Abstract
Background
There is growing evidence that mast cells (MCs) play a role in knee osteoarthritis (OA). H1-antihistamines block H1-receptors of histamine, which is an important mediator of MCs. There is a lack of data on whether H1-antihistamines can influence OA. We hypothesized that the use of H1-antihistamines may be linked to the reduced prevalence of knee OA.
Methods
Baseline data from the Osteoarthritis Initiative cohort were analysed cross-sectionally. Unadjusted and adjusted logistic regression models were performed to compare the prevalence of knee OA in H1-antihistamine users and non-users. Generalized estimating equations were used to adjust for the correlation between knees. Knee OA was defined as (1) Kellgren-Lawrence (KL) grade ≥ 2 or total joint replacement or (2) KL grade ≥ 2 and joint space narrowing or total joint replacement.
Results
The analysed sample consisted of 8545 knees (664 knees of H1-antihistamine users and 7881 knees of H1-antihistamine non-users). The use of H1-antihistamines was associated with reduced prevalence of knee OA in unadjusted and adjusted models using both the first (adjusted OR, 0.77; 95% CI, 0.62, 0.96; P < 0.02) and second (adjusted OR, 0.75; 95% CI, 0.62, 0.93; P < 0.008) definitions of knee OA.
Conclusions
H1-antihistamines are associated with a reduced prevalence of knee OA. The findings indicate that this class of drugs should be further evaluated for possible structure-modifying properties in knee OA.
Keywords: Osteoarthritis, Knee osteoarthritis, Outcomes research
Background
Mast cells (MCs) have long been considered to be inflammatory cells involved primarily in parasitic infections and allergic reactions. In recent years, an emergent role has been described for MCs in various chronic inflammatory diseases, including cancers [1], rheumatoid arthritis [2], atherosclerosis, obesity and diabetes [3]. In osteoarthritis (OA), MCs are prevalent in synovial tissue, and their presence is associated with structural damage [4]. The primary action of histamine H1-receptor blockers (H1-antihistamines) is to block the effects of histamine on its specific receptors. In addition, many H1-antihistamines have anti-inflammatory effects and are able to stabilize the membranes of MC, leading to decreased release of multiple mediators of MC [5].
Therefore, H1-antihistamines might be a candidate class of drugs to prevent and treat OA. Currently, there is a lack of data on the effects of H1-antihistamines in knee OA in humans. In one exploratory study, H1-antihistamines were linked to decreased progression and less pain in knee OA [6].
We hypothesized that H1-antihistamine use may be linked to reduced prevalence of knee OA. To test this hypothesis, we evaluated the cross-sectional association between the use of oral H1-antihistamines and radiographic knee OA using the data from the publicly available Osteoarthritis Initiative (OAI) cohort.
Methods
In these cross-sectional analyses we compared the knees of OAI participants taking H1-antihistamines at baseline with the knees of control participants. For the present study, we used longitudinal data obtained from the OAI, which is publicly available at http://oai.epi-ucsf.org. Specific datasets used were “MIF00” (version 0.2.2), “AllClinical00” (version 0.2.2), “Enrollees00 (version 17)”, “kXR_SQ_BU00” (version 0.6), and “Outcomes99” (version 8). The detailed information about the OAI protocol can be found elsewhere [7]. The OAI cohort consists of a progression subcohort (patients with symptomatic tibiofemoral knee OA, n = 1390), an incidence subcohort (subjects with increased risk of OA, n = 3284) and a reference control subcohort (n = 122). In this analysis we used the cross-sectional baseline data from both the progression and incidence subcohorts. The main inclusion criteria were the following: age between 45 and 79 years for both subcohorts, symptomatic tibiofemoral knee OA for the progression subcohort, and the presence of established or putative risk factors for incident knee OA for the incidence subcohort. The OAI subjects were recruited and enrolled between February 2004 and May 2006 at four recruitment centres in the United States. This study received ethical approval from each recruitment centre. All participants provided written informed consent. The prespecified sample size was 5000 women and men (4000 in the incidence subcohort, 800 in the progression subcohort). The sample size was expected to provide an adequate number of knees with incident and worsening OA-related structural and clinical changes to achieve the primary aims of the OAI study.
Clinical measures
Height was measured in millimetres using a calibrated, wall-mounted stadiometer. The measurement was performed twice with the subject in light clothing, without shoes, and during inspiration. Body weight was measured in kilograms with a calibrated, standard balance beam scale. The measurement was performed twice with the subject in light clothing without shoes, heavy jewellery or wallets. Body mass index (BMI) was calculated based on weight (in kg) divided by height (in cm) squared. Smoking history and education status were assessed using self-administered questionnaires. Prior knee surgery, family history of knee replacement, and Physical Activity Scale for the Elderly (PASE) were evaluated using interview.
To acquire information about the use of H1-antihistamines, a medication inventory method was used wherein the participants brought in all of the medications they were currently taking, and the brand name, generic name or active ingredients were recorded and matched to an entry in an online medication dictionary [8]. Only participants who reported taking H1-antihistamines for more than 1 year prior to baseline were included in the analyses.
Radiographic assessment
Posteroanterior weight-bearing knee radiographs were performed annually using a Synaflexer frame (Synarc, San Francisco, CA, USA), which allowed a fixed, standardized and reproducible knee position. X-ray interpretation was performed centrally at Boston University by three readers. In case of a disagreement about the presence of radiographic OA, the reading was adjudicated by a panel of three readers. A consensus reading was achieved when at least two of the three readers agreed.
The initial incidence and progression OAI subcohort assignments were based primarily on Kellgren-Lawrence (KL) readings at the OAI clinical centres. Because further central assessments could provide different grading results, assignment to incidence and progression subcohorts might not reflect a participant’s knee OA status at baseline.
In the OAI, there were two definitions of knee OA based on central reading: (1) KL grade ≥ 2 or total joint replacement and (2) KL grade ≥ 2 and joint space narrowing (JSN) or total joint replacement. We used both definitions as outcomes of our study.
Statistical analysis
Continuous variables are presented as mean (SD), and categorical variables as number (percent). Although most of the continuous variables were not normally distributed, we used mean (SD) as the preferred statistic even in the setting of non-normally distributed data [9]. Unadjusted and adjusted logistic regression models were used to assess the association between the use of H1-antihistamines and radiographic knee OA. Generalized estimating equations (GEEs) were used to adjust for the correlation between knees. GEE allows for not using imputation methods for the missing data, because the participants with missing data are not excluded from the analysis [10]. The models were adjusted for BMI, age, race, sex, smoking history, education status, history of prior knee surgery, family history of knee replacement, PASE and subcohort assignment. These potential confounders were selected on the basis of literature and clinical plausibility. The adjustment for the subcohort assignment was carried out because the inclusion criteria for both subcohorts were different.
Sensitivity analyses
We performed sensitivity analyses using the definition of knee OA as a KL score ≥ 2 (not including knee replacement in the definition).
Results
A sample of 4273 OAI participants (8545 knees) was analysed, and 332 of them (664 knees) used histamine H1-receptor blockers at baseline (Fig. 1). The vast majority of H1-antihistamine users were taking second-generation H1-antihistamines. The compared groups consisted of middle-aged, overweight participants. There were 1518 (25%) knees with radiographic OA in the incidence subcohort and 1537 (61.4%) knees with radiographic OA in the progression subcohort using the first definition of knee OA. According to the second definition of knee OA, there were 2028 (33.6%) and 1834 (73.3%) knees with radiographic OA in the incidence and progression subcohorts, respectively.
Fig. 1.
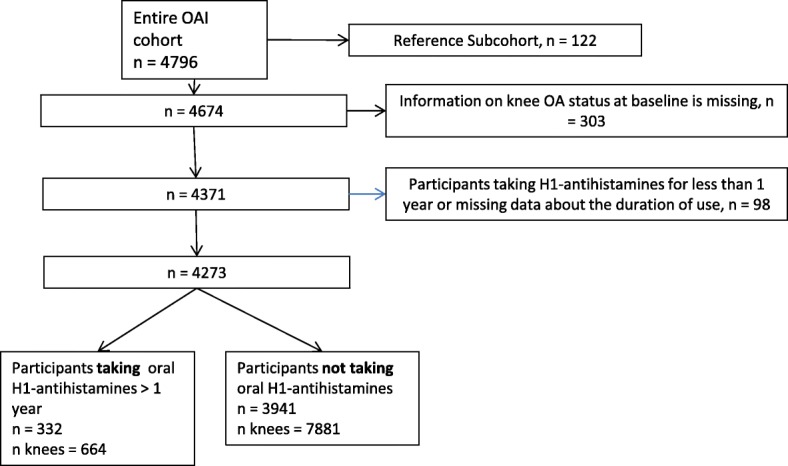
Flowchart of participants selected for the analyses
In relative terms, the prevalence of radiographic knee OA was 15.5% and 10.8% lower in the H1-antihistamine users group using the first and second definitions of radiographic knee OA, respectively. This difference was due to a lower proportion of patients with bilateral knee OA in the H1-antihistamine users group (26.5% and 17% less patients with bilateral knee OA using first and second definitions of knee OA, respectively) (Table 1).
Table 1.
Characteristics of participants taking and not taking histamine H1-receptor antagonists
| Participants taking H1-antihistamines | Participants not taking H1- antihistamines | |
|---|---|---|
| No. of participants | 332 | 3941 |
| No. of knees | 664 | 7881 |
| Age, yr | 59.84 (8.17) | 61.45 (9.23) |
| Sex | ||
| Female | 94 (28.3) | 1712 (43.4) |
| Male | 238 (71.7) | 2229 (56.6) |
| BMI, kg/m2 | 29.18 (4.86) | 28.64 (4.79) |
| Race | ||
| Other non-white | 3 (0.9) | 64 (1.6) |
| White or Caucasian | 261 (78.6) | 3156 (80.1) |
| Black or African American | 64 (19.3) | 685 (17.4) |
| Asian | 4 (1.2) | 32 (0.8) |
| History of knee surgery | 73 (22.0) | 936 (23.8) |
| Family history of knee OA | 60 (18.1) | 611 (15.5) |
| PASE | 160.38 (82.74) | 161.68 (82.23) |
| Education | ||
| Less than high school graduate | 6 (1.8) | 136 (3.5) |
| High school graduate | 38 (11.4) | 489 (12.4) |
| Some college | 79 (23.8) | 939 (23.8) |
| College graduate | 63 (19.0) | 846 (21.5) |
| Some graduate school | 28 (8.4) | 319 (8.1) |
| Graduate degree | 116 (34.9) | 1183 (30.0) |
| Smoking history | ||
| Never | 180 (54.2) | 2056 (52.2) |
| Current | 19 (5.7) | 242 (6.1) |
| Former | 129 (38.9) | 1585 (40.2) |
| Current but never regular | 2 (0.6) | 6 (0.2) |
| WOMAC subscales | ||
| WOMAC pain, right knee | 2.93 (3.45) | 2.42 (3.12) |
| WOMAC pain, left knee | 2.70 (3.65) | 2.28 (3.33) |
| WOMAC function, right knee | 9.75 (11.28) | 7.75 (10.19) |
| WOMAC function, left knee | 9.92 (12.52) | 7.92 (11.02) |
| KL, knees (%) | ||
| 0 | 256 (38.6) | 2904 (36.8) |
| 1 | 138 (20.8) | 1386 (17.6) |
| 2 | 176 (26.5) | 2129 (27.0) |
| 3 | 71 (10.7) | 1135 (14.4) |
| 4 | 18 (2.7) | 272 (3.5) |
| Patients with knee OA, defined as KL ≥ 2 with JSN, or knee replacement (%) | 145 (43.67) | 1913 (48.54) |
| Unilateral, no. of patients (%) | 87 (26.2) | 974 (24.7) |
| Bilateral, no. of patients (%) | 58 (17.5) | 939 (23.8) |
| Patients with knee OA, defined as KL ≥ 2, or knee replacement (%) | 181 (54.52) | 2318 (58.84) |
| Unilateral, patients (%) | 92 (27.7) | 1044 (26.5) |
| Bilateral, patients (%) | 89 (26.8) | 1274 (32.3) |
| JSN, medial compartment, knees (%) | ||
| 0 | 441 (66.4) | 4985 (63.3) |
| 1 | 154 (23.2) | 1734 (22.0) |
| 2 | 58 (8.7) | 912 (11.6) |
| 3 | 6 (0.9) | 195 (2.5) |
| JSN, lateral compartment, knees (%) | ||
| 0 | 608 (91.6) | 7162 (90.9) |
| 1 | 26 (3.9) | 340 (4.3) |
| 2 | 13 (2.0) | 245 (3.1) |
| 3 | 12 (1.8) | 79 (1.0) |
| Knee replacement | 5 (0.8) | 53 (0.7) |
| H1-antihistamines | ||
| Second-generation | ||
| Fexofenadine | 177 (53.31) | – |
| Cetirizine | 85 (25.6) | – |
| Desloratadine | 39 (11.75) | – |
| Loratadine | 23 (6.93) | – |
| First-generation | ||
| Diphenhydramine | 7 (2.11) | – |
| Chlorpheniramine | 4 (1.2) | – |
| Promethazine | 5 (1.51) | – |
| Brompheniramine | 1 (0.3) | – |
| Cyproheptadine | 1 (0.3) | – |
| Dexbrompheniramine | 1 (0.3) | – |
| Phenyltoloxamine | 1 (0.3) | – |
| Pyrilamine | 1 (0.3) | – |
| Duration of H1-antihistamine use | ||
| 1–3 yr | 136 (40.96) | |
| 3–5 yr | 92 (27.71) | |
| More than 5 yr | 104 (31.33) | |
| Study endpoints | ||
| Radiographic knee OA, defined as KL ≥ 2 with JSN, or knee replacement, knees (%) | 203 (30.6) | 2852 (36.2) |
| Radiographic knee OA, defined as KL ≥ 2, or knee replacement, knees (%) | 270 (40.7) | 3592 (45.6) |
Abbreviations: OA Osteoarthritis, BMI Body mass index, KL Kellgren-Lawrence grade, PASE Physical Activity Scale for the Elderly, WOMAC Western Ontario and McMaster Universities Osteoarthritis Index, JSN Joint space narrowing (grades 0–3)
Data are presented as the mean (SD) or number (%). Possible ranges for WOMAC pain score are 0–20. Possible ranges for WOMAC function score are 0–68
In the regression analyses, the use of H1-antihistamines was associated with lower prevalence of radiographic knee OA using both definitions in either crude or adjusted analyses (Table 2). The sensitivity analysis did not change the direction and significance of our results shown in Table 2.
Table 2.
Association between the use of histamine H1-receptor antagonists and prevalence of radiographic knee OA
| Non-adjusted models | Adjusted models | |||||
|---|---|---|---|---|---|---|
| OR | 95% CI | P value | OR | 95% CI | P value | |
| Radiographic knee OA, defined as KL ≥ 2 with JSN | 0.78 | 0.63 to 0.95 | 0.014 | 0.77 | 0.62 to 0.96 | 0.02 |
| Radiographic knee OA, defined as KL ≥ 2 | 0.81 | 0.67 to 0.99 | 0.041 | 0.75 | 0.62 to 0.93 | 0.008 |
Note. The models were adjusted for BMI, race, age, gender, Physical Activity scale for the elderly (PASE), history of knee surgery, family history of knee OA, smoking status, education, and Subcohort assignment. OR – odds ratio, CI – confidence interval
Discussion
In cross-sectional analyses of OAI data, H1-antihistamines were associated with decreased prevalence of radiographic knee OA. Our study had the following important limitations: a cross-sectional design and a lack of precise information about the duration and dose of antihistamine use. The presence of inclusion criteria may limit the generalizability of the results.
Our data are in line with an exploratory, hypothesis-generating study performed on longitudinal OAI data. In this study, antihistamine users, defined as those using antihistamines at the first four annual visits, showed a signal for reduced changes in joint space width during 36-month follow-up. The authors did not evaluate statistical significance and did not perform adjustment for possible confounders [6]. In contrast, our analysis was cross-sectional, we used a dichotomous outcome measure of radiographic knee OA, and our models were adjusted for multiple confounders. Thus, our data may be considered as an initial line of evidence that antihistamines may influence knee OA.
The demonstrated association between H1-antihistamine use and the decreased prevalence of knee OA may be explained in several ways. First, it is tempting to speculate that H1-antihistamines prevent knee OA by stabilizing the membranes of MC and blocking the effects of histamine, which is their major mediator.
The role of MCs in OA was suggested more than 20 years ago by several studies showing elevation of MCs and histamine levels in synovial fluids and synovial tissues from patients with OA [11, 12]. These findings were confirmed recently, and a trend towards an association between the number of MCs and increased KL grade was also found [4].
MCs are capable to produce a plethora of mediators which are released upon different stimuli via degranulation, secretion and exocytosis. Mediators stored in MC granules are represented by amines, proteoglycans, proteases, lysosomal enzymes, and cytokines [13]. Most of these compounds may be involved in the pathogenesis of OA. Thus, histamine induced an increase in the proliferation of human articular chondrocytes [14] and upregulated production of matrix metalloproteinase (MMP)-13 and MMP-3 by these cells via H1-receptors [15]. MC-derived polyamines, which are naturally occurring, positively charged polycations, are able to promote chondrocyte differentiation, which may result in OA [16]. MC-produced chymase has potent pro-inflammatory properties and plays a key role in MMP-9 and MMP-2 activation [17]. A recent meta-analysis showed a significant link between serum levels of MMP-9, MMP-2 and OA, suggesting a contribution of these MMPs to the pathogenesis of OA [18]. MCs have also been shown to synthesize, store and release nerve growth factor (NGF) [19], which is a promising new target for the treatment of pain in OA [20]. A recent study showed that NGF-induced production of prostaglandin D2 in joint MCs is critical for developing pain in OA [21]. One pilot observational study showed improvement of pain in patients with OA receiving therapy with anti-immunoglobulin E treatment, whose main effect is MC stabilization [22]. In addition, H1-antihistamines have been demonstrated to exert anti-inflammatory properties via down-regulation of nuclear factor-κB and suppression of secretion of various pro-inflammatory cytokines, including tumour necrosis factor-α, interleukin (IL)-6, IL-8 and granulocyte-macrophage colony-stimulating factor, by different cell types [23–25]. Thus, a growing body of data indicates that MCs and their mediators contribute to the pathogenesis of OA. Another way of suppressing MCs may be the use of peroxisome proliferator-activated receptor-α (PPARα) agonists [26]. Clinical improvement in erosive hand OA treated with the PPARα agonist fenofibrate in a small study [27] may in part be explained by the reduced activation of MCs.
Second, the findings may be due to inherent limitations of cross-sectional studies and the presence of unidentified confounders. There is a need of prospective studies evaluating the effects of H1-antihistamines on knee OA. Longitudinal observational studies may provide initial evidence that should be tested further in randomized controlled studies. It is agreed that to prevent several kinds of biases in the longitudinal analyses of observational cohort studies, one needs to employ a ‘new-user’ design that evaluates persons who were treatment-naïve at baseline and started the treatment of interest only after enrolment [28]. We were not able to perform this kind of analysis, because it would require a significantly larger sample size than that of the OAI dataset to gain sufficient statistical power using the selected knee OA outcomes. The strengths of this analysis are that it is based on a large population with a well-defined cohort, and we used standardised and reproducible procedures for knee radiograph acquisition, as well as an extensive adjudication process to determine KL and JSN grades.
Conclusions
H1-antihistamines are associated with decreased prevalence of knee OA. In view of the absence of effective structure-modifying drugs for OA and the emerging role of MCs in OA, our findings provide an impetus for further studies evaluating H1-antihistamines in OA.
Acknowledgements
The OAI is a public-private partnership comprising five contracts (N01-AR-2-2258, N01-AR-2-2259, N01-AR-2-2260, N01-AR-2-2261, N01-AR-2-2262) funded by the National Institutes of Health (NIH), a branch of the Department of Health and Human Services, and conducted by the OAI study investigators. Private funding partners include Merck Research Laboratories, Novartis Pharmaceuticals Corporation, GlaxoSmithKline and Pfizer, Inc. Private sector funding for the OAI is managed by the Foundation for the National Institutes of Health. This article was prepared using an OAI public use dataset and does not necessarily reflect the opinions or views of the OAI investigators, the NIH or the private funding partners. We thank Peter Mittwede, MD, PhD, from Edanz Group (www.edanzediting.com/ac) for editing a draft of the manuscript.
Availability of data and materials
The datasets generated and/or analysed during the present study are available from the Osteoarthritis Initiative website (https://oai.epi-ucsf.org/).
Abbreviations
- GEE
Generalized estimating equation
- IL
Interleukin
- JSN
Joint space narrowing
- KL
Kellgren-Lawrence
- MC
Mast cells
- MMP
Matrix metalloproteinase
- NGF
Nerve growth factor
- OA
Osteoarthritis
- OAI
Osteoarthritis Initiative
- PASE
Physical Activity Scale for the Elderly
- PPARα
Peroxisome proliferator-activated receptor-α
Authors’ contributions
Both authors contributed to the conception and design of the study and to the interpretation of the findings. IS conducted the statistical analyses. Both authors assisted in drafting the manuscript, and both of them read and approved the final draft.
Ethics approval and consent to participate
The OAI was approved by the Institutional Review Board of the University of California, San Francisco (UCSF), and its affiliates. All participants provided informed consent to participate in the OAI study.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Ivan Shirinsky, Phone: +73832282547, Email: ivan.shirinsky@gmail.com.
Valery Shirinsky, Phone: +73832282547, Email: valery.shirinsky@gmail.com.
References
- 1.Varricchi G, Galdiero MR, Loffredo S, Marone G, Iannone R, Granata F. Are mast cells MASTers in cancer? Front Immunol. 2017;8:424. doi: 10.3389/fimmu.2017.00424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kritas SK, Saggini A, Varvara G, Murmura G, Caraffa A, Antinolfi P, Toniato E, Pantalone A, Neri G, Frydas S, et al. Mast cell involvement in rheumatoid arthritis. J Biol Regul Homeost Agents. 2013;27(3):655–660. [PubMed] [Google Scholar]
- 3.Shi GP, Bot I, Kovanen PT. Mast cells in human and experimental cardiometabolic diseases. Nat Rev Cardiol. 2015;12(11):643–658. doi: 10.1038/nrcardio.2015.117. [DOI] [PubMed] [Google Scholar]
- 4.de Lange-Brokaar BJ, Kloppenburg M, Andersen SN, Dorjee AL, Yusuf E, Herb-van Toorn L, Kroon HM, Zuurmond AM, Stojanovic-Susulic V, Bloem JL, et al. Characterization of synovial mast cells in knee osteoarthritis: association with clinical parameters. Osteoarthritis Cartilage. 2016;24(4):664–671. doi: 10.1016/j.joca.2015.11.011. [DOI] [PubMed] [Google Scholar]
- 5.Levi-Schaffer F, Eliashar R. Mast cell stabilizing properties of antihistamines. J Invest Dermatol. 2009;129(11):2549–2551. doi: 10.1038/jid.2009.256. [DOI] [PubMed] [Google Scholar]
- 6.Driban JB, Lo GH, Eaton CB, Lapane KL, Nevitt M, Harvey WF, McCulloch CE, McAlindon TE. Exploratory analysis of osteoarthritis progression among medication users: data from the Osteoarthritis Initiative. Ther Adv Musculoskelet Dis. 2016;8(6):207–219. doi: 10.1177/1759720X16664323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.The osteoarthritis initiative protocol for the cohort study. http://oai.epi-csf.org/datarelease/docs/StudyDesignProtocol.pdf. Accessed 24 May 2018.
- 8.Pahor M, Chrischilles EA, Guralnik JM, Brown SL, Wallace RB, Carbonin P. Drug data coding and analysis in epidemiologic studies. Eur J Epidemiol. 1994;10(4):405–411. doi: 10.1007/BF01719664. [DOI] [PubMed] [Google Scholar]
- 9.Lydersen S. Statistical review: frequently given comments. Ann Rheum Dis. 2015;74(2):323–325. doi: 10.1136/annrheumdis-2014-206186. [DOI] [PubMed] [Google Scholar]
- 10.Twisk J, de Vente W. Attrition in longitudinal studies: how to deal with missing data. J Clin Epidemiol. 2002;55(4):329–337. doi: 10.1016/S0895-4356(01)00476-0. [DOI] [PubMed] [Google Scholar]
- 11.Dean G, Hoyland JA, Denton J, Donn RP, Freemont AJ. Mast cells in the synovium and synovial fluid in osteoarthritis. Br J Rheumatol. 1993;32(8):671–675. doi: 10.1093/rheumatology/32.8.671. [DOI] [PubMed] [Google Scholar]
- 12.Renoux M, Hilliquin P, Galoppin L, Florentin I, Menkes CJ. Release of mast cell mediators and nitrites into knee joint fluid in osteoarthritis--comparison with articular chondrocalcinosis and rheumatoid arthritis. Osteoarthritis Cartilage. 1996;4(3):175–179. doi: 10.1016/S1063-4584(96)80013-6. [DOI] [PubMed] [Google Scholar]
- 13.Moon TC, Befus AD, Kulka M. Mast cell mediators: their differential release and the secretory pathways involved. Front Immunol. 2014;5:569. doi: 10.3389/fimmu.2014.00569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tetlow LC, Woolley DE. Histamine stimulates the proliferation of human articular chondrocytes in vitro and is expressed by chondrocytes in osteoarthritic cartilage. Ann Rheum Dis. 2003;62(10):991–994. doi: 10.1136/ard.62.10.991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tetlow LC, Woolley DE. Histamine stimulates matrix metalloproteinase-3 and -13 production by human articular chondrocytes in vitro. Ann Rheum Dis. 2002;61(8):737–740. doi: 10.1136/ard.61.8.737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Facchini A, Borzi RM, Olivotto E, Platano D, Pagani S, Cetrullo S, Flamigni F. Role of polyamines in hypertrophy and terminal differentiation of osteoarthritic chondrocytes. Amino Acids. 2012;42(2–3):667–678. doi: 10.1007/s00726-011-1041-9. [DOI] [PubMed] [Google Scholar]
- 17.Tchougounova E, Lundequist A, Fajardo I, Winberg JO, Abrink M, Pejler G. A key role for mast cell chymase in the activation of pro-matrix metalloprotease-9 and pro-matrix metalloprotease-2. J Biol Chem. 2005;280(10):9291–9296. doi: 10.1074/jbc.M410396200. [DOI] [PubMed] [Google Scholar]
- 18.Zeng GQ, Chen AB, Li W, Song JH, Gao CY. High MMP-1, MMP-2, and MMP-9 protein levels in osteoarthritis. Genet Mol Res. 2015;14(4):14811–14822. doi: 10.4238/2015.November.18.46. [DOI] [PubMed] [Google Scholar]
- 19.Leon A, Buriani A, Dal Toso R, Fabris M, Romanello S, Aloe L, Levi-Montalcini R. Mast cells synthesize, store, and release nerve growth factor. Proc Natl Acad Sci U S A. 1994;91(9):3739–3743. doi: 10.1073/pnas.91.9.3739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schnitzer TJ, Marks JA. A systematic review of the efficacy and general safety of antibodies to NGF in the treatment of OA of the hip or knee. Osteoarthritis Cartilage. 2015;23(Suppl 1):S8–17. doi: 10.1016/j.joca.2014.10.003. [DOI] [PubMed] [Google Scholar]
- 21.Sousa-Valente J, Calvo L, Vacca V, Simeoli R, Arevalo JC, Malcangio M. Role of TrkA signalling and mast cells in the initiation of osteoarthritis pain in the monoiodoacetate model. Osteoarthritis Cartilage. 2018;26(1):84–94. doi: 10.1016/j.joca.2017.08.006. [DOI] [PubMed] [Google Scholar]
- 22.Aquili A, Farinelli L, Bottegoni C, Antonicelli L, Gigante A. The effect of anti-IgE therapy in knee osteoarthritis: a pilot observational study. J Biol Regul Homeost Agents. 2017;31(4 Suppl 1):1–5. [PubMed] [Google Scholar]
- 23.Carayol N, Crampette L, Mainprice B, Ben-Soussen P, Verrecchia M, Bousquet J, Lebel B. Inhibition of mediator and cytokine release from dispersed nasal polyp cells by mizolastine. Allergy. 2002;57(11):1067–1070. doi: 10.1034/j.1398-9995.2002.23452.x. [DOI] [PubMed] [Google Scholar]
- 24.Matsubara M, Tamura T, Ohmori K, Hasegawa K. Histamine H1 receptor antagonist blocks histamine-induced proinflammatory cytokine production through inhibition of Ca2+−dependent protein kinase C, Raf/MEK/ERK and IKK/IκB/NF-κB signal cascades. Biochem Pharmacol. 2005;69(3):433–449. doi: 10.1016/j.bcp.2004.10.006. [DOI] [PubMed] [Google Scholar]
- 25.Lippert U, Moller A, Welker P, Artuc M, Henz BM. Inhibition of cytokine secretion from human leukemic mast cells and basophils by H1- and H2-receptor antagonists. Exp Dermatol. 2000;9(2):118–124. doi: 10.1034/j.1600-0625.2000.009002118.x. [DOI] [PubMed] [Google Scholar]
- 26.Sugiyama H, Nonaka T, Kishimoto T, Komoriya K, Tsuji K, Nakahata T. Peroxisome proliferator-activated receptors are expressed in human cultured mast cells: a possible role of these receptors in negative regulation of mast cell activation. Eur J Immunol. 2000;30(12):3363–3370. doi: 10.1002/1521-4141(2000012)30:12<3363::AID-IMMU3363>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- 27.Shirinsky IV, Shirinsky VS. Treatment of erosive osteoarthritis with peroxisome proliferator-activated receptor alpha agonist fenofibrate: a pilot study. Rheumatol Int. 2014;34(5):613–616. doi: 10.1007/s00296-013-2766-4. [DOI] [PubMed] [Google Scholar]
- 28.Ray WA. Evaluating medication effects outside of clinical trials: new-user designs. Am J Epidemiol. 2003;158(9):915–920. doi: 10.1093/aje/kwg231. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated and/or analysed during the present study are available from the Osteoarthritis Initiative website (https://oai.epi-ucsf.org/).


