Abstract
Background:
The xanthine oxidase inhibitor allopurinol improves endothelial function in different populations, including patients with chronic heart failure (CHF). Its effect on arterial stiffness parameters is less clear. We investigated the effect of short-term low-dose allopurinol therapy on arterial stiffness in Saudi patients with stable mild-moderate CHF.
Methods:
A prospective, randomized, double-blind, placebo-controlled study was performed on 73 patients with mild-moderate CHF. In all, 36 patients were randomized to allopurinol 300 mg daily for 3 months, while 37 patients were randomized to placebo. Arterial stiffness parameters, aortic pulse wave velocity (Ao-PWV) and heart rate corrected augmentation index (c-AIx), were assessed before and after treatment along with serum uric acid.
Results:
A total of 66 patients completed the study. Both groups were matched for age, sex, severity of heart failure, and arterial stiffness. Compared with placebo, allopurinol recipients had a significant fall in uric acid concentration from 6.31 ± 1.4 (SD) mg/dL to 3.81 ± 1.2 (P < .001). Despite that, there was no significant change in arterial stiffness parameters between allopurinol and placebo groups. Post-treatment Ao-PWV was 9.79 ± 2.6 m/s in the allopurinol group and 10.07 ± 3.4 m/s in the placebo group, P = .723. Post-treatment c-AIx was 24.0% ± 9.1% and 22.0% ± 9.9%, respectively, P = .403.
Conclusions:
We have shown that allopurinol significantly reduced uric acid concentration in Saudi patients with CHF but was not associated with a change in arterial stiffness. Our cohort of patients had worse arterial stiffness values at baseline, which might make them more resistant to change using our study regimen.
The study has been registered with the International Standard Randomized Controlled Trial Number registry with an identifier number of ISRCTN58980230.
Keywords: Chronic heart failure, allopurinol, arterial stiffness, pulse wave velocity, augmentation index
Introduction
Endothelial dysfunction, characterized by reduced bioavailability of nitric oxide, is an important step in the progression of atherosclerosis1-5 and has an important prognostic value for cardiovascular (CV) events,6 whether assessed in coronary arteries5,7,8 or peripheral conduit arteries.9-15 In patients with chronic heart failure (CHF), endothelial dysfunction has been documented16-18 with loss of vascular oxidative balance to be the likely underlying mechanism.19 Oxidative stress was documented in such patients, via increased xanthine oxidase enzyme activity and reduced extracellular superoxide dismutase (ecSOD) activity, with the activity of both enzymes closely correlated with flow-dependent dilatation findings in radial artery.19 Endothelial dysfunction in patients with CHF was found to be an independent predictor of major CV events20-22 and mortality.23-25
Pulse wave velocity (PWV) is commonly used as a direct and a “gold standard” measure of arterial stiffness; a measure of the viscoelastic properties of the arterial wall. Aortic pulse wave velocity (Ao-PWV) in particular (carotid-femoral) has a prognostic significance for CV outcomes in the general population beyond traditional CV risk factors. In 2014, an individual data meta-analysis on 17 635 subjects showed that CV events increased by 30% per 1-SD increase of Ao-PWV (95% confidence interval [CI], 1.18-1.43) after adjustment for traditional risk factors.26 Factors regulating arterial stiffness include vessel wall structure, distending arterial pressure, and smooth muscle tone determined by endothelial function.27 That explains the co-existence of endothelial dysfunction and arterial stiffness in healthy population,28,29 in patients with different CV risk factors30-33 and in CHF.34
As xanthine oxidase activity has been found to be upregulated in CHF, recent clinical studies were designed to study the effect of allopurinol, a xanthine oxidase inhibitor on endothelial function in patients with different CV risk factors. Only a few studies assessed the short-term effect of allopurinol on endothelial function in patients with mild-moderate CHF,35-40 with positive results. Based on these findings and as endothelial dysfunction has been associated with arterial stiffness, we hypothesize that allopurinol 300 mg add-on therapy for 3 months to clinically stable patients with CHF will result in reduction of arterial stiffness parameters; Ao-PWV and augmentation index (AIx). A secondary end point included its effect on exercise capacity, as skeletal muscle dysfunction partially attributed to oxidative stress contributes to exercise intolerance in heart failure.41
Methods
Study design
A prospective, randomized, double-blind, placebo-controlled study was planned to enroll patients (age > 18 years) with clinically stable, compensated, CHF (New York Heart Association [NYHA] functional class I-III). Documentation of left ventricular systolic dysfunction was done by biplane Simpson method.42 Clinical stabilization of cardiac condition and medications for at least 3 months before enrollment was required. Patients were excluded if they had any of the following: serum creatinine > 2 mg/dL or creatinine clearance of <60 mL/min, admission to hospital within the previous 3 months due to decompensated heart failure or new ischemic event, uncontrolled blood pressure (BP) (>160/100 mm Hg), on concomitant antioxidant vitamins, immunosuppressive therapy, or with contraindication to allopurinol therapy. The study protocol was approved by the research ethics committee—Deanship of scientific research, Imam Abdulrahman Bin Faisal University, Dammam, Saudi Arabia.
Study protocol
Suitable patients were identified; the designed protocol involves 4 visits as demonstrated in the study flowchart (Figure 1).
Figure 1.
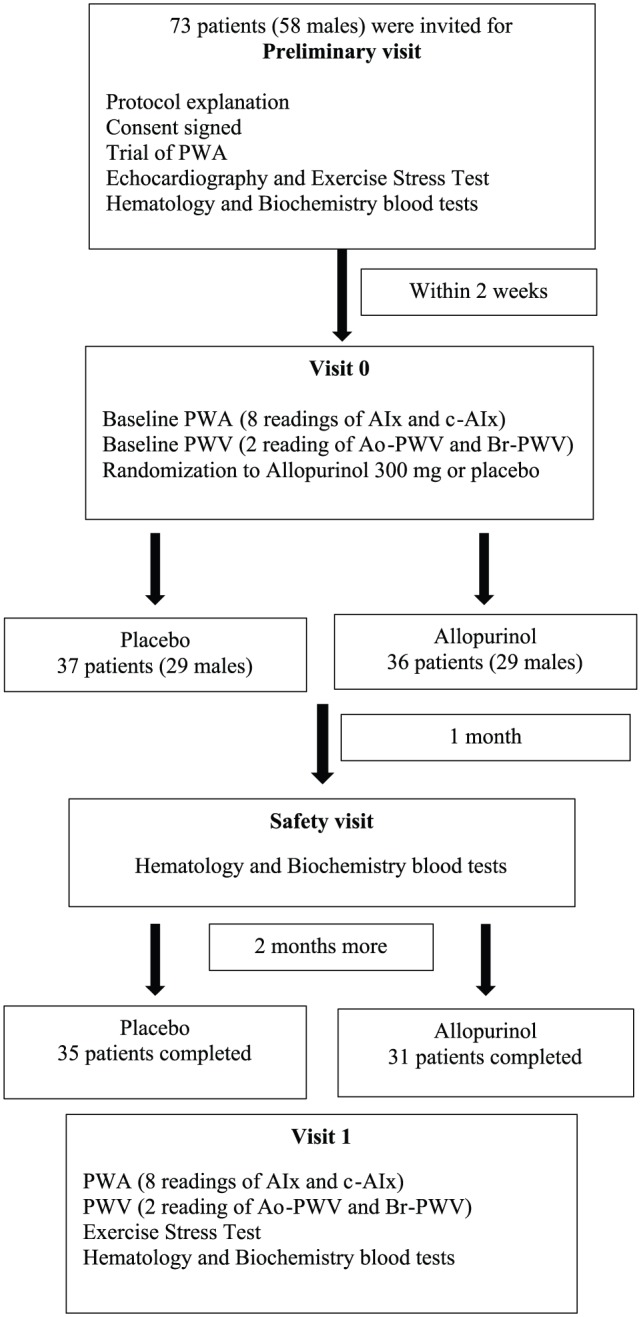
Study flowchart. AIx indicates augmentation index; c-AIx, heart rate corrected augmentation index; Ao-PWV, aortic pulse wave velocity; Br-PWV, brachial pulse wave velocity; PWA, pulse wave analysis.
Preliminary visit
Patients were asked to report to King Fahd Hospital of the University in a fasting state (12 hours). An informed written consent with both Arabic and English explanation of the study was given to all patients, and signed by those who have agreed to take part. Then patients were introduced to SphygmoCor CPV System, V9 software, AtCor Medical Pty Ltd, Sydney, Australia. Few readings of AIx were taken for acclimatization (all performed by MMA). However, they were not used in the analysis. Echocardiography using Vivd E9 Machine from GE, USA (including 2D/M-mode and Doppler studies) was done followed by exercise stress test using Schiller cardiovert CS-200 stress system from Schiller, USA. Baseline hematologic and biochemical screening with renal, liver function tests, C-reactive protein, uric acid, lipid profile, and fasting blood sugar were requested.
Visit 0 (within 2 weeks of preliminary visit)
After confirming the suitability of the patients, they reported in a fasting state. Water and morning medications were allowed. After 30 minutes of supine rest, 2 readings of blood pressure were taken by CARESCAPE V100 vital signs monitor with Dinamap technology, GE El Seif Healthcare Arabia, Riyadh, Saudi Arabia. Pulse wave analysis (PWA) started by performing 8 readings of AIx followed by 2 readings for Ao-PWV and brachial pulse wave velocity (Br-PWV). Each patient received either 300 mg allopurinol (Apo-Allopurinol-APOTEX, Canada) or placebo. Block randomization with a block size of 4 was used and blocking sequence was masked form the investigators.
Additional (safety) visit (1 month after randomization)
Patients were seen to check for any side effects and they had repeat blood tests.
Visit 1 (3 months after randomization)
Patients reported in a fasting state for a similar protocol as in visit 0. Repeat-exercise stress test was performed along with hematologic and biochemical screening.
Discontinuation of treatment was allowed for patients who were intolerant of treatment, who developed an abnormal finding in their blood tests after 1 month, or who voluntarily withdrew from the study.
Assessment of arterial stiffness
The SphygmoCor system is a computerized, non-invasive, portable, and simple-to-use device to analyze pulse pressure waveform. It has 2 assessment facilities: PWA and PWV.
Pulse wave velocity (expressed as meter/second) was determined by measuring the time delay between a characteristic point on the 2 pressure waveforms: the carotid and femoral arteries in the case of Ao-PWV that are known distance apart (measured on the surface of the body). The characteristic point is commonly taken as the foot of the wave; marked with green dot in PWV report (Figure 2). Brachial pulse wave velocity involves 2 different points: the carotid and radial arteries. In this study protocol, the average of 2 readings was taken for Ao-PWV and Br-PWV.
Figure 2.
Aortic pulse wave velocity report.
Pulse wave analysis provides AIx as the most widespread measure of wave reflection.43 It can be derived from the central pressure waveform that is analyzed and 2 systolic peaks are determined. The first systolic peak (P1) is defined as the forward pressure wave created by ventricular contraction, and the second (P2) is due to the reflected pressure wave. Augmentation index can be defined as (P2 - P1) / PP, where PP denotes the systolic–diastolic pressure difference (expressed as percentage of the pulse pressure). The value is inversely proportional with heart rate, and so heart rate corrected AIx (c-AIx) at 75 beats/min was used. In this study protocol, the average of 8 readings was taken.
Uric acid measurements
Uric acid was analyzed by Dimension EXL 200 integrated chemistry system, Siemens Healthineers, Saudi Arabia.
Exercise stress test
The modified Bruce protocol was performed according to the American Heart Association’s guidelines for clinical exercise testing44 with an electrically driven treadmill. Electrocardiogram was monitored continuously and blood pressure was checked manually at baseline and at 3-minute intervals with a calibrated Trimline mercury sphygmomanometer by PyMah Corp. Somerville, New Jersey, USA. Standardized instructions were given on how to perform the test and patients were instructed to exercise until fatigue or dyspnea prevents him or her from continuing or until instructed to stop. Metabolic equivalent (MET) was calculated and used to describe the functional capacity or exercise tolerance of an individual. It is a simple, practical, and easily understood procedure for expressing the energy cost of physical activities as a multiple of the resting metabolic rate.45
Study outcome measures
The primary outcome in this study was a change in Ao-PWV. Secondary outcomes include a change in other arterial stiffness parameters, such as heart rate corrected augmentation index (c-AIx) and Br-PWV. Change in exercise capacity assessed by MET was also assessed.
Compliance was assessed by pill count. Patients were asked to bring their pill bottles at the end of the study. The number of pills taken was calculated as number of pill provided – number of pills returned. Percent compliance was calculated as actual pills taken / number of pills provided × 100%.
Statistical analysis
Baseline characteristics are reported as mean ± SD for continuous variables and as a number and percentage for categorical variables. Two-sample t test and chi-square test were applied to compare continuous and categorical variables, respectively, between study groups. Lipid profile data were positively skewed and so they were log transformed prior to statistical testing to satisfy the normality assumption for t tests. For the primary and secondary study outcomes, the change from baseline was calculated by paired t test within the same group and by independent-sample t tests between study groups. All statistical analyses and normality testing was done by Minitab Statistical Software, V17, State College, Pennsylvania, USA.
Sample size
The primary end point for sample size calculation was Ao-PWV. From a previous work (Manal Alem, unpublished data, 2005), we had a SD of 0.938 m/s variability in PWV assessment using an average of 2 readings taken in 2 occasions, 2 weeks apart in a group of 16 hypertensive patients. That means we needed 29 patients in every group to detect 1 m/s change in PWV with 80% power (α = .05).
Results
Population characteristics
A total of 73 patients were randomized, 37 were in the placebo arm and 36 were in allopurinol arm. The 2 groups were matched for age and sex with 29 men in both groups (Table 1). There was a significant difference in body mass index (BMI) between the 2 groups: 31.5 ± 4.1 in placebo group and 26.4 ± 5.2 in allopurinol group (P < .001). The cause of heart failure was mainly due to ischemic heart disease which contributed to 94.4% of the cases in the allopurinol group vs 78.4% in the placebo group (P = .046). The incidence of systemic hypertension and type 2 diabetes mellitus in the 2 groups was matched (Table 1). The severity of heart failure was matched between the groups, with most patients being NYHA functional class II (78% of participants). Fractional shortening and ejection fraction were matched between the 2 groups. Neither study groups was hyperuricemic (Table 1). There were no significant baseline differences in peripheral/central blood pressure, heart rate, or arterial stiffness parameters (Table 2).
Table 1.
Baseline characteristics according to study group.
| Characteristic | Placebo (n = 37) |
Allopurinol (n = 36) |
P |
|---|---|---|---|
| Age (yr) | 55.3 ± 13 | 56.6 ± 10.1 | .632 |
| Male sex | 29 (78.3%) | 29 (80.5%) | .818 |
| BMI | 31.5 ± 4.1 | 26.4 ± 5.2 | <.001 |
| Cause of CHF | |||
| IHD | 29 (78.4%) | 34 (94.4%) | .046 |
| HHD | 4 | 2 | .414 |
| DCM | 2 | 0 | — |
| Valvular | 1 | 0 | — |
| DM related CM | 1 | 0 | — |
| Hypertension | 10 | 10 | .943 |
| Diabetes mellitus | 27 | 19 | .074 |
| NYHA functional class | |||
| 1 | 4 | 2 | |
| 2 | 29 (78.4%) | 28 (77.8%) | .585 |
| 3 | 4 | 6 | |
| Fractional shortening (%) | 26.6 ± 10.3 | 24.9 ± 11.6 | .497 |
| Ejection fraction | |||
| 1 >55% | 15 | 11 | |
| 2 mildly reduced (45%-54.9%) | 6 | 9 | .753 |
| 3 moderately reduced (30%-44.9%) | 12 | 12 | |
| 4 severely reduced (<30%) | 4 | 4 | |
| History of smoking | |||
| Active | 13 | 5 | |
| Ex-smoker | 8 | 9 | .103 |
| Never | 16 | 22 | |
| Uric acid (mg/dL) | 6.09 ± 1.9 | 6.31 ± 1.4 | .580 |
| aSerum cholesterol (mg/dL) | 150.6 ± 1.2 | 155.2 ± 1.3 | .562 |
| aSerum triglyceride (mg/dL) | 136.7 ± 1.6 | 117 ± 1.7 | .172 |
| aHDL-cholesterol (mg/dL) | 36.5 ± 1.3 | 39.6 ± 1.3 | .164 |
| aLDL-cholesterol (mg/dL) | 86.6 ± 1.3 | 90.2 ± 1.4 | .554 |
| Risk factor (cholesterol/HDL) | 4.3 ± 1.4 | 4.1 ± 1.2 | .524 |
| Exercise stress test | |||
| Number of participants | 18 | 11 | |
| MET | 8.66 ± 2.23 | 8.74 ± 3.41 | .736 |
Abbreviations: BMI, body mass index; CHF, chronic heart failure; CM, cardiomyopathy; DCM, dilated cardiomyopathy; DM, diabetes mellitus; IHD, ischemic heart disease; HDL, high-density lipoprotein; HHD, hypertensive heart disease; LDL, low-density lipoprotein; MET, metabolic equivalent; NYHA, New York Heart Association.
Values are expressed as mean ± SD or number (percentage of patients).
Lipid profile data are geometrical means obtained from log transformation of the raw data.
Table 2.
Baseline hemodynamics and arterial stiffness parameters according to study group.
| Parameter | Placebo (n = 37) |
Allopurinol (n = 36) |
P |
|---|---|---|---|
| Peripheral SBP (mm Hg) | 132.7 ± 18.5 | 127.5 ± 18.8 | .238 |
| Peripheral DBP (mm Hg) | 70.9 ± 10.2 | 74.0 ± 10.7 | .207 |
| Peripheral MAP (mm Hg) | 94.1 ± 12.3 | 94.6 ± 12.4 | .863 |
| Peripheral PP (mm Hg) | 61.4 ± 14.3 | 52.9 ± 15.3 | .016 |
| HR (beat/min) | 67.9 ± 11.4 | 69.0 ± 13.2 | .713 |
| Central SBP (mm Hg) | 121.1 ± 18.7 | 117.4 ± 18.8 | .397 |
| Central DBP (mm Hg) | 72.0 ± 10.9 | 74.6 ± 11.1 | .305 |
| Central MAP (mm Hg) | 92.5 ± 12.7 | 92.6 ± 12.8 | .981 |
| Central PP (mm Hg) | 49.2 ± 14.8 | 42.8 ± 15.2 | .071 |
| Aortic PWV (m/s) | 9.99 ± 3.0 | 9.57 ± 2.7 | .555 |
| Heart rate corrected c-AIx (%) | 21.8 ± 10.5 | 24.6 ± 9.6 | .238 |
| Augmentation index AIx (%) | 25.9 ± 11.4 | 27.8 ± 12.8 | .505 |
| Brachial PWV (m/s) | 9.35 ± 1.4 | 9.33 ± 1.5 | .947 |
Abbreviations: AIx, augmentation index; c-AIx, heart rate corrected augmentation index; DBP, diastolic blood pressure; HR, heart rate; MAP, mean blood pressure; PP, systolic–diastolic pressure difference; PWV, pulse wave velocity; SBP, systolic blood pressure.
Values are expressed as mean ± SD.
Cardiac medication
Both groups were treated with optimal heart failure medications and matched with no significant difference (Table 3).
Table 3.
Baseline cardiac medications.
| Drug | Placebo (n = 37) |
Allopurinol (n = 36) |
P |
|---|---|---|---|
| Angiotensin converting enzyme inhibitors | 19 | 18 | .908 |
| Angiotensin receptor blockers | 15 | 10 | .251 |
| Beta blockers | 34 | 33 | .972 |
| Statins | 27 | 30 | .285 |
| Thiazide diuretics | 6 | 6 | .959 |
| Loop diuretics | 16 | 18 | .563 |
| Spironolactone | 11 | 13 | .562 |
| Aspirin | 32 | 34 | .248 |
| Clopidogrel | 16 | 20 | .293 |
| Warfarin | 3 | 3 | .972 |
| Calcium channel blockers | 7 | 8 | .727 |
| Digoxin | 3 | 0 | .081 |
| Nitroglycerin | 11 | 12 | .740 |
| Insulin | 8 | 6 | .591 |
| Oral hypoglycemic agents | 19 | 15 | .407 |
Chi-square test for association was used.
Response to study medication
A total of 66 patients completed the trial; 35 in the placebo arm and 31 patients in allopurinol arm. Two patients withdrew from the placebo group due to side effects related to placebo tablets (dizziness in one and itchiness in the other). Five patients withdrew from the allopurinol group: 3 left the country for immigration reasons, 1 had an acute exacerbation of heart failure and was admitted, and the last refused to continue the trial without giving any reasons. In response to study medication, patients in the allopurinol group who completed the trial had a significant drop in their uric acid concentration from 6.25 ± 1.4 mg/dL to 3.81 ± 1.2 mg/dL (P < .001), while patients in the placebo group did not have any significant change, baseline uric acid was 6.11 ± 1.9 mg/dL and 6.21 ± 1.8 mg/dL after treatment (P = .514). Comparison of uric acid change between the 2 groups was also statistically significant with P < .001 (Table 4). Despite that significant drop in uric acid concentration, all arterial stiffness parameters, Ao-PWV, c-AIx, and Br-PWV, remained unchanged. Their values in the allopurinol group were 9.79 ± 2.65 m/s, 24.0% ± 9.12%, and 8.98 ± 1.10 m/s, respectively, at the end of the study period (Figure 3). Changes in lipid profile, arterial stiffness, peripheral, as well as central blood pressure data in response to treatment are presented in Table 4.
Table 4.
Changes from baseline according to treatment.
| Characteristic | Placebo (n = 35) |
Allopurinol (n = 31) |
P |
|---|---|---|---|
| Uric acid (mg/dL) | 0.10 ± 0.9 | −2.44 ± 1.6 | <.001 |
| aSerum cholesterol (mg/dL) | 1.74 ± 23.62 | −6.24 ± 34.69 | .385 |
| aSerum triglyceride (mg/dL) | −18.86 ± 79.41 | −36.3 ± 63.34 | .481 |
| aHDL-cholesterol (mg/dL) | −2.62 ± 13.99 | −5.94 ± 21.93 | .657 |
| aLDL-cholesterol (mg/dL) | −1.36 ± 17.99 | −2.33 ± 22.42 | .916 |
| Risk factor (cholesterol/HDL) | −0.10 ± 0.83 | −0.10 ± 0.60 | .937 |
| Aortic PWV (m/s) | 0.28 ± 1.61 | 0.20 ± 1.79 | .865 |
| Heart rate corrected c-AIx (%) | −0.28 ± 5.14 | −1.32 ± 5.77 | .443 |
| Augmentation index AIx (%) | −1.38 ± 5.96 | −1.06 ± 6.37 | .835 |
| Brachial PWV (m/s) | −0.45 ± 1.22 | −0.15 ± 1.24 | .350 |
| Exercise stress test | |||
| Number of participants | 18 | 11 | |
| MET | −0.17 ± 1.95 | −0.68 ± 3.21 | .642 |
| Peripheral SBP (mm Hg) | −2.47 ± 11.9 | −0.05 ± 16.7 | .505 |
| Peripheral DBP (mm Hg) | −0.61 ± 7.7 | −0.48 ± 8.5 | .948 |
| Peripheral MAP (mm Hg) | −1.37 ± 9.2 | −0.16 ± 11.0 | .632 |
| Peripheral PP (mm Hg) | −2.77 ± 8.96 | 0.39 ± 15.62 | .327 |
| HR (beat/min) | 1.63 ± 9.1 | 0.23 ± 11.2 | .581 |
| Central SBP (mm Hg) | −3.19 ± 11.1 | 0.79 ± 18.8 | .309 |
| Central DBP (mm Hg) | −0.34 ± 8.44 | 0.53 ± 9.70 | .699 |
| Central MAP (mm Hg) | −1.30 ± 9.53 | 0.94 ± 13.1 | .436 |
| Central PP (mm Hg) | −2.87 ± 7.72 | 0.26 ± 14.42 | .286 |
Abbreviations: AIx, augmentation index; c-AIx, heart rate corrected augmentation index; DBP, diastolic blood pressure; HDL, high-density lipoprotein; HR, heart rate; LDL, low-density lipoprotein; MAP, mean blood pressure; MET, metabolic equivalent; PP, systolic–diastolic pressure difference; PWV, pulse wave velocity; SBP, systolic blood pressure.
Values are expressed as absolute change from baseline ± SD (post drug treatment – baseline).
Statistical tests for the lipid profile data were performed on log transformed data although the difference data shown used the raw data.
Figure 3.
Dot plot showing change in uric acid, c-AIx, and Ao-PWV in response to study medication. The augmentation index is scaled to fit the figure so that units on the vertical axis are 0.1 of the actual percent values. The filled squares with error bars adjacent to dots represent the means and bootstrap generated 95% confidence intervals. Filled circles represent allopurinol and open circles represent placebo. Augmentation index used is c-AIx that is heart rate corrected augmentation index; Ao-PWV, aortic pulse wave velocity.
A further analysis was done to explore if allopurinol had a tendency for different effects based on age, sex, or baseline Ao-PWV. A median split for age was taken at 56 years; there were 36 in the younger group and 37 in the older group. For sex, 58 were men and 15 women. A median split for Ao-PWV at 9.05 m/s resulted in 34 patients in each group. A fixed effects analysis of variance was done with the change in Ao-PWV as the dependent variable. Independent variables were median split for age, sex, baseline Ao-PWV, and drug treatment. No interactions with drug treatment were detected in the analysis for the variables: split age (P = .637), sex (P = .862), split baseline Ao-PWV (P = .677). A simple effects analysis for the split baseline Ao-PWV confirmed the latter finding that no statistical difference in changes in Ao-PWV was found for placebo versus allopurinol for those patients with low baseline Ao-PWV, t(28) = .807, P = .426, and for those patients with high baseline Ao-PWV, t(26) = 0.984, P = .334. In summary, these analyses indicate that allopurinol had little or no effect even when comparing young with old, men with women, and low with high baseline Ao-PWV.
Compliance was calculated from 28 patients in every group who returned their pill containers at the end of the study. It was comparable between the 2 groups; 91.2% ± 9.3% in the placebo group and 86.5% ± 12.6% in allopurinol group (P = .114).
Exercise stress test
Eighteen patients in the placebo group and 11 patients in the allopurinol group completed exercise stress test. Metabolic equivalents (MET) were matched between the 2 groups at baseline (Table 1). Metabolic equivalents at the end of study were 8.48 ± 3.08 in the placebo group and 8.05 ± 2.91 in the allopurinol group (P = .756). Comparison of the changes from baseline according to treatment is presented in Table 4. The reason behind the small number of participants was personal reluctance to perform the test in 4 patients, the remaining subjects had other reasons such as osteoarthritis (10), vertigo/ dizziness (4), left ventricular thrombus/left bundle branch block (LBBB) (3), frail state (3), neurologic deficient (2), leg pain (1), broken arm (1), and others/missing data (9).
Discussion
To the best of our knowledge, this is the first study to assess the effect of allopurinol on arterial stiffness parameters in CHF patients. We have shown that allopurinol produced a 38% reduction in uric acid from baseline in the allopurinol group that was statistically significant with the specified dose and duration. Our figure appears to be higher than the 33.8% reduction reported in a recent meta-analysis that assessed the efficacy of urate lowering therapies in gout patients using a similar regimen.46 Despite that, allopurinol did not affect any of the parameters tested; Ao-PWV, c-AIx, and Br-PWV (Table 4). In the literature, allopurinol effect on endothelial function to start with has been documented with encouraging results in heterogeneous groups of different patient’s populations with variable baseline uric acid concentrations.47-50 Looking at the studies that included patients with CHF only, only a few studies tested the effect of allopurinol therapy on endothelial function in CHF patients and demonstrated a beneficial effect with the same dose we used (300 mg/day) but for a shorter period.35-40 But these studies did not test its effect on arterial stiffness. Five of these studies35-39 tested allopurinol effect for a maximum period of 1 month on older populations than our cohort and with a higher baseline serum uric acid. Perhaps the patient populations in these 5 studies had a relatively high level of oxidative stress for allopurinol to exhibit its antioxidant activity and improve endothelial function accordingly. These studies further demonstrated its ability to decrease markers of oxidative stress35-37; a property that was thought to be independent of uric acid reduction.37 The sixth study by Xiao et al tested the same dose and duration of allopurinol as in our study and on a population closer to our cohort in age and baseline uric acid. However, extrapolating from that study to our population would not be possible due to their study design (open-label) and risk of bias (lack of placebo).40
This study examined arterial stiffness which can be determined by smooth muscle tone influenced by vasoactive substances released from the endothelium, distending arterial pressure and vessel wall structure.27 However, arterial stiffness results primarily from arteriosclerosis, principally a disease of the media, related to normal or accelerated aging rather than atherosclerosis; principally a disease of the intima, affecting the vessel in a patchy and not uniform manner.51 Regarding endothelial function, few studies in the literature assessed allopurinol effect on both endothelial function and arterial stiffness represented by c-AIx. Using different patient populations, they obtained variable results. Three studies demonstrated beneficial effects on both phenomena in patients with coronary artery disease52,53 and chronic kidney disease.54 Whereas one study demonstrated a beneficial effect on endothelial function only in patients with type 2 diabetes mellitus,55 another did not demonstrate any beneficial effects on either phenomena in such patients.56 By comparing the baseline arterial stiffness figures in these patient populations and ours, we have shown that our Saudi population had worse arterial stiffness figures compared with the populations included in 3 of these studies.53,54,56 This raises the question that our study population did not respond to such therapy with the given dose and duration because they differ from those other populations, perhaps in genetically relevant ways. Epidemiologic studies assessing arterial stiffness indices in Saudi population are not available to assess the severity and predict the reversibility of this phenomenon in relation to age, sex, and serum uric acid. In fact, looking at arterials stiffness data from Framingham Heart study that included 4140 participants, it showed interestingly that for every 1 mg/dL rise in serum uric acid, there will be associated 0.06 m/s rise in Ao-PWV.57 It is important to note that individuals in the fourth quartile (based on serum uric acid) had a mean age of 40 years, serum uric acid of 7.3 mg/dL, a mean Ao-PWV of 7.5 m/s, and an AIx of 2.92%, while our Saudi population in this study had a mean age of 55 years, serum uric acid of 6 mg/dL, a mean Ao-PWV of 10.0 m/s, and as AIx of 23.2%. Considering differences in co-morbidities, our population might have an irreversible status of arterial stiffness with the intervention used in this study.
The second factor contributing to arterial stiffness is distending arterial pressure and such an effect was eliminated in our study as allopurinol did not modify peripheral or central blood pressure significantly, despite an associated small reduction in peripheral systolic and diastolic blood pressure that was reported in a meta-analysis on patients receiving allopurinol therapy.58 Vessel wall structure is the third factor contributing to arterial stiffness and is less likely to change over a 3-month period. What might contribute to that are any possible changes in lipid profile due to allopurinol therapy. Such an effect was reported after 3 months therapy with conflicting results in the literature,59-61 but not in our cohort (Table 4).
Accordingly, demonstrating a beneficial effect of allopurinol on endothelial function (via more dynamic methods of assessment) in a certain population can be independent from its effect on arterial stiffness (which can be slower to develop especially if we exclude blood pressure alteration). Finally, if we hypothesized that allopurinol will have a beneficial effect on arterial stiffness in our population via its antioxidant activity and effect on endothelial function, then it is not surprising that there was no improvement in exercise tolerance in those participants who were tested. This finding aligns with other studies that tested the effect of allopurinol on exercise capacity in CHF.62,63
Limitations
Our study included a relatively small number of patients, but a power calculation indicated this was sufficient for our required effect size. The assessment of arterial stiffness indices with an adequate number of readings to guarantee reproducible results is time-consuming, in addition to exercise stress testing, which would make additional assessment of endothelial function technically difficult. Assessment of markers of oxidative stress could have been useful had they been done. The third limitation was the relatively short period for the intervention to allow arterial structural changes in response to allopurinol therapy.
Conclusions
Allopurinol is an old drug with a promising effect on arterial structure and function. Small-scale human studies have demonstrated that short-term use can benefit endothelial function in patients with CHF. However, this is the first study to test the extension of such a benefit to arterial stiffness parameters for a longer period of time. Despite a marked reduction of uric acid in our study, we did not demonstrate any benefit of allopurinol on arterial stiffness parameters in our patient population. Future studies using a longer treatment time or higher dose could allow any beneficial effects of allopurinol on arterial structure and function to be seen.
Acknowledgments
We are thankful to all of our patients who participated in the clinical trial. We would like to thank Dr Muammar Abu Sheikha from Jazeera Pharmaceutical Industries, Dammam, Saudi Arabia, for supplying the placebo tablets. We would like to thank Ph. Abdullah Al Asmari, Director of Medical Supply, King Fahd Hospital of the University for coordinating with Thimar Al Jazirah Corporation regarding Allopurinol supply from APOTEX/Canada. Findings from this study were presented in an oral presentation session for late-breakers abstracts at the annual meeting of the European Society of Hypertension (June 13, 2015). This study was completed at King Fahd Hospital of the University, Al Khobar, Saudi Arabia. This is the university hospital for Imam Abdulrahman Bin Faisal University, Dammam, Saudi Arabia.
Footnotes
Funding:The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This clinical trial was funded by deanship of scientific research at Imam Abdulrahman Bin Faisal University, Dammam, Saudi Arabia (grant 20100065).
Declaration Of Conflicting Interests:The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Author Contributions: All the authors have read and approved the final manuscript. All authors have read and approved the data presented in the manuscript.
ORCID iD: Manal M Alem  https://orcid.org/0000-0001-8447-792X
https://orcid.org/0000-0001-8447-792X
References
- 1. Ludmer PL, Selwyn AP, Shook TL, et al. Paradoxical vasoconstriction induced by acetylcholine in atherosclerotic coronary arteries. N Engl J Med. 1986;315:1046–1051. [DOI] [PubMed] [Google Scholar]
- 2. Celermajer DS, Sorensen KE, Gooch VM, et al. Non-invasive detection of endothelial dysfunction in children and adults at risk of atherosclerosis. Lancet. 1992;340:1111–1115. [DOI] [PubMed] [Google Scholar]
- 3. Celermajer DS, Sorensen KE, Bull C, Robinson J, Deanfield JE. Endothelium-dependent dilation in the systemic arteries of asymptomatic subjects relates to coronary risk factors and their interaction. J Am Coll Cardiol. 1994;24:1468–1474. [DOI] [PubMed] [Google Scholar]
- 4. Reddy KG, Nair RN, Sheehan HM, Hodgson JM. Evidence that selective endothelial dysfunction may occur in the absence of angiographic or ultrasound atherosclerosis in patients with risk factors for atherosclerosis. J Am Coll Cardiol. 1994;23:833–843. [DOI] [PubMed] [Google Scholar]
- 5. Suwaidi JA, Hamasaki S, Higano ST, Nishimura RA, Holmes DR, Jr, Lerman A. Long-term follow-up of patients with mild coronary artery disease and endothelial dysfunction. Circulation. 2000;101:948–954. [DOI] [PubMed] [Google Scholar]
- 6. Endemann DH, Schiffrin EL. Endothelial dysfunction. J Am Soc Nephrol. 2004;15:1983–1992. [DOI] [PubMed] [Google Scholar]
- 7. Halcox JP, Schenke WH, Zalos G, et al. Prognostic value of coronary vascular endothelial dysfunction. Circulation. 2002;106:653–658. [DOI] [PubMed] [Google Scholar]
- 8. Schachinger V, Britten MB, Zeiher AM. Prognostic impact of coronary vasodilator dysfunction on adverse long-term outcome of coronary heart disease. Circulation. 2000;101:1899–1906. [DOI] [PubMed] [Google Scholar]
- 9. Heitzer T, Schlinzig T, Krohn K, Meinertz T, Munzel T. Endothelial dysfunction, oxidative stress, and risk of cardiovascular events in patients with coronary artery disease. Circulation. 2001;104:2673–2678. [DOI] [PubMed] [Google Scholar]
- 10. Gokce N, Keaney JF, Jr, Hunter LM, et al. Predictive value of noninvasively determined endothelial dysfunction for long-term cardiovascular events in patients with peripheral vascular disease. J Am Coll Cardiol. 2003;41:1769–1775. [DOI] [PubMed] [Google Scholar]
- 11. Gokce N, Keaney JF, Jr, Hunter LM, Watkins MT, Menzoian JO, Vita JA. Risk stratification for postoperative cardiovascular events via noninvasive assessment of endothelial function: a prospective study. Circulation. 2002;105:1567–1572. [DOI] [PubMed] [Google Scholar]
- 12. Fichtlscherer S, Breuer S, Zeiher AM. Prognostic value of systemic endothelial dysfunction in patients with acute coronary syndromes: further evidence for the existence of the “vulnerable” patient. Circulation. 2004;110:1926–1932. [DOI] [PubMed] [Google Scholar]
- 13. Modena MG, Bonetti L, Coppi F, Bursi F, Rossi R. Prognostic role of reversible endothelial dysfunction in hypertensive postmenopausal women. J Am Coll Cardiol. 2002;40:505–510. [DOI] [PubMed] [Google Scholar]
- 14. Perticone F, Ceravolo R, Pujia A, et al. Prognostic significance of endothelial dysfunction in hypertensive patients. Circulation. 2001;104:191–196. [DOI] [PubMed] [Google Scholar]
- 15. Neunteufl T, Heher S, Katzenschlager R, et al. Late prognostic value of flow-mediated dilation in the brachial artery of patients with chest pain. Am J Cardiol. 2000;86:207–210. [DOI] [PubMed] [Google Scholar]
- 16. Kubo SH, Rector TS, Bank AJ, Williams RE, Heifetz SM. Endothelium-dependent vasodilation is attenuated in patients with heart failure. Circulation. 1991;84:1589–1596. [DOI] [PubMed] [Google Scholar]
- 17. Drexler H, Hayoz D, Munzel T, et al. Endothelial function in chronic congestive heart failure. Am J Cardiol. 1992;69:1596–1601. [DOI] [PubMed] [Google Scholar]
- 18. Bank AJ, Lee PC, Kubo SH. Endothelial dysfunction in patients with heart failure: relationship to disease severity. J Card Fail. 2000;6:29–36. [DOI] [PubMed] [Google Scholar]
- 19. Landmesser U, Spiekermann S, Dikalov S, et al. Vascular oxidative stress and endothelial dysfunction in patients with chronic heart failure: role of xanthine-oxidase and extracellular superoxide dismutase. Circulation. 2002;106:3073–3078. [DOI] [PubMed] [Google Scholar]
- 20. Fischer D, Rossa S, Landmesser U, et al. Endothelial dysfunction in patients with chronic heart failure is independently associated with increased incidence of hospitalization, cardiac transplantation, or death. Eur Heart J. 2005;26:65–69. [DOI] [PubMed] [Google Scholar]
- 21. Heitzer T, Baldus S, von KY, Rudolph V, Meinertz T. Systemic endothelial dysfunction as an early predictor of adverse outcome in heart failure. Arterioscler Thromb Vasc Biol. 2005;25:1174–1179. [DOI] [PubMed] [Google Scholar]
- 22. de Berrazueta JR, Guerra-Ruiz A, Garcia-Unzueta MT, et al. Endothelial dysfunction, measured by reactive hyperaemia using strain-gauge plethysmography, is an independent predictor of adverse outcome in heart failure. Eur J Heart Fail. 2010;12:477–483. [DOI] [PubMed] [Google Scholar]
- 23. Meyer B, Mortl D, Strecker K, et al. Flow-mediated vasodilation predicts outcome in patients with chronic heart failure: comparison with B-type natriuretic peptide. J Am Coll Cardiol. 2005;46:1011–1018. [DOI] [PubMed] [Google Scholar]
- 24. Katz SD, Hryniewicz K, Hriljac I, et al. Vascular endothelial dysfunction and mortality risk in patients with chronic heart failure. Circulation. 2005;111:310–314. [DOI] [PubMed] [Google Scholar]
- 25. Shechter M, Matetzky S, Arad M, Feinberg MS, Freimark D. Vascular endothelial function predicts mortality risk in patients with advanced ischaemic chronic heart failure. Eur J Heart Fail. 2009;11:588–593. [DOI] [PubMed] [Google Scholar]
- 26. Ben-Shlomo Y, Spears M, Boustred C, et al. Aortic pulse wave velocity improves cardiovascular event prediction: an individual participant meta-analysis of prospective observational data from 17,635 subjects. J Am Coll Cardiol. 2014;63:636–646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Wilkinson IB, Franklin SS, Cockcroft JR. Nitric oxide and the regulation of large artery stiffness: from physiology to pharmacology. Hypertension. 2004;44:112–116. [DOI] [PubMed] [Google Scholar]
- 28. Wilkinson IB, MacCallum H, Cockcroft JR, Webb DJ. Inhibition of basal nitric oxide synthesis increases aortic augmentation index and pulse wave velocity in vivo. Br J Clin Pharmacol. 2002;53:189–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. McEniery CM, Wallace S, Mackenzie IS, et al. Endothelial function is associated with pulse pressure, pulse wave velocity, and augmentation index in healthy humans. Hypertension. 2006;48:602–608. [DOI] [PubMed] [Google Scholar]
- 30. Ravikumar R, Deepa R, Shanthirani C, Mohan V. Comparison of carotid intima-media thickness, arterial stiffness, and brachial artery flow mediated dilatation in diabetic and nondiabetic subjects (The Chennai Urban Population Study [CUPS-9]). Am J Cardiol. 2002;90:702–707. [DOI] [PubMed] [Google Scholar]
- 31. Nigam A, Mitchell GF, Lambert J, Tardif JC. Relation between conduit vessel stiffness (assessed by tonometry) and endothelial function (assessed by flow-mediated dilatation) in patients with and without coronary heart disease. Am J Cardiol. 2003;92:395–399. [DOI] [PubMed] [Google Scholar]
- 32. Jadhav UM, Kadam NN. Non-invasive assessment of arterial stiffness by pulse-wave velocity correlates with endothelial dysfunction. Indian Heart J. 2005;57:226–232. [PubMed] [Google Scholar]
- 33. Wiesmann F, Petersen SE, Leeson PM, et al. Global impairment of brachial, carotid, and aortic vascular function in young smokers: direct quantification by high-resolution magnetic resonance imaging. J Am Coll Cardiol. 2004;44:2056–2064. [DOI] [PubMed] [Google Scholar]
- 34. Ramsey MW, Goodfellow J, Jones CJ, Luddington LA, Lewis MJ, Henderson AH. Endothelial control of arterial distensibility is impaired in chronic heart failure. Circulation. 1995;92:3212–3219. [DOI] [PubMed] [Google Scholar]
- 35. Farquharson CA, Butler R, Hill A, Belch JJ, Struthers AD. Allopurinol improves endothelial dysfunction in chronic heart failure. Circulation. 2002;106:221–226. [DOI] [PubMed] [Google Scholar]
- 36. Doehner W, Schoene N, Rauchhaus M, et al. Effects of xanthine oxidase inhibition with allopurinol on endothelial function and peripheral blood flow in hyperuricemic patients with chronic heart failure: results from 2 placebo-controlled studies. Circulation. 2002;105:2619–2624. [DOI] [PubMed] [Google Scholar]
- 37. George J, Carr E, Davies J, Belch JJ, Struthers A. High-dose allopurinol improves endothelial function by profoundly reducing vascular oxidative stress and not by lowering uric acid. Circulation. 2006;114:2508–2516. [DOI] [PubMed] [Google Scholar]
- 38. Tousoulis D, Andreou I, Tsiatas M, et al. Effects of rosuvastatin and allopurinol on circulating endothelial progenitor cells in patients with congestive heart failure: the impact of inflammatory process and oxidative stress. Atherosclerosis. 2011;214:151–157. [DOI] [PubMed] [Google Scholar]
- 39. Greig D, Alcaino H, Castro PF, et al. Xanthine-oxidase inhibitors and statins in chronic heart failure: effects on vascular and functional parameters. J Heart Lung Transplant. 2011;30:408–413. [DOI] [PubMed] [Google Scholar]
- 40. Xiao J, Deng SB, She Q, et al. Allopurinol ameliorates cardiac function in non-hyperuricaemic patients with chronic heart failure. Eur Rev Med Pharmacol Sci. 2016;20:756–761. [PubMed] [Google Scholar]
- 41. Tsutsui H, Ide T, Hayashidani S, et al. Enhanced generation of reactive oxygen species in the limb skeletal muscles from a murine infarct model of heart failure. Circulation. 2001;104:134–136. [DOI] [PubMed] [Google Scholar]
- 42. Lang RM, Badano LP, Mor-Avi V, et al. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr. 2015;28:1.e14–39.e14. [DOI] [PubMed] [Google Scholar]
- 43. Nichols WW, O’rourke MF, Vlachopoulos C. Wave reflection. In: Nichols WW, O’rourke MF, Vlachopoulos C, eds. McDonald’s Blood Flow in Arteries: Theoretical, Experimental, and Clinical Principles. 6th ed. New York, NY: CRC Press (Taylor & Francis Group); 2011:195–223. [Google Scholar]
- 44. Gibbons RJ, Balady GJ, Beasley JW, et al. ACC/AHA guidelines for exercise testing. A Report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Committee on Exercise Testing). J Am Coll Cardiol. 1997;30:260–311. [DOI] [PubMed] [Google Scholar]
- 45. Jette M, Sidney K, Blumchen G. Metabolic equivalents (METS) in exercise testing, exercise prescription, and evaluation of functional capacity. Clin Cardiol. 1990;13:555–565. [DOI] [PubMed] [Google Scholar]
- 46. Borghi C, Perez-Ruiz F. Urate lowering therapies in the treatment of gout: a systematic review and meta-analysis. Eur Rev Med Pharmacol Sci. 2016;20:983–992. [PubMed] [Google Scholar]
- 47. Higgins P, Dawson J, Lees KR, McArthur K, Quinn TJ, Walters MR. Xanthine oxidase inhibition for the treatment of cardiovascular disease: a systematic review and meta-analysis. Cardiovasc Ther. 2012;30:217–226. [DOI] [PubMed] [Google Scholar]
- 48. Kanbay M, Siriopol D, Nistor I, et al. Effects of allopurinol on endothelial dysfunction: a meta-analysis. Am J Nephrol. 2014;39:348–356. [DOI] [PubMed] [Google Scholar]
- 49. Xin W, Mi S, Lin Z. Allopurinol therapy improves vascular endothelial function in subjects at risk for cardiovascular diseases: a meta-analysis of randomized controlled trials. Cardiovasc Ther. 2016;34:441–449. [DOI] [PubMed] [Google Scholar]
- 50. Cicero AFG, Pirro M, Watts GF, Mikhailidis DP, Banach M, Sahebkar A. Effects of allopurinol on endothelial function: a systematic review and meta-analysis of randomized placebo-controlled trials. Drugs. 2018;78:99–109. [DOI] [PubMed] [Google Scholar]
- 51. Tsioufis C, Vlachopoulos C. Imaging biomarkers: carotid intima media thickness and aortic stiffness as predictors of cardiovascular disease. In: Nilsson PM, Olsen MH, Laurent S, eds. Early Vascular Aging. Förlag: Elsevie; 2015:225–238. [Google Scholar]
- 52. Rajendra NS, Ireland S, George J, Belch JJ, Lang CC, Struthers AD. Mechanistic insights into the therapeutic use of high-dose allopurinol in angina pectoris. J Am Coll Cardiol. 2011;58:820–828. [DOI] [PubMed] [Google Scholar]
- 53. Rekhraj S, Gandy SJ, Szwejkowski BR, et al. High-dose allopurinol reduces left ventricular mass in patients with ischemic heart disease. J Am Coll Cardiol. 2013;61:926–932. [DOI] [PubMed] [Google Scholar]
- 54. Kao MP, Ang DS, Gandy SJ, et al. Allopurinol benefits left ventricular mass and endothelial dysfunction in chronic kidney disease. J Am Soc Nephrol. 2011;22:1382–1389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Dawson J, Quinn T, Harrow C, et al. Allopurinol and nitric oxide activity in the cerebral circulation of those with diabetes: a randomized trial. Diabetes Care. 2009;32:135–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Szwejkowski BR, Gandy SJ, Rekhraj S, et al. Allopurinol reduces left ventricular mass in patients with type 2 diabetes and left ventricular hypertrophy. J Am Coll Cardiol. 2013;62:2284–2293. [DOI] [PubMed] [Google Scholar]
- 57. Mehta T, Nuccio E, McFann K, Madero M, Sarnak MJ, Jalal D. Association of uric acid with vascular stiffness in the Framingham heart study. Am J Hypertens. 2015;28:877–883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Agarwal V, Hans N, Messerli FH. Effect of allopurinol on blood pressure: a systematic review and meta-analysis. J Clin Hypertens (Greenwich). 2013;15:435–442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Shelmadine B, Bowden RG, Wilson RL, Beavers D, Hartman J. The effects of lowering uric acid levels using allopurinol on markers of metabolic syndrome in end-stage renal disease patients: a pilot study. Anadolu Kardiyol Derg. 2009;9:385–389. [PubMed] [Google Scholar]
- 60. Bowden RG, Shelmadine BD, Moreillon JJ, Deike E, Griggs JO, Wilson RL. Effects of uric acid on lipid levels in CKD patients in a randomized controlled trial. Cardiol Res. 2013;4:56–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Ziga N, Becic F. Allopurinol effect on values of lipid profile fractions in hyperuricemic patients diagnosed with metabolic syndrome. Mater Sociomed. 2013;25:167–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Gavin AD, Struthers AD. Allopurinol reduces B-type natriuretic peptide concentrations and haemoglobin but does not alter exercise capacity in chronic heart failure. Heart. 2005;91:749–753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Givertz MM, Anstrom KJ, Redfield MM, et al. Effects of xanthine oxidase inhibition in hyperuricemic heart failure patients: the xanthine oxidase inhibition for hyperuricemic heart failure patients (EXACT-HF) study. Circulation. 2015;131:1763–1771. [DOI] [PMC free article] [PubMed] [Google Scholar]




