Abstract
Development of reading skills has been shown to be tightly linked to phonological processing skills and to some extent to speech perception abilities. Although speech perception is also known to play a role in reading development, it is not clear which processes underlie this connection. Using event-related potentials (ERPs) we investigated the speech processing mechanisms for common and uncommon sound contrasts (/ba/-/da/-/ga/ and /ata/-/at:a/) with respect to the native language of school-age children in Finland and the United States. In addition, a comprehensive behavioral test battery of reading and phonological processing was administered. ERPs revealed that the children could discriminate between the speech sound contrasts (place of articulation and phoneme length) regardless of their native language. No differences emerged between the Finnish and US children in their change detection responses. However, the brain responses to the phoneme length contrast correlated robustly with reading scores in the US children with larger responses being linked to poorer reading skills. Finnish children also showed correlations between the reading and phonological measures and ERP responses, but the pattern of results was not as clear as for the US children. The results indicate that speech perception is linked to reading skills and this link is more robust for uncommon speech sound contrasts.
Keywords: children, cross-linguistic, dyslexia, EEG, event-related potentials, phonology, reading, speech
Introduction
Development of reading skills has been shown to be tightly linked to facility with phonological processing skills (e.g. Anthony & Francis, 2005; Bradley & Bryant, 1978; Goswami & Bryant, 1990; Melby-Lervåg, Lyster, & Hulme, 2012; Wagner & Torgesen, 1987). Reading skills have also been previously linked to speech perception abilities (e.g., McBride-Chang, 1995; Mody et al., 1997). Here we set out to investigate the relationship between speech sound processing, using brain event-related potentials (ERPs), and reading skills in two different languages, English and Finnish.
Speech perception is known to play a role in reading development, but it is not clear which processes underlie this connection. Deficient speech processing measured with ERPs as well as discrimination and categorization tasks is related to reading failure in individuals with dyslexia (e.g., Hämäläinen et al., 2009; Leppänen et al., 2002; McBride-Chang, 1995; Richardson et al., 2003; Schulte-Körne et al., 1998), and atypical specialization to phoneme contrasts of one’s own native language is related to deficient reading skills (Serniclaes et al., 2004). It is also known that speech perception abilities change during the life course as a result of spoken language exposure (Kuhl et al., 2008).
For studying speech representations in the brain, the electroencephalogram (EEG) technique offers an objective measure at any age (for reviews, see Bishop, 2007; Conboy et al., 2008; Friederici, 2005; Kuhl, 2004; Schulte-Körne & Bruder, 2010). Two event-related potentials (ERPs) derived from EEG have been shown to index auditory and speech sound discrimination accuracy: mismatch negativity (MMN) and late discriminative negativity (LDN) (Cheour et al., 2001; Näätänen et al., 2010). The amplitude of MMN to speech stimuli is also modulated by longer-term experience with speech sound representations, leading to larger responses for native contrasts as compared to non-native contrasts (Kirmse et al., 2008; Näätänen et al., 1997; Winkler et al., 1999). The attenuated MMN to non-native contrasts is likely due to top-down processes such as tuning of native speech sound categories and the consequent drop of discrimination accuracy for within-category speech contrasts (Näätänen et al., 1997; Kuhl et al., 2008).
There is less clear picture on how native and non-native speech sounds affect the LDN. Several alternatives for the functional significance of this ERP component has been put forward, for example that it would reflect preattentive cognitive evaluation of the stimuli (Ceponiene et al., 2004; Jakoby et al., 2011) or the formation of memory representations of the stimuli (Barry et al., 2009). The LDN seems to be related to the success in learning a foreign language (Jakoby et al., 2011; Shestakova et al., 2003) and the capacity to learn new words as reflected by a non-word repetition task (Barry et al., 2009). These studies indicate that LDN reflects an important processing stage in speech perception.
The strength of these ERP responses also differs between children with dyslexia and typically developing individuals (e.g., Hämäläinen et al., 2013; Lohvansuu et al., 2014; Maurer, Bucher, Brem, & Brandeis, 2003; Schulte-Körne, Deimel, Bartling, & Remschmidt, 1998). Also other ERP responses which are generated as a consequence of afferent activation and the related processing, have been found to differentiate between individuals with reading problems and with typical reading skills (e.g., Helenius et al., 2002; Hämäläinen et al., 2013; Lohvansuu et al., 2014). For example, in children, the obligatory ERPs form a series of responses termed P1, N2 (or N250) and N4 named after their latency and order (e.g., Ceponiene et al., 2005). Moreover, infants at high familial risk for dyslexia already show abnormal brain responses to the contrasts of their native language (Leppänen et al., 1999; 2002; van Zuijen et al., 2013) as well as to speech sounds occurring rarely in the mother tongue (Guttorm et al., 2001; 2005; 2010). Furthermore, among typically developing children, correlations between neural responses to speech, reading and phonological skills have been observed (Bonte et al., 2007; Espy et al., 2004; Kuuluvainen et al., 2016; Parviainen et al., 2011).
The first aim of this study was therefore to examine how longer-term exposure to native language affects the brain responses generated by detection of deviant speech sounds embedded in a stream of repeated speech sounds in both English speaking and Finnish speaking school aged children. We hypothesized that language-group differences would emerge particularly for the phoneme length contrast. The phoneme length contrast is not semantically distinctive in English whereas it is in Finnish. Supporting our hypothesis, earlier studies have observed amplitude differences in MMN responses to phoneme length changes in Finnish and German speaking adults (e.g., Kirmse et al., 2008). For the other speech sound contrasts we used, i.e. consonant-vowel syllables with place-of-articulation changes (see the methods), the change-detection ERP responses were expected to be larger for sounds that are common than sounds that are uncommon in the Finnish language, although to a lesser degree due to English language exposure of almost all Finnish children.
The second aim of the study was to examine whether the ERP responses to speech sounds that are common or uncommon in one’s native language were associated with reading skills or skills that are highly predictive of reading accuracy and speed (i.e., phonological awareness, rapid naming, verbal short-term memory) as previous studies have shown such associations for native speech sound processing (e.g., Bonte et al., 2007; Kuuluvainen et al., 2016).
Methods
Participants
The Finnish children were recruited at the Central Finland area via the local day-care centers, schools and a learning disability clinic. Altogether 41 children (20 girls, 21 boys) participated in the EEG study at the age of 10.3 – 12.5 years (at the 4th grade in school). They had been screened for exclusion criteria (learning disabilities other than dyslexia, neurological disorders, medication, head injuries, hearing problems). There were 4 children with a diagnosis of dyslexia and additional nine children had reading scores below −1.25 standard deviations (SD) at the 2nd grade (however, at the 4th grade only five of them had reading scores below −1.25 SDs). These children were included in the final sample of 38 children with successful EEG data acquisition.
The children from the USA were recruited from the greater New Haven CT region via local advertisements. Altogether 76 children participated in the EEG study at the age of 4.9 – 12.2 years (kindergarten to 5th grade in school) who had been screened for exclusion criteria (neurological disorders, medication, head injuries, hearing problems). Some children were excluded based on the diagnosis of attention deficit disorder (9), minor brain dysfunction (6), and specific language impairment (3). There were 6 children with a diagnosis of dyslexia who were included in the final sample. Good EEG data were obtained for the experiment with English speech sounds for 54 children and for the experiment with Finnish speech sounds for 44 children.
Behavioral measures
Finland
All behavioral assessments were conducted in June-November, while at the end of 4th grade and start of 5th grade in two testing sessions, to characterize the reading level, phonological skills and verbal working memory of the children.
Working memory
Series of numbers both forward and backward from the Wechsler Intelligence Scale for Children - Third edition (WISC-III) was used (Wechsler, 1991).
Reading in Finnish
Six reading tests were used. Standardized test of word list reading (Lukilasse; Häyrinen et al., 1999), number of correctly read words in 45 sec was used as the score; non-word list reading based on TOWRE (Torgesen et al., 1999), number of correctly read non-words in 45 sec was used as the score; standardized test of sentence reading where the children had to read a sentence and match it with the related correct picture (Lindeman, 1998), number of read sentences in 120 sec was used as the score; text reading (Puolakanaho et al., 2008), number of correctly read words in 1 minute was used as the score; pseudoword text reading (Eklund et al., 2015), number of correctly read words and total reading time were used as the scores; lexical decision task where the children silently read words and had to decide whether the word had a meaning or not, number of correct decisions was used as the score.
Writing in Finnish
Writing 4-syllabic words from dictation, number of correct items out of 10 was used as the score; Writing 4-syllabic pseudowords from dictation, number of correct items out of 12 was used as the score.
Phonological processing
Phoneme deletion task requiring children to delete a specified phoneme from 1–3 syllabic non-words, number of correct items out of 18 was used as the score; phoneme length perception where the child heard through headphones two non-words and had to decide whether they were the same or different (Hämäläinen et al., 2009), number of correct items out of 22 was used as the score; non-word repetition task from the Neuropsychological test battery (NEPSY; Korkman, Kirk, Kemp, 1998), number of correct items out of 16 was used as the score.
Rapid naming
Rapid automatized naming task (RAN: Objects Letters (Denckla & Rudel, 1976). Total matrix completion time (in seconds) was used as a measure.
The United States
All behavioral assessments were conducted in year round, and children participated in two testing sessions to characterize reading level, phonological skills and verbal working memory. Standard scores from all tests were used in the analyses.
The Comprehensive Test of Phonological Achievement (CTOPP) (Wagner et al., 1999), subtests of Phonological Awareness (Elision, Blending Words, and Blending Nonwords) were administered to determine awareness and access to the speech sound structure. Subtests of Phonological Memory (Memory for Digits and Nonword Repetition) and Rapid Naming were also administered to assess phonological encoding in working memory and speed of lexical retrieval.
The Tests of Word Reading Efficiency (TOWRE) (Torgesen et al., 1999) is comprised of two subtests requiring the speeded reading of real English words (Sight Word Efficiency) and of pseudowords (Phonemic Decoding Efficiency). For both subtests, the items are ordered from easiest to most difficult, and the examinee is asked to read as many items as possible in 45 seconds. Total score across the subtests was also calculated.
The Gray Oral Reading Test–3 (GORT-3) (Widerholdt, 1992) was used as an assessment of reading proficiency. Participants read aloud stories of increasing difficulty, followed by answering questions about the stories. This test measures oral reading ability in the domains of accuracy in terms of word pronunciation, fluency, and comprehension.
The Peabody Picture Vocabulary Test-III (PPVT-III) was used to measure lexical/vocabulary skills (Dunn & Dunn, 1997). In the PPVT, stimulus words are provided for which an individual must select the corresponding picture from a field of four. The PPVT-IV is arranged to provide words of increasing difficulty.
The WASI (Psychological Corporation, 1999) is administered as a measure of IQ. The four subtests of the WASI—Vocabulary, Block Design, Similarities, and Matrix Reasoning are used to measure various facets of intelligence, including verbal knowledge, spatial reasoning, and visual information processing. Vocabulary and Similarities subtests compose Verbal IQ. Block Design and Matrix Reasoning subtests make up the Performance IQ.
Stimuli and procedure
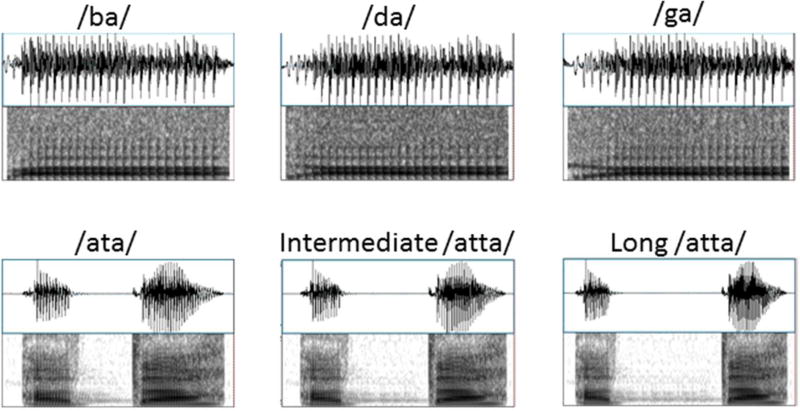
Three passive oddball experiments were run for all children with English (/ba/, /da/, /ga/), Finnish (/ata/, intermediate /atta/ and long /atta/), and Taiwan Mandarin (/fau/ with three different lexical tones) speech stimuli (see Table 1 for details of the English and Finnish stimuli). Place-of-articulation contrast was used in the English experiment with the consonant-vowel syllable /ba/ as the standard sound and /da/ and /ga/ syllables as the deviant sounds, and stop consonant (silent gap) length contrast was used in the Finnish experiment with the pseudoword /ata/ as the standard sound (short consonant length) and an intermediate /atta/ and long /atta/ sounds as the deviant sounds (8.3 % probability for each deviant in each experiment). The long /atta/ sound was clearly categorized as having a long consonant length, whereas the intermediate /atta/ was in the long category but close to the category border (Richardson et al., 2003). The experiments were run in the same order (English, Finnish, Chinese) for all children. The experiment with the Taiwan Mandarin stimuli was not carried out to all children due to fatigue and is therefore not reported here. In each experiment the standard sound was repeated 1000 times and two deviants were repeated each 125 times. The stimuli were presented in a pseudorandom order such that there were always at least 2 standard stimulus presentations between each deviant stimulus.
Table 1.
Details of the speech stimuli used in the experiments.
| English | Finnish | |||||
|---|---|---|---|---|---|---|
| /ba/ | /da/ | /ga/ | /ata/ | intermediate /atta/ |
long /atta/ | |
| Stimulus type | standard | deviant | deviant | standard | deviant | deviant |
| Contrast | place-of-articulation | place-of-articulation | place-of-articulation | gap duration (95 ms) | gap duration (155 ms) | gap duration (255 ms) |
| Total duration (ms) | 251 | 251 | 251 | 298 | 359 | 440 |
Stimuli were presented at a comfortable hearing level through a loudspeaker situated approximately 80 cm above the participant with 75 – 82 dB(C). The onset-to-onset stimulus onset asynchrony (SOA) was 1220 ms in each experiment which caused the offset-to-onset inter-stimulus interval (ISI) to vary according to the length of the stimuli.
During the experiment the participants sat in an armchair watching a muted movie and were asked not to pay attention to the presented speech stimuli. The ERP measurement lasted altogether ca. 1.5 hours. Breaks were provided when necessary.
EEG acquisition
In both sites, the EEG data were collected with an Electric Geodesics Inc. (EGI) EEG-system and NetStation 4.2 software (http://www.egi.com/). Ag-AgCl electrodes with the EGI 128-channel Hydrocel sensor net were used with Cz as the reference channel during recording. The sampling rate was 500 Hz. The EEG was filtered online with a highpass filter of 0.1 and a lowpass filter of 200 Hz. Electrode impedances were pursued to be set below 50 kΩ in the beginning of the experiment. During the experiment, the quality of the data was monitored and the electrode impedances were adjusted when necessary.
Data analysis
The EEG data was analyzed using BESA Research 6.0 (BESA GmbH, Gräfelfing, Germany). Channels showing continuously bad data were interpolated using the spherical spline method (3.0 channels on average for the Finnish sample, 5.3 for the US sample). The EEG data was offline filtered using zero-phase 0.5 Hz (12dB/Oct) highpass and 30 Hz (24 dB/Oct) lowpass. ICA (Infomax on 20–120 sec time window containing at least 2 blinks) was used for correcting eye blink artifacts in the data. For averaging the epoch length was −200 – 1020 ms with 200 ms prestimulus baseline. Artifact rejection criteria were 175 µV (maximum minus minimum amplitude) within the whole epoch and 75 µV for fast transient amplitude changes. See Table 2 for accepted number of epochs after the artifact rejection. The groups differed in the number of epochs (US children having less epochs after artifact rejection than Finnish children; all ps < 0.006) when tested with independent samples t-tests.
Table 2.
Average epoch numbers (standard deviantiations) included in event-related potential averaging after artifact rejection for the Finnish (N=38) and US (N=54 for Ba-Da-Ga, N=44 for Ata-Atta) samples.
| PreDa standard |
PreGa standard |
Da deviant |
Ga deviant |
Pre int /atta/ standard |
Pre long /atta/ standard |
Intermediate /atta/ deviant |
Long /atta/ deviant |
|
|---|---|---|---|---|---|---|---|---|
| Finnish | 100.3 (18.8) | 101.0 (19.6) | 102.5 (18.3) | 100.0 (19.5) | 94.2 (12.8) | 93.0 (14.3) | 94.5 (13.9) | 92.1 (14.3) |
| US | 73.2 (22.5) | 72.4 (21.9) | 73.0 (21.9) | 72.3 (22.9) | 80.0 (20.5) | 80.6 (21.3) | 80.5 (20.7) | 80.9 (19.8) |
Due to the large number of behavioral reading measures in Finnish, Principal axis factoring (based on correlation matrix, Varimax rotation, and Bartlett factor scores) was used for the six reading variables yielding two factors: reading accuracy and reading speed explaining 49 % and 29 % of the total variance, respectively. In addition, the scores of the two writing tasks were summed, and the time (in seconds) in the two RAN tasks was averaged.
ERPs to the deviant stimuli and the pre-deviant standard stimuli were examined. When examining the responses to the pre-deviant standard stimuli only those results similar to both pre-deviant standards are reported for each experiment. The responses to the pre-deviant standard stimuli were examined separately to have comparable signal-to-noise ratio (same number of trials) to the deviant stimulus responses. To test the effect of native language exposure, the ERP responses were compared between the Finnish and US samples in BESA Statistics 2.0 using non-parametric permutation statistics and clustering (time points and electrodes) that is based on initial independent samples t-tests. All analyses used a channel neighbor distance of 3 cm, and time window 0 – 998 ms. The clustering (time points and electrodes) is used to control for Type I error (see Maris & Oostenveld, 2007). The number of permutations was 1000. To control for the effects of age, it was entered as a covariate into BESA Statistics 2.0 using permutation statistics based on one-way ANOVA (between groups comparison). For completeness, we report if the group effects were affected by the age covariate. To examine the effects of speech sound discrimination on reading skills the ERP data (each channel and each time point for each type of stimulus) was correlated with the behavioral measures of reading and reading related skills in BESA Statistics 2.0. The correlations were also corrected for multiple comparisons of channels and time points using permutation statistics and data clustering as implemented in BESA Statistics 2.0. Therefore, the time windows and channel clusters were data-driven and not defined a priori.
Results
Cognitive skills
Descriptive statistics of the cognitive skill measures are presented in Tables 3 and 4.
Table 3.
Descriptive statistics of the cognitive skill measures for the Finnish children (N=38).
| Mean | Standard deviation |
Range | |
|---|---|---|---|
| Age (years) | 11.08 | 0.51 | 10.3 – 12.5 |
| Digit span, raw score | 11.6 | 2.8 | 8 – 21 |
| Word list reading (Lukilasse), correct items | 45.9 | 12.0 | 19 – 71 |
| Text reading, correct items | 119.1 | 4.8 | 104 – 124 |
| Non-word text reading, correct items | 43.6 | 11.4 | 19–66 |
| Sentence reading, correct items | 37.8 | 10.8 | 14–57 |
| Lexical decision, correct items | 143.8 | 4.5 | 131–150 |
| Writing, words, correct items | 5.7 | 2.5 | 0–10 |
| Writing, non-words, correct items | 8.3 | 2.1 | 3–12 |
| Phoneme deletion, correct items | 14.8 | 3.2 | 4–18 |
| Non-word repetition, correct items | 9.7 | 2.0 | 4–14 |
| Rapid automatic naming, objects, time (sec) | 49.6 | 8.8 | 33–72 |
| Rapid automatic naming, letters, time (sec) | 28.9 | 7.4 | 16–52 |
| Phoneme length perception, correct items | 16.9 | 2.6 | 11–21 |
Table 4.
Descriptive statistics of the cognitive skill measures for the US children.
| Mean | Standard deviation | Range | |
|---|---|---|---|
| Age (years), N=54 | 7.97 | 1.67 | 4.9–11.2 |
| Phonological awareness, N=46 | 107.4 | 15.6 | 85–151 |
| Phonological memory, N=48 | 96.3 | 9.9 | 79–127 |
| Rapid automatic naming, N=21 | 102.6 | 12.6 | 85–124 |
| Grey oral reading test, accuracy, N=42 | 9.1 | 4.3 | 1–17 |
| Grey oral reading test, comprehension, N=42 | 11.8 | 3.4 | 5–19 |
| Grey oral reading test, fluency, N=42 | 10.1 | 4.3 | 1–18 |
| Tests of word reading efficiency, total, N=43 | 107.6 | 20.3 | 66–146 |
| Tests of word reading efficiency, phonemic, N=43 | 106.8 | 16.9 | 79–140 |
| Tests of word reading efficiency, sight, N=43 | 105.9 | 18.1 | 59–138 |
| Peabody picture vocabulary test, N=52 | 113.4 | 11.1 | 95–139 |
| Performance IQ, N=43 | 109.6 | 14.2 | 79–138 |
| Verbal IQ, N=44 | 110.7 | 13.7 | 61–151 |
ERP results
Differences between responses to the deviant and standard stimuli
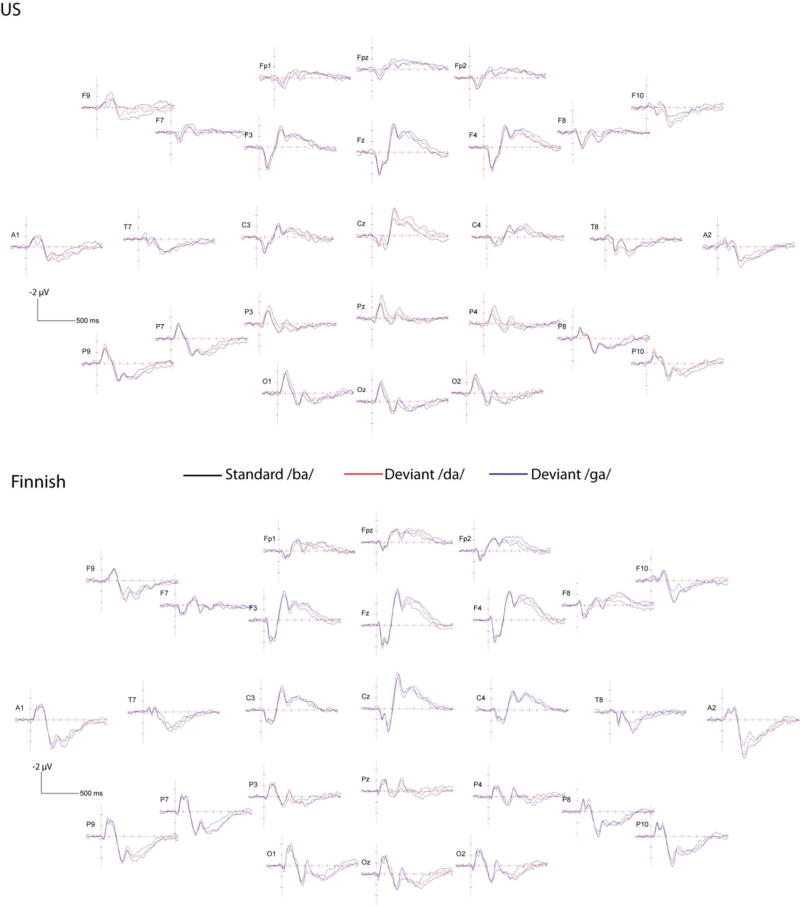
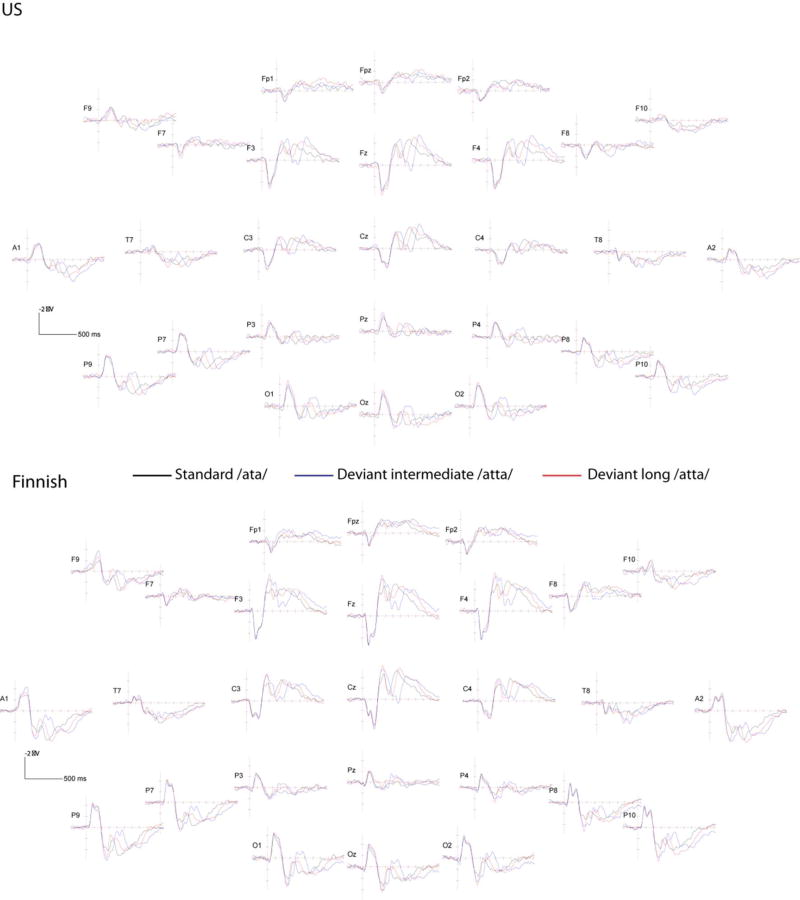
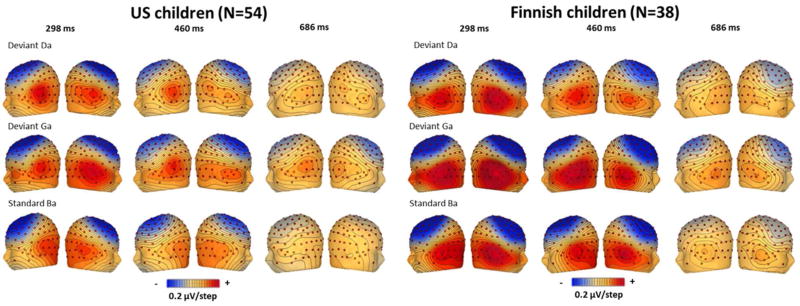
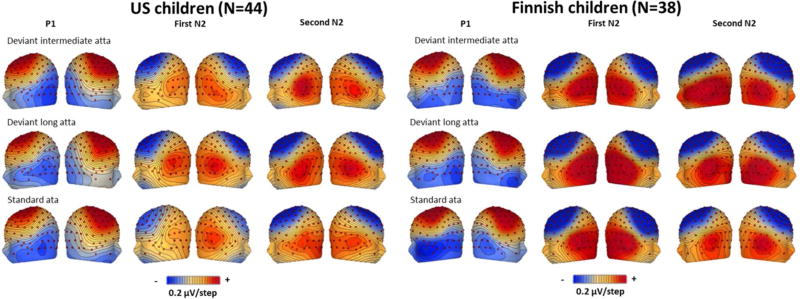
To ascertain that the children were able to detect the differences between the stimuli, the responses to the deviant and standard stimuli were compared. Differences were found for all conditions and in both samples, except between those to the deviant /da/ and standard /ba/ in the US sample. See below details of the stimulus differences in Tables 5 and 6, and Figures 2 and 3 for the ERP waveforms, and Figures 4 and 5 for the topographies.
Table 5.
Summary of the statistical differences between the responses to the deviant and standard stimuli in the Finnish sample (N=34).
| Cluster number |
p-value | Cohen’s d | 95 % confidence interval |
Topography | Time window (ms) |
Event-related potential components |
|
|---|---|---|---|---|---|---|---|
| Da vs. ba | 1 | <0.0001 | 0.99 | 0.46–1.23 | Parietal | 238–400 | N250/MMN |
| 2 | <0.005 | 0.61 | 0.12–0.78 | Temporal – fronto-central | 416–738 | LDN | |
| 3 | <0.009 | −0.76 | −1.02–(−0.26) | Left parieto-occipital | 502–750 | LDN | |
| 4 | <0.042 | −0.60 | −1.17–(−0.17) | Left fronto-central | 260–386 | N250 | |
| Ga vs. ba | 1 | <0.0001 | 0.66 | 0.17–0.91 | Fronto-central | 274–950 | MMN+LDN |
| 2 | <0.0001 | −0.74 | −0.75–(−0.18) | Parietal | 400–876 | LDN | |
| 3 | <0.042 | −0.73 | −1.79–(−0.43) | Right temporal | 252–440 | N250 | |
| Intermediate atta vs. ata | 1 | <0.0001 | 0.73 | 0.27–1.14 | Fronto-central | 556–826 | N250/LDN to second syllable |
| 2 | <0.0001 | −0.77 | −1.18–(−0.31) | Fronto-central | 254–514 | Atta4 larger N250, P1_2 | |
| 3 | <0.003 | −0.60 | −1.14–(−0.16) | Parieto-occipital | 500–798 | LDN more positive for atta4 | |
| 4 | <0.004 | 0.93 | 0.55–1.59 | Fronto-central | 174–286 | larger N250 for atta4 | |
| 5 | <0.004 | 0.68 | 0.31–1.52 | Parieto-occipital/left temporal | 318–474 | earlier N250 for ata1 | |
| 6 | <0.014 | −0.79 | −1.77–(−0.49) | Parieto-occipital/right temporal | 198–280 | larger N250 for atta4 | |
| Long atta vs. ata | 1 | <0.0001 | −1.83 | −2.58–(−1.56) | Fronto-central until 592 ms then parieto-occipital | 362–998 | P1_2 and N250_2 for atta8 |
| 2 | <0.0001 | 1.14 | 0.61–1.41 | Fronto-central | 560–998 | N250_2 for atta8 | |
| 3 | <0.002 | 0.87 | 0.66–2.09 | Parieto-occipital | 366–542 | P1_2 for atta8 | |
| 4 | <0.009 | 1.03 | 0.66–1.67 | Fronto-central | 212–364 | larger N250 for atta8 | |
| 5 | <0.014 | −0.74 | −1.83–(−0.45) | Parieto-occipital | 174–370 | larger N250 for atta8 |
Note. Cohen’s d and 95 % confidence intervals were calculated for the channel and time window showing maximal differences in the cluster-based permutation statistic.
Table 6.
Summary of the statistical differences between the responot significantes to the deviant and standard stimuli in the US sample (N=54 for Ba-Da-Ga, N=44 for Ata-Atta).
| Cluster number |
p-value | Cohen’s d | 95 % confidence intervals |
Topography | Time window (ms) |
Event-related potential components |
|
|---|---|---|---|---|---|---|---|
| Ga vs. ba | 1 | <0.0001 | 0.69 | 0.30–1.04 | Parieto-occipital – Frontro-central | 80–492; 494–998 | P1, N250, LDN |
| 2 | <0.027 | −0.67 | −1.23–(−0.34) | Frontal | 102–476 | P1 | |
| 3 | <0.036 | −0.57 | −1.35–(−0.27) | Left temporal | 270–736 | LDN | |
| Intermediat e atta vs. ata | 1 | <0.001 | 0.85 | 0.39–1.14 | Frontro-central | 488–876 | N250 to 2nd syllable in atta |
| 2 | <0.003 | −0.79 | −1.12–(−0.34) | Left temporo-occipital | 488–994 | N250 to 2nd syllable in atta | |
| 3 | <0.006 | −1.17 | −1.80–(−0.85) | Frontro-central | 340–476 | N250 to 2nd syllable in ata | |
| 4 | <0.020 | 0.80 | 0.64–2.06 | Parieto-occipital | 364–470 | N250 to 2nd syllable in ata | |
| 5 | <0.012 | −1.02 | −1.81–(−0.75) | Frontro-central | 182–364 | larger N250 for atta | |
| Long atta vs. ata | 1 | <0.0001 | 1.11 | 1.01–2.23 | Frontro-central until 372 ms then parieto-occipital | 212–546 | larger N250 for atta and P1 to 2nd syllable in atta |
| 2 | <0.0001 | −1.86 | −2.61–(−1.65) | Frontro-central | 330–534 | N250 to 2nd syllable in ata | |
| 3 | <0.002 | 0.99 | 0.46–1.13 | Frontro-central | 540–956 | N250 to 2nd syllable in atta | |
| 4 | <0.004 | −0.55 | −1.11–(−0.15) | Parieto-occipital | 528–868 | N250 to 2nd syllable in atta | |
| 5 | <0.038 | −0.37 | −0.95–0.06 | Parieto-occipital | 224–374 | larger N250 for atta |
Note. Cohen’s d and 95 % confidence intervals were calculated for the channel and time window showing maximal differences in the cluster-based permutation statistic.
Figure 2.
ERP waveforms in response to the English stimuli in the US sample of children (above; N=54) and in the Finnish sample of children (below; N=38). Black line is the response to the standard /ba/ stimulus, blue line the deviant /da/ stimulus, and red line the deviant /ga/ stimulus. ERP montage has been transformed to the standard 10-10 electrode positions and re-referenced to the average reference. Horizontal line marks 100 ms and vertical line marks 1 µV, negative voltages are plotted up.
Figure 3.
ERP waveforms in response to the Finnish stimuli in the US sample of children (above; N=44) and in the Finnish sample of children (below; N=38). Black line is the response to the standard /ata/ stimulus, blue line to the deviant intermediate /atta/ stimulus, and red line the deviant long /atta/ stimulus. ERP montage has been transformed to the standard 10-10 electrode positions and re-referenced to the average reference. Horizontal line marks 100 ms and vertical line marks 1 µV, negative voltages are plotted up.
Figure 4.
Topographic maps of the voltages in the English speech sound experiment. Time points correspond to: 298 ms = MMN, 460 ms = N4, 686 ms = LDN. The contour lines represent 0.2 µV per line, red is positive voltages, blue negative voltages.
Figure 5.
Topographic maps of the voltages in the Finnish speech sound experiment. The components correspond to time points: P1 = 160 ms, first N2 = 288/274/328 ms, second N2 = 452/520/588 ms for standard ata, deviant intermediate atta, and deviant long atta, respectively. The contour lines represent 0.5 µV per line, red is positive voltages, blue negative voltages.
Differences between Finnish and US samples
To test if there are language specific differences in the neural responses, we compared ERP amplitudes between the groups. First, the group differences were examined for the responses to the standard and deviant sounds separately in order to see processing differences at the level of exogenous responses. There were general group differences that resulted from larger responses starting with the N250 for all stimuli in the Finnish children compared to the US children, see Table 7 for a summary of these differences. When age was used as a covariate the significant amplitude differences between the groups remained.
Table 7.
Summary of the statistical Differences between the ERP responses of the Finnish (N=38) and the US children (N=54 for Ba-Da-Ga, N=44 for Ata-Atta).
| Cluster number |
p-value | Cohen’s d | 95 % confidence interval |
Topography | Time window (ms) |
Event-related potential components |
|
|---|---|---|---|---|---|---|---|
| Ba-Da-Ga | |||||||
| da | 1 | <0.0001 | 1.19 | 0.77–1.64 | Temporo-occipital | 190–712 | N250 and N4 |
| 2 | <0.002 | −0.87 | −1.17–(−0.40) | Fronto-central | 340–714 | N4/LDN | |
| 3 | <0.024 | −1.20 | −1.76–(−0.86) | Fronto-central | 194–330 | N250 | |
| ga | 1 | <0.002 | 1.26 | 1.02–2.06 | Parieto-occipital | 90–520 | P1 and N4 |
| 2 | <0.017 | −1.03 | −2.00–(−0.82) | Fronto-central | 118–334 | N250 | |
| 3 | <0.019 | −0.92 | −1.32–(−0.49) | Fronto-central | 344–660 | N4/LDN | |
| pre-da standard | 1 | <0.006 | −0.97 | −1.26–(−0.51) | Fronto-central | 178–694 | N250 and N4 |
| 2 | <0.042 | 0.77 | 0.47–1.63 | Left temporal | 196–676 | N250 and N4 | |
| pre-ga standard | 1 | <0.022 | −0.85 | −1.55–(−0.53) | Fronto-central | 180–406 | N250 |
| 2 | <0.015 | 0.87 | 0.56–1.66 | Right temporal | 180–724 | N250 and N4 | |
| Difference wave: da | 1 | not significant | - | - | |||
| Difference wave: ga | 1 | <0.043 | 0.60 | 0.18–1.00 | Parieto-occipital | 576–886 | LDN |
| Ata-Atta | |||||||
| intermediate atta | 1 | <0.0001 | −0.93 | −1.41–(−0.51) | Fronto-central | 154–998s | N250 for 1st syllable, P1, 250 for 2nd syllable |
| 2 | <0.003 | 1.17 | 0.79–1.73 | Temporo-occipital | 184–998 | N250 for 1st syllable, P1, 250 for 2nd syllable | |
| long atta | 1 | <0.005 | −1.02 | −1.14–(−0.46) | Fronto-central | 386–998 | P1, N250 for 2nd syllable |
| 2 | <0.012 | −1.18 | −1.92–(−0.89) | Fronto-central | 84–366 | P1, N250 for 1st syllable | |
| 3 | <0.019 | 1.14 | 1.06–2.34 | Temporo-occipital | 180–364 | P1, N250 for 1st syllable | |
| 4 | <0.036 | 1.01 | 0.81–2.03 | Temporo-occipital | 638–998 | N250/LDN for 2nd syllable | |
| pre intermediate atta standard | 1 | <0.0001 | −1.26 | −1.45–(−0.71) | Fronto-central | 182–892 | N250 for 1st syllable, P1, N250 for 2nd syllable |
| 2 | <0.0001 | 1.06 | 0.61–1.47 | Temporo-occipital | 168–988 | N250 for 1st syllable, P1, N250 for 2nd syllable | |
| pre long atta standard | 1 | <0.0001 | −1.32 | −1.70–(−0.86) | Fronto-central | 138–704 | N250 for 1st syllable, P1, N250 for 2nd syllable |
Note. Cohen’s d and 95 % confidence intervals were calculated for the channel and time window showing maximal differences in the cluster-based permutation statistic.
Second, the group differences were examined for the difference waves (response to the deviant minus response to the standard) in order to examine processing related to change detection mechanisms that have been linked to discrimination abilities in previous literature (e.g., Näätänen et al., 2010). When examining the difference waves, the cluster-based test showed one cluster where the Finnish and the US children differed for the response to the deviant /ga/ sound. This difference was most prominent at parieto-occipital electrodes at 576 – 886 ms (p<0.043). The US children had larger negative going responses at the parietal electrodes than the Finnish children while Finnish children had larger positive going responses at the occipital electrodes. When age was entered as a covariate to the analysis this group difference became non-significant. Therefore, it is not discussed further.
Correlations
The second goal of the study was to examine if the brain responses to common and uncommon speech sound contrasts would be linked to reading abilities. Below the associations between the amplitudes of exogenous and change detection responses are correlated with the reading measures.
Correlations to common speech sound stimuli
In the Finnish sample the ERP amplitudes for intermediate and long /atta/s showed significant correlations with phonological measures (digit span, phoneme deletion, non-word repetition) and reading accuracy, as shown in Table 8. Larger negative amplitudes were associated with better performance in the tasks. However, the scalp areas and time windows with largest correlations varied depending on the variables. There were no significant correlations between the ERP amplitudes and cognitive skill measures in the US sample for the common speech sound stimuli (/ba/, /da/, /ga/).
Table 8.
Summary of the correlations between the ERP and cognitive skill measures in the Finnish sample (N=38).
| ERP stimuli | Cognitive measure | Topography | Time window (ms) |
Pearson correlation coefficient |
95 % confiden ce interval |
p-value |
|---|---|---|---|---|---|---|
| Ga | Reading speed | Right fronto-central | 664–998 | −0.478 | −0.692–(−0.187) | <0.004 |
| Ga | Reading accuracy | Fronto-central | 2–250 | −0,617 | −0.782–(−0.370) | <0.026 |
| Ga | Phoneme deletion | Parieto-occipital | 200–370 | 0,625 | 0.381–0.787 | <0.046 |
| Da | Reading speed | Parieto-occipital | 480–722 | −0.487 | −0.698–(−0.199) | <0.036 |
| Intermediate Atta | Digit span | Fronto-central | 372–816 | −0.442 | −0.667–(−0.142) | <0.049 |
| Difference wave: Ga | Rapid automatic naming, time | Temporo-occipital | 434–662 | 0.598 | 0.345–0.771 | <0.030 |
| Difference wave: intermediate Atta | Phoneme deletion | Right parieto-occipital | 200–466 | −0.662 | −0.810–(−0.434) | <0.019 |
| Difference wave: intermediate Atta | Non-word repetition | Central | 308–746 | −0.525 | −0.723–(−0.247) | <0.010 |
| Difference wave: long Atta | Reading accuracy | Fronto-central | 514–788 | −0.557 | −0.744–(−0.289) | p<0.020 |
Correlations to uncommon speech sound stimuli
In the Finnish sample the response to the deviant /ga/ sound correlated with reading speed, accuracy, and phoneme deletion scores. For reading speed the correlation indicated faster reading speed with larger response, for reading accuracy better accuracy with smaller (more negative) amplitudes, for phoneme deletion better phoneme deletion skill with larger positive amplitudes. There was also a correlation between the response to the deviant /da/ sound and reading speed indicating faster reading speed with larger response. When difference waves were examined response to the deviant /ga/ showed correlation with RAN indicating slow rapid naming with large positive voltages. Table 8 shows again that the scalp areas and time windows with maximal correlations vary depending on the behavioral measure.
In contrast, in the US sample systematic and robust correlations were found between the responses to the uncommon speech sounds (/ata/, intermediate and long /atta/s) and reading skills (Table 9). Poor performance in GORT reading accuracy, fluency and comprehension as well as TOWRE reading tasks was linked to larger ERP amplitudes for all variables. The time windows where the correlations were observed encompassed all ERP components starting from P1 generated by the first syllable of the stimulus at the fronto-central channels and at the parieto-occipital channels starting from P1 generated by the second syllable of the stimulus.
Table 9.
Summary of the correlations between the ERP and cognitive skill measures in the US sample.
| Stimulus | Cognitive measure | Topography | Time window (ms) |
Pearson correlatio n coeffient |
95 % confiden ce interval |
cluster p-value |
|---|---|---|---|---|---|---|
| Intermediate Atta | GORT accuracy (N=34) | Fronto-central | 156–998 | 0.683 | 0.449–0.830 | <0.008 |
| Intermediate Atta | GORT accuracy (N=34) | Parieto-occipital | 412–816 | −0.557 | −0.753–(−0.270) | <0.019 |
| Intermediate Atta | GORT fluency (N=34) | Fronto-central | 136–998 | 0.618 | 0,353–0,791 | <0.014 |
| Intermediate Atta | GORT fluency (N=34) | Parieto-occipital | 412–744 | −0.538 | −0.741–(−0.245) | <0.045 |
| Intermediate Atta | GORT comprehension (N=34) | Fronto-central | 136–998 | 0.613 | 0.347–0.788 | <0.017 |
| Intermediate Atta | GORT comprehension (N=34) | Parieto-occipital | 412–744 | −0.532 | −0.737–(−0.236) | <0.047 |
| Long Atta | GORT accuracy (N=34) | Fronto-central | 120–998 | 0.701 | 0.476–0.840 | <0.001 |
| Long Atta | GORT fluency (N=34) | Fronto-central | 124–998 | 0.689 | 0.457–0.833 | <0.001 |
| Long Atta | GORT comprehension (N=34) | Fronto-central | 158–998 | 0.728 | 0.517–0.855 | <0.001 |
| Long Atta | GORT comprehension (N=34) | Right temporal | 408–910 | −0.604 | −0.782–(−0.334) | <0.048 |
| Intermediate Atta | TOWRE phonemic (N=35) | Fronto-central | 186–826 | 0.628 | 0.373–0.795 | <0.013 |
| Long Atta | TOWRE phonemic (N=35) | Fronto-central | 166–872 | 0.624 | 0.368–0.793 | <0.001 |
| Long Atta | TOWRE phonemic (N=35) | Right parieto-occipital | 158–468 | −0.609 | −0.784–(−0.347) | <0.046 |
| Intermediate Atta | TOWRE sight word (N=35) | Right parieto-occipital | 228–998 | −0.468 | −0.693–(−0.159) | <0.001 |
| Intermediate Atta | TOWRE sight word (N=35) | Fronto-central | 182–990 | 0.569 | 0.291–0.759 | <0.002 |
| Long Atta | TOWRE sight word (N=35) | Fronto-central | 70–430 | 0.636 | 0.385–0.800 | <0.005 |
| Long Atta | TOWRE sight word (N=35) | Fronto-central | 448–902 | 0.464 | 0.155–0.690 | <0.010 |
| Long Atta | TOWRE sight word (N=35) | Right parieto-occipital | 116–604 | −0.657 | −0.813–(−0.415) | <0.018 |
| Long Atta | TOWRE sight word (N=35) | Right temporal | 564–996 | −0.546 | −0.744–(−0.260) | <0.036 |
| Difference wave: Intermediate Atta | GORT accuracy (N=34) | Fronto-central | 242–998 | 0.726 | 0.515–0.855 | <0.003 |
| Difference wave: Intermediate Atta | GORT accuracy (N=34) | Parieto-occipital | 240–662 | −0.738 | −0.861–(−0.533) | <0.003 |
| Difference wave: Intermediate Atta | GORT fluency (N=34) | Fronto-central | 242–998 | 0.494 | 0.187–0.713 | <0.002 |
| Difference wave: Intermediate Atta | GORT fluency (N=34) | Parieto-occipital | 240–662 | −0.692 | −0.835–(−0.462) | <0.024 |
| Difference wave: Intermediate Atta | GORT comprehension (N=34) | Fronto-central | 2–998 | 0.697 | 0.470–0.838 | <0.002 |
| Difference wave: Intermediate Atta | GORT comprehension (N=34) | Parieto-occipital | 40–672 | −0.627 | −0.796–(−0.366) | <0.003 |
| Difference wave: Long Atta | GORT accuracy (N=34) | Fronto-central - parieto-occipital | 320–998 | 0.497 | 0.191–0.715 | <0.005 |
| Difference wave: Long Atta | GORT accuracy (N=34) | Frontal - fronto-central-occipital | 34–354 | 0.601 | 0.330–0.781 | <0.036 |
| Difference wave: Long Atta | GORT fluency (N=34) | Fronto-central | 314–998 | 0.533 | 0.237–0.738 | <0.005 |
| Difference wave: Long Atta | GORT fluency (N=34) | Parieto-occipital | 36–354 | 0.679 | 0.442–0.827 | <0.036 |
| Difference wave: Long Atta | GORT comprehension (N=34) | Fronto-central | 298–998 | 0.731 | 0.522–0.857 | <0.005 |
| Difference wave: Long Atta | GORT comprehension (N=34) | Parieto-occipital | 408–998 | −0.580 | −0.768–(−0.301) | <0.003 |
| Difference wave: Intermediate Atta | TOWRE sight word (N=35) | Parieto-occipital | 280–554 | −0.586 | −0.769–(−0.314) | <0.013 |
| Difference wave: Intermediate Atta | TOWRE sight word (N=35) | Parieto-occipital | 658–998 | −0.561 | −0.754–(−0.281) | <0.015 |
| Difference wave: Intermediate Atta | TOWRE sight word (N=35) | Left fronto-central | 324–748 | 0.484 | 0.180–0.704 | <0.030 |
| Difference wave: Long Atta | TOWRE sight word (N=35) | Fronto-central - parieto-occipital | 464–998 | 0.258 | −0.082–0.545 | <0.002 |
Note. Correlation coefficient and 95 % confidence intervals are calculated from the maximal channel at the time window indicated by the cluster-based permutation statistic.
The difference waves showed similar correlation pattern for GORT reading accuracy, fluency and comprehension: the larger the amplitude the poorer the reading starting from the MMN time window or even earlier. Correlations were also found between the difference wave amplitudes and TOWRE sight word and phonemic reading skills. Difference wave for the intermediate /atta/ correlated significantly with TOWRE sight word reading and had three correlation clusters: parieto-occipital channels at 280–554 ms, the more negative the voltage the better the reading score; parieto-occipital channels at 658–998 ms, the more negative the voltage the better the reading score; left fronto-central channels at 324–748 ms, the more positive the voltage the better the reading score. Difference wave for the long deviant /atta/ correlated significantly with TOWRE sight word reading score showing a variable topography for the maximal correlations depending on the latency: starting at the fronto-central channels at 464 ms changing to parieto-occipital channels at 800 ms and continuing until 998 ms, the more positive the voltage the better the reading score.
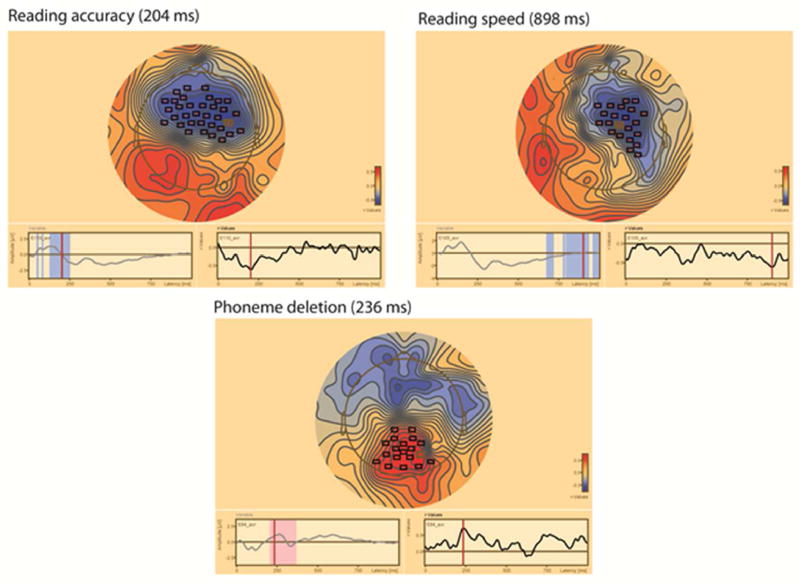
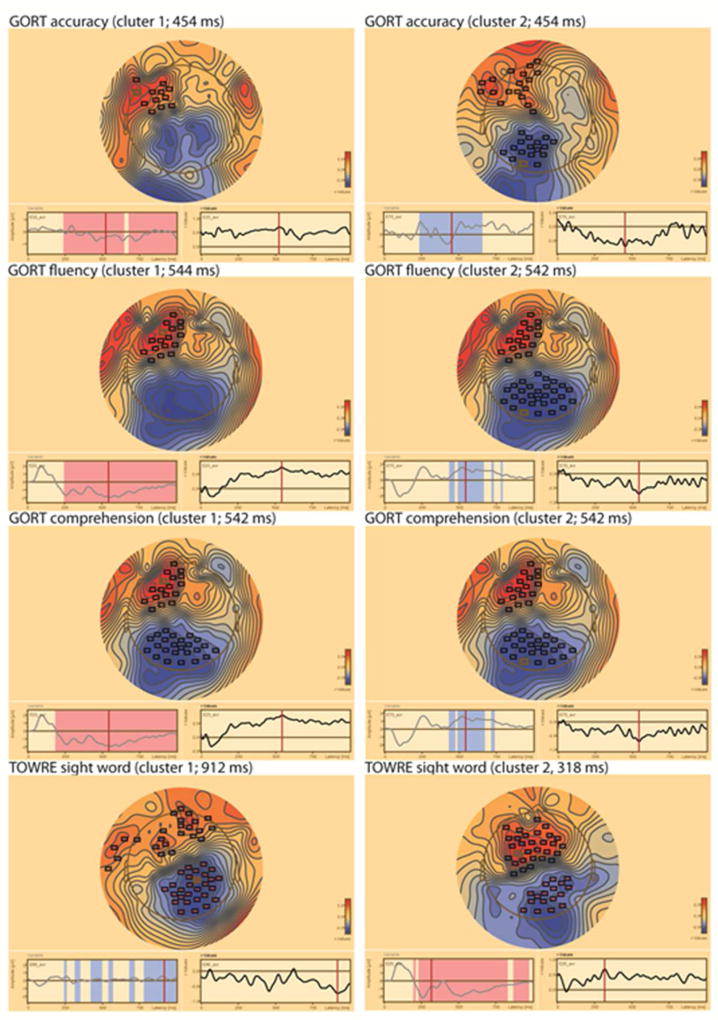
See Figures 6 and 7 for examples of the correlation coefficient topographies between the ERP measures and cognitive skill test scores.
Figure 6.
Topographic distribution of the correlation coefficient values between the deviant /ga/ stimulus and cognitive measures in the Finnish sample (N=38). Channel clusters with statistically significant values are marked with boxes in the topography. In the topography plot, red color denotes positive coefficients and blue negative coefficients. Topography is plotted at the highest coefficient values (time point indicated in parenthesis after the cognitive measure). Left bottom: Time windows associated with the channel cluster are marked with red and blue on the ERP waveform from the channel with maximal correlation. Right bottom: correlation coefficients for each time point at the maximal channel.
Figure 7.
Topographic distribution of the correlation coefficient values between the intermediate deviant /atta/ stimulus and reading test scores, for TOWRE (N=35), and for GORT (N=34) in the US sample. Channel clusters with statistically significant values (1st cluster at left column, 2nd cluster at right column) are marked with boxes. In the topography plot, red denotes positive coefficients and blue negative coefficients. Topography is plotted at the highest coefficient values (time point indicated in parenthesis after the reading test name). Left bottom: Time windows associated with the channel cluster are marked with red and blue on the ERP waveform from the channel with maximal correlation. Right bottom: correlation coefficients for each time point at the maximal channel.
Discussion
We set out to examine whether exposure to one’s native language would result in differential brain responses in school-age children, and whether these responses would be associated with reading skills as predicted by research on dyslexia and phonological processing. The Finnish and the US children differed in ERP amplitude for all stimulus types with the Finnish sample having larger responses. However, after including age as a covariate in our model, no group differences were observed for the difference waves for the Finnish or English stimuli indicating that long-term language exposure did not cause robust differences in ERP responses related to detection of speech sound changes. Critically, associations between the ERPs and reading measures in both the Finnish and the US children were found, mainly to the ERPs elicited by the speech sounds that were uncommon in the native language.
Exposure to native language was hypothesized to enhance the processing of the speech sounds common in each language. This was not found in the current study. There might be two possible explanations for the lack of clear group differences for change detection responses. First, the Finnish children had studied English at school already for two years and have most likely been exposed to English in the environment. In addition, although the /ba/-/da/-/ga/ contrasts do not form semantically distinctive minimal word pairs in Finnish, all of these stop consonants are part of the Finnish phonology. Second, the Finnish speech sound contrast with a stop consonant length change (as in /ata/-/atta/) involves a shift in the major energy peak in the stimulus. This leads to large differences in afferent activation and obligatory responses between the stimuli and therefore makes the examination of the difference wave between the short /ata/ and the long /atta/ sounds difficult to interpret. The heavy overlap with the obligatory responses could diminish the native language effect on the discriminatory ERP responses.
We also hypothesized that the ERP responses elicited by speech processing would be associated with phonological skills and reading skills based on theories on impaired reading skills (e.g., Elbro, 1998; Elbro & Jensen, 2005; Espy et al., 2004; Mody et al., 1997). In the current study, the correlations between the ERP responses and cognitive test scores, mainly reading scores, were most robust in the US sample and for the ERP responses to the stimulus contrasts that are uncommon to English (phoneme length). The larger the responses to the uncommon speech stimulus the poorer were the reading skills. This would be in line with theories on longer-term phonological representations being important for reading acquisition and development (e.g., Vellutino et al., 2004) and with the effects of perceptual narrowing to native speech sounds during the first year of life (Tsao et al., 2004; Kuhl et al., 2008). Less efficient perceptual narrowing to the native speech sounds has been shown to be correlated with poorer later language skills (Tsao et al., 2004; Kuhl et al., 2008) and increased discrimination accuracy of non-native speech sound contrasts to be linked with reading problems (Serniclaes et al., 2004). Therefore, in the current study, it is likely that the larger ERP responses to the speech sound contrasts uncommon to English would reflect poorly developed longer-term phonological representations in children.
The Finnish sample showed also association between the ERP measures and phonological measures for both types of speech stimuli (common and uncommon to Finnish). Interestingly the correlation between the cognitive measures and the ERP amplitudes to the phonemic length contrasts was opposite in the Finnish sample compared to the US sample. This suggests that better change detection of native language features (i.e., larger ERP amplitude) would be linked to better reading and reading-related cognitive skills whereas better change detection of non-native language features would be linked to poorer reading skills. On the other hand, in the Finnish children the direction of the association for the voiced stop consonant contrasts that are uncommon in the Finnish language was opposite to that found for the US children: the Finnish children showed better cognitive performance with larger change detection responses for /ba/-/ga/ contrast. The time window of the correlation seemed to correspond to that of MMN (phoneme deletion) and LDN (reading speed), but the topography pattern of the correlations were not typical for the MMN response. For the Finnish children, at the age of 12 years there could already be many intervening variables, for example exposure to English language via TV, music, internet, gaming and school, affecting the ERP amplitudes that could obscure the effects of the English stimuli as uncommon sounds and therefore also affect the associations with cognitive skill measures. Also, the English stimulus contrasts used are a part of the Finnish phonology, though they occur relatively rarely, and therefore might not be processed as non-native. These could have had an effect on the different correlation pattern between the US and Finnish samples.
The orthographic differences between Finnish and English could also affect the strength of the associations between the ERP measures and reading skills. The reading processes in the transparent Finnish language could rely less on phonological processes than in the opaque English language (Ziegler et al., 2010). This is reflected, for example, in previous results showing stronger association between phonological skills and reading in opaque orthographies than in transparent orthographies particularly at later school age (e.g., Georgiou et al., 2008; Ziegler et al., 2010). It is possible that the ERP measures are more closely linked to phonological abilities and therefore stronger associations between ERP measures and reading were found in the English speaking children.
Most previous studies that have found associations between ERP measures and reading or reading-related skills have examined individuals with dyslexia and/or using sounds from only one language (e.g., Hämäläinen et al., 2013; Lohvansuu et al., 2014; Schulte-Körne et al., 1998). Other studies examining typically developing children have also found associations between ERP responses to non-linguistic sounds measured in young children and reading skills at school-age (Espy et al., 2004) and ERP responses to native speech sounds, phonological skills and prereading skills in kindergarten children (Kuuluvainen et al., 2016). Also, associations between infant ERPs to non-speech sounds and later language skills have been observed in both typically developing children and children at risk for language problems (Choudhury & Benasich, 2011). These studies suggest that the associations between ERP responses and reading related skills are more readily observable for native speech sound contrasts earlier in development than at school-age. This could be due to the ongoing changes of the phonological representations that can be larger at younger ages than in the older school-age children.
The majority of the significant correlations were found for the deviant sounds in wide time windows encompassing both the obligatory P1 and N250 responses as well as the change detection responses MMN and LDN. However, no associations were found between the ERP responses to the standard sounds and cognitive skill measures. This suggests that general level encoding, not just change detection and sound discrimination related processes, of the rarely presented speech sounds is associated with reading skills. This was somewhat unexpected because previous studies suggest that particularly the MMN and LDN responses would be sensitive to exposure to different languages (e.g., Jakoby et al., 2011; Näätänen et al., 1997; Shestakova et al., 2003; Winkler et al., 1999). However, most previous studies have not examined the associations between MMN, LDN and reading ability as continuous variables or have examined only the effect of language exposure, and not reading skills, on these ERPs. Previous studies on ERP responses in individuals with dyslexia, on the other hand, have also shown associations between obligatory N250 responses and reading or pre-reading skills in children (Hämäläinen et al., 2013; Hämäläinen et al., 2015) and N1 response and reading in adults (Helenius et al., 2002).
In order to specifically examine the contribution of MMN and LDN in the correlations, the obligatory responses should be controlled for using reversed standard and deviant probabilities or a mixture of stimuli occurring with equal probabilities to the deviant stimuli in the oddball experiment (e.g., Jacobsen & Schröger, 2001; Lohvansuu et al., 2013; Schröger & Wolff, 1996). Due to time limitations and endurance of the child participants such control experiments were not carried out in the current study.
An additional interesting finding was the larger responses of the Finnish children compared to the US children for all of the stimuli. This enhancement was particularly prominent at the N250 time window while it was not present at the P1 time window. This indicates that the cause for the larger responses is not related to technical issues in the EEG measurements but to differences in the two samples. The cause for the larger responses cannot, however, be solved based on the variables available from the current datasets.
There were two differences between the samples that could have affected the results. First, the Finnish children were older than the US children. However, age covariate did not eliminate the group differences, and therefore it is unlikely to be the primary cause for the larger ERPs in the US children. Second, despite the same analysis pipeline for both of the datasets there were more trials left in the Finnish data than in the US data. Poorer signal-to-noise ratio usually leads to larger ERP responses, but here the Finnish children had larger responses and slightly better signal-to-noise ratio based on the trial numbers and therefore this is an unlikely explanation for the results.
Overall, our results show that processing of uncommon speech sound contrasts with respect to native language is associated with reading skills. This is in line with earlier studies on young children showing that less efficient specialization to the native language can be associated with poorer language skills (Tsao et al., 2004; Kuhl et al., 2008) and with reading problems (Serniclaes et al., 2004). Our results also support the link between speech perception, phonological skills and reading skills particularly in opaque orthographies.
Figure 1.
Waveforms and spectrograms of the English (above) and Finnish (below) stimuli used in the experiments.
Acknowledgments
This study was supported by the Academy of Finland (profiling action “Multilete” #292 466), European Union H2020 MSCA-ITN-2014-ETN Programme, “Advancing brain research in children’s developmental neurocognitive disorders” -project (ChildBrain, #641652), NIH grants: P01 HD HD001994 “Nature and acquisition of the speech code and reading”, PI: C. Fowler; R01 HD 48830 Neurobiological Foundations of Reading (Dis)ability, PI: K. Pugh R03 HD053409 & R03 HD053409 “Neurocognitive development in RD children with/without general cognitive deficits”, PI: N. Landi.
References
- Adams MJ. Beginning to read: Thinking and learning about print. MIT press; 1994. [Google Scholar]
- Anthony JL, Francis DJ. Development of phonological awareness. Current Directions in Psychological Science. 2005;14:255–259. [Google Scholar]
- Barry JG, Hardiman MJ, Bishop DV. Mismatch response to polysyllabic nonwords: A neurophysiological signature of language learning capacity. PloS one. 2009;4:e6270. doi: 10.1371/journal.pone.0006270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Best CC, McRoberts GW. Infant perception of non-native consonant contrasts that adults assimilate in different ways. Language and Speech. 2003;46:183–216. doi: 10.1177/00238309030460020701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop DVM. Using mismatch negativity to study central auditory processing in developmental language and literacy impairments: where are we, and where should we be going? Psychological Bulletin. 2007;133:651. doi: 10.1037/0033-2909.133.4.651. [DOI] [PubMed] [Google Scholar]
- Bonte ML, Poelmans H, Blomert L. Deviant neurophysiological responses to phonological regularities in speech in dyslexic children. Neuropsychologia. 2007;45:1427–1437. doi: 10.1016/j.neuropsychologia.2006.11.009. [DOI] [PubMed] [Google Scholar]
- Bradley L, Bryant P. Difficulties in auditory organisation as a possible cause of reading backwardness. Nature. 1978;271:746–747. doi: 10.1038/271746a0. [DOI] [PubMed] [Google Scholar]
- Čeponienė R, Alku P, Westerfield M, Torki M, Townsend J. ERPs differentiate syllable and nonphonetic sound processing in children and adults. Psychophysiology. 2005;42:391–406. doi: 10.1111/j.1469-8986.2005.00305.x. [DOI] [PubMed] [Google Scholar]
- Cheour M, Korpilahti P, Martynova O, Lang AH. Mismatch negativity and late discriminative negativity in investigating speech perception and learning in children and infants. Audiology and Neurotology. 2001;6:2–11. doi: 10.1159/000046804. [DOI] [PubMed] [Google Scholar]
- Choudhury N, Benasich AA. Maturation of auditory evoked potentials from 6 to 48 months: prediction to 3 and 4 year language and cognitive abilities. Clinical Neurophysiology. 2011;122:320–338. doi: 10.1016/j.clinph.2010.05.035. [DOI] [PubMed] [Google Scholar]
- Conboy BT, Rivera-Gaxiola M, Silva-Pereyra J, Kuhl PK. Event-related potential studies of early language processing at the phoneme, word, and sentence levels. Early Language Development. 2008;5:23–64. [Google Scholar]
- Denckla MB, Rudel RG. Rapid ‘automatized’ naming (RAN): Dyslexia differentiated from other learning disabilities. Neuropsychologia. 1976;14:471–479. doi: 10.1016/0028-3932(76)90075-0. [DOI] [PubMed] [Google Scholar]
- Eklund K, Torppa M, Aro M, Leppänen PH, Lyytinen H. Literacy Skill Development of Children With Familial Risk for Dyslexia Through Grades 2, 3, and 8. Journal of Educational Psychology. 2015;107:126–140. [Google Scholar]
- Elbro C. When reading is “readn” or somthn. Distinctness of phonological representations of lexical items in normal and disabled readers. Scandinavian Journal of Psychology. 1998;39:149–153. doi: 10.1111/1467-9450.393070. [DOI] [PubMed] [Google Scholar]
- Elbro C, Jensen MN. Quality of phonological representations, verbal learning, and phoneme awareness in dyslexic and normal readers. Scandinavian Journal of Psychology. 2005;46:375–384. doi: 10.1111/j.1467-9450.2005.00468.x. [DOI] [PubMed] [Google Scholar]
- Espy KA, Molfese DL, Molfese VJ, Modglin A. Development of auditory event-related potentials in young children and relations to word-level reading abilities at age 8 years. Annals of Dyslexia. 2004;54:9–38. doi: 10.1007/s11881-004-0002-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friederici AD. Neurophysiological markers of early language acquisition: from syllables to sentences. Trends in Cognitive Sciences. 2005;9:481–488. doi: 10.1016/j.tics.2005.08.008. [DOI] [PubMed] [Google Scholar]
- Georgiou GK, Parrila R, Papadopoulos TC. Predictors of word decoding and reading fluency across languages varying in orthographic consistency. Journal of Educational Psychology. 2008;100(3):566. [Google Scholar]
- Goswami U, Bryant P. Phonological skills and learning to read. Hove: Lawrence Erlbaum; 1990. [Google Scholar]
- Guttorm TK, Leppänen PHT, Hämäläinen JA, Eklund KM, Lyytinen H. Newborn event-related potentials predict poorer pre-reading skills in children at risk for dyslexia. Journal of Learning Disabilities. 2010;43:391–401. doi: 10.1177/0022219409345005. [DOI] [PubMed] [Google Scholar]
- Guttorm TK, Leppänen PHT, Poikkeus AM, Eklund KM, Lyytinen P, Lyytinen H. Brain event-related potentials (ERPs) measured at birth predict later language development in children with and without familial risk for dyslexia. Cortex. 2005;41:291–303. doi: 10.1016/s0010-9452(08)70267-3. [DOI] [PubMed] [Google Scholar]
- Guttorm TK, Leppänen PHT, Richardson U, Lyytinen H. Event-related potentials and consonant differentiation in newborns with familial risk for dyslexia. Journal of Learning Disabilities. 2001;34:534–544. doi: 10.1177/002221940103400606. [DOI] [PubMed] [Google Scholar]
- Helenius P, Salmelin R, Richardson U, Leinonen S, Lyytinen H. Abnormal auditory cortical activation in dyslexia 100 msec after speech onset. Journal of Cognitive Neuroscience. 2002;14:603–617. doi: 10.1162/08989290260045846. [DOI] [PubMed] [Google Scholar]
- Hämäläinen JA, Leppänen PHT, Eklund K, Thomson J, Richardson U, Guttorm TK, Lyytinen H. Common variance in amplitude envelope perception tasks and their impact on phoneme duration perception and reading and spelling in Finnish children with reading disabilities. Applied Psycholinguistics. 2009;30:511–530. [Google Scholar]
- Hämäläinen JA, Guttorm TK, Richardson U, Alku P, Lyytinen H, Leppänen PHT. Auditory Event-Related Potentials Measured in Kindergarten Predict Later Reading Problems at School Age. Developmental neuropsychology. 2013;38:550–566. doi: 10.1080/87565641.2012.718817. [DOI] [PubMed] [Google Scholar]
- Hämäläinen JA, Lohvansuu K, Ervast L, Leppänen PH. Event-related potentials to tones show differences between children with multiple risk factors for dyslexia and control children before the onset of formal reading instruction. International Journal of Psychophysiology. 2015;95:101–112. doi: 10.1016/j.ijpsycho.2014.04.004. [DOI] [PubMed] [Google Scholar]
- Häyrinen T, Serenius-Sirve S, Korkman M. Lukilasse [Lukilasse-test battery for screening reading, spelling, and arithmetics] Helsinki: Psykologien Kustannus; 1999. [Google Scholar]
- Helenius P, Salmelin R, Service E, Connolly JF, Leinonen S, Lyytinen H. Cortical activation during spoken-word segmentation in nonreading-impaired and dyslexic adults. Journal of Neuroscience. 2002;22:2936–2944. doi: 10.1523/JNEUROSCI.22-07-02936.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobsen T, Schröger E. Is there pre-attentive memory-based comparison of pitch? Psychophysiology. 2001;38:723–727. [PubMed] [Google Scholar]
- Jakoby H, Goldstein A, Faust M. Electrophysiological correlates of speech perception mechanisms and individual differences in second language attainment. Psychophysiology. 2011;48:1517–1531. doi: 10.1111/j.1469-8986.2011.01227.x. [DOI] [PubMed] [Google Scholar]
- Kirmse U, Ylinen S, Tervaniemi M, Vainio M, Schröger E, Jacobsen T. Modulation of the mismatch negativity (MMN) to vowel duration changes in native speakers of Finnish and German as a result of language experience. International Journal of Psychophysiology. 2008;67:131–143. doi: 10.1016/j.ijpsycho.2007.10.012. [DOI] [PubMed] [Google Scholar]
- Korkman M, Kirk U, Kemp S. NEPSY - lasten neuropsykologinen tutkimus. Psykologien Kustannus Oy; Helsinki: 1998. [Google Scholar]
- Kuhl PK. Early language acquisition: cracking the speech code. Nature Reviews Neuroscience. 2004;5:831–843. doi: 10.1038/nrn1533. [DOI] [PubMed] [Google Scholar]
- Kuhl PK, Tsao FM, Liu HM. Foreign-language experience in infancy: Effects of short-term exposure and social interaction on phonetic learning. Proceedings of the National Academy of Sciences. 2003;100:9096–9101. doi: 10.1073/pnas.1532872100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhl PK, Conboy BT, Coffey-Corina S, Padden D, Rivera-Gaxiola M, Nelson T. Phonetic learning as a pathway to language: new data and native language magnet theory expanded (NLM-e) Philosophical Transactions of the Royal Society of London B: Biological Sciences. 2008;363:979–1000. doi: 10.1098/rstb.2007.2154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhl PK. Brain mechanisms in early language acquisition. Neuron. 2010;67:713–727. doi: 10.1016/j.neuron.2010.08.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuuluvainen S, Alku P, Makkonen T, Lipsanen J, Kujala T. Cortical speech and non-speech discrimination in relation to cognitive measures in preschool children. European Journal of Neuroscience. 2016;43:738–750. doi: 10.1111/ejn.13141. [DOI] [PubMed] [Google Scholar]
- Landerl K, Ramus F, Moll K, Lyytinen H, Leppänen PH, Lohvansuu K, Kunze S. Predictors of developmental dyslexia in European orthographies with varying complexity. Journal of Child Psychology and Psychiatry. 2013;54:686–694. doi: 10.1111/jcpp.12029. [DOI] [PubMed] [Google Scholar]
- Leppänen PH, Pihko E, Eklund KM, Lyytinen H. Cortical responses of infants with and without a genetic risk for dyslexia: II. Group effects. Neuroreport. 1999;10:969–973. doi: 10.1097/00001756-199904060-00014. [DOI] [PubMed] [Google Scholar]
- Leppänen PHT, Richardson U, Pihko E, Eklund KM, Guttorm TK, Aro M, Lyytinen H. Brain responses to changes in speech sound durations differ between infants with and without familial risk for dyslexia. Developmental Neuropsychology. 2002;22:407–422. doi: 10.1207/S15326942dn2201_4. [DOI] [PubMed] [Google Scholar]
- Lindeman J. Allu-Ala-asteen lukutesti [Reading test for primary school] Turku, Finland: University of Turku; 1998. [Google Scholar]
- Lohvansuu K, Hämäläinen JA, Tanskanen A, Bartling J, Bruder J, Honbolygó F, Leppänen PH. Separating mismatch negativity (MMN) response from auditory obligatory brain responses in school-aged children. Psychophysiology. 2013;50:640–652. doi: 10.1111/psyp.12048. [DOI] [PubMed] [Google Scholar]
- Lohvansuu K, Hämäläinen JA, Tanskanen A, Ervast L, Heikkinen E, Lyytinen H, Leppänen PHT. Enhancement of brain event-related potentials to speech sounds is associated with compensated reading skills in dyslexic children with familial risk for dyslexia. International Journal of Psychophysiology. 2014;94:298–310. doi: 10.1016/j.ijpsycho.2014.10.002. [DOI] [PubMed] [Google Scholar]
- Maris E, Oostenveld R. Nonparametric statistical testing of EEG-and MEG-data. Journal of neuroscience methods. 2007;164:177–190. doi: 10.1016/j.jneumeth.2007.03.024. [DOI] [PubMed] [Google Scholar]
- Maurer U, Bucher K, Brem S, Brandeis D. Altered responses to tone and phoneme mismatch in kindergartners at familial dyslexia risk. Neuroreport. 2003;14:2245–2250. doi: 10.1097/00001756-200312020-00022. [DOI] [PubMed] [Google Scholar]
- McBride-Chang C. Phonological processing, speech perception and reading disability: An integrative review. Educational Psychologist. 1995;30:109–121. [Google Scholar]
- Melby-Lervåg M, Lyster SAH, Hulme C. Phonological skills and their role in learning to read: a meta-analytic review. Psychological Bulletin. 2012;138:322. doi: 10.1037/a0026744. [DOI] [PubMed] [Google Scholar]
- Mody M, Studdert-Kennedy M, Brady S. Speech perception deficits in poor readers: auditory processing or phonological coding? Journal of Experimental Child Psychology. 1997;64:199–231. doi: 10.1006/jecp.1996.2343. [DOI] [PubMed] [Google Scholar]
- Näätänen R, Lehtokoski A, Lennest M, Luuki A, Alliki J, Sinkkonen J, Alho K. Language-specific phoneme representations revealed by electric and magnetic brain responses. Nature. 1997;385:432–434. doi: 10.1038/385432a0. [DOI] [PubMed] [Google Scholar]
- Näätänen R, Astikainen P, Ruusuvirta T, Huotilainen M. Automatic auditory intelligence: An expression of the sensory–cognitive core of cognitive processes. Brain research reviews. 2010;64:123–136. doi: 10.1016/j.brainresrev.2010.03.001. [DOI] [PubMed] [Google Scholar]
- Parviainen T, Helenius P, Poskiparta E, Niemi P, Salmelin R. Speech perception in the child brain: Cortical timing and its relevance to literacy acquisition. Human Brain Mapping. 2011;32:2193–2206. doi: 10.1002/hbm.21181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puolakanaho A, Ahonen T, Aro M, Eklund K, Leppänen PH, Poikkeus AM, Lyytinen H. Developmental links of very early phonological and language skills to second grade reading outcomes: Strong to accuracy but only minor to fluency. Journal of Learning Disabilities. 2008;41:353–70. doi: 10.1177/0022219407311747. [DOI] [PubMed] [Google Scholar]
- Richardson U, Leppänen PH, Leiwo M, Lyytinen H. Speech perception of infants with high familial risk for dyslexia differ at the age of 6 months. Developmental neuropsychology. 2003;23:385–397. doi: 10.1207/S15326942DN2303_5. [DOI] [PubMed] [Google Scholar]
- Schulte-Korne G, Bruder J. Clinical neurophysiology of visual and auditory processing in dyslexia: A review. Clinical Neurophysiology. 2010;121:1794–1809. doi: 10.1016/j.clinph.2010.04.028. [DOI] [PubMed] [Google Scholar]
- Schulte-Körne G, Deimel W, Bartling J, Remschmidt H. Auditory processing and dyslexia: evidence for a specific speech processing deficit. Neuroreport. 1998;9:337–340. doi: 10.1097/00001756-199801260-00029. [DOI] [PubMed] [Google Scholar]
- Schröger E, Wolff C. Mismatch response of the human brain to changes in sound location. NeuroReport. 1996;7:3005–3008. doi: 10.1097/00001756-199611250-00041. [DOI] [PubMed] [Google Scholar]
- Serniclaes W, Van Heghe S, Mousty P, Carré R, Sprenger-Charolles L. Allophonic mode of speech perception in dyslexia. Journal of Experimental Child Psychology. 2004;87:336–361. doi: 10.1016/j.jecp.2004.02.001. [DOI] [PubMed] [Google Scholar]
- Seymour PH, Aro M, Erskine JM. Foundation literacy acquisition in European orthographies. British Journal of Psychology. 2003;94:143–174. doi: 10.1348/000712603321661859. [DOI] [PubMed] [Google Scholar]
- Share DL. On the Anglocentricities of current reading research and practice: the perils of overreliance on an" outlier" orthography. Psychological bulletin. 2008;134:584. doi: 10.1037/0033-2909.134.4.584. [DOI] [PubMed] [Google Scholar]
- Shestakova A, Huotilainen M, Ceponiene R, Cheour M. Event-related potentials associated with second language learning in children. Clinical Neurophysiology. 2003;114:1507–1512. doi: 10.1016/s1388-2457(03)00134-2. [DOI] [PubMed] [Google Scholar]
- Schulte-Körne G, Deimel W, Bartling J, Remschmidt H. Auditory processing and dyslexia: evidence for a specific speech processing deficit. Neuroreport. 1998;9:337–340. doi: 10.1097/00001756-199801260-00029. [DOI] [PubMed] [Google Scholar]
- Torgeson JK, Wagner RK, Rashotte CA. Test of Word Reading Efficiency (TOWRE) Austin, TX: ProEd; 1999. [Google Scholar]
- Tsao FM, Liu HM, Kuhl PK. Speech perception in infancy predicts language development in the second year of life: A longitudinal study. Child development. 2004;75:1067–1084. doi: 10.1111/j.1467-8624.2004.00726.x. [DOI] [PubMed] [Google Scholar]
- Vellutino FR, Fletcher JM, Snowling MJ, Scanlon DM. Specific reading disability (dyslexia): what have we learned in the past four decades? Journal of child psychology and psychiatry. 2004;45:2–40. doi: 10.1046/j.0021-9630.2003.00305.x. [DOI] [PubMed] [Google Scholar]
- Wagner RK, Torgesen JK. The nature of phonological processing and its causal role in the acquisition of reading skills. Psychological Bulletin. 1987;101:192–212. [Google Scholar]
- Wagner RK, Torgesen JK, Rashotte CA. Comprehensive Test of Phonological Processing: CTOPP. Austin, TX: Pro-Ed; 1999. [Google Scholar]
- Wechsler D. WISC-III: Wechsler intelligence scale for children: Manual. Psychological Corporation; 1991. [Google Scholar]
- Winkler I, Lehtokoski A, Alku P, Vainio M, Czigler I, Csepe V, Iivonen A. Pre-attentive detection of vowel contrasts utilizes both phonetic and auditory memory representations. Cognitive Brain Research. 1999;7:357–369. doi: 10.1016/s0926-6410(98)00039-1. [DOI] [PubMed] [Google Scholar]
- Ziegler JC, Bertrand D, Toth D, Csepe V, Reis A, Faisca L, Blomert L. Orthographic depth and its impact on universal predictors of reading: A cross-language investigation. Psychological Science. 2010;21:551–559. doi: 10.1177/0956797610363406. [DOI] [PubMed] [Google Scholar]
- van Zuijen TL, Plakas A, Maassen BA, Maurits NM, van der Leij A. Infant ERPs separate children at risk of dyslexia who become good readers from those who become poor readers. Developmental science. 2013;16:554–563. doi: 10.1111/desc.12049. [DOI] [PubMed] [Google Scholar]