatants was determined by ELISA. *P < 0.05, ***P < 0.001 versus vehicle-treated condition. Data are mean ± SD.
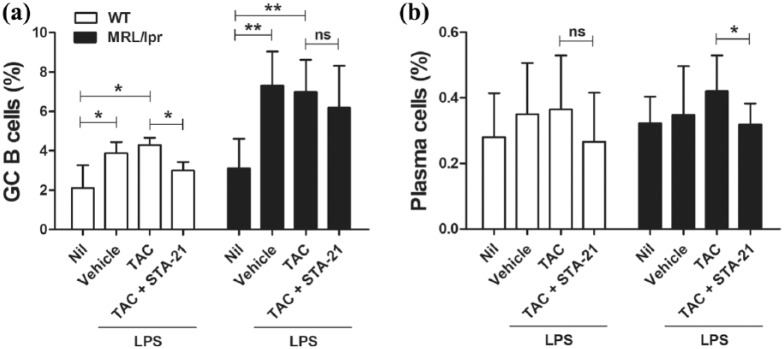
TAC with STA-21 suppressed GC B cells and plasma cells
B cells play an important role in the pathogenesis of lupus. B cells can differentiate into antibody-secreting plasmablasts and plasma cells that produce cytokines and chemokines and can function as antigen-presenting cells. Follicular Th (Tfh) cells are required for the proliferation and maturation of B cells in GCs.7 To investigate the effect of TAC on populations of B220+GL-7+ GC B cells and B220–CD138+ plasma cells, isolated splenocytes of WT or lupus-prone MRL/lpr mice were cultured with TAC in the presence of LPS for 3 days. TAC treatment did not affect populations of GC B cells and plasma cells (Figure 5). However, treatment with TAC and STA-21 reduced the percentage of GC B cells in WT mice (P < 0.05; Figure 5(a)). Treatment with TAC and STA-21 also reduced the percentage of plasma cells in MRL/lpr mice (P < 0.05; Figure 5(b)).
Figure 5.
Combination therapy with TAC and STA-21 reduced the population of GC B cells and plasma cells. Splenocytes of WT or lupus-prone MRL/lpr mice (n = 5) were cultured with TAC (1 nM) in the presence of LPS (100 ng/mL) for 3 days. After 3 days, cells were stained with antibodies against (a) B220+GL-7+ GC B cells and (b) B220–CD138+ plasma cells for flow cytometric analysis. *P < 0.05, **P < 0.01. Data are mean ± SD.
Discussion
This study aimed to determine whether TAC regulates effector T cells, GC B cells, and plasma cells in mice. In this study, we demonstrated that treatment with TAC suppressed the population of Th1, Th2, and Th17 cells, as well as the production of IFN-γ and IL-17A in WT and lupus-prone NZB/WF1, Roquinsan/san, and MRL/lpr mice. TAC also reduced the population of Treg cells; however, combination treatment with STA-21, a potent STAT3 inhibitor, increased the population of Treg cells. TAC and STA-21 suppressed the population of GC B cells and plasma cells in a synergistic manner.
Upon antigen stimulation, CD4+ T cells can differentiate into various subsets of helper T cells, which have distinct functions and cytokine profiles, depending on the cytokine milieu during activation. Th17 cells, which are characterized by their expression of IL-17A, IL-17F, and IL-21, were recently identified and can be distinguished from Th1 and Th2 cells.8 Treg cells have an immunoregulatory function and play an opposite role from that of Th17 cells, by maintaining self-tolerance.9 TAC reduces the number of Th17 and Th1 cells and preserves the number of Treg and Th2 cells among human CD4+ cells.10 In renal transplant recipients, TAC has been shown to significantly suppress the expression of adhesion molecules and costimulatory ligands, as well as the Th1/Th2 response.11 There have been few reports of the effects of TAC on the regulation of effector T cells in SLE. In 6-month-old NZB/WF1 mice, TAC inhibited Th1-related cytokine and immunoglobulin-2a anti-DNA antibody production.12 In this study, we confirmed that TAC treatment of splenocytes from WT or lupus-prone NZB/WF1, Roquinsan/san, and MRL/lpr mice reduced the population of Th1, Th2, and Th17 cells, as well as the production of related cytokines. Furthermore, the population of Treg cells was also suppressed by TAC. This result is reasonable because TAC inhibits IL-2 production in T lymphocytes and IL-2 is critical for Treg function.13 Combination therapy with STA-21 could enhance the increase in the population of Treg cells. In our previous study, we found that STA-21 induces expansion of Treg cells in vivo and in vitro by inducing STAT5 phosphorylation in CD4 cells.6 It is possible that STA-21 exerts an additive effect with TAC in terms of Treg cell induction. Further studies are needed to define the mechanism of action of TAC and STA-21 in this process.
Maturation of the antibody response, as well as memory B cell and plasma cell differentiation, occurs in GCs. GCs are important sites where B cells proliferate and undergo class switching, somatic hypermutation, and affinity maturation within B-cell follicles in secondary lymphoid organs. GC reactions produce long-lived antibody-secreting plasma cells and memory B cells.14 In this study, TAC treatment did not affect GC B cells or plasma cells in WT or lupus-prone mice. Importantly, we identified synergistic effects of TAC and STA-21, in terms of suppressing GC B cells and plasma cells in WT and lupus-prone mice. STAT3 signaling in B cells is essential for GC formation and GC B cell maintenance, as well as for production of IgG and plasma cell differentiation in MRL/lpr lupus-prone mice.15 Further investigation is needed to understand the mechanism underlying Treg induction by TAC and STA-21.
In conclusion, our data are the first to demonstrate additive effects of TAC and the STAT3 inhibitor STA-21 in WT and lupus-prone mice. Combination treatment with TAC and STA-21 increased the population of Treg cells compared to treatment with TAC alone. In addition, STA-21 reduced the population of GC B cells and plasma cells. These findings suggest that the application of TAC with a STAT3 signal inhibitor may be a promising treatment for SLE.
Acknowledgments
M.-L.C. and S.-H.P. contributed equally to this work.
Footnotes
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: This study was funded by Astellas Pharma Korea, Inc. and was supported by a grant from the Korea Health Technology R&D Project through the Korea Health Industry Development Institute (KHIDI), funded by the Ministry of Health & Welfare, Republic of Korea (HI15C1062).
References
- 1. Tsokos GC. (2011) Systemic lupus erythematosus. New England Journal of Medicine 365: 2110–2121. [DOI] [PubMed] [Google Scholar]
- 2. Liu Z, Davidson A. (2011) BAFF and selection of autoreactive B cells. Trends in Immunology 32: 388–394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Shlomchik MJ, Craft JE, Mamula MJ. (2001) From T to B and back again: Positive feedback in systemic autoimmune disease. Nature Reviews Immunology 1: 147–153. [DOI] [PubMed] [Google Scholar]
- 4. Dheer D, Jyoti PN, Gupta R. (2017) Tacrolimus: An updated review on delivering strategies for multifarious diseases. European Journal of Pharmaceutical Science 114: 217–227. [DOI] [PubMed] [Google Scholar]
- 5. Ishii S, Miwa Y, Otsuka K, et al. (2015) Influence of renal complications on the efficacy and adverse events of tacrolimus combination therapy in patients with systemic lupus erythematosus (SLE) during a maintenance phase: A single-centre, prospective study. Lupus Science & Medicine 2: e000091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Park JS, Kwok SK, Lim MA, et al. (2014) STA-21, a promising STAT-3 inhibitor that reciprocally regulates Th17 and Treg cells, inhibits osteoclastogenesis in mice and humans and alleviates autoimmune inflammation in an experimental model of rheumatoid arthritis. Arthritis & Rheumatology 66: 918–929. [DOI] [PubMed] [Google Scholar]
- 7. Craft JE. (2012) Follicular helper T cells in immunity and systemic autoimmunity. Nature Reviews Rheumatology 8: 337–347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Bettelli E, Carrier Y, Gao W, et al. (2006) Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature 441: 235–238. [DOI] [PubMed] [Google Scholar]
- 9. Zhu J, Yamane H, Paul WE. (2010) Differentiation of effector CD4 T cell populations. Annual Review of Immunology 28: 445–489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Miroux C, Morales O, Ghazal K, et al. (2012) In vitro effects of cyclosporine A and tacrolimus on regulatory T-cell proliferation and function. Transplantation 94: 123–131. [DOI] [PubMed] [Google Scholar]
- 11. Weimer R, Melk A, Daniel V, et al. (2000) Switch from cyclosporine A to tacrolimus in renal transplant recipients: Impact on Th1, Th2, and monokine responses. Human Immunology 61: 884–897. [DOI] [PubMed] [Google Scholar]
- 12. Sugiyama M, Funauchi M, Yamagata T, et al. (2004) Predominant inhibition of Th1 cytokines in New Zealand black/white F1 mice treated with FK506. Scandinavian Journal of Rheumatology 33: 108–114. [DOI] [PubMed] [Google Scholar]
- 13. Minguillon J, Morancho B, Kim SJ, et al. (2005) Concentrations of cyclosporin A and FK506 that inhibit IL-2 induction in human T cells do not affect TGF-beta1 biosynthesis, whereas higher doses of cyclosporin A trigger apoptosis and release of preformed TGF-beta1. Journal of Leukocyte Biology 77: 748–758. [DOI] [PubMed] [Google Scholar]
- 14. Victora GD, Nussenzweig MC. (2012) Germinal centers. Annual Review of Immunology 30: 429–457. [DOI] [PubMed] [Google Scholar]
- 15. Ding C, Chen X, Dascani P, et al. (2016) STAT3 signaling in B cells is critical for germinal center maintenance and contributes to the pathogenesis of murine models of lupus. Journal of Immunology 196: 4477–4486. [DOI] [PMC free article] [PubMed] [Google Scholar]