Abstract
Oxa-dibenzocyclooctynes (ODIBO, 2a–c) are prepared by photochemical decarbonylation of corresponding cyclopropenones (photo-ODIBO, 1a–c). While photo-ODIBO does not react with azides, ODIBO is one of the most reactive cyclooctynes exhibiting rates of cycloaddition over 45 M−1s−1 in aqueous solutions. ODIBO is stable under ambient conditions and has low reactivity towards thiols. Photo-ODIBO survives heating up to 160°C and does not react with thiols.
Numerous methods for the efficient immobilization, labeling, or ligation of various substrates are based on Cu(I) – catalyzed acetylene-azide cycloaddition (CuAAC).1 Use of cytotoxic copper catalyst, however, somewhat limits the utility of CuAAC.2 Recently discovered strain-promoted cycloaddition (SPAAC) of azides to cyclooctynes,3 dibenzocyclooctynes,4 aza-dibenzocyclooctynes,5 and thia-cycloalkynes6 offers a bio-compatible catalyst-free version of the azide click reaction.7
The rate of the 1,3-dipolar cycloaddition of the parent cyclooctyne8 is too slow (~0.001 M−1s−1)3c for the majority of practical applications and the search for cycloalkynes with higher reactivity is one of the major goals in the development of SPAAC technology. Electronic activation of cyclooctynes can be achieved by placing electron-withdrawing groups in the propargylic position. While one fluorine substituent has limited effect on reactivity (e.g., MOFO, k ~ 4.3×10−3 M−1s−1),3c difluorinated cyclooctyne (DIFO)3c reacts 40 – 60 times faster than the parent compound. Alternatively, the cyclooctyne reactivity can be enhanced by the modification of its structural parameters, as was demonstrated on the examples of benzocyclooctyne (COMBO, k ~ 0.24 M−1s−1),9 dibenzocyclooctynes (DIBO k ~ 5.7×10−2 M−1s−1)10 and bicycle[6.1.0]non-4-yne (BCN, k ~ 0.14 M−1s−1).5d High reactivity achieved by the combination of both approaches in difluorobenzocyclooctyne (DIFBO, k ~ 0.22 M−1s−1), is, unfortunately, accompanied by low stability, as DIFBO undergoes trimerization in solution or in solid state.11 Introduction of a sulfur atom in DIFBO structure enhances the stability of cycloalkynes but also decreases the reactivity of thia-DIFBO (1.4×10−2 M−1s−1).6 Azacycloalkynes, e.g., azadibenzocyclooctyne (ADIBO/DIBAC, k ~ 0.29 – 0.42 M−1s−1)5b,c and azadibenzocyclooctynone (BARAC, k ~ 0.96 M−1s−1),5a are currently the most reactive cycloalkynes available. It is important to note that enhancement of azide reactivity in cycloalkynes is often accompanied by the increased susceptibility to nucleophilic attack. Most of the cyclooctynes tested in our lab underwent rapid hydration in PBS buffer at 60°C. Cyclooctynes containing electron-withdrawing substituents are also known to react with cytosolic thiols and cysteine residues in proteins.12 Thus, DIFO reacts with cysteine at a rate similar to that observed in cycloaddition with azides.12a
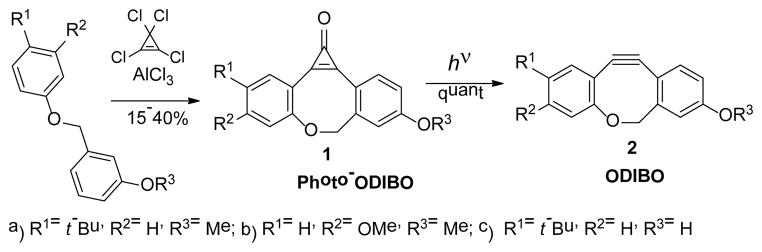
Photochemical generation of reactive cyclooctynes permits spatial and temporal control of the click reaction. Cyclopropenones are especially suitable for this purpose as they do not react with azides and possess excellent thermal stability.10,13 We report here the use of a cyclopropenone route for the first ever preparation of oxa-dibenzocyclooctyne (ODIBO, 2, Scheme 1).
Scheme 1.
Synthesis of ODIBO
Preparation of cyclooctynes used in SPAAC usually requires lengthy multistep syntheses. ODIBO, on the other hand, is prepared in two steps starting from appropriately substituted phenyl benzyl ether. The Friedel-Crafts alkylation of the latter with tetracholorocyclopropenone followed by hydrolysis gives cyclopropenone (photo-DIBO, 1) in a moderate yield. Subsequent irradiation of 1 with UV light results in quantitative formation of ODIBO (2, Scheme 1).14
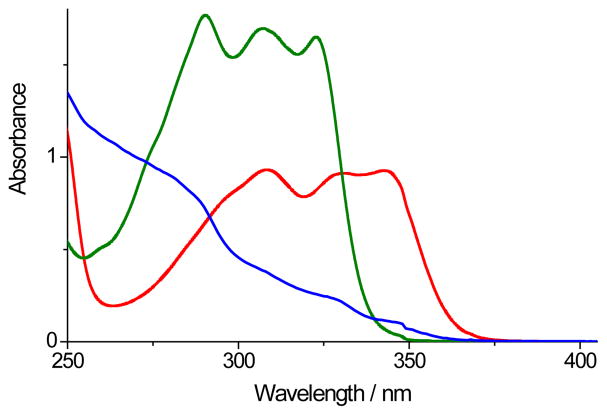
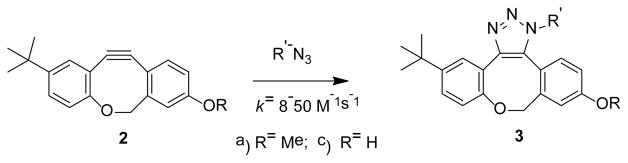
Cyclopropenones 1a–c are colorless crystalline compounds that have long shelf life if stored protected from light. Cyclopropenone 1a melts at 160–163°C without decomposition and survives 6 h refluxing in o-dichlorobenzene. 1a was also quantitatively recovered after 2 h incubation at 90°C in aqueous solutions, as well as in biphosphate or TRIS buffers. Cyclopropenones 1a–c do not react with organic azides or thiols under ambient conditions. UV spectra of methanol solutions of cyclopropenones 1a–c contain three close-lying intense bands (λmax = 308, 330 and 343 nm, logε ~ 4.5, Fig. 1). Irradiation of photo-DIBO 1a–c with 350 nm light results in efficient decarbonylation of the starting material (Φ=0.16), which could be observed by the bleaching of the 343 nm band, and the quantitative formation of ODIBO 2a–c (Scheme 1). Cyclooctyne 2 absorbance bands are blue-shifted in relation to cyclopropenone precursor (λmax = 290, 308 and 322 nm, logε ~ 4.2, Fig. 1). This feature allows for convenient monitoring of the decarbonylation reaction of 1. ODIBO 2a–c can be stored for months in a neat form (yellowish oil) at temperatures ~ 4°C and are stable in aqueous PBS solutions below 50°C. At higher temperatures ODIBO undergoes slow hydration, e.g., at 75°C in PBS:DMSO (7 : 3) half-lifetime of 2a is 1.5 h. ODIBO has relatively low reactivity towards endogenous thiols: the half lifetime of ODIBO 2a in PBS solution in the presence of 10 mM of glutathione is 8.2 h at room temperature. On the other hand, ODIBO shows exceptional reactivity towards organic azides (vide infra). SPAAC of cyclooctynes, with the exception of symmetric BCN, produces a mixture of two isomeric triazoles, the products of head-to-head and head-to-tail cycloadditions. This phenomenon often complicates spectral analysis of the resulting products. According to chromatographic data, cycloaddition of azides to t-butyl substituted ODIBO derivatives 2a,c produces only one regioisomer of triazole 3 (Scheme 2). The triazole produced in a preparative scale reaction of 2a with benzyl azide shows only one set of signals supporting the regioselectivety of the addition. We assume that less sterically hindered isomer of triazole 3 is produced (Scheme 2).
Fig. 1.
UV spectra of 115 μM methanol solutions of photo-ODIBO (1a, red line), ODIBO (2a, green line), and triazol (3a, blue line).
Scheme 2.
Regioselective reaction of ODIBO 2a,c with azides
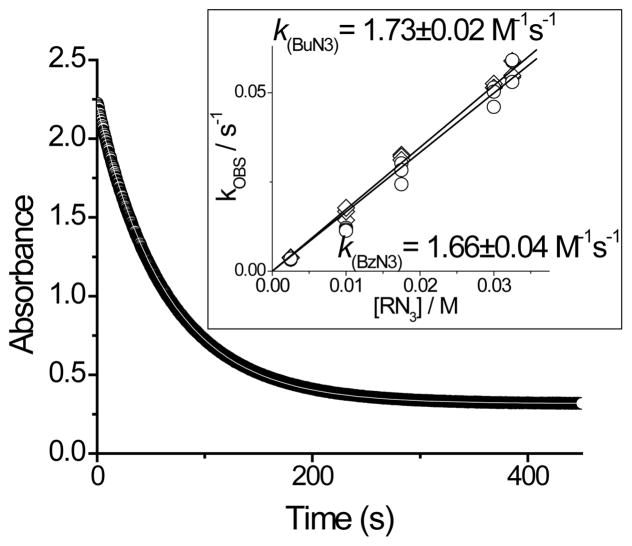
The accurate rate measurements of ODIBO 2a–c reaction with organic azides were conducted by UV spectroscopy at 25±0.1°C in methanol and in aqueous solutions. Reactions were conducted under pseudo-first order conditions, using 20 fold or higher excess of azides. The consumption of starting material was monitored by following the decay of the characteristic 322 nm absorbance of 2a–c. The experimental data fits the single exponential equation well. Linear dependence of the observed pseudo-first order rate constants on azide concentration was analyzed by the least squares method to obtain the bimolecular rate constants (Table 1, Fig. 2 and Fig. S114).
Table 1.
Bimolecular rate constants for the reaction of ODIBO 2a–c with benzyl azide in various solvents.
| ODIBO | Solvent | Azide | Rate (M−1s−1) |
|---|---|---|---|
| 2a | MeOH | Benzyl azide | 1.66±0.04 |
| 2a | MeOH | n-Butyl azide | 1.77±0.02 |
| 2a | MeOH | TEG-azide | 1.96±0.03 |
| 2a | Water-MeOH-THFa | Benzyl azide | 45.1±2.6 |
| 2a | Water-MeOH-THFa | n-Butyl azide | 28.6±1.3 |
| 2a | Water-THF (7:3) | TEG-azide | 7.2±0.1 |
| 2b | MeOH | Benzyl azide | 2.22±0.01b |
| 2c | MeOH | TEG-azide | 1.7±0.8 |
| 2c | Water-MeOH (7:3) | TEG-azide | 8.91±0.07 b |
| 2c | Water-MeOH (95:5) | TEG-azide | 11.3±0.2b |
65% Water, 20% MeOH, 15% THF;
Evaluated from rate measurements conducted at a single azide concentration
Fig. 2.
Reaction of 0.115 mM ODIBO 2a with 2.5 mM BzN3 in MeOH at 25°C. The insert illustrates the linear dependence of the observed rates on azide concentration.
In methanol ODIBO 2a,c react with azides almost two times faster than the current most reactive SPAAC reagent BARAC5a (Table 1).). Direct comparison of the reactivity of various cyclooctynes, however, should be done with caution since the rate of the cycloaddition reaction is commonly measured using inherently less accurate NMR technique14 and solvents of different polarity (CD3CN for BARAC). Bulky t-butyl substituent in cyclooctynes 2a,c does not significantly affect the rate of the cycloaddition as sterically less hindered analog 2b shows similar reactivity (Table 1).
While SPAAC ligation methods were specifically designed for biological applications, which implies aqueous solutions, the reactivity of cyclooctynes is usually studied in organic solvents. In a handful of cases where the kinetics of SPAAC was measured in mostly aqueous solutions, presence of water has significantly enhanced the rate of the reaction, from mere 16% for ADIBO/DIBAC5b to more than 100% for BCN3d to a 13-fold increase for DIBO.4 This rate-enhancing effect was also observed in present work for ODIBO. In 65–70% aqueous solutions15 ODIBO reacts 5 – 28 times faster with organic azides than in methanol (Table 1). The reaction still follows the first order law well and the rate shows linear dependence on azide concentration (Fig. S114).
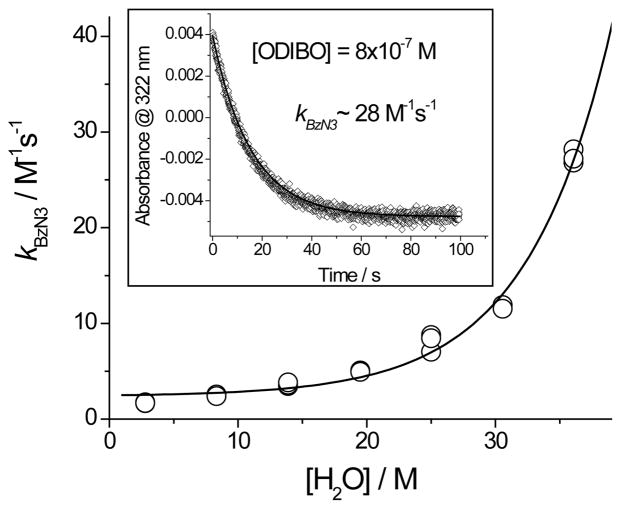
To explore the water concentration effect on the rate of ODIBO click reactions further, we have measured the rate of the disappearance of ODIBO 2a (115 μM) in the presence of 2.5 mM of benzyl azide in aqueous methanol with variable water content. Second order rate constants calculated from the observed pseudo-first order rate constants show smooth non-linear increase with the rise in water concentration (Fig 3). Conservative extrapolation of this trend suggests that in wholly aqueous solution azide cycloaddition to ODIBO could proceed with the rates in excess of 2–3×102 M−1s−1. The acceleration of SPAAC reaction in aqueous solution is apparently caused by higher polarity and/or donor-acceptor interaction with the solvent. However, it is also possible that rate enhancement is caused by the formation of an inhomogeneous solution, such as the microemulsion of azide or aggregation of ODIBO, at higher water concentrations. While the smooth monotonic increase of the reaction rates shown in Figure 3 and linear dependence of pseudo-first order rate constant on azide concentration (Fig. S114) do not support such a hypothesis, we conducted two additional experiments to get further insight in the reactivity-enhancement effect of water. Under the extreme dilution conditions, with the concentration of ODIBO 2a at 800 nM the observed rate of the reaction with benzyl azide in water-methanol-THF mixture (13 : 4 : 3) was virtually identical to that recorded at 150 times higher concentration of the substrate (Insert in Figure 3). This observation shows that solubility of ODIBO does not cause rate enhancement in aqueous solutions. Next, we explored the reaction of ODIBO 2c with water soluble azide, 1-amino-8-azido-3,5-dioxaoctane (TEG-Azide). The formation of triazole proceeded progressively faster on the way from methanol solutions to almost neat water albeit effect was less pronounced than for aromatic azide (Table 1).
Fig. 3.
Dependence of the rates constants for the reaction of ODIBO 1a with benzyl azide on water contents in aqueous methanol. (Bimolecular rate constants were evaluated from a rate measured at a 2.5 mM of benzyl azide and 115 μM of 1a). Insert shows the kinetic trace recorded at 800 nM of ODIBO 2a.
Conclusions
Oxa-dibenzocyclooctyne (ODIBO, 2) exhibits unsurpassed reactivity in metal-free acetylene-azide cycloaddition. The rate of the reaction dramatically increases in aqueous solution, making this compound very suitable for rapid labeling applications in biochemistry. High reactivity of ODIBO towards organic azides is combined with good aqueous stability and low susceptibility to nucleophilic attack. These properties should reduce or eliminate non-specific binding, known for other SPAAC reagents. The photochemical precursor of ODIBO, in which the triple bond is masked as cyclopropenone functionality (Photo-ODIBO, 1) does not react with azides, thus allowing for spatially and temporally – resolved labeling. In addition, Photo-ODIBO possesses great thermal stability and can survive heating in excess of 160° C. Our current work is focused on the optimization of the preparative procedures and conjugation of ODIBO with biotin and fluorescent dyes for cell-labeling experiments.
Supplementary Material
Footnotes
Electronic Supplementary Information (ESI) available: Experimental procedures; synthesis and characterization of new compounds. See DOI: 10.1039/b000000x/
References
- 1.Kolb HC, Finn MG, Sharpless KB. Angew Chem, Int Ed. 2001;40:2004. doi: 10.1002/1521-3773(20010601)40:11<2004::AID-ANIE2004>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]; Hein JE, Fokin VV. Chem Soc Rev. 2010;39:1302. doi: 10.1039/b904091a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gaetke LM, Chow CK. Toxicology. 2003;189:147. doi: 10.1016/s0300-483x(03)00159-8. [DOI] [PubMed] [Google Scholar]; Burrows CJ, Muller JG. Chem Rev. 1998;98:1109. doi: 10.1021/cr960421s. [DOI] [PubMed] [Google Scholar]; Wang Q, Chan TR, Hilgraf R, Fokin VV, Sharpless KB, Finn MG. J Am Chem Soc. 2003;125:3192. doi: 10.1021/ja021381e. [DOI] [PubMed] [Google Scholar]
- 3.a) Baskin JM, Prescher JA, Laughlin ST, Agard NJ, Chang PV, Miller IA, Lo A, Codelli JA, Bertozzi CR. Proc Natl Acad Sci USA. 2007;104:16793. doi: 10.1073/pnas.0707090104. [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Sletten EM, Bertozzi CR. Org Lett. 2008;10:3097. doi: 10.1021/ol801141k. [DOI] [PMC free article] [PubMed] [Google Scholar]; c) Codelli JA, Baskin JM, Agard NJ, Bertozzi CR. J Am Chem Soc. 2008;130:11486. doi: 10.1021/ja803086r. [DOI] [PMC free article] [PubMed] [Google Scholar]; d) Dommerholt J, Schmidt S, Temming R, Hendriks LJA, Rutjes FPJT, van Hest JCM, Lefeber DJ, Friedl P, van Delft FL. Angew Chem Int Ed. 2010;49:9422. doi: 10.1002/anie.201003761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ning X, Guo J, Wolfert MA, Boons G-J. Angew Chem, Int Ed. 2008;47:2253. doi: 10.1002/anie.200705456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.a) Jewett JC, Sletten EM, Bertozzi CR. J Am Chem Soc. 2010;132:3688. doi: 10.1021/ja100014q. [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Debets MF, van Berkel SS, Schoffelen S, Rutjes FPJT, van Hest JCM, van Delft FL. Chem Commun. 2010;46:97. doi: 10.1039/b917797c. [DOI] [PubMed] [Google Scholar]; c) Kuzmin A, Poloukhtine A, Wolfert M, Popik VV. Bioconjugate Chem. 2010;21:2076. doi: 10.1021/bc100306u. [DOI] [PubMed] [Google Scholar]
- 6.de Almeida G, Sletten EM, Nakamura H, Palaniappan KK, Bertozzi CR. AngewChem Int Ed. 2012;51:2443. doi: 10.1002/anie.201106325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.a) Ess DH, Jones GO, Houk KN. Org Lett. 2008;10:1633. doi: 10.1021/ol8003657. [DOI] [PubMed] [Google Scholar]; b) Jewett JC, Bertozzi CR. Chem Soc Rev. 2010;39:1272. doi: 10.1039/b901970g. [DOI] [PMC free article] [PubMed] [Google Scholar]; c) Debets MF, van der Doelen CWJ, Rutjes FPJT, van Delft FL. ChemBioChem. 2010;11:1168. doi: 10.1002/cbic.201000064. [DOI] [PubMed] [Google Scholar]; d) Debets MF, van Berkel SS, Dommerholt J, Dirks AJ, Rutjes AFPJT, van Delft FL. Acc Chem Res. 2011;44:805. doi: 10.1021/ar200059z. [DOI] [PubMed] [Google Scholar]; e) Sletten EM, Bertozzi CR. Acc Chem Res. 2011;44:666. doi: 10.1021/ar200148z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Krebs A, Wilke J. Top Curr Chem. 1983;109:189. [Google Scholar]
- 9.Varga BR, Kállay M, Hegyi K, Béni S, Kele P. Chem. 2012;18:822. doi: 10.1002/chem.201102329. [DOI] [PubMed] [Google Scholar]
- 10.Poloukhtine AA, Mbua NE, Wolfert MA, Boons G-J, Popik VV. J Am Chem Soc. 2009;131:15769. doi: 10.1021/ja9054096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sletten EM, Nakamura H, Jewett JC, Bertozzi CR. J Am Chem Soc. 2010;132:11799. doi: 10.1021/ja105005t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.a) Beatty KE, Fisk JD, Smart BP, Lu YY, Szychowski J, Hangauer MJ, Baskin JM, Bertozzi CR, Tirrell DA. ChemBioChem. 2010;11:2092. doi: 10.1002/cbic.201000419. [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Chang PV, Prescher JA, Sletten EM, Baskin JM, Miller IA, Agard NJ, Lo A, Bertozzi CR. Proc Natl Acad Sci USA. 2010;107:1821. doi: 10.1073/pnas.0911116107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Orski SV, Poloukhtine AA, Arumugam S, Mao L, Popik VV, Locklin J. J Am Chem Soc. 2010;132:11024. doi: 10.1021/ja105066t. [DOI] [PubMed] [Google Scholar]
- 14.Electronic Supplementary Information
- 15.Organic co-solvent was necessary for ensuring homogeneity of the azide solution.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.