Abstract
Objective
Congenital bicuspid aortic valve (BAV) is distinctly associated with the development of ascending aortopathy in adulthood, portending risk of both ascending aortic aneurysm and dissection. Our previous work implicated deficiency in oxidative stress response as a mediator of the BAV-associated aortopathy. We hypothesize that reactive oxygen species generation invokes elevated local oxidative tissue damage in ascending aorta of patients with BAV.
Methods
Ascending aortic specimens were obtained from patients undergoing elective aortic replacement and/or aortic valve replacement and during heart transplant operations. Levels of superoxide anion were measured via high-pressure liquid chromatography–based detection of 2-hydroxyethidium in aortic specimens. Lipid peroxidation and enzymatic activity of superoxide dismutase and peroxidase were quantified in aortic specimens.
Results
Superoxide anion production was elevated in aortic specimens from patients with nonaneurysmal BAV (n = 59) compared with specimens from patients with the morphologically normal tricuspid aortic valve (TAV, n = 38). Total superoxide dismutase activity was similar among aortic specimens from patients with TAV versus BAV (n = 27 and 26, respectively), whereas peroxidase activity was increased in aortic specimens from patients with BAV compared with specimens from patients with TAV (n = 14 for both groups). Lipid peroxidation was elevated in aortic specimens from BAV patients compared with TAV patients (n = 14 and 11, respectively).
Conclusions
Superoxide anion accumulation and increased lipid peroxidation demonstrate that, despite increased peroxidase activity, the ascending aortopathy of patients with BAV involves oxidative stress. In addition, the absence of increased superoxide dismutase activity in BAV specimens indicates a deficiency in antioxidant defense. This suggests that the characteristic smooth muscle cell loss observed in BAV aortopathy may be a consequence of superoxidemediated cell damage.
Keywords: aortic aneurysm, oxidative stress, vascular biology, bicuspid aortic valve
Graphical abstract
Increased superoxide anion and oxidative damage in the proximal aorta of patients with bicuspid aortic valve.
Bicuspid aortic valve (BAV) is the most prevalent congenital heart malformation1 and is associated with an increased risk of developing thoracic aortic aneurysm (TAA) or dissection in the ascending thoracic aorta.2 Risk stratification for aortic catastrophe among patients with BAV is challenging because the cause of BAV formation and the upstream effector molecules and consequent mediating pathways of the associated aortopathy are not understood fully.
Oxidative stress has been implicated in idiopathic TAA,3 Marfan syndrome,4 and abdominal aortic aneurysms.5 However, the young age-related incidence of TAA formation in the patient with BAV, asymmetric dilation pattern localized to the proximal ascending aorta, the lack of multiorgan involvement, and the noninflammatory nature of the pathology indicate that the pathophysiology of BAV-associated aortopathy is distinct from that of the abdominal aorta, idiopathic TAAs, and TAAs arising in patients with connective tissue disorders. Oxidative stress response has been proposed by our group as a mediator of BAV-associated aortopathy from observations of increased susceptibility to oxidative stress-induced cell death of primary medial smooth muscle cells (SMCs) from patients with BAV compared with age-matched controls with normal-caliber aorta.6 These findings led us to hypothesize that reactive oxygen species (ROS) generation invokes elevated local oxidative tissue damage in ascending aortic specimens of patients with BAV.
In the present study, we sought to investigate superoxide anion (O2•−) and lipid peroxidation levels in ascending aortic specimens of patients with BAV ex vivo. We also examined superoxide dismutase (SOD) activity, a major ROS defense mechanism, as well as peroxidase activity in BAV specimens. Collectively, our results suggest that an environment of heightened oxidative stress and consequent oxidative damage to the ascending aorta contribute to BAV-associated aortopathy.
METHODS
Tissue Collection
Human ascending aortic specimens (n = 130) were collected, with approval of the institutional review board (#PRO07020120 – approved on February 26, 2015) and informed patient consent, from patients undergoing elective aortic replacement due to aneurysm or aortic valve replacement in the absence of an aneurysm. All procedures performed were in accordance with institutional guidelines. Nonaneurysmal ascending aortic specimens were collected from local heart transplant donor and recipient patients with tricuspid aortic valve (TAV) and no history of aortic disease or aortic valve disease, with approval of the institutional research board and informed patient consent or approval from the Center for Organ Recovery and Education. Patient demographics, age, sex, aortic diameter, degree of aortic insufficiency and/or stenosis, valve morphology (tricuspid or bicuspid) and relevant comorbidities (eg, hypertension and smoking) were recorded carefully and are displayed in Table 1. Ascending aortic specimens were categorized as aneurysmal when the maximal orthogonal aortic diameter exceeded 42 mm. Patients clinically diagnosed with a known connective tissue disorder, such as Marfan, Ehlers-Danlos, and Loeys-Dietz syndrome, were excluded from this study.
TABLE 1.
Demographics for all patients enrolled in the study
| TAV
|
BAV
|
|||
|---|---|---|---|---|
| NA | TAA | NA | TAA | |
| n, M/F | 27 (20/6)* | 28 (18/10) | 22 (15/7) | 53 (43/10) |
|
| ||||
| Age, y | 59.5 ± 16 | 64.0 ± 10 | 56 ± 14† | 56 ± 11† |
|
| ||||
| Diameter, mm | ≤42 | 52.0 ± 5.2 | 38 ± 4.28†,‡ | 49.0 ± 4.3†,§ |
|
| ||||
| Aortic index | ≤2.20 | 2.60 ± 0.52 | 1.82 ± 0.27†,‡ | 2.34 ± 0.38†,§ |
|
| ||||
| HTN | 67% | 79% | 68% | 71% |
|
| ||||
| Smoking | 83% | 67% | 57% | 55% |
|
| ||||
| AI | ||||
| 1+ | 6% | 26% | 29% | 26% |
| 2-3+ | 25% | 30% | 10% | 14% |
| 4+ | 6% | 15% | 19% | 12% |
|
| ||||
| AS | ||||
| Mild | 0% | 0% | 0% | 8% |
| Mod | 0% | 0% | 5% | 16% |
| Severe | 15% | 9% | 71 %†,‖ | 49%†,‖ |
Data are expressed as median ± SD (age and diameter), or as percentage (HTN, smoking, AI, and AS). TAV, Tricuspid aortic valve; BAV, bicuspid aortic valve; NA, nonaneurysmal; TAA, thoracic aortic aneurysm; n, number of patients enrolled; M, male; F, female; HTN, hypertension; AI, aortic insufficiency; AS, aortic stenosis; Mod, moderate.
The sex of 1 patient was unknown.
Indicate P < .05 versus TAV-TAA, BAV-NA, and TAV-NA, respectively, as assessed by χ2 test or a MannWhitney U test.
The diameter of 7 patients was unknown.
BAV morphotype was assessed intraoperatively in almost all cases, according to the Sievers classification.7 Among the 22 patients with nonaneurysmal BAV, 17 (77%) presented withatype 1 BAV (15 type 1L/R, 1 type 1R/N, 1 type 1L/N) and 1 patient (4.5%) exhibited a type 2 BAV morphotype. The majority (60%) of the 53 patients with aneurysmal BAV presented with a type 1 BAV (including 26 type 1L/R, 5 type 1 R/N, and 1 type 1L/N) whereas 4 patients (7.5%) exhibited a type 0 BAV. The frequency of BAV morphotypes is consistent with previous work from our laboratory8 and others.7,9
On excision, aortic specimens were placed in cold saline and transported to the laboratory, where samples of smaller size were sectioned for various assay. An effort was made to maintain the time elapsed between specimen excision and harvesting constant. Samples from similar regions of the aorta were used for each assay to adequately compare the data. The adventitial layer was stripped carefully from the tunica media and the intima was gently scraped away. Medial specimens were processed as described herein for various assays.
High-Pressure Liquid Chromatography (HPLC)- Based Detection of 2-Hydroxyethidium (2-OH-E+)
2-OH-E+ has been shown to be the lone reaction product of O2•− with dihydroethidium (DHE), making its detection via HPLC the most sensitive and accurate method to determine O2•− generation in biological specimens.10 A portion (35-40 mg, 1-2 cm2) of fresh aortic media was incubated in 10 mmol/L DHE (Sigma-Aldrich, St Louis, Mo) in phosphate-buffered saline (PBS; Life Technologies, Carlsbad, Calif) for 30 minutes in the dark at 37°C with 5% CO2 and humidity. The DHE-labeled tissue was then snap-frozen in liquid nitrogen and homogenized with the gentleMACS Dissociator (Miltenyi Biotec Inc, San Diego, Calif). The total homogenate was further passed through a 28.5-gauge needle in 150 μL 0.1 % Triton X-100 in PBS. Twenty microliters of the homogenate was reserved for total protein determination with the BCA Protein Assay Kit (Pierce, Rockford, Ill). One hundred microliters of the homogenate was diluted 1:1 in extraction buffer (0.2 mol/L perchloric acid in methanol) and incubated on ice for 2 hours. The samples were centrifuged at 20,000g for 30 minutes at 4°C. Samples (120 μL) were then diluted 1:1 in 1 M phosphate buffer (0.7 mol/L potassium phosphate monobasic and 0.3 mol/L phosphoric acid, pH 2.6), followed by centrifugation at 20,000g for 15 minutes at 4° C. The supernatant (200 μL) was subjected to HPLC analysis for electrochemical detection (ESA CoulArray 5600) of 2-OH-E+. An ether-linked phenyl column (100 mm × 4.6 mm) was used with 2 mobile phases (Solution A: 50 mmol/L phosphate buffer in 90% water and 10% acetonitrile; Solution B: 50 mmol/L phosphate buffer in 40% water and 60% acetonitrile), and a gradient elution to increase the acetonitrile concentration from 25% to 60% over 10 minutes at a flow rate of 0.75 mL/min. The areas of the corresponding peaks were assessed with ESA CoulArray software (SelectScience, Bath, UK). The limit of detection for this assay was determined from a standard curve and established at 20 fmol. The concentration of 2-OH-E+ in each sample was calculated with a standard curve and normalized to the protein concentration of the tissue lysate. Specimens with undetectable 2-OH-E+ were assigned values at the limit of detection (20 fmol) to enable statistical analyses on all specimens interrogated. As such, 11 of a total of 97 samples were assigned this limit of detection.
Quantification of SOD and Peroxidase Activity
For determination of antioxidant activities, snap-frozen aortic media tissue samples were homogenized in cold lysis buffer (20 mmol/L HEPES buffer solution, pH 7.2, 1 mmol/L ethylene glycol-bis(β-aminoethyl ether)-N,N,N′,N′-tetraacetic acid, 210 mmol/L mannitol, and 70 mmol/L sucrose; all Sigma-Aldrich) for SOD activity and in PBS (Ca2+/Mg2+-free) for the peroxidase assay with the gentleMACS Dissociator (Miltenyi Biotec Inc). Total SOD activity was determined according to the manufacturer’s instructions (Cayman Chemical, Ann Arbor, Mich). Peroxidase activity was quantified by a modification of the method of Weydert and Cullen.11 To summarize, homogenized medial specimens were diluted 1:1 in PBS and absorbance was measured at 240 nm every 8 seconds for 24 seconds after the addition of 30 mmol/L H2O2. Peroxidase activity (K) was calculated as previously described.11 SOD and peroxidase activity levels were normalized to total protein content as determined by the Bradford method (Bio-Rad, Hercules, Calif).
Detection of Lipid Peroxidation
To assess the oxidative damage at the tissue level, lipid peroxidation was assessed by quantification of 8-iso-prostaglandin F2α, resulting from the nonenzymatic peroxidation of arachidonic acid in membrane phospholipids.12 Aliquots of aortic tissue lysates were prepared as described previously for the SOD and peroxidase activity assays and used to determined amount of lipid peroxidation using the Oxiselect 8-iso-prostaglandin F2α ELISA kit (Cell BioLabs Inc, San Diego, Calif) according to the manufacturer’s instructions. The amount of 8-iso-prostogladin F2α was measured from aortic tissue lysate against a standard curve. Results were further normalized to the protein concentration and expressed as pg/mg protein of tissue lysate.
Statistical Analyses
All results mentioned in the text are expressed as mean ± standard error of the mean and are represented in figures as median and interquartile range with errors bars representing 90th and 10th percentiles. Data in Table 1 are expressed as median ± standard deviation. Experimental outliers were identified and excluded from analysis with the Outlier Labeling Rule according to Hoaglin and Iglewicz13 and Tukey. Statistical tests were performed using SPSS, version 22 (IBM Corp, Armonk, NY). Comparisons between group demographics were assessed by χ2 test or a MannWhitney U test. All experimental endpoints were compared in SPSS for the 4 patient cohorts with the nonparametric Kruskal-Wallis test. When experimental endpoints were compared between TAV versus BAV specimens, the Mann-Whitney U nonparametric test was performed. A P value of less than .05 was considered statistically significant.
RESULTS
Levels of O2•− Are Increased in the Aortic Media of BAV Specimens
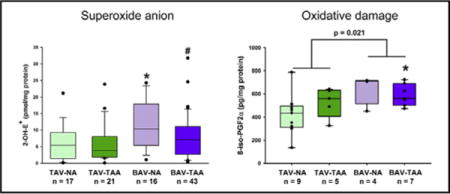
Quantification of 2-OH-E+, the specific oxidation product of DHE by O2•−, showed that aortic specimens from nonaneurysmal BAV have the highest level of O2•− (Figure 1, 11.7 ± 1.86 pmol/pg protein). The level of O2•− in nonaneurysmal BAV specimens was increased compared with O2•− levels from nonaneurysmal TAV and aneurysmal BAV aortic specimens (Figure 1, 6.41 ± 1.30 pmol/mg, P = .041 and 7.80 ± 1.02 pmol/mg, P = .042, respectively).
FIGURE 1.
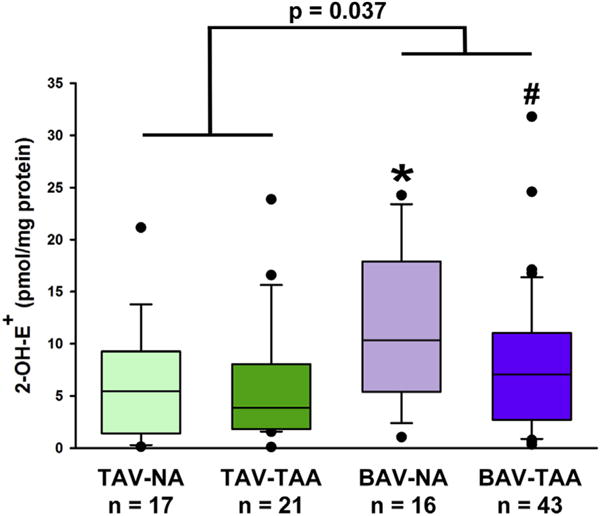
Detection of O2•− generation. HPLC-based detection of 2-OH-E+ in aortic media showing a significant increase in O2•− in the ascending aorta of patients with BAV. Box plots depict the median (middle line of the box), first (lower line of the box), and third (upper line of the box) quartiles with errors bars representing 90th and 10th percentiles. Data points distributed below the 10th percentile and above the 90th percentile are indicated by a closed circle. *Indicates P < .05 from TAV- NA and #Indicates P < .05 from BAV-NA using a Kruskal-Wallis test. P = .037 indicates a statistically significant difference between all BAV specimens and all TAV specimens using a Mann-Whitney U nonparametric test. 2-OH-E+, 2-hydroxyethidium; TAV-NA, tricuspid aortic valve-nonaneurysmal; TAV-TAA, tricuspid aortic valve-thoracic aortic aneurysm; BAV-NA, bicuspid aortic valve-nonaneurysmal; BAV-TAA, bicuspid aortic valve-thoracic aortic aneurysm.
Specimens From Patients With BAV Exhibit Similar SOD Activity and Increased Peroxidase Activity
Total SOD activity was quantified in nonaneurysmal and patients with aneurysmal BAV and TAV and found to be similar among the 4 patient cohorts (Figure 2, A). Conversely, aortic specimens from patients with nonaneurysmal BAV exhibited increased peroxidase activity compared with nonaneurysmal TAV specimens (Figure 2, B, 15.9 ± 2.29 K/mg vs 7.18 ± 1.14 K/mg, respectively, P = .023). When combined, BAV specimens exhibited a greater peroxidase activity compared with TAV specimens independent of the presence of aneurysm (Figure 2, B, 15.4 ± 1.87 K/mg vs 7.69 ± 1.15 K/mg, respectively, P < .002).
FIGURE 2.
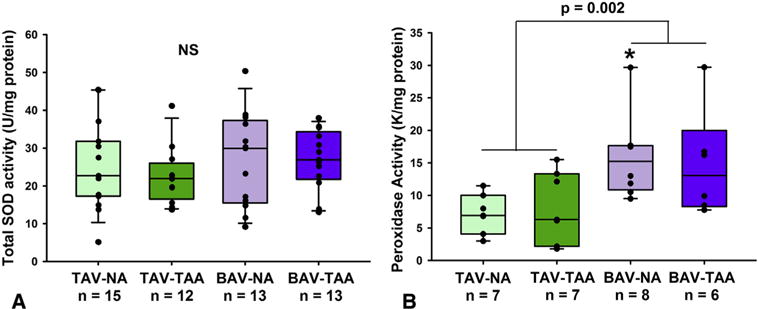
Detection of antioxidant activity in aortic media. A, Total SOD activity was similar among aortic specimens from patients with aneurysmal and nonaneurysmal BAV and TAV. B, Peroxidase activity was found to be increased in aortic specimens from patients with BAV compared with TAV specimens. Box plots depict the median (middle line of the box), first (lower line of the box), and third (upper line of the box) quartiles with errors bars representing 90th and 10th percentiles. All data points are indicated by a closed circle. *Indicates P < .05 fromTAV-NA. In (B) P = .002 was obtained using a Mann-Whitney nonparametric U test comparing all TAV versus all BAV specimens. SOD, Superoxide dismutase; TAV-NA, tricuspid aortic valve-nonaneurysmal; TAV-TAA, tricuspid aortic valve-thoracic aortic aneurysm; BAV-NA, bicuspid aortic valve-nonaneurysmal; BAV-TAA, bicuspid aortic valve-thoracic aortic aneurysm; NS, not significant using a Kruskal-Wallis test.
Lipid Peroxidation Is Increased in Aortic Specimens From Patients With BAV
We assessed lipid peroxidation as a marker of cellular damage due to oxidative stress. Quantification of 8-iso-prostaglandin F2α revealed increased lipid peroxidation in the aortic media of aneurysmal patients with BAV compared with specimens from nonaneurysmal patients with TAV (Figure 3, 589 ± 35.4 pg/mg vs 420 ± 60.1 pg/mg, respectively, P = .023). Levels of 8-iso-prostaglandin F2α were elevated in all BAV specimens compared with all TAV specimens, irrespective of the presence of aneurysm (Figure 3, 610 ± 32.0 pg/mg vs 458 ± 44.7 pg/mg, P = .021).
FIGURE 3.
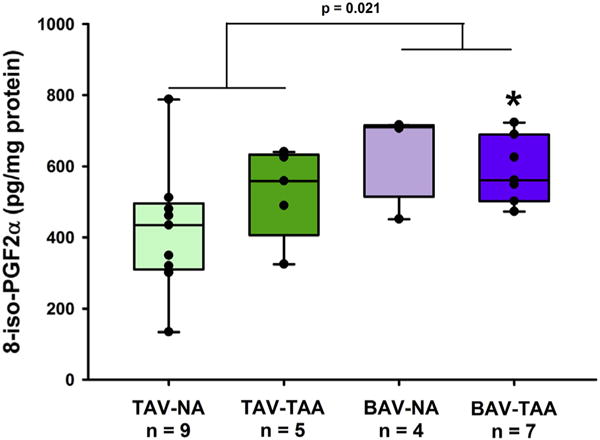
Lipid peroxidation in aortic media. Elevated oxidative damage in aortic specimens from patients with BAV was revealed by increased levels of 8-iso-PGF2α detected by ELISA. Box plots depict the median (middle line of the box), first (lower line of the box), and third (upper line of the box) quartiles with errors bars representing 90th and 10th percentiles. All data points are indicated by a closed circle. *Indicates P < .05 from TAV-NA via Kruskal-Wallis. A P value of .021 was obtained using a Mann-Whitney nonparametric U test comparing all TAV versus all BAV specimens. 8-iso-PGF2α, 8-iso-prostaglandin F2α; TAV-NA, tricuspid aortic valve-nonaneurysmal; TAV-TAA, tricuspid aortic valve-thoracic aortic aneurysm; BAV-NA, bicuspid aortic valve-nonaneurysmal; BAV-TAA, bicuspid aortic valve-thoracic aortic aneurysm.
DISCUSSION
In the setting of ascending aortic aneurysm, it is accepted widely that vessel dilatation and loss of biomechanical integrity is a consequence of cystic medial degeneration, a phenomenon involving noninflammatory SMC loss and elastin fragmentation in the aortic media.14 However, aneurysms arising in the BAV population are distinct from those presenting in patients with connective tissue disorders, such as the Marfan syndrome, and from degenerative TAA occurring in patients with the morphologically normal TAV.15 Specifically, the cause of BAV remains unknown, with no single gene or set of genes identified as being responsible for the abnormal valve development and the associated aortopathy.16 From an epidemiologic standpoint, patients with BAV present for aortic replacement approximately 10 to 15 years younger than patients with TAV with degenerative aneurysms, and the frequency of TAA is estimated to be approximately 10%, whereas TAA in patients with TAV occurs at a frequency of less than 0.02% .17–19 In addition, it is likely that all patients with BAV have a certain degree of ascending aortopathy because they do exhibit a greater ascending aortic diameter compared with TAV patients,19–21 even when matched for variables such as sex, age, or degree or valvulopathy.22 The distinct manifestations of BAV- versus TAV-associated aortopathy is further supported by our previous work at the tissue, cellular, and molecular levels showing distinct biomechanical properties associated with differences in extracellular matrix maturity and architecture23–27 and altered SMC response to oxidative stress6,28 in the BAV versus TAV aortopathy.
Despite an abundance of literature describing oxidative stress in several cardiovascular pathologies, there have been relatively few reports focusing on the involvement of ROS in thoracic aortic diseases. In this study, we have uncovered elevated O2•− levels along with demonstrating cellular oxidative damage in the thoracic aortic media of BAVaortas compared with TAVaortas. Interestingly, this increase in oxidative stress was detected in both aneurysmal and nonaneurysmal BAV specimens but was not noted in aneurysmal TAV specimens. This finding suggests that oxidative stress may play a prominent role in the onset and progression of BAV-associated aortopathy, whereas it may be less involved in the pathophysiology of degenerative aneurysms. In addition, O2•− levels were significantly greater in nonaneurysmal BAV specimens compared with aneurysmal BAV specimens. This may indicate that O2•− plays a more prominent role in the early stages of BAV aortopathy than in the later stages. Further investigation is required to determine the exact link between elevated O2•− and the changes in extracellular matrix (ECM) composition and microarchitecture revealed by our group in specimens collected from earlier stages of BAV aortopathy.24
Several investigators have examined the involvement of oxidative stress in animal models of TAA. For example, Fbn1C1039G/+ mice, a mouse model of Marfan syndrome, developed TAAs29 and exhibited down-regulation of SOD expression amidst up-regulation of the expression of inducible nitric oxide synthase, NADPH oxidase (NOX) subunits, and xanthine oxidase, accompanied by increased lipid peroxidation.30 In addition, elevated levels of homocysteine and protein carbonyl contents were found in the serum of patients with Marfan syndrome along with a decrease in total antioxidant capacity.4 Although the exact role of hyperhomocysteinemia in human Marfan disease is unclear, it has been shown to cause increased deposition of collagen and degradation of elastin in the mouse aorta, ultimately leading to altered vessel biomechanics evidenced by reduced aortic flow velocity.31 In view of the paucity of studies focused on oxidative stress in patients presenting with TAA, determining whether O2•− is a cause or a consequence of the BAV-associated aortopathy remains unknown and is warranted.
We previously uncovered a marked susceptibility to oxidative stress among SMCs from BAV-associated aortopathy specimens by demonstrating a deficiency of basal and stress-induced expression of the antioxidant metallothionein,6 a finding that was corroborated by others.32 Although peroxidase activity was found to be elevated in aortic specimens from patients with BAV, total SOD activity remained similar between patient cohorts in the present study. These results are in line with our recent study showing that the expression of the 3 SOD isoforms is either similar (Sod1) or decreased (Sod2 and Sod3) in the R region of the BAV ascending aorta, which corresponds to the aortic segment proximally adjacent to the right coronary sinus.8 In a smaller cohort of aneurysmal patients with BAV, it recently was reported that the protein level of SOD3 was decreased in the ascending aorta and associated with decreased phosphorylation level of 2 targets of SOD3, namely Erk1/2 and Akt, compared with nonaneurysmal TAV specimens.33 The same study also revealed similar levels of SOD3 expression in specimens of degenerative aneurysms and healthy aorta.33 In parallel, recent work from Branchetti and colleagues34 described down-regulation of SOD1 and SOD2 at the mRNA level in the aortic media of patient with degenerative aneurysms. Despite these recent reports focused on the expression of SODs in BAV and TAV aneurysmal aortic specimens, the enzymatic activity of SODs has not been reported previously. We found that total SOD activity was similar, suggesting an inadequate defensive response by SMCs to the increase in O2•− levels. The relative contributions of SOD1, SOD2, and SOD3 isoforms in overall SOD enzymatic activity in BAV aortic specimens will require further study.
Our data indicate that despite activation of the antioxidant enzyme peroxidase, we detected increased levels of 8-iso-PGF2α, which are indicative of oxidative damage in BAV aortic specimens and could negatively affect cellular function. Isoprostane result from the nonenzymatic peroxidation of arachidonic acid in membrane phospholipids, thus affecting the integrity of all cell membranes.12,35,36 Lipid peroxidation was observed in aneurysmal BAV specimens despite similar levels of 2-OH-E+ in these specimens. This discrepancy could be explained by the fact that other ROS such as hydrogen peroxide, hydroxyl radical, or hydroxyperoxyl radical may be involved in later stages of BAV aortopathy. Regardless of the nature of the ROS involved, oxidative damage in form of lipid peroxidation was detected in our aneurysmal BAV specimens. Lipid peroxidation has been associated with cardiovascular disease including atherosclerosis,37 coronary artery disease,38 and aortic insufficiency.39 At the cellular level, increased lipid peroxidation is known to perturb the homeostasis of the plasma membrane, leading to cell death in several cell types including vascular SMCs.40 In the BAV aortic specimens, the high levels of 8-iso-PGF2α could thus contribute to SMC loss during cystic medial degeneration. Furthermore, mishandling of O2•− and H2O2 in the BAV aorta may adversely influence SMC behavior, as shown in the study by Branchetti and colleagues,34 where treatment of human aortic SMCs with H2O2 resulted in alteration of cell phenotype. In addition, we previously reported that SMCs isolated from BAV aortic specimens have reduced cell viability under oxidative stress conditions when compared to SMCs from TAV aortic specimens.6 This observation was attributed to the lower levels of the antioxidant metallothionein measured in BAVaortic specimens,6 since a similar reduced cell viability under oxidative stress conditions was observed in aortic SMCs isolated from metallothionein knockout mice.28 Collectively, these studies indicate an imbalance of ROS-related mechanisms in the aortic wall of patients with BAV.
To date, the main sources of O2•− production in SMCs, including NOXs and xanthine oxidase, have not been investigated in human aortic specimens. However, in a mouse model of TAA induced by angiotensin II infusion, increased expression of the NOX subunit p22phox was measured and found to colocalize with matrix metalloproteinase activity in the aortic media.41 More recently, we identified endothelial nitric oxide synthase (eNOS) as one probable source of O2•− in the ascending aorta.42 Although eNOS has been implicated previously in the pathophysiology of BAV-associated aortopathy,42–44 involvement in development or progression of the associated aortopathy is mechanistically unclear. Regional down-regulation of eNOS expression has been reported in BAV aortic specimens,43 and single-nucleotide polymorphisms have been identified in patients with aneurysmal BAV.44 Work from our laboratory has revealed increased eNOS expression in aortic intima-media specimens isolated from patients with BAV.42 Despite this finding, nitric oxide bioavailability was not found to be concordantly elevated. Since uncoupling of eNOS has been associated with a lack of NO bioavailability and O2•− generation in the aorta,42 we surmise that eNOS is a likely source of O2•− production in the BAV aorta.
In conclusion, our data reveal an environment of heightened oxidative stress associated with impaired oxidative defense and increased lipid peroxidation in the aortic wall in the setting of BAV-associated aortopathy. The influence of oxidative stress on medial SMCs and on the medial matrix microarchitecture and biomechanical integrity in the aortic wall of patients with BAV is the focus of our ongoing work.
Limitations of the Study
A limitation to our study is the limited number of aortic specimens for certain patient subsets. Specifically, we were unable to further delineate the role of valvulopathy (aortic valve regurgitation and/or aortic valve stenosis) or BAV morphotype in the different parameters measured in our study. Therefore, severe aortic valve regurgitation and/or stenosis were potential confounding factors in our experiments. Despise these clinical limitations, we recently have demonstrated that aortic valve regurgitation influenced Sod gene expression in aneurysmal specimens isolated from patients with TAV, whereas aortic valve stenosis had no effect.8
Clinical Implications of the Study
Despite the fact that BAV is the most common cardiac anomaly, occurring in 1% to 2% of the population, the cellular and molecular mechanisms underlying the associated aortopathy remain largely unknown. We propose a role for oxidative stress in the BAV-associated aortopathy, whereby O2•− and possibly other ROS lead to oxidative damages in the aortic wall. Despite an increase in the peroxidase component of the antioxidant response, the specific antioxidant defense against O2•− (ie, superoxide dismutase) was not increased in aortic BAV specimens. Because oxidative stress is a well-known disruptor of ECM homeostasis, we suggest that oxidative stress is involved in the ECM derangements associated with BAVaortopathy. Understanding the exact link between oxidative stress and ECM in the context of BAV aortopathy may lead to the discovery of new therapeutic targets to prevent and/or delay aneurysm formation and/or rupture in patients with BAV. In addition, with the development of imaging methods allowing for in vivo detection of oxidative stress,45–47 our work may support the need to design new diagnostic tools that would utilize oxidative stress as a marker of BAV aortopathy.
Central Message.
Levels of superoxide anion and oxidative damages are elevated in the ascending aorta of patients with bicuspid aortic valve.
Perspective.
In patients with bicuspid aortic valve (BAV), the proximal aorta is characterized by heightened superoxide anion production and oxidative damage. This oxidative environment may adversely impact cellular and matrix homeostasis and could explain the unique manifestation of BAV aortopathy. Identifying the impact of oxidative stress on local biological parameters may offer a novel perspective for the management of BAV aortopathy.
Acknowledgments
The authors gratefully acknowledge Deborah Cleary for technical assistant in patient specimen preparation, bioassay performance, and related analysis and Kristin Valchar and Julie Schreiber for assistance with institutional review board protocols and informed patient consent. We thank Forozan Navid for help with aortic specimen acquisition and Dr Andrew Althouse at the Heart and Vascular Institute for assistance with statistical analyses.
Research reported in this publication was supported by the National Heart, Lung and Blood Institute of the National Institutes of Health, grant HL109132 (TGG).
Abbreviations and Acronyms
- BAV
bicuspid aortic valve
- DHE
dihydroethidium
- ECM
extracellular matrix
- eNOS
endothelial nitric oxide synthase
- NOX
NADPH oxidase
- O2•−
superoxide anion
- 2-OH-E+
2-hydroxyethidium
- PBS
phosphate-buffered saline
- ROS
reactive oxygen species
- SMC
smooth muscle cells
- SOD
superoxide dismutase
- TAA
thoracic aortic aneurysm
- TAV
tricuspid aortic valve
Footnotes
Conflict of Interest Statement
Authors have nothing to disclose with regard to commercial support.
References
- 1.Roberts WC. The congenitally bicuspid aortic valve. A study of 85 autopsy cases. Am J Cardiol. 1970;26:72–83. doi: 10.1016/0002-9149(70)90761-7. [DOI] [PubMed] [Google Scholar]
- 2.Ward C. Clinical significance of the bicuspid aortic valve. Heart. 2000;83:81–5. doi: 10.1136/heart.83.1.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ejiri J, Inoue N, Tsukube T, Munezane T, Hino Y, Kobayashi S, et al. Oxidative stress in the pathogenesis of thoracic aortic aneurysm: protective role of statin and angiotensin II type 1 receptor blocker. Cardiovasc Res. 2003;59:988–96. doi: 10.1016/s0008-6363(03)00523-6. [DOI] [PubMed] [Google Scholar]
- 4.Fiorillo C, Becatti M, Attanasio M, Lucarini L, Nassi N, Evangelisti L, et al. Evidence for oxidative stress in plasma of patients with Marfan syndrome. Int J Cardiol. 2010;145:544–6. doi: 10.1016/j.ijcard.2010.04.077. [DOI] [PubMed] [Google Scholar]
- 5.Xiong W, Mactaggart J, Knispel R, Worth J, Zhu Z, Li Y, et al. Inhibition of reactive oxygen species attenuates aneurysm formation in a murine model. Atherosclerosis. 2009;202:128–34. doi: 10.1016/j.atherosclerosis.2008.03.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Phillippi JA, Klyachko EA, Kenny JP, Eskay MA, Gorman RC, Gleason TG. Basal and oxidative stress-induced expression of metallothionein is decreased in ascending aortic aneurysms of bicuspid aortic valve patients. Circulation. 2009;119:2498–506. doi: 10.1161/CIRCULATIONAHA.108.770776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sievers HH, Schmidtke C. A classification system for the bicuspid aortic valve from 304 surgical specimens. J Thorac Cardiovasc Surg. 2007;133:1226–33. doi: 10.1016/j.jtcvs.2007.01.039. [DOI] [PubMed] [Google Scholar]
- 8.Phillippi JA, Hill JC, Billaud M, Green BR, Kotlarczyk MP, Gleason TG. Bicuspid aortic valve morphotype correlates with regional antioxidant gene expression profiles in the proximal ascending aorta. Ann Thorac Surg. 2017;140:79–87. doi: 10.1016/j.athoracsur.2016.10.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sievers HH, Stierle U, Mohamed SA, Hanke T, Richardt D, Schmidtke C, et al. Toward individualized management of the ascending aorta in bicuspid aortic valve surgery: the role of valve phenotype in 1362 patients. J Thorac Cardiovasc Surg. 2014;148:2072–80. doi: 10.1016/j.jtcvs.2014.04.007. [DOI] [PubMed] [Google Scholar]
- 10.Zielonka J, Vasquez-Vivar J, Kalyanaraman B. Detection of 2-hydroxyethidium in cellular systems: a unique marker product of superoxide and hydroethidine. Nat Protoc. 2008;3:8–21. doi: 10.1038/nprot.2007.473. [DOI] [PubMed] [Google Scholar]
- 11.Weydert CJ, Cullen JJ. Measurement of superoxide dismutase, catalase and glutathione peroxidase in cultured cells and tissue. Nat Protoc. 2010;5:51–66. doi: 10.1038/nprot.2009.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Griendling KK, Touyz RM, Zweier JL, Dikalov S, Chilian W, Chen YR, et al. Measurement of reactive oxygen species, reactive nitrogen species, and redox-dependent signaling in the cardiovascular system: a scientific statement From the American Heart Association. Circ Res. 2016;119:e39–75. doi: 10.1161/RES.0000000000000110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hoaglin DC, Iglewicz B. Fine-tuning some resistant rules for outlier labeling. J Am Stat Assoc. 1987;82:1147–9. [Google Scholar]
- 14.Wu D, Shen YH, Russell L, Coselli JS, LeMaire SA. Molecular mechanisms of thoracic aortic dissection. J Surg Res. 2013;184:907–24. doi: 10.1016/j.jss.2013.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gleason TG. Heritable disorders predisposing to aortic dissection. Semin Thorac Cardiovasc Surg. 2005;17:274–81. doi: 10.1053/j.semtcvs.2005.06.001. [DOI] [PubMed] [Google Scholar]
- 16.Elefteriades JA, Farkas EA. Thoracic aortic aneurysm clinically pertinent controversies and uncertainties. J Am Coll Cardiol. 2010;55:841–57. doi: 10.1016/j.jacc.2009.08.084. [DOI] [PubMed] [Google Scholar]
- 17.Olsson C, Thelin S, Stahle E, Ekbom A, Granath F. Thoracic aortic aneurysm and dissection: increasing prevalence and improved outcomes reported in a nationwide population-based study of more than 14,000 cases from 1987 to 2002. Circulation. 2006;114:2611–8. doi: 10.1161/CIRCULATIONAHA.106.630400. [DOI] [PubMed] [Google Scholar]
- 18.Michelena HI, Khanna AD, Mahoney D, Margaryan E, Topilsky Y, Suri RM, et al. Incidence of aortic complications in patients with bicuspid aortic valves. JAMA. 2011;306:1104–12. doi: 10.1001/jama.2011.1286. [DOI] [PubMed] [Google Scholar]
- 19.Sabet HY, Edwards WD, Tazelaar HD, Daly RC. Congenitally bicuspid aortic valves: a surgical pathology study of 542 cases (1991 through 1996) and a literature review of 2,715 additional cases. Mayo Clin Proc. 1999;74:14–26. doi: 10.4065/74.1.14. [DOI] [PubMed] [Google Scholar]
- 20.Michelena HI, Desjardins VA, Avierinos JF, Russo A, Nkomo VT, Sundt TM, et al. Natural history of asymptomatic patients with normally functioning or minimally dysfunctional bicuspid aortic valve in the community. Circulation. 2008;117:2776–84. doi: 10.1161/CIRCULATIONAHA.107.740878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tzemos N, Therrien J, Yip J, Thanassoulis G, Tremblay S, Jamorski MT, et al. Outcomes in adults with bicuspid aortic valves. JAMA. 2008;300:1317–25. doi: 10.1001/jama.300.11.1317. [DOI] [PubMed] [Google Scholar]
- 22.Keane MG, Wiegers SE, Plappert T, Pochettino A, Bavaria JE, Sutton MG. Bicuspid aortic valves are associated with aortic dilatation out of proportion to coexistent valvular lesions. Circulation. 2000;102(19 suppl 3):III35–9. doi: 10.1161/01.cir.102.suppl_3.iii-35. [DOI] [PubMed] [Google Scholar]
- 23.Tsamis A, Phillippi JA, Koch RG, Krawiec JT, D’Amore A, Watkins SC, et al. Extracellular matrix fiber microarchitecture is region-specific in bicuspid aortic valve-associated ascending aortic aneurysms. J Thorac Cardiovasc Surg. 2016;151:1718–28.e5. doi: 10.1016/j.jtcvs.2016.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Phillippi JA, Green BR, Eskay MA, Kotlarczyk MP, Hill MR, Robertson AM, et al. Mechanism of aortic medial matrix remodeling is distinct in patients with bicuspid aortic valve. J Thorac Cardiovasc Surg. 2014;147:1056–64. doi: 10.1016/j.jtcvs.2013.04.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tsamis A, Phillippi JA, Koch RG, Pasta S, D’Amore A, Watkins SC, et al. Fiber micro-architecture in the longitudinal-radial and circumferential-radial planes of ascending thoracic aortic aneurysm media. J Biomech. 2013;46:2787–94. doi: 10.1016/j.jbiomech.2013.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pichamuthu JE, Phillippi JA, Cleary DA, Chew DW, Hempel J, Vorp DA, et al. Differential tensile strength and collagen composition in ascending aortic aneurysms by aortic valve phenotype. Ann Thorac Surg. 2013;96:2147–54. doi: 10.1016/j.athoracsur.2013.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pasta S, Phillippi JA, Gleason TG, Vorp DA. Effect of aneurysm on the mechanical dissection properties of the human ascending thoracic aorta. J Thorac Cardiovasc Surg. 2012;143:460–7. doi: 10.1016/j.jtcvs.2011.07.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Phillippi JA, Eskay MA, Kubala AA, Pitt BR, Gleason TG. Altered oxidative stress responses and increased type I collagen expression in bicuspid aortic valve patients. Ann Thorac Surg. 2010;90:1893–8. doi: 10.1016/j.athoracsur.2010.07.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pereira L, Lee SY, Gayraud B, Andrikopoulos K, Shapiro SD, Bunton T, et al. Pathogenetic sequence for aneurysm revealed in mice underexpressing fibrillin-1. Proc Natl Acad Sci USA. 1999;96:3819–23. doi: 10.1073/pnas.96.7.3819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yang HH, van Breemen C, Chung AW. Vasomotor dysfunction in the thoracic aorta of Marfan syndrome is associated with accumulation of oxidative stress. Vasc Pharmacol. 2010;52:37–45. doi: 10.1016/j.vph.2009.10.005. [DOI] [PubMed] [Google Scholar]
- 31.Steed MM, Tyagi N, Sen U, Schuschke DA, Joshua IG, Tyagi SC. Functional consequences of the collagen/elastin switch in vascular remodeling in hyperho-mocysteinemic wild-type, eNOS−/−, and iNOS−/− mice. Am J Physiol. 2010;299:L301–11. doi: 10.1152/ajplung.00065.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Folkersen L, Wagsater D, Paloschi V, Jackson V, Petrini J, Kurtovic S, et al. Unraveling divergent gene expression profiles in bicuspid and tricuspid aortic valve patients with thoracic aortic dilatation: the ASAP study. Mol Med. 2011;17:1365–73. doi: 10.2119/molmed.2011.00286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Arcucci A, Ruocco MR, Albano F, Granato G, Romano V, Corso G, et al. Analysis of extracellular superoxide dismutase and Akt in ascending aortic aneurysm with tricuspid or bicuspid aortic valve. Eur J Histochem. 2014;58:2383. doi: 10.4081/ejh.2014.2383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Branchetti E, Poggio P, Sainger R, Shang E, Grau JB, Jackson BM, et al. Oxidative stress modulates vascular smooth muscle cell phenotype via CTGF in thoracic aortic aneurysm. Cardiovasc Res. 2013;100:316–24. doi: 10.1093/cvr/cvt205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hill BG, Haberzettl P, Ahmed Y, Srivastava S, Bhatnagar A. Unsaturated lipid peroxidation-derived aldehydes activate autophagy in vascular smooth-muscle cells. Biochem J. 2008;410:525–34. doi: 10.1042/BJ20071063. [DOI] [PubMed] [Google Scholar]
- 36.Cabre A, Girona J, Vallve JC, Heras M, Masana L. Cytotoxic effects of the lipid peroxidation product 2,4-decadienal in vascular smooth muscle cells. Atherosclerosis. 2003;169:245–50. doi: 10.1016/s0021-9150(03)00196-5. [DOI] [PubMed] [Google Scholar]
- 37.Holvoet P, Collen D. Oxidation of low density lipoproteins in the pathogenesis of atherosclerosis. Atherosclerosis. 1998;137(suppl):S33–8. doi: 10.1016/s0021-9150(97)00305-5. [DOI] [PubMed] [Google Scholar]
- 38.Holvoet P, Mertens A, Verhamme P, Bogaerts K, Beyens G, Verhaeghe R, et al. Circulating oxidized LDL is a useful marker for identifying patients with coronary artery disease. Arterioscler Thromb Vasc Biol. 2001;21:844–8. doi: 10.1161/01.atv.21.5.844. [DOI] [PubMed] [Google Scholar]
- 39.Shimoni S, Bar I, Zilberman L, George J. Autoantibodies to oxidized low-density lipoprotein in patients with aortic regurgitation: association with aortic diameter size. Cardiology. 2014;128:54–61. doi: 10.1159/000357835. [DOI] [PubMed] [Google Scholar]
- 40.Fruhwirth GO, Moumtzi A, Loidl A, Ingolic E, Hermetter A. The oxidized phospholipids POVPC and PGPC inhibit growth and induce apoptosis in vascular smooth muscle cells. Biochim Biophys Acta. 2006;1761:1060–9. doi: 10.1016/j.bbalip.2006.06.001. [DOI] [PubMed] [Google Scholar]
- 41.Ejiri J, Inoue N, Tsukube T, Munezane T, Hino Y, Hunter GC. Oxidative stress in human abdominal aortic aneurysmal and occlusive disease. Cardiovasc Res. 1999;59:39–45. [Google Scholar]
- 42.Kotlarczyk MP, Billaud M, Green BR, Hill JC, Shiva S, Kelley EE, et al. Regional disruptions in endothelial nitric oxide pathway associated with bicuspid aortic valve. Ann Thorac Surg. 2016;102:1274–81. doi: 10.1016/j.athoracsur.2016.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mohamed SA, Radtke A, Saraei R, Bullerdiek J, Sorani H, Nimzyk R, et al. Locally different endothelial nitric oxide synthase protein levels in ascending aortic aneurysms of bicuspid and tricuspid aortic valve. Cardiol Res Pract. 2012;2012:165957. doi: 10.1155/2012/165957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pisano C, Maresi E, Balistreri CR, Candore G, Merlo D, Fattouch K, et al. Histological and genetic studies in patients with bicuspid aortic valve and ascending aorta complications. Interact Cardiovasc Thorac Surg. 2012;14:300–6. doi: 10.1093/icvts/ivr114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Prunty MC, Aung MH, Hanif AM, Allen RS, Chrenek MA, Boatright JH, et al. In vivo imaging of retinal oxidative stress using a reactive oxygen species-activated fluorescent probe. Invest Ophthalmol Vis Sci. 2015;56:5862–70. doi: 10.1167/iovs.15-16810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tanase M, Urbanska AM, Zolla V, Clement CC, Huang L, Morozova K, et al. Role of carbonyl modifications on aging-associated protein aggregation. Sci Rep. 2016;6:19311. doi: 10.1038/srep19311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Khoo NK, Cantu-Medellin N, St Croix C, Kelley EE. In vivo immuno-spin trapping: imaging the footprints of oxidative Stress. Curr Protoc Cytom. 2015;74:12.42.1–11. doi: 10.1002/0471142956.cy1242s74. [DOI] [PMC free article] [PubMed] [Google Scholar]


