Short abstract
Objective
To investigate brain morphometric changes in medication-overuse headache with excessive intake of caffeine-containing combination analgesics.
Materials and methods
We recruited 32 medication-overuse headache patients overusing caffeine-containing combination analgesics and 26 normal controls with matched sex and age. Magnetic resonance T1-weighted images were processed by automatic volume algorithm of brain regions over the whole brain according to the neuromorphometrics template. We explored the volume differences between groups and correlation with clinical variables.
Results
Medication-overuse headache patients demonstrated decreased volume in cerebellum, optic chiasm, and increased volume in right lateral orbital gyrus, left calcarine, bilateral middle occipital gyrus, right superior parietal lobe, and right temporal transverse gyrus compared with normal controls. The increased volume was primarily contributed by patients of lower headache frequency (10–20 days/month) and with no psychological comorbidities. In regression analyses, the volume of bilateral middle occipital gyrus had negative association with migraine duration, and the volume of right lateral orbital gyrus and right superior parietal lobe was negatively correlated with number of medications per month.
Conclusions
Volume changes of brain regions involved in affective and cognitive processing, visual and auditory perception, and pain sensory/discrimination suggested a particular role of those regions in the pathogenesis of medication-overuse headache overusing caffeine-containing combination analgesics. Morphometric changes in multiple visual processing areas and volume gain in lower headache frequency and less anxiety and depression may be specific features related to overusing caffeine-containing combination analgesics.
Keywords: Morphometric, volume, brain, magnetic resonance imaging, medication-overuse headache, caffeine-containing combination analgesics, visual processing areas
Introduction
Medication-overuse headache (MOH) is a common disabling disorder.1 It is defined as headache occurring on 15 or more days per month as a consequence of regular overuse of acute or symptomatic headache medication for more than three months.2 The term “regular overuse” means taking ergots, triptans, opioids, combination analgesics, or multiple drug classes at least 10 days and taking simple analgesics at least 15 days per month.2 Simple analgesics and triptans were the most often overused medication in European countries, while ergotamines were most widely overused in Latin America and India.3–5 However, in China, combination analgesics containing acetaminophen and/or nonsteroidal anti-inflammatory drugs (NSAIDS) and caffeine were the most commonly overused acute headache medication.6
As an analgesic adjuvant in the acute treatment of primary headache, caffeine can improve antinociception primarily through nonselective antagonism of the adenosine A1, A2A, and A2B receptors resulting in vasoconstrictive effects.7 Besides, caffeine has excitatory effects on the central nervous system and chronic intake of caffeine can develop into a dependence syndrome.8 Caffeine withdrawal may trigger headache attack as well as other withdrawal syndrome.9 Therefore, pathophysiology and treatment strategy of MOH overusing caffeine-containing combination analgesics (CCCA) might be different from other kinds of overused medication.
The pathophysiological mechanisms underlying MOH are poorly understood. Advanced imaging studies have demonstrated alterations in the structure and function of atypical regions that participate in pain processing and addiction.10–12 However, previous imaging studies enrolled MOH patients with various types of overused medicine and no structural imaging studies focused on a certain type of overused medicine. Different types of overused medicine might be associated with differences in structure and function of brain regions.
In this study, we aimed to investigate the structural alteration for MOH overusing CCCA. We hypothesize that the volume of certain brain regions may change in a peculiar pattern for MOH patients overusing CCCA and the volume change may be associated with some clinical variables. To address this hypothesis, we prospectively obtained high resolution structural images from 32 MOH patients and 26 normal controls (NC) to calculate the volume differences of brain regions over the whole brain using automatic volume algorithm according to the neuromorphometrics template. We then performed subgroup analyses, correlation analyses, and linear regression analyses to determine the association of headache variables, anxiety, depression, and cognitive variables with the abnormal brain regions.
Materials and methods
Subjects
In total, 32 MOH patients were enrolled from the International Headache Center, Department of Neurology, Chinese PLA General Hospital. The inclusion criteria were as follows: (1) diagnosis of 8.2 MOH, and 1.1 migraine without aura based on the International Classification of Headache Disorders, third edition (beta version); (2) no migraine preventive medication used in the past three months; and (3) right handed. The exclusion criteria were as follows: (1) with any chronic disorders, including hypertension, diabetes mellitus, cardiovascular diseases, cerebrovascular disorders, neoplastic diseases, connective tissue diseases, other subtypes of headache, chronic somatic pain, severe anxiety or depression preceding the onset of headache, psychiatric diseases, etc.; (2) with alcohol, nicotine, or other substance abuse; (3) with psychotic disorder and regular use of a psychoactive or hormone medication. A total of 26 NC were recruited from the hospital’s staff and local community. NC should also be right handed and never have had any primary headache disorders or other types of headache in the past year, and the exclusion criteria were the same with MOH’s exclusion criteria. General demographic and headache information (e.g., migraine duration, headache feature, laterality, extent, frequency, accompanying symptoms, MOH duration, number of medication per day and per month) were registered and evaluated in our headache database. All the patients were given with the Visual Analogue Scale (VAS) to assess headache extent. All the participants were evaluated by the Hamilton Anxiety Scale (HAMA),13 the Hamilton Depression Scale (HAMD),14 and the Montreal Cognitive Assessment (MoCA) Beijing Version (www.mocatest.org).
Imaging protocols were identical for all the subjects. Alcohol, nicotine, caffeine, and other substances were avoided for at least 12 h before MRI examination. This study protocol was approved by the local institutional review board, and written informed consents were obtained from all participants according to the approval of the ethics committee.
MRI acquisition
All MRI data were acquired on a GE 3.0T MR system (DISCOVERY MR750, GE Healthcare, Milwaukee, WI, USA) and a conventional eight-channel quadrature head coil was used. First, conventional T2-weighted imaging and T1 fluid-attenuated inversion recovery weighted imaging were acquired to exclude the subjects with obvious structural damage and T2-visible lesion. Second, the brain structural images were obtained by a three-dimensional T1-weighted fast spoiled gradient recalled echo sequence generating 180 contiguous axial slices (repetition time (TR) = 6.3 ms, echo time (TE) = 2.8 ms, flip angle = 15°, field of view (POV) = 25.6 cm × 25.6 cm, matrix = 256 × 256, slice thickness = 1 mm, number of acquisition (NEX) = 1).
Magnetic resonance image processing
All the data were preprocessed with Computational Anatomy (CAT, http://www.neuro.uni-jena.de/cat/), which is based on Statistical Parametric Mapping 12 (http://www.fil.ion.ucl.ac.uk/spm/) and MATLAB 7.6 (The Mathworks, Natick, MA, USA). The image processing included following steps (Figure 1): (1) all the structural image origin (Figure 1(a)) was set at the anterior commissure (AC) (Figure 1(b)); (2) the mutual information affine registration with tissue probability was used to achieve approximate alignment, and ICBM space template—East Asian brains were used to perform affine regularization; (3) the high-dimensional DARTEL registration was performed to generate normalized images (Figure 1(c)), and then brain tissue was segmented into gray matter (Figure 1(d)), white matter (Figure 1(e)); and (4) the neuromorphometrics template (Figure 1(f)) was used to extract the volume of each brain region.
Figure 1.
Imaging processing steps.
Statistical analysis
The statistical analysis was performed by using IBM SPSS 22. The age, migraine duration, MOH duration, headache frequency (headache days per month), VAS, numbers of medication per month (NM), HAMA, HAMD, and MoCA were performed with independent samples t test, and sex was performed with chi-square test. The significant differences of each brain-region volume between MOH group and NC group were computed using general linear model (independent univariate t test with age, sex, total intracranial volume (TIV) as covariates). Patients were further subdivided into lower frequency (LF) group (10–20 headache days/month) and higher frequency (HF) group (21–30 headache days/month), with or without definite anxiety (HAMA ≥ 14 or <14), with or without definite depression (HAMD ≥ 20 or <20), with or without cognitive impairment (MoCA<26 or ≥26) to evaluate volume differences between subgroups and in comparison with NC using General Linear Model (one-way analysis of variance with age, sex, and TIV as covariates followed by pairwise comparison using Bonferroni correction method). The Pearson’s or Spearman’s correlation analyses (depending on whether data were two continuous variables in normal distribution or not) were applied between volume of positive brain regions and the clinical variables in LF and HF subgroups, respectively. Multiple linear regressions (stepwise method) were performed to find association of clinical variables (migraine duration, MOH duration, headache frequency, VAS, NM, HAMA, HAMD, MoCA) with the volume of abnormal brain regions for all the MOH patients.
Results
Demography and clinical characteristics of the participants
In total, 32 MOH patients (25 females and 7 males) and 26 NC (20 females and 6 males) were enrolled. The demographic data and clinical profiles are presented in Table 1. There was no significant difference for age and sex between MOH and NC. However, significantly increased HAMA and HAMD scores and decreased MoCA score were identified in MOH compared with NC (p < 0.05).
Table 1.
Demographics and headache profiles of the participants.
| Clinical variables | MOH | NCs | p |
|---|---|---|---|
| Sex (F/M) | 32(25/7) | 26(20/6) | 0.58 |
| Age (yrs) | 41.97 ± 8.66 | 42.00 ± 11.28 | 0.99 |
| HAMA | 17.53 ± 7.62 | 2.34 ± 1.50 | 0.00 |
| HAMD | 18.22 ± 10.12 | 1.12 ± 1.03 | 0.00 |
| MoCA | 24.03 ± 3.97 | 28.19 ± 1.96 | 0.00 |
| Migraine duration (yrs) | 17.47 ± 7.50 | ||
| Duration of medication overuse (yrs) | 4.89 ± 5.28 | ||
| Headache frequency (days per month) | 26.56 ± 5.13 | ||
| VAS (0–10) | 7.81 ± 1.47 | ||
| Number of medications per month | 128.9 ± 110.5 | ||
| Medication compositions (patients N (%)) | |||
| Amidazophin + caffeine | 8(25%) | ||
| Paracetamol + amidazophin + caffeine | 16(50%) | ||
| Paracetamol + aspirin + caffeine | 6(18.8%) | ||
| Ibuprofen + amidazophin + caffeine | 1(3.1%) | ||
| Paracetamol + amidazophin + aspirin + caffeine | 1(3.1%) |
MOH: medication-overuse headache; NCs: normal controls; HAMA: Hamilton Anxiety Scale; HAMD: Hamilton Depression Scale; MoCA: Montreal Cognitive Assessment; VAS: Visual Analogue Scale; yrs: years; N: number.
The most frequently overused combination analgesics were composed of paracetamol + amidazophin + caffeine (50%), followed by amidazophin + caffeine (25%) and paracetamol + aspirin + caffeine (18.8%). Therefore, amidazophin and paracetamol were the most frequently used analgesics in combination analgesics.
The clinical variables (age, sex, migraine duration, MOH duration, headache frequency, VAS, number of medications per month) did not differ significantly between MOH subgroups according to whether there’s definite anxiety, definite depression, and cognitive impairment. The clinical profiles of subgroups according to different headache frequencies are shown in Table 2. Sex constitution differs between LF and HF groups (p = 0.047). Patients in LF group were younger (t = −3.07, p = 0.016) and had shorter migraine duration (t = −2.24, p = 0.033) than patients in HF group. There were no differences regarding MOH duration, VAS, number of medications per month, HAMA, HAMD, and MoCA between LF and HF subgroups (p > 0.05).
Table 2.
Clinical profiles of subgroups by different headache frequency.
| Clinical variables | LF | HF | p |
|---|---|---|---|
| Sex (F/M) | 8(4/4) | 24(21/3) | 0.026 |
| Age (yrs) | 34.75 ± 7.01 | 44.37 ± 7.88 | 0.005 |
| HAMA | 15.37 ± 6.99 | 18.25 ± 7.83 | 0.364 |
| HAMD | 15.00 ± 11.12 | 19.29 ± 9.77 | 0.306 |
| MoCA | 25.63 ± 1.99 | 23.50 ± 4.33 | 0.194 |
| Migraine duration (yrs) | 12.63 ± 6.93 | 19.08 ± 7.10 | 0.033 |
| MOH duration (yrs) | 3.25 ± 1.39 | 5.44 ± 5.98 | 0.318 |
| VAS (0–10) | 7.50 ± 1.41 | 7.92 ± 1.50 | 0.496 |
| Number of medications per month | 96.25 ± 78.73 | 139.79 ± 118.64 | 0.343 |
| Total intracranial volume (ml) | 1571.49 ± 201.12 | 1420.78 ± 66.53 | 0.003 |
LF: lower frequency (10–20 headache days per month); HF: higher frequency (21–30 headache days per month); F/M: female/male; HAMA: Hamilton Anxiety Scale; HAMD: Hamilton Depression Scale; MoCA: Montreal Cognitive Assessment; MOH: medication-overuse headache; VAS: Visual Analogue Scale; yrs: years.
Brain-region volume differences between MOH and NC
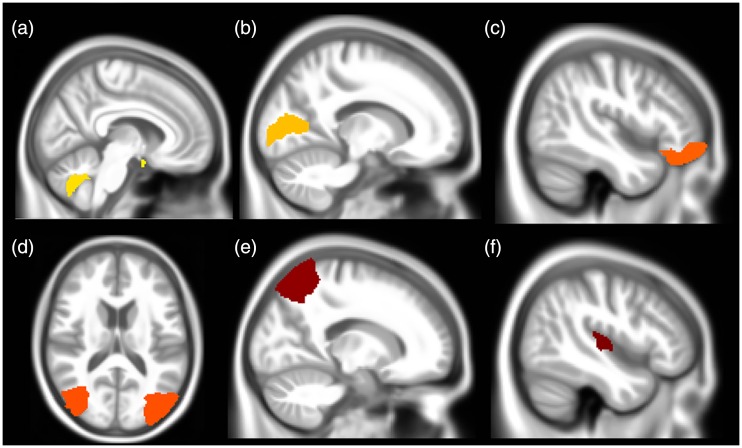
In total, 142 regions were obtained for volume analyses for each participant. Total brain volume was of no difference between groups. The MOH patients displayed decreased volume in left cerebellar vermal lobules VIII-X, optic chiasm, and increased volume in left calcarine, left and right middle occipital gyrus, right superior parietal lobe, right temporal transverse gyrus, and right lateral orbital gyrus (Table 3 and Figure 2).
Table 3.
Volume differences of abnormal brain regions between MOH and NC.
| Brain regions | MOH (ml) | NC (ml) | b | p |
|---|---|---|---|---|
| Optic chiasm | 0.006 ± 0.001 | 0.009 ± 0.001 | −0.003 | 0.031 |
| Left cerebellar vermal lobules VIII-X | 0.958 ± 0.015 | 1.009 ± 0.016 | −0.051 | 0.023 |
| Left calcarine | 3.316 ± 0.078 | 3.006 ± 0.087 | 0.309 | 0.011 |
| Right lateral orbital gyrus | 2.110 ± 0.046 | 1.942 ± 0.051 | 0.168 | 0.017 |
| Left middle occipital gyrus | 5.766 ± 0.117 | 5.386 ± 0.130 | 0.379 | 0.035 |
| Right middle occipital gyrus | 4.942 ± 0.093 | 4.497 ± 0.103 | 0.445 | 0.002 |
| Right superior parietal lobe | 9.594 ± 0.173 | 8.948 ± 0.192 | 0.646 | 0.016 |
| Right temporal transverse gyrus | 1.150 ± 0.026 | 1.070 ± 0.029 | 0.081 | 0.045 |
| Total intracranial volume | 1458.5 ± 129.7 | 1457.1 ± 136.3 | 0.969 |
Note: Adjusted mean volume of abnormal brain regions controlled for age, sex, and total intracranial volume. MOH: medication-overuse headache; NC: normal control.
Figure 2.
The brain regions with volume alteration in patients of medication-overuse headache compared to normal controls. (a) Decreased volume in optic chiasm and left cerebellar vermal lobules VII X. (b) to (f) Increased volume in left calcarine, right lateral orbital gyrus, bilateral middle occipital gyrus, right superior parietal lobe, and right temporal transverse gyrus, respectively.
Brain-region volume differences in subgroup analyses
As shown in Figure 3, the subgroups analyses found a tendency that the volume gain was mainly contributed by patients without definite anxiety, depression, cognitive impairment, and of lower headache frequency except that of right superior parietal gyrus. The volume loss in left cerebellar vermal lobules VIII-X was also significant only in the subgroups without psychological comorbidities. The volume-gain regions tended to decrease and volume-loss region tended to increase but did not reach statistical significance in patients with psychological comorbidities and of higher headache frequency compared to those with no definite psychological comorbidities and of lower headache frequency. The results of subgroup analyses of different headache frequencies did not differ even adjusted by migraine duration.
Figure 3.
Brain-region volume differences of subgroup analyses. (a) Volume alteration in patients with (HAMA (1)) or without (HAMA (0)) definite anxiety compared with NC. (b) Volume differences in patients with (HAMD (1)) or without (HAMD (0)) definite depression compared with NC. (c) Volume differences in patients with (MoCA (1)) or without (MoCA (0)) cognitive impairment in comparison with NC. NC. (d) Volume differences in subgroups of different headache frequency compared with NC. NC: normal controls; HAMA: Hamilton Anxiety Scale score; HAMD: Hamilton Depression Scale score; MoCA: Montreal cognitive Assessment score; HF: headache frequency; L: left; R: right. * p < 0.05 in comparison with NC.
Correlation of clinical variables and abnormal brain regions
In the LF subgroup of MOH patients, correlation analyses found that the volume of left calcarine and right middle occipital gyrus had positive correlation with the gender of male. The volume of left cerebellar vermal lobules VIII-X had negative correlation with age. No correlation was found between the brain regions and other clinical variables.
Table 4 showed the correlation analyses in HF subgroup. Migraine duration was negatively associated with right lateral orbital gyrus and left middle occipital gyrus. Headache frequency was negatively associated with left calcarine and left middle occipital gyrus. Number of medications per month was negatively correlated with right superior parietal gyrus. HAMA was negatively correlated with left middle occipital gyrus. All the positive brain regions except optic chiasm had positive correlation with total intracranial volume.
Table 4.
Correlation analyses of brain-region volume with clinical variables in high frequency group.
| Items | L cerebellar vermal lobules VIII-X | L calcarine | R lateral orbital gyrus | L middle occipital gyrus | R middle occipital gyrus | R superior parietal gyrus | R temporal transverse gyrus | |
|---|---|---|---|---|---|---|---|---|
| Sex | r | 0.553 | 0.413 | |||||
| p | 0.001 | 0.019 | ||||||
| Age | r | −0.431 | −0.445 | −0.359 | ||||
| p | 0.014 | 0.011 | 0.044 | |||||
| Migraine duration (yrs) | r | −0.427 | −0.352 | |||||
| p | 0.015 | 0.048 | ||||||
| Headache frequency | r | −0.486 | −0.368 | −0.404 | ||||
| p | 0.005 | 0.038 | 0.022 | |||||
| Number of medication per month | r | −0.390 | ||||||
| p | 0.027 | |||||||
| HAMA | r | −0.425 | ||||||
| p | 0.015 | |||||||
| Total intracranial volume | r | 0.546 | 0.556 | 0.479 | 0.697 | 0.634 | 0.516 | 0.707 |
| p | 0.001 | 0.001 | 0.006 | 0.000 | 0.000 | 0.003 | 0.000 |
Note: Only the values of p < 0.05 were listed in the Pearson’s or Spearman’s correlation analyses. L: left; R: right. HAMA: Hamilton Anxiety Scale score.
In the regression analyses of all the MOH patients (Figure 4), migraine duration had negative association with the volume of left and right middle occipital gyrus and the number of medications per month had negative association with volume of right superior parietal lobe and lateral orbital gyrus. No correlation was found for brain regions of abnormal volume with MOH duration, headache frequency, VAS, HAMA, HAMD, or MoCA in the regression models.
Figure 4.
Correlation of clinical variables with brain regions in regression models (p < 0.05). (a) and (b) Negative association of migraine duration with bilateral middle occipital gyrus. (c) and (d) Negative association of number of medications per month with right superior parietal lobe and lateral orbital gyrus.R: right; L: left.
Discussion
This is the first study to analyze brain-region volume changes of whole brain for MOH patients overusing CCCA. MOH patients demonstrated decreased volume in cerebellum and optic chiasm, and increased volume in brain regions related to affective/cognitive processing, visual and auditory perception, and pain sensory/discrimination. The increased volume was primarily contributed by patients without anxiety, depression, or cognitive impairment, and of lower headache frequency (10–20 days/month). Correlation analyses found negative correlation of migraine duration and number of medications per month with some of these brain regions.
Previous voxel-based morphometry (VBM)12 or surface-based morphometry15 studies analyzed gray matter volume (GMV) or cortical thickness based on clusters but whether the more macroscopic volume of brain regions altered was unknown. Other volume studies adopted focal region of interest11 and could not reflect the more widespread volume alteration over the whole brain. Our study calculated intracranial volume by an automatic whole-brain algorithm of the brain regions extracted according to the neuromorphometrics template. By this method, we can find the volume alteration of each brain region throughout the whole brain compared with NC. The MOH patients with overused CCCA presented the most common MOH subtype in China and some other countries in Asia.6,16 Compared to previous studies with various overused medicine, our participants were more simply constituted and may minimize confounding factors caused by different kinds of overused medicine.
In contrast to previous VBM studies which commonly detected decreased GMV in the cortex,17 most of the positive brain regions in our studies had increased region volume, especially in the lower frequency subgroups and patents without psychological or cognitive comorbidities. Several points may contribute to this difference: Firstly, VBM studies detected focal microstructure change by the number of clusters and both increased and decreased clusters may occur simultaneously in a same brain region. Therefore, the VBM results and brain region volume may not be parallel. Secondly, MOH patients containing those overusing triptan or other kinds of medications may lead to a different pattern of volume alteration. Thirdly, the volume dynamically reduced during disease progression suggesting that the volume differences only reflected one stage of disease.
Although most studies reported decreased volume or thickness of regions related to pain, a few studies also found increased cortical thickness or volume. Thicker cortical areas have been reported in somatosensory cortex,18,19 middle frontal gyrus,19 motion-processing visual areas,20 left hippocampal21 in episodic migraine (EM). GMV increase was reported in amygdale, putamen in chronic migraine.22 For MOH, increased cortifical gyrification was observed in fusiform cortex, medial temporal regions, right occipital pole15 and increased GMV was found in periaqueductal gray matter, thalamus, and ventral striatum.11,23
One study found significantly thicker cortex of postcentral gyri and temporal lobe in high frequency group (8–14 days/month) and NC than low frequency group (<2 days/month) in EM.24 Another study found that increased thickness of bilateral postcentral gyrus positively correlated with disease duration and headache frequency in migraine.19 These findings implicated that some cortical thickness of migraine may reduce in early stage of migraine and thicken in more advanced stage. In our study, the volume of most abnormal brain regions increased in lower frequency (10–20 days/month) of MOH and in patients without anxiety or depression (Figure 3) but decreased with disease progression indicating that cortical volume may reach to peak at early stage of migraine chronicity but decrease with even more headache attacks and number of medications as well as accompanied psychological disorders (Table 4 and Figure 4). The LF subgroup did not show any correlation of headache or psychological variables with abnormal brain regions, perhaps because of limited sample number. Hippocampus and amygdala volumes were reported to have a similar pattern of dynamic change related to migraine frequency.25
The mechanisms of GMV changes are unclear so far. GM changes were considered to be related to neuronal or glial cell genesis or degeneration; increases/decreases in cell size, spine, or synapse turnover; neurogenic inflammation or excitotoxicity; changes in blood flow or interstitial fluids, and may reflect a combination of these.26 The volume or density of a brain region may increase and decrease in different headache frequency,24,25 during and between migraine attacks,27 and response to treatment28 suggesting complicated structural plasticity. It is hard to specify why the GMV had such changes related to the clinical variables. The clinical varieties and dynamic volume changes may lead to the diversities of altered brain areas in previous studies.
Pain is thought to be due to complex central processing of ascending signals and descending modulatory pathways.29 Dura-sensitive thalamic neurons have been identified in animal models to project in multiple directions into anatomically distinct cortical areas of affect, motor capacity, visual and auditory perception, spatial orientation, memory retrieval, and olfaction, which proposed a framework for conceptualizing migraine headache and its associated symptoms.30 Imaging studies also identified altered structure and function in some of the abovementioned regions for migraine and MOH patients.17,31 In our study, we found altered volume in cerebellum and regions related to affect and cognitive processing (right lateral orbital gyrus), visual (left calcarine, bilateral middle occipital gyrus, right superior parietal lobe, optic chiasm), and auditory (right temporal transverse gyrus) perception, and pain sensory (right superior parietal lobe) dimensions of migraine. Cerebellum is involved in processing sensorimotor, cognitive, and affective information.32 Cerebellum volume decrease was also found in chronic migraine patients.12 Orbital gyrus, as the core of the mesocorticolimbic dopaminergic circuit (reward pathways), plays important roles in affect modulation, reward learning, decision making, and drug addiction.33,34 Orbital gyrus has shown lower metabolism, altered activity and volume decrease in MOH patients12,23,35 in previous studies. The volume of orbital gyrus in this study was negatively correlated with number of medications per month, indicating an important role in the pathogenesis of MOH.
In agreement with a previous migraine study,20 we found altered volume in visual association processing areas. The occipital lobe is the visual information processing center and is responsible for part of language, action feeling, and abstract concepts based on the functional connection to the frontal-parietal network in task goals and the presence of irrelevant information.36 Alteration of these structures may present as photophobia, light sensitivity, deficits of visual information processing and visual attention in migraine. Altered volume and functional activity of middle occipital gyrus have been reported in generalized anxiety disorder,37 MOH,12 and dependence problems such as gambling disorder.38 Cortical spreading depression (CSD) is considered as the mechanism of migraine aura, however, migraine without aura could also be the result of a “silent” CSD-like event initiated from occipital cortex.39,40 Therefore, the altered visual information processing areas might be the result of repeated migraine attack and/or accompanied mood/cognitive dysfunction in MOH, but vice versa is also possible. Interestingly, we found decreased volume of optic chiasm. A previous study adopted diffusion tensor imaging to study the white matter of migraine patients and revealed significantly lower fractional anisotropy values in the white matter subjacent to area V3A, in superior colliculus and the lateral geniculate nucleus, indicating loss of myelin or increased axonal diameter with increased myelin of the white matter involved in visual processing.20 The nonimage forming visual pathway has been defined to arise from the optic chiasm to the pulvinar, and from the pulvinar to several associative cortical brain regions. Such pathway may allow photic signals to converge on a thalamic region to be selectively activated during migraine headache and provide an anatomical substrate for exacerbation of migraine headache by light in the human.41
The prominent finding in the volume alteration of multiple visual processing regions may be related to the overused type of medicine in this study. Caffeine can accentuate global processing of visual attention on the hierarchical shape task and improve rapid visual information processing accuracy.42,43 Besides, acetylsalicylic acid decreased latency and reaction time of visually evoked event-related potentials, possibly via the serotonergic transmitter system.44 NSAIDs showed neuroprotective and memory enhancing effect against chlorpromazine induced Parkinson’s model.45 The increased volume of visual processing regions at lower frequency of medication overuse and negative association with migraine duration supported that lower frequency of caffeine and analgesics may improve visual cognitive processing but excessive overuse of them may remodel the brain possibly due to desensitization of receptors.
Our study has some limitations. First, we did not compare the brain structural differences of overusing CCCA with other types of medications since very few patients overused other medications in China. We need longer time to recruit those patients. Second, EM patients were not included in this study. Dynamic volume changes can be better presented with different frequency of headache attack and medication intake. Third, functional study was not included in this study. Multimodel imaging analyses may be considered in future studies to explore the pathogenesis of MOH more comprehensively. Fourth, longitudinal study is needed to reveal the structural alteration after drug withdrawal.
Conclusions
Brain regions involved in affective and cognitive processing, visual and auditory perception, and pain sensory/discrimination were found to have volume alteration. The altered volume of multiple brain regions related to visual processing may be a specific feature in our study. Volume increase of most abnormal brain regions occurred in patients with lower headache frequency (10–20 days/month), less anxiety/depression, no cognitive impairment, and some had negative correlation with headache variables, which may reflect complicated neural plasticity of MOH overusing CCCA.
Authors’ Contributions
Category 1: (a) Conception and design: Shengyuan Yu. (b) Acquisition of data: Xiaoyan Chen, Zhiye Chen, Mengqi Liu, and Zhao Dong. (c) Analysis and interpretation of data: Xiaoyan Chen and Zhiye Chen. Category 2: (a) Drafting the article: Xiaoyan Chen and Zhiye Chen. (b) Revising it for intellectual content: Shengyuan Yu. All authors read and approved the final manuscript.
Declaration of Conflicting Interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by Hainan Provincial Natural Science Foundation of China (818MS153), the Special Financial Grant from the China Postdoctoral Science Foundation (2014T70960), the Foundation for Medical and Health Sci & Tech innovation Project of Sanya (2016YW37) and the Nursery Technology Innovation Fund of Chinese PLA General Hospital (12KMM39).
References
- 1.Group GBDNDC. Global, regional, and national burden of neurological disorders during 1990–2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet Neurol 2017; 16: 877–897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Headache Classification Committee of the International Headache Society. The International Classification of Headache Disorders, 3rd edition. Cephalalgia 2018; 38: 1–211. [DOI] [PubMed] [Google Scholar]
- 3.Find NL, Terlizzi R, Munksgaard SB, Bendtsen L, Tassorelli C, Nappi G, Katsarava G, Lainez M, Goicochea MT, Shand B, Fadic R, Spadafora S, Pagani M, Jensen R; for COMOESTAS Consortium. Medication overuse headache in Europe and Latin America: general demographic and clinical characteristics, referral pathways and national distribution of painkillers in a descriptive, multinational, multicenter study. J Headache Pain 2015; 17: 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kluonaitis K, Petrauskiene E, Ryliskiene K. Clinical characteristics and overuse patterns of medication overuse headache: retrospective case-series study. Clin Neurol Neurosurg 2017; 163: 124–127. [DOI] [PubMed] [Google Scholar]
- 5.Ravishankar K. Medication overuse headache in India. Cephalalgia 2008; 28: 1223–1226. [DOI] [PubMed] [Google Scholar]
- 6.Dong Z, Chen X, Steiner TJ, Di H, He M, Dai W, Pan M, Zhang M, Liu R, Yu S. Medication-overuse headache in China: clinical profile, and an evaluation of the ICHD-3 beta diagnostic criteria. Cephalalgia 2015; 35: 644–651. [DOI] [PubMed] [Google Scholar]
- 7.Lipton RB, Diener HC, Robbins MS, Garas SY, Patel K. Caffeine in the management of patients with headache. J Headache Pain 2017; 18: 107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cappelletti S, Piacentino D, Sani G, Aromatario M. Caffeine: cognitive and physical performance enhancer or psychoactive drug? Curr Neuropharmacol 2015; 13: 71–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Juliano LM, Huntley ED, Harrell PT, Westerman AT. Development of the caffeine withdrawal symptom questionnaire: caffeine withdrawal symptoms cluster into 7 factors. Drug Alcohol Depend 2012; 124: 229–234. [DOI] [PubMed] [Google Scholar]
- 10.Chanraud S, Di Scala G, Dilharreguy B, Schoenen J, Allard M, Radat F. Brain functional connectivity and morphology changes in medication-overuse headache: clue for dependence-related processes? Cephalalgia 2014; 34: 605–615. [DOI] [PubMed] [Google Scholar]
- 11.Chen Z, Chen X, Liu M, Liu S, Ma L, Yu S. Volume gain of periaqueductal gray in medication-overuse headache. J Headache Pain 2017; 18: 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lai TH, Chou KH, Fuh JL, Lee PL, Kung YC, Lin CP, Wang SJ. Gray matter changes related to medication overuse in patients with chronic migraine. Cephalalgia 2016; 36: 1324–1333. [DOI] [PubMed] [Google Scholar]
- 13.Maier W, Buller R, Philipp M, Heuser I. The Hamilton Anxiety Scale: reliability, validity and sensitivity to change in anxiety and depressive disorders. J Affect Disord 1988; 14: 61–68. [DOI] [PubMed] [Google Scholar]
- 14.Hamilton M. Development of a rating scale for primary depressive illness. Br J Soc Clin Psychol 1967; 6: 278–296. [DOI] [PubMed] [Google Scholar]
- 15.Riederer F, Schaer M, Gantenbein AR, Luechinger R, Michels L, Kaya M, Kollias S, Sándor PS. Cortical alterations in medication-overuse headache. Headache 2017; 57: 255–265. [DOI] [PubMed] [Google Scholar]
- 16.Kanki R, Nagaseki Y, Sakai F. Medication-overuse headache in Japan. Cephalalgia 2008; 28: 1227–1228. [DOI] [PubMed] [Google Scholar]
- 17.Jia Z, Yu S. Grey matter alterations in migraine: a systematic review and meta-analysis. Neuroimage Clin 2017; 14: 130–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.DaSilva AF, Granziera C, Snyder J, Hadjikhani N. Thickening in the somatosensory cortex of patients with migraine. Neurology 2007; 69: 1990–1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kim JH, Kim JB, Suh SI, Seo WK, Oh K, Koh SB. Thickening of the somatosensory cortex in migraine without aura. Cephalalgia 2014; 34: 1125–1133. [DOI] [PubMed] [Google Scholar]
- 20.Granziera C, DaSilva AF, Snyder J, Tuch DS, Hadjikhani N. Anatomical alterations of the visual motion processing network in migraine with and without aura. PLoS Med 2006; 3: e402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hubbard CS, Khan SA, Keaser ML, Mathur VA, Goyal M, Seminowicz DA. Altered brain structure and function correlate with disease severity and pain catastrophizing in migraine patients. eNeuro 2014; 1: e20.14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Neeb L, Bastian K, Villringer K, Israel H, Reuter U, Fiebach JB. Structural gray matter alterations in chronic migraine: implications for a progressive disease? Headache 2017; 57: 400–416. [DOI] [PubMed] [Google Scholar]
- 23.Riederer F, Marti M, Luechinger R, Lanzenberger R, von Meyenburg J, Gantenbein AR, Pirrotta R, Gaul C, Kollias S, Sándor PS. Grey matter changes associated with medication-overuse headache: correlations with disease related disability and anxiety. World J Biol Psychiatr 2012; 13: 517–525. [DOI] [PubMed] [Google Scholar]
- 24.Maleki N, Becerra L, Brawn J, Bigal M, Burstein R, Borsook D. Concurrent functional and structural cortical alterations in migraine. Cephalalgia 2012; 32: 607–620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liu HY, Chou KH, Lee PL, Fuh JL, Niddam DM, Lai KL, Hsiao FJ, Lin YY, Chen WT, Wang SJ, Lin CP. Hippocampus and amygdala volume in relation to migraine frequency and prognosis. Cephalalgia 2017; 37: 1329–1336. [DOI] [PubMed] [Google Scholar]
- 26.May A. Experience-dependent structural plasticity in the adult human brain. Trends Cogn Sci (Regul Ed) 2011; 15: 475–482. [DOI] [PubMed] [Google Scholar]
- 27.Coppola G, Di Renzo A, Tinelli E, Iacovelli E, Lepre C, Di Lorenzo C, Di Lorenzo G, Di Lenola D, Parisi V, Serrao M, Pauri F, Fiermonte G, Bianco F, Pierelli F. Evidence for brain morphometric changes during the migraine cycle: a magnetic resonance-based morphometry study. Cephalalgia 2015; 35: 783–791. [DOI] [PubMed] [Google Scholar]
- 28.Riederer F, Gantenbein AR, Marti M, Luechinger R, Kollias S, Sandor PS. Decrease of gray matter volume in the midbrain is associated with treatment response in medication-overuse headache: possible influence of orbitofrontal cortex. J Neurosci 2013; 33: 15343–15349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Quintero GC. Advances in cortical modulation of pain. J Pain Res 2013; 6: 713–725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Noseda R, Jakubowski M, Kainz V, Borsook D, Burstein R. Cortical projections of functionally identified thalamic trigeminovascular neurons: implications for migraine headache and its associated symptoms. J Neurosci 2011; 31: 14204–14217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schwedt TJ, Chong CD. Medication overuse headache: pathophysiological insights from structural and functional brain MRI research. Headache 2017; 57: 1173–1178. [DOI] [PubMed] [Google Scholar]
- 32.Timmann D, Drepper J, Frings M, Maschke M, Richter S, Gerwig M, Kolb FP. The human cerebellum contributes to motor, emotional and cognitive associative learning. A review. Cortex 2010; 46: 845–857. [DOI] [PubMed] [Google Scholar]
- 33.Kahnt T, Chang LJ, Park SQ, Heinzle J, Haynes JD. Connectivity-based parcellation of the human orbitofrontal cortex. J Neurosci 2012; 32: 6240–6250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lucantonio F, Stalnaker TA, Shaham Y, Niv Y, Schoenbaum G. The impact of orbitofrontal dysfunction on cocaine addiction. Nat Neurosci 2012; 15: 358–366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fumal A, Laureys S, Di Clemente L, Boly M, Bohotin V, Vandenheede M, Coppola G, Salmon E, Kupers R, Schoenen J. Orbitofrontal cortex involvement in chronic analgesic-overuse headache evolving from episodic migraine. Brain 2006; 129: 543–550. [DOI] [PubMed] [Google Scholar]
- 36.Chadick JZ, Gazzaley A. Differential coupling of visual cortex with default or frontal-parietal network based on goals. Nat Neurosci 2011; 14: 830–832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Moon CM, Jeong GW. Functional neuroanatomy on the working memory under emotional distraction in patients with generalized anxiety disorder. Psychiatr Clin Neurosci 2015; 69: 609–619. [DOI] [PubMed] [Google Scholar]
- 38.Meng YJ, Deng W, Wang HY, Guo WJ, Li T, Lam C, Lin X. Reward pathway dysfunction in gambling disorder: a meta-analysis of functional magnetic resonance imaging studies. Behav Brain Res 2014; 275: 243–251. [DOI] [PubMed] [Google Scholar]
- 39.Cao Y, Welch KM, Aurora S, Vikingstad EM. Functional MRI-BOLD of visually triggered headache in patients with migraine. Arch Neurol 1999; 56: 548–554. [DOI] [PubMed] [Google Scholar]
- 40.Woods RP, Iacoboni M, Mazziotta JC, Brief R. Bilateral spreading cerebral hypoperfusion during spontaneous migraine headache. N Engl J Med 1994; 331: 1689–1692. [DOI] [PubMed] [Google Scholar]
- 41.Maleki N, Becerra L, Upadhyay J, Burstein R, Borsook D. Direct optic nerve pulvinar connections defined by diffusion MR tractography in humans: implications for photophobia. Hum Brain Mapp 2012; 33: 75–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Giles GE, Mahoney CR, Brunye TT, Taylor HA, Kanarek RB. Caffeine and theanine exert opposite effects on attention under emotional arousal. Can J Physiol Pharmacol 2017; 95: 93–100. [DOI] [PubMed] [Google Scholar]
- 43.Haskell CF, Kennedy DO, Milne AL, Wesnes KA, Scholey AB. The effects of L-theanine, caffeine and their combination on cognition and mood. Biol Psychol 2008; 77: 113–122. [DOI] [PubMed] [Google Scholar]
- 44.Austermann M, Grotemeyer KH, Evers S, Rodding D, Husstedt IW. The influence of acetylsalicylic acid on cognitive processing: an event-related potentials study. Psychopharmacology (Berl) 1998; 138: 369–374. [DOI] [PubMed] [Google Scholar]
- 45.Naeem S, Ikram R, Khan SS, Rao SS. NSAIDs ameliorate cognitive and motor impairment in a model of parkinsonism induced by chlorpromazine. Pakistan J Pharma Sci 2017; 30: 801–808. [PubMed] [Google Scholar]