Abstract
Background:
Several studies have demonstrated harm associated with using erythropoiesis-stimulating agents (ESA) to achieve higher hemoglobin (Hb) levels. Subsequently, more conservative use of ESAs has changed anemia therapy in patients with chronic renal failure.
Objective:
The objectives were to identify transfusion rates in hemodialysis (HD) patients during the first year of therapy, to identify factors associated with the probability of transfusion, describe reasons for the transfusions, and identify the Hb values associated with each transfusion. An exploratory objective was to describe the age of red blood cell transfusions.
Design:
This was a multicenter prospective observational cohort study.
Setting:
There were 12 study sites in 5 Canadian provinces. The study was performed from 2012 to 2014.
Methods:
The study patients were adult incident chronic HD patients in these centers. Patients with acute kidney injury, peritoneal dialysis, and planned transfer to satellite units were excluded. Patients had to receive at least 1 month of chronic HD to be eligible. Data for 3 months prior to HD were obtained by retrospective chart review. Prospectively, charts were reviewed monthly for 12 months for data abstraction.
Results:
There were 314 patients enrolled and 79.9% completed 12 month follow-up. Ninety-four (29.9%) patients received at least 1 unit of blood. During the first 90 days, the transfusion episode rate was 148.4 per 100 patient-years compared with 62.6 per 100 patient-years post 90 days. The most frequent indication was a low Hb value (92%) with gastrointestinal bleeding, surgical blood loss, and fatigue accounting for 9.9%, 8.6%, and 4.5%, respectively. Some patients had >1 indication. The mean Hb values prior to transfusion episodes ranged from 75.3 to 78.6 g/L. Cox regression analysis on time to first transfusion and time to first hospitalization/death both showed an association with inpatient initiation of HD. Some 37.5% initiated HD as an inpatient and differed from those starting as an outpatient. They had less predialysis care and laboratory data suggested more inflammation. The mean and median ages of the blood units transfused were 24.9 (SD = 10.0) and 23 days (interquartile range = 17-33).
Conclusions:
This work reported the blood transfusion rate in incident HD patients in Canada during a period associated with conservative ESA prescription. The major indication for transfusion was a low Hb rather than clinical symptoms. Initiation of HD as an inpatient was independently associated with the probability of receiving a blood transfusion. These findings require further investigation.
Keywords: hemodialysis, erythropoiesis-stimulating agent, transfusion
Abrégé
Contexte:
Plusieurs études ont fait état de lésions associées à l’utilisation d’agents stimulant l’érythropoïèse (ASE) pour hausser le taux d’hémoglobine (Hb). Dès lors, une utilisation plus conservatrice des ASE a modifié le traitement de l’anémie chez les patients atteints d’insuffisance rénale chronique.
Objectifs de l’étude:
L’étude visait à i) établir les taux de transfusion sanguine chez les patients hémodialysés au cours de la première année de traitement; ii) cerner les facteurs associés à la probabilité de recourir à une transfusion sanguine; iii) connaître les raisons de la transfusion; et iv) caractériser le taux d’hémoglobine au moment de l’intervention. En outre, un objectif exploratoire consistait à déterminer l’âge des érythrocytes transfusés.
Type d’étude:
Il s’agit d’une étude de cohorte observationnelle prospective multicentrique.
Cadre de l’étude:
L’étude s’est tenue entre 2012 et 2014 sur douze sites répartis dans cinq provinces canadiennes.
Méthodologie:
Les patients adultes hémodialysés des centres participants ont été recrutés pour l’étude. Ont été exclus les patients atteints d’insuffisance rénale aiguë, les patients traités par dialyse péritonéale et les patients à être transférés vers une unité satellite. Pour être admissible, le patient devait recevoir un traitement d’hémodialyse continu pendant au moins un mois. On a rétrospectivement tiré des dossiers médicaux les données des trois mois précédant l’hémodialyse, puis on a extrait les données des dossiers médicaux chaque mois sur un an.
Résultats:
Un total de 314 patients a participé à l’étude et 79,9 % d’entre eux ont complété les 12 mois de suivi. Sur cette période, 94 patients (29,9 %) ont reçu au moins une transfusion sanguine. Au cours des 90 premiers jours, le taux d’épisodes transfusionnels était de 148,4 pour 100 années-patients, comparativement à 62,6 pour 100 années-patients pour le reste de l’étude. La raison la plus fréquente de recourir à une transfusion était un faible taux d’Hb (92 % des cas); les cas de saignements gastro-intestinaux, de perte de sang périchirurgicale et de fatigue comptaient quant à eux pour 9,9 %, 8,6 % et 4,5 % respectivement. Certains patients cumulant plus d’une indication. Le taux d’Hb moyen prétransfusion variait de 75,3 à 78,6 g/L. Une analyse de régression de Cox sur le temps écoulé jusqu’à la première transfusion et jusqu’à la première hospitalisation (ou le décès) du patient a montré une corrélation avec l’initiation d’un traitement d’hémodialyse chez les patients hospitalisés. Les sujets qui avaient initié leur traitement d’hémodialyse alors qu’ils étaient hospitalisés (37,5 %) ont reçu moins de soins prédialyse et présentaient davantage d’inflammation que les sujets qui avaient commencé leurs traitements d’hémodialyse en tant que patient externe. Enfin, l’âge moyen et l’âge médian des érythrocytes transfusés étaient de 24,9 jours (ÉT : 10,0) et de 23 jours (EIQ : 17 à 23).
Conclusion:
Notre étude a permis de connaître le taux de transfusions sanguines dans une population de patients hémodialysés canadiens au cours d’une période correspondant à une prescription conservatrice d’ASE. On a observé que la principale raison de transfusion était un faible taux d’Hb et non des symptômes cliniques. Enfin, une hémodialyse amorcée en cours d’hospitalisation a été associée à une probabilité accrue de transfusion sanguine. Nos constatations devraient faire l’objet d’études plus approfondies.
What was known before
Data regarding transfusion rates following more conservative use of ESAs is limited to data from the United States, mostly from billing data and excluding hospitalized patients. There are few data addressing the reasons for transfusion and no data evaluating associations with transfusion probability. There are no data about the age of red blood cells transfused in this population. Only 1 US study addresses transfusions during the first 90 days of HD. There are no data regarding transfusion practice in Canada following more conservative ESA therapy.
What this adds
We have a credible estimate of transfusion rates post conservative use of ESA during the first year of therapy in the incident chronic HD population in Canada, divided into the early less stable 90 days and the more stable 90 to 365 days. The indication for transfusion appears based on the Hb value rather than clinical symptoms with mean Hb values less than 80 g/L being the indication. Inpatient initiation of HD is associated with an increased probability of transfusion and/or hospitalization. The association with increased markers of inflammation could be the subject of future research. The age of transfused red blood cells transfused in this population is new data but the clinical relevance is unknown.
Introduction
Hemodialysis (HD) patients in Canada received frequent blood transfusions prior to the approved availability of erythropoiesis-stimulating agents (ESA) in 1990.1 A randomized clinical study which compared outcomes among prevalent HD patients randomized to placebo (hemoglobin [Hb]: 74 g/L), epoetin alfa with a target Hb of 95 to 105 g/L, and epoetin alfa with a target Hb of 115 to 130 g/L was reported in 1990.2 Among the 40 subjects randomized to placebo, 23 were transfused during the 6-month follow-up compared with 2 of 78 randomized to receive erythropoietin alfa. Quality of life was significantly improved in those treated with ESA.2
Hb targets and the dose of ESA had increased until several studies demonstrated harm associated with higher Hb targets.3,4 This led to changes in Kidney Disease: Improving Global Outcomes (KDIGO) guidelines5 that recommended more conservative use of ESA. The Canadian Society of Nephrology Commentary on these guidelines6 suggested that, with the exception of patients with a history of malignancy, stroke, or recent transient ischemic attacks, the recommended Hb target should be 100 to 110 g/L, with 95 to 115 g/L being an acceptable range.
There was concern that more restrictive use of ESA might lead to increased use of transfusions. Another concern was that older red blood cell (RBC) transfusions might be associated with worse clinical outcomes.7 However, there are no data addressing this in patients requiring hemodialysis.
The majority of data addressing transfusion rates in HD patients are based on claims data from the United States that appear to underestimate the transfusion rate, especially among hospitalized patients.8-12 There are few data addressing the transfusion rates among incident HD patients during the less stable first 90 days of HD. In addition, few data address the context of a transfusion episode (clinical indication, prescriber, site of transfusion, and the Hb value at which transfusion occurs). There are few data identifying the clinical factors associated with transfusion, and there are no data regarding the age of RBCs transfused in HD patients.
We have conducted a prospective observational study among incident HD patients in Canada between 2012 and 2014. The primary objective was to identify the rate of transfusion of RBC units and transfusion episodes during the first year of therapy. The secondary objectives were to identify factors associated with the probability of experiencing a transfusion episode, describe the reasons for transfusion, and identify the Hb value associated with transfusion episodes. An exploratory objective was to describe the age of RBC transfusions during the first year of HD therapy. This exploratory objective was based on the concerns raised by Zimring7 in 2013 in which the potential adverse effects of the biochemical changes which occur in stored blood were critically reviewed.
Methods
This was a multicenter prospective observational study of incident chronic HD patients in 10 centers in Canada. Screening of all potential patients occurred 21 days (±14 days) after the initiation of HD. Eligibility was determined at the completion of the screening period.
The study protocol was approved by the Research Ethics Board (REB) in each center. The written patient informed consent form, approved by the REB, contained adequate explanation of the aims, methods, anticipated benefits and potential hazards of the study. A signed copy of the informed consent was included in the patient’s medical record.
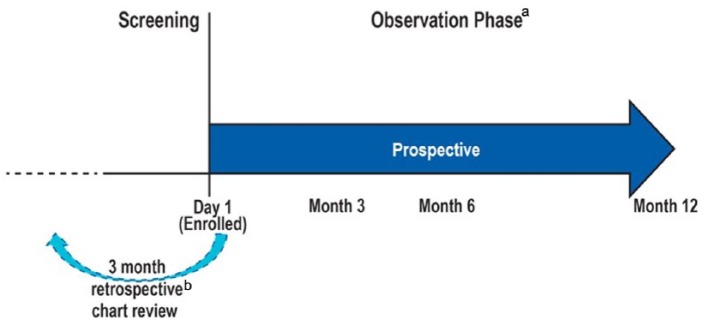
Patients who met the eligibility criteria were enrolled (no later than 6 weeks after the initiation of chronic HD treatment) and followed for up to 12 months following enrollment. Data for the 3 months prior to initiation of chronic HD were obtained by chart review (Figure 1). The charts were from the predialysis clinic in each center and were reviewed by the study coordinator in each center. The data collected were predefined and collected in the case report form. This form was reviewed by the study monitor to ensure that all data fields were completed.
Figure 1.
Study design.
aChart review and data abstraction.
bCollect key information predialysis.
The eligibility criteria were adults (≥18 years of age) diagnosed with chronic kidney disease (CKD) and initiating chronic HD, defined as requiring at least 4 consecutive weeks of HD occurring at least twice weekly. Exclusion criteria were acute renal failure, peritoneal dialysis or planning to transfer to peritoneal dialysis within 2 months, living donor kidney transplant planned within 2 months, and receiving chronic HD for more than 6 weeks prior to screening. Patients who planned to transfer to satellite units were excluded for logistical reasons.
Retrospective data for the 3 months prior to the initiation of chronic HD were retrieved by chart review. Prospectively, charts were reviewed monthly and data were abstracted. Where data regarding the reason for transfusion were not clear, the study coordinator in each center followed up with the prescriber.
The data abstracted retrospectively included pre-HD care, transfusion history, use of ESA and iron, and type of vascular access. The data abstracted prospectively included information on transfusion history, indication for the transfusion, Hb value prior to transfusion, monthly routine laboratory data, serum ferritin, ESA prescription, intravenous iron prescription, hospitalization, and death. For each unit of blood transfused, the blood type and age of the RBC transfusion were recorded.
Statistical Methods
A sample size of 400 was planned based on the level of precision of ±14 (half width of the 95% confidence interval) around the estimated rate of transfusion per 100 patient-years of exposure. Because of a slower-than-anticipated rate of recruitment, the sample size was truncated to 314 patients, providing 284 patient-years of follow-up. Given the achieved sample size of 314 subjects with 284 patient-years of follow-up, the level of precision around the actual estimated rate is ±14.2.
The rate of transfusion was calculated as the number of units transfused per 100 patient-years of follow-up with Poisson 95% confidence limits. A transfusion episode was defined as one or more units of blood transfused in a 24-hour period. Transfusion rates were calculated for the first 90 days after initiation of chronic HD and post 90 days. The cumulative incidence rates of transfusion were calculated with death as a competing risk.13 Missing data were not imputed.
The age of RBC units transfused was summarized as mean and median age. Summary statistics were also calculated on age of RBC by blood type. A post hoc comparison of mean age by blood type was also explored.
Hospitalization and death were examined for patients with and without a transfusion. Hospitalizations for dialysis initiation or for dialysis access issues within the first week following initiation of HD were excluded. The nonrandomized nature of patients with and without transfusion and the immortal time bias (that a patient cannot be hospitalized or transfused after death) was also assessed using epidemiologic methods14 to adjust for any bias. The cumulative incidence of hospitalization was calculated with death as a competing risk13 categorizing patients as having a transfusion prior to hospitalization or not.
To assess associations of incident transfusion episodes with demographic and clinical characteristics, Cox regression models were fit on time to first transfusion, censoring for death, and end of follow-up. Cox regression was also fit on time to first hospitalization or death including baseline and disease characteristics as well as a time varying covariate for transfusion within 30 days prior to the hospitalization. All Cox models were selected by first including covariates with P values < .1 from univariate models. The variables used in the univariate models are shown in Table 1. Final models were assessed for collinearity and were selected based on the Akaike information criterion statistic.15
Table 1.
Baseline Demographic and Clinical Data.
| Total (N = 314) |
||
|---|---|---|
| n | % | |
| Age | ||
| < 65 y | 152 | 48.4 |
| ≥ 65 y | 162 | 51.6 |
| Sex | ||
| Female | 110 | 35.0 |
| Male | 204 | 65.0 |
| Race | ||
| White | 234 | 74.5 |
| Asian | 38 | 12.1 |
| Other | 42 | 13.4 |
| CKD | ||
| Diabetes | 140 | 44.6 |
| Vascular disease | 52 | 16.6 |
| Glomerulonephritis | 52 | 16.6 |
| Other/unknown | 70 | 22.3 |
| Pre-HD care | ||
| Yes | 276 | 87.9 |
| No | 38 | 12.1 |
| Duration pre-HD care | ||
| < 3 months | 27 | 9.8 |
| 3-12 months | 42 | 15.2 |
| ≥ 12 months | 207 | 75.0 |
| Access at initiation HD | ||
| AV fistula | 99 | 31.5 |
| Semipermanent catheter | 171 | 54.5 |
| Graft | 14 | 4.5 |
| Temporary catheter | 42 | 13.4 |
| Patient status at initiation | ||
| Inpatient | 118 | 37.6 |
| Outpatient | 196 | 62.4 |
| Prior transfusion | ||
| Yes | 57 | 18.2 |
| No | 257 | 81.8 |
Note. CKD = chronic kidney disease; HD = hemodialysis.
A post hoc analysis examining the subgroups of patients who initiated HD as an inpatient versus outpatient was explored using chi-squared comparisons for categorical variables and t test for continuous variables.
All P values are nominal with no adjustment for multiplicity. All analyses were done using SAS V9.2.
Results
There were 326 patients screened and 314 enrolled in this study constituting the full analysis set. A total of 251 (79.9%) patients completed at least 12 months follow-up. Among the 63 who did not complete 12 months follow-up, 21 died and 15 switched to peritoneal dialysis. Other reasons for incomplete follow-up included 7 kidney transplants, 8 with recovery of renal function, and other categories accounting for the remaining 12 patients.
The baseline characteristics of the study population, the predialysis care, vascular access at initiation of chronic HD, and site of initiation are described in Table 1. The mean age was 63.6 years.
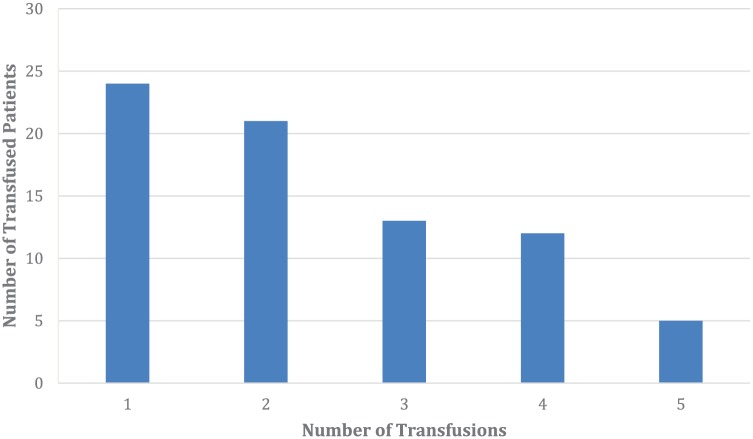
There were 94 (29.9%) patients who received at least 1 unit of blood. There were 383 units of blood transfused during 283.3 patient-years of follow-up. A frequency distribution of units of blood transfused, up to 5 units, is shown in Figure 2. An additional 19 patients received more than 5 units of blood, 18 of which were equally distributed over frequencies of 6 to 16 transfusions.
Figure 2.
Frequency distribution of units of blood transfused among patients who received at least 1 unit of blood (n = 94), up to 5 units.
Note. An additional 19 patients received more than 5 units (see text).
During the first 90 days, there were 168 units of blood transfused over 76.1 patient-years for a rate of 220.6 (188.5, 256.6) units per 100 patient-years. The transfusion episode rate was 148.4 (122.3, 178.4) episodes per 100 patient-years (Table 2).
Table 2.
Transfusion Summary by the First 90 Days and Post 90 Days After Initiation of Chronic Hemodialysis.
| First 90 days | Post 90 days | |
|---|---|---|
| Number of patient-years | 76.1 | 207.6 |
| Number of RBC units transfused | 168 | 215 |
| Rate per 100 patient-years follow-up (95% CI) | 220.6 (188.5-256.6) | 103.6 (90.2-118.4) |
| Number of transfusion episodes | 113 | 130 |
| Rate per 100 patient-years follow-up (95% CI) | 148.4 (122.3-178.4) | 62.6 (52.3-74.3) |
Note. RBC = red blood cell; CI = confidence interval.
After 90 days, there were 215 units of blood transfused over 207.6 patient-years for a rate of 103.6 (90.2-118.4) units per 100 patient-years follow-up. The transfusion episode rate was 62.6 (52.3-74.3) episodes per 100 patient-years (Table 2).
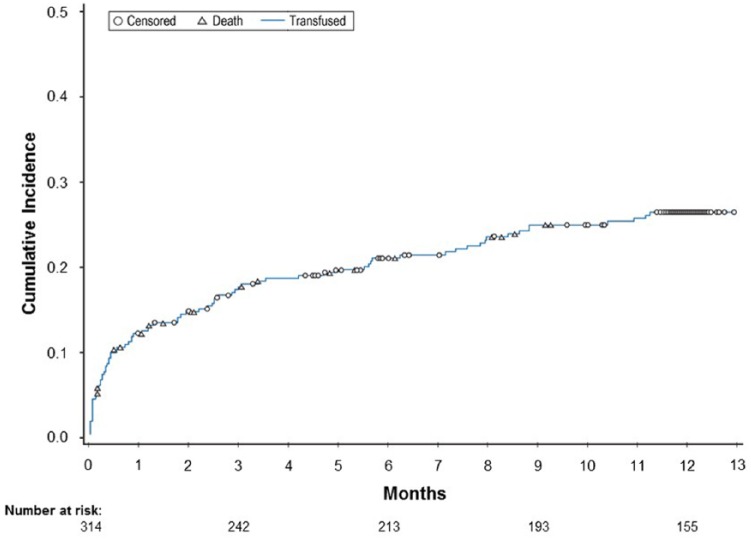
Figure 3 shows the time to first unit of blood transfused, with death as a competing risk and censored for end of follow-up. The probability of a first transfusion was high during the first 90 days after initiation of chronic HD and decreased thereafter. The multivariate Cox regression model found the only variables associated with the probability of transfusion were increased age hazard ratio ([HR] 95% CI) = 1.02 (1.00-1.03) and inpatient status at initiation HR (95% CI) = 2.8 (1.9-4.3).
Figure 3.
Time to first transfusion censoring for death and end of follow-up.
Most transfusions were requested by nephrologists (70%) with surgeons and cardiologists accounting for 10% and 5%, respectively. About 65% of the transfusions occurred in the dialysis unit while 35% occurred in an inpatient setting.
The most frequent indication for a transfusion episode was a low Hb value (92.2%) with gastrointestinal bleeding, perisurgical blood loss, and fatigue accounting for 9.9%, 8.6%, and 4.5%, respectively. Cardiovascular symptoms accounted for 2.1%. Some patients had multiple indications for transfusion.
The mean Hb values (SD) prior to the first, second, and third transfusion episodes were 78.6 g/L (11.1), 75.3g/L (10.5), and 75.9 g/l (10.3).
Among the 220 patients never transfused, the proportion prescribed ESA during the study varied between 81% and 85.3% while those transfused had a slightly higher proportion, varying between 88.1% and 94.0%. Intravenous iron prescription, among those never transfused varied between 71.4% and 76.7% compared with 65.9% and 74.0% among those transfused. There were no temporal trends. Table 3 shows the mean Hb and mean serum ferritin values at 3 to 12 months follow-up.
Table 3.
Mean Hemoglobin (g/L) and Serum Ferritin Values (µg/L).
| Month | Hb (g/L) |
Ferritin (µg/L) |
||
|---|---|---|---|---|
| Transfused (n = 94) | Not transfused (n = 220) | Transfused (n = 94) | Not transfused (n = 220) | |
| 3 | 105.9 | 106.4 | 349 | 297 |
| 6 | 103.7 | 108.9 | 490 | 341 |
| 9 | 105.6 | 108.4 | 451 | 399 |
| 12 | 105.7 | 108.7 | 504 | 566 |
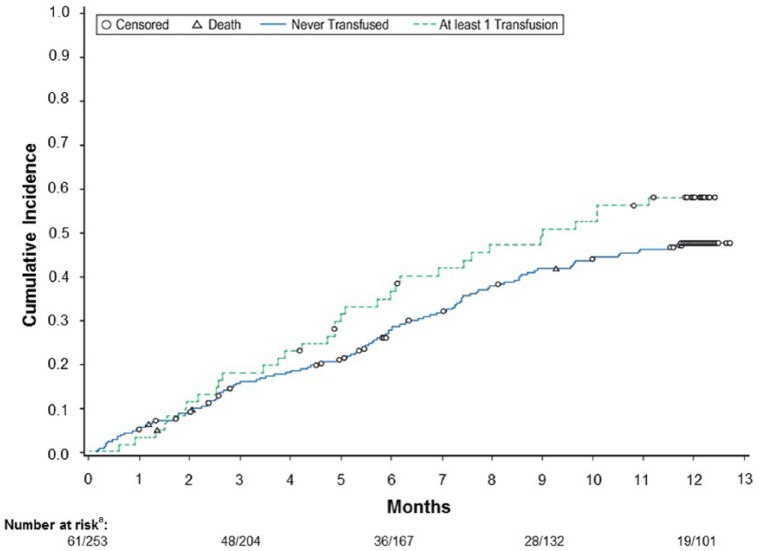
The cumulative incidence of first hospitalization with death as a competing risk is greater for subjects who had received a transfusion than for subjects who were never transfused (Figure 4). The Cox regression model showed that the risk of first hospitalization or death is increased with transfusion within 30 days prior to the event HR (95% CI) = 4.6 (2.8-7.5) and inpatient HD initiation HR (95% CI) = 1.44 (1.03-2.01).
Figure 4.
Cumulative incidence of hospitalization with death as a competing risk, categorizing patients as having or not having a transfusion prior to hospitalization.
an/n represents number of cases for transfused and nontransfused groups, respectively. Patients who had an RCB transfusion after hospitalization (n = 33) are included in the nontransfused group.
Inpatient initiation of chronic HD was associated with both the time to first transfusion and time to first hospitalization/death. A post hoc exploration of the variables which differ between subjects initiating chronic HD as inpatient compared with outpatient are shown in Tables 4 and 5. Patients initiating chronic HD as an inpatient were less likely to have received pre-HD care, and for those who received pre-HD care, patients initiating chronic HD as an inpatient had a shorter duration of care. Patients initiating as an inpatient were less likely to have an AV fistula and more likely to have a semipermanent or temporary hemodialysis catheter (Table 4). Patients initiating chronic HD as an inpatient had lower serum albumin, higher serum ferritin, higher C-reactive protein, and lower Hb values than those starting as an outpatient (Table 5).
Table 4.
Comparison Between Subjects Initiating Chronic Hemodialysis as an Inpatient Compared With Outpatient.
| Outpatient (n= 196) | Inpatient (n = 118) | P | ||
|---|---|---|---|---|
| Pre-HD care | No | 10 (5%) | 28 (24%) | <.001 |
| Yes | 186 (95%) | 90 (76%) | ||
| Duration (months) | 1-3 | 27 (14%) | 38 (32%) | <.001 |
| 3-12 | 29 (15%) | 13 (11%) | ||
| >12 | 140 (71%) | 67 (57%) | ||
| Vascular access | Fistula | 76 (39%) | 16 (14%) | < .001 |
| Semi-Perm | 96 (49%) | 70 (60%) | ||
| Temporary | 15 (8%) | 15 (13%) | ||
| Other | 9 (4%) | 17 (13%) | ||
| Prior transfusion | No | 176 (90%) | 81 (69%) | <.001 |
| Yes | 20 (10%) | 37 (31%) |
Table 5.
Comparison Baseline Laboratory Values Between Subjects Initiating Chronic Hemodialysis as an Inpatient Compared With Outpatient.
| n | Outpatient | Inpatient | P | |
|---|---|---|---|---|
| Albumin (g/L) | 267 | 34.4 | 29.3 | <.001 |
| Ferritin (µg/L) | 238 | 221.2 | 617.3 | .038 |
| CRP (mg/L) | 80 | 2.0 | 5.7 | .005 |
| Hb (g/L) | 301 | 97.1 | 91.4 | .001 |
Note. CRP= C-reactive protein.
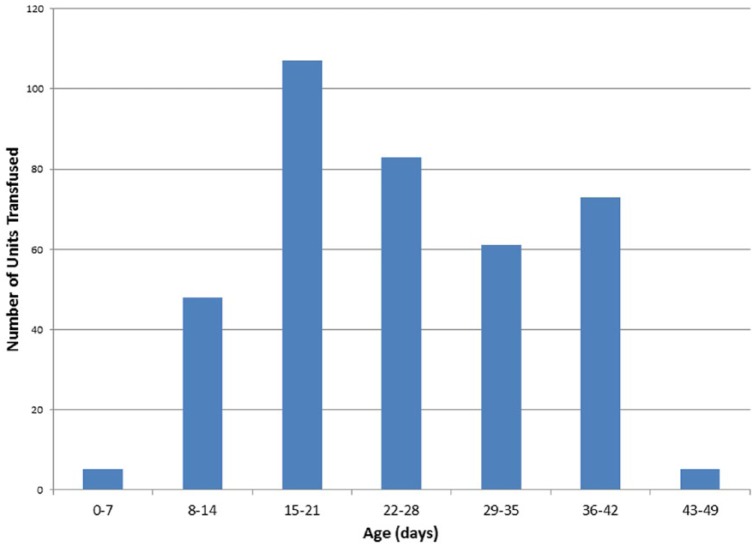
There were 381 units of blood transfused with a known blood type. The majority were type O (51.2%). The next most frequent was type A (31.2%). The distribution of the age of the transfused blood by unit (all types combined) is shown in Figure 5. The mean and median age of the RBC units transfused were 24.9 (SD = 10.0) and 23 days (interquartile range [IQR] = 17-33). The mean age of transfused blood type O was 22.1 days (SD = 9.2) compared with all non–type O blood at 28.2 days (SD = 9.1) (P < .001).
Figure 5.
Frequency distribution of age of red blood cell transfusions.
Discussion
The transfusion episode rate during the first 90 days was 148.4 episodes per 100 patient-years. There is one other study which addresses the transfusion rate during the first 90 days of HD. Wang et al16 studied older patients undergoing incident HD covered by fee-for-service Medicare in the United States. Between 2011 and 2013, the transfusion episode rate was 148.4 (145.6-151.2) per 100 patient-years. This point estimate is identical to that in our study.
The number of transfusion episodes post 90 days was 62.6 episodes per 100 patient-years. Our data were collected between 2012 and 2014. Data from the United States for prevalent patients have been reported for the years 2005 to 2012.8-12 The rates have varied from 23 to 43 episodes per 100 patient-years.8-11 These reports have used data from Medicare claims and the authors acknowledge probable underreporting, particularly for transfusions given while hospitalized. Recently, Chertow et al reported a rate of 30 per 100 patient-years in 2005 with an increase to 37 in 2012.12 Wang et al16 showed transfusion episode rates of 87.9, 76.5, and 71.6 per 100 patient-years for days 90-180, 181-270, and 271-360 days post initiation of HD.
The most frequent indication for a transfusion episode was a low Hb value with clinical indications being much lower. Whitman et al17 used adaptive choice-based cognitive analysis to explore decision-making for transfusion of chronic HD patients in the United States. The absolute Hb value was the most important factor, accounting for 29% of decision-making, followed by functional status at 16% and cardiovascular comorbidities at 12%. The reasons for transfusion are consistent with the findings in our study. Some 92% of respondents would transfuse when the Hb value was ≤75 g/L with most providers being averse to transfusion when the Hb was >85 g/L.17
The impact of allowing Hb values to decrease to 75 to 80 g/L on quality of life was not addressed in our study. Data from the TREAT study4 reported no important difference in quality of life estimates comparing median Hb values of 125 g/L to 106 g/L. The data are not informative for Hb values of 75 to 80 g/L. Reanalysis of the Canadian Erythropoietin Study Group results18,19 showed that correction of Hb values from a mean of 74 g/L to 102 g/L or 117 g/L was associated with statistically significant improvement in fatigue, exercise tolerance, and physical function.
There was an increase in serum ferritin values over time for both those transfused and those never transfused with values > 500 µg/L in both groups at 12 months (Table 4). The progressive increase in serum ferritin values during the first year of HD is did not differ between those transfused and not transfused. Although we collected data on whether patients were prescribed IV iron or not, we had not collected information on dose and were unable to explore this further.
Initiation of chronic HD as an inpatient was associated with shorter time to first transfusion and first hospitalization. Those who had initiated HD as an inpatient had less predialysis care, and laboratory data suggest increased inflammation in these patients. However, 76% of those initiating HD as an inpatient had received predialysis care and 57% had been referred >12 months before initiation of HD. The proportion initiating HD as an inpatient was 39.6%, similar to the proportion reported in Canada in 2006.20
Transfusion of older RBCs has been associated with increased morbidity and mortality following cardiac surgery.21 A recent randomized controlled trial22 showed no difference in inhospital mortality between those randomized to receive usual storage time RBC transfusions (23.6 days) compared with those with short-term storage (13.0 days). There have been no studies in hemodialysis patients. However, the biologic changes that occur in stored RBCs might have an adverse effect on HD patients. This includes production of free Hb which can scavenge nitric oxide and production of asymmetric dimethylarginine which is known to inhibit nitric oxide synthesis.7 This could impact HD patients in whom endothelial dysfunction is thought to be due to reduced generation and bioavailability of nitric oxide.23
The mean and median ages of the transfused RBCs in our study were 24.9 and 23.0 days, respectively. There are no other data in the hemodialysis population to which this can be compared. The frequency distribution (Figure 5) and the IQR of 17 to 33 shows that many patients received older blood. The adverse outcomes in the observational study of patients with cardiovascular disease21 were in those who had received blood transfusions with the longest quartile of storage time (23-42 days).
The strengths of this study include the prospective observational design with clearly defined transfusion events, reasons for transfusion, Hb values triggering transfusion, and information regarding ESA and IV iron prescription with hemoglobin and serum ferritin values over time. A limitation of the study is that it is hypothesis generating rather than hypothesis testing. We did not collect data on ESA or IV iron dose and were underpowered to evaluate any association between the age of RBCs transfused and clinical outcomes.
In conclusion, the key findings were as follows : Documen-tation of a credible estimate of transfusion rates during the first year of chronic HD with differentiation between the early high transfusion rates during the first 90 days and the more stable period post 90 days; identification of the reasons for transfusion, site of transfusion, and Hb trigger values for transfusion; documentation of the age of RBC transfusions; identification of inpatient initiation as being associated with both time to first transfusion and time to first hospitalization or death; and identification of increased markers of inflammation as being associated with inpatient initiation.
These data will provide baseline information for studies of strategies to reduce transfusion rates and will provide a credible reference for changes over time. Future research could address the impact of low Hb values on quality of life and the impact of identification of patients with increased inflammatory markers during predialysis care.
Acknowledgments
The Investigators for this study: Aminu K. Bello, University of Alberta Hospital, Edmonton, AB; Christine M. Ribic, St. Joseph’s Healthcare, Hamilton, ON; Serge H. Cournoyer, Hôpital Charles LeMoyne, Greenfield Park, QC; Mercedes Kiaii, St. Paul’s Hospital, Vancouver, BC; Martine LeBlanc, Hôpital Maisonneuve-Rosemont, Montreal, QC; Norman Muirhead, University Hospital, London Health Sciences Centre, London, ON; Brenda R. Hemmelgarn, Foothills Medical Centre, Calgary, AB; Gihad Nesrallah, Humber River Hospital, Toronto, ON; Steven D. Soroka, QEII Health Sciences Centre, Halifax, NS; Paul Tam, The Scarborough Hospital, Scarborough, ON. Lieven P. Billen of Amgen Inc assisted in the preparation of this article.
Footnotes
Ethics Approval and Consent to Participate: The study protocol was approved by the Research Ethics Board (REB) in each center. The written patient informed consent form, approved by the REB, contained adequate explanation of the aims, methods, anticipated benefits and potential hazards of the study. A signed copy of the informed consent was included in the patient’s medical record.
Consent for Publication: All authors consent to the publication of this study.
Availability of Data and Materials: Available upon request.
Declaration of Conflicting Interests: The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: Christine M. Ribic has received research grants from Astellas, Leo, Pfizer, and Amgen and is a consultant to Astellas, Leo, Pfizer, and Alexion. Serge H. Cournoyer has received research grants from Amgen, Rockwell, Bayer, GSK, and Abbvie. He is a consultant to Amgen, Sanofi, and Otsuka and is a member of the speaker’s bureau for Otsuka. Melanie Poulin-Costello is a former employee of Amgen Inc and has stock in Amgen Inc. David N. Churchill is a consultant to Amgen, Capsule Communication, and Indegene and owns stock in Amgen, Baxter, and Pfizer. Norman Muirhead has received research grants from Janssen, Amgen, Pfizer, GSK, and BMS. The remaining authors have no conflicts of interest to declare.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was funded by Amgen Canada Inc.
References
- 1. Churchill DN, Taylor DW, Cook RJ, et al. Canadian hemodialysis morbidity study. Am J Kidney Dis. 1992;19:214-234. [DOI] [PubMed] [Google Scholar]
- 2. Canadian Erythropoietin Study Group. Association between recombinant human erythropoietin and quality of life and exercise capacity of patients receiving haemodialysis. Br Med J. 1990;300:573-578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Singh AK, Szczech L, Tang KL, et al. Correction of anemia with epoetin alfa in chronic kidney disease. N Engl J Med. 2006;355:2085-2098. [DOI] [PubMed] [Google Scholar]
- 4. Pfeffer MA, Burdmann EA, Chen CY, et al. A trial of darbepoetin alfa in type 2 diabetes and chronic kidney disease. N Engl J Med. 2009;361:2019-2032. [DOI] [PubMed] [Google Scholar]
- 5. McMurray JJV, Parfrey PS, Adamson JW, et al. Kidney Disease: Improving Global Outcomes (KDIGO) anemia work group. KDIGO clinical practice guideline for anemia in chronic kidney disease. Kidney Int suppl. 2012;2:279-335. [Google Scholar]
- 6. Moist LM, Troyanov S, White CT, et al. Canadian society of nephrology commentary on the 2012 KDIGO clinical practice guideline for anemia in CKD. Am J Kidney Dis. 2013;62:860-873. [DOI] [PubMed] [Google Scholar]
- 7. Zimring JC. Fresh versus old blood: are there differences and do they matter? Hematology Am Soc Hematol Educ Program. 2013;2013:651-655. [DOI] [PubMed] [Google Scholar]
- 8. Molony JT, Monda KL, Suying L, et al. Effects of epoetin alfa titration practices, implemented after changes to product labeling, on hemoglobin levels, transfusion use, and hospitalization rates. Am J Kidney Dis. 2016;68:266-276. [DOI] [PubMed] [Google Scholar]
- 9. Wetmore JB, Peng Y, Monda KL, et al. Trends in anemia management practices in patients receiving hemodialysis and peritoneal dialysis: a retrospective cohort analysis. Am J Nephrol. 2015;41:345-361. [DOI] [PubMed] [Google Scholar]
- 10. Cappell KA, Shreay S, Cao Z, Varker HV, Paoli CJ, Gitlin M. Red blood cell (RBC) transfusion rates among US chronic dialysis patients during changes to Medicare end-stage renal disease (ESRD) reimbursement systems and erythropoiesis stimulating agent (ESA) labels. BMC Nephrol. 2014;15:116-127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Liu J, Suying L, Gilbertson DT, Monda KL, Bradbury BD, Collins AJ. Development of a standardized transfusion ratio as a metric for evaluating dialysis facility anemia management practices. Am J Kidney Dis. 2014;64:608-615. [DOI] [PubMed] [Google Scholar]
- 12. Chertow GM, Liu J, Monda KL, et al. Epoetin alfa and outcomes in dialysis amid regulatory and payment reform. J Am Soc Nephrol. 2016;27:3129-3138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kim HT. Cumulative incidence in competing risks data and competing risks regression analysis. Clin Cancer Res. 2007;13:559-565. [DOI] [PubMed] [Google Scholar]
- 14. Suissa S. Immortal time bias in observational studies of drug effects. Pharmacoepidemiol Drug Saf. 2007;16:241-249. [DOI] [PubMed] [Google Scholar]
- 15. Ronchetti E. Robust model selection in regression. Stat Probab Lett. 1985;3:21-23. [Google Scholar]
- 16. Wang C, Kane R, Levenson M, et al. Association between changes in CMS reimbursement policy and drug labels for erythrocyte-stimulating agents with outcomes for older patients undergoing hemodialysis covered by fee-for-service Medicare. JAMA Intern Med. 2016;176:1818-1825. [DOI] [PubMed] [Google Scholar]
- 17. Whitman CB, Shreay S, Gitlin M, van Oijen MGH, Spiegel BM. Clinical factors and the decision to transfuse chronic dialysis patients. Clin J Am Soc Nephrol. 2013;8:1942-1951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Keown PA, Churchill DN, Poulin-Costello Lei L, et al. Dialysis patients treated with Epoetin alfa show improved anemia symptoms: a new analysis of the Canadian Erythropoietin Study Group trial. Hemodial Int. 2010;14:168-173. [DOI] [PubMed] [Google Scholar]
- 19. Muirhead N, Keown PA, Churchill DN, et al. Dialysis patients treated with epoetin alfa show improved exercise tolerance and physical function: a new analysis of the Canadian Erythropoietin Study Group trial. Hemodial Int. 2011;15:87-94. [DOI] [PubMed] [Google Scholar]
- 20. Mendelssohn DC, Curtis B, Yeates K, et al. Suboptimal initiation of dialysis with and without early referral to a nephrologist. Nephrol Dial Transplant. 2011;26:2959-2965. [DOI] [PubMed] [Google Scholar]
- 21. Eikelboom JW, Cook RJ, Liu Y, Heddle NM. Duration of red cell storage before transfusion and in-hospital mortality. Am Heart J. 2010;159:737-743. [DOI] [PubMed] [Google Scholar]
- 22. Heddle NM, Cook RJ, Arnold DM, et al. Effect of short-term vs. long-term blood storage on mortality after transfusion. N Engl J Med. 2016;375:1937-1945. [DOI] [PubMed] [Google Scholar]
- 23. Ueda S, Yamagishi S-I, Kaida Y, Okuda S. Asymmetric dimethylarginine may be a missing link between cardiovascular disease and chronic kidney disease. Nephrology. 2007;12:582-590. [DOI] [PubMed] [Google Scholar]