Abstract
Purpose:
Melanoma is a lethal skin cancer with unmet clinical needs for targeted imaging and therapy. Nanoscale materials conjugated with targeting components have shown great potential to improve tumor delivery efficiency while minimizing undesirable side effects in vivo. Herein, we proposed to develop targeted nanoparticles for melanoma theranostics.
Method:
In this work, gold nanocages (AuNCs) were conjugated with α-melanocyte-stimulating hormone (α-MSH) peptide and radiolabeled with 64Cu for melanocortin 1 receptor-(MC1R) targeted positron emission tomography (PET) in a mouse B16/F10 melanoma model.
Results:
Their controlled synthesis and surface chemistry enabled well-defined structure and radiolabeling efficiency. In vivo pharmacokinetic evaluation demonstrated comparable organ distribution between the targeted and nontargeted AuNCs. However, micro-PET/computed tomography (CT) imaging demonstrated specific and improved tumor accumulation via MC1R-mediated delivery. By increasing the coverage density of α-MSH peptide on AuNCs, the tumor delivery efficiency was improved.
Conclusion:
The controlled synthesis, sensitive PET imaging, and optimal tumor targeting suggested the potential of targeted AuNCs for melanoma theranostics.
Keywords: gold nanocage, positron emission tomography, melanoma, melanocortin 1 receptor, α-melanocyte-stimulating hormone
Introduction
Skin cancer is the fifth most common cancer in the United States.1 Of various dermatologic malignancies, melanoma accounts for only 1% of all the incidence but approximately 75% of the death in the case of skin cancer.2 With the improvement in treatment strategies, malignant melanoma can be effectively treated upon early detection. However, the prognosis upon onset of metastasis is poor, as reflected by a 5-year survival rate of only 13% once distant malignancy has occurred.3 Thus, early and sensitive detection of melanoma is of paramount importance in improving prognosis and patient survival. In addition, malignant melanoma is extraordinarily resistant to chemotherapy, immunotherapy, and external beam radiation therapy. Thus, new therapeutics are urgently needed for melanoma.
Nanoparticles, due to the size-promoted enhanced permeability and retention effect in leaky tumor vasculature, have been widely used for cancer imaging and therapy. Moreover, the high surface area to volume ratio of nanoparticles also enables the conjugation of targeting ligands, loading/encapsulation of therapeutic payloads, and labeling of diagnostic elements. Nanoparticles have been widely studied as multivalent platforms for cancer theranostics.4-6 Of them, the gold nanoparticles with photothermal capability hold great promise as a theranostic platform for image-guided cancer therapy.7-11 In our previous studies, we have examined the pharmacokinetics, tumor targeting, and intratumoral distribution of a variety of gold nanostructures with different sizes and shapes.12-15 Gold nanocage (AuNCs), due to their efficient photothermal conversion and favorable biodistribution, are a unique type of nanostructure for targeted cancer nanomedicine.12,13,15,16 Among the various targets assessed in melanoma detection, melanocortin 1 receptor (MC1R) has been widely studied due to its critical role in the incidence and progression of melanoma, making it a valid biomarker for the diagnosis of not only primary tumors but also metastasis in distant organs.17,18 By conjugating [Nle4, D-Phe7]-α-melanocyte-stimulating hormone (NDP-α-MSH), Lu et al have demonstrated receptor-mediated active targeting and efficient photothermal ablation of melanoma with gold nanoshells in a murine tumor model.19 However, the imaging potential of these targeted nanostructures for melanoma has not been fully realized as a reference to guide the treatment. Previously, we studied the photoacoustic imaging of melanoma using NDP-α-MSH-conjugated AuNCs (ca 60 nm in size) in a mouse B16 model showing sensitive and specific detection of tumors.20 However, the pharmacokinetics and tumor targeting efficiency of AuNCs need further optimization to enhance its theranostic potential.
Herein, we prepared AuNCs of approximately 40 nm in size for MC1R-targeted imaging in a mouse B16/F10 melanoma model. Through 64Cu radiolabeling, we examined the organ distribution profile of AuNCs and compared the tumor imaging efficiency of AuNCs conjugated with various amounts of NDP-α-MSH peptide.
Experimental
Chemicals and Reagents
Orthopyridyldisulfide-polyethylene glycol-N-hydroxysuccinimide (OPSS-PEG-SVA, MW ≈ 5000) and poly(ethylene glycol)monomethyl ether thiol (MW ≈ 5000) were both obtained from Laysan Bio (Arab, Alabama). S-2-(4-Aminobenzyl)-1,4,7,10-tetraazacyclododecane tetraacetic acid (NH2-Bn-DOTA) was purchased from Macrocyclics (Dallas, Texas). [Nle4, D-Phe7-Lys(Dde)]-α-MSH [NDP-MSH (Dde)-α-NH2] peptide was customized by CPC Scientific (Sunnyvale, California). Other chemicals and reagents, including poly(vinylpyrrolidone) (PVP, MW ≈ 55 000), gold (III) chloride trihydrate (HAuCl4.3H2O), ascorbic acid, sodium chloride (NaCl), and phosphate-buffered saline (PBS) were obtained from Sigma-Aldrich (St. Louis, Missouri). All chemicals were used as received unless specified.
Synthesis of Gold Nanocages
Gold nanocages covered with PVP were prepared using a galvanic replacement reaction between Ag nanocubes and HAuCl4 following a previously reported protocol.21 The reaction was frequently monitored by measuring the localized surface plasmon resonance (LSPR) peaks with UV-Vis spectroscopy (Cary 50 spectrometer, Varian, Palo Alto, California) during the reaction. The reaction was stopped when the LSPR reached approximately 800 nm. After washing with saturated NaCl solution, the AuNCs were collected by centrifugation at 15 000×g for 15 minutes, and purified by washing with MilliQ water 3 times.
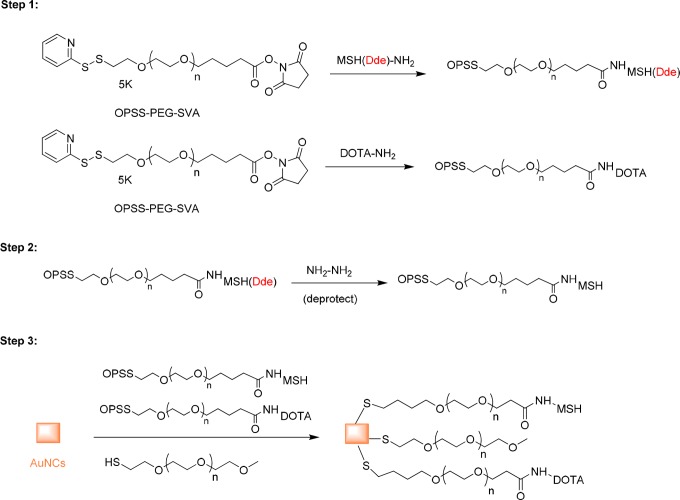
Preparation of OPPS-PEG-MSH
The OPSS-PEG-MSH molecule was prepared by coupling the primary amine group on the NDP-α-MSH peptide to OPSS-PEG-SVA (Figure 1). The amine-reactive OPSS-PEG-SVA was mixed with NDP-α-MSH (Dde)-NH2 peptide in PBS (pH 7.4) at a molar ratio of 1:5 and allowed to react at 4°C overnight. The solution containing the OPSS-PEG-MSH(Dde) conjugate was desalted with a centrifuge filter (Amicon, 3 kDa) and washed with MilliQ water. The concentrated solution was recovered and lyophilized. The resulting solid was added to 2% hydrazine in N,N-dimethylformamide (DMF).19 After 1 hour incubation at room temperature, the OPPS-PEG-MSH was precipitated by adding diethyl ether and collected by centrifugation at 22 000×g for 5 minutes. The supernatant was removed, and the remaining pellet was washed with diethyl ether. The pellet was further dried by air and stored at −20°C for future use.
Figure 1.
Scheme for the preparation of AuNC-PEG-MSH.
Preparation of OPPS-PEG-DOTA
The OPSS-PEG-DOTA molecule was prepared by coupling the primary amine group on DOTA-NH2 with OPSS-PEG-SVA. The amine-reactive OPSS-PEG-SVA was mixed with DOTA-NH2 in pH 7.4 PBS buffer at a molar ratio of 1:5 and allowed to react at 4°C overnight. The solution containing the OPSS-PEG-DOTA was desalted with a centrifuge filter (Amicon, 3 kDa) and washed with MilliQ water.
Conjugation of PEG, PEG-DOTA, and PEG-MSH to AuNCs
The MSH peptide conjugated AuNCs (MSH-PEG-AuNCs) were prepared by adding AuNCs solution to a mixture of OPSS-PEG-MSH, OPPS-PEG-DOTA, and HS-PEG-OMe (MW ≈ 5000; OPSS-PEG-MSH:OPPS-PEG-DOTA:HS-PEG-OMe = 1:4:4) with a total PEG to AuNCs molar ratio of 105 to 1 (Figure 1). The mixture was allowed to incubate at 4°C overnight on a shaker. The conjugated AuNCs were purified by centrifugation at 11 000×g for 15 minutes. The pellet was redispersed in MilliQ water and centrifuged again. For AuNCs conjugated with a high density of MSH, the same conjugation strategy was used but with an elevated molar ratio of OPSS-PEG-MSH versus other PEG components (OPSS-PEG-MSH:OPPS-PEG-DOTA:HS-PEG-OMe = 1:1:1).
Comparison of Photothermal Effects of Nontargeted AuNCs-PEG and Targeted AuNCs-PEG-MSH
Aqueous suspensions of the nontargeted AuNCs-PEG and targeted AuNCs-PEG-MSH at identical concentrations were placed on a 96-well plate and irradiated with a diode laser (λ = 808 nm, Power Technology, Alexander, Arkansas) directly from above at a power density of 1.1 W/cm2. The temperature changes were monitored using a NIR camera (ICI7320, Infrared Camera, Beaumont, Texas) placed on top of the suspension at 6-second intervals. The average temperature of the suspension was determined from the thermograph using IR Flash software (Infrared Camera, version 2.10) at each time point.
Radiolabeling of AuNCs-PEG-MSH and AuNCs-PEG
64Cu (t1/2 = 12.7 h, β+ =17%, β− = 40%) was produced on the Washington University Medical School CS-15 cyclotron by the 64Ni (p, n) 64Cu nuclear reaction. AuNCs-PEG-MSH or AuNCs-PEG (about 6 pmol) were incubated with 185 MBq 64Cu2+ in 0.1 M ammonium acetate buffer (pH 5.5) at 45°C for 1 hour to achieve maximal labeling-specific activity. The 64Cu-AuNCs-PEG-MSH or 64Cu-AuNCs-PEG were dissolved in MilliQ water and purified by centrifugation at 15 000×g for 15 minutes followed by filtration through a 0.22 μmol/L syringe filter. After ethylenediaminetetraacetic acid (EDTA, 10 mmol/L in 50 mmol/L pH 7.4 phosphate buffer) challenge, the radiochemical purity of radiolabeled AuNCs was measured by radio instant thin layer chromatography (radio-iTLC, Bioscan, Washington, DC).
Biodistribution of 64Cu-AuNCs-PEG-MSH or 64Cu-AuNCs-PEG
All animal studies were performed in compliance with guidelines set forth by the NIH Office of Laboratory Animal Welfare and approved by the Washington University Animal Studies Committee. Biodistribution studies were performed in male C57BL/6 mice weighing 20 to 25 g (n = 4, Charles River Laboratory, Wilmington, Massachusetts) with 0.37 MBq (0.057 pmol) of 64Cu-AuNCs-PEG-MSH or 64Cu-AuNCs-PEG in 100 μL of saline (APP Pharmaceuticals, Schaumburg, Illinios) injected via the tail vein. The mice were anesthetized with inhaled isoflurane during tracer injection and reanesthetized before euthanasia by cervical dislocation at each time point. Organs of interest were collected, weighed, and counted with a Beckman 8000 gamma counter (Beckman, Brea, California). Standards were prepared and measured along with the samples to calculate the percentage of the injected dose per gram of tissue (%ID/g).10,22
Positron Emission Tomography/Computed Tomography of 64Cu-AuNCs-PEG-MSH
The B16/F10 cell line was obtained from the American Type Culture Collection (ATCC, Manassas, Virginia). The cells were cultured in Dulbecco’s Modified Eagle’s medium supplemented with 10% fetal bovine serum and 1% penicillin/streptomycin and incubated at 37°C in a humidified atmosphere of 5% CO2. Ten days after 5 ×105 B16/F10 cells were implanted, nude mice were anesthetized with isoflurane and injected with 3.7 MBq (0.57 pmol) of 64Cu-AuNCs-PEG-MSH in 100 μL of saline via the tail vein. MicroPET scans were performed on either microPET Focus 220 (Siemens, Malvern, Pennslyvania) or Inveon PET/CT system (Siemens) at 4, 24, and 48 hours postinjection. The competitive receptor blocking study was performed by coinjection of nonradiolabeled AuNCs-PEG-MSH and 64Cu-AuNCs-PEG-MSH with a molar ratio of 35:1. The PET images were corrected for attenuation, scatter, normalization, and camera dead time and coregistered with CT images. Both PET scanners were cross-calibrated periodically. The PET images were reconstructed with the maximum a posteriori (MAP) algorithm and analyzed by ASIPro (Siemens Medical solutions, Knoxville, Tennessee). The tumor uptake of the radiolabeled AuNCs was calculated as %ID/g in 3-dimensional regions of interest.
Histology and Immunohistochemistry
Sections of tumor in 5 μm thickness were cut from paraformaldehyde-fixed (24 hours), paraffin-embedded specimens. The sections were deparaffinized and rehydrated through a series of xylenes and graded alcohols before undergoing antigen retrieval pretreatment (10 mmol/L Tris, 1 mmol/L EDTA, 0.05% Tween, pH 9.0, for 10 minutes). They were treated with 0.3% H2O2 for 30 minutes, followed by blocking serum for 1 hour to prevent nonspecific binding (Vectastain, Vector Laboratories, Burlingame, California). The sections were then incubated overnight at 4°C with primary antibody (anti-melanin, 1:100 in blocking serum, Santa Cruz Biotechnology, Dallas, Texas). Secondary antibody was applied (Vector Laboratories), and blue color development was achieved using an alkaline phosphatase-based immunostaining kit (Vector Laboratories).
Statistical Analysis
Group variation is described as the mean (standard deviation). Group comparisons were made using the student t test. The significance level in all tests was P ≤ .05. GraphPad Prism v. 6.04 (La Jolla, California) was used for all statistical analyses.
Results and Discussion
Synthesis and Characterization of AuNCs-PEG-MSH
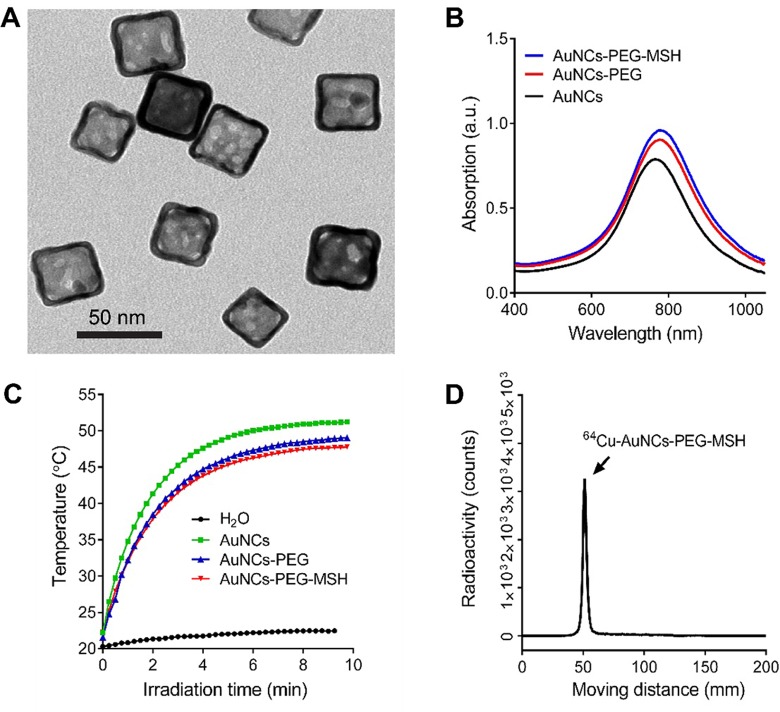
Previously, we used 50-nm AuNCs conjugated with [Nle4, D-Phe7]-α-melanocyte-stimulating hormone (NDP-α-MSH) peptide for melanoma photoacoustic imaging and showed sensitive and specific detection of MC1R in a mouse B16 model.20 However, the large size of AuNCs led to unfavorable in vivo pharmacokinetics and high uptake by the mononuclear phagocyte system (MPS: liver, and spleen). Thus, we prepared MC1R-targeted AuNCs-PEG-MSH using AuNCs with an edge length of 35 nm and a wall thickness of 3.6 nm (Figure 2A) by following the procedure outlined in Figure 1. Due to the well-defined structure and inherent optical properties of AuNCs, the surface modification did not change the LSPR peak of the AuNCs, which was illustrated by the almost identical LSPR peaks around 790 nm for the unmodified AuNCs, AuNCs-PEG, and AuNCs-PEG-MSH shown in Figure 2B.20 Moreover, as shown in Figure 2C, the 3 samples of nanocages also displayed comparable efficiencies for the photothermal effect to convert light to heat, suggesting no aggregation during PEGylation.19 Following our previous strategy for quantifying the PEGylation density,13 the number of NDP-α-MSH peptides conjugated with the AuNCs was estimated to be approximately 1800 copies per AuNC. These nanocages were then efficiently radiolabeled with 64Cu2+ through the DOTA chelator conjugated with the AuNCs, enabling high-specific activity (6.5 MBq/pmol) and trace amount (0.057 and 0.57 pmol for biodistribution and PET imaging, respectively) administration for in vivo applications. After centrifugal purification, the radiochemical purity of 64Cu-AuNCs-PEG-MSH (> 95% pure) was confirmed by radio-iTLC (Figure 2D) prior to intravenous injection.
Figure 2.
Characterization of gold nanocages. A, Transmission electron microscopy image of AuNC-PEG-MSH. B, UV-Vis spectra of unmodified gold nanocages (AuNCs), AuNCs-PEG, and AuNCs-PEG-MSH showing similar LSPR peaks. C, Plots of temperatures as a function of irradiation time for suspension of H2O, AuNCs, AuNCs-PEG, AuNCs-PEG-MSH. The concentration (0.025 nmol/L) of each suspension had the same maximum extinction intensity. The laser power density was 1.1 W/cm3. D, Instant radio-thin layer chromatogram (iTLC) of purified 64Cu-AuNCs-PEG-MSH.
Biodistribution of AuNCs-PEG-MSH
Biodistribution studies of the purified 64Cu-AuNCs-PEG-MSH and 64Cu-AuNCs-PEG were conducted in wild-type C57BL/6 mice at 4 and 24 hours postinjection. As shown in Figure 3, the targeted and nontargeted nanocages showed comparable in vivo organ distribution profiles at the 2 time points. Both nanocages showed high blood retentions around 20% ID/g (20.30 ± 1.84%ID/g for 64Cu-AuNCs-PEG and 19.89 ± 2.52%ID/g for 64Cu-AuNCs-PEG-MSH) at 4 hours, followed by rapid decrease to less than 2%ID/g at 24 hours, which was also verified by the variations in other blood pool organs including heart and lungs. The high blood retention of both targeted and nontargeted nanocages indicates high efficiency of surface modification and is consistent with our previous study for nontargeted nanocages.13 Although the uptake in MPS system was at least 2 times less than their bigger counterpart,13 due to the heavy metal nature and sizes,23,24 both nanocages still had significant liver and spleen accumulation but slightly different patterns. In contrast to the slightly increased liver accumulations (12.4 ± 2.66%ID/g at 4 hours to 19.2 ± 0.91%ID/g at 24 hours) of 64Cu-AuNCs-PEG, the hepatic uptake of 64Cu-AuNCs-PEG-MSH was relatively stable during the 24-hour study (15.1 ± 3.92%ID/g and 17.6 ± 1.77%ID/g at 4 hours and 24 hours, respectively). Although the spleen accumulations of both nanocages were comparable to those in liver at 4 hours, both significantly increased at 24-hour time point (86.9 ± 10.7%ID/g and 90.3 ± 10.9%ID/g for 64Cu-AuNCs-PEG-MSH and 64Cu-AuNCs-PEG, respectively, P < .0001 for both, n = 4/group), which was also consistent with our previous report about the size effect of nanoparticles in spleen accumulation.25,26 Owing to the similar in vivo pharmacokinetics of the 2 nanocages, the nontargeted 64Cu-AuNCs-PEG could serve as a useful control to study the tumor targeting effect enabled by the conjugation of α-MSH peptide.
Figure 3.
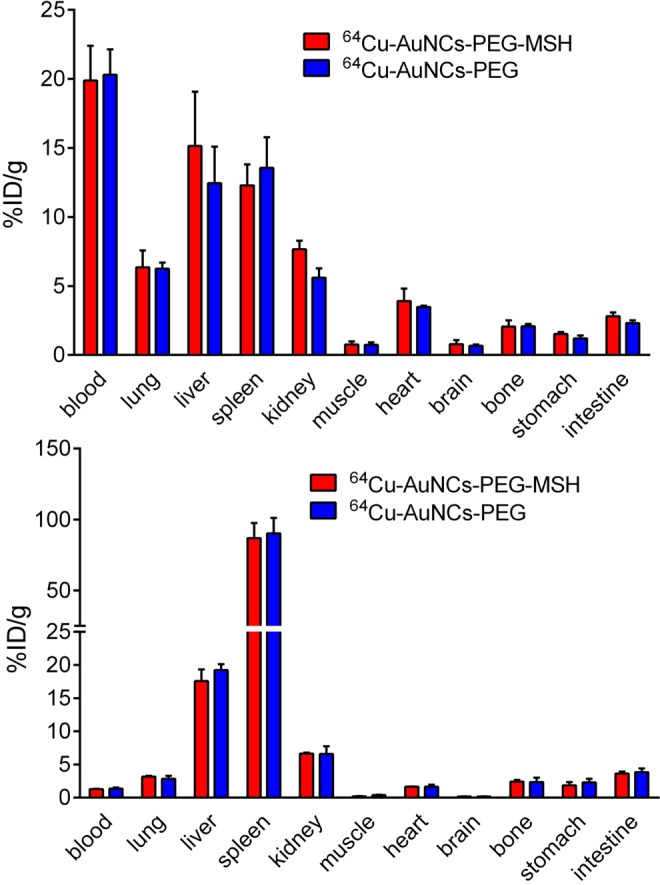
In vivo biodistribution of 64Cu-AuNCs-PEG-MSH and 64Cu-AuNCs-PEG in wild-type C57BL/6 mice at 4 (top panel) and 24 hours (bottom panel) (n = 4/group) postintravenous injection.
Positron Emission Tomography/Computed Tomography Imaging of AuNCs-PEG-MSH
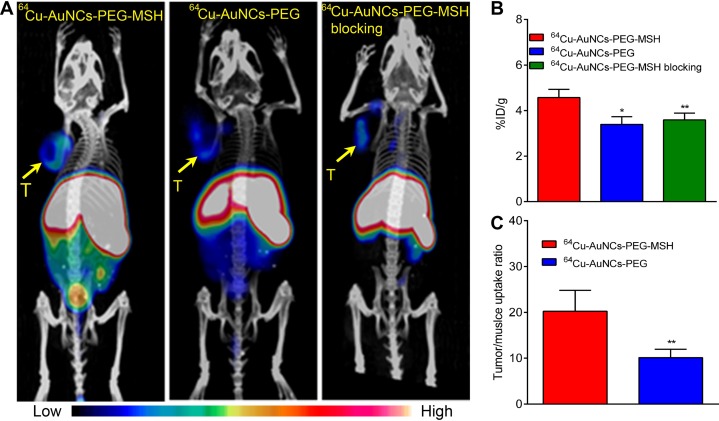
Positron emission tomography/computed tomography images of 64Cu-AuNCs-PEG-MSH showed tumor accumulation at 24 hours postinjection in a B16/F10 mouse melanoma model (Figure 4A). The high uptakes in liver and spleen were also consistent with the biodistribution studies. Quantitative tumor uptake analysis demonstrated 4.57 ± 0.36%ID/g (n = 4/group) accumulation within the tumor, comparable to the results acquired with targeted gold nanospheres.27 The nontargeted 64Cu-AuNCs-PEG showed weak signals within the tumor, confirmed by the significantly lower tumor accumulation (3.40 ± 0.34%ID/g, P < .001, n = 4/group). Through competitive receptor blocking, the tumor accumulation of 64Cu-AuNCs-PEG-MSH was significantly decreased (3.59 ± 0.30%ID/g, P < .001, n = 4/group), showing no statistical difference from that of 64Cu-AuNCs-PEG, demonstrating the targeting specificity of 64Cu-AuNCs-PEG-MSH. A further analysis of the tumor localization showed that the tumor/muscle uptake ratio of the targeted nanocages was 20.26 ± 4.54 (n = 4/group), twice of that acquired using the nontargeted nanocages (10.11 ± 1.84, P < .001, n = 4/group), indicating the advantage of targeted AuNCs for cancer diagnosis.
Figure 4.
A, Representative positron emission tomography/computed tomography (PET/CT) images of 64Cu-AuNCs-PEG-MSH, 64Cu-AuNCs-PEG, and competitive receptor blocking in mice bearing B16/F10 melanoma at 24 hours postinjection. The blocking study was carried by coinjection of nonradiolabeled AuNCs-PEG-MSH in excess (molar ratio of AuNCs-PEG-MSH to 64Cu-AuNCs-PEG-MSH at 40:1; yellow Arrow T, Tumor). B, Quantitative tumor uptakes of 64Cu-AuNCs-PEG-MSH, 64Cu-AuNCs-PEG, and blocking studies at 24 hours. (n = 4/group). C, Tumor-to-muscle ratio of 64Cu-AuNCs-PEG-MSH and 64Cu-AuNCs-PEG at 24 hours (n = 4 / group). *P < .05, **P < .001.
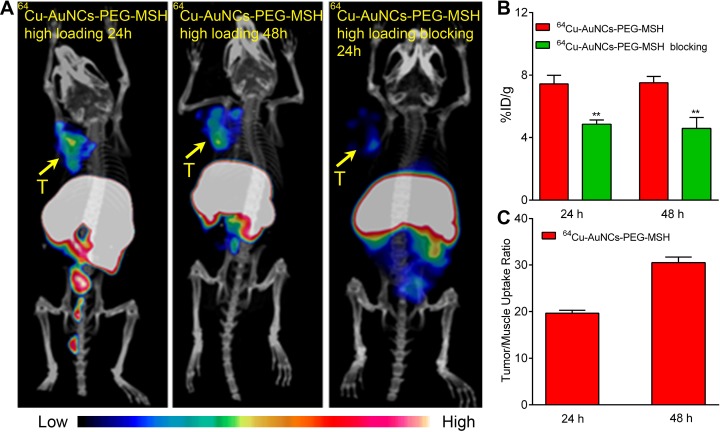
To further improve the targeting efficiency of 64Cu-AuNCs-PEG-MSH in vivo, the amount of NDP-α-MSH peptide conjugated with the surface of AuNCs was increased to 5400 copies/AuNC by increasing the molar ratio of OPPS-PEG-MSH to HS-PEG-OMe during the conjugation reaction. Interestingly, the increased conjugation of targeting NDP-α-MSH peptide also led to increased uptake in the B16/F10 tumors, as demonstrated in Figure 5A. The quantitative analysis showed the tumor localization was 7.43 ± 0.55%ID/g (n = 4/group) at 24 hours and remained stable up to 48 hours (7.52 ± 0.40%ID/g, n = 4/group). Both were twice as much as that acquired with nontargeted 64Cu-AuNCs-PEG (P < .001, n = 4/group). Through the coinjection of nonradiolabeled AuNCs-PEG-MSH in excess amount, the tumor uptake was significantly blocked (4.58 ± 0.69%ID/g, P < .001, n = 4/group), suggesting the targeting specificity. Additionally, the elevated tumor uptake also led to improved tumor/muscle contrast ratio, which were 19.67 ± 0.60 at 24 hours (n = 4/group) and further increased to 30.48 ± 1.27 at 48 hours (n = 4/group), which will help to better localize the tumor for photothermal treatment and reduce the side effect in the future. Furthermore, the improved targeting sensitivity and specificity may be particularly useful for detecting small metastatic lesions in distant organs such as liver due to the stable background hepatic accumulation during the 24-hour study.
Figure 5.
A, Representative positron emission tomography/computed tomography (PET/CT) images of 64Cu-AuNCs-PEG-MSH with high loading of α-melanocyte-stimulating hormone (α-MSH) peptide and competitive receptor blocking studies in mice-bearing B16/F10 melanoma at 24 and 48 hours postinjection. The competitive blocking studies were carried with coinjection of non-radiolabeled AuNCs-PEG-MSH conjugated with a high loading of α-MSH peptide in excess amount (molar ratio of AuNCs-PEG-MSH: 64Cu-AuNCs-PEG-MSH = 40:1; T, tumor). B, Quantitative tumor uptake analysis at 24 and 48 hours (n = 4/group). C, Tumor-to-muscle ratio of 64Cu-AuNCs-PEG-MSH with high α-MSH peptide at 24 and 48 hours (n = 4/group). *P < .005, **P < .001.
Currently, there are a lot of effort using combined strategy for melanoma treatment such as integrating immunotherapy, BRAF inhibitor, and radiotherapy, which has shown significant efficacy in patients. However, due to the lack of noninvasive imaging approach, it is challenging to fulfill the goal of personalized therapy. The AuNCs developed herein has the potential to serve as a multivalent platform to integrate the imaging and therapy together for melanoma theranostics by loading/encapsulating small molecule inhibitors, radiolabeling with 67Cu/64Cu for MSH peptide-directed treatment following PET guidance.28-31
Characterization of MC1R Receptor
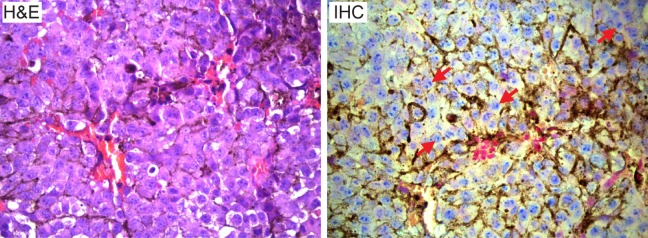
Histopathological analysis of B16/F10 tumor and characterization of MC1R receptor was also performed in the collected tumor tissues. As shown in Figure 6, the hematoxylin and eosin staining showed a high level of large and polynucleated tumor cells in the B16/F10 tumors collected at 10 days postimplant. Immunohistochemical staining of the tumor demonstrated the high expression of MC1R receptor in malignant melanocytes across the tumor (Figure 6), which is consistent with high targeting effect of 64Cu-AuNCs-PEG-MSH.
Figure 6.
Hematoxylin and eosin (H&E) staining of B16/F10 tumor tissue showing large and polynucleated tumor cells. Immunohistochemical staining of MC1R receptor in B16/F10 tumor. Tumor cells are stained with MC1R (blue) and counterstained with nuclear fast red (pink). The brown color indicates melanin expression (marked by arrows). All panels are at ×400.
Conclusion
In summary, we have demonstrated the synthesis of MSH-conjugated AuNCs with well-defined structure and surface chemistry for MC1R-targeted imaging in a mouse B16/F10 melanoma model. The high radiolabeling-specific activity of AuNCs ensured the trace amount administration for in vivo applications. Pharmacokinetic evaluation of 64Cu-AuNCs-PEG-MSH showed high initial blood circulation, followed by fast clearance for low background. Positron emission tomography/computed tomography imaging of the MC1R-targeted 64Cu-AuNCs-PEG-MSH demonstrated sensitive and specific detection of MC1R in the B16/F10 tumors, confirmed by the overexpression of MC1R in tumor cells. By increasing the loading of targeting α-MSH peptide onto the surface of AuNCs, the tumor targeting efficiency was also increased, indicating the effectiveness of this targeting strategy in improving tumor delivery for potential photothermal therapy. However, there are also some limitations associated with the current studies. The biodistribution profile of 64Cu-AuNCs-PEG-MSH needs to be improved to reduce MPS system accumulation and minimize any potential toxicity concerns. The targeting efficiency needs to be further enhanced for future photothermal treatment studies.
Footnotes
Author’s Note: Yongfeng Zhao and Bo Pang contributed equally to this work.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported in part by a grant from NIH (R01 CA138527) and startup funds from the Georgia Institute of Technology.
ORCID iD: Yongjian Liu  http://orcid.org/0000-0002-1118-1535
http://orcid.org/0000-0002-1118-1535
References
- 1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2017. CA: Cancer J Clin. 2017;67(1):7–30. [DOI] [PubMed] [Google Scholar]
- 2. Giljohann DA, Seferos DS, Daniel WL, Massich MD, Patel PC, Mirkin CA. Gold nanoparticles for biology and medicine. Angew Chem Int Ed. 2010;49(19):3280–3294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Garbe C, Eigentler TK, Keilholz U, Hauschild A, Kirkwood JM. Systematic review of medical treatment in melanoma: current status and future prospects. Oncologist. 2011;16(1):5–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Berciano-Guerrero MA, Montesa-Pino A, Castaneda-Penalvo G, Munoz-Fernandez L, Rodriguez-Flores J. Nanoparticles in melanoma. Curr Med Chem. 2014;21(32):3701–3716. [DOI] [PubMed] [Google Scholar]
- 5. You S, Luo J, Grossniklaus HE, Gou ML, Meng K, Zhang Q. Nanomedicine in the application of uveal melanoma. Int J Ophthalmol. 2016;9(8):1215–1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Iyer AK, Khaled G, Fang J, Maeda H. Exploiting the enhanced permeability and retention effect for tumor targeting. Drug Discov Today. 2006;11(17-18):812–818. [DOI] [PubMed] [Google Scholar]
- 7. Hu M, Chen J, Li Z-Y, et al. Gold nanostructures: engineering their plasmonic properties for biomedical applications. Chem Soc Rev. 2006;35(11):1084–1094. [DOI] [PubMed] [Google Scholar]
- 8. Pissuwan D, Valenzuela SM, Cortie MB. Therapeutic possibilities of plasmonically heated gold nanoparticles. Trends Biotechnol. 2006;24(2):62–67. [DOI] [PubMed] [Google Scholar]
- 9. Bhise K, Sau S, Alsaab H, Kashaw SK, Tekade RK, Iyer AK. Nanomedicine for cancer diagnosis and therapy: advancement, success and structure-activity relationship. Ther Deliv. 2017;8(11):1003–1018. [DOI] [PubMed] [Google Scholar]
- 10. Pang B, Zhao Y, Luehmann H, et al. 64Cu-Doped PdCu@Au Tripods: a multifunctional nanomaterial for positron emission tomography and image-guided photothermal cancer treatment. ACS Nano. 2016;10(3):3121–3131. [DOI] [PubMed] [Google Scholar]
- 11. Deng H, Zhong Y, Du M, et al. Theranostic self-assembly structure of gold nanoparticles for NIR photothermal therapy and X-Ray computed tomography imaging. Theranostics. 2014;4(9):904–918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Wang Y, Black KCL, Luehmann H, et al. A comparison study of gold nanohexapods, nanorods, and nanocages for photothermal cancer treatment. ACS Nano. 2013;7(3):2068–2077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Wang Y, Liu Y, Luehmann H, et al. Evaluating the pharmacokinetics and in vivo cancer targeting capability of au nanocages by positron emission tomography imaging. ACS Nano. 2012;6(7):5880–5888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Wang Y, Liu Y, Luehmann H, et al. Radioluminescent gold nanocages with controlled radioactivity for real-time in vivo imaging. Nano Lett. 2013;13(2):581–585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Black KCL, Wang Y, Luehmann HP, et al. Radioactive 198Au-doped nanostructures with different shapes for in vivo analyses of their biodistribution, tumor uptake, and intratumoral distribution. ACS Nano. 2014;8(5):4385–4394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Moon GD, Choi S-W, Cai X, et al. A new theranostic system based on gold nanocages and phase-change materials with unique features for photoacoustic imaging and controlled release. J Am Chem Soc. 2011;133(13):4762–4765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Rosenkranz AA, Slastnikova TA, Durymanov MO, Sobolev AS. Malignant melanoma and melanocortin 1 receptor. Biochemistry (Mosc). 2013;78(11):1228–1237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Miao Y, Benwell K, Quinn TP. 99mTc- and 111In-labeled α-Melanocyte-stimulating hormone peptides as imaging probes for primary and pulmonary metastatic melanoma detection. J Nucl Med. 2007;48(1):73–80. [PubMed] [Google Scholar]
- 19. Lu W, Xiong C, Zhang G, et al. Targeted photothermal ablation of murine melanomas with melanocyte-stimulating hormone analog–conjugated hollow gold nanospheres. Clin Cancer Res. 2009;15(3):876–886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kim C, Cho EC, Chen J, et al. In vivo molecular photoacoustic tomography of melanomas targeted by bioconjugated gold nanocages. ACS Nano. 2010;4(8):4559–4564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Skrabalak SE, Au L, Li X, Xia Y. Facile synthesis of Ag nanocubes and Au nanocages. Nat Protoc. 2007;2(9):2182–2190. [DOI] [PubMed] [Google Scholar]
- 22. Liu Y, Ibricevic A, Cohen JA, et al. Impact of hydrogel nanoparticle size and functionalization on in vivo behavior for lung imaging and therapeutics. Mol Pharm. 2009;6(6):1891–1902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Choi HS, Liu W, Misra P, et al. Renal clearance of quantum dots. Nat Biotechnol. 2007;25(10):1165–1170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Lipka J, Semmler-Behnke M, Sperling RA, et al. Biodistribution of PEG-modified gold nanoparticles following intratracheal instillation and intravenous injection. Biomaterials. 2010;31(25):6574–6581. [DOI] [PubMed] [Google Scholar]
- 25. Jørgensen JT, Persson M, Madsen J, Kjær A. High tumor uptake of 64Cu: implications for molecular imaging of tumor characteristics with copper-based PET tracers. Nucl Med Biol. 2013;40(3):345–350. [DOI] [PubMed] [Google Scholar]
- 26. Zhao Y, Sultan D, Detering L, et al. Copper-64-alloyed gold nanoparticles for cancer imaging: improved radiolabel stability and diagnostic accuracy. Angew Chem Int Ed Engl. 2014;53(1):156–159. [DOI] [PubMed] [Google Scholar]
- 27. Lu W, Xiong C, Zhang R, et al. Receptor-mediated transcytosis: a mechanism for active extravascular transport of nanoparticles in solid tumors. J Control Release. 2012;161(3):959–966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Novak-Hofer I, Schubiger PA. Copper-67 as a therapeutic nuclide for radioimmunotherapy. Eur J Nucl Med Mol Imaging. 2002;29(6):821–830. [DOI] [PubMed] [Google Scholar]
- 29. Stokes WA, Binder DC, Jones BL, et al. Impact of immunotherapy among patients with melanoma brain metastases managed with radiotherapy. J Neuroimmunol. 2017;313:118–122. [DOI] [PubMed] [Google Scholar]
- 30. Geukes Foppen MH, Boogerd W, Blank CU, van Thienen JV, Haanen JB, Brandsma D. Clinical and radiological response of BRAF inhibition and MEK inhibition in patients with brain metastases from BRAF-mutated melanoma. Melanoma Res. 2018;28(2):126–133. [DOI] [PubMed] [Google Scholar]
- 31. Hecht M, Meier F, Zimmer L, et al. Clinical outcome of concomitant vs interrupted BRAF inhibitor therapy during radiotherapy in melanoma patients. Br J Cancer. 2018;118(6):785–792. [DOI] [PMC free article] [PubMed] [Google Scholar]