Abstract
We studied the effect of myosin inhibitors, N-benzyl-p-toluenesulfonamide (BTS), blebbistatin, and butanedione monoxime (BDM) on X-ray diffraction patterns from rabbit psoas fibers under relaxing and contracting conditions. The first two inhibitors suppressed the contractile force almost completely at a 100 μM concentration, and a similar effect was obtained at 50 mM for BDM. However, still substantial changes were observed in the diffraction patterns upon calcium-activation of inhibited muscle fibers. (1) The 2nd actin layer-line reflection was enhanced normally, indicating that calcium binding to troponin and the subsequent movement of tropomyosin are not inhibited, (2) the myosin layer-line reflections became much weaker, and (3) the 1,1/1,0 intensity ratio of the equatorial reflections was increased. The observations (2) and (3) indicate that, even in the presence of the inhibitors at a saturating concentration, myosin heads leave the helix on the thick filaments and approach the thin filaments. Interestingly, the d1,0 spacing of the filament lattice remained unchanged upon activation of inhibited fibers, in contrast to the case of normal activation in which the spacing is decreased. This suggests that the normal activated myosin heads exert a pull in both axial and radial directions, but in the presence of the inhibitors, the pull is suppressed, and as a result, the heads simply bind to actin without exerting any force. The results support the idea that the inhibitors do not block the myosin binding to actin, but block the step of force-producing transition of the bound actomyosin complex.
Keywords: blebbistatin, BTS, BDM, synchrotron radiation, small-molecule inhibitor
Significance.
X-ray diffraction and mechanical measurements indicate that, in the presence of saturating concentrations of small-molecule myosin inhibitors (BTS, BDM, blebbistatin), many of the Ca-induced activation processes occur normally in rabbit skeletal muscle. The results support the idea that these inhibitors primarily block the force-producing transition after myosin binding to actin.
N-benzyl-p-toluenesulfonamide (BTS) and blebbistatin are recently found small-molecule inhibitors that can specifically suppress the functions of myosin-II at micromolar concentrations [1–4]. BTS is specific to the fast skeletal isoform of myosin-II [1,2], but blebbistatin is effective for a wider variety of myosin-II isoforms. Because of this, blebbistatin has been widely used to test whether myosin-II is involved in various cellular processes. Biochemical and crystallographic studies shed some light on the mechanism of inhibition: Blebbistatin binds in a hydrophobic pocket at the apex of the 50-kDa cleft of the myosin motor domain [5], stabilizing the structure in the ‘transition’ state preceding the force-producing step after actin binding [6]. BTS is also reported to inhibit the release of inorganic phosphate (Pi) from myosin after ATP hydrolysis, a step coupled to the force producing step [2]. It is clear from these studies, therefore, that the two inhibitors do not exert their actions by simply eliminating the actomyosin interaction, and the consequences of the inhibition for the mechanical properties of muscle fibers are not straightforward.
Here, to characterize the inhibited state of muscle fibers, we recorded the changes of X-ray diffraction patterns upon calcium-activation of rabbit skeletal muscle fibers in the presence of these inhibitors, and compared them with those from control fibers or fibers in the presence of the traditional inhibitor, butanedione monoxime (BDM, [7–9]). BDM is also reported to block the force-producing step of the actomyosin interaction [10–15]. The published studies about the effects of myosin inhibitors on the X-ray diffraction patterns focus on two points: One is their effects on the helical order of the thick filaments in the relaxed state, and the other is their effects on the number of myosin heads attached to actin.
It is known that, in relaxed rabbit skeletal muscle fibers, the helix of the myosin heads on the thick filament is disordered at low temperatures (~0°C), while at higher temperature (~20°C), it is ordered. As a result, clearer myosin layer-line reflections are observed at higher temperatures [16]. Initially, this result was interpreted to reflect the temperature-dependent shift of the equilibrium between the M·ATP and M·ADP·Pi states of myosin (where M stands for myosin), the latter being favored at higher temperatures [17]. Later studies show that it is rather the ‘closed’ conformation of myosin head that gives rise to a good helical order. The M·ADP·Pi state is one of the states that favor the ‘closed’ conformation, but this conformation is also favored by the presence of so-called phosphate analogs, i.e., aluminofluoride and beryllium fluoride [18], and a myosin inhibitor blebbistatin [19], resulting in an increased helical order. An increased helical order is also observed in the presence of BDM [20]. Here the ‘closed’ conformation refers to the one consisting of “the switch-2 element of myosin head being in a position that interacts with the γ-phosphate-binding pocket; open conformation, the switch-2 loop being rotated away ~4 Å from the γ-phosphate-binding pocket, facilitating phosphate release” (quoted from ref. [19]).
As for the number of myosin heads attached to actin, the inhibitors are expected to increase the population of the myosin heads attached to actin in a low force A·M·ADP·Pi form (where A stands for actin) because of their properties to block the release of Pi from that actomyosin complex. Earlier results of the effect of BDM on X-ray diffraction patterns support this expectation [21,22]. These studies show that the intensity ratio of the 1,1 and 1,0 equatorial reflections (I1,1/I1,0 ratio, reflecting the number of myosin heads attached to or staying in the vicinity of the thin filaments) is greater than expected from the force level in muscle fibers activated in the presence of BDM. However, a conflicting result was obtained by Griffiths et al. [23], who showed that the I1,1/I1,0 ratio approached linearly to the relaxed level as the BDM concentration is increased. From this result they concluded that BDM simply reduces the number of attached myosin heads.
In the present study, we examined the effects of the two potent small-molecule myosin inhibitors, blebbistatin and BTS, on the diffraction patterns from rabbit skeletal muscle fibers, in addition to weaker BDM. A special emphasis is placed on the changes of the diffraction patterns upon calcium-activation. Blebbistatin and BTS were applied to fibers at a 100 μM concentration, which is much higher than their half-maximal inhibitory concentrations (IC50). Upon calcium-activation, however, still substantial changes were observed in the myosin-based reflections. From these results, it is evident that the effects of these inhibitors are not simply to reduce the number of myosin heads attached to actin.
Materials and methods
Material
Animal experiments were conducted under approval of the institutional animal experiment committee. Skinned muscle fibers were prepared from the psoas muscle of Japanese white rabbit as described [24]. Briefly, small bundles of muscle fibers (a few millimeters in diameter) were excised from the psoas muscle, and were treated with a relaxing solution containing 1% Triton X-100 for ~10 minutes on ice. After thoroughly washing with a relaxing solution, the bundles were placed in a 50% mixture of the relaxing solution and glycerol, and kept in a refrigerator overnight. After this, the bundles were placed in a freezer at −20°C for long-term storage. A few days before X-ray experiments, single fibers were pulled out of the bundle, and 30 fibers were mounted in a single specimen chamber, as described [25]. The sarcomere length was adjusted to the full filament overlap length (2.5 μm) by He-Ne laser diffraction. For mechanical measurements, single fibers or bundles of 2 single fibers were used.
Solutions
The composition of the solutions was as described [24]. The relaxing solution contained 80 mM K-propionate, 20 mM imidazole, 10 mM EGTA, 4 mM ATP, 5 mM MgCl2, 20 mM phosphocreatine and 300 U/ml creatine phosphokinase (C3755, Sigma-Aldrich, USA) (pH=7.2). In the pre-activating solution, the concentration of EGTA was reduced to 0.2 mM. The activating solution contained 10.1 mM CaCl2 in addition to the components of the relaxing solution. BTS was obtained from Sigma Rare Chemicals (USA) or Tokyo Chemical Industry Co. (Japan), and (S)-(−)-blebbistatin was obtained from Toronto Research Chemicals (Canada) or Calbiochem (USA). BDM was obtained from Sigma-Aldrich. When necessary, these chemicals were added to the three solutions described above.
Mechanical measurements
The isometric force and responses to step stretches (~1% fiber length) were recorded as described [24]. The force transducer was of a semiconductor type (AE801, Akers, Norway), and the stretches were applied by using an optical scanner (G-120D, General Scanning, USA). The data were recorded by using the data acquisition system (USB-6210, National Instruments, USA). The response to a step stretch was fitted to a single exponential decay function, and the amplitude of response was defined as the value extrapolated to the moment of stretch. The temperature of experiments was 6–8°C.
X-ray diffraction experiments
Static diffraction patterns were recorded in the BL45XU beamline of SPring-8, Hyogo, Japan [26], by using a cooled charge-coupled device (CCD) camera (1000×1018 pixels, model C4880 or 1024×1024 pixels, model C4792-98, Hamamatsu Photonics, Japan) in combination with an image intensifier (VP5445-mod, Hamamatsu Photonics) as described [25]. The wavelength of X-rays was 0.09 nm or 0.1 nm. The camera length was ~2 m. The X-ray diffraction patterns recorded under the same experimental conditions were summed, the four quadrants were averaged, and the background scattering was subtracted as described [27,28]. The patterns were recorded at 6–8°C.
Statistical Analysis
Statistical analyses (column statistics and t-test) were performed by using a commercial software package (Prism, GraphPad, USA).
Results
Mechanical measurements
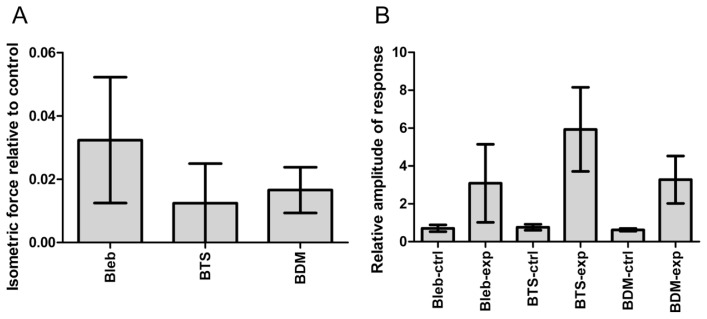
The three inhibitors, BTS, blebbistatin and BDM, had very similar effects on the isometric force and responses to step stretches at concentrations of 100 μM, 100 μM and 50 mM, respectively (Figs. 1 and 2). The calcium-activated isometric force was suppressed to ~2% of control in the presence of these inhibitors. When calcium-activated, the amplitude of the force response of control fibers to a ~1% stretch was 0.5–0.9 Po (Po = isometric force immediately before stretch). The inhibited fibers also showed clear force responses, and their amplitudes were much greater than in control fibers, after normalizing to the level of isometric force (2–7 Po). The force responses of relaxed fibers were virtually nonexistent, either in the presence or absence of inhibitors. These observations suggest that a greater number of myosin cross-bridges are formed than expected from the isometric force level in the presence of these inhibitors.
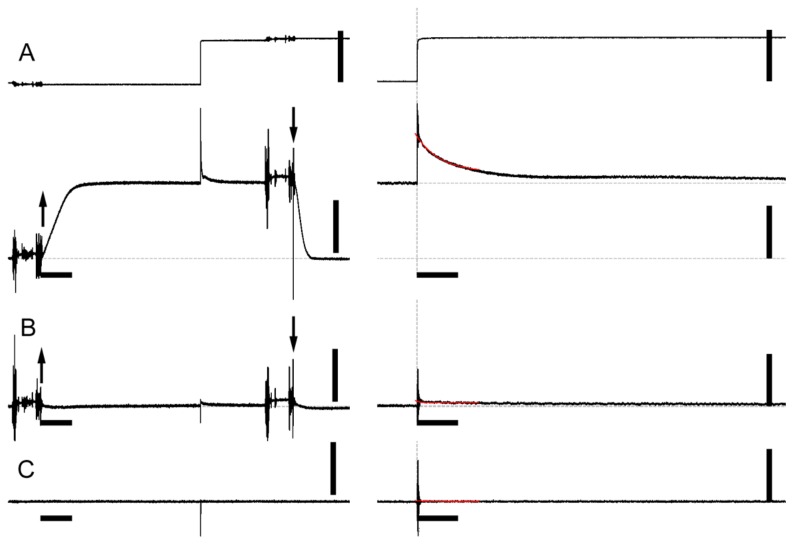
Figure 1.
Force response of skinned rabbit psoas muscle fibers to a step stretch in the presence or absence of inhibitors. (A), control, calcium-activated (pCa=4.0); (B), 100 μM blebbistatin, calcium-activated; (C), control, relaxed. Upper trace, length (A only); lower trace, force. Left traces, slower time scale; right traces, faster time scale. Time scale bars indicate 2 s and 50 ms in the left and right panels, respectively. The length and force scale bars indicate 50 μm and 0.5 mN, respectively (fiber length, ~5 mm). The upward and downward arrows indicate the solution exchanges from the pre-activating to activating solutions, and from the activating to relaxing solutions, respectively. The traces shown here were taken from the same muscle fiber.
Figure 2.
Summary of the mechanical properties of skinned rabbit psoas fibers in the presence of inhibitors. (A), the level of isometric force developed in the activating solution (pCa=4.0), relative to the value before addition of the inhibitor. Bleb=100 μM blebbistatin; BTS=100 μM N-benzyl-P-toluenesuifonamide; BDM=50 mM butanedione monoxime. The values indicate the mean±S.D. (n=4). (B), Amplitude of force response to a 1% step stretch of activated rabbit psoas fibers, before and after addition of inhibitors. The values are relative to the level of isometric force. ctrl=before addition of inhibitor; exp=after addition of inhibitor. The values indicate the mean±S.D. (n=4). The concentrations of the inhibitors are the same as in (A).
X-ray diffraction
By analyzing the equatorial reflections, one can obtain information about the radial force exerted by the myosin heads (the 1,0 lattice spacing, d1,0), and about the movement of myosin heads from the thick filament backbone towards the thin filaments (the 1,1/1,0 intensity ratio). The results of analysis are summarized in Figure 3.
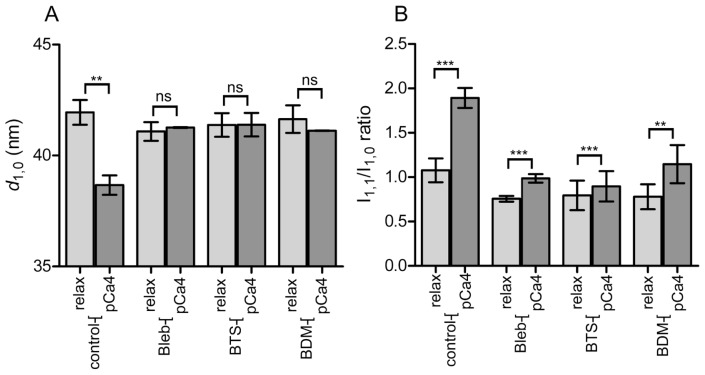
Figure 3.
Summary of analysis of X-ray equatorial reflections of skinned rabbit skeletal muscle fibers in the presence or absence of inhibitors. (A), The 1,0 lattice spacing (d1,0), obtained by Gaussian fitting of the reflection intensity profile after subtraction of the background (assumed exponential). (B), The 1,1/1,0 equatorial reflection intensity ratio from the same fibers. In both (A) and (B), the values are the mean±S.D. (n=6 sets of fibers for blebbistatin and n=4 for others). In most cases, 6 fibers were mounted in a single set, and this was sufficient for recording the equatorial reflections. The results of the paired t-test are also shown. ns=non-significant; and the levels of significance are; *, p<0.05; **, p<0.01; ***, p<0.001.
The d1,0 spacing in the relaxed state was 41–42 nm, regardless of the presence or absence of inhibitors. In control, the d1,0 spacing was reduced to ~38.7 nm upon calcium-activation, and the reduction is statistically highly significant (p<0.01). In the presence of inhibitors, however, no significant change was observed in the d1,0 spacing upon calcium-activation (Fig. 3A).
As for the 1,1/1,0 intensity ratio, it is well known that upon calcium-activation, the ratio is increased as the mass of myosin head approaches the thin filaments [29]. Confirming this, the intensity ratio increased from 1.08 to 1.89 upon calcium-activation of control fibers. In the presence of inhibitors, significant increases were observed in the ratio (p<0.01 in all three inhibitors), although the extent of increase was smaller than in control (Fig. 3B). This indicates that, although the activated force was dramatically suppressed, there is still substantial movement of myosin heads towards the thin filaments.
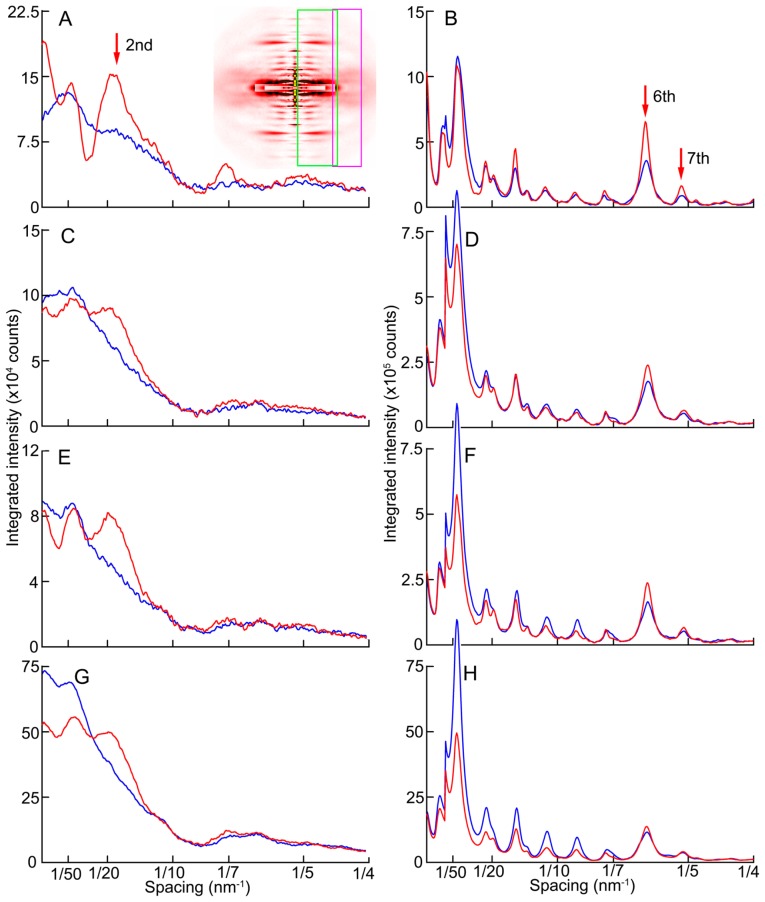
Effects of the inhibitors were also examined for the layer-line and meridional reflections (Figs. 4–6). It is immediately noticed from the difference map (Fig. 5, right column) that the calcium-induced enhancement of the 2nd and 6th actin layer lines occurs normally in the presence of inhibitors. The former reports mainly the movement of tropomyosin molecules on the thin filaments [30,31] and the latter reports the conformational change of actin monomers upon activation [25,32,33]. These changes indicate that the regulatory system on the thin filaments as well as actin is activated normally in the presence of the inhibitors. In the intensity profiles (Fig. 6), one may consider that the enhancement is weaker in the presence of inhibitors. However, the activation of the thin filament is known to occur in two steps, and the calcium-activated thin filament is further activated by the strongly-bound myosin heads [34]. In the presence of inhibitors, very few strongly-bound heads exist (only a few percent of normal). Considering this, it should be considered that the process of calcium-induced thin filament activation takes place normally in the presence of the inhibitors.
Figure 4.
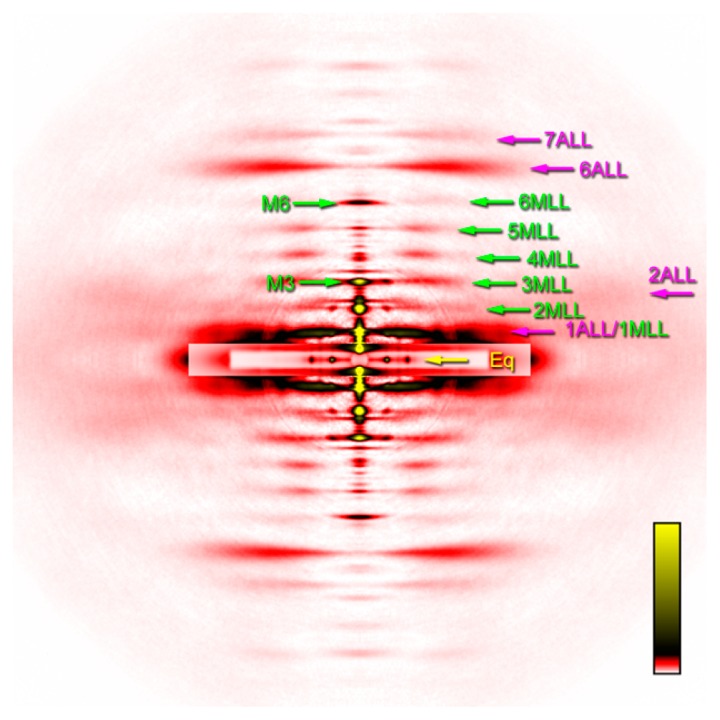
Two-dimensional diffraction pattern summed from 4 sets of 30 muscle fibers. MLL, myosin layer-line reflection; ALL, actin layer-line reflection. The number is the order of reflection. The 1st actin layer-line reflection at 1/36 nm and the 1st myosin reflection at 1/42.9 nm are partially overlapped. Eq, equatorial reflections. Of the two prominent reflections, the inner one is the 1,0 and the outer one is the 1,1. M3 and M6 are the myosin meridional reflections at 1/14.3 nm and 1/7.2 nm, respectively. The pattern was recorded in the presence of 100 μM blebbistatin at pCa=4.0 at 6–8°C.
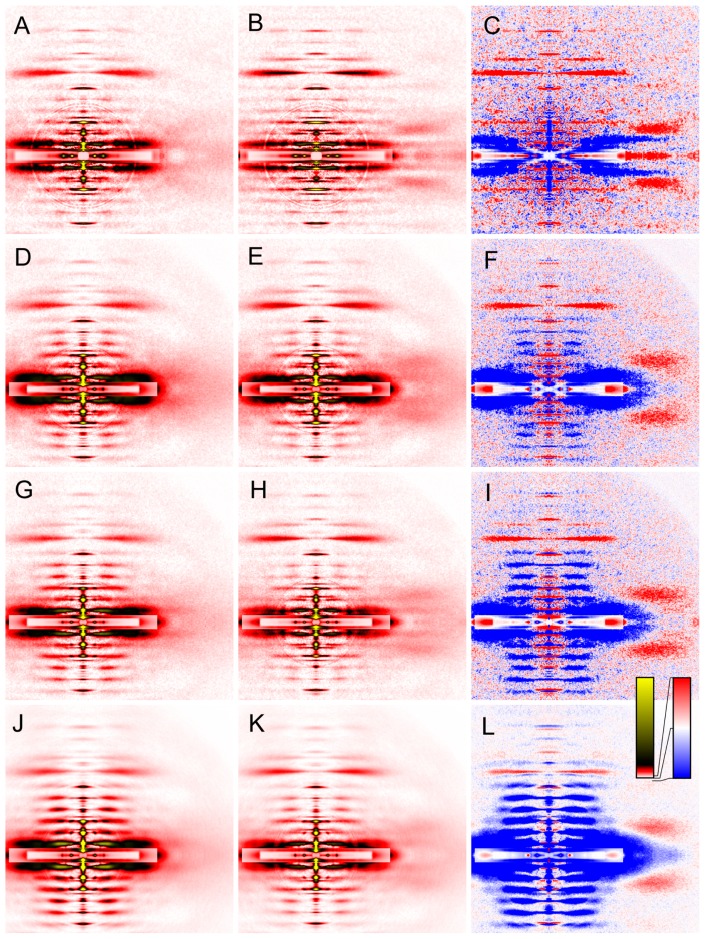
Figure 5.
Effect of calcium-activation on two-dimensional diffraction patterns from skinned rabbit psoas fibers in the presence or absence of inhibitors at 6–8°C. (A, B, C), control; (D, E, F), 100 μM BTS; (G, H, I), 50 mM BDM; (J, K, L), 100 μM blebbistatin. (A, D, G, J), relaxed; (B, E, H, K), calcium-activated; (C, F, I, L), difference between calcium-activated and relaxed patterns. The red color indicates that the intensities are increased upon calcium-activation. The blue area, decreased. The pattern in (K) is identical to the one in Figure 4. Patterns were summed for up to 4 sets of 30 muscle fibers.
Figure 6.
Changes of intensity profiles along the meridian upon calcium-activation. Taken from the diffraction patterns in Figure 5. (A, B), control; (C, D), 100 μM BTS; (E, F), 50 mM BDM; (G, H), 100 μM blebbistatin. (A, C, E, G), taken from the region containing the 2nd actin layer-line reflections (magenta box in the inset); (B, D, F, H), taken from the region containing the 6th and 7th actin layer-line reflections (green box in the inset of A). The blue and red curves represent the relaxed and calcium-activated states, respectively.
In vertebrate skeletal muscles, a series of clear myosin layer-line reflections are observed in the relaxed state, indicating that the myosin heads form well-registered helices on the thick filament backbone. During contraction, these reflections are weakened [35]. As stated in Introduction, the myosin layer-line reflections in relaxed mammalian muscle are weak at low temperature, indicating that the helix of myosin heads is disordered. When activated, no further decrease of intensities was observed in control fibers (Fig. 5A–C). As has been reported, the inhibitors restored the intensities of the myosin layer-line reflections in the relaxed state at low temperatures, but the extent of restoration seems to depend on inhibitors. BTS had the weakest effect (Fig. 5D). The effect of BDM was moderate (Fig. 5G), but its effect was somewhat variable. The effect of blebbistatin was the strongest of all (Fig. 5K). When these fibers were calcium-activated in the presence of inhibitors, the myosin layer-line reflections were weakened, as they are in uninhibited fibers at higher temperatures or in living frog muscle. The myosin layer-line reflections are not abolished completely, because, the force-producing step being blocked, the equilibrium among major reaction intermediates is expected to retain a substantial population in the M·ADP·Pi state that favors the helical order.
It is of interest to note that both the 3rd and 6th myosin meridional reflections, often called M3 (1/14.3 nm−1) and M6 (1/7.2 nm−1), respectively, shifted inwards along the meridian upon calcium-activation, even when the active force was almost completely abolished by the inhibitors. This indicates that the shift of the reflection (normally taken to indicate thick-filament elongation) is not caused by the elongation of the thick filaments by active force. In the case of M3, the spacing changed from 14.35±0.01 to 14.42±0.00 nm (mean±S.D., n=6; the same applies to the following statistics). This is a 0.5% increase, and is smaller than the 1.5% increase reported for intact muscle fibers [36]. Similarly, the M6 spacing changed from 7.278±0.003 to 7.316±0.004 nm, being also a 0.5% increase.
The integrated intensities of these meridional reflections decreased upon calcium-activation. The M3 and M6 decreased to 75±7% and 78±7%, respectively. This is contrary to the case of normally activated fibers, and this may be because the configuration of myosin heads in the presence of inhibitors is different from the normal one (less perpendicular to the filament axis).
In addition these reflections were spread along the equator, as they are during normal contraction. This spread is ascribed to the loss of lateral register of the thick filaments [37].
Discussion
In this study, we examined the effects of three small-molecule myosin inhibitors (BTS, blebbistatin and BDM) on the X-ray diffraction patterns and mechanics of skinned rabbit skeletal muscle fibers. A special focus was placed on their effects on the process of calcium-activation of these fibers.
Effects on mechanical properties
At the low temperatures used in this study, BTS, blebbistatin and BDM similarly suppressed the generation of active contractile force almost entirely, but not completely at concentrations of 100 μM, 100 μM and 50 mM, respectively. Approximately, 2–3% of active force remained at these concentrations. For BTS, this extent of remaining force agrees with the description by Cheung et al. [1], but this is greater than expected from the half maximal inhibitory concentration (IC50, 3 μM for BTS [1] and 1.6 μM for blebbistatin [38]). With this IC50 value for BTS, the residual force at 100 μM should be of the order of 10−10 of control (For BDM, the IC50 value is reported to be ~10 mM [39]). The persistence of the residual force may be because the forward rate constant of the target reaction step is not completely zero for inhibitor-bound myosin molecule. If so, it is expected that, once the target reaction step has passed, the following force-generating steps proceed normally.
The results of the mechanical experiment showed that the response to a 1% step stretch was much greater in the presence of inhibitors than in control, when normalized to the level of active force before stretch (Figs. 1, 2). This implies that, in addition to the small fraction of myosin heads that have entered the normal force-producing process, there exists an increased population of myosin heads that are attached to actin but do not produce force. This observation agrees with the results of biochemical studies, showing that these inhibitors block the force-producing transition rather than the step of myosin binding to actin (see Introduction).
It is generally considered that the stiffness of activated muscle fibers is proportional to the number of myosin heads bound to the thin filaments (e.g., Ford et al. [40]). A smaller decrease of muscle fiber stiffness than the active force has already been reported for BTS by Pinniger et al. [41], and they concluded that the higher stiffness relative to force was due to the presence of myosin heads attached in a pre-power-stroke state. The deviation from the linear relationship between force and stiffness may be explained by the presence of thick- and thin-filament compliance [42], but by using a fast-stretch protocol that is not affected by filament compliance, Colombini et al. [43] concluded that there was an increased population of non-force myosin cross-bridges in BTS-inhibited fibers.
If only the active myosin heads contribute to fiber stiffness, the stiffness would approach zero as the number of active myosin heads is reduced, whether or not the myofilaments are compliant. Unlike most of the other studies, the present experiments were conducted at a saturating concentration of BTS or blebbistatin, and the active force was very small. Nevertheless, disproportionally large force responses were recorded, and this is not explained by the presence of filament compliance. Therefore, the present results support the idea of an increased population of no- or low-force myosin cross-bridges. The decay of the force response was slow (Fig. 1B), indicating slow attachment/detachment kinetics. Therefore the low-force myosin cross-bridges present here are not like the weak-binding fast-equilibrium cross-bridges observed at low ionic strength [44], but rather like the slower ones whose population is increased in the presence of inorganic phosphate [24] or during shortening [45]. Nocella et al. [46] referred to the possibility of calcium-dependent stiffening of connectin (titin), but it is irrelevant to the results, because the present study was conducted at a short sarcomere length at which connectin remains slack.
Effects on X-ray diffraction patterns
Also in the X-ray diffraction experiments, the equatorial 1,1/1,0 intensity ratio showed a significant increase upon calcium-activation of inhibited fibers (Fig. 3b). The intensity change of X-ray reflection is proportional to the squares of the changes of the structure factor and the number of participating molecules as well. Therefore, the 2–3% population of myosin heads that have entered the force-producing processes would hardly affect the intensity ratio. Therefore, the changes of the intensity ratio also suggest the presence of a substantial myosin population that approaches the thin filaments upon calcium-activation.
The observation of the enhanced myosin layer-line reflections confirmed the previous results that the inhibitors restored the helical order of myosin heads on the thick filament, in the relaxed state at low temperatures. Although the extent of inhibition of active force was all similar for the three inhibitors, the extent of action of helical order restoration seemed to vary from inhibitor to inhibitor. The effect of blebbistatin was strongest, while that of BTS was weakest. This result may indicate that the action to induce the ‘closed’ state of myosin heads (see Introduction) differs from inhibitor to inhibitor, even at the saturating levels of inhibitors.
The restored myosin layer-line reflections were all weakened after calcium-activation. This means that the helical order of the myosin heads is diminished upon calcium-activation. At 20°C, the layer-line reflections were even stronger in the relaxed state and their intensities were reduced dramatically upon calcium-activation (data not shown). This clearly shows that there is some interaction between myosin heads and the thin filaments after calcium-activation. If 2–3% of myosin heads were bound to actin, and the rest of the myosin heads still kept the helical order, there would be no major reduction in the intensities of the layer-line reflections. Taking the evidence from the equatorial and layer-line reflections together, the X-ray diffraction results support the idea that the inhibited myosin heads interact with actin in some way, without generating active force, in the presence of calcium.
The highly helically-ordered state of myosin heads as observed at high temperature is often called “super-relaxed state” [47]. In this state it is also known that the 1,1/1,0 intensity ratio is further lowered with respect to the value at lower temperature [48]. The effect of the inhibitors seems to mimic that of an increased temperature, and Wilson et al. [49] reported that blebbistatin stabilized myosin in the super-relaxed state. In the present study, upon addition of inhibitors, the restoration of the myosin layer-line reflections is accompanied by a decrease of the 1,1/1,0 intensity ratio (see Fig. 3B and compare the relaxed ratio in control and those in the presence of inhibitors). Then an alternative interpretation of the bar-graphs in Figure 3B is possible, i.e., the action of calcium-activation in the presence of inhibitors is simply to bring back the myosin state from the super-relaxed to the normal-relaxed (disordered) states at this low temperature. However, this resemblance between the relaxed state in control and the calcium-activated state in the presence of inhibitors is considered to be superficial. In the absence of inhibitors, lowering the temperature is considered to shift the equilibrium between the M·ATP and M·ADP·Pi states toward the former, causing the disorder of myosin heads [16]. It is very unlikely that the calcium-induced activation of the thin filaments causes this equilibrium shift (reversal of the normal actomyosin reaction steps) without influencing actin-myosin interaction. It would be more natural to consider that, by making the myosin binding site open on actin, calcium promotes the forward transition from the M·ADP·Pi state to A·M·ADP·Pi state.
The high helical order of myosin heads is commonly observed in the relaxed mammalian and amphibian muscles. In this sense, the helically-ordered state is not “super-relaxed” but it is “normal-relaxed”. Rather, it is the disordered state at lower temperatures with a reversed equilibrium that is unusual.
It is worth noting that the inward shift of the myosin meridional reflections also occurred upon calcium-activation of inhibited fibers. In uninhibited fibers the shift of the M3 and M6 reflections corresponds to a ~1.5% elongation of the thick filaments, and higher-order reflections also show comparable changes [36]. If it comes from the real elongation of the thick filaments, it is too large to be explained by the force-induced strain of the thick filaments. Therefore it has been argued that the large shift of the peak position reflects a systematic perturbation of the helical repeat of the myosin heads [50–53]. The present study clearly indicates that a similar shift occurs in inhibited fibers in which active force is almost completely suppressed, and the results indicate that this process occurs normally in the inhibited fibers.
Conclusion
To summarize, many of the processes that occur in normal calcium-activated muscle fibers seem to occur normally in fibers inhibited by saturating concentrations of small-molecule myosin inhibitors. The present results generally support the idea that these inhibitors block the force-producing transition of attached myosin heads. As a result, the population of low-force myosin cross-bridges is increased. This low-force form is considered to be in equilibrium with the detached form, but the detached form is not identical with the form in the relaxed fibers.
Acknowledgement
We thank Dr. T. Hikima, RIKEN, for his help at the beamline. The X-ray diffraction study was performed under approval of SPring-8 Proposal Review Committee (Proposal Nos. 2004A0583-NL2a-np, 2005B0267, 2006A1427, 2013A1431, 2013B1333, 2017A1214, 2017B1212).
Footnotes
Conflicts of Interest
The author declares that he has no conflict of interest.
Author Contribution
H. I. designed and performed experiments, analyzed data and wrote manuscript.
References
- 1.Cheung A, Dantzig JA, Hollingwirth S, Baylor SM, Goldman YE, Mitchison TJ, et al. A small-molecule inhibitor of skeletal muscle myosin II. Nat Cell Biol. 2002;4:83–88. doi: 10.1038/ncb734. [DOI] [PubMed] [Google Scholar]
- 2.Shaw MA, Ostap ME, Goldman YE. Mechanism of inhibition of skeletal muscle actomyosin in N-benzyl-p-toluenesulfonamide. Biochemistry. 2003;42:6128–6135. doi: 10.1021/bi026964f. [DOI] [PubMed] [Google Scholar]
- 3.Straight AF, Cheung A, Limouze J, Chen I, Westwood NJ, Sellers JR, et al. Dissecting temporal and spatial control of cytokinesis with a myosin II inhibitor. Science. 2003;299:1743–1747. doi: 10.1126/science.1081412. [DOI] [PubMed] [Google Scholar]
- 4.Limouze J, Straight AF, Mitchison T, Sellers JR. Specificity of blebbistatin, an inhibitor of myosin II. J Muscle Res Cell Motil. 2004;25:337–341. doi: 10.1007/s10974-004-6060-7. [DOI] [PubMed] [Google Scholar]
- 5.Allingham JS, Smith R, Rayment I. The structural basis of blebbistatin inhibition and specificity for myosin II. Nat Struct Mol Biol. 2005;12:378–379. doi: 10.1038/nsmb908. [DOI] [PubMed] [Google Scholar]
- 6.Ramamurthy B, Yengo CM, Straight AF, Mitchison TJ, Sweeney HL. Kinetic mechanism of blebbistatin inhibition of nonmuscle myosin IIB. Biochemistry. 2004;43:14832–14839. doi: 10.1021/bi0490284. [DOI] [PubMed] [Google Scholar]
- 7.Horiuti K, Higuchi H, Umazume Y, Konishi M, Okazaki O, Kurihara S. Mechanism of action of 2,3-butanedione 2-monoxime on contraction of frog skeletal muscle fibres. J Muscle Res Cell Motil. 1988;9:156–164. doi: 10.1007/BF01773737. [DOI] [PubMed] [Google Scholar]
- 8.Higuchi H, Takemori S. Butanedione monoxime suppresses contraction and ATPase activity of rabbit skeletal muscle. J Biochem. 1989;105:638–643. doi: 10.1093/oxfordjournals.jbchem.a122717. [DOI] [PubMed] [Google Scholar]
- 9.Bagni MA, Cecchi G, Colomo F, Garzella P. Effects of 2,3-butanedione monoxime on the crossbridge kinetics in frog single muscle fibres. J Muscle Res Cell Motil. 1992;13:516–522. doi: 10.1007/BF01737994. [DOI] [PubMed] [Google Scholar]
- 10.Lenart TD, Tanner JW, Goldman YE. 2,3-butanedione monoxime (BDM) suppresses cross-bridge reattachment following laser photolysis of caged ATP. Biophys J. 1989;55:260a. [Google Scholar]
- 11.Herrmann C, Wray J, Travers F, Barman T. Effect of 2,3-butanedione monoxime on myosin and myofibrillar ATPases. An example of an uncompetitive inhibitor. Biochemistry. 1992;31:12227–12232. doi: 10.1021/bi00163a036. [DOI] [PubMed] [Google Scholar]
- 12.McKillop DFA, Fortune NS, Ranatunga KW, Geeves MA. The influence of 2,3-butanedione 2-monoxime (BDM) on the interaction between actin and myosin in solution and in skinned muscle fibres. J Muscle Res Cell Motil. 1994;15:309–318. doi: 10.1007/BF00123483. [DOI] [PubMed] [Google Scholar]
- 13.Zhao Y, Kawai M. BDM affects nucleotide binding and force generation steps of the cross-bridge cycle in rabbit psoas muscle fibers. Am J Physiol. 1994;266:C437–C447. doi: 10.1152/ajpcell.1994.266.2.C437. [DOI] [PubMed] [Google Scholar]
- 14.Zhao L, Naber N, Cooke R. Muscle cross-bridges bound to actin are disordered in the presence of 2,3-butanedione monoxime. Biophys J. 1995;68:1980–1990. doi: 10.1016/S0006-3495(95)80375-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Regnier M, Morris C, Homsher E. Regulation of the cross-bridge transition from a weakly to strongly bound state in skinned rabbit muscle fibers. Am J Physiol. 1995;269:C1532–C1539. doi: 10.1152/ajpcell.1995.269.6.C1532. [DOI] [PubMed] [Google Scholar]
- 16.Wray JS. Structure of relaxed myosin filaments in relation to nucleotide state in vertebrate skeletal muscle. J Muscle Res Cell Motil. 1987;8:62a. [Google Scholar]
- 17.Xu S, Gu J, Rhodes T, Belknap B, Rosenbaum G, Offer G, et al. The MADPPi state is required for helical order in the thick filaments of skeletal muscle. Biophys J. 1999;77:2665–2676. doi: 10.1016/s0006-3495(99)77101-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zoghbi ME, Woodhead JL, Craig R, Padrón R. Helical order in tarantula thick filaments requires the “closed” conformation of the myosin head. J Mol Biol. 2004;342:1223–1236. doi: 10.1016/j.jmb.2004.07.037. [DOI] [PubMed] [Google Scholar]
- 19.Xu S, White HD, Offer GW, Yu LC. Stabilization of helical order in the thick filaments by blebbistatin: further evidence of coexisting multiple conformations of myosin. Biophys J. 2009;96:3673–3681. doi: 10.1016/j.bpj.2009.01.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yamaguchi M, Kimura M, Li ZB, Ohno T, Takemori S, Hoh JF, et al. X-ray diffraction analysis of the effects of myosin regulatory light chain phosphorylation and butanedione monoxime on skinned skeletal muscle fibers. Am J Physiol Cell Physiol. 2016;310:C692–C700. doi: 10.1152/ajpcell.00318.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yagi N, Takemori S, Watanabe M, Horiuti K, Amemiya Y. Effects of 2,3-butanedione monoxime on contraction of frog skeletal muscle: an X-ray diffraction study. J Muscle Res Cell Motil. 1992;13:153–160. doi: 10.1007/BF01874152. [DOI] [PubMed] [Google Scholar]
- 22.Hoskins BK, Ashley CC, Pelc R, Rapp G, Griffiths PJ. Time-resolved equatorial X-ray diffraction studies of skinned muscle fibres during stretch and release. J Mol Biol. 1999;290:77–97. doi: 10.1006/jmbi.1999.2857. [DOI] [PubMed] [Google Scholar]
- 23.Griffiths PJ, Bagni MA, Colombini B, Amenitsch H, Bernstorff S, Funari S, et al. Effects of the number of actin-bound S1 and axial force on X-ray patterns of intact skeletal muscle. Biophys J. 2006;90:975–984. doi: 10.1529/biophysj.105.068619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Iwamoto H. Strain sensitivity and turnover rate of low force cross-bridges in contracting skeletal muscle fibers in the presence of phosphate. Biophys J. 1995;68:243–250. doi: 10.1016/S0006-3495(95)80180-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Iwamoto H, Oiwa K, Suzuki T, Fujisawa T. X-ray diffraction evidence for the lack of stereospecific protein interactions in highly activated actomyosin complex. J Mol Biol. 2001;305:863–874. doi: 10.1006/jmbi.2000.4334. [DOI] [PubMed] [Google Scholar]
- 26.Fujisawa T, Inoue K, Oka T, Iwamoto H, Uruga T, Kumasaka T, et al. Small-angle X-ray scattering station at the SPring-8 RIKEN beamline. J Appl Cryst. 2000;33:797–800. [Google Scholar]
- 27.Iwamoto H, Wakayama J, Fujisawa T, Yagi N. Static and dynamic X-ray diffraction recordings from living mammalian and amphibian skeletal muscles. Biophys J. 2003;85:2492–2506. doi: 10.1016/s0006-3495(03)74672-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Iwamoto H, Inoue K, Yagi N. Fast X-ray recordings reveal dynamic action of contractile and regulatory proteins in stretch-activated insect flight muscle. Biophys J. 2010;99:184–192. doi: 10.1016/j.bpj.2010.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Haselgrove JC, Huxley HE. X-ray evidence for radial cross-bridge movement and for the sliding filament model in actively contracting skeletal muscle. J Mol Biol. 1973;77:549–568. doi: 10.1016/0022-2836(73)90222-2. [DOI] [PubMed] [Google Scholar]
- 30.Huxley HE. Structural changes in the actin- and myosin-containing filaments during contraction. Cold Spring Harbor Symp Quant Biol. 1973;37:361–376. [Google Scholar]
- 31.Parry DAD, Squire JM. Structural role of tropomyosin in muscle regulation: Analysis of the X-ray diffraction patterns from relaxed and contracting muscles. J Mol Biol. 1973;75:33–55. doi: 10.1016/0022-2836(73)90527-5. [DOI] [PubMed] [Google Scholar]
- 32.Wakabayashi K, Ueno Y, Amemiya Y, Tanaka H. Intensity changes of actin-based layer lines from frog skeletal muscles during an isometric contraction. Adv Exp Med Biol. 1988;226:353–367. [PubMed] [Google Scholar]
- 33.Yagi N, Matsubara I. Changes in the 5.9 nm actin layer-line on activation of frog skeletal muscles. Adv Exp Med Biol. 1988;226:369–380. [PubMed] [Google Scholar]
- 34.Lehrer SS, Morris EP. Dual effects of tropomyosin and troponin-tropomyosin on actomyosin subfragment-1 ATPase. J Biol Chem. 1982;257:8073–8080. [PubMed] [Google Scholar]
- 35.Huxley HE, Brown W. The low-angle X-ray diagram of vertebrate striated muscle and its behaviour during contraction and rigor. J Mol Biol. 1967;30:383–434. doi: 10.1016/s0022-2836(67)80046-9. [DOI] [PubMed] [Google Scholar]
- 36.Huxley HE, Stewart A, Sosa H, Irving T. X-ray diffraction measurements of the extensibility of actin and myosin filaments in contracting muscle. Biophys J. 1994;67:2411–2421. doi: 10.1016/S0006-3495(94)80728-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Huxley HE, Faruqi AR, Kress M, Bordas J, Koch MHJ. Time-resolved X-ray diffraction studies of the myosin layer-line reflections during muscle contraction. J Mol Biol. 1982;158:637–684. doi: 10.1016/0022-2836(82)90253-4. [DOI] [PubMed] [Google Scholar]
- 38.Kovács M, Tóth J, Heténtyi C, Málnási-Csizmadia A, Sellers JR. Mechanism of blebbistatin inhibition of myosin II. J Biol Chem. 2004;279:35557–35563. doi: 10.1074/jbc.M405319200. [DOI] [PubMed] [Google Scholar]
- 39.Fryer MW, Neering IR, Stephenson DG. Effects of 2,3-butanedione monoximime on the contractile activation properties of fast- and slow-twitch rat muscle fibres. J Physiol (Lond) 1988;407:53–75. doi: 10.1113/jphysiol.1988.sp017403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ford LE, Huxley AF, Simmons RM. The relation between stiffness and filament overlap in stimulated frog muscle fibres. J Physiol (Lond) 1981;311:219–249. doi: 10.1113/jphysiol.1981.sp013582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pinniger GJ, Bruton JD, Westerblad H, Ranatunga KW. Effects of a myosin-II inhibitor (N-benzyl-p-toluene sulphonamide, BTS) on contractile characteristics of intact fast-twitch mammalian muscle fibres. J Muscle Res Cell Motil. 2005;26:135–141. doi: 10.1007/s10974-005-2679-2. [DOI] [PubMed] [Google Scholar]
- 42.Linari M, Piazessi G, Lombardi V. The effect of myofilament compliance on kinetics of force generation by myosin motors in muscle. Biophys J. 2009;96:583–592. doi: 10.1016/j.bpj.2008.09.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Colombini B, Nocella M, Bagni MM, Griffiths PJ, Cecchi G. Is the cross-bridge stiffness proportional to tension during muscle fiber activation? Biophys J. 2010;98:2582–2590. doi: 10.1016/j.bpj.2010.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Brenner B, Schoenberg M, Chalovich JM, Greene LE, Eisenberg G. Evidence for cross-bridge attachment in relaxed muscle at low ionic strength. Proc Natl Acad Sci USA. 1982;79:7288–7291. doi: 10.1073/pnas.79.23.7288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Iwamoto H. Evidence for increased low force cross-bridge population in shortening skinned skeletal muscle fibers: Implications for actomyosin kinetics. Biophys J. 1995;69:1022–1035. doi: 10.1016/S0006-3495(95)79977-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nocella M, Cecchi G, Bagni MM, Colombini B. Force enhancement after stretch in mammalian muscle fiber: no evidence of cross-bridge involvement. Am J Physiol Cell Physiol. 2014;307:C1123–C1129. doi: 10.1152/ajpcell.00290.2014. [DOI] [PubMed] [Google Scholar]
- 47.Stewart MA, Franks-Skiba K, Chen S, Cooke R. Myosin ATP turnover rate is a mechanism involved in thermongenesis in resting skeletal muscle fibers. Proc Natl Acad Sci USA. 2010;107:430–435. doi: 10.1073/pnas.0909468107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bershitsky SY, Koubassova NA, Bennett PM, Ferenczi MA, Shestakov DA, Tsaturyan AK. Myosin heads contribute to the maintenance of filament order in relaxed rabbit muscle. Biophys J. 2010;99:1827–1834. doi: 10.1016/j.bpj.2010.06.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wilson C, Naber N, Pate E, Cooke R. The myosin inhibitor blebbistatin stabilizes the super-relaxed state in skeletal muscle. Biophys J. 2014;107:1637–1646. doi: 10.1016/j.bpj.2014.07.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Haselgrove JC. X-ray evidence for conformational changes in the myosin filaments of vertebrate striated muscle. J Mol Biol. 1975;92:113–143. doi: 10.1016/0022-2836(75)90094-7. [DOI] [PubMed] [Google Scholar]
- 51.Yagi N, O’Brien EJ, Matsubara I. Changes of thick filament structure during contraction of frog striated muscle. Biophys J. 1981;33:121–137. doi: 10.1016/S0006-3495(81)84876-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bordas J, Diakun GP, Diaz FG, Lewis RA, Lowy J, Mant GR, et al. Two-dimensional time-resolved X-ray diffraction studies of live isometrically contracting frog sartorius muscle. J Muscle Res Cell Motil. 1993;14:311–324. doi: 10.1007/BF00123096. [DOI] [PubMed] [Google Scholar]
- 53.Wakabayashi K, Sugimoto Y, Tanaka H, Ueno Y, Takezawa Y, Amemiya Y. X-ray diffraction evidence for the extensibility of actin and myosin filaments during muscle contraction. Biophys J. 1994;67:2422–2435. doi: 10.1016/S0006-3495(94)80729-5. [DOI] [PMC free article] [PubMed] [Google Scholar]