Abstract
Prostate-specific membrane antigen (PSMA) is a promising target for imaging diagnostics and targeted radionuclide therapy (theranostics) of prostate cancer and its metastases. There is increasing evidence of encouraging response rates and a low toxicity profile of radioligand therapy (RLT) of metastatic castration-resistant prostate cancer using 177Lu-labeled PSMA ligands. In this article, we review the current status of diagnostics and therapy using radiolabeled PSMA ligands. We also suggest protocols for patient selection criteria and conduct of PSMA-based RLT. Challenges and opportunities of PSMA theranostics are discussed.
Keywords: 177Lu-PSMA, prostate cancer, radioligand therapy, mCRPC, PSMA
Introduction
Most deaths related to prostate cancer (PC) are due to advanced disease, which results from any combination of lymphatic, blood, or contiguous local spread. Targeted radionuclide therapy is an attractive and quickly developing therapy option for a variety of cancers, such as lymphoma, melanoma, and neuroendocrine tumor.1-4 Prostate-specific membrane antigen (PSMA), also known as folate hydrolase I or glutamate carboxypeptidase II, is a type II, 750-amino acid transmembrane protein (100-120 kDa), which is anchored in the cell membrane of prostate epithelial cells. The PSMA is highly expressed on prostate epithelial cells and strongly upregulated in PC. The PSMA expression seems correlated to androgen independence, the presence of metastases, and PC progression.5 Despite its name, PSMA is not specific to the prostate gland and is expressed in other normal (eg, salivary glands, duodenal mucosa, subset of proximal renal tubular cells, and subpopulation of neuroendocrine cells in the colonic crypts) and neoplastic (eg, subtypes of transitional cell carcinoma, renal cell carcinoma, colon carcinoma, and peritumoral and endotumoral endothelial cell of neovasculature) tissues.6-8 There is no known natural ligand for PSMA, and the reasons for its upregulation in PC remains unknown. The PSMA ligands undergo constitutive cell internalization by PSMA, which, therefore, represents an appealing molecular target for theranostics in metastatic PC.9
PSMA-Based Imaging
Anti-PSMA Antibodies
The first commercialized anti-PSMA antibody was 111In-capromab-pendetide (ProstaScint, AYTU Bioscience, Englewood, USA),10 which was approved by the US Food and Drug Administration in 1996. However, ProstaScint was only able to bind to the intracellular epitope of PSMA and was therefore not capable of visualizing viable PC cells leading to poor clinical performance.11
In contrast to 111In-capromab-pendetide, J591 binds to the extracellular domain of PSMA. Labeled with 111In, J591 can be used for scintigraphy12 and when labeled with 89Zr for positron emission tomography (PET).13,14 Recently, 89Zr-Df-IAB2M was introduced as an anti-PSMA minibody.15 Despite good tumor detection rates and contrast in scintigraphy,16 J591 as well as other radiolabeled antibodies present with limitations such as low tumor penetrability, long delay between injection and imaging, nonspecific accumulation associated with inflammation, and high radiation exposure due to the necessity of radionuclides with long half-lives due to the long plasma half-life of antibodies.
Small-Molecule PSMA Inhibitors
Small-molecule inhibitors have been developed over the past 2 decades.17-27 The basic chemical structure of the majority of small-molecule PSMA ligands consists of glutamate–urea–lysine dimers. This structure derives from hydroxyphosphinyl derivatives described by Jackson et al in 1996.17 Another significant step forward was the development of urea-based inhibitors by Kozikowski et al21,23 as well as the inhibition of PSMA by phosphonamidothionate derivatives of glutamic acid.28 One attribute of small-molecule inhibitors that has a significant impact on imaging and therapy is the feature that these ligands are internalized into the cells after binding to PSMA.
Small-Molecule PSMA Inhibitors for Scintigraphy
123I- (MIP)-1072 and 123I-MIP-1095 were the first small-molecule PSMA inhibitors that were introduced into the clinic in 2008 by Molecular Insight Pharmaceuticals, Inc (MIP, now a subsidiary of Progenics Pharmaceuticals Inc, Cambridge, Massachusetts, USA).24,29 These ligands demonstrated for the first time the enormous potential of PSMA ligands for imaging and therapy of PC.25,30 The subsequent replacement of 123I by 131I led to the first human therapy with PSMA ligands.31,32 However, the widespread availability and use of 99mTc made the development of 99mTc-labeled PSMA ligands a desirable pursuit. MIP-1404 and MIP-1405 were the first 99mTc-labeled PSMA ligands, which were introduced into the clinic in 2010.26,33 Although both ligands presented with promising results,25 99mTc-MIP-1404 was chosen to enter further clinical studies due to its lower urinary excretion.34,35 Currently, 99mTc-MIP-1404 is the subject of a phase 3 clinical trial designed to evaluate its sensitivity and specificity to detect PC compared to histopathology (ClinicalTrials.gov Identifier: NCT02615067).
PSMA Ligands for PET Imaging
The development of PSMA ligands for PET imaging dates back to as early as 2002. The first tracer published was 11C-MCG which was used in animal studies.22 The clinical breakthrough of PET imaging with PSMA ligands was achieved with the invention of 68Ga-PSMA-11 (also called HBED-CC, HBED, PSMA-HBED, or Prostamedix), which demonstrated excellent characteristics.36-38 Since its clinical introduction in May 2011, PET imaging with 68Ga-PSMA-11 has spread rapidly worldwide and is regarded as a significant step forward in the diagnosis of recurrent PC.38-44 The first publications indicated that this novel method is significantly superior compared to alternative methods used for the detection of recurrent PC.36,38 Later publications confirmed the high sensitivity and specificity of 68Ga-PSMA-11 PET/computed tomography (CT).38-44 68Ga-PSMA-11 PET/CT is usually conducted at 1 hour post injection (p.i.) according to its first described clinical setup.37 However, experience showed that most PC lesions present with increased uptake and contrast at later imaging timings (3 hours p.i.), despite the relatively short half-life of 68 Ga.37 Later publications confirmed these findings.45-47 Alternative PSMA ligands such as 18F-DCFPyL, 68Ga-PSMA-617, 68Ga-PSMA I&T, or 124I-MIP-1095 demonstrated similar characteristics.31,48-50
Several studies with large patient cohorts have analyzed the sensitivity of 68Ga-PSMA-11 PET/CT as well as possibly interacting factors such as prostate specific antigen (PSA), injected amount of tracer, androgen deprivation therapy (ADT), or Gleason Score (GS).39-41 As expected, the probability to detect tumor lesions (sensitivity) increased with the PSA value. At PSA values less than 0.5 ng/mL, the reported detection probability was about 50%. This rate is assumed to be the highest among all methods for imaging recurrent PC. However, as shown in Figure 1, a continuous increase in PSA level does not always correlate with an increase in tumor detection. Even in the subcohort of patients with PSA levels above 10 ng/mL, not all patients presented with a pathologic 68Ga-PSMA-11 PET/CT. Absent or low PSMA expression as well as small tumor size could be possible reasons for this observation. No association was found between the probability of a pathologic scan and either of age, injected tracer activity, faster PSA doubling times, or faster PSA velocity. No significant association but only a tendency was found between higher GS and a higher probability of a pathologic scan.39,40 In contrast to GS, an ongoing ADT at the time of the scans was significantly associated with a pathologic 68Ga-PSMA-11 PET/CT39,40 although such a therapy can cause a reduction in tumor size as well as PSA values. Both of these effects usually entail a negative impact on tumor detection. It is known from preclinical literature that ADT can increase the PSMA expression in PC cells.51,52 On the other hand, another reason for the fact that patients with ADT more often showed pathological findings in the 68Ga-PSMA-11-PET/CT could be that ADT is often started in cases of advanced tumor stages. According to the current knowledge, there is no need to pause an ADT prior to a PSMA ligand scan.
Figure 1.
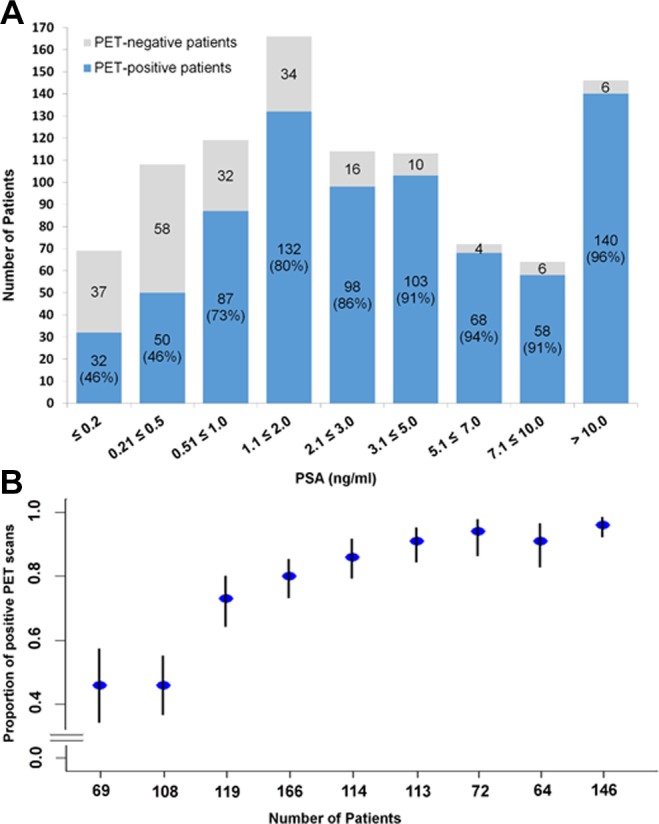
Probability of a pathologic 68Ga-PSMA-11 PET/CT as histogram (A) and plot of the rates of pathologic scans with confidence intervals (B) depending on PSA (ng/mL) in a cohort of 971 patients. Reprinted from Afshar-Oromieh et al40 with the permission of the authors. Ct indicates computed tomography; PET, positron emission tomography; PSMA, prostate-specific membrane antigen.
The PSMA is not specific for PC. Since the late 1990s, it is also well-known that the neovasculature of many solid tumors may express PSMA as well.53 This is reflected by multiple case reports describing PET-positive nonprostatic tissues.8 However, in case of recurrent PC, 68Ga-PSMA-11 PET/CT has showed an excellent specificity.39,54,55
Recently, 18F-labeled PSMA-ligands were clinically introduced with promising results such as DCFBC, DCFPyL, or PSMA-1007.48,56-58 They all show promising first results. In theory, 18F-labeled tracers present with advantages such as higher physical resolution and high amounts of 18F deriving from cyclotrons. However, future studies will show whether these theoretical advantages can outweigh the faster pharmacokinetics and excellent tumor contrast of 68Ga-PSMA-11.
PSMA-Targeted Therapy
After rather unsuccessful therapy with 90Y-CYT-356 monoclonal antibody (mAb) that binds to the intracellular domain of PSMA,59 the phase 1 and 2 clinical trials utilizing radiolabeled (177Lu or 90Y) PSMA mAb J591, which targets the extracellular domain and then gets internalized through endocytosis, have shown promising results,16,60-62 however, with higher ratio of irreversible hematological toxicity. Tagawa et al60 treated 47 patients with 177Lu-PSMA mAb J591. They reported grade 4 thrombocytopenia in 46.8% of patients (29.8% received platelet transfusions), and a total of 25.5% patients experienced grade 4 neutropenia. Monoclonal antibodies are large molecules and therefore show poor permeability in solid tumors and slow clearance from the circulation, which is probably the cause of grade 4 hematotoxicity. Instead of mAb, Maresca et al described the design and synthesis of a series of small-molecule inhibitors of PSMA.24 First clinical experiences with PSMA-based radionuclide treatment using 131I-labeled PSMA ligands showed promising results with mild hematotoxicity and a PSA decrease >50% in 60% of treated patients with PC.31,32 The 131I has a long half-life of 8.02 days and with maximum β-particle range of 2.4 mm in soft tissue. Due to the γ-emitting properties and long half-life, 131I is less attractive from a radiation safety point of view. The 177Lu has a half-life of 6.7 days and low-energy β-particles emission with a mean range of 0.7 mm and maximum range of 2.1 mm in soft tissue.63
Current Status of PSMA-Targeted Radioligand Therapy
Table 1 shows a list of current therapy molecules already administered in humans. In the first cohort of 10 patients, mild side effects and considerable rates of PSA response were reported after 1 cycle of radioligand therapy (RLT) with 177Lu PSMA-617.64 A decline in PSA was observed in 70% of patients, with more than 50% decline in a few patients. Another recently published study showed the safety and favorable therapy response in a larger cohort of 24 patients who received up to 2 cycles of 177Lu PSMA-617. Twenty-two patients received 2 cycles of RLT. Eight weeks after the second cycle of 177Lu PSMA-617 therapy, 15 (68.2%) of 22 patients experienced a PSA decline compared to the baseline PSA value, of who 15 experienced a decline of more than 30% and 13 a decline of more than 50%.65 Relevant hematotoxicity (grade 3) occurred during the observation period (within 2 months after the last cycle) in 2 patients only. Apart from minor grade 1 or 2 hematotoxicity, the majority of patients did not have any hematotoxicity during the observation period. No relevant nephrotoxicity or hepatotoxicity was observed. The overall survival benefit of RLT in comparison to a historical control group could be demonstrated in another investigation.66 The estimated median survival was 29.4 weeks in the group treated with 177Lu PSMA-617, which was noticeably longer than survival in a historical control group with 19.7 weeks (hazard ratio: 0.44; 95% confidence interval: 0.20-0.95; P = .031).
Table 1.
PSMA Ligands for Therapy.
| Ligand | Radionuclide | Statusa |
|---|---|---|
| MIP-1095 | Iodine-131 | Compassionate use |
| PSMA-I&T | Lutetium-177 | Compassionate use |
| J591 | Lutetium-177 | Phase I |
| PSMA-617 | Lutetium-177 | Phase I/phase II/phase III |
Abbreviations: MIP, Molecular Insight Pharmaceuticals; PSMA, prostate-specific membrane antigen.
a Status of use according clinicaltrials.gov.
Baum et al presented similar results using 177Lu PSMA-I&T67 treating 56 patients with metastasized PC at an early stage of castration resistance. Any PSA decline and a decline of ≥50% were reported in 80% and a median overall survival of 13.7 months. In this cohort, prior chemotherapy was performed in 25% of patients. Prior therapy with abiraterone was performed in 21% and prior enzalutamide was given in 11% of patients, whereas in the prior study mentioned earlier, pretreatments with chemotherapy, abiraterone, and enzalutamide were administered in 78%, 75%, and 86% of patients, respectively.66
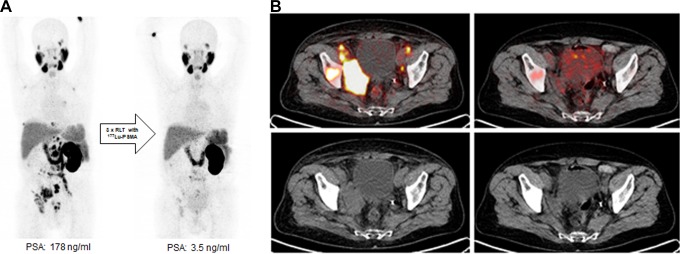
Results of the abovementioned studies were confirmed by the findings of the German multicenter study including 145 patients with metastatic castration-resistant PC. Biochemical response was defined by the PC work group as a PSA decline ≥50%.68 A PSA decline ≥50% and any PSA decline were reported in 45% and 60% of the patients.69 The presence of visceral metastases and an alkaline phosphatase ≥220 U/L were negative predictors of therapy response. Adverse events were analyzed according to common toxicity criteria. Grade 3 to 4 toxicities such as anemia, leukopenia, and thrombocytopenia were reported in 10%, 3%, and 4% of patients treated with 177Lu PSMA-617. Mild or transient xerostomia were reported in 8% of the patients. Repeated cycles of 177Lu PSMA-617 RLT can be performed after radionuclide therapy using 223Ra dichloride, without a higher probability of hematotoxicity.69 These results about the safety and toxicity were confirmed by another study including 49 patients who were treated with 3 cycles of 177Lu-PSMA-617. The patients were separated into 2 groups subjected to a history of prior therapy with 223Ra. Group 1 included 20 patients who had received therapy with 223Ra prior to 177Lu-PSMA-617 therapy. Group 2, which was the control group, comprised 29 patients without any history of a bone-targeted radionuclide therapy. No grade 4 hematotoxicity was observed in the entire study population. There were no significant differences between the 2 groups regarding leukopenia and thrombocytopenia.70 Figure 2A and B shows a case of a 66-year-old patient with metastasized castration-resistant PC with an excellent response to 177Lu PSMA-617 RLT with history of prior 223Ra dichloride therapy.
Figure 2.
A, 68Ga-PSMA11-PET images of a 66-year-old patient with castration-resistant prostate cancer pretreated with docetaxel, abiraterone, and 6 cycles of 223Radium. Maximum intensity projection (MIP) on the left side shows multiple bone and lymph node lesions. Patient was treated with 3 cycles of 177Lu-PSMA-617 radioligand therapy with a cumulative activity of 13.5 GBq (reduced activity because of single kidney). The MIP images on the right side show significant reduction in prostate-specific membrane antigen (PSMA)-positive lesions in correlation with a PSA decline of 99%. B, Fused images of 68Ga-PSMA11-positron emission tomography/computed tomography (PET/CT) images in the upper row show a significant reduction in PSMA-positive lesions. Low-dose CT images (lower row) show a significant volume reduction in soft tissue lesions (especially in the right pelvis).
Although there is a specific renal binding of PSMA-ligands, no grade 3 to 4 toxicities were reported after the RLT. Only low-grade renal toxicity has been reported so far.71 Table 2 presents reported toxicities of 177Lu-PSMA RLT.
Table 2.
Safety of 177Lu-PSMA radioligand therapy in the literature.
| Hematotoxicity CTCAE grade 3/4 | ||||||
|---|---|---|---|---|---|---|
| Reference | n | Hb (%) | WBC (%) | Plt (%) | Xerostomia | Nonhematologic AE |
| Ahmadzadehfar et al.64 | 10 | 10 | 0 | 0 | 0 | mild nausea, fatigue |
| Rahbar et al.66 | 28 | 11 | 0 | 0 | 14 | mild nausea |
| Ahmadzadehfar et al.65 | 24 | 9 | 0 | 0 | 8.7 | mild nausea |
| Baum et al.67 | 56 | 0 | 0 | 0 | 3.5 | na |
| Kratochwil et al.72 | 30 | 3.3 | 0 | 3.3 | 6.7 | mild nausea, fatigue |
| Rahbar et al.73 | 82 | 2.8 | 0 | 0 | 8.5 | mild nausea |
| Heck et al.74 | 22 | 0 | 0 | 0 | 37 | fatigue, appetite loss |
| Rahbar et al.69 | 145 | 10 | 3 | 4 | 8 | mild nausea |
| Bräuer et al.75 | 59 | 18 | 3 | 3 | 25 | nausea, fatigue |
Abbreviations: CTCAE: common toxicity criteria of adverse events, Hb: haemoglobin, WBC: white blood cells, Plt: platlets.
Yordanova et al analyzed changes in creatinine, cyctatin C, glomerular filtration rate, and tubular function assessed by 99mTc-MAG3 renal scintigraphy in 55 patients receiving at least 3 (range 3-6) consecutive cycles of 177Lu PSMA-617 every 8 weeks. Significant negative predictor for renal function were age (>65 years), arterial hypertension, and prior renal failure.
Two recently published studies75,76 analyzed overall survival in patients receiving up to 8 cycles of 177Lu PSMA-617 RLT. Any PSA decline after the first cycle was a significant prognosticator of survival in both studies (68 vs 33 weeks reported by the first mentioned study and 59 vs 28 weeks reported by the second-listed study). These results could be confirmed by a larger 2-center study in patients pretreated with at least 1 line of chemotherapy and abiraterone and/or enzalutamide.77 The PSA decline of at least 20.87% was the optimal parameter in predicting improved overall survival in the multivariate analysis. The same study group also showed that in patients who do not respond to the first cycle, further therapy cycles should be performed, because nearly one-third of the patients showed a delayed response after additional therapy cycles.78
According to currently available results, 177Lu PSMA-617 is a new and promising therapy option for patients with metastatic PC, but it has not yet reached clinical approval. It should therefore mostly be offered to patients as a salvage therapy; however, a more upfront role of RLT with PSMA ligands leading to regulatory approval would seem prudent.
In the following, we summarize the current therapy protocol most widely used.
Indications for RLT With 177Lu-PSMA-617
metastatic castrate-resistant PC with PSMA-positive metastatic disease upon PSMA-PET or single-photon emission computed tomography (SPECT) imaging;
- after initial hormone therapy (luteinizing hormone-releasing hormone agonists/antagonists):
- progressive disease, despite newly developed hormone therapies (abiraterone / enzalutamide)
- progressive disease, despite (first line or second line) chemotherapy (docetaxel and cabazitaxel) or patient not suitable for chemotherapy
not suitable for 223Ra-dichloride due to visceral metastases or diffuse bone marrow metastases; and
decision for salvage PSMA therapy at the institutional interdisciplinary tumor board.
Preparation Prior to RLT
PSMA-PET or SPECT imaging to verify PSMA positive lesions.
Renal scintigraphy to evaluate renal function and rule out obstructive dysfunction, which should be treated prior to the RLT
Prerequisites for RLT
White blood cells ≥ 2 tsd/µL.
Hb ≥ 8 g/dL, in the case of symptomatic anemia a red blood cell transfusion should precede the therapy. The RLT with 177Lu-PSMA-617 may have a positive effect on bone marrow depression because of tumor regression in bone marrow.79
Platelets ≥ 75 × 109/L.
Creatinine < 2 mg/dL.
Eastern Cooperative Oncology Group ≤2.
Proposed Protocol
Cooling the salivary glands from 30 minutes before and up to 4 hours after the 177Lu-PSMA-617 injection for reducing the risk of salivary glands radiation injuries.
Using urinary catheter in incontinent patients in the first 48 hours for avoiding any contamination.
A dose of 6 to 7.4 GBq 177Lu-PSMA-617.
Injection intravenously as a slow bolus (over about 30 seconds) followed by 1000 mL Ringer or NaCl.
Four to 6 cycles of the RLT every 6 to 8 weeks.
Follow-Up After RLT
The PSMA-PET or SPECT imaging before first cycle to confirm PSMA-positive metastatic disease and after the last cycle (interim imaging should also be performed if needed).
Laboratory test after RLT should be performed every 2 weeks: blood cell counts, creatinine, and liver panel.
Decision to pursue the RLT depending on the imaging and clinical situation.
Future Perspectives and Remaining Issues
The PSMA-based imaging and RLT is a theranostic approach, which is currently applied in metastatic castration–resistant PC. Radioligand therapy with 177Lu-PSMA-617 seems to be a promising treatment for metastatic patients with PC. Despite very encouraging early results, several issues will need to be resolved to clarify and establish the exact role of PSMA-based theranostics in PC. With regard to imaging, currently a number of different agents are available, and the most suitable agent from the point of view of optimal imaging characteristics, availability, access, and cost will need to be identified. Similar notion holds for RLT with β- or α-particles and the specific clinical situations, which may be best amenable for either type of particles or both. Using this therapy, as a combination with newer antiandrogenic drugs such as abiraterone and enzalutamide, at earlier stages of the therapy must be evaluated in future studies. Larger prospective multicenter studies are needed to evaluate the potential of RLT among and with other available therapy options. Moreover, the long-term results and toxicities must be analyzed after longer follow-up periods. We have to point out that while preliminary protocols have been devised, both the imaging and the therapy protocols will evolve in time as experience with PSMA theranostics grows. An expanded understanding of the effects of various treatments on PSMA expression will be needed to facilitate therapy response assessment. It has been recognized that ADT increases PSMA expression which is not only relevant in recognition of posttreatment PSMA PET imaging changes but also suggests the possibility of synergistic therapeutic effect of androgen deprivation and PSMA-based RLT. Determination of the impact of PSMA theranostics on various outcome measures and comparative cost–benefit analysis will need investigations.
Footnotes
Declaration of Conflicting Interests: The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: The University of Muenster received consulting fees from ABX Advanced Biochemical Compounds, Radeberg, Germany for K.R.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: Kambiz Rahbar, MD  http://orcid.org/0000-0002-4591-4055
http://orcid.org/0000-0002-4591-4055
References
- 1. Kraeber-Bodere F, Bodet-Milin C, Rousseau C, et al. Radioimmunoconjugates for the treatment of cancer. Semin Oncol. 2014;41(5):613–622. [DOI] [PubMed] [Google Scholar]
- 2. Mier W, Kratochwil C, Hassel JC, et al. Radiopharmaceutical therapy of patients with metastasized melanoma with the melanin-binding benzamide 131I-BA52. J Nucl Med. 2014;55(1):9–14. [DOI] [PubMed] [Google Scholar]
- 3. van der Zwan WA, Bodei L, Mueller-Brand J, de Herder WW, Kvols LK, Kwekkeboom DJ. GEPNETs update: radionuclide therapy in neuroendocrine tumors. Eur J Endocrinol. 2015;172(1):R1–R8. [DOI] [PubMed] [Google Scholar]
- 4. Bodei L, Kidd M, Paganelli G, et al. Long-term tolerability of PRRT in 807 patients with neuroendocrine tumours: the value and limitations of clinical factors. Eur J Nucl Med Mol Imaging. 2015;42(1):5–19. [DOI] [PubMed] [Google Scholar]
- 5. Santoni M, Scarpelli M, Mazzucchelli R, et al. Targeting prostate-specific membrane antigen for personalized therapies in prostate cancer: morphologic and molecular backgrounds and future promises. J Biol Regul Homeost Agents. 2014;28(4):555–563. [PubMed] [Google Scholar]
- 6. Silver DA, Pellicer I, Fair WR, Heston WD, Cordon-Cardo C. Prostate-specific membrane antigen expression in normal and malignant human tissues. Clin Cancer Res. 1997;3(1):81–85. [PubMed] [Google Scholar]
- 7. Spatz S, Tolkach Y, Jung K, et al. Comprehensive evaluation of prostate-specific membrane antigen expression in the vasculature of renal tumors: implications for imaging studies and prognostic role. J Urol. 2018;199(2):370–377. [DOI] [PubMed] [Google Scholar]
- 8. Sheikhbahaei S, Afshar-Oromieh A, Eiber M, et al. Pearls and pitfalls in clinical interpretation of prostate-specific membrane antigen (PSMA)-targeted PET imaging. Eur J Nucl Med Mol Imaging. 2017;44(12):2117–2136. [DOI] [PubMed] [Google Scholar]
- 9. Haberkorn U, Eder M, Kopka K, Babich JW, Eisenhut M. New strategies in prostate cancer: prostate-specific membrane antigen (PSMA) ligands for diagnosis and therapy. Clin Cancer Res. 2016;22(1):9–15. [DOI] [PubMed] [Google Scholar]
- 10. Rosenthal SA, Haseman MK, Polascik TJ. Utility of capromab pendetide (ProstaScint) imaging in the management of prostate cancer. Tech Urol. 2001;7(1):27–37. [PubMed] [Google Scholar]
- 11. Wilkinson S, Chodak G. The role of 111indium-capromab pendetide imaging for assessing biochemical failure after radical prostatectomy. J Urol. 2004;172(1):133–136. [DOI] [PubMed] [Google Scholar]
- 12. Vallabhajosula S, Kuji I, Hamacher KA, et al. Pharmacokinetics and biodistribution of 111In- and 177Lu-labeled J591 antibody specific for prostate-specific membrane antigen: prediction of 90Y-J591 radiation dosimetry based on 111In or 177Lu? J Nucl Med. 2005;46(4):634–641. [PubMed] [Google Scholar]
- 13. Holland JP, Divilov V, Bander NH, Smith-Jones PM, Larson SM, Lewis JS. 89Zr-DFO-J591 for immunoPET of prostate-specific membrane antigen expression in vivo. J Nucl Med. 2010;51(8):1293–1300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Pandit-Taskar N, O’Donoghue JA, Durack JC, et al. A Phase I/II Study for analytic validation of 89Zr-J591 ImmunoPET as a molecular imaging agent for metastatic prostate cancer. Clin Cancer Res. 2015;21(23):5277–5285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Pandit-Taskar N, O’Donoghue JA, Ruan S, et al. First-in-human imaging with 89Zr-Df-IAB2 M Anti-PSMA minibody in patients with metastatic prostate cancer: pharmacokinetics, biodistribution, dosimetry, and lesion uptake. J Nucl Med. 2016;57(12):1858–1864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Bander NH, Milowsky MI, Nanus DM, Kostakoglu L, Vallabhajosula S, Goldsmith SJ. Phase I trial of 177lutetium-labeled J591, a monoclonal antibody to prostate-specific membrane antigen, in patients with androgen-independent prostate cancer. J Clin Oncol. 2005;23(21):4591–4601. [DOI] [PubMed] [Google Scholar]
- 17. Jackson PF, Cole DC, Slusher BS, et al. Design, synthesis, and biological activity of a potent inhibitor of the neuropeptidase N-acetylated alpha-linked acidic dipeptidase. J Med Chem. 1996;39(2):619–622. [DOI] [PubMed] [Google Scholar]
- 18. Jackson PF, Slusher BS. Design of NAALADase inhibitors: a novel neuroprotective strategy. Curr Med Chem. 2001;8(8):949–957. [DOI] [PubMed] [Google Scholar]
- 19. Luthi-Carter R, Barczak AK, Speno H, Coyle JT. Molecular characterization of human brain N-acetylated alpha-linked acidic dipeptidase (NAALADase). J Pharmacol Exp Ther. 1998;286(2):1020–1025. [PubMed] [Google Scholar]
- 20. Tiffany CW, Lapidus RG, Merion A, Calvin DC, Slusher BS. Characterization of the enzymatic activity of PSM: comparison with brain NAALADase. Prostate. 1999;39(1):28–35. [DOI] [PubMed] [Google Scholar]
- 21. Kozikowski AP, Nan F, Conti P, et al. Design of remarkably simple, yet potent urea-based inhibitors of glutamate carboxypeptidase II (NAALADase). J Med Chem. 2001;44(3):298–301. [DOI] [PubMed] [Google Scholar]
- 22. Pomper MG, Musachio JL, Zhang J, et al. 11C-MCG: synthesis, uptake selectivity, and primate PET of a probe for glutamate carboxypeptidase II (NAALADase). Mol Imaging. 2002;1(2):96–101. [DOI] [PubMed] [Google Scholar]
- 23. Kozikowski AP, Zhang J, Nan F, et al. Synthesis of urea-based inhibitors as active site probes of glutamate carboxypeptidase II: efficacy as analgesic agents. J Med Chem. 2004;47(7):1729–1738. [DOI] [PubMed] [Google Scholar]
- 24. Maresca KP, Hillier SM, Femia FJ, et al. A series of halogenated heterodimeric inhibitors of prostate specific membrane antigen (PSMA) as radiolabeled probes for targeting prostate cancer. J Med Chem. 2009;52(2):347–357. [DOI] [PubMed] [Google Scholar]
- 25. Barrett JA, Coleman RE, Goldsmith SJ, et al. First-in-man evaluation of 2 high-affinity PSMA-avid small molecules for imaging prostate cancer. J Nucl Med. 2013;54(3):380–387. [DOI] [PubMed] [Google Scholar]
- 26. Hillier SM, Maresca KP, Lu G, et al. 99mTc-labeled small-molecule inhibitors of prostate-specific membrane antigen for molecular imaging of prostate cancer. J Nucl Med. 2013;54(8):1369–1376. [DOI] [PubMed] [Google Scholar]
- 27. Eder M, Eisenhut M, Babich J, Haberkorn U. PSMA as a target for radiolabelled small molecules. Eur J Nucl Med Mol Imaging. 2013;40(6):819–823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Rodriguez CE, Lu H, Martinez AR, Hu Y, Brunelle A, Berkman CE. Inhibition of glutamate carboxypeptidase II by phosphonamidothionate derivatives of glutamic acid. J Enzyme Inhib. 2001;16(4):359–365. [PubMed] [Google Scholar]
- 29. Hillier SM, Maresca KP, Femia FJ, et al. Preclinical evaluation of novel glutamate-urea-lysine analogues that target prostate-specific membrane antigen as molecular imaging pharmaceuticals for prostate cancer. Cancer Res. 2009;69(17):6932–6940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Hillier SM, Kern AM, Maresca KP, et al. 123I-MIP-1072, a small-molecule inhibitor of prostate-specific membrane antigen, is effective at monitoring tumor response to taxane therapy. J Nucl Med. 2011;52(7):1087–1093. [DOI] [PubMed] [Google Scholar]
- 31. Zechmann CM, Afshar-Oromieh A, Armor T, et al. Radiation dosimetry and first therapy results with a (124)I/ (131)I-labeled small molecule (MIP-1095) targeting PSMA for prostate cancer therapy. Eur J Nucl Med Mol Imaging. 2014;41(7):1280–1292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Afshar-Oromieh A, Haberkorn U, Zechmann C, et al. Repeated PSMA-targeting radioligand therapy of metastatic prostate cancer with 131I-MIP-1095. Eur J Nucl Med Mol Imaging. 2017;44(6):950–959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Lu G, Maresca KP, Hillier SM, et al. Synthesis and SAR of (9)(9)mTc/Re-labeled small molecule prostate specific membrane antigen inhibitors with novel polar chelates. Bioorg Med Chem Lett. 2013;23(5):1557–1563. [DOI] [PubMed] [Google Scholar]
- 34. Vallabhajosula S, Osborne J, Nikolopoulou A, et al. PSMA targeted SPECT imaging biomarker to detect local and metastatic prostate cancer (PCa): Phase I studies with 99mTc-MIP-1404. J Nucl Med. 2013;54(suppl 2):281. [Google Scholar]
- 35. Goffin K, Joniau S, Tenke P, et al. A phase 2 study of 99mTc-trofolastat (MIP-1404) to identify prostate cancer (PCa) in high-risk patients (pts) undergoing radical prostatectomy (RP) and extended pelvic lymph node (ePLN) dissection: an interim analysis. J Nucl Med. 2014;55(suppl 1):15.24263087 [Google Scholar]
- 36. Afshar-Oromieh A, Haberkorn U, Eder M, Eisenhut M, Zechmann CM. [68 Ga]Gallium-labelled PSMA ligand as superior PET tracer for the diagnosis of prostate cancer: comparison with 18F-FECH. Eur J Nucl Med Mol Imaging. 2012;39(6):1085–1086. [DOI] [PubMed] [Google Scholar]
- 37. Afshar-Oromieh A, Malcher A, Eder M, et al. PET imaging with a [68 Ga]gallium-labelled PSMA ligand for the diagnosis of prostate cancer: biodistribution in humans and first evaluation of tumour lesions. Eur J Nucl Med Mol Imaging. 2013;40(4):486–495. [DOI] [PubMed] [Google Scholar]
- 38. Afshar-Oromieh A, Zechmann CM, Malcher A, et al. Comparison of PET imaging with a (68)Ga-labelled PSMA ligand and (18)F-choline-based PET/CT for the diagnosis of recurrent prostate cancer. Eur J Nucl Med Mol Imaging. 2014;41(1):11–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Afshar-Oromieh A, Avtzi E, Giesel FL, et al. The diagnostic value of PET/CT imaging with the (68)Ga-labelled PSMA ligand HBED-CC in the diagnosis of recurrent prostate cancer. Eur J Nucl Med Mol Imaging. 2015;42(2):197–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Afshar-Oromieh A, Holland-Letz T, Giesel FL, et al. Diagnostic performance of 68Ga-PSMA-11 (HBED-CC) PET/CT in patients with recurrent prostate cancer: evaluation in 1007 patients. Eur J Nucl Med Mol Imaging. 2017;44(8):1258–1268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Eiber M, Maurer T, Souvatzoglou M, et al. Evaluation of Hybrid (6)(8)Ga-PSMA Ligand PET/CT in 248 Patients with Biochemical Recurrence After Radical Prostatectomy. J Nucl Med. 2015;56(5):668–674. [DOI] [PubMed] [Google Scholar]
- 42. Schwenck J, Rempp H, Reischl G, et al. Comparison of 68Ga-labelled PSMA-11 and 11C-choline in the detection of prostate cancer metastases by PET/CT. Eur J Nucl Med Mol Imaging. 2017;44(1):92–101. [DOI] [PubMed] [Google Scholar]
- 43. Morigi JJ, Stricker PD, van Leeuwen PJ, et al. Prospective comparison of 18F-fluoromethylcholine versus 68Ga-PSMA PET/CT in prostate cancer patients who have rising PSA after curative treatment and are being considered for targeted therapy. J Nucl Med. 2015;56(8):1185–1190. [DOI] [PubMed] [Google Scholar]
- 44. Pfister D, Porres D, Heidenreich A, et al. Detection of recurrent prostate cancer lesions before salvage lymphadenectomy is more accurate with (68)Ga-PSMA-HBED-CC than with (18)F-Fluoroethylcholine PET/CT. Eur J Nucl Med Mol Imaging. 2016;43(8):1410–1417. [DOI] [PubMed] [Google Scholar]
- 45. Afshar-Oromieh A, Hetzheim H, Kubler W, et al. Radiation dosimetry of (68)Ga-PSMA-11 (HBED-CC) and preliminary evaluation of optimal imaging timing. Eur J Nucl Med Mol Imaging. 2016;43(9):1611–1620. [DOI] [PubMed] [Google Scholar]
- 46. Afshar-Oromieh A, Sattler LP, Mier W, et al. The clinical impact of additional late PET/CT imaging with 68Ga-PSMA-11 (HBED-CC) in the diagnosis of prostate cancer. J Nucl Med. 2017;58(5):750–755. [DOI] [PubMed] [Google Scholar]
- 47. Pfob CH, Ziegler S, Graner FP, et al. Biodistribution and radiation dosimetry of (68)Ga-PSMA HBED CC-a PSMA specific probe for PET imaging of prostate cancer. Eur J Nucl Med Mol Imaging. 2016;43(11):1962–1970. [DOI] [PubMed] [Google Scholar]
- 48. Szabo Z, Mena E, Rowe SP, et al. Initial evaluation of [(18)F]DCFPyL for Prostate-Specific Membrane Antigen (PSMA)-Targeted PET imaging of prostate cancer. Mol Imaging Biol. 2015;17(4):565–574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Afshar-Oromieh A, Hetzheim H, Kratochwil C, et al. The theranostic PSMA Ligand PSMA-617 in the diagnosis of prostate cancer by PET/CT: biodistribution in humans, radiation dosimetry, and first evaluation of tumor lesions. J Nucl Med. 2015;56(11):1697–1705. [DOI] [PubMed] [Google Scholar]
- 50. Herrmann K, Bluemel C, Weineisen M, et al. Biodistribution and radiation dosimetry for a probe targeting prostate-specific membrane antigen for imaging and therapy. J Nucl Med. 2015;56(6):855–861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Evans MJ, Smith-Jones PM, Wongvipat J, et al. Noninvasive measurement of androgen receptor signaling with a positron-emitting radiopharmaceutical that targets prostate-specific membrane antigen. Proc Natl Acad Sci U S A. 2011;108(23):9578–9582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Hope TA, Truillet C, Ehman EC, et al. 68Ga-PSMA-11 PET Imaging of response to androgen receptor inhibition: first human experience. J Nucl Med. 2017;58(1):81–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Chang SS. Overview of prostate-specific membrane antigen. Rev Urol. 2004;6(suppl 10):S13–S18. [PMC free article] [PubMed] [Google Scholar]
- 54. Herlemann A, Wenter V, Kretschmer A, et al. 68Ga-PSMA positron emission tomography/computed tomography provides accurate staging of lymph node regions prior to lymph node dissection in patients with prostate cancer. Eur Urol. 2016;70(4):553–557. [DOI] [PubMed] [Google Scholar]
- 55. Maurer T, Gschwend JE, Rauscher I, et al. Diagnostic efficacy of (68)Gallium-PSMA positron emission tomography compared to conventional imaging for lymph node staging of 130 consecutive patients with intermediate to high risk prostate cancer. J Urol. 2016;195(5):1436–1443. [DOI] [PubMed] [Google Scholar]
- 56. Mease RC, Dusich CL, Foss CA, et al. N-[N-[(S)-1,3-Dicarboxypropyl]carbamoyl]-4-[18F]fluorobenzyl-L-cysteine, [18F]DCFBC: a new imaging probe for prostate cancer. Clin Cancer Res. 2008;14(10):3036–3043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Wondergem M, van der Zant F, Knol R, Lazarenko S, Pruim J, de Jong IJ. 18F-DCFPyL PET/CT in the detection of prostate cancer at 60 and 120 minutes; detection rate, image quality, activity kinetics and biodistribution. J Nucl Med. 2017;58(11):1797–1804. [DOI] [PubMed] [Google Scholar]
- 58. Cardinale J, Schafer M, Benesova M, et al. Preclinical evaluation of 18F-PSMA-1007, a new prostate-specific membrane antigen ligand for prostate cancer imaging. J Nucl Med. 2017;58(3):425–431. [DOI] [PubMed] [Google Scholar]
- 59. Deb N, Goris M, Trisler K, et al. Treatment of hormone-refractory prostate cancer with 90Y-CYT-356 monoclonal antibody. Clin Cancer Res. 1996;2(8):1289–1297. [PubMed] [Google Scholar]
- 60. Tagawa ST, Milowsky MI, Morris M, et al. Phase II study of Lutetium-177-labeled anti-prostate-specific membrane antigen monoclonal antibody J591 for metastatic castration-resistant prostate cancer. Clin Cancer Res. 2013;19(18):5182–5191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Vallabhajosula S, Goldsmith SJ, Hamacher KA, et al. Prediction of myelotoxicity based on bone marrow radiation-absorbed dose: radioimmunotherapy studies using 90Y- and 177Lu-labeled J591 antibodies specific for prostate-specific membrane antigen. J Nucl Med. 2005;46(5):850–858. [PubMed] [Google Scholar]
- 62. Vallabhajosula S, Goldsmith SJ, Kostakoglu L, Milowsky MI, Nanus DM, Bander NH. Radioimmunotherapy of prostate cancer using 90Y- and 177Lu-labeled J591 monoclonal antibodies: effect of multiple treatments on myelotoxicity. Clin Cancer Res. 2005;11(19 pt 2):7195s–7200s. [DOI] [PubMed] [Google Scholar]
- 63. Kam BL, Teunissen JJ, Krenning EP, et al. Lutetium-labelled peptides for therapy of neuroendocrine tumours. Eur J Nucl Med Mol Imaging. 2012;39(suppl 1):S103–S112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Ahmadzadehfar H, Rahbar K, Kurpig S, et al. Early side effects and first results of radioligand therapy with (177)Lu-DKFZ-617 PSMA of castrate-resistant metastatic prostate cancer: a two-centre study. EJNMMI Res. 2015;5(1):114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Ahmadzadehfar H, Eppard E, Kurpig S, et al. Therapeutic response and side effects of repeated radioligand therapy with 177Lu-PSMA-DKFZ-617 of castrate-resistant metastatic prostate cancer. Oncotarget. 2016;7(11):12477–12488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Rahbar K, Bode A, Weckesser M, et al. Radioligand therapy with 177Lu-PSMA-617 as a novel therapeutic option in patients with metastatic castration resistant prostate cancer. Clin Nucl Med. 2016;41(7):522–528. [DOI] [PubMed] [Google Scholar]
- 67. Baum RP, Kulkarni HR, Schuchardt C, et al. 177Lu-Labeled prostate-specific membrane antigen radioligand therapy of metastatic castration-resistant prostate cancer: safety and efficacy. J Nucl Med. 2016;57(7):1006–1013. [DOI] [PubMed] [Google Scholar]
- 68. Scher HI, Morris MJ, Stadler WM, et al. Trial design and objectives for castration-resistant prostate cancer: updated recommendations from the prostate cancer clinical trials working group 3. J Clin Oncol. 2016;34(12):1402–1418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Rahbar K, Ahmadzadehfar H, Kratochwil C, et al. German multicenter study investigating 177Lu-PSMA-617 radioligand therapy in advanced prostate cancer patients. J Nucl Med. 2017;58(1):85–90. [DOI] [PubMed] [Google Scholar]
- 70. Ahmadzadehfar H, Zimbelmann S, Yordanova A, et al. Radioligand therapy of metastatic prostate cancer using 177Lu-PSMA-617 after radiation exposure to 223Ra-dichloride. Oncotarget. 2017;8(33):55567–55574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Yordanova A, Becker A, Eppard E, et al. The impact of repeated cycles of radioligand therapy using [177Lu]Lu-PSMA-617 on renal function in patients with hormone refractory metastatic prostate cancer. Eur J Nucl Med Mol Imaging. 2017;44(9):1473–1479. [DOI] [PubMed] [Google Scholar]
- 72. Kratochwil C, Giesel FL, Stefanova M, et al. PSMA-targeted radionuclide therapy of metastatic castration-resistant prostate cancer with 177Lu-Labeled PSMA-617. J Nucl Med. 2016;57(8):1170–6. [DOI] [PubMed] [Google Scholar]
- 73. Rahbar K, Schmidt M, Heinzel A, et al. Response and tolerability of a single dose of 177Lu-PSMA-617 in patients with metastatic castration-resistant prostate cancer: A multicenter retrospective analysis. J Nucl Med. 2016;57(9):1334–8. [DOI] [PubMed] [Google Scholar]
- 74. Heck MM, Retz M, Tauber R, et al. [PSMA-targeted radioligand therapy in prostate cancer]. Urologe A. 2017;56(1):32–9. [DOI] [PubMed] [Google Scholar]
- 75. Brauer A, Grubert LS, Roll W, et al. 177Lu-PSMA-617 radioligand therapy and outcome in patients with metastasized castration-resistant prostate cancer. Eur J Nucl Med Mol Imaging. 2017;44(10):1663–1670. [DOI] [PubMed] [Google Scholar]
- 76. Ahmadzadehfar H, Wegen S, Yordanova A, et al. Overall survival and response pattern of castration-resistant metastatic prostate cancer to multiple cycles of radioligand therapy using [177Lu]Lu-PSMA-617. Eur J Nucl Med Mol Imaging. 2017;44(9):1448–1454. [DOI] [PubMed] [Google Scholar]
- 77. Rahbar K, Boegemann M, Yordanova A, et al. PSMA targeted radioligandtherapy in metastatic castration resistant prostate cancer after chemotherapy, abiraterone and/or enzalutamide. A retrospective analysis of overall survival. Eur J Nucl Med Mol Imaging. 2018;45(1):12–19. [DOI] [PubMed] [Google Scholar]
- 78. Rahbar K, Bogeman M, Yordanova A, et al. Delayed response after repeated (177)Lu-PSMA-617 radioligand therapy in patients with metastatic castration resistant prostate cancer. Eur J Nucl Med Mol Imaging. 2018;45(2):243–246. [DOI] [PubMed] [Google Scholar]
- 79. Schlenkhoff CD, Gaertner F, Essler M, Schmidt M, Ahmadzadehfar H. Positive influence of 177Lu PSMA-617 therapy on bone marrow depression caused by metastatic prostate cancer. Clin Nucl Med. 2016;41(6):478–478. [DOI] [PubMed] [Google Scholar]