Abstract
Anthrax, caused by the spore-forming bacterium Bacillus anthracis, is a zoonotic disease that affects humans and animals throughout the world. In North America, anthrax outbreaks occur in livestock and wildlife species. Vaccine administration in wildlife is untenable; the most effective form of management is surveillance and decontamination of carcasses. Successful management is critical because untreated carcasses can create infectious zones increasing risk for other susceptible hosts. We studied the bacterium in a re-emerging anthrax zone in southwest Montana. In 2008, a large anthraxepizootic primarily affected a domestic bison (Bison bison) herd and the male segment of a free-ranging elk (Cervus elaphus) herd in southwestern Montana. Following the outbreak, we initiated a telemetry study on elk to evaluate resource selection during the anthrax season to assist with anthrax management. We used a mixed effects generalized linear model (GLM) to estimate resource selection by male elk, and we mapped habitat preferences across the landscape. We overlaid preferred habitats on ecological niche model-based estimates of B. anthracis presence. We observed significant overlap between areas with a high predicted probability of male elk selection and B. anthracis potential. These potentially risky areas of elk and B. anthracis overlap were broadly spread over public and private lands. Future outbreaks in the region are probable, and this analysis identified the spatial extent of the risk area in the region, which can be used to prioritize anthrax surveillance.
Keywords: anthrax, Bacillus anthracis, disease, elk, Montana, resource selection function, zoonosis
Infectious diseases affecting wildlife and domestic animals can present challenges to wildlife conservation and human health (Fouchier et al. 2004, Proffitt et al. 2011, Alexander et al. 2012, Bagamian et al. 2013). Understanding wildlife disease ecology is related to animal behavior and movement; an animal must encounter a pathogen (e.g., ingestion, inhalation, inoculation by a vector, or physical contact) for transmission to take place and movement behaviors can facilitate these interactions. Analyzing specific resources that are selected for, or avoided, using a resource selection function modeling framework has been employed for linking wildlife movement to disease transmission (Brook and McLachlan 2009, Kilpatrick et al. 2009, Proffitt et al. 2011). This approach may be particularly useful in disease systems, such as anthrax, where the host contacts infectious material on the landscape.
Anthrax, caused by the spore-forming bacterium Bacillus anthracis, is an indirectly transmitted disease that can be transmitted through encountering infectious material in the environment (e.g., soil, vegetation, bones). The ability of the bacteria to persist on the landscape is related to climatic and soil conditions (Van Ness 1971, Hugh-Jones and Blackburn 2009). When an animal dies from anthrax, spores from the carcass may contaminate the area leading to exposure or infection in susceptible hosts that interact with the site (Blackburn et al. 2014b, Turner et al. 2014). In geographic areas that support B. anthracis, it is possible to have a landscape with a widespread distribution of infectious sites that are potentially attractive to grazing species (Turner et al. 2014). Therefore, defining where B. anthracis can persist on the landscape, and characterizing how wildlife use these areas is an important part of understanding transmission risk and identifying places where wildlife could become infected with anthrax. Quantifying anthrax risk is critically important because anthrax outbreaks in wildlife and livestock have significant and wide ranging impacts including agricultural economic losses, conservation concerns, wildlife management challenges, and public health threats (Turner et al. 2013).
Recent anthrax outbreaks have been reported in free-ranging plains bison (Bison bison; Shury et al. 2009), white tailed deer (Odocoileus virginianus; Blackburn and Goodin 2013, Blackburn et al. 2014b), and elk (Cervus elaphus; Hugh-Jones and Blackburn 2009, Blackburn et al. 2014a) in North America. The outbreak in elk occurred in southwest Montana during summer 2008. This outbreak was of particular concern because it was the first reported outbreak in a wild elk population in North America and occurred in a region that has not reported anthrax in decades (Blackburn et al. 2014a). During this outbreak, domestic plains bison and free-ranging male elk died from anthrax. There were significant economic losses attributed to material used to decontaminate carcasses, loss of livestock, and the loss of mature male elk.
Management and surveillance strategies for anthrax in wild ungulate populations are challenging because vaccination, the primary means of control in livestock, is untenable in wildlife (Hugh-Jones and De Vos 2002), and carcasses are difficult to find (Bellan et al. 2013). Proactive surveillance of wildlife populations during the anthrax season is important to the prevention of future outbreaks. Identifying cases early and destroying or decontaminating carcasses can reduce the quantity of spores released from the carcass into the environment, and may reduce future exposures. Knowledge of high-risk anthrax zones is essential for effective surveillance and ecological niche models are useful in predicting the potential geographic distribution of B. anthracis (Blackburn et al. 2007). Ecological niche models have been used to map B. anthracis across several landscapes (Blackburn et al. 2007, Joyner et al. 2010, Mullins et al. 2011, 2013). Quantifying spatial overlap of B. anthracis and wildlife populations, or transmission risk, is an important step for defining priority areas for surveillance.
The purpose of this study was to assist with anthrax surveillance in southwest Montana by identifying landscapes where male elk are likely at the highest risk of B. anthracis exposure. Our objectives were to 1) estimate male elk resource selection during the anthrax season, 2) identify areas where the environment supports the persistence of B. anthracis, 3) quantify the degree of overlap between male elk and B. anthracis presence, and 4) identify stakeholders in an effort to assist with management and surveillance strategies.
STUDY AREA
Our study area was defined as the Northern Madison Study Area, which is a 1,652-km2 region in the Madison range in southwest Montana (Atwood 2006). The study area includes an approximately 300-km2 privately owned ranch managed for grazing bison and wildlife conservation and was the site of the 2008 anthrax outbreak (Fig. 1). Our study focused on summer months (Jun–Aug) because this is the historical anthrax season in the region (Blackburn et al. 2014a). Bison fences limit bison to the private ranch but do not prohibit the movement of elk or other cervids across property boundaries. The study area is bordered by the Gallatin National Forest to the south, Gallatin River to the east, and Madison River to the west. The study area is 27% shrubland, 31% grasslands, 36% coniferous forests, and 5% deciduous forests. The southern portion of the study area is dominated by steep forested terrain and the eastern portion is gentler sloped grassland and shrubland. Elevations in the study area range from 1,334 m to 3,352 m in the high peaks region. Land ownership includes 39% United States Forest Service (USFS) land, 4% state land, and 57% privately owned land. There are hunting opportunities on some regions of public and private land in the study area. Carnivores in the region include grizzly bears (Ursus arctos horribilis), American black bears (Ursus americanus), coyotes (Canis latrans), mountain lions (Puma concolor), and wolves (Canis lupus; Atwood 2006, Blackburn et al. 2014a). Ungulates present in the study area include elk, mule deer (Odocoileus hemionus), white-tailed deer, moose (Alces alces; Atwood 2006), and pronghorn (Antilocapra americana) in the northern portion of the study area.
Figure 1.
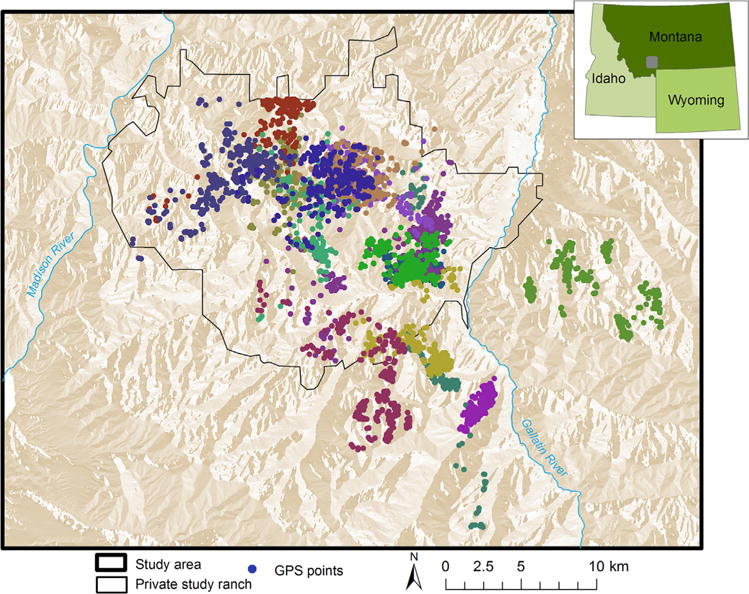
Study site in southwestern Montana, USA with telemetry global positioning system (GPS) points collected from male elk during the anthrax risk period (Jun–Aug) for 2010–2012. Each individual male elk is represented with a different shade. The privately owned ranch where the 2008 anthrax epizootic took place is outlined in black.
METHODS
Telemetry and Environmental Data
We captured 14 adult male elk on the ranch using chemical immobilization delivered from helicopter and fitted with global positioning system (GPS) collars (model GPS3300L; Lotek, Newmarket, ON, Canada) in April 2010 (n = 10) and January 2012 (n = 4). We captured individuals across the private ranch, and we made efforts for capture locations to be distributed across the private ranch. Collars were programmed to record GPS fixes every 30 minutes for 1–3 years. To estimate male elk resource selection during the anthrax season, we included location data collected from 1 June to 31 August from 2010 to 2012. Elk capture protocols were developed by Montana Fish, Wildlife and Parks (FWP) and approved by the University of Florida Institutional Animal Care and Use Committee (UF IACUC #201105751 to JKB).
We evaluated 4 landscape factors potentially affecting male elk resource selection: land cover type, slope, aspect, and elevation. We selected these covariates based on their relationship to male elk habitat selection in other studies. Multiple studies have found that male elk select forested land cover during summer months (Altmann 1951, Unsworth et al. 1998, McCorquodale 2003, Brook and McLachlan 2009). We defined 2 land cover types based on the 2010 Montana Spatial Data Infrastructure (MSDI) land cover dataset (http://geoinfo.montanastatelibrary.org/data/msdi/landuse/, accessed 31 Jul 2013): forested and non-forested. We included elevation to test the hypothesis that male elk select higher elevations during summer months (Altmann 1951, McCorquodale 2003). We estimated a digital elevation model (DEM) using the United States Geological Survey (USGS) National Elevation Dataset (NED) with a 30-m resolution (http://ned.usgs.gov/, accessed 31 Jul 2013). A relationship between slope and male elk habitat selection during summer months has also been reported (Unsworth et al. 1998, McCorquodale 2013), and we derived a slope raster surface from the DEM using ArcGIS 10.1 (Environmental Systems Research Institute, Inc., Redlands, CA). Proffitt et al. (2011) identified aspect as a significant predictor of female elk resource selection on our study area, and we derived an aspect raster surface from the DEM using ArcGIS 10.1. We categorized aspect as southerly (134–224°) and not southerly (0–135°, 225–360°; Proffitt et al. 2011).
Multiple studies have established a relationship between proximity to roads and male elk habitat selection (Edge et al. 1987, Unsworth et al. 1998, Rowland et al. 2005, McCorquodale 2013, Montgomery et al. 2013), and we evaluated 3 different road covariates: distance to primary, secondary, and tertiary roads. We categorized roads based on intensity of use following the methods of Montgomery et al. (2013). Primary roads included National Highway System (NHS) interstate, NHS non-interstate, and state roads. We derived road variables from a USFS road map, MSDI road data, and a map of ranch roads provided by ranch staff. Secondary roads included main ranch roads and county roads. Tertiary roads included 2-track and logging roads. We generated a 30-m raster surface for each of the 3 road categories using the Euclidean distance tool in ArcGIS 10.1.
The effect of wolves on male elk habitat selection has been discussed in multiple studies (Laundré et al. 2001, Creel et al. 2005, Hebblewhite et al. 2005, Christianson and Creel 2008), and their effect on elk resource selection appears to vary across study sites. A wolf pack has been established in the study area since 2002 (Blackburn et al. 2014a). To map wolf risk, we estimated the density of wolf kills reported through ongoing efforts to monitor interactions and predation by wolf of elk and bison. Routine spring and summer surveys of the private study ranch are conducted to identify wolf kills through the presence of scavengers, wolf tracking, and radio telemetry of wolves collared in the area. For this study, we limited wolf data to kills between April and August during 2010–2013, months when ranch roads were accessible for surveys. To estimate wolf predation risk for the study area, we calculated densities of kill locations from 2010 to 2013 using kernel density estimation in ArcGIS 10.1 (Fotheringham et al. 2000); we calculated bandwidth settings following Blackburn et al. (2014c) with the number of kills used as the sample size.
Resource Selection Model Development
We employed a use-versus-availability framework to estimate a resource selection function (RSF; Manly et al. 2002). We were interested in the selection of resources at the landscape scale, which corresponds to second-order selection defined by Johnson (1980) and more recently by Meyer and Thuiller (2006). Second-order selection is a comparison of population-level seasonal range (available habitat) and individual seasonal ranges (used habitat; DeCesare et al. 2012). We randomly selected 4 GPS locations/collared individual/day to represent used locations under the assumption that GPS locations were representative of each collared individuals’ seasonal range composition. We selected 5 available points/used point from within a seasonal, herd-level 100% minimum convex polygon.
We employed a generalized linear mixed effect model, which includes fixed and random effects, to estimate resource selection. Gillies et al. (2006) suggested employing mixed effect models to account for random variation in sampling, and we included a random intercept for individual and year. The random intercept is calculated as the difference between the mean intercept for all groups and the intercept for the individual group (Gillies et al. 2006). We selected the final model using only fixed effects, and added random effects to the final model.
We screened variables prior to inclusion in model development for correlation with other variables in the data set (Pearson’s correlation coefficient |r| ≥ 0.7), and significance in univariate analyses (P < 0.1). We standardized all continuous variables to allow for a direct comparison between model coefficients. We pooled data across all individuals and years for model creation. There was evidence that each of our covariates could affect male elk resource selection during summer; however, the combination of covariates that best described resource selection at our study site was not known. We generated a model list including all additive combinations of our covariates (Womack et al. 2013): land cover, elevation, slope, aspect, secondary roads, tertiary roads, and wolf predation. We fit a generalized linear fixed effect model to each combination of covariates:
where w(x) is the relative probability of a pixel being selected, β0 is the intercept, and β1 is the estimated coefficient for variable X1. If β > 1, a preference for that resource is indicated, and a β < 1 indicates avoidance of that resource (Manly et al. 2002). An RSF is not equivalent to a true probability and Boyce (2006) recommend employing the exponential form of w(x), which is interpreted as the odds ratio. We generated fixed effect RSFs for each model in the list. We used Akaike’s Information Criterion (AIC) to rank models by their ability to describe empirical data at the population level (Burnham and Anderson 2002). The AIC score identifies the most parsimonious model, which has the fewest variables explaining the greatest amount of variation (Thompson and Lee 2000). We ranked models using the difference in AIC between the model of interest and the model with the smallest AIC (DAIC; Anderson et al. 2000, Burnham and Anderson 2002) and calculated Akaike weights (wi; Burnham and Anderson 2002) to identify a suite of competing models (Sittler et al. 2015). We defined competing models as all those required for Σwi to be >0.95 because the best model identified with an AIC approach is not necessarily representative of landscape use (Sittler et al. 2015).
To select the final model, we compared the predictive accuracies of competing models (Sittler et al. 2015) using a 5-fold cross validation approach to determine the relationship between predicted selection and use (Boyce et al. 2002). We randomly selected and withheld 20% of used GPS points from model creation. We employed the logistic equation, using unstandardized model coefficients, to calculate and map the RSF values for each pixel. We split RSF values into 10 quantiles that were adjusted for area (Boyce et al. 2002) so each bin represented an equal proportion of the landscape, with 1 corresponding to the lowest relative probability of selection and 10 corresponding to the highest. We overlaid the excluded used points on the RSF surface, and calculated the number of used points within each of the 10 bins. We employed the Spearman rank correlation coefficient (rs) to compare the bin rank and number of used points per bin (Boyce et al. 2002). We calculated the average correlation coefficient across 5 iterations. We selected the final model by comparing the average correlation coefficients across competing models. A stronger relationship between bin number and number of points/bin indicates a stronger relationship between predicted selection and use. We mapped the final model across the study area at a 30-m resolution using the same methods employed for model validation: solving the logistic equation with unstandardized model coefficients and splitting RSF values into 10 equal area bins.
B. anthracis Ecological Niche Models
To define the geographic distribution of B. anthracis, we used an ecological niche modeling approach to model infected carcass locations identified during the 2008 outbreak. For this study, we use the genetic algorithm for rule-set prediction (GARP; Stockwell 1999). The methodology is described in detail for modeling B. anthracis elsewhere (Blackburn et al. 2007, Mullins et al. 2011). In those studies, mean normalized difference vegetation index (NDVI) and NDVI amplitude were among the most important variables for predicting B. anthracis. For this study, we developed a coverage set of continuous environmental variables including 16-day moderate resolution imaging spectroradiometer (MODIS) images from June to August 2008 to derive raster coverages of NDVI mean, minimum, maximum, range, and standard deviation, distance to streams, elevation, and slope. We also performed a tasseled-cap transformation in ERDAS IMAGINE (Hexagon Geospatial, Norcross, GA) on a Landsat image from August 2008 to derive the wetness band (blue band). B. anthracis has affinity to moist, low lying soils (Van Ness 1971, Hugh-Jones and Blackburn 2009), which can be detected by the wetness band, elevation, and slope. We resampled all variables to 30 m to match the RSF resolution. We mapped GPS coordinates of bison, elk, and deer carcasses from the 2008 outbreak and filtered coordinates for spatially unique locations (defined as 1 point/ 30-m cell; Joyner et al. 2010). We randomly divided spatially unique carcass locations into 75% training and 25% post hoc testing subsets using Geostatistical Analyst in ArcGIS 10.1. We constructed a GARP experiment in DesktopGARP 1.1.3 (University of Kansas Center for Research, Lawrence, KS) using the environmental coverages and the 75% training subset. We used an internal data split of 75% training/25% testing for model building and internal testing to optimize rules within GARP. We developed 200 models using all 4 rule types (logit, range, negated range, and atomic), convergence limit 0.01, and 1,000 iterations. We employed the best subset procedure with a 10% omission cutoff and 50% omission cutoff to optimize model selection and arrive at a best subset of 10 models (Anderson et al. 2003). We used omission (false negative; carcass sites omitted by models), commission (false positive; proportion of the landscape predicted present), and an area under the curve (AUC) estimate to evaluate model accuracy following McNyset (2005). We limited model building and accuracy metrics to the ranch (Fig. 1), the boundary of the 2008 surveillance effort, and projected the models onto our larger study area to compare with RSF estimates of male elk habitat.
To map B. anthracis, we summated the 10 models in the best subset using the raster calculator in ArcGIS 10.1. We chose 3 cutoffs for B. anthracis presence to compare to elk resource selection. The conservative cutoff defined presence as agreement between 9 or 10 models in the best subset (from the summation). The moderate cutoff defined presence as agreement between 6 and 10 best models. The liberal approach defined presence as any pixel predicted by ≥1 of the 10 best models.
B. anthracis and Male Elk Spatial Overlap
We generated a map of the relative probability of spatial overlap between male elk and B. anthracis presence for each cutoff: conservative, moderate, and liberal. We classified an RSF value ≥ 5, which corresponds to the upper 50% area adjusted quartile, as high probability of elk habitat use. We overlaid high probability elk habitat (RSF ≥ 5) with regions predicted as pathogen presence. Owner parcel data for the study area was downloaded from the Montana state library geographic information services (http://nris.mt.gov/gis/, accessed 13 Aug 2014) to explore the relationship between anthrax risk and land ownership. We collapsed the data into 3 ownership categories: USFS, state, and privately owned land. We calculated the area of each land ownership type that comprised the high probability RSF surface, B. anthracis presence surface, and overlap between high RSF and B. anthracis presence.
RESULTS
We used GPS collar data from 13 mature male elk collected during the 2010 (n = 10 individuals), 2011 (n = 2 individuals from 2010), and 2012 (n = 3 individuals) anthrax seasons in the analysis (Table S1). All GPS fixes were an average of 6,170 m from primary roads, 3,228 m from secondary roads, and 984 m from tertiary roads. Approximately 60% of GPS fixes occurred in the forested land cover category. The average elevation of GPS fixes was 1,940 m, average slope of used points was 12.8°, and 14% of used points had a southerly aspect.
There were no variables in the data set with a Pearson’s correlation coefficient |r| ≥ 0.7. Primary roads was not significant in a univariate analysis (P = 0.8) and was not included in model list development. We identified 7 competing models from our model list based on Σwi > 0.95 (Table 1). The average Spearman rank correlation coefficient between bin number and number of used points/bin across 5 iterations for competing models ranged from rs = 0.358 to rs = 0.847.
Table 1.
Competing models (Σwi > 0.95) predicting male elk resource selection during the anthrax risk period (Jun–Aug) in the northern Madison study area, southwest Montana, USA during 2010–2012, derived from the model list of all additive combinations of covariates. For each model, we report model covariates, change in Akaike’s Information Criterion (DAIC), AIC weights (wi), and the average Spearman rank correlation coefficients (rs), which measures the relationship between model prediction and use, across 5 folds.
| Model | ΔAIC | wi | rs |
|---|---|---|---|
| Elevation + slope + forest + tertiary road + secondary road + wolf | 0.00 | 0.322 | 0.672 |
| Elevation + slope + forest + secondary road + wolf | 1.00 | 0.189 | 0.405 |
| Elevation + slope + aspect + forest + tertiary road + secondary road + wolf | 1.35 | 0.159 | 0.593 |
| Elevation + slope + aspect + forest + secondary road + wolf | 2.25 | 0.101 | 0.358 |
| Elevation + slope + forest + tertiary road + secondary road | 2.24 | 0.102 | 0.847 |
| Elevation + slope + aspect + forest + tertiary road + secondary road | 3.46 | 0.055 | 0.612 |
| Elevation + slope + forest + secondary road | 3.61 | 0.051 | 0.418 |
We selected the model with the structure elevation + slope + forest + tertiary road + secondary road as the final model based the strength of the relationship between predicted and used habitat, as compared to the other competing models. Closer proximity to tertiary roads, farther proximity to secondary roads, forested land cover (compared to shrubland and grasslands), gentler slopes, and lower elevations were all significantly associated with a higher probability of male elk resource selection during the anthrax season (Table 2 and Fig. 2). The private land in the central portion of the study area and the public lands to the southeast had the highest predicted relatively probability of male elk selection. The high use area, defined by the RSF bin ≥ 5, covered an area of 1,056 km2 (Table 3).
Table 2.
Standardized coefficient estimates and 95% confidence intervals for covariates included in the final resource selection function model for male elk in the northern Madison study area, southwest Montana, USA during the anthrax risk period (Jun–Aug), derived from male elk locations during 2010–2012.
| 95% CI
|
|||
|---|---|---|---|
| Variable | Estimate | Lower | Upper |
| Intercept | −2.0724a | −2.1255 | −2.0200 |
| Distance to tertiary roads | −0.0352b | −0.0731 | 0.0024 |
| Distance to secondary roads | 0.1660a | 0.1263 | 0.2058 |
| Forest (as compared to grasslands and shrublands) | 0.8490a | 0.7750 | 0.9233 |
| Slope | −0.1794a | −0.2160 | −0.1429 |
| Elevation | −0.2602a | −0.3089 | −0.2117 |
Indicates significance with 99% confidence.
Indicates significance with 90% confidence.
Figure 2.
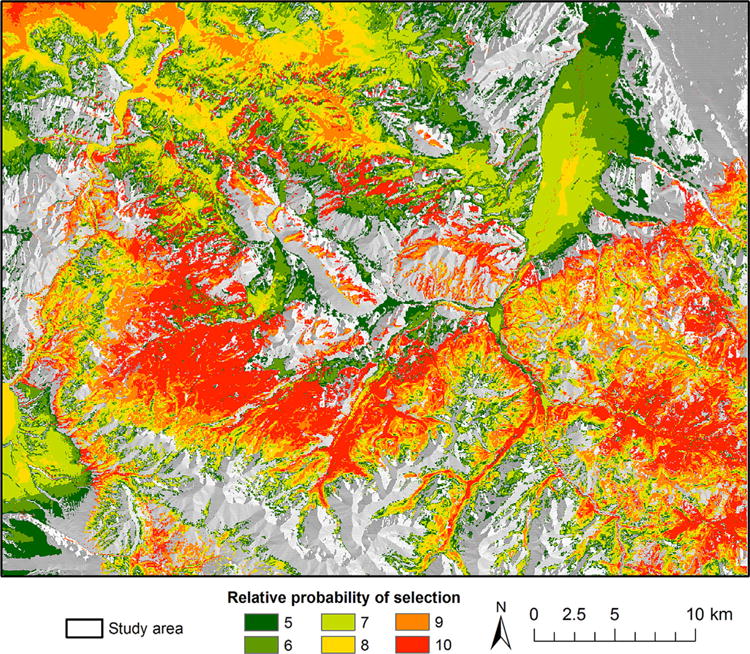
The relative probability of male elk resource selection in the northern Madison study area, southwest Montana, USA during the anthrax risk period (Jun–Aug) for 2010–2012. The top 5 out of 10 bins are displayed, and higher resource selection values reflect higher predicted relative probability of selection.
Table 3.
Proportion of area classified as high risk for male elk exposure to Bacillus anthracis in each land ownership classification, southwest Montana, USA based on elk locations in 2010–1012 and anthrax presence in 2008. We report the area of land with a resource selection function (RSF) bin score ≥ 5 for each land ownership. We also report the area of land predicted to support B. anthracis persistence for each land ownership category based on 3 calculations of predicted presence (conservative, moderate, and liberal). We report the area of land characterized by overlap between high RSF values and B. anthracis presence for each type of land ownership.
|
B. anthracis presence
|
Elk RSF ≥ 5, B. anthracis presence overlap
|
||||||
|---|---|---|---|---|---|---|---|
| Land ownership area (km2) | Elk RSF ≥ 5 | Conservative | Moderate | Liberal | Conservative | Moderate | Liberal |
| Private land | 579.03 | 342.45 | 445.98 | 615.70 | 195.81 | 253.3 | 354.9 |
| Montana state land | 37.71 | 10.94 | 14.88 | 23.37 | 6.64 | 9.08 | 13.28 |
| U.S. Forest Service | 439.63 | 20.02 | 38.01 | 86.35 | 16.45 | 32.16 | 72.09 |
| Total area (km2) | 1,056.36 | 373.41 | 498.86 | 725.43 | 218.90 | 294.54 | 440.27 |
The ecological niche modeling process reached convergence of accuracy (0.01) prior to reaching the maximum iteration setting (1,000). The overall predictive accuracy was high (average omission 4.7%), with an AUC of 0.74, confirming a reasonable model (Table 4). Several regions of the study area were predicted to be supportive of B. anthracis for each definition of presence (Fig. 3). The conservative cutoff predicted presence in the northeast and eastern border of the study area. An additional region of presence was identified across the center of the study area, and expanding to the northeast boundary. The moderate cutoff also predicted B. anthracis presence in the central and northeast portions of the study area. The liberal cutoff predicted potential B. anthracis presence across the majority of the study area, and particularly the central and northeast regions. B. anthracis presence was predicted for some portion of private, state, and USFS land at each cutoff (Table 3); the greatest area of predicted presence was on privately owned land (Table 3).
Table 4.
Anthrax carcass sample sizes and accuracy metrics for the ecological niche modeling experiment to estimate the potential geographic distribution of Bacillus anthracis on the landscape in the northern Madison study area, southwestern Montana, USA, based on a summer 2008 anthrax outbreak.
| Metric | Model specifications |
|---|---|
| N to build models | 85a |
| N to test models (independent) | 29 |
| Total omission | 3.4 |
| Average omission | 4.7 |
| Total commission | 62.92 |
| Average commission | 41.41 |
| AUCb | 0.7416c,d |
N was divided into 75% training and 25% testing at each model iteration.
AUC = area under curve.
Z = 8.72 (P < 0.001).
SE = 0.0530.
Figure 3.
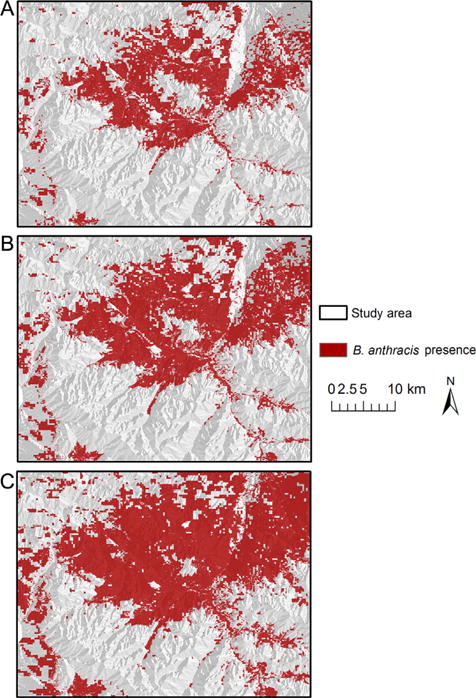
The potential distribution of Bacillus anthracis in the North Madison Study Area, southwest Montana, USA predicted from an ecological niche model experiment based on carcasses collected during the summer 2008 outbreak. We used 3 cutoffs to predict the potential distribution of B. anthracis: conservative (A), moderate (B), and liberal (C).
For the conservative B. anthracis cutoff, areas with the highest probability of male elk contacting the pathogen were distributed across the study area (Fig. 4). The north central portion of the study area was predicted as high risk of overlap between male elk and B. anthracis. Additional areas along the eastern and western boundaries of the study area were also identified as high-risk areas. The moderate cutoff of high-risk regions was spatially similar to the conservative approach, but the areas of high risk were larger. The liberal cutoff of presence identified additional portions of the southeast border of the study area as the highest probability of elk coming into contact with B. anthracis. Predicted spatial overlap between male elk and B. anthracis occurred on public and privately owned land (Fig. 5 and Table 3).
Figure 4.
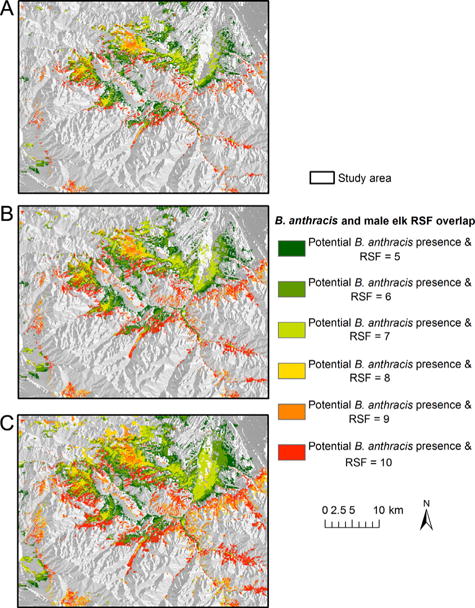
The predicted relative probability of male elk and Bacillus anthracis spatial overlap during the anthrax risk period in the North Madison study area, southwest Montana, USA based on elk locations from June through August 2010–2012 and recovered carcasses during a summer 2008 anthrax outbreak using 3 definitions of B. anthracis presence: conservative (A), moderate (B), and liberal (C). Higher resource selection function (RSF) values reflect higher predicted relative probability of selection.
Figure 5.
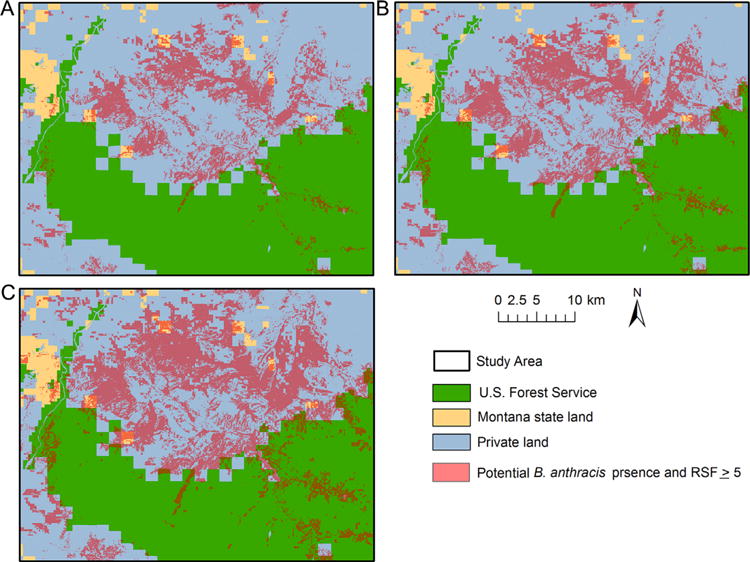
The predicted areas of male elk and Bacillus anthracis spatial overlap across land ownerships during the anthrax risk period (Jun–Aug) in the North Madison study area, southwest Montana, USA based on elk locations 2010–2012 and recovered carcasses during a summer 2008 anthrax outbreak. The male elk distribution included all areas with a resource selection function (RSF) value ≥ 5. B. anthracis distribution was defined using 3 definitions: conservative (A), moderate (B), and liberal (C).
DISCUSSION
Anthrax is underreported, underdiagnosed, and is undervalued as a public health concern (Fasanella et al. 2010, Bagamian et al. 2013). The 2008 outbreak was the first reported anthrax in southwest Montana for several decades (Blackburn et al. 2014a) and the first reported outbreak in wild elk in North America. There were extensive regions on the landscape where preferred male elk habitat overlapped with potential B. anthracis presence. These regions of elk anthrax risk were distributed across private, state, and federal land, which has important implications for anthrax management in the region.
During the anthrax risk period (defined here as Jun–Aug) male elk selected areas closer to tertiary roads, farther from secondary roads, with gentler slopes, dominated by forested land cover types, and with lower elevations. The preference for tertiary roads could be related to the high density of such roads throughout the study area. Tertiary roads are 2-track and logging roads that get minimal use, and it is plausible that male elk use tertiary roads for travel, particularly in steep or heavily forested landscapes. The avoidance of secondary roads is in line with other studies that report male elk avoiding heavily traveled roads during summer (Montgomery et al. 2013). Male elk in other studies have shown an avoidance of primary roads (Montgomery et al. 2013), but distance to primary roads was not significant in our analysis. The disparity in our findings could be attributed to a high average distance of used habitat to primary roads (6,000 m) that was too far to trigger an avoidance response. Male elk selection of higher elevations and forested land cover during summer could potentially be related to avoidance of summer heat. Male elk also selected gentler slopes compared to the available slopes in the study area. Wolf predation risk and aspect were not primary influences of male elk distributions, and our wolf predation risk and aspect covariates were not included in the top-ranked model. Wolf kill location data were limited to the private ranch where the 2008 outbreak occurred (Fig. S2), and it is possible that this data set was not representative of wolf predation across the study area. Fine scale predation behavior and occupancy data across the study area would provide a more thorough understanding of the relationship between elk and wolves. Areas of high predicted use were primarily identified on USFS land, and private land.
The predicted distribution of male elk during the anthrax risk period had a high predictive accuracy across our study site, but predictive accuracy was not evaluated beyond our study area. Other studies have reported that RSF maps applied to a larger landscape had decreased predictive accuracy (Proffitt et al. 2011). This trend has been related to variation in available habitat, and seasonal variations in climatic or environmental conditions (Mysterud and Ims 1998). Obtaining movement data from male elk during summer in other anthrax zones would provide an opportunity to determine the ability to extrapolate these findings, and explore differences in risk under different landscape compositions. We hypothesize that our findings would be relevant in landscapes with similar habitat characteristics.
Our ecological niche modeling experiment predicted significant portions of the study area as suitable for pathogen persistence. Although there were differences in the predicted B. anthracis spatial distribution under the conservative, moderate, and liberal definition of pathogen presence, each definition is plausible because all cutoffs were derived from the 10 best models in the ecological niche model experiment. This result suggests that B. anthracis may persist beyond the previously documented cases of 2008 and other areas of western Montana may be suitable for B. anthracis.
The current definitions of B. anthracis presence, derived from mortality data, may underestimate anthrax intensity (Bellan et al. 2013) and the geographic extent of outbreaks (Bagamian et al. 2013). Efforts since 2008 to control the disease in the bison herd have included limiting bison grazing in the areas of greatest mortality in 2008, which may have partially reduced cases in the subsequent years since the outbreak; however, determining this is difficult. Underestimates of the extent or intensity of disease can be attributed to the challenges associated with locating and confirming anthrax carcasses across remote landscapes (Blackburn et al. 2014a,b). This trend could be particularly relevant to our study area where high probability male elk distribution predictions include significant portions of heavily forested, steep terrain where infected carcasses are likely to be undetected. In wood bison (B. bison athabascae) outbreaks in northern Canada, anthrax mortalities in remote and heavily forested landscapes were cited as one of the primary challenges to anthrax surveillance in the region (Nishi et al. 2002). Heightened surveillance in the region would enhance our understanding of the spatial extent of anthrax risk; however, conducting surveillance in these landscapes would be logistically challenging. Targeting surveillance with our risk maps may improve anthrax carcass detections. Likewise, these maps can be used to define livestock vaccination priority areas in the study area.
Our results suggest that livestock in the region, on public and private land, are likely using landscapes supportive of B. anthracis. This is a significant concern because wildlife outbreaks can affect unvaccinated livestock when there is overlap in space use in regions that can support B. anthracis persistence (Bengis et al. 2002). For example, in 1997, there was a significant anthrax outbreak in southwest Texas that primarily affected white-tailed deer with hypothesized spillover to neighboring livestock populations (Hugh-Jones and De Vos 2002), which was confirmed in the region in 2009 and 2010 (Blackburn et al. 2014b). There is also the potential for spillover to other susceptible wildlife species in the region. We estimated an area of 20–86 km2 (conservative to liberal cutoff) of public land that was predicted to support B. anthracis persistence. These findings highlight that disease risk in this emerging anthrax region could be a multi-species concern with the capacity to affect wildlife and livestock on public and private lands. Increased monitoring of susceptible wildlife and livestock on public and private land, and reporting spatial locations of disease occurrence should be implemented to relate our predictions to actual transmission dynamics on the landscape.
Anthrax outbreaks can lead to severe economic losses, related to the loss of livestock, loss of hunted wildlife, and outbreak cleanup and management. An anthrax outbreak on public lands in late summer, a situation predicted as plausible by our modeling results, could also have implications for hunters during the archery season including a rapid die off of ungulate populations and public health concerns for hunters. These findings reflect the need for a collaborative approach between all stakeholders to successfully implement anthrax surveillance and management for male elk in the region. There has been a call for collaborative elk management in the region between public and private organizations in recent years in response to challenges related to hunter access, population management (Proffitt et al. 2013), and brucellosis control (Cross et al. 2010). This study reiterates the need for collaboration between public and private organizations as it relates to disease control.
MANAGEMENT IMPLICATIONS
Our prediction of male elk overlap with the distribution of B. anthracis provides risk maps useful for targeting anthrax surveillance efforts in southwestern Montana. These high-risk regions should be prioritized for carcass detection efforts. We also recommend a collaborative approach to anthrax surveillance and management between federal, state, and private land owners because anthrax could pose a risk to multiple wildlife hosts, livestock, and potentially archery hunters on public and private land in this emerging anthrax zone.
Supplementary Material
Acknowledgments
Extensive logistical support was provided by Turner Enterprises and the ranch personnel throughout the study. We thank I. T. Kracalik, M. Traught, and M. C. Blackburn for field work support. We thank the Montana FWP Region 3 staff for animal capture and collar recovery support. This work was partially funded by the Emerging Pathogens Institute, University of Florida. L. R. M. was partially supported by the College of Liberal Arts and Sciences, University of Florida.
Footnotes
SUPPORTING INFORMATION
Additional supporting information may be found in the online version of this article at the publisher’s website.
Associate Editor: Scott McCorquodale.
Contributor Information
LILLIAN R. MORRIS, Department of Geography, Spatial Epidemiology and Ecology Research Laboratory, Emerging Pathogens Institute, University of Florida, Gainesville, FL 32611, USA
KELLY M. PROFFITT, Montana Fish Wildlife and Parks, 1400 South 19th Avenue, Bozeman, MT 59718, USA
VALPA ASHER, Turner Enterprises, 1123 Research Drive, Bozeman, MT, USA.
JASON K. BLACKBURN, Department of Geography, Spatial Epidemiology and Ecology Research Laboratory, Emerging Pathogens Institute, University of Florida, Gainesville, FL 32611, USA.
LITERATURE CITED
- Alexander KA, Blackburn JK, Vandewalle ME, Pesapane R, Baipoledi EK, Elzer PH. Buffalo, bush meat, and the zoonotic threat of brucellosis in Botswana. PLoS ONE. 2012;7:e32842. doi: 10.1371/journal.pone.0032842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altmann M. Social behavior of elk, Cervus canadensis nelsoni, in the Jackson Hole area of Wyoming. Behaviour. 1951;4:116–142. [Google Scholar]
- Anderson DR, Burnham KP, Thompson WL. Null hypothesis testing: problems, prevalence, and an alternative. Journal of Wildlife Management. 2000;64:912–923. [Google Scholar]
- Anderson RP, Lew D, Peterson AT. Evaluating predictive models of species’ distributions: criteria for selecting optimal models. Ecological Modelling. 2003;162:211–232. [Google Scholar]
- Atwood TC. Dissertation. Utah State University; Logan, USA: 2006. Wolves, coyotes, elk, and mule deer: predator-prey behavioral interactions in southwestern Montana. [Google Scholar]
- Bagamian KH, Alexander KA, Hadfield TL, Blackburn JK. Ante- and postmortem diagnostic techniques for anthrax: rethinking pathogen exposure and the geographic extent of the disease in wildlife. Journal of Wildlife Diseases. 2013;49:786–801. doi: 10.7589/2013-05-126. [DOI] [PubMed] [Google Scholar]
- Bellan SE, Gimenez O, Choquet R, Getz WM. A hierarchical distance sampling approach to estimating mortality rates from opportunistic carcass surveillance data. Methods in Ecology and Evolution. 2013;4:361–369. doi: 10.1111/2041-210x.12021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bengis RG, Kock RA, Fischer J. Infectious animal diseases: the wildlife/livestock interface. Revue Scientifique et Technique-Office International des Épizooties. 2002;21:53–66. doi: 10.20506/rst.21.1.1322. [DOI] [PubMed] [Google Scholar]
- Blackburn JK, Asher V, Stokke S, Hunter DL, Alexander KA. Dances with anthrax: wolves (Canis lupus) kill anthrax bacteremic plains bison (Bison bison bison) in southwestern Montana. Journal of Wildlife Diseases. 2014a;50:393–396. doi: 10.7589/2013-08-204. [DOI] [PubMed] [Google Scholar]
- Blackburn JK, Goodin DG. Differentiation of springtime vegetation indices associated with summer anthrax epizootics in west Texas, USA, deer. Journal of Wildlife Diseases. 2013;49:699–703. doi: 10.7589/2012-10-253. [DOI] [PubMed] [Google Scholar]
- Blackburn JK, Hadfield TL, Curtis AJ, Hugh-Jones ME. Spatial and temporal patterns of anthrax in white-tailed deer, Odocoileus virginianus, and hematophagous flies in west Texas during the summertime anthrax risk period. Annals of the Association of American Geographers. 2014c;104:939–958. [Google Scholar]
- Blackburn JK, McNyset KM, Curtis A, Hugh-Jones ME. Modeling the geographic distribution of Bacillus anthracis, the causative agent of anthrax disease, for the contiguous United States using predictive ecologic niche modeling. American Journal of Tropical Medicine and Hygiene. 2007;77:1103–1110. [PubMed] [Google Scholar]
- Blackburn JK, Van Ert M, Mullins JC, Hadfield TL, Hugh-Jones ME. The necrophagous fly anthrax transmission pathway: empirical and genetic evidence from wildlife epizootics. Vector-Borne and Zoonotic Diseases. 2014b;14:576–583. doi: 10.1089/vbz.2013.1538. [DOI] [PubMed] [Google Scholar]
- Boyce MS. Scale for resource selection functions. Diversity and Distributions. 2006;12:269–276. [Google Scholar]
- Boyce MS, Vernier PR, Nielsen SE, Schmiegelow FK. Evaluating resource selection functions. Ecological Modelling. 2002;157:281–300. [Google Scholar]
- Brook RK, McLachlan SM. Transdisciplinary habitat models for elk and cattle as a proxy for bovine tuberculosis transmission risk. Preventive Veterinary Medicine. 2009;91:197–208. doi: 10.1016/j.prevetmed.2009.05.021. [DOI] [PubMed] [Google Scholar]
- Burnham KP, Anderson DR. Model selection and multimodel inference: a practical information-theoretic approach. Springer; New York, New York, USA: 2002. [Google Scholar]
- Christianson D, Creel S. Risk effects in elk: sex-specific responses in grazing and browsing due to predation risk from wolves. Behavioral Ecology. 2008;19:1258–1266. [Google Scholar]
- Creel S, Winnie J, Jr, Maxwell B, Hamlin K, Creel M. Elk alter habitat selection as an antipredator response to wolves. Ecology. 2005;86:3387–3397. [Google Scholar]
- Cross PC, Heisey DM, Scurlock BM, Edwards WH, Ebinger MR, Brennan A. Mapping brucellosis increases relatie to elk density using hierarchical Bayesian models. PLoS One. 2010;5:e10322. doi: 10.1371/journal.pone.0010322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeCesare NJ, Hebblewhite M, Schmiegelow F, Hervieux D, McDermid GJ, Neufeld L, Bradley M, Whittington J, Smith KG, Morgantini LE. Transcending scale dependence in identifying habitat with resource selection functions. Ecological Applications. 2012;22:1068–1083. doi: 10.1890/11-1610.1. [DOI] [PubMed] [Google Scholar]
- Edge WD, Marcum CL, Olson-Edge SL. Summer habitat selection by elk in western Montana: a multivariate approach. Journal of Wildlife Management. 1987;51:844–851. [Google Scholar]
- Fasanella A, Galante D, Garofolo G, Jones MH. Anthrax undervalued zoonosis. Veterinary Microbiology. 2010;140:318–331. doi: 10.1016/j.vetmic.2009.08.016. [DOI] [PubMed] [Google Scholar]
- Fotheringham AS, Brunsdon C, Charlton M. Quantitative geography: perspectives on spatial data analysis. Sage; London, United Kingdom: 2000. [Google Scholar]
- Fouchier RA, Schneeberger PM, Rozendaal FW, Broekman JM, Kemink SA, Munster V, Kuiken T, Rimmelzwaan GF, Schutten M, van Doornum GJ. Avian influenza A virus (H7N7) associated with human conjunctivitis and a fatal case of acute respiratory distress syndrome. Proceedings of the National Academy of sciences of the United States of America. 2004;101:1356–1361. doi: 10.1073/pnas.0308352100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillies CS, Hebblewhite M, Nielsen SE, Krawchuk MA, Aldridge CL, Frair JL, Saher DJ, Stevens CE, Jerde CL. Application of random effects to the study of resource selection by animals. Journal of Animal Ecology. 2006;75:887–898. doi: 10.1111/j.1365-2656.2006.01106.x. [DOI] [PubMed] [Google Scholar]
- Hebblewhite M, Merrill E, McDonald T. Spatial decomposition of predation risk using resource selection functions: an example in a wolf-elk predator-prey system. Oikos. 2005;111:101–111. [Google Scholar]
- Hugh-Jones M, Blackburn J. The ecology of Bacillus anthracis. Molecular Aspects of Medicine. 2009;30:356–367. doi: 10.1016/j.mam.2009.08.003. [DOI] [PubMed] [Google Scholar]
- Hugh-Jones M, De Vos V. Anthrax and wildlife. Revue Scientifique et Technique-Office International des Epizooties. 2002;21:359–384. doi: 10.20506/rst.21.2.1336. [DOI] [PubMed] [Google Scholar]
- Johnson DH. The comparison of usage and availability measurements for evaluating resource preference. Ecology. 1980;61:65–71. [Google Scholar]
- Joyner TA, Lukhnova L, Pazilov Y, Temiralyeva G, Hugh-Jones ME, Aikimbayev A, Blackburn JK. Modeling the potential distribution of Bacillus anthracis under multiple climate change scenarios for Kazakhstan. PLoS ONE. 2010;5:e9596. doi: 10.1371/journal.pone.0009596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilpatrick AM, Gillin CM, Daszak P. Wildlife-livestock conflict: the risk of pathogen transmission from bison to cattle outside Yellowstone National Park. Journal of Applied Ecology. 2009;46:476–485. [Google Scholar]
- Laundré JW, Hernández L, Altendorf KB. Wolves, elk, and bison: reestablishing the “landscape of fear” in Yellowstone National Park, USA. Canadian Journal of Zoology. 2001;79:1401–1409. [Google Scholar]
- Manly B, McDonald L, Thomas D, McDonald T, Erickson W. Resource selection by animals: statistical analysis and design for field studies. Kluwer, Nordrecht; The Netherlands: 2002. [Google Scholar]
- McCorquodale SM. Sex-specific movements and habitat use by elk in the Cascade Range of Washington. Journal of Wildlife Management. 2003;67:729–741. [Google Scholar]
- McCorquodale SM. A brief review of the scientific literature on elk, roads, & traffic. Washington Department of Fish and Wildlife; Olympia, USA: 2013. [Google Scholar]
- McNyset K. Use of ecological niche modelling to predict distributions of freshwater fish species in Kansas. Ecology of Freshwater Fish. 2005;14:243–255. [Google Scholar]
- Meyer CB, Thuiller W. Accuracy of resource selection functions across spatial scales. Diversity and Distributions. 2006;12:288–297. [Google Scholar]
- Montgomery RA, Roloff GJ, Millspaugh JJ. Variation in elk response to roads by season, sex, and road type. Journal of Wildlife Management. 2013;77:313–325. [Google Scholar]
- Mullins JC, Garofolo G, Van Ert M, Fasanella A, Lukhnova L, Hugh-Jones ME, Blackburn JK. Ecological niche modeling of Bacillus anthracis on three continents: evidence for genetic-ecological divergence? PLoS ONE. 2013;8:e72451. doi: 10.1371/journal.pone.0072451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullins J, Lukhnova L, Aikimbayev A, Pazilov Y, Van Ert M, Blackburn JK. Ecological niche modelling of the Bacillus anthracis A1.a sub-lineage in Kazakhstan. BMC Ecology. 2011;11:32. doi: 10.1186/1472-6785-11-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mysterud A, Ims RA. Functional responses in habitat use: availability influences relative use in trade-off situations. Ecology. 1998;79:1435–1441. [Google Scholar]
- Nishi JS, Dragon DC, Elkin BT, Mitchell J, Ellsworth TR, Hugh-Jones ME. Emergency response planning for anthrax outbreaks in bison herds of northern Canada. Annals of the New York Academy of Sciences. 2002;969:245–250. doi: 10.1111/j.1749-6632.2002.tb04386.x. [DOI] [PubMed] [Google Scholar]
- Proffitt KM, Gude JA, Hamlin KL, Garrott RA, Cunningham JA, Grigg JL. Elk distribution and spatial overlap with livestock during the brucellosis transmission risk period. Journal of Applied Ecology. 2011;48:471–478. [Google Scholar]
- Proffitt KM, Gude JA, Hamlin KL, Messer MA. Effects of hunter access and habitat security on elk habitat selection in landscapes with a public and private land matrix. Journal of Wildlife Management. 2013;77:514–524. [Google Scholar]
- Rowland MM, Wisdom MJ, Johnson BK, Penninger MA. Effects of roads on elk: implications for management in forested ecosystems. In: Wisdom MJ, editor. The Starkey Project: a synthesis of long-term studies of elk and mule deer Reprinted from the 2004 Transactions of the North American Wildlife and Natural Resources Conference. Alliance Communications Group; Lawrence, Kansas, USA: 2005. pp. 42–52. [Google Scholar]
- Shury TK, Frandsen D, O’Brodovich L. Anthrax in free-ranging bison in the Prince Albert National Park area of Saskatchewan in 2008. The Canadian Veterinary Journal. 2009;50:152. [PMC free article] [PubMed] [Google Scholar]
- Sittler KL, Parker KL, Gillingham MP. Resource separation by mountain ungulates on a landscape modified by fire. Journal of Wildlife Management. 2015;79:591–604. [Google Scholar]
- Stockwell D. The GARP modelling system: problems and solutions to automated spatial prediction. International Journal of Geographical Information Science. 1999;13:143–158. [Google Scholar]
- Thompson WL, Lee DC. Modeling relationships between landscape-level attributes and snorkel counts of Chinook salmon and steelhead parr in Idaho. Canadian Journal of Fisheries and Aquatic Sciences. 2000;57:1834–1842. [Google Scholar]
- Turner WC, Imologhome P, Havarua Z, Kaaya GP, Mfune JK, Mpofu ID, Getz WM. Soil ingestion, nutrition and the seasonality of anthrax in herbivores of Etosha National Park. Ecosphere. 2013;4:art13. [Google Scholar]
- Turner WC, Kausrud KL, Krishnappa YS, Cromsigt JP, Ganz HH, Mapaure I, Cloete CC, Havarua Z, Küsters M, Getz WM. Fatal attraction: vegetation responses to nutrient inputs attract herbivores to infectious anthrax carcass sites. Proceedings of the Royal Society B: Biological Sciences. 2014;281:20141785. doi: 10.1098/rspb.2014.1785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unsworth JW, Kuck L, Garton EO, Butterfield BR. Elk habitat selection on the Clearwater National Forest, Idaho. Journal of Wildlife Management. 1998;62:1255–1263. [Google Scholar]
- Van Ness GB. Ecology of anthrax. Science. 1971;172:1303–1307. doi: 10.1126/science.172.3990.1303. [DOI] [PubMed] [Google Scholar]
- Womack KM, Amelon SK, Thompson FR., III Resource selection by Indiana bats during the maternity season. Journal of Wildlife Management. 2013;77:707–715. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


