Abstract
We hypothesized that tobacco usage is an independent prognostic factor in patients with myelodysplastic syndromes (MDS). To evaluate the impact of tobacco usage in this population, we identified patients diagnosed with MDS in our Center’s MDS database and reviewed individual charts retrospectively. Of the 767 MDS patients identified, 743 patients (97%) had a known tobacco usage history. Given that the majority of tobacco users were smokers, we stratified patients as having never smoked (never-smoker group) versus current or former smokers (ever-smoker group). Greater than 60% of ever-smokers were risk stratified as having low or intermediate-1 (int-1) risk at diagnosis based on the International Prognostic Scoring System for MDS. In patients with lower-risk MDS, we found that ever-smokers had an increased proportion of poor-risk karyotypes (8.8%) compared with never-smokers (2.4%) (P = 0.003). The adverse effect of smoking was greatest in the low-risk and int-1-risk groups, where median overall survival was 69 months (95% CI 42–96) in never-smokers versus 48 months (95% CI 41–55) in ever-smokers (P = 0.006). The median overall survival for never-smokers, former smokers, and current smokers was 69 months (95% CI 42–96), 50 months (95% CI 43–57), and 38 months (95% CI 23–53), respectively, in patients risk stratified as lower-risk MDS (P = 0.01). Our findings suggest that tobacco usage negatively impacts overall survival in patients with lower-risk MDS.
Keywords: MDS, Myelodysplastic syndromes, Tobacco
1. Introduction
Myelodysplastic syndromes (MDS) encompass a heterogeneous spectrum of hematopoietic malignancies characterized by morphologic dysplasia and bone marrow failure, with a (AML) transformation [1,2]. Treatment goals for MDS include improvement of quality of life, reversal of symptomatic cytopenias, and prevention and potential delay of AML transformation. Risk stratification by clinically predictive models is utilized to assist in determining therapeutic decisions. The most widely utilized model is the International Prognostic Scoring System (IPSS) [3]; more recently, several newer models have also been validated as prognostic tools [4–6]. However, in addition to a patient’s inherent disease status, assessment of an individual’s comorbidities and behavioral factors may have a significant impact on patient outcomes. Because MDS is a disease seen predominantly with advancing age, these factors may require further consideration in addition to disease-specific risk assessments [7].
Current or former tobacco usage has been both well recognized and established as being associated with an increased risk for developing several malignancies. Several epidemiologic studies suggest a link between history of smoking tobacco and risk of MDS [8–10]. Recent meta-analysis investigating cigarette smoking and the development of MDS suggests that there is an association between ever-smokers and increased risk of developing MDS [11]. However, there has been limited evaluation of tobacco usage with MDS-related outcomes; to our knowledge, only one other study has been published that addresses this issue. Data from the analysis conducted by Ma and colleagues suggest that cigarette smoking in patients with low-risk MDS is associated with increased mortality [12]. Thus, we were interested in further investigating the impact of tobacco usage on outcome among lower- and higher-risk MDS patients and conduct here a retrospective analysis addressing this question.
2. Methods
Patients were identified through the MDS database at the Moffitt Cancer Center (MCC). Individual charts were subsequently reviewed for variables associated with MDS diagnosis. The primary objective was to evaluate the role of prior or current tobacco usage at time of presentation to MCC as a prognostic factor for overall survival (OS) and to assess risk of progression to AML. Variables collected included demographics, karyotype, serum ferritin, red blood cell transfusion dependence, and treatment with azanucleosides. IPSS risk stratification and MD Anderson risk stratification were calculated as previously reported [3,4]. Briefly, the IPSS is calculating by determining a cumulative score based on values or ranges within each of the following parameters: percentage of marrow blasts (<5%, 5–10%, 11–20%, 21–30%), karyotype (good, intermediate, poor), and number of peripheral cytopenias (0–1, or 2–3). The MD Anderson risk stratification is calculating similarly by determining a cumulative score based on values or ranges within each of the following factors: performance status (Zubrod performance status 0–1, ≥2), patient age (<60, 60–64, ≥65), degree of peripheral thrombocytopenia (platelets (K/μl) <30, 30–49, 50–199, ≥200) degree of peripheral anemia (hemoglobin (G/DL) <12.0, ≥12.0), percentage of bone marrow blasts (<5, 5–10, 11–29), presence of leukocytosis (white blood count (K/μl) ≤20.0, >20.0), karyotype (chromosome 7 abnormalities or >3 abnormalities, all other abnormalities), and history of prior transfusions of either red blood cells or platelets (yes or no).
Tobacco use status was obtained through the MCC registry database and subsequently used for this study. History of tobacco usage was obtained by cancer registry abstractors who locate information on smoking status from a variety of places within the medical record, including but not limited to (1) the patient’s self-reported smoking status obtained through patient questionnaire at time of consultation and (2) physician notes. These parameters were subsequently entered into the MCC registry database and coded into categories of tobacco usage. Because a majority of our patients used smoking tobacco (i.e., cigarettes), patients were grouped into the following categories: never-smoker, former-smoker, and current-smoker. Never-smokers were defined as patients with self-reported history of never having any prior cigarette usage. Categorization as a former and current smoker was again based on patient self-reported history and included patients who had a history or currently smoked less than 1 pack-per-day to greater than 2 packs-per-day. Because the number of patients utilizing smokeless tobacco was limited, we included these patients with the patients who used smoking tobacco. Therefore, this group will be referred to as “smokers” in our analysis. Additionally, former- and current-tobacco users were categorized together as “ever-smokers.”
Chi-square test and t-test were used to compare baseline characteristics between groups. Kaplan–Meier estimates were used to calculate OS from time of referral to MCC. Log-rank test was used for comparison of the Kaplan–Meier survival estimates between different groups. Cox proportional hazards regression was used for multivariable analysis. Statistical significance was defined as a P value <0.5 using two-tailed tests. All analyses were conducted using SPSS version 19.0 software.
3. Results
Between January 2001 and December 2009, 767 patients were identified in the MCC MDS database at the time of this analysis. Of these patients, tobacco usage history at presentation to MCC was known in 743 patients. These patients were subsequently stratified based on their tobacco usage status (current, former, or never), with 256 patients stratified in the never-smoker group and 487 stratified in the ever-smoker group (current and former smokers). Tobacco use included current cigarette smokers (n = 70), former tobacco users (n = 399), cigar/pipe users (n = 16), and snuff/chew users (n = 2). Table 1 summarizes the baseline characteristics. When we analyzed patients categorized by IPSS with low-risk and intermediate-1 (int-1)-risk MDS, we observed a significantly greater proportion of poor-risk karyotype in ever-smokers (8.8%) versus never-smokers (2.4%) (P = 0.003).
Table 1.
Baseline characteristics based on smoking status.
| Never-smokers (n = 256) |
Ever-smokers (n = 487) |
P-value | |
|---|---|---|---|
| Age | |||
| <60 years | 74 (28.9%) | 118 (24.2%) | 0.1 |
| ≥60 years | 182 (71.1%) | 369 (75.8%) | |
| WHO classification | |||
| RA | 54 (21.1%) | 108 (22.2%) | 0.06 |
| RARS | 28 (10.9%) | 63 (12.9%) | |
| RCMD | 61 (23.8%) | 80 (16.4%) | |
| RAEB | 96 (37.5%) | 213 (43.7%) | |
| Del 5q | 7 (2.7%) | 15 (3.1%) | |
| Karyotype | |||
| Good | 160 (62.5%) | 275 (56.5%) | 0.1 |
| Intermediate | 36 (14.1%) | 80 (16.4%) | |
| Poor | 42 (16.4%) | 109 (22.4%) | |
| Missing | 18 (7%) | 23 (4.7%) | |
| MD Anderson risk | |||
| Low | 61 (23.8%) | 95 (19.5%) | 0.35 |
| Intermediate-1 | 85 (33.2%) | 147 (30.2%) | |
| Intermediate-2 | 48 (18.8%) | 102 (20.9%) | |
| High | 35 (13.7%) | 88 (18.1%) | |
| Missing | 27 (10.5%) | 55 (11.3%) | |
| RBC-TD | |||
| Yes | 116 (45.3%) | 230 (47.7%) | 0.7 |
| HMA treatment | |||
| Yes | 115 (49.9%) | 248 (50.9%) | 0.07 |
| IPSS | |||
| Low | 55 (24%) | 87 (20.3%) | 0.6 |
| Intermediate-1 | 113 (49.3%) | 209 (48.7%) | |
| Intermediate-2 | 49 (21.4%) | 106 (24.7%) | |
| High | 12 (5.5%) | 27 (6.3%) | |
| Ferritin ≥1000 ng/mL | 61 (35.9%) | 104 (31.8%) | 0.2 |
HMA, hypomethylating agent; IPSS, International Prognostic Scoring System; RA, refractory anemia; RARS, refractory anemia with ringed sideroblasts; RCMD, refractory cytopenia with multilineage dysplasia; RAEB, refractory anemia with excess blasts; RBC-TD, red blood cell transfusion dependence; WHO, World Health Organization.
With a median follow-up duration from diagnosis of MDS of 55 months [95% confidence interval (CI) 50.5–59.6], we found that the median OS for never-smokers was 48 months (95% CI 37–59) compared to 35 months (95% CI 29–41) in ever-smokers (P = 0.01) (Fig. 1). The adverse effect of smoking was noted to be greatest in patients with lower-risk MDS, defined as having low- or int-1-risk by IPSS. In these patients, the median OS was 69 months (95% CI 42–96) in never-smokers compared to 48 months (95% CI 41–55) in ever-smokers (P = 0.006) (Fig. 2). The median OS was 69 months (95% CI 42–96), 50 months (95% CI 43–57), and 38 months (95% CI 23–53), respectively, in the never-smoker, former-smoker, and current smoker groups in lower-risk MDS (P = 0.01) (Fig. 3). No difference in OS was observed in patients with higher-risk MDS (intermediate-2 or high risk based on IPSS) with a median OS of 22 months (95% CI 12–32) in never-smokers and 18 months (95% CI 14–22) in the ever-smoker group (P = 0.89).
Fig. 1.
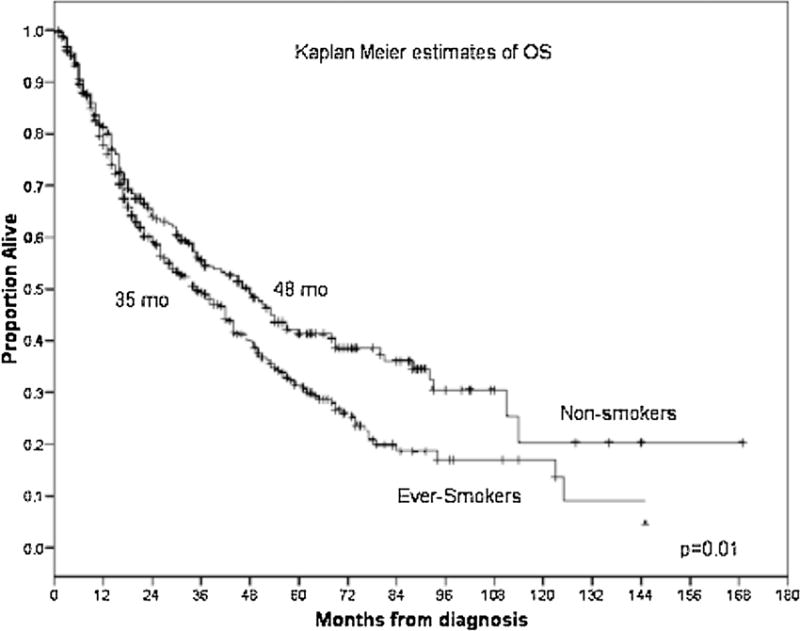
Kaplan–Meier estimates of overall survival (OS) according to smoking status in patients with myelodysplastic syndrome (MDS).
Fig. 2.
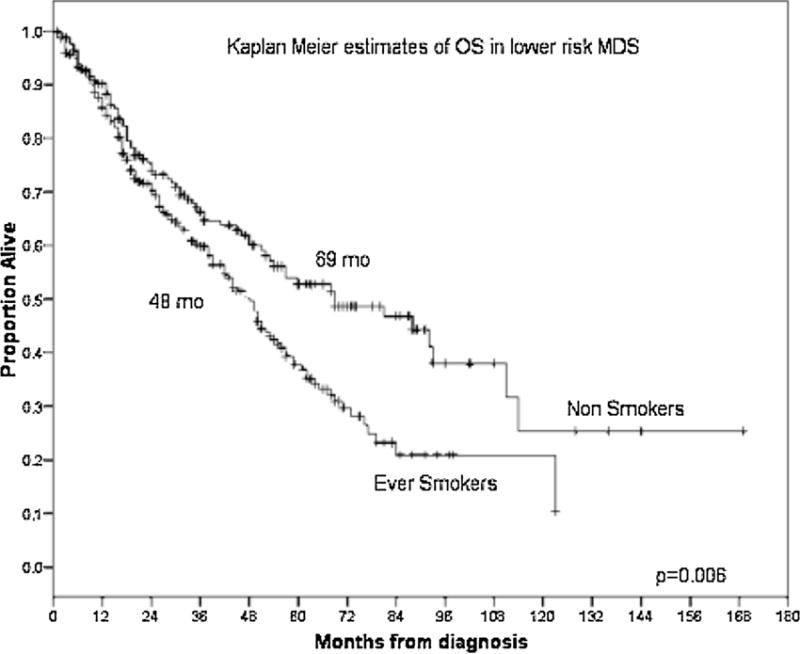
Kaplan–Meier estimates of OS according to smoking status in lower-risk MDS by IPSS.
Fig. 3.
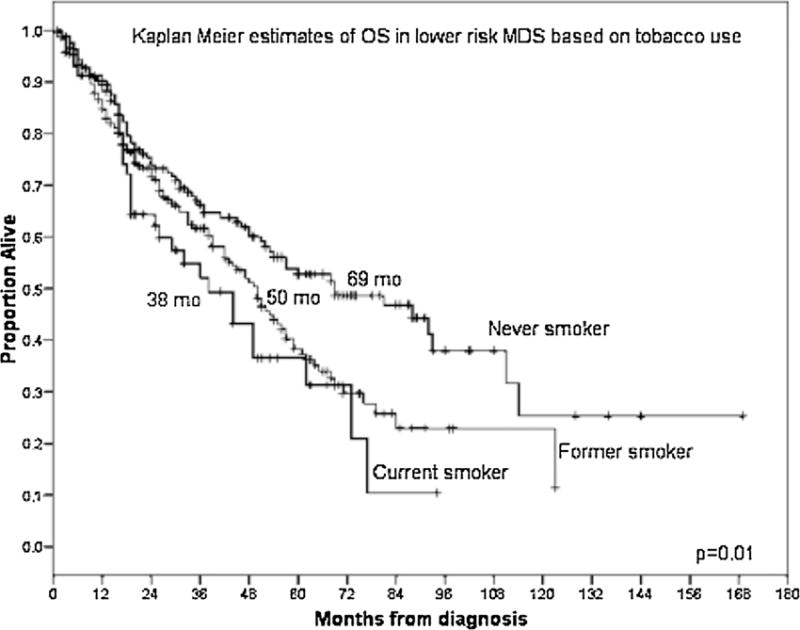
Kaplan–Meier estimates of OS according to smoking status (never, former, or current smokers) in lower-risk MDS by IPSS.
We further explored the association of smoking and karyotype amongst patients with lower-risk MDS. Cytogenetic features and risk stratification as defined by the IPSS differed significantly among never-smokers, former smokers, and current smokers (P = 0.12). The majority of lower-risk MDS patients had good risk cytogenetics as defined by the IPSS, with 80% of never-smokers (n = 134), 75% of former smokers (n = 177), and 83% of current smokers (n = 48) stratified into this karyotype category. Intermediate risk karyotype was noted in 16% of never-smokers (n = 26), 16% of former smokers (n = 37), and 12% of current smokers (n = 7). Poor risk karyotypic features were noted in 2% of never-smokers (n = 4), 10% of former smokers (n = 23), and 5% of current smokers (n = 3). An adverse impact of smoking was observed in good- and intermediate-risk karyotype but not in poor-risk karyotype. Among patients with good-risk karyotype, the median OS in never-smokers was 57 months (95% CI 42–73) compared to 45 in months in ever-smokers (95% CI 37–53) (P = 0.03). In the intermediate-risk karyotype group, the median OS was 51 months (95% CI 29–73) for never-smokers compared to 20 months (95% CI 12–28 months) in ever-smokers (P = 0.09). Finally, for the poor-risk karyotype group, the median OS was 17 months (95% CI 7–27) compared to 18 months (95% CI 15–21) for never-smokers and ever-smokers, respectively (P = 0.5).
Regarding AML transformation, in patients with lower-risk MDS, the rate of AML transformation was 18.2% in ever-smokers versus 9.5% in never-smokers (P = 0.04). No differences in rate of AML transformation based on tobacco usage were observed in higher-risk MDS patients. Finally, we aimed to stratify patients based on azanucleoside therapy; 38% of never-smokers (n = 64), 50% of former smokers (n = 118), and 45% (n = 26) of current smokers received a hypomethylating agent (P = 0.7). Based on azanucleo-side therapy, we found that, in patients with lower-risk MDS, the impact of tobacco usage was seen predominantly in those patients who did not receive azanucleosides therapy. The median OS for patients who did not receive azanucleosides was 50 months (95% CI 44–56) for ever-smokers compared to 111 months (95% CI 57–165) for never-smokers (P = 0.002). In contrast, patients with lower-risk MDS who received azanucleosides had median OS results of 42 months (95% CI 33–51) for ever-smokers versus 51 months (95% CI 31–71) for never-smokers (P = 0.67). In multivariable Cox regression analysis in lower-risk MDS patients, tobacco usage predicted inferior OS [Hazard ratio 1.49 (95% CI 1.1–2.0)] (P = 0.009) after adjustment for MD Anderson risk stratification. When further adjusting by MD Anderson risk stratification, age at diagnosis, and tobacco usage, both MD Anderson risk stratification (P = 0.000; Hazard ratio 1.62 (95% CI 1.4–1.9)) and tobacco usage (P = 0.006; Hazard ratio 1.49 (95% CI 1.1–2.0)) were predictive for inferior OS in multivariate analysis.
4. Discussion
The employment of currently available prognostic models for those diagnosed with MDS has been validated to assist in the estimation of OS and progression of AML in MDS [3–6,13]. Although each scoring system has some variability, these models prognosticate MDS based primarily on inherent intrinsic disease attributes, such as percentage of bone marrow blasts. Additional independent disease related factors have also been reported as independent prognostic variables, and with continued advancement in molecular testing, biologic prognostic factors will enhance our understanding of the disease [14–21]. Prior studies demonstrate that underlying co-morbidities and performance status are also well-established prognostic factors. Our aim was to evaluate patient- or “host”-specific features that may contribute to MDS-related outcomes, specifically, the influence of habitual and behavioral factors. Therefore, we aimed to determine the association between tobacco usage and disease outcomes.
To our knowledge, this is the largest study to address impact of tobacco usage on outcome in a cohort of MDS patients. Ma and colleagues, when investigating the impact of tobacco use among 616 patients, reported poor outcomes in patients who smoked, particularly in those with low-risk MDS by IPSS [12]. Our study confirms these findings in a larger cohort. We also demonstrate that not only is OS worse in lower-risk MDS patients who were ever-smokers, but we were able to further segregate low-risk MDS patients into never-, former-, and current-smokers, each with distinct and statistically different OS rates. The higher frequency of poor-risk karyotypes and AML progression among tobacco users in lower-risk patients suggests that tobacco exposure may influence the disease-specific biological potential. The effect seen from tobacco usage was independent of the global MD Anderson Score, which has been reported to refine the prognostic value of the IPSS [6]. Tobacco usage was seen to have a predominant effect on OS in those patients who did not receive treatment with azanucleosides. Although these results are intriguing, the difference noted in this study may reflect the bias of the treating physician and the presence of higher-risk features in those who were treated with azanucleosides compared to those who were not. Whether behavior modifications including discontinuation of tobacco usage may improve outcomes is an intriguing concept. The variable OS rate between former smokers and current smokers suggests that decreased tobacco exposure may affect MDS-related mortality; further studies in regard to modification of these factors are required.
The pathophysiologic mechanism by which tobacco usage may play a role in MDS is less clear. Cigarette smoke has been shown to be a primary exposure source of known carcinogen benzene, and increased benzene blood levels have been reported in smokers [22]. In an epidemiologic study by Korte et al., approximately 10–50% of smoking-induced total leukemia mortality and up to 60% of smoking-related AML mortality were suggested to be related to benzene exposure [23]. Animal models have demonstrated that the deficiency of cytosolic protein NAD(P)H: quinone oxidoreductase-1 (NQO1) may be associated with myeloid pathology, such as myeloid hyperplasia [24,25]. Additionally, NQO1 deficiency in conjunction with vitamin C deficiency may be a risk factor for developing MDS [25]. Recent analysis by Seastone et al. [26] noted a greater number of molecular abnormalities in MDS patients, particularly related to histone modification highlighting potential tobacco-mediated molecular pathogenesis of MDS. Additionally, tobacco-related changes may also predispose patients to specific molecular abnormalities such as methylation and further study of tobacco users who have also received hypomethylating agents for treatment may give us further insight.
Limitations of our study include its retrospective study design, which also precluded us from confirmation of physician-reported or patient self-reported tobacco usage. Outcomes based on magnitude of smoking (i.e., pack-years) and from cessation of smoking in ever-smokers could also be valuable in future prospective evaluations. Additionally, we did not evaluate the association between tobacco usage and other comorbidities, some of which may stimulate inflammatory processes triggering alterations in the bone marrow microenvironment. Further studies will also benefit from evaluation of smoking-related comorbidities, such as cancers and chronic disease, which are known sequela of tobacco usage that could contribute to alternative smoking-related outcomes. In this study, we included a limited number of patients who reported using smokeless tobacco into our “ever-smoker” cohort, and we agree that these other forms of tobacco may have different effects. Further prospective validation of tobacco usage as a prognostic variable is needed. Furthermore, evaluation of behavioral modification on disease status and prognosis is warranted.
Acknowledgments
We thank Rasa Hamilton (Moffitt Cancer Center) for editorial assistance.
Funding source
None.
Footnotes
Data were presented at the American Society of Hematology 53rd Annual Meeting, December 2011, San Diego, California (Abstract #3790).
Conflict of interest
None.
Disclosures
None.
Contributions: RSK was responsible for research design. All authors contributed to acquisition, analysis, or interpretation of data, drafting and revising the paper, and approval of the final version.
References
- 1.Bennett JM, Komrokji R, Kouides P. The myelodysplastic syndromes. In: Abeloff MD, Armitage JO, Niederhuber JE, editors. Clinical oncology. New York: Churchill Livingstone; 2004. pp. 2849–81. [Google Scholar]
- 2.Bennett JM, Komrokji RS. The myelodysplastic syndromes: diagnosis, molecular biology and risk assessment. Hematology (Amsterdam, Netherlands) 2005;10(Suppl 1):258–69. doi: 10.1080/10245330512331390311. [DOI] [PubMed] [Google Scholar]
- 3.Greenberg P, Cox C, Lebeau MM, Fenaux P, Morel P, Sanz G, et al. International scoring system for evaluating prognosis in myelodysplastic syndromes. Blood. 1997;89:2079–88. [PubMed] [Google Scholar]
- 4.Kantarjian H, O’Brien S, Ravandi F, Cortes J, Shan J, Bennett JM, et al. Proposal for a new risk model in myelodysplastic syndrome that accounts for events not considered in the original International Prognostic Scoring System. Cancer. 2008;113:1351–61. doi: 10.1002/cncr.23697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mishra A, Corrales-Yepez M, Al Ali NA, Kharfan-Dabaja M, Padron E, Zhang L, et al. Validation of the revised International Prognostic Scoring System in treated patients with myelodysplastic syndromes. Am J Hematol. 2013;88:566–70. doi: 10.1002/ajh.23454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Komrokji RS, Corrales-Yepez M, Al Ali N, Kharfan-Dabaja M, Padron E, Fields T, et al. Validation of the MD Anderson Prognostic Risk Model for patients with myelodysplastic syndrome. Cancer. 2012;118:2659–64. doi: 10.1002/cncr.26567. [DOI] [PubMed] [Google Scholar]
- 7.Della Porta MG, Malcovati L, Strupp C, Ambaglio I, Kuendgen A, Zipperer E, et al. Risk stratification based on both disease status and extra-hematologic comorbidities in patients with myelodysplastic syndrome. Haematologica. 2011;96:441–9. doi: 10.3324/haematol.2010.033506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Björk J, Albin M, Mauritzson N, Strömberg U, Johansson B, Hagmar L. Smoking and myelodysplastic syndromes. Epidemiology. 2000;11:285–91. doi: 10.1097/00001648-200005000-00010. [DOI] [PubMed] [Google Scholar]
- 9.Ma X, Lim U, Park Y, Mayne ST, Wang R, Hartge P, et al. Obesity, lifestyle factors, and risk of myelodysplastic syndromes in a large US cohort. Am J Epidemiol. 2009;169:1492–9. doi: 10.1093/aje/kwp074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Du Y, Fryzek J, Sekeres MA, Taioli E. Smoking and alcohol intake as risk factors for myelodysplastic syndromes (MDS) Leuk Res. 2012;34:1–5. doi: 10.1016/j.leukres.2009.08.006. [DOI] [PubMed] [Google Scholar]
- 11.Tong H, Hu C, Yin X, et al. A meta-analysis of the relationship between cigarette smoking and incidence of myelodysplastic syndrome. PLOS ONE. 2013;8:e67537. doi: 10.1371/journal.pone.0067537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ma X, Wang R, Galili N, Mayne ST, Wang SA, Yu H, et al. Cigarette smoking shortens the survival of patients with low-risk myelodysplastic syndromes. Cancer Causes Control. 2011;22:623–9. doi: 10.1007/s10552-011-9735-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Malcovati L, Germing U, Kuendgen A, Della Porta MG, Pascutto C, Inv-ernizzi R, et al. Time-dependent prognostic scoring system for predicting survival and leukemic evolution in myelodysplastic syndromes. J Clin Oncol. 2007;25:3503–10. doi: 10.1200/JCO.2006.08.5696. [DOI] [PubMed] [Google Scholar]
- 14.Guillermo S, Benet N, Esperanza S, et al. Independent impact of iron overload and transfusion dependency on survival and leukemic evolution in patients with myelodysplastic syndrome. ASH Annu Meet Abstr. 2008;112(11):640. [Google Scholar]
- 15.Della Porta MG, Malcovati L, Boveri E, Travaglino E, Pietra D, Pascutto C, et al. Clinical relevance of bone marrow fibrosis and CD34-positive cell clusters in primary myelodysplastic syndromes. J Clin Oncol. 2009;27:754–62. doi: 10.1200/JCO.2008.18.2246. [DOI] [PubMed] [Google Scholar]
- 16.Neumann F, Gattermann N, Barthelmes HU, Haas R, Germing U. Levels of beta 2 microglobulin have a prognostic relevance for patients with myelodysplastic syndrome with regard to survival and the risk of transformation into acute myelogenous leukemia. Leuk Res. 2009;33(2):232–6. doi: 10.1016/j.leukres.2008.06.003. [DOI] [PubMed] [Google Scholar]
- 17.Wimazal F, Sperr WR, Kundi M, Vales A, Fonatsch C, Thalhammer-Scherrer R, et al. Prognostic significance of serial determinations of lactate dehydrogenase (LDH) in the follow-up of patients with myelodysplastic syndromes. Ann Oncol. 2008;19:970–6. doi: 10.1093/annonc/mdm595. [DOI] [PubMed] [Google Scholar]
- 18.Kosmider O, Gelsi-Boyer V, Cheok M, Graber S, Della-Valle V, Picard F, et al. TET2 mutation is an independent favorable prognostic factor in myelodysplastic syndromes (MDSs) Blood. 2009;114:3285–91. doi: 10.1182/blood-2009-04-215814. [DOI] [PubMed] [Google Scholar]
- 19.Gelsi-Boyer V, Trouplin V, Roquain J, Adélaïde J, Carbuccia N, Esterni B, et al. ASXL1 mutation is associated with poor prognosis and acute transformation in chronic myelomonocytic leukaemia. Br J Haematol. 2010;151:365–75. doi: 10.1111/j.1365-2141.2010.08381.x. [DOI] [PubMed] [Google Scholar]
- 20.Bejar R, Stevenson K, Abdel-Wahab O, Galili N, Nilsson B, Garcia-Manero G, et al. Clinical effect of point mutations in myelodysplastic syndromes. N Engl J Med. 2011;364:2496–506. doi: 10.1056/NEJMoa1013343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kolquist KA, Schultz RA, Furrow A, Brown TC, Han JY, Campbell LJ, et al. Microarray-based comparative genomic hybridization of cancer targets reveals novel, recurrent genetic aberrations in the myelodysplastic syndromes. Cancer Genet. 2011;204:603–28. doi: 10.1016/j.cancergen.2011.10.004. [DOI] [PubMed] [Google Scholar]
- 22.Chambers DM, Ocariz JM, McGuirk MF, Blount BC. Impact of cigarette smoking on volatile organic compound (VOC) blood levels in the U.S. population: NHANES 2003-2004. Environ Int. 2011;37:1321–8. doi: 10.1016/j.envint.2011.05.016. [DOI] [PubMed] [Google Scholar]
- 23.Korte JE, Hertz-Picciotto I, Schulz MR, Ball LM, Duell EJ. The contribution of benzene to smoking-induced leukemia. Environ Health Perspect. 2000;108:333–9. doi: 10.1289/ehp.00108333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Long DJ, 2nd, Gaikwad A, Multani A, Pathak S, Montgomery CA, Gon-zalez FJ, et al. Disruption of the NAD(P)H:quinone oxidoreductase 1 (NQO1) gene in mice causes myelogenous hyperplasia. Cancer Res. 2002;62:3030–6. [PubMed] [Google Scholar]
- 25.Das A, Dey N, Ghosh A, Das T, Chatterjee IB. NAD(P)H: quinone oxidoreductase 1 deficiency conjoint with marginal vitamin C deficiency causes cigarette smoke induced myelodysplastic syndromes. PLoS One. 2011;6:e20590. doi: 10.1371/journal.pone.0020590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Seastone DJ, Mukherjee S, Otrock ZK, Elson P, Keng MK, Przychodzen B, et al. Distinct pattern of genomic changes associated with smoking in patients with myelodysplastic syndromes (MDS) (abstract) Blood. 2013;122:660. [Google Scholar]


